Sterols from the Madagascar Sponge Fascaplysinopsis sp.
Abstract
:1. Introduction
2. Results and Discussion
- - 7.1% of conventional mono-unsaturated Δ5-sterols including cholesta-5,24-dien-3β-ol (or desmosterol). The latter constitutes near half of the Δ5-sterols (3.2%).
- - 18.1% of conventional mono-unsaturated Δ7-sterols represented by cholest-7-en-3β-ol (10.5%) and 24-methylcholesta-7,22E-dien-3β-ol (7.6%).
- - 61.0% of known di-unsaturated nucleus Δ5,7-sterols with a preponderance of 24-methylcholesta-5,7,22E-trien-3β-ol (19.5%), cholesta-5,7,22E-trien-3β-ol (12.2%), cholesta-5,7-dien-3β-ol (9.9%) and 24-ethylcholesta-5,7-dien-3β-ol (8.4%).
3. Experimental Section
3.1. Sponge material
3.2. Extraction and isolation of sterols
3.3. Analysis of sterols
3.4. Free radical scavenging activity
3.5. Antioxidant activity
4. Conclusions
Acknowledgements
References
- Bishara, A; Rudi, A; Aknin, M; Neumann, D; Ben-Califa, N; Kashman, Y. Salarins A and B and Tulearin A: new cytotoxic sponge-derived macrolides. Org Lett 2008, 10, 153–156. [Google Scholar]
- Bishara, A; Rudi, A; Aknin, M; Neumann, D; Ben-Califa, N; Kashman, Y. Salarin C, a new cytotoxic sponge-derived nitrogenous macrolide. Tetrahedron Lett 2008, 49, 4355–4358. [Google Scholar]
- Bishara, A; Rudi, A; Aknin, M; Neumann, D; Ben-Califa, N; Kashman, Y. Salarins D-J, seven new nitrogenous macrolides from the madagascar sponge Fascaplysinopsis sp. Tetrahedron 2010, 66, 4339–4345. [Google Scholar]
- Bishara, A; Rudi, A; Goldberg, I; Aknin, M; Kashman, Y. Tulearin A, B and C; structure and absolute configuration. Tetrahedron Lett 2010, 50, 3820–3822. [Google Scholar]
- Bishara, A; Rudi, A; Aknin, M; Neumann, D; Ben-Califa, N; Kashman, Y. Taumycins A and B, two bioactive lipodepsipeptides from the Madagascar sponge Fascaplysinopsis sp. Org Lett 2008, 10, 4307–4309. [Google Scholar]
- Bishara, A; Rudi, A; Goldberg, I; Aknin, M; Neumann, D; Ben-Califa, N; Kashman, Y. Tausalarin C: a new bioactive marine sponge-derived nitrogenous bismacrolide. Organic Lett 2009, 11, 3538–3541. [Google Scholar]
- Klein, D; Braekman, JC; Daloze, D; Hoffmann, L; Castillo, G; Demoulin, V. Madangolide and laingolide A, two novel macrolides from Lyngbia bouillonii (cyanobacteria). J Nat Prod 1999, 62, 934–936. [Google Scholar]
- Klein, D; Braekman, JC; Daloze, D; Hoffmann, L; Castillo, G; Demoulin, V. Laingolide, a novel 15-membered macrolide from Lyngbia bouillonii (cyanophyceae). Tetrahedron Lett 1996, 37, 7519–7520. [Google Scholar]
- Bergmann, W; Burke, DC. Contributions to the study of marine products. XXXIX. The nucleosides of sponges III. Spongothymidine and spongouridine. J Org Chem 1955, 20, 1501–1507. [Google Scholar]
- Wang, G. Diversity and biotechnological potential-associated microbial consortia. J Ind Microbiol Biotechnol 2006, 33, 545–551. [Google Scholar]
- Thomas, TRA; Kavlekar, DP; Lokabharathi, PA. Marine drugs from sponge-microbe association - A review.
