Screening for Elevated Blood Lead Levels in Children: Assessment of Criteria and a Proposal for New Ones in France
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Data Collection
2.2. Statistical Analyses
2.2.1. Sensitivity and Specificity of the Current Lead Risk Questionnaire
2.2.2. Logistic Regression Modeling
2.2.3. Performance Evaluation of Multivariable Models
2.2.4. Internal Validation of Performance of the Better Model
3. Results
3.1. Sensitivity and Specificity of the Current Lead Risk Questionnaire in Identifying BLLs Higher than 50 µg/L
| Self-Reported Exposure Questions | Observed Responses in Saturn-Inf Sample (n = 3831) * | Estimated Prevalence in French Children <7 Years (N = 5102,537) | Observed Prevalence of BLLs ≥ 50 µg/L in Saturn-Inf Sample (n = 67) * | |
|---|---|---|---|---|
| n | % (95% CI) | n | % | |
| Child recently arrived in France (<1 year) | ||||
| No | 3802 | 99.8 (99.6; 99.9) | 65 | 98.5 |
| Yes | 10 | 0.2 (0.1; 0.4) | 1 | 1.5 |
| Child residing with someone who has been lead-poisoned | ||||
| No | 3333 | 88.0 (86.0; 89.7) | 59 | 88.1 |
| Yes | 3 | 0.04 (0.01; 0.1) | 0 | 0 |
| Don’t know | 436 | 12.0 (10.3; 13.9) | 8 | 10.9 |
| Adult occupation involves lead exposure | ||||
| No | 2778 | 86.8 (84.9; 88.5) | 42 | 82.3 |
| Yes | 461 | 13.2 (11.5; 15.0) | 9 | 17.6 |
| Child drinking tap water where housing has lead pipes | ||||
| No | 1770 | 49.4 (45.5; 53.8) | 23 | 34.3 |
| Yes | 66 | 2.2 (1.3; 3.6) | 4 | 6,0 |
| Don’t know | 1928 | 48.4 (44.8; 51.9) | 40 | 59.7 |
| Child living in pre-1949 housing with peeling paint inside | ||||
| No | 2465 | 71.8 (65.5; 78.6) | 36 | 57.1 |
| Yes | 163 | 4.8 (3.6; 6.2) | 5 | 7.9 |
| Don’t know | 1064 | 23.4 (20.2; 27.0) | 22 | 34.9 |
| Child living in pre-1949 housing with recent renovation work inside | ||||
| No | 2379 | 70.5 (62.6; 79.2) | 33 | 53.2 |
| Yes | 290 | 7.5 (6.1; 9.4) | 9 | 14.5 |
| Don’t know | 972 | 22.0 (19.1; 25.0) | 20 | 32.3 |
| Child living in pre-1949 housing and having tendency to scrape off or nibble paint | ||||
| No | 2589 | 75.5 (68.7; 82.6) | 36 | 55.4 |
