Bioadhesive Controlled Metronidazole Release Matrix Based on Chitosan and Xanthan Gum
Abstract
:1. Introduction
2. Results and Discussion
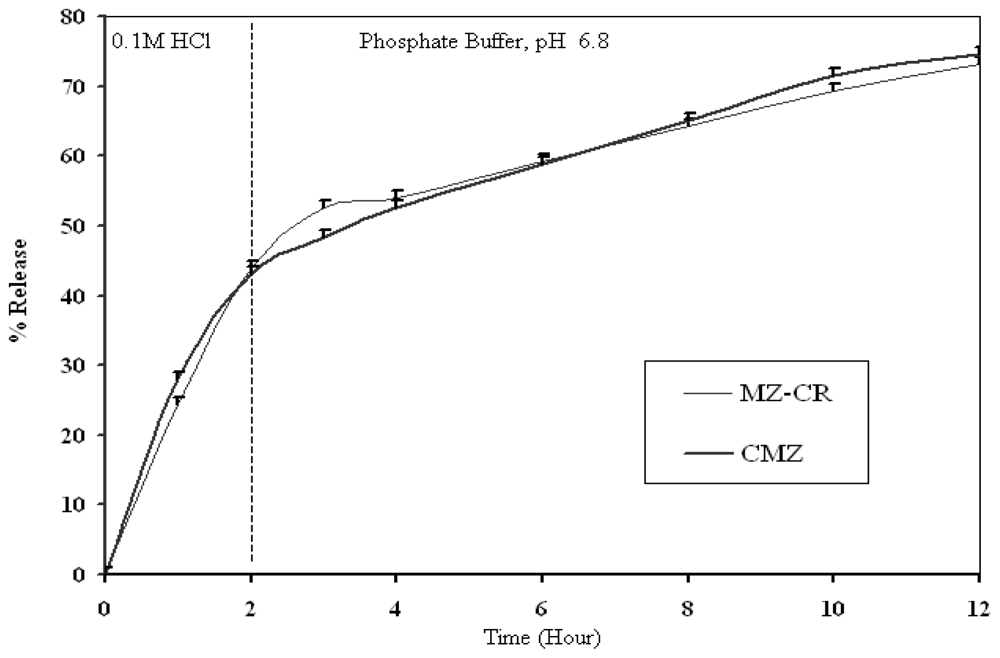
2.1. In vitro release study
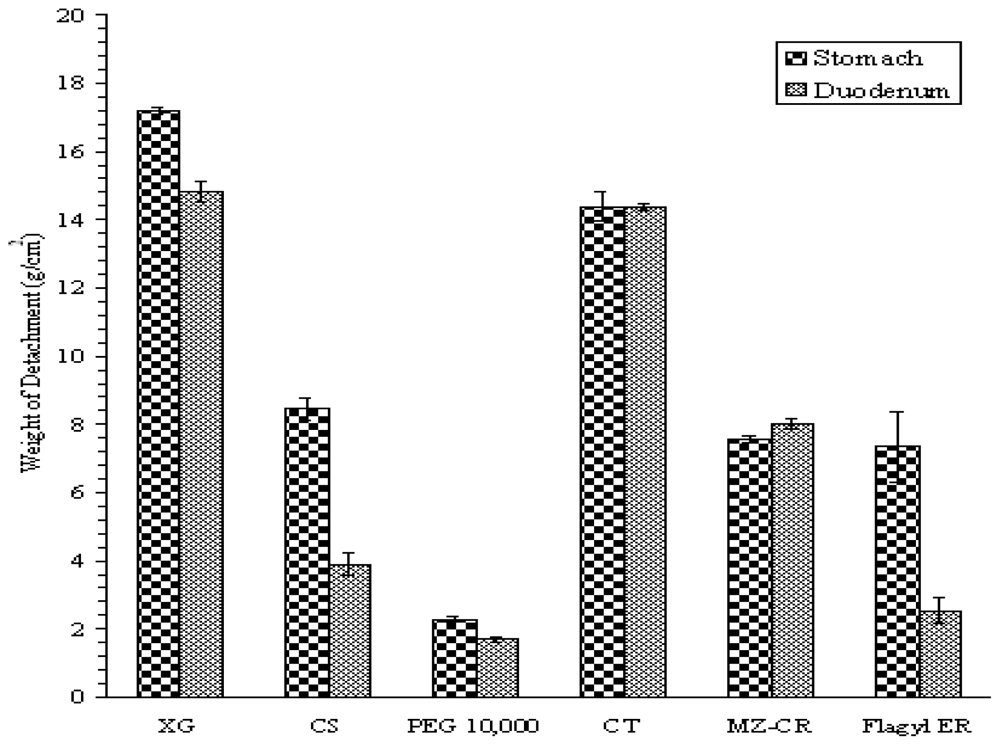
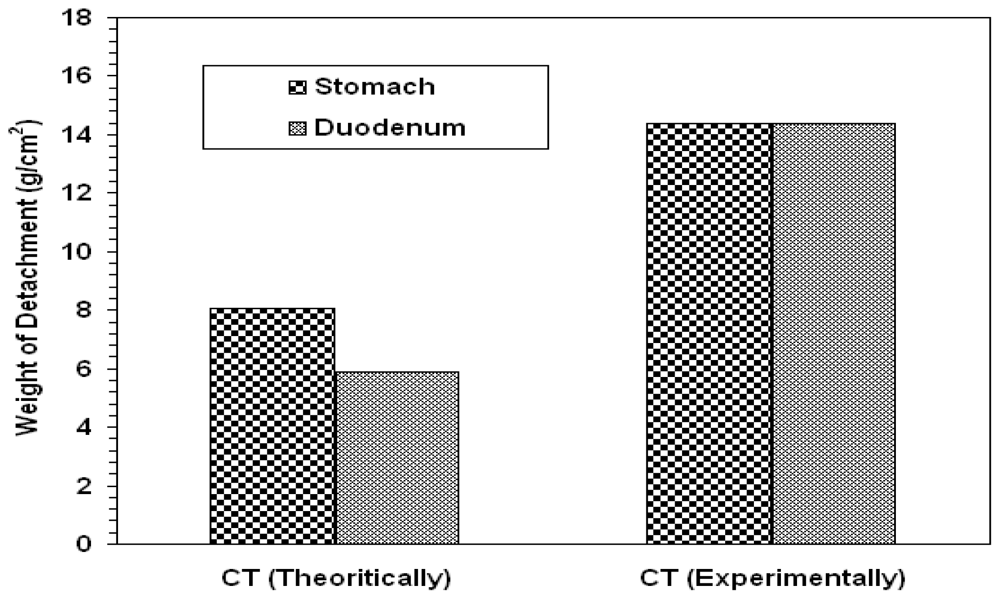
2.1.1. Bioadhesion
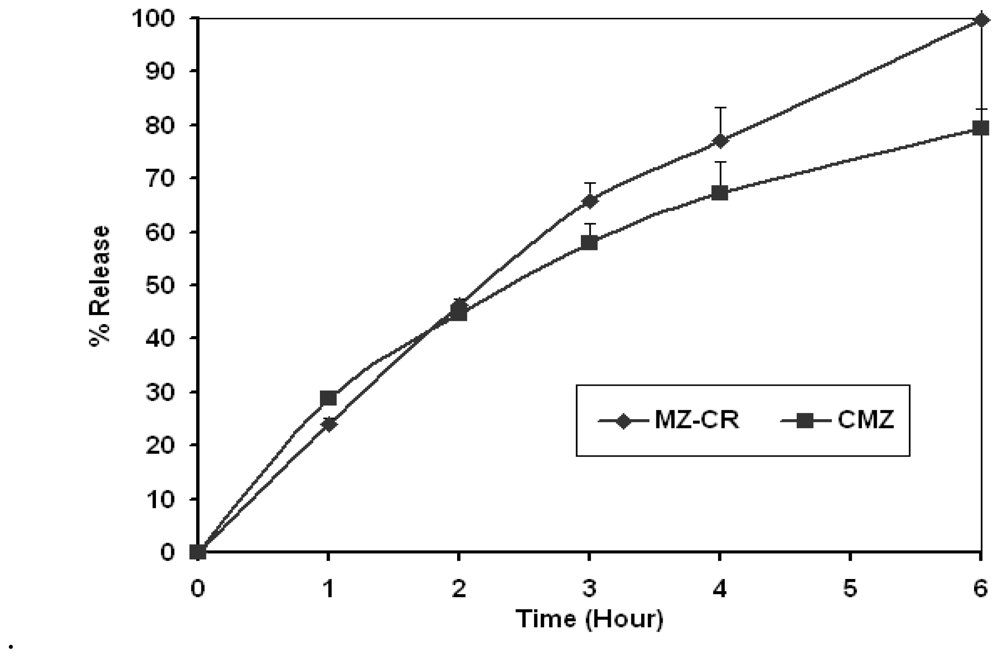
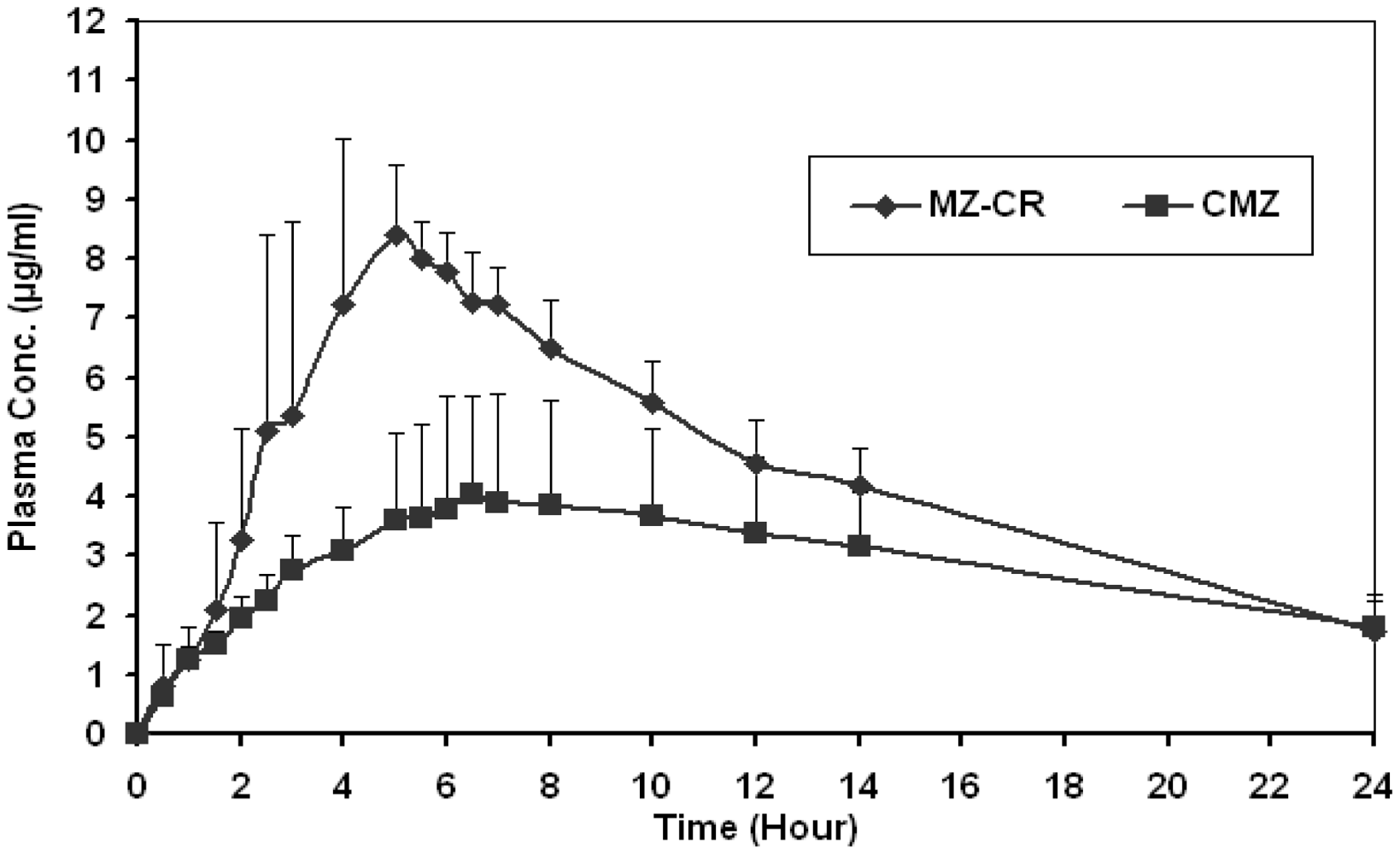
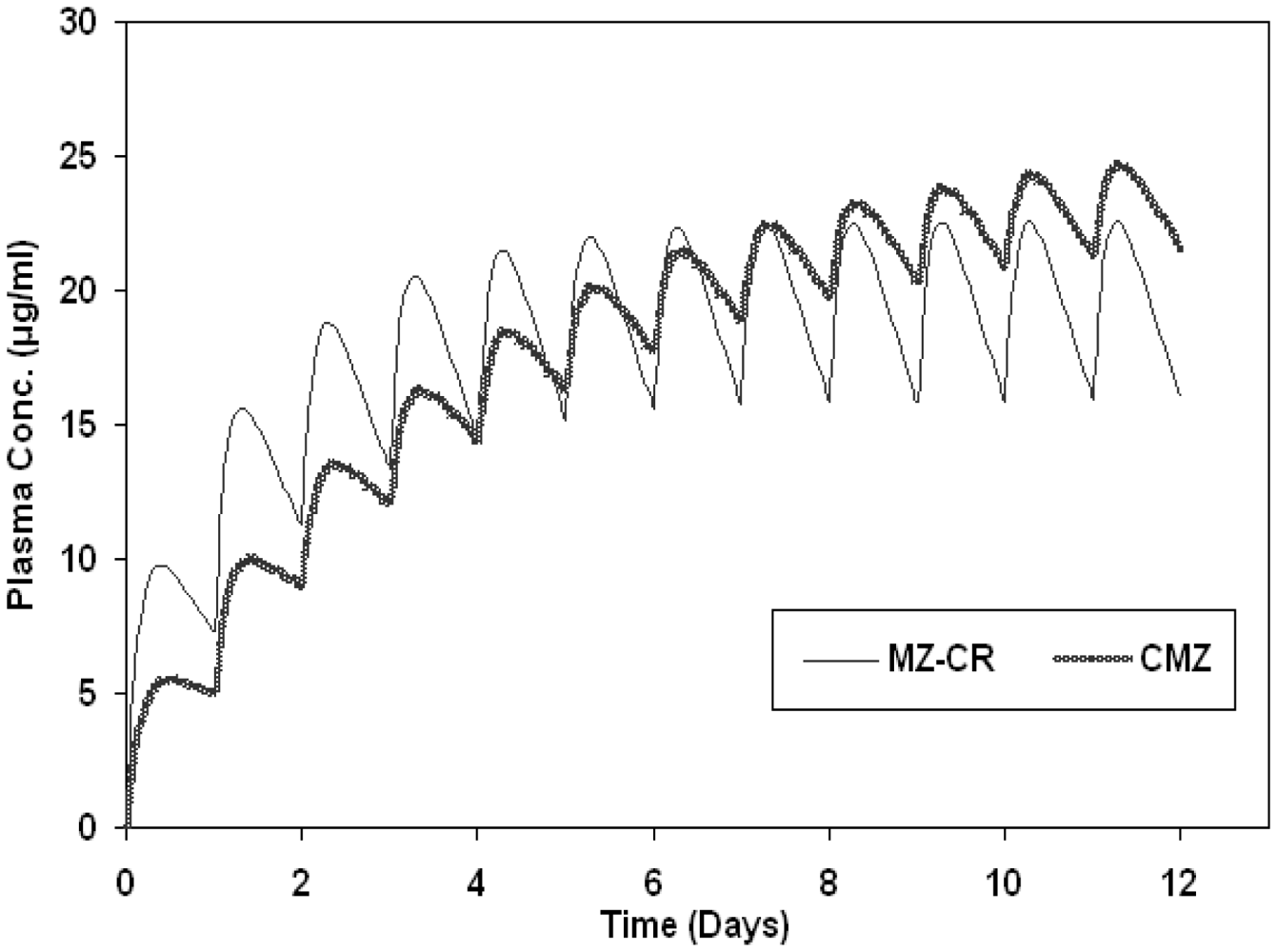
2.2. In vivo study
3. Experimental
3.1. Materials
3.2. Methods
3.2.1. Preparation of MZ controlled release preparation (MZ-CR)
3.2.2. In vitro dissolution test
3.2.3. Similarity factor
3.2.4. In vitro bioadhesion test
3.2.5. In vivo assessment of MZ controlled release dosage form
3.2.6. Determination of metronidazole in plasma
3.2.7. Pharmacokinetic analysis
3.2.7.1. Evaluation of bioavailability
3.2.7.2. Statistical considerations
4. Conclusions
References
- Dhopeshwarkar, V; Zata, J. Evaluation of xanthan gum in the preparation of sustained release matrix tablets. Drug Dev Ind Pharm 1993, 19, 999–1017. [Google Scholar]
- Siepmann, J; Peppas, NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev 2001, 48, 139–157. [Google Scholar]
