Meat Nutritional Value and Exoskeleton Valorisation of Callinectes sapidus from Three Sites of Biological and Ecological Interest in Morocco: Scientific Insights Toward a Management Strategy in the Mediterranean Sea
Abstract
1. Introduction
2. Results
2.1. Morphological and Yield Characteristics of Callinectes Sapidus
2.2. Physicochemical Characterization of Meat and Exoskeleton
2.2.1. Meat
2.2.2. Exoskeleton
2.3. Chemical Extraction and Characterization of Chitin and Chitosan
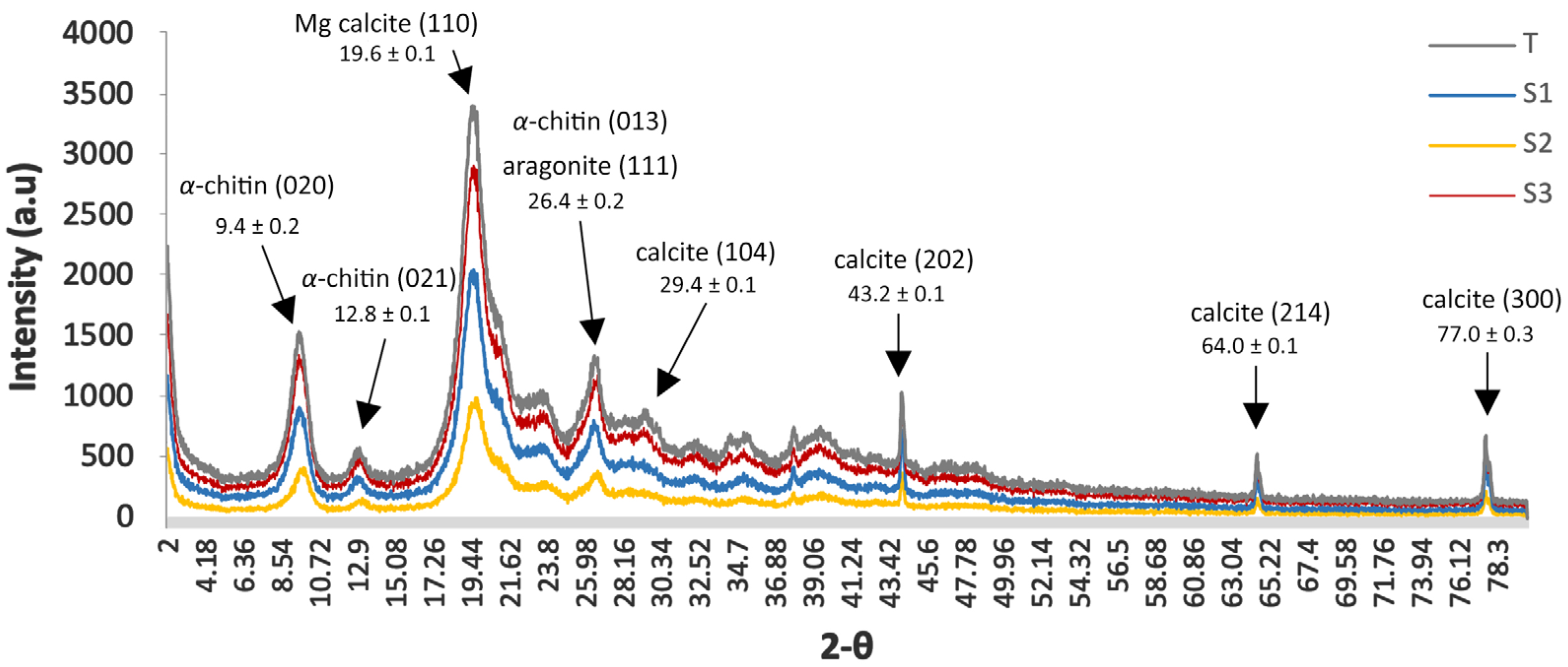
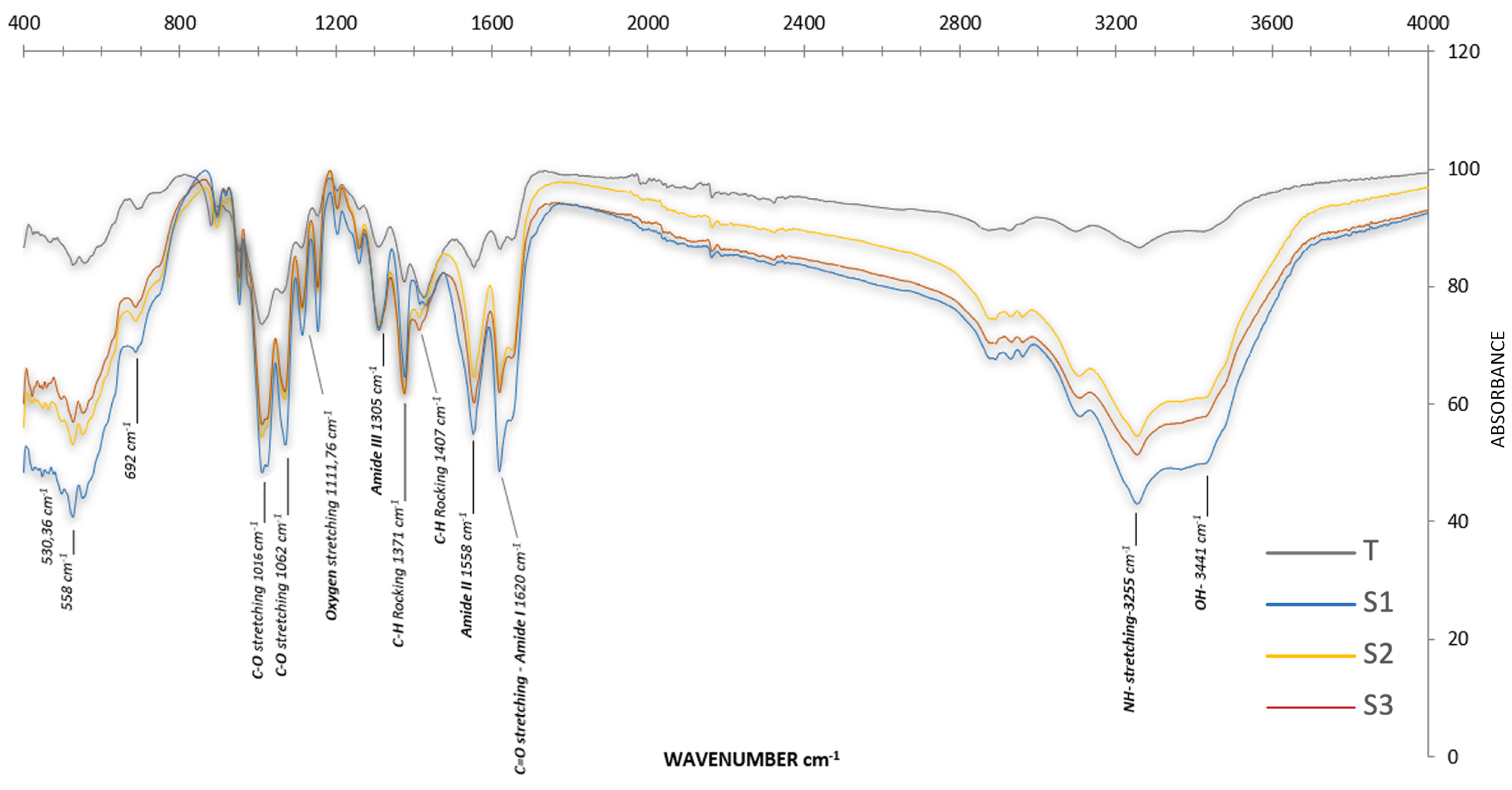
2.3.1. Physicochemical Characterization of Chitin
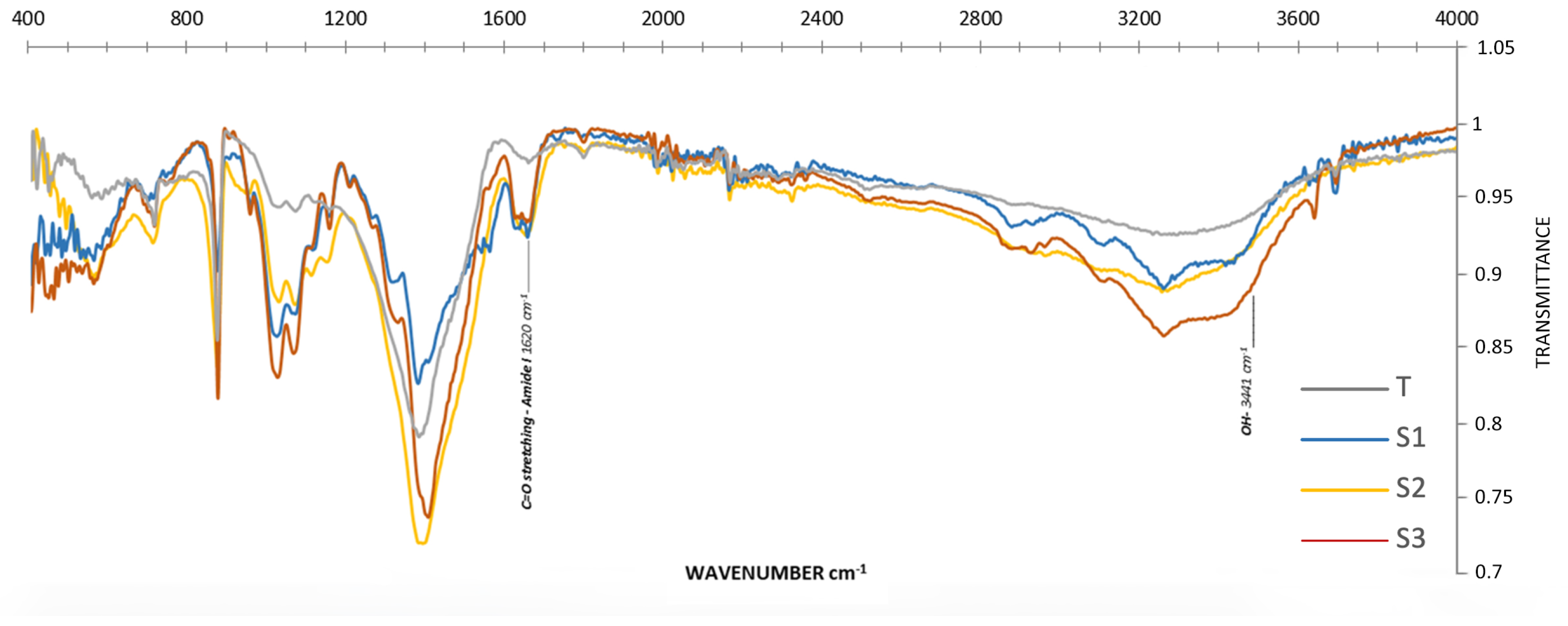
2.3.2. Physicochemical Characterization of Chitosan
2.3.3. Degree of Deacetylation (DDA) of Chitosan
