Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications
Abstract
:Abbreviations
| AA | arachidonic acid |
| ALA | α-linolenic acid |
| DHA | docosahexaenoic acid |
| DW | dry weight |
| EPA | eicosapentaenoic acid |
| FA | fatty acid |
| FAME | fatty acid methyl ester |
| GC-MS | gas chromatography-mass spectrometry |
| GLA | γ-linolenic acid |
| LA | linoleic acid |
| MUFA | monounsaturated fatty acid |
| PCA | principal component analysis |
| PUFA | polyunsaturated fatty acid |
| SFA | saturated fatty acid |
| VLCPUFA | very long chain polyunsaturated fatty acid |
1. Introduction
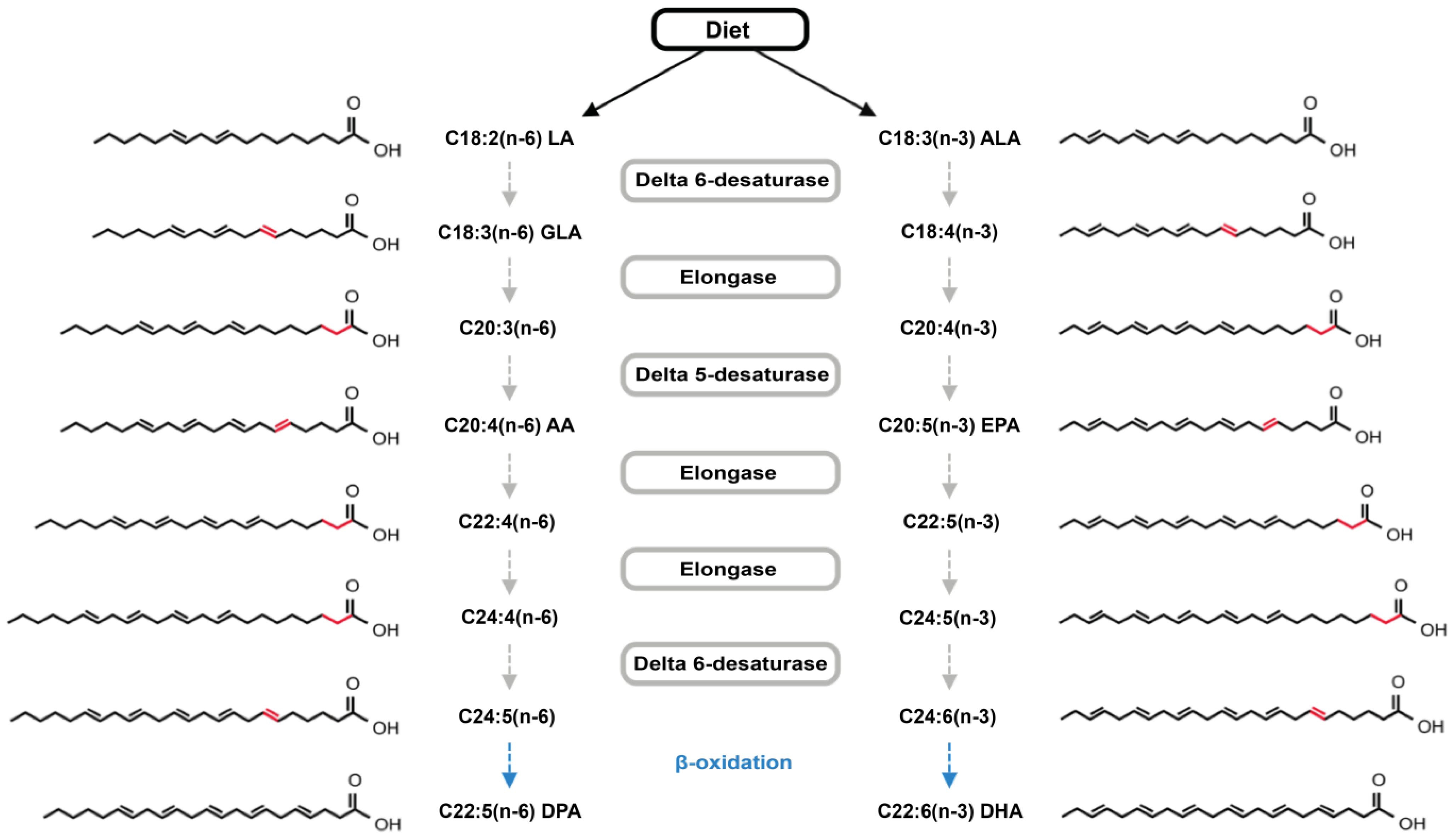
2. Results and Discussion
2.1. FAME Concentration
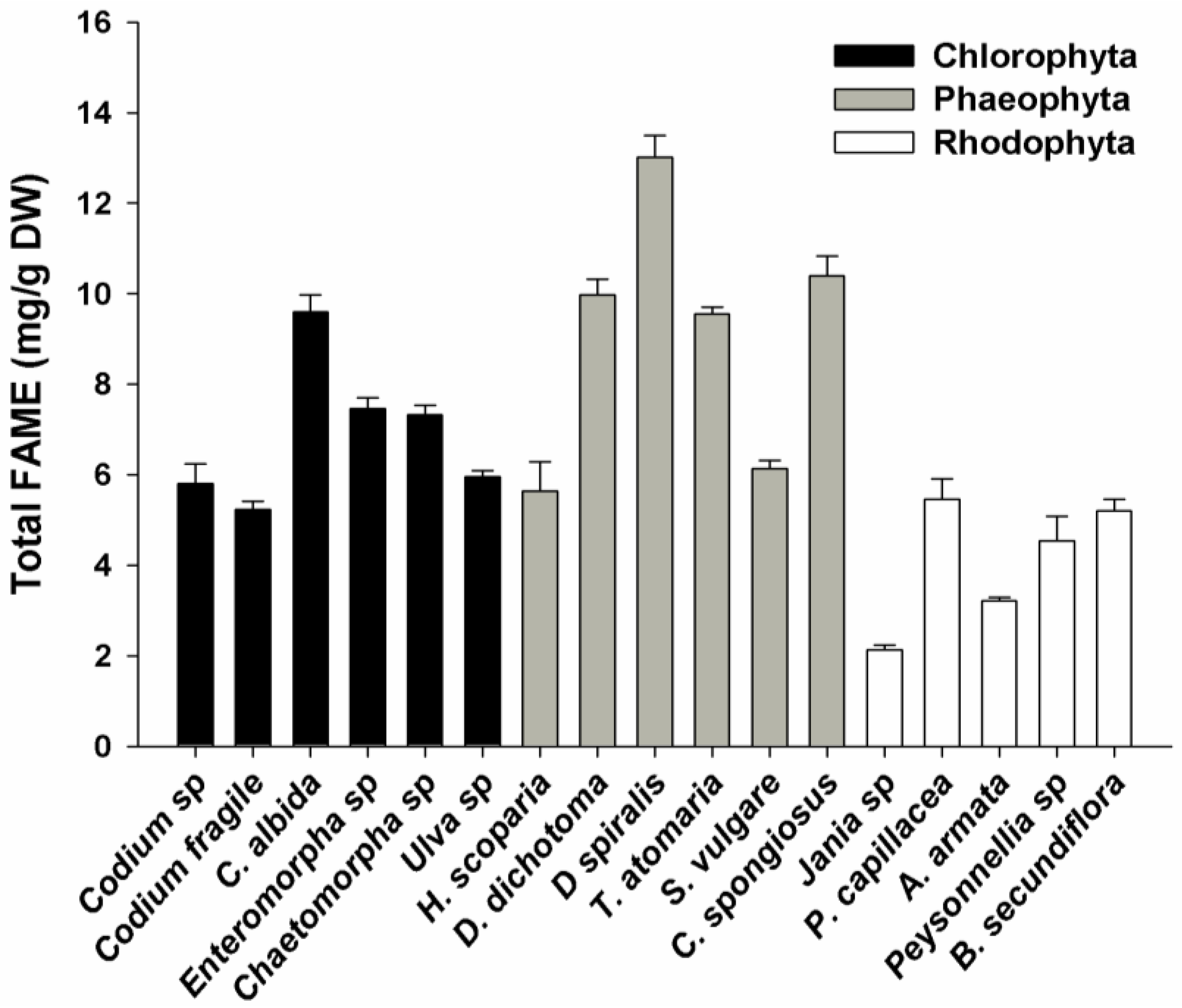
2.2. FAME Profile
2.2.1. Chlorophyta
| Fatty acid (%) | Codium sp. | Codium fragile | Cladophora albida | Enteromorpha sp. | Chaetomorpha sp. | Ulva sp. |
|---|---|---|---|---|---|---|
| C10:0 | 0.42 ± 0.02 | n.d. | n.d. | n.d. | n.d. | n.d. |
| C12:0 | 2.86 ± 0.15 | 1.53 ± 0.14 | 0.21 ± 0.01 | 0.39 ± 0.01 | 0.58 ± 0.02 | n.d. |
| C14:0 | 4.42 ± 0.09 | 3.29 ± 0.18 | 12.48 ± 0.04 | 2.74 ± 0.04 | 21.74 ± 0.24 | 2.28 ± 0.03 |
| C15:0 | 0.32 ± 0.01 | n.d. | 0.56 ± 0.02 | 0.90 ± 0.02 | 0.38 ± 0.01 | 0.49 ± 0.01 |
| C16:0 | 32.75 ± 1.31 | 40.73 ± 0.83 | 33.04 ± 0.52 | 52.66 ± 0.80 | 33.24 ± 0.86 | 50.11 ± 0.34 |
| C17:0 | 0.27 ± 0.01 | n.d. | 0.26 ± 0.01 | 0.28 ± 0.01 | n.d. | n.d. |
| C18:0 | 1.34 ± 0.07 | 1.51 ± 0.06 | 1.28 ± 0.19 | 1.80 ± 0.01 | 1.03 ± 0.09 | 1.14 ± 0.02 |
| C20:0 | 0.98 ± 0.10 | 1.01 ± 0.05 | 0.38 ± 0.01 | 2.08 ± 0.04 | n.d. | n.d. |