- Taylor, MW; Radax, R; Steger, D; Wagner, M. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev 2007, 71, 295–347. [Google Scholar]
- Radjassa, OK; Martens, T; Grossart, H; Brinkhoff, T; Sabdono, A; Simmon, M. Antagonistic activity of a marine bacterium Pseudoalteromonas luteoviolacea TAB4.2 associated with coral Acroporas sp. J Biol Sci 2007, 7, 239–246. [Google Scholar]
- Hentschel, U; Hopke, J; Horn, M; Friedrich, AB; Wagner, M; Hacker, J; Moore, BS. Molecular evidence for a uniform microbial community in sponges from different oceans. Appl Environ Microbiol 2002, 68, 4431–4440. [Google Scholar]
- Proksch, P; Edrada, RA; Ebel, R. Drugs from the seas: current status and microbiological implications. Appl. Biotechnol. Microbiol. 2002, 59, 125–134. [Google Scholar]
- Gillan, FT; Stoilov, IL; Thompson, JE; Hogg, RW; Wilkinson, CR; Djerassi, C. Fatty acids as biological markers for bacterial symbionts in sponges. Lipids 1988, 23, 139–145. [Google Scholar]
- Barnathan, G; Genin, E; Velosaotsy, NE; Kornprobst, JM; Al-Lihaibi, S; Al-Solfyani, A; Nongonierma, R. Phospholipid fatty acids and sterols of two Cinachyrella sponges from the Saudi Arabian Red Sea: comparison with Cinachyrella species from other origins. Comp Biochem Pysiol Part B 2003, 135, 297–308. [Google Scholar]
- Khan, AS; Goad, L. The sterol constituents and Δ5,7-sterols content of some bivalve mollusks. Comp Biochem Physiol 1983, 76b, 569–573. [Google Scholar]
- Goad, LJ; Akihisa, T. Analysis of Sterols; Blackie Academic and Professional: London, UK, 2008. [Google Scholar]
- Elenkov, I; Milkova, T; Andreev, S; Popov, S. Sterol composition and biosynthesis in the Black Sea sponge Dysidea fragilis. Comp Biochem Physiol 1994, 107B, 547–551. [Google Scholar]
- Arreguin-Espinosa, R; Arreguin, B; Hernandez-Santoyo, A; Rodriguez-Romero, A. Sterol composition and biosynthesis in the sponge Spheciospongia vesparia. J Chem Technol Biotechnol 1998, 72, 245–248. [Google Scholar]
- Marquez, DM; Martinez, A. Antileishmanial epidioxysterols from the Colombian marine sponge Ircinia campana are oxidation products from naturally occurring Δ5,7 sterols. Vitae, Revista de la Facultad de Quimica Farmaceutica 2007, 14, 61–66. [Google Scholar]
- Popov, S; Stoilov, I; Demirev, P. Gas chromatographic/Mass spectrometric method for the identification of Δ5,7 sterols in sterol mixtures. Biomed Mass Spectrom 1984, 11, 608–610. [Google Scholar]
- Erpenbeck, D; van Soest, RWM. Status and perspective of sponge chemosystematics. Mar Biotechnol 2007, 9, 2–19. [Google Scholar]
