Evaluation of Three Chitin Metal Silicate Co-Precipitates as a Potential Multifunctional Single Excipient in Tablet Formulations
Abstract
:1. Introduction
2. Results and Discussion
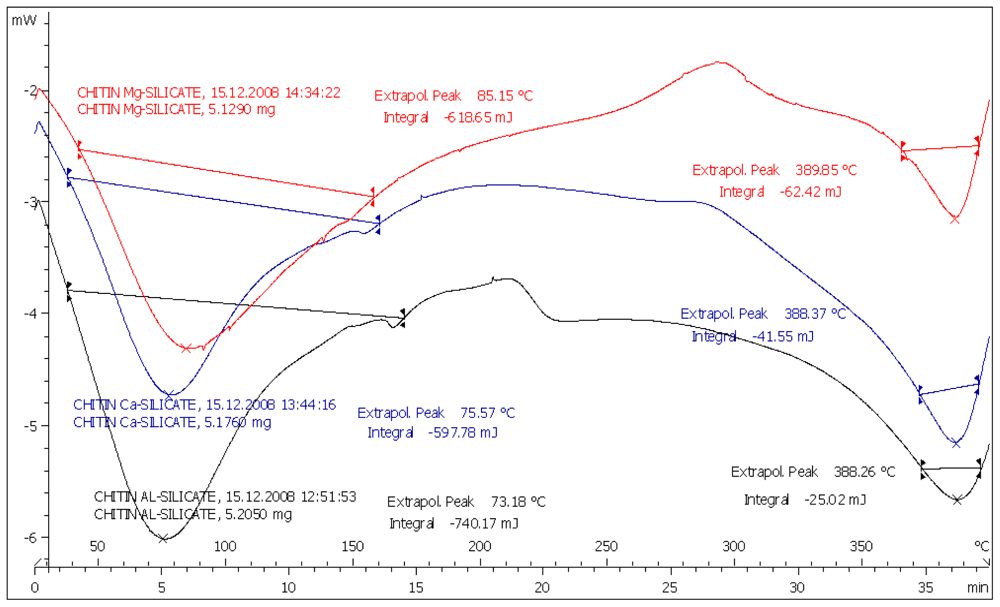
2.1. Preparation and Characterization of Metal Silicate and Their Compacts
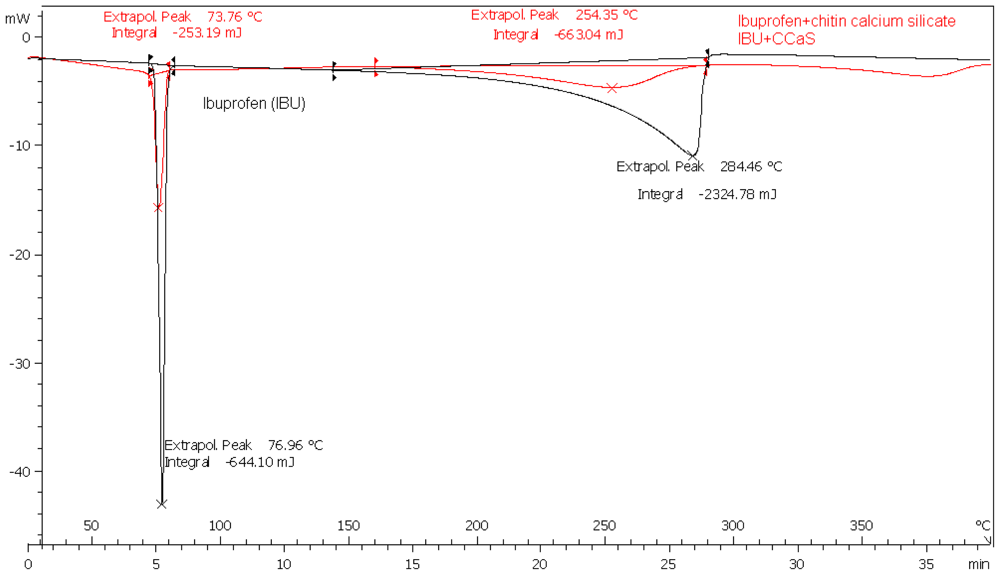
2.2. Drug Excipient Compatibility
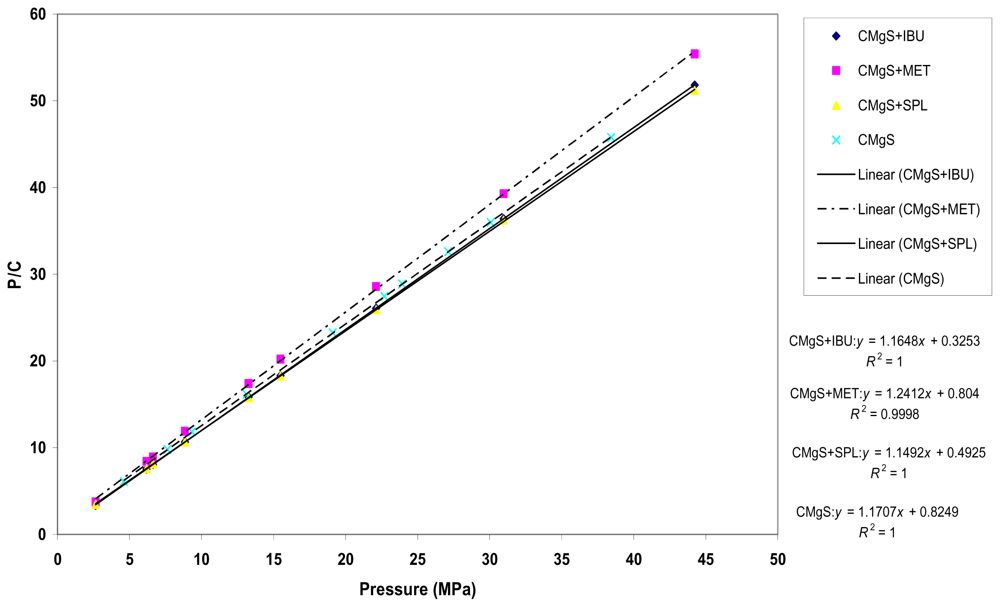
2.3. Compression Studies Analysis
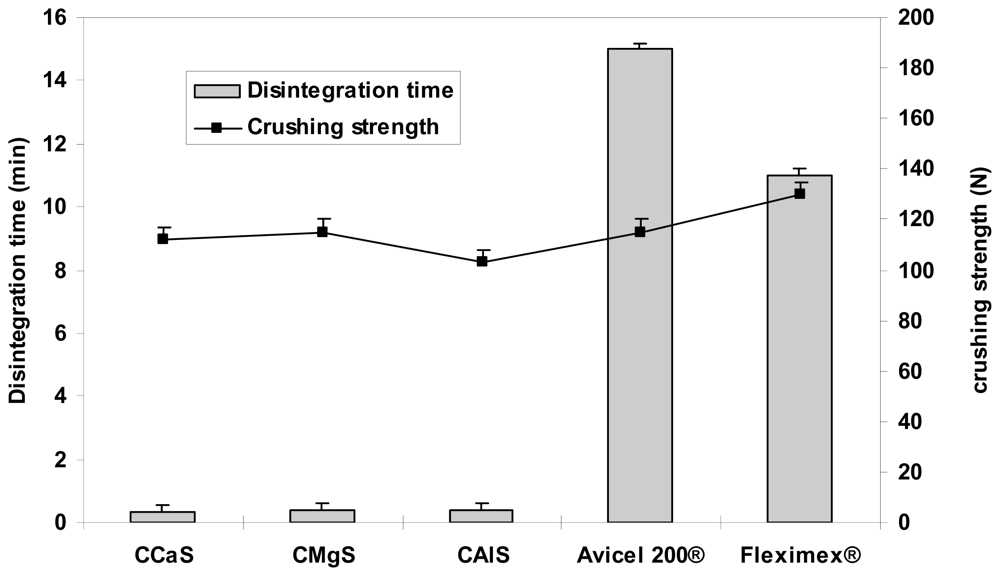
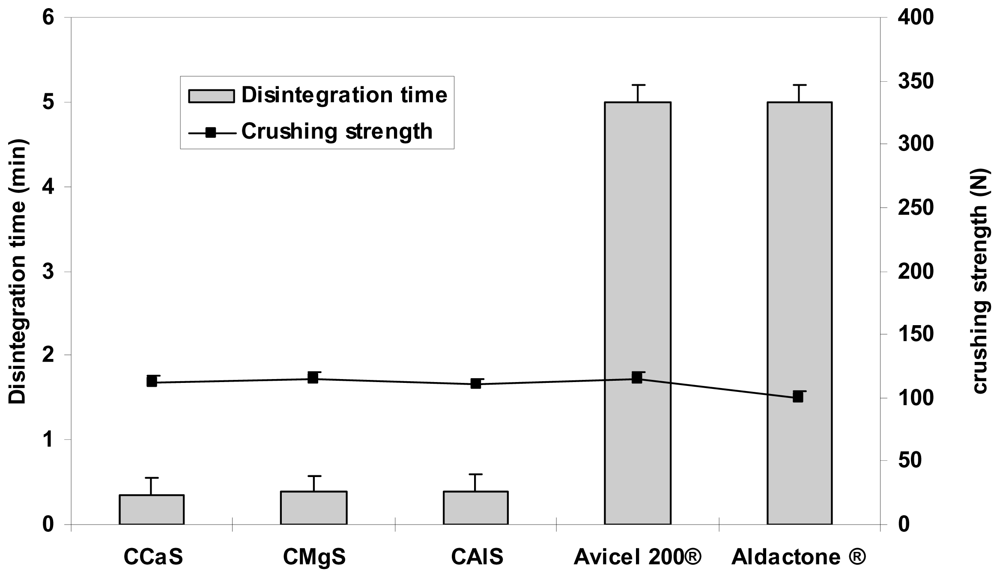
2.4. Physical Properties of the Tablets
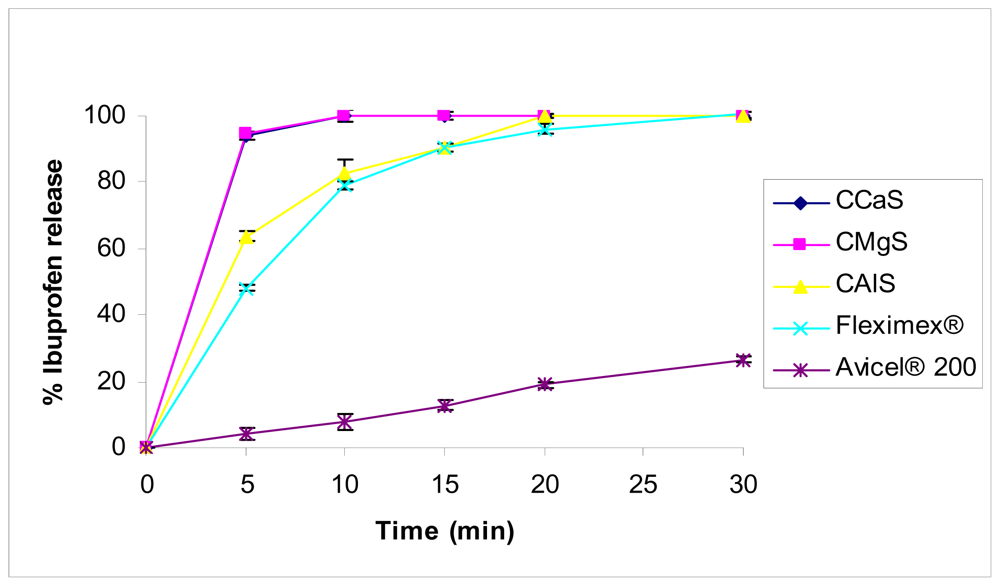
2.5. Drug Release Studies
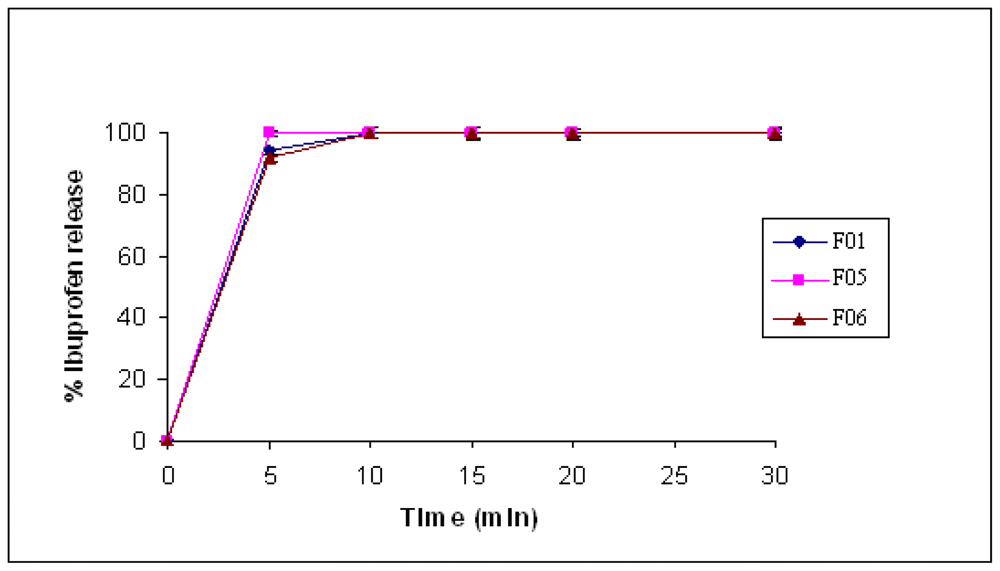
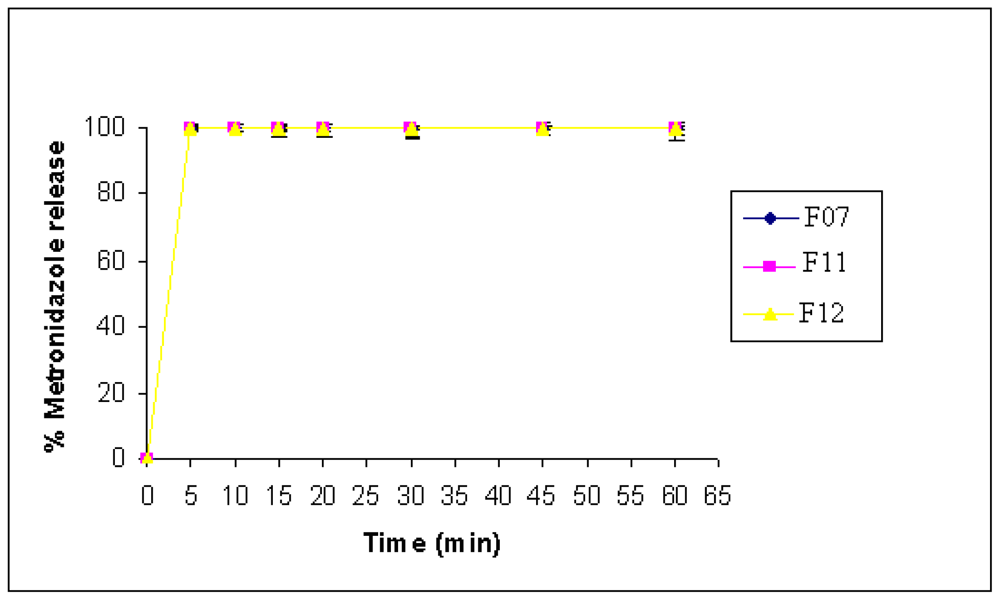
2.6. Tablets Prepared by Wet Granulation
3. Experimental
3.1. Materials
3.2. Methods
3.2.1. Preparation of Three Chitin Metal Silicates (CMS): Chitin-Ca, -Mg and -Al Silicate Co Precipitates
3.2.2. Characterization of the Compacts