| Yes | 46 | 0.9 (0.6; 1.5) | 5 | 7.7 |
| Don’t know | 1084 | 23.6 (20.4; 27.3) | 24 | 36.9 |
| TP | FP | FN | TN | Missing Data (Don’t Knows and Missing) | Sensitivity (95%CI) * | Specificity (95%CI) * |
|---|---|---|---|---|---|---|
| 23 | 831 | 17 | 1402 | 1558 | 0.51 (0.26; 0.75) | 0.66 (0.62; 0.70) |
3.2. Multivariable Predictive Models
| Self-Report Exposure Questions | Observed Cases in BLLs ≥ 44 µg/L (n = 107) | Estimated Prevalence (%) in BLLs ≥ 44 µg/L | OR | 95% CI | ||
|---|---|---|---|---|---|---|
| Child recently arrived in France (<1 year) | 2 | 2.3 | 11.6 | 0.3 | 455.5 | |
| Child having tendency to scrape off or nibble paint | 16 | 11.6 | 0.7 | 0.2 | 2.7 | |
| Adult occupation involves lead exposure | 16 | 7.6 | 0.6 | 0.3 | 1.4 | |
| Lead pipes | Type of drinking water | |||||
| No | Bottled water | 24 | 29.2 | Reference level | ||
| No | Tap water | 19 | 28.1 | 0.8 | 0.3 | 2.0 |
| Yes | Bottled water | 2 | 0.5 | 0.1 | 0 | 1.1 |
| Yes | Tap water | 4 | 6.9 | 32.6 | 1.7 | 626.6 |
| Don’t know | Bottled water | 23 | 12.6 | 0.3 | 0.1 | 1.1 |
| Don’t know | Tap water | 35 | 22.6 | 1.5 | 0.3 | 8.4 |
| Housing age | Peeling paint inside the dwelling | |||||
| Built post-1949 | No | 32 | 22.1 | Reference level | ||
| Built post-1949 | Yes | 4 | 4.4 | 2.9 | 0.5 | 18.2 |
| Built pre-1949 | No | 20 | 18.4 | 3.6 | 1.0 | 12.2 |
| Built pre-1949 | Yes | 11 | 12.0 | 0.8 | 0.1 | 7.2 |
| Don’t know | No | 23 | 36.9 | 6.2 | 1.3 | 29.6 |
| Don’t know | Yes | 10 | 6.3 | 0.3 | 0.0 | 4.7 |
| Housing age | Renovation works inside the dwelling | |||||
| Built post-1949 | No | 27 | 18.4 | Reference level | ||
| Built post-1949 | Yes | 11 | 8.5 | 1.2 | 0.4 | 3.7 |
| Built pre-1949 | No | 18 | 19.9 | 3.6 | 1.0 | 12.2 |
| Built pre-1949 | Yes | 13 | 10.2 | 0.8 | 0.2 | 4.2 |
| Don’t know | No | 25 | 34.2 | 6.2 | 1.3 | 29.6 |
| Don’t know | Yes | 8 | 8.7 | 0.6 | 0.1 | 3.3 |
| Variables | Observed Cases for BLLs > 44 µg/L (n = 107) | Estimated Prevalence (%) for BLLs > 44 µg/L | OR | 95% CI | ||
|---|---|---|---|---|---|---|
| Gender of child | ||||||
| Male | 60 | 47.8 | Reference level | |||