- Davaran, S; Rashidi, MR; Khani, A. Synthesis of chemically cross-linked hydroxypropyl methyl cellulose hydrogels and their application in controlled release of 5-amino salicylic acid. Drug Dev Ind Pharm 2007, 33, 881–887. [Google Scholar]
- Paul, W; Sharma, CP. Chitosan, A drug carrier for the 21st century. STP Pharma Sci 2000, 10, 5–22. [Google Scholar]
- Lindner, WD; Lippold, BC. Drug release from hydrocolloid embeddings with high or low susceptibility to hydrodynamic stress. Pharm Res 1995, 12, 1781–1785. [Google Scholar]
- Rajabi-Siahboomi, AR; Bowtell, RW; Mansfield, P; Davies, MC. Structure and behavior in hydrophilic matrix sustained release dosage forms: 4. Studies of water mobility and diffusion coefficients in the gel layer of hpmc tablets using NMR imaging. Pharm Res 1996, 13, 376–380. [Google Scholar]
- Am Ende, M; Bell, L; Peppas, N; Massimo, G; Colombo, P. Measurement of the swelling force in ionic polymeric networks: II. Swelling force and disintegration of controlled release dosage formulations using ph-sensitive components. Int J Pharm 1995, 120, 33–40. [Google Scholar]
- Harland, R; Dubernet, C; Benoît, J; Peppas, N. A model of dissolution-controlled, diffusional drug release from non-swellable polymeric microspheres. J Control Rel 1988, 7, 207–215. [Google Scholar]