3.2.2.1. Compatibility Study
3.2.2.2. Characterization of Compacts by Heckel and Kawkita Plots
3.2.3. Tablet Preparation
3.2.3.1. Preparation of Tablets by Direct Compression Method
3.2.3.2. Preparation of Tablets by Wet Granulation Using Chitin-Mg Silicate
3.2.4. Tablet Characterization
3.2.4.1. Tablet Thickness
3.2.4.2. Drug Content Uniformity
3.2.4.3. Dissolution Test
3.3. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Silverstein, I. Excipient GMP Quality Standards One Is Enough. Pharm Technol 2002, 25, 46–52. [Google Scholar]
- Armstrong, NA. Functionality Related Tests for Excipients. Int J Pharm 1997, 155, 1–5. [Google Scholar]
- Van de Waterbeemd, H; Testa, B. Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability, 2 ed; Wiley-VCH: New York, NY, USA, 2008. [Google Scholar]
- Jackson, K; Young, D; Pant, S. Drug-excipient interactions and their affect on absorption. Pharm Sci Technol Today 2000, 3, 336–345. [Google Scholar]
- Monedero Perales, MC; Munoz-Ruiz, A; Velasco Antequera, MV; Munoz Munoz, N; Jimenez-Castellanos, MR. Comparative Tableting and Microstructural Properties of a New Starch for Direct Compression. Drug Develop Ind Pharm 1996, 22, 689–695. [Google Scholar]
- Hwang, R; Peck, GR. A Systematic Evaluation of the Compression and Tablets Characteristics of Various Types of Microcrystalline Cellulose. Pharm Technol 2001, 24, 112–132. [Google Scholar]
- Reimerdes, D; Aufmuth, KP. Tabletting with Co-processed Lactose-Cellulose Excipients. Manuf Chem 1992, 63, 21–24. [Google Scholar]
- Lourdes Garzo’n, M; Villafuerte, L. Compactibility of mixtures of calcium carbonate and microcrystalline cellulose. Int J Pharm 2002, 231, 33–41. [Google Scholar]
- Patel, V; Patel, M; Patel, R. Chitosan: A Unique Pharmaceutical Excipient. Drug Deliv Technol 2005, 6, 30–40. [Google Scholar]