| Female | 47 | 52.2 | 2.4 | 1.2 | 5.0 | |
| Child recently arrived in France (<1 year) | ||||||
| No | 104 | 97.7 | Reference level | |||
| Yes | 2 | 2.3 | 6.4 | 0 | 1366.7 | |
| Mother’s country of birth | ||||||
| France | 73 | 69.9 | Reference level | |||
| Other country | 33 | 30.1 | 2.9 | 1.2 | 6.8 | |
| Parents smoke indoors | ||||||
| No | 74 | 75.7 | Reference level | |||
| <1 h/day | 13 | 12.0 | 3.1 | 0.9 | 10.4 | |
| 1–2 h/day | 6 | 2.6 | 0.6 | 0.2 | 2.2 | |
| 2–5 h/day | 6 | 2.2 | 0.1 | 0 | 0.6 | |
| >5 h/day | 7 | 7.5 | 8.6 | 2.7 | 27.5 | |
| Lead pipes | Type of drinking water | |||||
| No | Bottled water | 24 | 29.2 | Reference level | ||
| No | Tap water | 19 | 28.1 | 1.0 | 0.3 | 3.4 |
| Yes | Bottled water | 2 | 0.5 | 0.1 | 0 | 1.5 |
| Yes | Tap water | 4 | 6.9 | 16.6 | 0.2 | 1321.7 |
| Don’t know | Bottled water | 23 | 12.6 | 0.2 | 0.1 | 0.9 |
| Don’t know | Tap water | 35 | 22.6 | 1.1 | 0.2 | 6.0 |
| Housing age | Peeling paint inside the dwelling | |||||
| Built post-1949 | No | 32 | 22.1 | Reference level | ||
| Built post-1949 | Yes | 4 | 4.4 | 3.1 | 0.4 | 25.0 |
| Built pre-1949 | No | 20 | 18.4 | 3.1 | 1.1 | 8.5 |
| Built pre-1949 | Yes | 11 | 12.0 | 0.8 | 0.1 | 11.0 |
| Don’t know | No | 23 | 36.9 | 4.8 | 0.9 | 25.0 |
| Don’t know | Yes | 10 | 6.3 | 0.1 | 0 | 4.1 |
| Adult occupation involves lead exposure | ||||||
| No | Reference level | |||||
| Yes | 16 | 7.6 | 0.3 | 0.1 | 0.9 | |
| Occupancy rate (number of people/number of rooms) | ||||||
| Change from percentile 25 to percentile 75:0.7 to 1.1 | 0.6 | 0.3 | 1.1 | |||
| Change from percentile 25 to percentile 95:0.7 to 1.7 | 1.4 | 0.8 | 2.6 | |||
| Change from percentile 25 to percentile 99:0.7 to 3 | 3.6 | 2.0 | 6.4 | |||
| Proportion of substandard pre-1949 housing per cadastral section or municipality | ||||||
| Change from percentile 25 to percentile 75:0.8 to 6.0% | 5.0 | 4.8 | 5.3 | |||
| Change from percentile 25 to percentile 95:0.8 to 15.5% | 14.2 | 13.5 | 15.0 | |||
| Change from percentile 25 to percentile 99:0.8 to 29.3% | 27.7 | 26.3 | 29.1 | |||
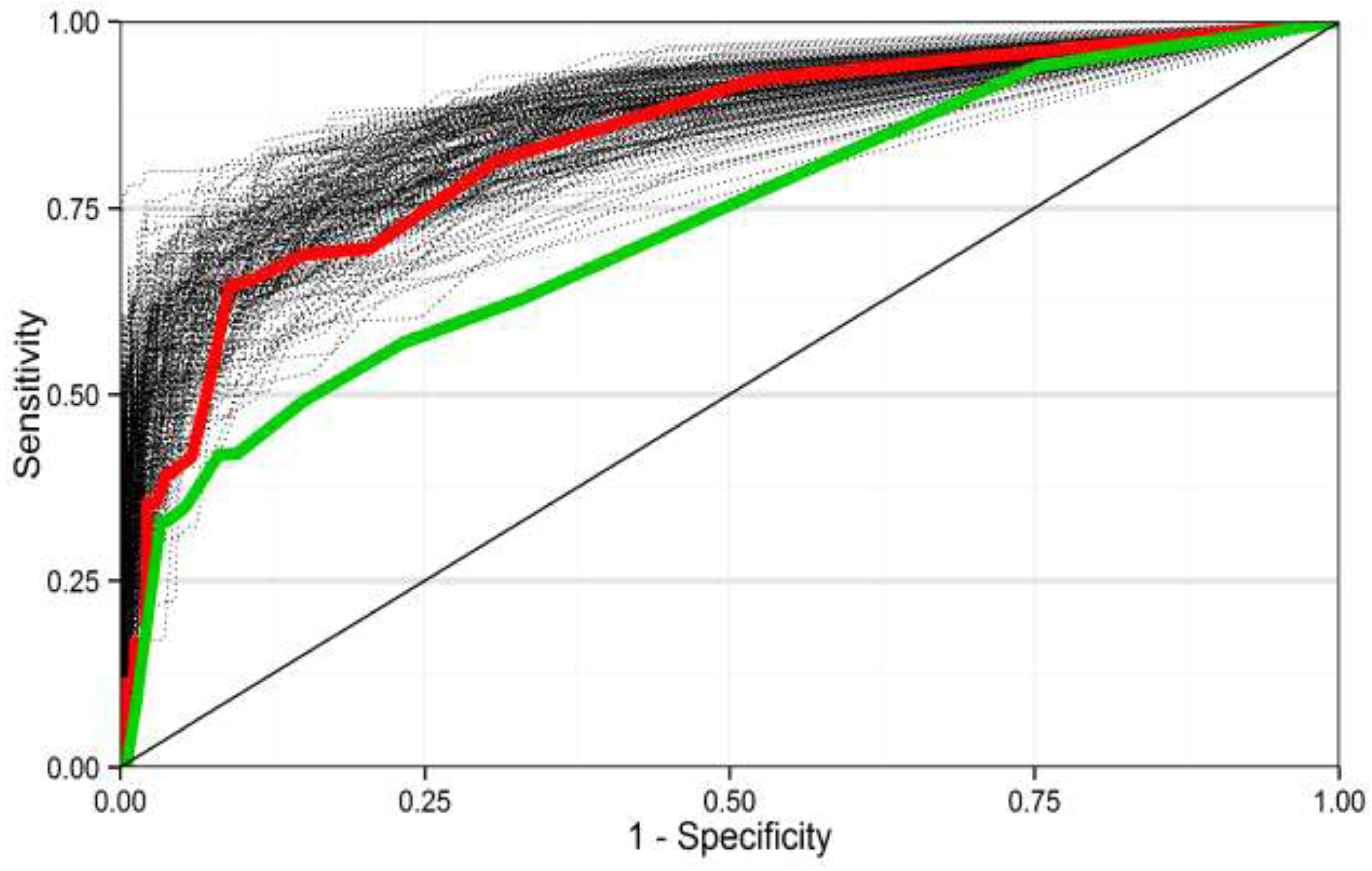