- Bergquist, PR; Karuso, P; Cambie, RC; Smith, DJ. Sterol composition and classification of the Porifera. Biochem Syst Ecol 1991, 19, 17–24. [Google Scholar]
- Dini, A; Sica, D; Boniforti, L. Two new Δ5,7-sterols from two spongidae sponges. Comp Biochem Physiol Part B 1984, 78, 741–744. [Google Scholar]
- De Rosa, M; Minale, L; Sodano, G. Metabolism in porifera - II. Distribution of sterols. Comp Biochem Physiol 1973, 46B, 823–837. [Google Scholar]
- De Rosa, S; Tommonaro, G; Slantchev, K; Stefano, K; Popov, S. Lipophylic metabolites from the marine sponge Ircinia muscarum and its cell culture. Mar Biol 2002, 140, 465–470. [Google Scholar]
- Sica, D; Picialli, V; Pronzato, R. Δ5,7-sterols from the sponges Ircinia pipetta and Dysidea avara. Identification of cholesta-5,7,24-trien-3-β-ol. Comp Biochem Physiol 1987, 88B, 293–296. [Google Scholar]
- Delseth, C; Tolela, L; Scheuer, PJ; Wells, RJ; Djerassi, C. 5α-24-Nor-cholestan-3β-ol and (24Z)-stigmasta-5,7,24(28)-trien-3-β-ol, two new marine sterols from the Pacific sponges Terpios zeteki and Dysidea herbacea. Helv Chim Acta 1979, 62, 101–109. [Google Scholar]
- Dogovic, NP; Spiteller, M; Gasic, MJ. Sterols from marine sponge Dysidea tupha. Bull Soc Chim Beograg 1979, 44, 321–324. [Google Scholar]
- De Rosa, S; Milone, A; Popov, S. Sterol composition of the sponge Fasciospongia cavernosa, from the Adriatic, Aegean and Tyrrhenian seas. Comp Biochem Physiol 1999, 123B, 235–239. [Google Scholar]
- Stoilov, IL; Bladocha-Moreau, M; Thompson, JE; Djerassi, C. Biosynthetic studies of marine lipids. 12. Biosynthesis in marine sponges of sterols possessing the Δ5,7-nucleus typical of fungi and the 24-alkyl side chain characteristic of plants. Tetrahedron 1987, 43, 2213–2222. [Google Scholar]
- John, V; Stoilov, IL; Djerassi, C; Karuso, P; Poiner, A; Scheuer, PJ. Biosynthetic studies on marine lipids. 20. Sequence of double bond introduction in the sponge sterol 24β-methyl cholesta- 5,7,22,25-tetraen-3β-ol. J Org Chem 1989, 54, 1642–1647. [Google Scholar]
- Zimmerman, MP; Thomas, FC; Thompson, JE; Djerassi, C; Streiner, H; Evans, E; Murphy, PT. The distribution of lipids and sterols in cell types from the marine sponge Pseudaxinyssa sp. Lipids 1989, 24, 210–216. [Google Scholar]
- Santalova, EA; Makarieva, TN; Gorshkova, IA; Dmitrenok, AS; Krasokhin, VB; Stonic, VA. Sterols from six marine sponges. Biochem Syst Ecol 2004, 32, 153–167. [Google Scholar]
