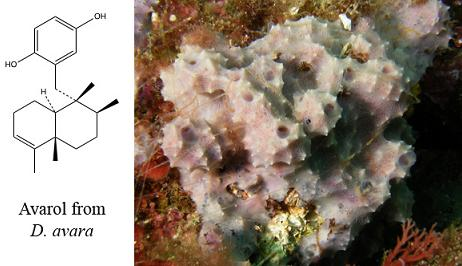
In Situ Aquaculture Methods for Dysidea avara (Demospongiae, Porifera) in the Northwestern Mediterranean
Abstract
:1. Introduction
2. Material and Methods
2.1. Culture experimental design
2.2. Monitoring
2.3. Toxicity analysis
2.4. Data analysis
3. Results
3.1. Survival
3.2. Growth
3.3. Toxicity
4. Discussion
4.1. Explant survival
4.2. Explant growth
4.3. Explants toxicity
5. Conclusions
Acknowledgements
- Samples Availability: Available from the authors.
References
- Becerro, MA; Thacker, RW; Turon, X; Uriz, MJ; Paul, VJ. Biogeography of sponge chemical ecology: Comparison of tropical and temperate defenses. Oecologia 2003, 135, 91–101. [Google Scholar]
- Martí, R; Fontana, A; Uriz, MJ; Cimino, G. Quantitative assessment of natural toxicity in sponges: Toxicity bioassay versus compound quantification. J. Chem. Ecol 2003, 29, 1307–1318. [Google Scholar]
- Martin, D; Uriz, MJ. Chemical bioactivity of Mediterranean benthic organisms against embryos and larvae of marine invertebrates. J. Exp. Mar. Biol. Ecol 1993, 173, 11–27. [Google Scholar]
- Uriz, MJ; Martin, D; Turon, X; Ballesteros, E; Hughes, R; Acebal, C. An approach to the ecological meaning of allelopathy in Mediterranean benthic communities. Mar. Ecol. Prog. Ser 1991, 70, 175–188. [Google Scholar]
- Sipkema, D; Franssen, MCR; Osinga, R; Tramper, J; Wijffels, RH. Marine sponges as pharmacy. Mar. Biotechnol 2005, 7, 142–162. [Google Scholar]
- Folmer, F; Schumacher, M; Jaspars, M; Dicato, M; Diederich, M. Nairne, GH, Ed.; Chemical ecology and medicinal chemistry of marine NF-κB inhibitors. In Aquatic Ecosystem Research Trends; NovaScience Publishers, Inc: Hauppauge, NY, USA, 2009; pp. 1–49. [Google Scholar]
- Müller, WEG; Maidhof, A; Zahn, RK; Schröder, HC; Gasic, MJ; Heidemann, D; Bernd, A; Kurelec, B; Eich, E; Sibert, G. Potent antileukemic activity of the novel cytostatic agent avarone and its analogues in vitro and in vivo. Cancer Res 1985, 45, 4822–4827. [Google Scholar]
- Amigo, M; Paya, M; Braza-Boils, A; De Rosa, S; Terencio, MC. Avarol inhibits TNF-alpha generation and NF-kappaB activation in human cells and in animal models. Life Sci 2008, 82, 256–264. [Google Scholar]
- Sladic, D; Gasic, MJ. Reactivity and biological activity of the marine sesquiterpene hydroquinone avarol and related compounds from sponges of the order Dictyoceratida. Molecules 2006, 11, 1–33. [Google Scholar]
- Loya, S; Hizi, A. The inhibition of human immunodeficiency virus type 1 reverse transcriptase by avarol and avarone derivates. FEBS Lett 1990, 269, 131–134. [Google Scholar]
- Müller, WEG; Schröder, HC. Cell biological aspects of HIV-1 infection: Effects of the anti-HIV-1 agent avarol. Int. J. Sports Med 1991, 12, 43–49. [Google Scholar]
- Pietschmann, R; Shatton, M; Schatton, W. Process for preparation of compositions with a high content in avarol and their use. E.U. Patent EP139119. 2004. Available online: http://www.freepatentsonline.com/EP1391197B1.html.