| C22:0 | 6.28 ± 0.54 | 10.98 ± 1.06 | 0.75 ± 0.08 | 3.99 ± 0.10 | 1.00 ± 0.23 | 5.01 ± 0.78 |
| C24:0 | 1.65 ± 0.15 | 3.32 ± 0.63 | 1.08. ± 0.01 | n.d. | 2.61 ± 0.17 | n.d. |
| ∑ SFA | 51.28 ± 1.44 | 62.37 ± 1.50 | 50.03 ± 0.56 | 64.85 ± 0.81 | 60.59 ± 0.94 | 59.04 ± 0.85 |
| C16:1n-7 | 3.34 ± 0.16 | 5.41 ± 0.17 | 13.90 ± 0.09 | 6.36 ± 0.07 | 2.83 ± 0.02 | 11.81 ± 0.14 |
| C18:1n-9c | 9.15 ± 0.04 | 1.49 ± 0.18 | 12.51 ± 0.02 | 9.08 ± 0.06 | 8.47 ± 0.03 | 5.51 ± 0.07 |
| C18:1n-9t | 0.89 ± 0.19 | 0.40 ± 0.94 | 0.79 ± 0.05 | 0.87 ± 0.10 | 0.30 ± 0.13 | n.d. |
| C20:1n-9 | 0.21 ± 0.04 | n.d. | 0.18 ± 0.01 | 0.31 ± 0.01 | 0.24 ± 0.01 | n.d. |
| C22:1n-9 | n.d. | n.d. | 0.35 ± 0.04 | 0.90 ± 0.02 | n.d. | n.d. |
| ∑ MUFA | 13.59 ±0.45 | 15.29 ± 0.97 | 27.73 ± 0.11 | 17.52 ± 0.14 | 11.84 ± 0.14 | 17.31 ± 0.15 |
| C16:2n-6 | 3.15 ± 0.10 | 1.40 ± 0.16 | 2.46 ± 0.01 | 0.83 ± 0.01 | 0.70 ± 0.02 | n.d. |
| C18:2n-6 | 12.23 ± 0.48 | 9.21 ± 0.32 | 15.54 ± 0.22 | 10.04 ± 1.20 | 24.55 ± 0.32 | 5.65 ± 0.11 |
| C20:2n-6 | n.d. | n.d. | n.d. | n.d. | 0.61 ± 0.01 | n.d. |
| C16:3n-3 | 8.11 ± 0.39 | 5.92 ± 0.29 | n.d. | 0.50 ± 0.01 | n.d. | n.d. |
| C16:3n-6 | n.d. | n.d. | n.d. | n.d. | 0.86 ± 0.01 | n.d. |
| C18:3n-3 | n.d. | n.d. | n.d. | n.d. | n.d. | 16.51 ± 0.23 |
| C18:3n-6 | 3.45 ± 0.16 | n.d. | n.d. | n.d. | n.d. | n.d. |
| C20:3n-6 | 0.75 ± 0.03 | 0.91 ± 0.12 | n.d. | n.d. | n.d. | n.d. |
| C20:4n-6 | 6.03 ±0.58 | 3.41 ± 0.20 | 1.37 ± 0.07 | 2.76 ± 0.09 | n.d. | n.d. |
| C20:5n-3 | 1.40 ± 0.28 | 1.48 ± 0.17 | 2.02 ± 0.05 | 3.52 ± 0.06 | 0.85 ± 0.04 | 1.50 ± 0.04 |
| C22:6n-3 | n.d. | n.d. | 0.86 ± 0.03 | n.d. | n.d. | n.d. |