3. Discussion
3.1. Physicochemical Characterization of Meat and Exoskeleton
3.1.1. Influence of the Ecological Context on the Biochemical Profile of the Meat
3.1.2. Influence of the Ecological Context on the Exoskeleton Biochemical Profile
3.2. Chemical Extraction and Characterization of Chitin and Chitosan
3.2.1. Chitin
3.2.2. Chitosan
4. Materials and Methods
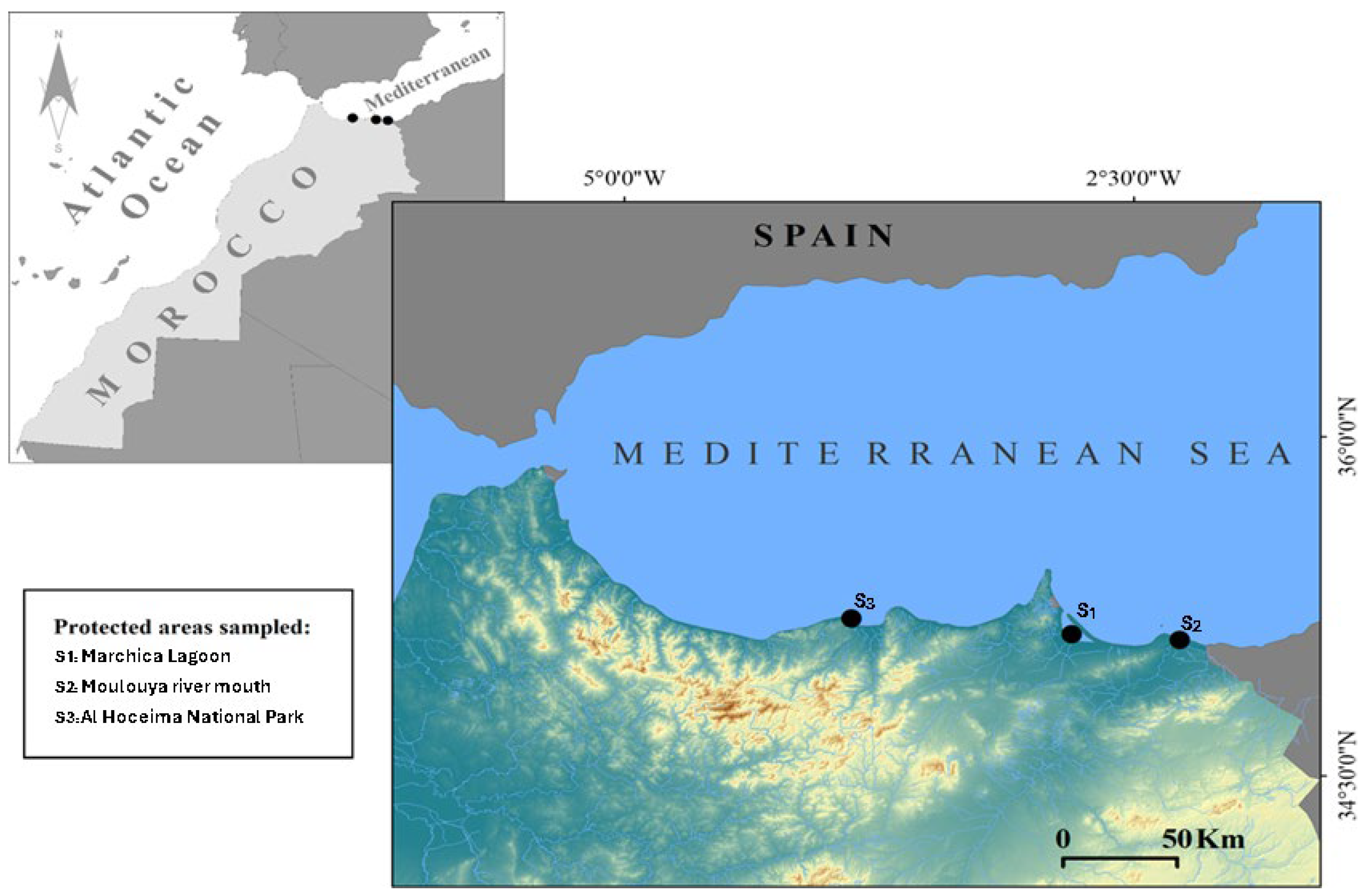
4.1. Sampling Localities and Species Identification
4.2. Preparation and Processing of the Crab Tissues
4.3. Physical Characterization of the Samples
4.3.1. Dry Matter Determination
4.3.2. Organic and Inorganic Matter Determination
4.4. Biochemical Characterization of Samples