- El-Barghouthi, M; Eftaiha, A; Rashid, I; Al-Remawi, M; Badwan, A. A Novel Superdisintegrating Agent Made from Physically Modified Chitosan with Silicon Dioxide. Drug Develop Ind Pharm 2008, 34, 373–383. [Google Scholar]
- Rashid, I; Al-Remawi, M; Eftaiha, A; Badwan, A. Chitin silicone dioxide coprecipitate as a novel superdisintegrant. J Pharm Sci 2008, 97, 4955–4969. [Google Scholar]
- Rashid, I; Daraghmeh, N; Al-Remawi, M; Leharne, S; Chowdhry, B; Badwan, A. Characterization of Chitin-Metal Silicates as Binding Superdisintegrants. J Pharm Sci 2009, 98, 4887–4901. [Google Scholar]
- Mura, P; Manderioli, A; Bramanti, G; Furlanetto, S; Pinzauti, S. Utilization of differential scanning calorimetry as a screening technique to determine the compatibility of ketoprofen with excipients. Int J Pharm 1995, 119, 71–79. [Google Scholar]
- Verma, R; Garg, S. Selection of excipients for extended release formulations of glipizide through drug–excipient compatibility testing. J Pharmceut Biomed 2005, 38, 633–644. [Google Scholar]
- Martins, E; Christiana, I; Olobayo, K. Effect of Grewia gum on the mechanical properties of Paracetamol tablet formulations. Afr J Pharm Pharmaco 2008, 2, 001–006. [Google Scholar]
- Odeku, OA; Patani, BO. Evaluation of dika nut mucilage (Irvingia gabonensis) as binding agent in metronidazole tablet formulations. Pharm Develop Technol 2005, 10, 439–446. [Google Scholar]
- Gohel, MC; Parikh, RK; Brahmbhatt, BK; Shah, AR. Improving the tablet characteristics and Dissolution Profile of Ibuprofen by Using a Novel Coprocessed Superdisintegrant. AAPS PharmSciTech 2007, 8. Article 13. [Google Scholar]
- Medina, MR; Kumar, V. Evaluation of cellulose II powders as a potential multifunctional excipient in tablet formulations. Int J Pharm 2006, 322, 31–35. [Google Scholar]
- RxList, The Internet Drug Index. In Aldactone®, (spironolactone) Tablets, USP; 2008; pp. 1–8.