| ∑ PUFA | 35.13 ± 0.91 | 22.34 ± 0.55 | 22.24 ± 0.24 | 17.64 ± 1.21 | 27.57 ± 0.33 | 23.65 ± 0.26 |
| ∑n-3 | 9.52 ± 0.48 | 7.40 ± 0.34 | 2.88 ± 0.05 | 4.02 ± 0.06 | 0.85 ± 0.04 | 18.00 ± 0.23 |
| ∑n-6 | 25.61 ± 0.78 | 14.93 ± 0.43 | 19.36 ± 0.23 | 13.62 ± 1.21 | 26.72 ± 0.32 | 5.65 ± 0.11 |
| ∑n-6/∑n-3 | 2.69 | 2.02 | 6.73 | 3.39 | 31.25 | 0.31 |
| PUFA/SFA | 0.68 | 0.36 | 0.44 | 0.27 | 0.46 | 0.40 |
2.2.2. Phaeophyta
| Fatty acid (%) | Halopteris scoparia | Dictyota dichotoma | Dictyota spiralis | Taonia atomaria | Sargassum vulgare | Cladostephus spongiosus |
|---|---|---|---|---|---|---|
| C10:0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| C12:0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| C14:0 | 6.84 ± 0.20 | 15.42 ± 0.31 | 14.00 ± 0.13 | 7.07 ± 0.21 | 6.33 ± 0.02 | 7.40 ± 0.05 |
| C15:0 | 0.43 ± 0.03 | 0.97 ± 0.06 | 0.73 ± 0.01 | 0.56 ± 0.01 | 0.62 ± 0.01 | 0.40 ± 0.01 |
| C16:0 | 24.36 ± 0.45 | 24.75 ± 0.32 | 21.69 ± 0.22 | 25.41 ± 0.97 | 31.23 ± 0.24 | 21.33 ± 0.35 |
| C17:0 | 0.37 ± 0.02 | n.d. | 0.23 ± 0.01 | 0.18 ± 0.01 | 0.22 ± 0.01 | 0.24 ± 0.01 |
| C18:0 | 1.92 ± 0.10 | 2.85 ± 0.08 | 2.43 ± 0.04 | 1.04 ± 0.21 | 1.62 ± 0.11 | 1.15 ± 0.03 |
| C20:0 | 0.98 ± 0.05 | 1.98 ± 0.12 | 1.12 ± 0.17 | 0.74 ± 0.09 | 0.94 ± 0.02 | 1.22 ± 0.04 |
| C22:0 | n.d. | n.d. | n.d. | 0.48 ± 0.05 | 1.38 ± 0.11 | n.d. |
| C24:0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| ∑ SFA | 34.89 ± 0.51 | 45.98 ± 0.47 | 40.20 ± 0.31 | 35.47 ± 1.02 | 42.34 ± 0.28 | 31.74 ± 0.35 |
| C16:1n-7 | 5.47 ± 0.09 | 15.49 ± 0.09 | 19.58 ± 0.12 | 8.09 ± 0.10 | 8.61 ± 0.11 | 5.72 ± 0.28 |