- Sandolo, C; Coviello, T; Matricardi, P; Alhaique, F. Characterization of polysaccharide hydrogels for modified drug delivery. Eur Biophys J 2007, 36, 693–700. [Google Scholar]
- Staniforth, JN; Baichwal, AR. Timerx: novel polysaccharide composites for controlled/programmed release of drugs in the gastrointestinal tract. Expert Opin Drug Deliv 2005, 2, 587–595. [Google Scholar]
- Baichwal, AR. Controlled release oxybutynin formulations. US Patent 5399359, 1995. [Google Scholar]
- Felt, O; Buri, P; Gurny, R. Chitosan: A unique polysaccharide for drug delivery. Drug Dev Ind Pharm 1998, 24, 979–993. [Google Scholar]
- Dodane, V; Vilivalam, V. Pharmaceutical applications of chitosan. Pharm Sci Technol Today 1998, 1, 246–253. [Google Scholar]
- Muzzarelli, RAA. Columbus, F, Ed.; Enhanced biochemical efficacy of oligomeric and partially depolymerized chitosans. In Chitosan: Manufacture, Properties and Usages; Nova Publishers: Hauppauge, NY, USA, 2010. [Google Scholar]
- Talukdar, MM; Vinckier, I; Moldenaers, P; Kinget, R. Rheological characterization of xanthan gum and hydroxypropylmethyl cellulose with respect to controlled-release drug delivery. J Pharm Sci 1996, 85, 537–540. [Google Scholar]
- El Gazayerly, ON. Release of Pentoxifylline from Xanthan Gum Matrix Tablets. Drug Dev Ind Pharm 2003, 29, 241–246. [Google Scholar]