- Paronen, P; Iilla, J. Porosity-Pressure Functions Pharmaceutical Powder Compaction Technology; Marcel Dekker Inc: New York, NY, USA, 1996; pp. 55–75. [Google Scholar]
- Itiola, OA. Compressional characteristics of three starches and the mechanical properties of their tablets. Pharm World J 1991, 8, 91–94. [Google Scholar]
- Kawakita, K; Ludde, KH. Some considerations on powder compression equations. Powder Technol 1971, 4, 61–68. [Google Scholar]


| Formula | Py | A | DA | A0 | D0 | DB |
|---|---|---|---|---|---|---|
| IBU(400 mg) + CMgS(300 mg) | 81.97 | 1.7 | 0.82 | 0.4811 | 0.38 | 0.44 |
| MET(500 mg) + CMgS(300 mg) | 86.21 | 1.53 | 0.78 | 0.3592 | 0.30 | 0.48 |
| SPL(25 mg) + CMgS(275 mg) | 103.10 | 1.19 | 0.70 | 0.2324 | 0.21 | 0.49 |
| Chitin Mg-silicate | 153.85 | 0.72 | 0.51 | 0.1641 | 0.15 | 0.36 |
| Formula | 1/ab | 1/a | a | 1/b | R2 |
|---|---|---|---|---|---|
| IBU + CMgS | 0.33 | 1.16 | 0.86 | 0.29 | 1.0000 |
| MET + CMgS | 0.80 | 1.24 | 0.80 | 0.36 | 0.9998 |
| SPL + CMgS | 0.49 | 1.15 | 0.87 | 0.43 | 1.0000 |
| CMgS | 0.82 | 1.17 | 0.85 | 0.69 | 1.0000 |
| Formula # | Drug | Tablet content (%) | Tablet thickness (mm) | Tablet friability (%) |
|---|---|---|---|---|
| F1 | IBU | 98 ± 2 | 0.71 ± 0.04 | 0.32 ± 0.05 |
| F2 | IBU | 101 ± 2 | 0.73 ± 0.05 | 0.42 ± 0.05 |
| F3 | IBU | 98 ± 03 | 0.74 ± 0.05 | 0.31 ± 0.05 |
| F4 | IBU | 100 ± 2 | 0.63 ± 0.04 | 0.40 ± 0.03 |
| F5 | IBU | 99 ± 2 | 0.74 ± 0.04 | 0.36 ± 0.05 |
| F6 | IBU | 101 ± 2 | 0.75 ± 0.04 | 0.33 ± 0.04 |
| F7 | MET | 98 ± 2 | 0.63 ± 0.04 | 0.29 ± 0.05 |
| F8 | MET | 99 ± 2 | 0.65 ± 0.04 | 0.38 ± 0.03 |
| F9 | MET | 98 ± 2 | 0.62 ± 0.04 | 0.35 ± 0.04 |
| F10 | MET | 101 ± 2 | 0.57 ± 0.04 | 0.36 ± 0.05 |
| F11 | MET | 99 ± 2 | 0.63 ± 0.04 | 0.42 ± 0.03 |
| F12 | MET | 101 ± 2 | 0.63 ± 0.04 | 0.39 ± 0.04 |
| F13 | SPL | 100 ± 2 | 0.38 ± 0.04 | 0.41 ± 0.05 |
| F14 | SPL | 98 ± 2 | 0.39 ± 0.04 | 0.44 ± 0.05 |
| F15 | SPL | 99 ± 2 | 0.40 ± 0.03 | 0.39 ± 0.05 |
| F16 | SPL | 100 ± 2 | 0.29 ± 0.05 | 0.47 ± 0.05 |
| Flexamex® | IBU | 101 ± 2 | 0.57 ± 0.05 | NR* |
| Dumozole® | MET | 99 ± 2 | 0.63 ± 0.05 | NR |
| Aldactone® | SPL | 98 ± 2 | 0.36 ± 0.04 | 0.56 ± 0.05 |
| Formula # | Crushing strength (N) | Disintegration time (min) |
|---|---|---|
| F05 (WIG, IBU) | 104 ± 5 | <1 min |