| C18:1n-9c | 5.57 ± 0.09 | 7.25 ± 0.06 | 7.57 ± 0.06 | 7.12 ± 0.21 | 6.08 ± 0.04 | 6.43 ± 0.18 |
| C18:1n-9t | 2.66 ± 0.25 | 1.24 ± 0.07 | 1.90 ± 0.04 | 0.97 ± 0.50 | 1.32 ± 0.02 | n.d. |
| C20:1n-9 | 0.40 ± 0.02 | 0.31 ± 0.01 | 0.29 ± 0.03 | 0.30 ± 0.08 | 0.56 ± 0.01 | n.d. |
| C22:1n-9 | n.d. | n.d. | n.d. | 0.85 ± 0.24 | 2.46 ± 0.06 | n.d. |
| ∑ MUFA | 14.09 ± 0.28 | 24.28 ± 0.13 | 29.34 ± 0.14 | 17.34 ± 0.61 | 19.03 ± 0.14 | 12.15 ± 0.33 |
| C16:2n-6 | n.d. | 0.44 ± 0.02 | n.d. | n.d. | n.d. | n.d. |
| C18:2n-6 | 20.35 ± 0.14 | 5.55 ± 0.02 | 6.05 ± 0.10 | 10.08 ± 0.32 | 7.59 ± 0.02 | 23.14 ± 0.26 |
| C16:3n-3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| C16:3n-6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| C18:3n-3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| C18:3n-6 | n.d. | 2.63 ± 0.20 | 3.38 ± 0.05 | 1.71 ± 0.08 | n.d. | 3.10 ± 0.03 |
| C20:3n-6 | 1.33 ± 0.09 | 2.60 ± 0.06 | 2.63 ± 0.14 | 2.14 ± 0.04 | 1.98 ± 0.29 | 1.62 ± 0.16 |
| C20:4n-6 | 13.96 ± 0.36 | 11.46 ± 0.59 | 18.40 ± 0.21 | 18.64 ± 0.11 | 18.64 ± 0.04 | 16.43 ± 0.13 |
| C20:5n-3 | 14.39 ± 0.25 | 6.57 ± 0.22 | n.d. | 13.55 ± 0.55 | 8.60 ± 0.12 | 11.46 ± 0.10 |
| C22:6n-3 | 0.99 ± 0.86 | n.d. | n.d. | 0.84 ± 0.03 | 1.50 ± 0.07 | n.d. |
| ∑ PUFA | 51.01 ± 0.98 | 29.74 ± 0.67 | 30.46 ± 0.28 | 47.19 ± 0.65 | 38.63 ± 0.32 | 56.11 ± 0.35 |
| ∑n-3 | 15.37 ± 0.89 | 6.57 ± 0.22 | n.d. | 14.40 ± 0.55 | 10.10 ± 0.14 | 11.46 ± 0.10 |
| ∑n-6 | 35.64 ± 0.40 | 23.16 ± 0.63 | 30.46 ± 0.28 | 32.79 ± 0.35 | 28.52 ± 0.29 | 44.65 ± 0.33 |
| ∑n-6/∑n-3 | 2.32 | 3.52 | n.a. | 2.28 | 2.82 | 3.89 |
| PUFA/SFA | 1.46 | 0.65 | 0.76 | 1.33 | 0.91 | 1.77 |