3.3. Performance of Predictive Models
4. Discussion
4.1. Sensitivity and Specificity of the Current Lead Risk Questionnaire in Screening BLLs Higher than 50 µg/L
4.2. Proposal for a New Lead Risk Questionnaire
4.3. Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bas, P.; Luzardo, O.P.; Pena-Quintana, L.; Gonzalez, J.E.; Pena, J.A.; Gracia, J.; Zumbado, M.; Boada, L.D. Determinants of blood lead levels in children: A cross-sectional study in the Canary Islands (Spain). Int. J. Hyg. Environ. Health 2012, 215, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Federal Environment Agency (Umweltbundesamt). German Environmental Survey for Children 2003/06-GerES IV-Human Biomonitoring Levels of Selected Substances in Blood and Urine of Children in Germany; Federal Environment Agency (Umweltbundesamt): Berlin, Germany, 2008. [Google Scholar]
- Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals, Update Tables; CDC: Atlanta, GA, USA, 2012.
- Health Canada. Second Report on Human Biomonitoring of Environmental Chemicals in Canada; Health Canada: Ottawa, ON, Canada, 2013. [Google Scholar]
- Hruba, F.; Stromberg, U.; Cerna, M.; Chen, C.; Harari, F.; Harari, R.; Horvat, M.; Koppova, K.; Kos, A.; Krskova, A.; et al. Blood, cadmium, mercury, and lead in children: An international comparison of cities in six European countries, and China, Ecuador, and Morocco. Environ. Int. 2012, 41, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, U.; Lundh, T.; Skerfving, S. Yearly measurements of blood lead in Swedish children since 1978: The declining trend continues in the petrol-lead-free period 1995–2007. Environ. Res. 2008, 107, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Etchevers, A.; Bretin, P.; Lecoffre, C.; Bidondo, M.L.; le Strat, Y.; Glorennec, P.; le Tertre, A. Blood lead levels and risk factors in young children in France, 2008–2009. Int. J. Hyg. Environ. Health 2014, 217, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on Lead in Food. Available online: http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/1570.pdf (accessed on 9 September 2015).