| F06 (WEG, IBU) | 75 ± 5 | <1 min |
| F11 (WIG, MET) | 71 ± 5 | <1 min |
| F12 (WEG, MET) | 43 ± 5 | <1 min |
| Formula # | Drug* | Process | CMgS* | CCaS* | CALS* | Avicel® 200* |
|---|---|---|---|---|---|---|
| F 1 | 400 (IBU) | DC | 300 | |||
| F 2 | 400 (IBU) | DC | 300 | |||
| F 3 | 400 (IBU) | DC | 300 | |||
| F 4 | 400 (IBU) | DC | 300 | |||
| F 5 | 400 (IBU) | WIG | 300 | |||
| F 6 | 400 (IBU) | WEG | 300 | |||
| F 7 | 500 (MET) | DC | 300 | |||
| F 8 | 500 (MET) | DC | 300 | |||
| F 9 | 500 (MET) | DC | 300 | |||
| F 10 | 500 (MET) | DC | 300 | |||
| F 11 | 500 (MET) | WIG | 200 | |||
| F 12 | 500 (MET) | WEG | 200 | |||
| F 13 | 25 (SPL) | DC | 275 | |||
| F 14 | 25 (SPL) | DC | 275 | |||
| F 15 | 25 (SPL) | DC | 275 | |||
| F 16 | 25 (SPL) | DC | 27 | |||
| Ingredient | IBU tablet | SPL tablet | MET Tablet |
|---|---|---|---|
| USP Apparatus | II | II | II |
| Dissolution medium (900 mL) | phosphate buffer pH7.2 | Water | 0.1 N HCl |
| Agitation rate (rpm) | 50 | 75 | 100 |
| Temperature (°C) | 37 | 37 | 37 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hamid, R.A.-S.; Al-Akayleh, F.; Shubair, M.; Rashid, I.; Al Remawi, M.; Badwan, A. Evaluation of Three Chitin Metal Silicate Co-Precipitates as a Potential Multifunctional Single Excipient in Tablet Formulations. Mar. Drugs 2010, 8, 1699-1715. https://doi.org/10.3390/md8051699
Hamid RA-S, Al-Akayleh F, Shubair M, Rashid I, Al Remawi M, Badwan A. Evaluation of Three Chitin Metal Silicate Co-Precipitates as a Potential Multifunctional Single Excipient in Tablet Formulations. Marine Drugs. 2010; 8(5):1699-1715. https://doi.org/10.3390/md8051699
Chicago/Turabian StyleHamid, Rana Al-Shaikh, Faisal Al-Akayleh, Mohammad Shubair, Iyad Rashid, Mayyas Al Remawi, and Adnan Badwan. 2010. "Evaluation of Three Chitin Metal Silicate Co-Precipitates as a Potential Multifunctional Single Excipient in Tablet Formulations" Marine Drugs 8, no. 5: 1699-1715. https://doi.org/10.3390/md8051699
APA StyleHamid, R. A.-S., Al-Akayleh, F., Shubair, M., Rashid, I., Al Remawi, M., & Badwan, A. (2010). Evaluation of Three Chitin Metal Silicate Co-Precipitates as a Potential Multifunctional Single Excipient in Tablet Formulations. Marine Drugs, 8(5), 1699-1715. https://doi.org/10.3390/md8051699