- Fukuda, M; Peppas, NA; Mcginity, JW. Properties Of sustained release hot-melt extruded tablets containing chitosan and xanthan gum. Int J Pharm 2006, 310, 90–100. [Google Scholar]
- Dumtriu, S; Chornet, E; Vidal, P. Poly-ionic insoluble hydrogels comprising xanthan gum and chitosan. US Patent 5620706, 1997. [Google Scholar]
- Moes, AJ. Gastroretentive dosage forms. Crit Rev Ther Drug Carrier Sys 1993, 10, 143–195. [Google Scholar]
- Miyazaki, S; Nakayama, A; Oda, M; Takada, M; Attwood, D. Drug release from oral mucosal adhesive tablets of chitosan and sodium alginate. Int J Pharm 1995, 118, 257–263. [Google Scholar]
- Miyazaki, S; Nakayama, A; Oda, M; Takada, M; Attwood, D. Chitosan and alginate based bioadhesive tablets for intraoral drug delivery. Biol Pharm Bull 1994, 17, 745–747. [Google Scholar]
- Nakamura, F; Ohta, R; Machida, Y; Nagai, T. In vitro and in vivo nasal mucoadhesion of some water-soluble polymers. Int J Pharm 1996, 134, 173–181. [Google Scholar]
- Badwan, AA; Al-Remawi, M. Pharmaceutical polymer composition for oral controlled-release delivery of terbutaline sulfate. US Patent 2008206334, 2008. [Google Scholar]
- Badwan, AA; Al-Remawi, M; Salem, M. Universal controlled-release composition comprising chitosan. European Patent EP1512394, 2008. [Google Scholar]