2.2.3. Rhodophyta
| Fatty acid (%) | Jania sp. | Pterocladiella capillacea | Asparagopsis armata | Peyssonnelia sp. | Bornetia secundiflora |
|---|---|---|---|---|---|
| C10:0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| C12:0 | n.d. | n.d. | 2.32 ± 0.09 | n.d. | 0.52 ± 0.01 |
| C14:0 | 4.25 ± 0.08 | 9.68 ± 0.10 | 21.67 ± 0.11 | 5.50 ± 0.17 | 10.29 ± 0.01 |
| C15:0 | 0.92 ± 0.01 | 0.41 ± 0.01 | 0.81 ± 0.02 | 0.60 ± 0.02 | 0.84 ± 0.17 |
| C16:0 | 44.44 ± 0.29 | 47.94 ± 0.64 | 53.21 ± 0.52 | 29.50 ± 0.41 | 32.93 ± 0.75 |
| C17:0 | n.d. | 0.39 ± 0.01 | 0.49 ± 0.02 | 0.61 ± 0.03 | 0.24 ± 0.01 |
| C18:0 | 1.94 ± 0.06 | 2.21 ± 0.04 | 2.81 ± 0.16 | 2.94 ± 0.09 | 1.33 ± 0.22 |
| C20:0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| C22:0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| C24:0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| ∑ SFA | 51.56 ± 0.31 | 60.62 ± 0.65 | 81.31 ± 0.56 | 39.15 ± 0.46 | 46.14 ± 0.82 |
| C16:1n-7 | 2.38 ± 0.07 | 3.15 ± 0.09 | 4.87 ± 0.92 | 3.45 ± 0.07 | 12.75 ± 0.26 |
| C18:1n-9c | 2.54 ± 0.03 | 3.33 ± 0.01 | 2.78 ± 0.19 | 3.08 ± 0.02 | 2.13 ± 0.09 |
| C18:1n-9t | 2.01 ± 0.01 | 1.97 ± 0.02 | 6.34 ± 0.12 | 1.91 ± 0.07 | 3.78 ± 0.36 |
| C20:1n-9 | 0.70 ± 0.01 | n.d. | n.d. | 0.42 ± 0.02 | n.d. |
| C22:1 n-9 | n.d. | n.d. | n.d. | n.d. | n.d. |
| ∑ MUFA | 7.64 ± 0.08 | 8.45 ± 0.10 | 13.99 ± 0.94 | 8.87 ± 0.11 | 18.66 ± 0.46 |
| C16:2n-6 | n.d. | n.d. | n.d. | n.d. | n.d. |
| C18:2n-6 | 2.37 ± 0.42 | 2.27 ± 0.05 | n.d. | 1.58 ± 0.08 | 1.64 ± 0.10 |
| C16:3n-3 | n.d. | n.d. | n.d. | n.d. | n.d. |
| C16:3n-6 | n.d. | n.d. | n.d. | n.d. | n.d. |