- Monograph on Health Effects of Low-Level Lead. Available online: https://ntp.niehs.nih.gov/ntp/ohat/lead/final/monographhealtheffectslowlevellead_newissn_508.pdf (accessed on 9 September 2015).
- French High Council for Public Health. Exposition au plomb: Détermination de Nouveaux Objectifs de Gestion; French High Council for Public Health: Paris, French, 2014. [Google Scholar]
- Agence Nationale D’accréditation et D’évaluation en Santé. Recommandations de la conference de consensus. In Intoxication par le Plomb de L’enfant et de la Femme Enceinte. Prévention et Prise en Charge Médico-Sociale; Agence Nationale D’accréditation et D’évaluation en Santé: Saint-Denis, France, 2003; pp. 1–33. [Google Scholar]
- Direction Générale de la Santé. Guide de Dépistage et de Prise en Charge de L’intoxication par le Plomb de L’enfant et de la Femme Enceinte; Ministère de la santé et des solidarités: Paris, France, 2006; pp. 1–31. [Google Scholar]
- Inserm, InVS. Saturnisme: Quelles Strategies de Dépistage Chez L’enfant? Les Editions Inserm: Paris, French, 2008; pp. 1–300. [Google Scholar]
- Glorennec, P.; Peyr, C.; Poupon, J.; Oulhote, Y.; Le Bot, B. Identifying sources of lead exposure for children, with lead concentrations and isotope ratios. J. Occup. Environ. Hyg. 2010, 7, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Lecoffre, C.; Ménard, E. Saturnisme Chez L’enfant. France 2008–2011, Résultats; Institut de Veille Sanitaire: Saint-Maurice, French, 2014; pp. 1–51. [Google Scholar]
- Oulhote, Y.; Le Bot, B.; Poupon, J.; Lucas, J.; Mandin, C.; Etchevers, A.; Zmirou-Navier, D.; Glorennec, P. Identification of sources of lead exposure in French children by lead isotope analysis: A cross-sectional study. Environ. Health 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Oulhote, Y.; le Tertre, A.; Etchevers, A.; Le Bot, B.; Lucas, J.P.; Mandin, C.; le Strat, Y.; Lanphear, B.; Glorennec, P. Implications of different residential lead standards on children’s blood lead levels in France: Predictions based on a national cross-sectional survey. Int. J. Hyg. Environ. Health 2013, 216, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.; Chen, J.T.; Waterman, P.D.; Soobader, M.J.; Subramanian, S.V.; Carson, R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US). J. Epidemiol. Community Health 2003, 57, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.L.; Dolinoy, D.C.; Overstreet, M.A. Mapping for prevention: GIS models for directing childhood lead poisoning prevention programs. Environ. Health Perspect. 2002, 110, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, A.; Staley, F.; Shire, J.; Muthukumar, S.; Kennedy, C.; Meyer, P.A.; Brown, M.J. Screening for lead poisoning: A geospatial approach to determine testing of children in at-risk neighborhoods. J. Pediatr. 2009, 154, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Kaplowitz, S.A.; Perlstadt, H.; D’Onofrio, G.; Melnick, E.R.; Baum, C.R.; Kirrane, B.M.; Post, L.A. The predictive value of self-report questions in a clinical decision rule for pediatric lead poisoning screening. Public Health Rep. 2012, 127, 375–382. [Google Scholar] [PubMed]
- Lumley, T. Complex Surveys; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Nagelkerke, N.J.D. A note on the general definition of the coefficient of determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Altman, D.G.; Royston, P. What do we mean by validating a prognostic model? Stat. Med. 2000, 19, 453–473. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Harrell, F.E., Jr.; Borsboom, G.J.; Eijkemans, M.J.; Vergouwe, Y.; Habbema, J.D. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol. 2001, 54, 774–781. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Interpreting and managing blood lead levels <10 µg/dL in children and reducing childhood exposures to lead: Recommendations of CDC’s Advisory Committee on Childhood Lead Poisoning Prevention. Recomm. Rep. 2007, 56, 1–14. [Google Scholar]