- Eftaiha, AF; El-Barghouthi, MI; Rashid, IS; Al-Remawi, MM; Saleh, AI; Badwan, AA. Compressibility and compactibility studies of chitosan, xanthan gum, and their mixtures. J Mater Sci 2009, 44, 1054–1062. [Google Scholar]
- Andrews, GP; Laverty, TP; Jones, DS. Mucoadhesive Polymeric Platforms For Controlled Drug Delivery. Eur J Pharm Biopharm 2009, 71, 505–518. [Google Scholar]
- Idkaidek, NM; Najib, NM. Enhancement of oral absorption of metronidazole suspension in humans. Eur J Pharm Biopharm 2000, 50, 213–216. [Google Scholar]
- Goodwin, A; Kersulyte, D; Sisson, G; Veldhuyzen van Zanten, SJ; Berg, DE; Hoffman, PS. Metronidazole resistance in helicobacter pylori is due to null mutations in a gene (rdxa) that encodes an oxygen-insensitive nadph nitroreductase. Mol Microbiol 1998, 28, 383–393. [Google Scholar]
- Bulck, K; Decostere, A; Gruntar, I; Baele, M; Krt, B; Ducatelle, R; Haesebrouck, F. In vitro antimicrobial susceptibility testing of helicobacter felis, H. Bizzozeronii, H. Salomoni. Antimicrob Agents Chemother 2005, 49, 2997–3000. [Google Scholar]
- Dagana, R; Klugmanb, KP; Craigc, WA; Baquerod, F. Evidence To support the rationale that bacterial eradication in respiratory tract infection is an important aim of antimicrobial therapy. J Antimicrob Chemother 2001, 47, 129–140. [Google Scholar]