| C18:3n-3 | n.d. | 0.93 ± 0.06 | n.d. | n.d. | n.d. |
| C18:3n-6 | n.d. | 1.01 ± 0.04 | n.d. | n.d. | 2.53 ± 0.02 |
| C20:3n-6 | n.d. | 1.14 ± 0.36 | n.d. | n.d. | n.d. |
| C20:4n-6 | 12.99 ± 0.13 | 10.33 ± 0.09 | 1.79 ± 0.34 | 26.59 ± 0.31 | 3.78 ± 0.10 |
| C20:5n-3 | 25.46 ± 0.53 | 15.26 ± 0.13 | 2.90 ± 0.15 | 18.52 ± 0.43 | 27.26 ± 0.64 |
| C22:6n-3 | n.d. | n.d. | n.d. | 4.86 ± 0.18 | n.d. |
| ∑ PUFA | 40.81 ± 0.69 | 30.94 ± 0.40 | 4.70 ± 0.38 | 51.98 ± 0.53 | 35.20 ± 0.66 |
| ∑n-3 | 25.46 ± 0.53 | 16.19 ± 0.14 | 2.90 ± 0.15 | 23.38 ± 0.47 | 27.26 ± 0.64 |
| ∑n-6 | 15.35 ± 0.44 | 14.74 ± 0.37 | 1.79 ± 0.34 | 28.60 ± 0.25 | 7.94 ± 0.14 |
| ∑n-6/∑n-3 | 0.60 | 0.91 | 0.62 | 1.92 | 0.29 |
| PUFA/SFA | 0.79 | 0.51 | 0.06 | 1.33 | 0.76 |
2.2.4. Multivariate Analysis
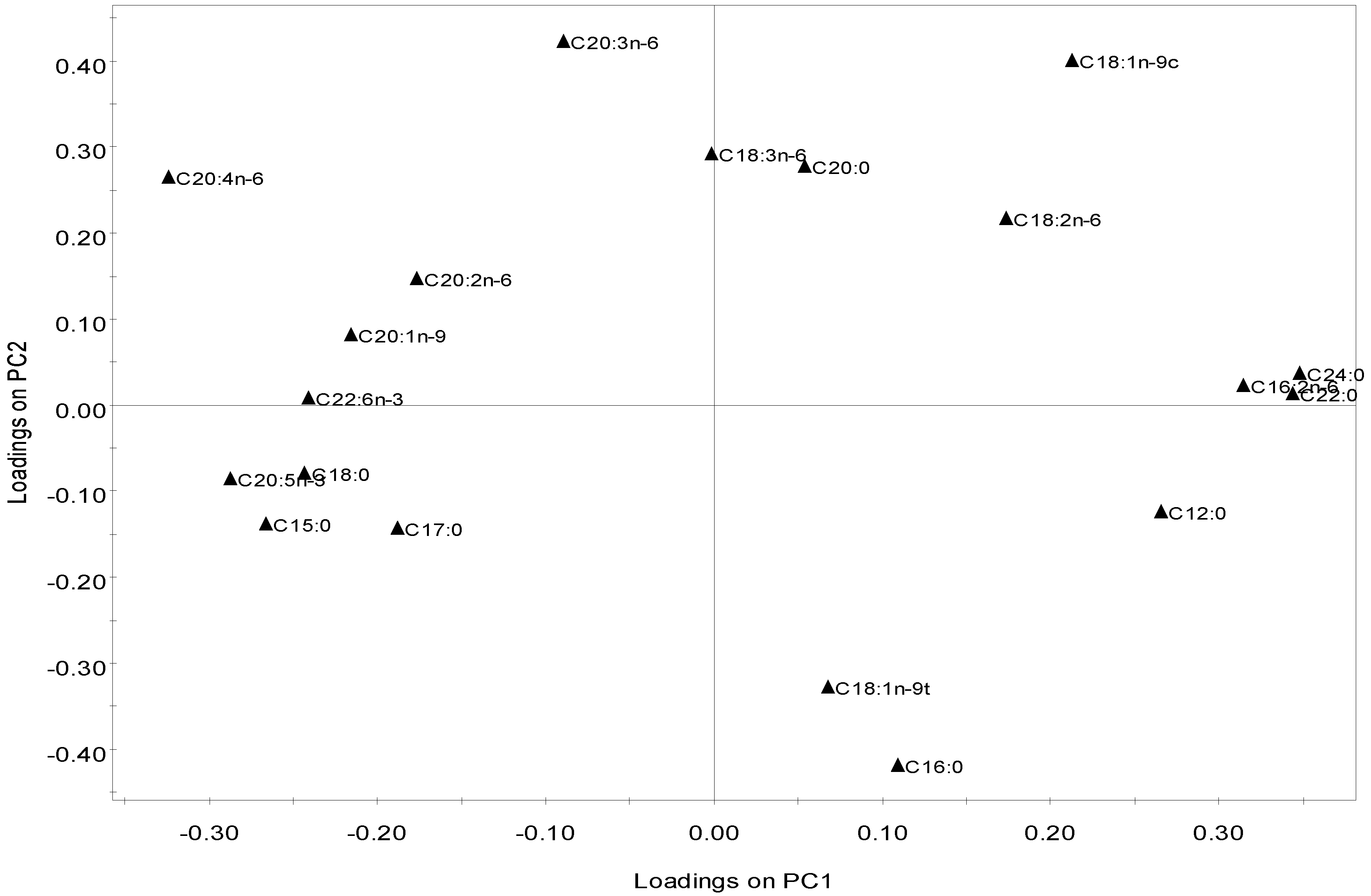
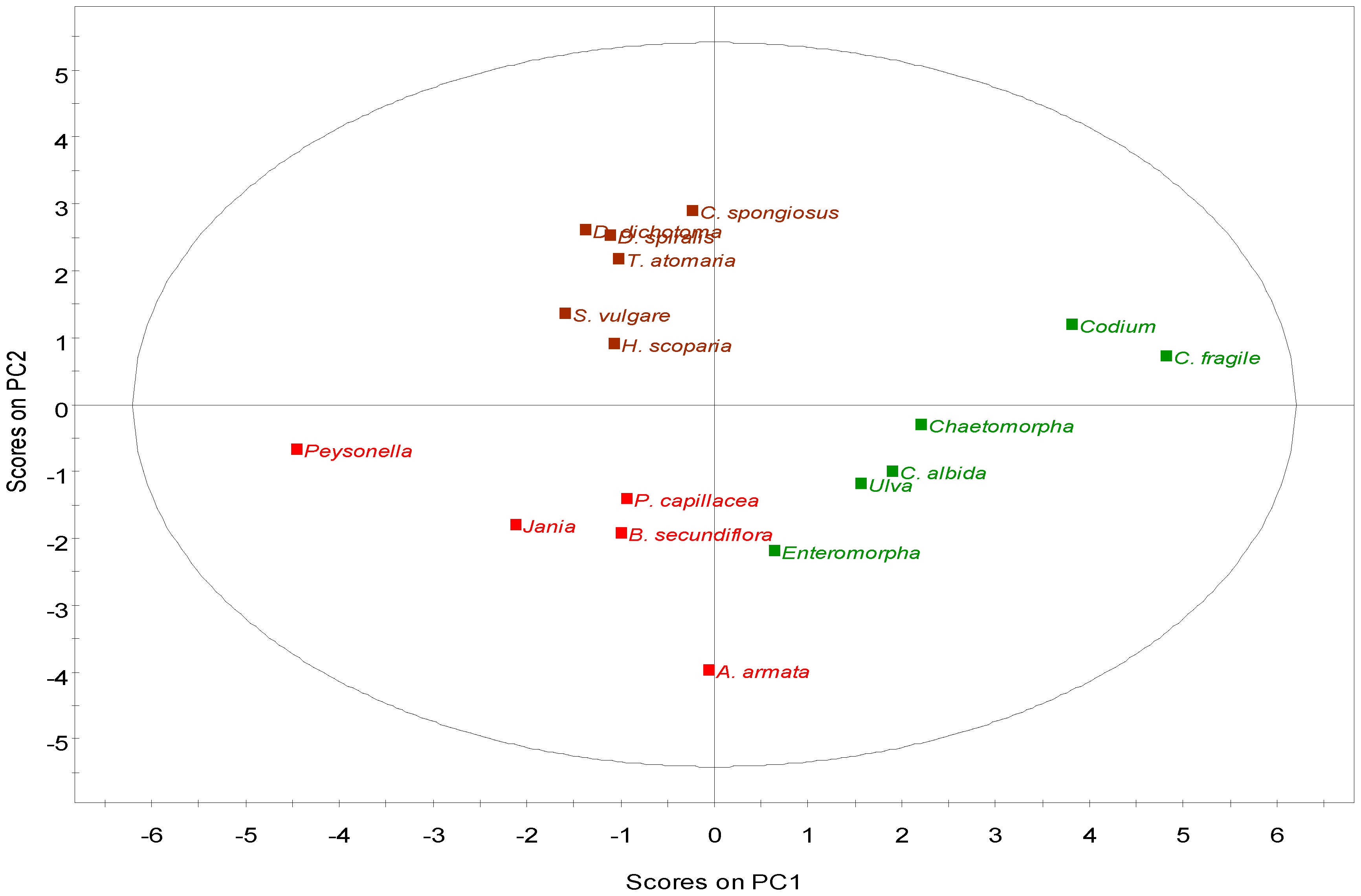
2.3. Nutritional and Pharmaceutical Applications
3. Experimental Section
3.1. Sampling and Processing of Macroalgae
3.2. FAME Preparation
3.3. Determination of FAME Profile by GC-MS
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Gill, I.; Valivety, R. Polyunsaturated fatty acids, part 1: Occurrence, biological activities and applications. Trends Biotechnol. 1997, 15, 401–409. [Google Scholar] [CrossRef]
- Radwan, S.S. Sources of C20-polyunsaturated fatty acids for biotechnological use. Appl. Microbiol. Biot. 1991, 35, 421–430. [Google Scholar]
- Ward, O.P.; Singh, A. Omega-3/6 fatty acids: alternative sources of production. Process Biochem. 2005, 40, 3627–3652. [Google Scholar] [CrossRef]