- De Rosa, S; De Caro, S; Iodice, C; Tommonaro, G; Stefanov, K; Popov, S. Development in primary cell culture of demosponges. J. Biotechnol 2003, 100, 119–125. [Google Scholar]
- Müller, WEG; Böhm, M; Batel, R; De Rosa, S; Tommonaro, G; Müller, IM; Schröder, HC. Application of cell culture for the production of bioactive compounds from sponges: Synthesis of avarol by primmorphs from Dysidea avara. J. Nat. Prod 2000, 63, 1077–1081. [Google Scholar]
- Müller, WEG; Wiens, M; Dell, T; Gamulin, V; Schröder, HC; Müller, IM. Bauplan of the Urmetazoa: basis for genetic complexity of Metazoa. Int. Rev. Cytol 2004, 235, 53–92. [Google Scholar]
- Pomponi, SA; Willoughby, R. Van Soest, RWM, Van Kempen, TMG, Braekman, JC, Eds.; Sponge cell culture for the production of bioactive metabolites. In Sponges in Time and Space; AA Balkema: Rotterdam, The Netherlands, 1994; pp. 395–400. [Google Scholar]
- Sipkema, D; Heilig, HGHJ; Akkermans, ADL; Osinga, R; Wijffels, RH. Sponge-cell culture? A molecular identification method for sponge cells. Mar. Biotechnol 2003, 5, 443–449. [Google Scholar]
- De Caralt, S; Otjens, H; Uriz, MJ; Wijffels, RH. Cultivation of sponge larvae: Settlement, survival and growth of juveniles. Mar. Biotechnol 2007, 9, 592–605. [Google Scholar]
- Sipkema, D; Osinga, R; Schatton, W; Mendola, D; Tramper, J; Wijffels, RH. Large-scale production of pharmaceuticals by marine sponges: Sea, cell or synthesis? Biotechnol. Bioeng 2005, 85, 239–247. [Google Scholar]
- Duckworth, AR. Farming soponges to supply bioactive metabolites and bath sponges: A review. Mar. Biotechnol 2009, 11, 669–679. [Google Scholar]
- Pronzato, R; Manconi, R. Mediterranean commercial sponges: Over 5000 years of natural history and cultural heritage. Mar. Ecol 2008, 29, 1–21. [Google Scholar]
- Cotte, J. Sponge culture. Bull. US Bur. Fish 1910, 28, 587–614. [Google Scholar]
- Moore, HF. A practical method of sponge culture. Bull. US Bur. Fish 1910, 28, 545–585. [Google Scholar]
- Verdenal, B; Vacelet, J. Rützler, K, Ed.; Sponge culture on vertical ropes in the Northwestern Mediterranean sea. In New Perspectives in Sponge Biology; Smithsonian Institution Press: Washington, DC, USA, 1990; pp. 416–424. [Google Scholar]
- Pronzato, R. Sponge fishing, disease and farming in the Mediterranean sea. Aquatic Conserv.: Mar. Freshw. Ecosyst 1999, 9, 485–493. [Google Scholar]
- Duckworth, AR; Battershill, CN. Developing farming structures for production of biologically active sponge metabolites. Aquaculture 1990, 217, 139–156. [Google Scholar]
- Duckworth, AR; Battershill, CN. Sponge aquaculture for the production of biologically active metabolites: The influence of farming protocols and environment. Aquaculture 2003, 221, 311–329. [Google Scholar]
- Van Treeck, P; Eisinger, M; Müller, J; Paster, M; Schuhmacher, H. Mariculture trials with Mediterranean sponge species. The exploitation of an old natural resource with sustainable and novel methods. Aquaculture 2003, 218, 439–455. [Google Scholar]
- Becerro, MA; Turon, X; Uriz, MJ. Natural variation of toxicity in encrusting sponge Crambe crambe (Schmidt) in relation to size and environment. J. Chem. Ecol 1995, 21, 1931–1946. [Google Scholar]
- Becerro, MA; Turon, X; Uriz, MJ. Chemically-mediated interactions in benthic organisms: The chemical ecology of Crambe crambe (Porifera, Poecilosclerida). Hydrobiologia 1997, 356, 77–89. [Google Scholar]
- Turon, X; Becerro, MA; Uriz, MJ. Seasonal patterns of toxicity in benthic invertebrates: The encrusting sponge Crambe crambe (Poecilosclerida). Oikos 1996, 75, 33–40. [Google Scholar]