- Binns, H.J.; LeBailly, S.A.; Poncher, J.; Kinsella, T.R.; Saunders, S.E. Is there lead in the suburbs? Risk assessment in Chicago suburban pediatric practices. Pediatrics 1994, 93, 164–171. [Google Scholar] [PubMed]
- Binns, H.J.; LeBailly, S.A.; Fingar, A.R.; Saunders, S. Evaluation of risk assessment questions used to target blood lead screening in Illinois. Pediatrics 1999, 103, 100–106. [Google Scholar] [CrossRef] [PubMed]
- France, E.K.; Gitterman, B.A.; Melinkovich, P.; Wright, R.A. The accuracy of a lead questionnaire in predicting elevated pediatric blood lead levels. Arch. Pediatr. Adolesc. Med. 1996, 150, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Ossiander, E.M. A systematic review of screening questionnaires for childhood lead poisoning. J. Public Health Manag. Pract. 2013, 19, E21–E29. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Wheeler, W.; Brown, M.J. Lead screening and prevalence of blood lead levels in children aged 1–2 years—Child blood lead surveillance system, United States, 2002–2010 and national health and nutrition examination survey, United States, 1999–2010. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 38–42. [Google Scholar]
- Kolossa-Gehring, M.; Becker, K.; Conrad, A.; Schroter-Kermani, C.; Schulz, C.; Seiwert, M. Environmental surveys, specimen bank and health related environmental monitoring in Germany. Int. J. Hyg. Environ. Health. 2012, 215, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C. Lead neurotoxicity and socioeconomic status: conceptual and analytical issues. Neurotoxicology 2008, 29, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.; Brown, M.J.; Kashtock, M.E.; Jacobs, D.E.; Whelan, E.A.; Rodman, J.; Schock, M.R.; Padilla, A.; Sinks, T. Lead exposures in U.S. Children, 2008: Implications for prevention. Environ. Health Perspect. 2008, 116, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Etchevers, A.; Le Tertre, A.; Lucas, J.P.; Bretin, P.; Oulhote, Y.; Le Bot, B.; Glorennec, P. Environmental determinants of different blood lead levels in children: A quantile analysis from a nationwide survey. Environ. Int. 2015, 74, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Nester, M.; de Vol, E.; Shinwari, N.; Al-Shahria, S. Determinants of blood lead levels in Saudi Arabian schoolgirls. Int. J. Occup. Environ. Health 1999, 5, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bretin, P.; Schapiro, E. Sources Inhabituelles D’intoxication par le Plomb Chez L’enfant et la Femme Enceinte; Institut de Veille Sanitaire: Saint-Maurice, France, 2006. [Google Scholar]
- Goswami, K. Eye cosmetic “surma”: Hidden threats of lead poisoning. Indian J. Clin. Biochem. 2013, 28, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, M.H.; White, F.; Agboatwalla, M.; Hozhabri, S.; Luby, S. Factors associated with elevated blood lead concentrations in children in Karachi, Pakistan. Bull. World Health Organ. 2002, 80, 769–775. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. Lead poisoning associated with use of traditional ethnic remedies—California, 1991–1992. MMWR Morb. Mortal. Wkly. Rep. 1993, 42, 521–524. [Google Scholar]
- Centers for Disease Control and Prevention. Lead poisoning associated with ayurvedic medications—Five states, 2000–2003. MMWR Morb. Mortal. Wkly. Rep. 2004, 53, 582–584. [Google Scholar]
- Centers for Disease Control and Prevention. Screening Young Children for Lead Poisoning: Guidance for State and Local Public Health Officials; Centers for Disease Control and Prevention: Atlanta, GA, USA, 1997.