- Huang, C.B.; Ebersole, J.L. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral Microbiol. 2010, 25, 75–80. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of high added-value compounds—a brief review of recent work. Biotechnol. Progr. 2011, 27, 597–613. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease effects on risk factors, molecular pathways and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Field, C.J.; Schley, P.D. Evidence for potential mechanisms for the effect of conjugated linoleic acid on tumor metabolism and immune function: Lessons from n-3 fatty acids. Am. J. Clin. Nutr. 2004, 79, 1190–1198. [Google Scholar]
- Das, M.; Zuniga, E.; Ojima, I. Novel taxoid-based tumor-targeting drug conjugates. Chim. Oggi 2009, 27, 54–56. [Google Scholar]
- Lindequist, U.; Schweder, T. Marine Biotechnology. In Biotechnology; Rehm, H.-J., Reed, G., Eds.; Wiley-VCH: Weinheim, Germany, 2001; Volume 10, pp. 441–484. [Google Scholar]
- Emken, E.A.; Adolf, R.O.; Gully, R.M. Dietary linoleic acid infuences desaturation and acylation of deuterium-labelled linoleic and linolenic acids in young adult males. Biochim. Biophys. Acta 1994, 1213, 277–288. [Google Scholar]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wootton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar]
- Burdge, G.C.; Finnegan, Y.E.; Minihane, A.M.; Williams, C.M.; Wootton, S.A. Effect of altered dietary n-3 fatty acid intake upon plasma lipid fatty acid composition, conversion of [13C] α-linolenic acid to longer-chain fatty acids and partitioning towards β-oxidation in older men. Br. J. Nutr. 2003, 90, 311–321. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461.