- Ferretti, C; Vacca, S; De Crucis, C; Marengo, B; Duckworth, AR; Manconi, R; Pronzato, R; Domenicotti, C. Growth dynamics and bioactivity variation of the Mediterranean demosponges Agelas ovoides (Agelasida, Agelasidae) and Petrosia ficiformis (Haplosclerida, Petrosiidae). Mar. Ecol 2009, 30, 327–336. [Google Scholar]
- Corriero, G; Longo, C; Mercurio, M; Marzano, CN; Lembo, G; Spedicato, MT. Rearing performance of Spongia officinalis on suspended ropes off the Southern Italian Coast (Central Mediterranean Sea). Aquaculture 2004, 238, 195–205. [Google Scholar]
- Wilkinson, CR; Vacelet, J. Transplantation of marine sponges to different conditions of light and current. J. Exp. Mar. Biol. Ecol 1979, 37, 91–104. [Google Scholar]
- De Caralt, S; Agell, G; Uriz, MJ. Long-term culture of sponges explants: Conditions enhancing survival and growth, and assessment of bioactivity. Biomol. Eng 2003, 20, 339–347. [Google Scholar]
- Duckworth, AR; Battershill, CN; Bergquist, PR. Influence of explant procedures and environmental factors on culture success of three sponges. Aquaculture 1997, 156, 251–267. [Google Scholar]
- Kaiser, KL; Ribo, JM. Photobacterium phosphoreum toxicity bioassay. II. Toxicity data compilation. Toxic. Assess 1988, 3, 195–237. [Google Scholar]
- Martí, R; Fontana, A; Uriz, MJ; Cimino, G. Quantitative assessment of natural toxicity in sponges: Toxicity bioassay versus compound quantification. J. Chem. Ecol 2003, 29, 1307–1318. [Google Scholar]
- Fox, GA. Scheiner, SM, Gurevitch, J, Eds.; Failure-time analysis: Emergence, flowering, survivorship, and other waiting times. In Design and Analysis of Ecological Experiments; Chapman and Hall: London, UK, 1993; pp. 113–137. [Google Scholar]
- Uriz, MJ; Turon, X; Becerro, MA; Galera, J. Feeding deterrence in sponges. The role of toxicity, physical defenses, energetic contents, and life-history stage. J. Exp. Mar. Biol. Ecol 1996, 205, 187–204. [Google Scholar]
- Mendola, D; De Caralt, S; Uriz, MJ; Van den End, F; Van Leeuwen, JL; Wijffels, RH. Environmental flow regimes for Dysidea avara sponges. Mar. Biotechnol 2008, 10, 622–630. [Google Scholar]
- De Voogd, NJ. An assessment of sponge mariculture potential in the Spermonde Archipelago, Indonesia. J. Mar. Biol. Ass. UK 2007, 87, 1777–1784. [Google Scholar]
- De Caralt, S; Uriz, MJ; Wijffels, RH. Grazing, differential size-class dynamics and survival of the Mediterranean sponge Corticium candelabrum. Mar. Ecol. Prog. Ser 2008, 360, 97–106. [Google Scholar]
- Blanquer, A; Uriz, MJ; Agell, G. Hidden diversity in sympatric sponges: adjusting life-history dynamics to share substrate. Mar. Ecol. Prog. Ser 2008, 371, 109–115. [Google Scholar]





© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Caralt, S.; Sánchez-Fontenla, J.; Uriz, M.J.; Wijffels, R.H. In Situ Aquaculture Methods for Dysidea avara (Demospongiae, Porifera) in the Northwestern Mediterranean. Mar. Drugs 2010, 8, 1731-1742. https://doi.org/10.3390/md8061731
De Caralt S, Sánchez-Fontenla J, Uriz MJ, Wijffels RH. In Situ Aquaculture Methods for Dysidea avara (Demospongiae, Porifera) in the Northwestern Mediterranean. Marine Drugs. 2010; 8(6):1731-1742. https://doi.org/10.3390/md8061731
Chicago/Turabian StyleDe Caralt, Sonia, Javier Sánchez-Fontenla, María J. Uriz, and Rene H. Wijffels. 2010. "In Situ Aquaculture Methods for Dysidea avara (Demospongiae, Porifera) in the Northwestern Mediterranean" Marine Drugs 8, no. 6: 1731-1742. https://doi.org/10.3390/md8061731
APA StyleDe Caralt, S., Sánchez-Fontenla, J., Uriz, M. J., & Wijffels, R. H. (2010). In Situ Aquaculture Methods for Dysidea avara (Demospongiae, Porifera) in the Northwestern Mediterranean. Marine Drugs, 8(6), 1731-1742. https://doi.org/10.3390/md8061731