- Ball, P; Baquero, F; Cars, O; File, T; Garau, J; Klugman, K; Low, DE; Rubinstein, E; Wise, R. Antibiotic therapy of community respiratory tract infections: Strategies for optimal outcomes and minimized resistance emergence. the consensus group on resistance and prescribing in respiratory tract infection. J Antimicrob Chemother 2002, 49, 31–40. [Google Scholar]
- Jacobs, MR. How can we predict bacterial eradication? Int J Inf Dis 2003, 7, 13–20. [Google Scholar]
- Craig, WA. Antimicrobial resistance issues of the future. Diag Micro Inf Dis 1996, 25, 213–217. [Google Scholar]
- Craig, WA. Pharmacokinetic/Pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Inf Dis 1998, 26, 1–12. [Google Scholar]
- Garau, J. Why do we need to eradicate pathogens in respiratory tract infection? Int J Inf Dis 2003, 7, 5–12. [Google Scholar]
- Lonks, JR; Garau, J; Gomez, L; Xercavins, M; Ochoa de Echagüen, A; Gareen, IF; Reiss, PT; Medeiros, AA. Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin resistant streptococcus pneumoniae. Clin Inf Dis 2002, 35, 556–564. [Google Scholar]
- US Food and Drug Administration, Code of Federal Register; 1995; Volume 60, pp. 61638–61642.