- Marszalek, J.R.; Lodish, H.F. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annu. Rev. Cell Dev. Biol. 2005, 21, 633–657. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerod, H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquacult. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Van Ginneken, V.J.; Helsper, J.P.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis. 2011, 10, 104. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Harwood, J.L. Effects of Environment on the Acyl Lipids of Algae and Higher Plants. In Structure, Function and Metabolism of Plant Lipids; Siegenthaler, P.A., Eichenberger, W., Eds.; Elsevier Science Press: Amsterdam, The Netherlands, 1984; pp. 543–550. [Google Scholar]
- Khotimchenko, S.V.; Vaskovsky, V.E.; Titlyanova, T.V. Fatty acids of marine algae from the Pacific Coast of North California. Bot. Mar. 2002, 45, 17–22. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Han, L.; Lou, Q. Fatty acids of some algae from the Bohai Sea. Phytochemistry 2002, 59, 157–161. [Google Scholar]
- Khotimchenko, S.V. Fatty acids of green macrophytic algae from the Sea of Japan. Phytochemistry 1993, 32, 1203–1207. [Google Scholar]
- Graeve, M.; Kattner, G.; Wiencke, C.; Karsten, U. Fatty acid composition of Arctic and Antarctic macroalgae: Indicator of phylogenetic and trophic relationships. Mar. Ecol. Prog. Ser. 2002, 231, 67–74. [Google Scholar] [CrossRef]
- Vaskovsky, V.E.; Khotimchenko, S.V.; Xia, B.; Hefang, L. Polar lipids and fatty acids of some marine macrophytes from the Yellow Sea. Phytochemistry 1996, 42, 1347–1356. [Google Scholar]
- Xu, X.-Q.; Tran, V.H.; Kraft, G.; Beardall, J. Fatty acids of six Codium species from southeast Australia. Phytochemistry 1998, 48, 1335–1339. [Google Scholar]
- Johns, R.B.; Nichols, P.D.; Perry, G.J. Fatty acid composition of ten marine algae from Australian waters. Phytochemistry 1979, 18, 799–802. [Google Scholar]
- Khotimchenko, S.V. Fatty acids of brown algae from the Russian Far East. Phytochemistry 1998, 49, 2363–2369. [Google Scholar]
- Khotimchenko, S.V.; Vaskovsky, V.E.; Przhemenetskaya, V.F. Distribution of eicosapentaenoic and arachidonic acids in different species of Gracilaria. Phytochemistry 1991, 30, 207–209. [Google Scholar]
- Narayan, B.; Miyashita, K.; Hosakawa, M. Comparative evaluation of fatty acid composition of different Sargassum (Fucales, Phaeophyta) species harvested from temperate and tropical waters. J. Aquat. Food Prod. Technol. 2004, 13, 53–70. [Google Scholar] [CrossRef]
- Galloway, A.W.E.; Britton-Simmons, K.H.; Duggins, D.O.; Gabrielson, P.W.; Brett, M.T. Fatty acid signatures differentiate marine macrophytes at ordinal and family ranks. J. Phycol. 2012, 48, 956–965. [Google Scholar] [CrossRef]
- Al-Hasan, R.H.; Hantash, F.M.; Radwan, S.S. Enriching marine macroalgae with eicosatetraenoic (arachidonic) and eicosapentaenoic acids by chilling. Appl. Microbiol. Biotechnol. 1991, 35, 530–535. [Google Scholar]
- Fleurence, J.; Gutbier, G.; Mabeau, S.; Leray, C. Fatty acids from 11 marine macroalgae of the French Brittany coast. J. Appl. Phycol. 1994, 6, 527–532. [Google Scholar] [CrossRef]
- Hanson, C.E.; Hyndes, G.A.; Wang, F.W. Differentiation of benthic marine primary producers using stable isotopes and fatty acids: Implications to food web studies. Aquat. Bot. 2010, 93, 114–122. [Google Scholar] [CrossRef]
- Bagga, D.; Wang, L.; Farias-Eisner, R.; Glaspy, J.A.; Reddy, S.T. Differential effects of prostaglandin derived from ω-6 and ω-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 1751–1756. [Google Scholar]
- Robinson, J.G.; Stone, N.J. Antiatherosclerotic and Antithrombotic Effects of Omega-3 Fatty Acids. Am. J. Cardiol. 2006, 98, 39–49. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Edidin, M. Polyunsaturated fatty acids and membrane organization: The balance between immunotherapy and susceptibility to infection. Chem. Phys. Lipids 2008, 153, 24–33. [Google Scholar] [CrossRef]
- Wang, J.; Luo, T.; Li, S.; Zhao, J. The powerful applications of polyunsaturated fatty acids in improving the therapeutic efficacy of anticancer drugs. Expert Opin. Drug Deliv. 2012, 9, 1–7. [Google Scholar]
- FAO Fisheries and Aquaculture Department, The State of World Fisheries and Aquaculture 2010; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; p. 197.
- Napier, J.A. The production of unusual fatty acids in transgenic plants. Annu. Rev. Plant Biol. 2007, 58, 295–319. [Google Scholar] [CrossRef]
- Luning, K.; Pang, S.J. Mass cultivation of seaweeds: Current aspects and approaches. J. Appl. Phycol. 2003, 15, 115–119. [Google Scholar] [CrossRef]
- Zemke-White, W.L.; Ohno, M. World seaweed utilisation: An end-of-century summary. J. Appl. Phycol. 1999, 11, 369–376. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper. No. 441; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; p. 118. [Google Scholar]
- Veena, C.K.; Josephine, A.; Preetha, S.P.; Varalakshmi, P. Beneficial role of sulfated polysaccharides from edible seaweed Fucus vesiculosus in experimental hyperoxaluria. Food Chem. 2007, 100, 1552–1559. [Google Scholar]
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 10, 1920-1935. https://doi.org/10.3390/md10091920
Pereira H, Barreira L, Figueiredo F, Custódio L, Vizetto-Duarte C, Polo C, Rešek E, Engelen A, Varela J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Marine Drugs. 2012; 10(9):1920-1935. https://doi.org/10.3390/md10091920
Chicago/Turabian StylePereira, Hugo, Luísa Barreira, Filipe Figueiredo, Luísa Custódio, Catarina Vizetto-Duarte, Cristina Polo, Eva Rešek, Aschwin Engelen, and João Varela. 2012. "Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications" Marine Drugs 10, no. 9: 1920-1935. https://doi.org/10.3390/md10091920
APA StylePereira, H., Barreira, L., Figueiredo, F., Custódio, L., Vizetto-Duarte, C., Polo, C., Rešek, E., Engelen, A., & Varela, J. (2012). Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Marine Drugs, 10(9), 1920-1935. https://doi.org/10.3390/md10091920