- Takao, T; Watanabe, N; Yagi, A; Sakata, K. A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci Biotech Biochem 1994, 58, 1780–1783. [Google Scholar]
- Pratt, DE; Miller, EE. A flavonoid antioxidant in Spanish peanuts (Arachia hypogeoa). J Am Oil Chem Soc 1984, 61, 1064–1067. [Google Scholar]
- Fromont, J; Kerr, S; Kerr, R; Riddle, M; Murphy, P. Chemotaxonomic relationships within, and comparison between, the orders Haplosclerida and Petrosida (Porifera: Demospongiae) using sterol complements. Biochem Syst Ecol 1994, 22, 735–752. [Google Scholar]

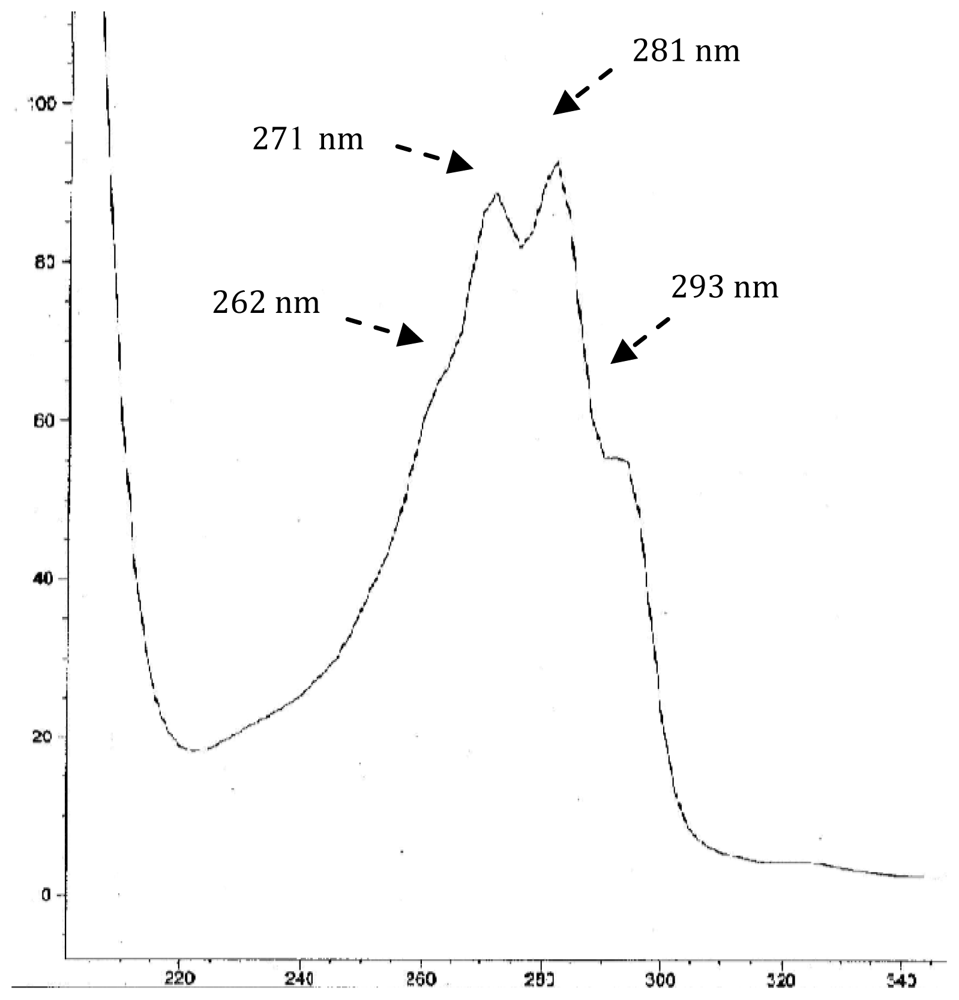


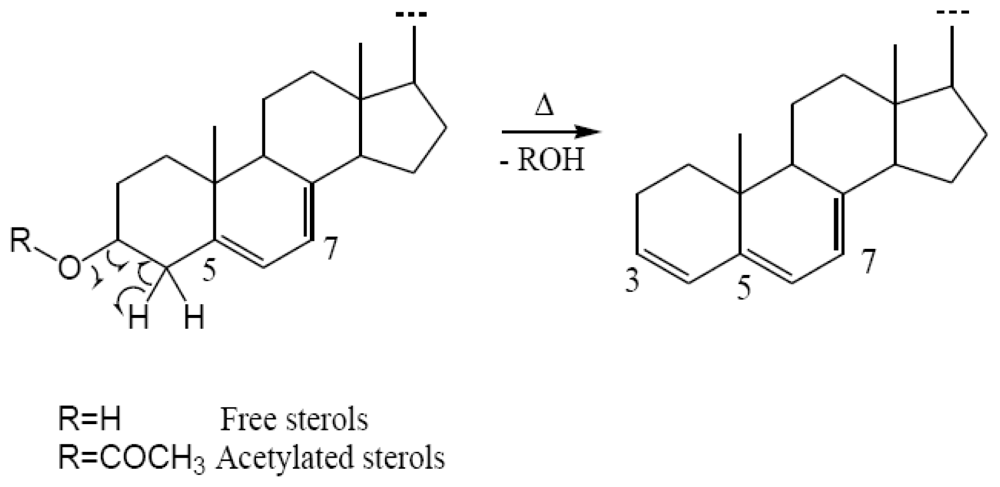


| Sterols | |||||
|---|---|---|---|---|---|
| Name | Molecular Mass | Short Désignation | RRTa | Composition (%) | |
| 1 | 24-Norcholesta-5,7,22E-trien-3β-ol | 368 | C26 Δ5,7,22 | 0.84 | 1.7 |
| 2 | Cholesta-5,22E-dien-3β-ol | 384 | C27 Δ5,22 | 0.94 | 1.5 |
| 3 | Cholesta-5,7,22E-trien-3β-ol | 382 | C27 Δ5,7,22 | 0.98 | 12.2 |
| 4 | Cholesta-5,24-dien-3β-ol | 384 | C27 Δ5,24 | 1.00 | 3.2 |
| 5 | Cholesta-5,7-dien-3β-ol | 384 | C27 Δ5,7 | 1.04 | 9.9 |
| 6 | Cholest-7-en-3β-ol | 386 | C27 Δ7 | 1.05 | 10.5 |
| 7 | 24ξ-Methylcholesta-5,7,22E-trien-3β-ol | 396 | C28 Δ5,7,22 | 1.09 | 19.5 |
| 8 | 24ξ-Methylcholesta-7,22E-dien-3β-ol | 398 | C28 Δ7,22 | 1.12 | 7.6 |
| 9 | Unknownc | 396 | C28 | 1.16 | 1.7 |
| 10 | 24ξ-Methylcholesta-5,7-dien-3β-ol | 398 | C28 Δ5,7 | 1.18 | 1.4 |
| 11 | 24ξ-Ethylcholesta-5,7,22-trien-3β-ol | 410 | C29 Δ5,7,22 | 1.22 | 3.5 |
| 12 | 24ξ-Ethylcholest-5-en-3β-ol | 414 | C29 Δ5 | 1.25 | 2.4 |
| 13 | 24ξ-Ethylcholesta-5,7,24(24’)-trien-3β-ol | 410 | C29Δ5,7,24(24’) | 1.28 | 1.1 |
| 14 | 24ξ-Ethylcholesta-5,7-dien-3β-ol | 412 | C29 Δ5,7 | 1.33 | 8.4 |
| 15 | Unknownd | 410 | C29 Δ5,7,22 | 1.35 | 3.3 |
| 16 | 24ξ-Methylcholesta-7-en-3β-ol | 414 | C29 Δ7 | 1.35 | tr b |
Side chain length | ||||||
|---|---|---|---|---|---|---|
| C26 | C27 | C28 | C29 | Total | ||
| Unsaturation in the nucleus | Δ5 | 4.7 | 2.4 | 7.1 | ||
| Δ7 | 10.5 | 7.6 | tr | 18.1 | ||
| Δ5,7 | 1.7 | 22.1 | 20.9 | 16.3 | 61.0 | |
| Total | 1.7 | 37.3 | 28.5 | 18.7 | 86.2 | |
| F1 | F2 | F3 | |
|---|---|---|---|
| DPPH test | − | + | − |
| β-carotene test | − | + | − |
| Sponges | Unsaturation in the nucleus | [Ref] | ||||
|---|---|---|---|---|---|---|
| Δ0 | Δ5 | Δ7 | Δ5,7 | |||
| Order Dictyoceratida | ||||||
| Family Spongidae | ||||||
| Coscinoderma sp. | + | [25] | ||||
| Hippospongia sp. | + | + | [25] | |||
| Ircinia campana | + | [22] | ||||