- Parodi, B; Russo, E; Caviglioli, G; Cafaggi, S. Development and characterization of a buccoadhesive dosage form of oxycodone hydrochloride. Drug Dev Ind Pharm 1996, 22, 445–450. [Google Scholar]
| MZ-CR | CMZ | |
|---|---|---|
| HCl | ||
| K | 29.48 | 25.37 |
| N | 0.8005 | 0.5789 |
| r2 | 0.992 | 0.988 |
| Phosphate Buffer, pH = 6.8 | ||
| K | 6.27 | 5.52 |
| N | 0.6654 | 0.7679 |
| r2 | 0.997 | 0.997 |
| Tmax | Cmax | T0.5 | AUC0-t | AUC0-∞ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vol. No. | T | R | T | R | T | R | T | R | T | R |
| 1 | 5.00 | 4.00 | 7.96 | 2.62 | 18.74 | 151.10 | 91.74 | 43.81 | 123.65 | 372.97 |
| 2 | 5.50 | 10.00 | 7.08 | 3.16 | 20.64 | 44.15 | 81.53 | 52.71 | 122.63 | 119.59 |
| 3 | 3.00 | 6.50 | 11.93 | 4.75 | 16.73 | 23.84 | 107.45 | 70.89 | 132.07 | 115.95 |
| 4 | 4.00 | 5.00 | 9.28 | 3.25 | 27.42 | 50.73 | 121.67 | 63.73 | 214.62 | 213.04 |
| 5 | 5.00 | 6.00 | 8.98 | 8.24 | 23.91 | 27.09 | 108.18 | 112.73 | 179.23 | 212.39 |
| 6 | 4.00 | 5.00 | 9.32 | 3.93 | 22.61 | 31.77 | 97.77 | 63.60 | 147.04 | 125.93 |
| 7 | 5.00 | 8.00 | 9.11 | 3.27 | 27.89 | 93.25 | 103.72 | 57.19 | 185.00 | 332.97 |
| 8 | 4.00 | 6.50 | 9.75 | 5.19 | 26.45 | 59.22 | 124.67 | 71.48 | 213.97 | 276.53 |
| G_Mean | 4.37 | 6.14 | 9.08 | 4.03 | 22.71 | 49.88 | 103.70 | 64.67 | 160.86 | 201.46 |
| STDEV | 0.82 | 1.90 | 1.41 | 1.81 | 4.13 | 42.98 | 14.44 | 20.66 | 38.50 | 99.44 |
| SEM | 0.29 | 0.67 | 0.50 | 0.64 | 1.46 | 15.20 | 5.11 | 7.30 | 13.61 | 35.16 |
| % CV | 18.50 | 29.87 | 15.33 | 42.09 | 17.91 | 71.46 | 13.81 | 30.82 | 23.36 | 44.96 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Eftaiha, A.F.; Qinna, N.; Rashid, I.S.; Al Remawi, M.M.; Al Shami, M.R.; Arafat, T.A.; Badwan, A.A. Bioadhesive Controlled Metronidazole Release Matrix Based on Chitosan and Xanthan Gum. Mar. Drugs 2010, 8, 1716-1730. https://doi.org/10.3390/md8051716
Eftaiha AF, Qinna N, Rashid IS, Al Remawi MM, Al Shami MR, Arafat TA, Badwan AA. Bioadhesive Controlled Metronidazole Release Matrix Based on Chitosan and Xanthan Gum. Marine Drugs. 2010; 8(5):1716-1730. https://doi.org/10.3390/md8051716
Chicago/Turabian StyleEftaiha, Ala’a F., Nidal Qinna, Iyad S. Rashid, Mayyas M. Al Remawi, Munther R. Al Shami, Tawfiq A. Arafat, and Adnan A. Badwan. 2010. "Bioadhesive Controlled Metronidazole Release Matrix Based on Chitosan and Xanthan Gum" Marine Drugs 8, no. 5: 1716-1730. https://doi.org/10.3390/md8051716
APA StyleEftaiha, A. F., Qinna, N., Rashid, I. S., Al Remawi, M. M., Al Shami, M. R., Arafat, T. A., & Badwan, A. A. (2010). Bioadhesive Controlled Metronidazole Release Matrix Based on Chitosan and Xanthan Gum. Marine Drugs, 8(5), 1716-1730. https://doi.org/10.3390/md8051716