- Kaplowitz, S.A.; Perlstadt, H.; Dziura, J.D.; Post, L.A. Behavioral and environmental explanations of elevated blood lead levels in immigrant children and children of immigrants. J. Immigr. Minor. Health 2015, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.L.; Gaitens, J.M.; Jacobs, D.E.; Strauss, W.; Nagaraja, J.; Pivetz, T.; Wilson, J.W.; Ashley, P.J. Exposure of U.S. children to residential dust lead, 1999–2004: II. The contribution of lead-contaminated dust to children’s blood lead levels. Environ. Health Perspect. 2009, 117, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.M.; Albalak, R.; Grosse, S.; Repace, J. Second-hand smoke exposure and blood lead levels in U.S. children. Epidemiology 2003, 14, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, A.; Garcia-Esquinas, E.; Fadrowski, J.J.; McLain, P.; Weaver, V.M.; Navas-Acien, A. Secondhand tobacco smoke: A source of lead exposure in US children and adolescents. Am. J. Public Health 2012, 102, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Spector, L.; Wickham, K.; Janis, G.; Winickoff, J.; Lindgren, B.; Murphy, S. Biomarker evidence of tobacco smoke exposure in children participating in lead screening. Am. J. Public Health 2013, 103, e54–e59. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.P.; Byrd, R.S.; Auinger, P.; Schaffer, S.J. Community characteristics associated with elevated blood lead levels in children. Pediatrics 1998, 101, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Using GIS to Assess and Direct Childhood Lead Poisoning Prevention; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2004.
- Kaplowitz, S.A.; Perlstadt, H.; Perlstadt, H.; Post, L.A. Comparing lead poisoning risk assessment methods: Census block group characteristics vs. zip codes as predictors. Public Health Rep. 2010, 125, 234–245. [Google Scholar] [PubMed]
- Roberts, J.R.; Hulsey, T.C.; Curtis, G.B.; Reigart, J.R. Using geographic information systems to assess risk for elevated blood lead levels in children. Public Health Rep. 2003, 118, 221–229. [Google Scholar] [CrossRef]
- Reissman, D.B.; Staley, F.; Curtis, G.B.; Kaufmann, R.B. Use of geographic information system technology to aid Health Department decision making about childhood lead poisoning prevention activities. Environ. Health Perspect. 2001, 109, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.P.; le Bot, B.; Glorennec, P.; Etchevers, A.; Bretin, P.; Douay, F.; Sebille, V.; Bellanger, L.; Mandin, C. Lead contamination in French children’s homes and environment. Environ. Res. 2012, 116, 58–65. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etchevers, A.; Glorennec, P.; Le Strat, Y.; Lecoffre, C.; Bretin, P.; Le Tertre, A. Screening for Elevated Blood Lead Levels in Children: Assessment of Criteria and a Proposal for New Ones in France. Int. J. Environ. Res. Public Health 2015, 12, 15366-15378. https://doi.org/10.3390/ijerph121214989
Etchevers A, Glorennec P, Le Strat Y, Lecoffre C, Bretin P, Le Tertre A. Screening for Elevated Blood Lead Levels in Children: Assessment of Criteria and a Proposal for New Ones in France. International Journal of Environmental Research and Public Health. 2015; 12(12):15366-15378. https://doi.org/10.3390/ijerph121214989
Chicago/Turabian StyleEtchevers, Anne, Philippe Glorennec, Yann Le Strat, Camille Lecoffre, Philippe Bretin, and Alain Le Tertre. 2015. "Screening for Elevated Blood Lead Levels in Children: Assessment of Criteria and a Proposal for New Ones in France" International Journal of Environmental Research and Public Health 12, no. 12: 15366-15378. https://doi.org/10.3390/ijerph121214989
APA StyleEtchevers, A., Glorennec, P., Le Strat, Y., Lecoffre, C., Bretin, P., & Le Tertre, A. (2015). Screening for Elevated Blood Lead Levels in Children: Assessment of Criteria and a Proposal for New Ones in France. International Journal of Environmental Research and Public Health, 12(12), 15366-15378. https://doi.org/10.3390/ijerph121214989