| Ircinia foetida | + | + | + | [26] | ||
| Ircinia muscarum | + | + | [27,28] | |||
| Ircinia pipetta | + | + | [29] | |||
| Ircinia sp. | + | + | [25] | |||
| Ircinia spinosula | + | [27] | ||||
| Ircinia variabilis | + | + | + | [26] | ||
| Hyattella intestinalis | + | + | [25] | |||
| Spongia nitens | + | [27] | ||||
| Spongia officinalis | + | [27] | ||||
| Family Dysideidae | ||||||
| Dysidea avara | + | + | [25,27] | |||
| Dysidea fragilis | + | + | + | [20] | ||
| Dysidea herbacea | + | + | + | + | [30] | |
| Dysidea sp. | + | + | [25] | |||
| Dysidea tupha | + | [31] | ||||
| Family Thorectidae | ||||||
| Cacospongia sp. | + | + | + | [25] | ||
| Fascaplysinopsis sp. | + | + | [25] | |||
| Fasciospongia carvernosa | + | + | + | [32] | ||
| Luffariella sp. | + | + | + | [25] | ||
| Psammocinia sp. | + | + | + | [25] | ||
| Thorectandra excavatus | + | + | [25] | |||
| Thorecta sp. | + | + | [25] | |||
| Side chains | without C-24 alkylation | with C-24 alkylation | total | |||
|---|---|---|---|---|---|---|
| Saturated | Δ5,7 | - | 9.2 | 1.4 | 8.4 | 19.7 |
| unsaturated | Δ5,7,22 | 1.7 | 12.2 | 19.5 | 6.8 | 40.2 |
| Δ5,7,24 | - | - | - | 1.1 | 1.1 | |
| total | 1.7 | 22.1 | 20.9 | 16.3 | 61.0 | |
| Zone | GPS coordinates | Specimens | Yield (%) |
|---|---|---|---|
| 1 | 22 32.958S–43°13.070E | 01 | 6.0% |
| 02 | 4.4% | ||
| 2 | 22°31.513S–43°12.904E | 03 | 5.2% |
| 3 | 22°30.954S–43°12.636E | 04 | 6.1% |
| 05 | 5.5% | ||
| 4 | 22°30.952S–43°12.558E | 06 | 3.1% |
| 5 | 22°30.766S–43°12.635E | 07 | 5.6% |
| 08 | 3.8% | ||
| 6 | 22°31.822S–43°12.939E | 09 | 4.0% |
| 10 | 5.2% |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aknin, M.; Gros, E.; Vacelet, J.; Kashman, Y.; Gauvin-Bialecki, A. Sterols from the Madagascar Sponge Fascaplysinopsis sp. Mar. Drugs 2010, 8, 2961-2975. https://doi.org/10.3390/md8122961
Aknin M, Gros E, Vacelet J, Kashman Y, Gauvin-Bialecki A. Sterols from the Madagascar Sponge Fascaplysinopsis sp. Marine Drugs. 2010; 8(12):2961-2975. https://doi.org/10.3390/md8122961
Chicago/Turabian StyleAknin, Maurice, Emmanuelle Gros, Jean Vacelet, Yoel Kashman, and Anne Gauvin-Bialecki. 2010. "Sterols from the Madagascar Sponge Fascaplysinopsis sp." Marine Drugs 8, no. 12: 2961-2975. https://doi.org/10.3390/md8122961
APA StyleAknin, M., Gros, E., Vacelet, J., Kashman, Y., & Gauvin-Bialecki, A. (2010). Sterols from the Madagascar Sponge Fascaplysinopsis sp. Marine Drugs, 8(12), 2961-2975. https://doi.org/10.3390/md8122961