4.4.1. Preparation of Extracts (Reducing Sugars, Proteins, and Lipids)
4.4.2. Reducing Sugars
4.4.3. Total Nitrogen and Proteins
4.4.4. Lipids
4.5. Statistical Analyses
4.6. Chitin and Chitosan Extraction and Characterization
4.6.1. Exoskeleton Preparation and Processing
4.6.2. Chitin Extraction
4.6.3. Deacetylation of Chitosan
4.6.4. Physicochemical Characterization of Chitin and Chitosan
- X-ray diffraction (XRD)
- Crystallinity index (CrI)
- Crystallite size (D)
- Degree of acetylation (DA) and of deacetylation (DDA)
4.7. Environmental Context of Sampling Sites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and Rising Economic Costs of Biological Invasions Worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Fristoe, T.S.; Chytrý, M.; Dawson, W.; Essl, F.; Heleno, R.; Kreft, H.; Maurel, N.; Pergl, J.; Pyšek, P.; Seebens, H.; et al. Dimensions of Invasiveness: Links between Local Abundance, Geographic Range Size, and Habitat Breadth in Europe’s Alien and Native Floras. Proc. Natl. Acad. Sci. USA 2021, 118, e2021173118. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N.; et al. Bioinvasion Impacts on Biodiversity, Ecosystem Services, and Human Health in the Mediterranean Sea. Aquat. Invasions 2022, 17, 308–352. [Google Scholar] [CrossRef]
- Virgili, R.; Tanduo, V.; Katsanevakis, S.; Terlizzi, F.; Villani, G.; Fontana, A.; Crocetta, F. The Miseno Lake (central-western Mediterranean Sea): An overlooked reservoir of non-indigenous and cryptogenic ascidians in a marine reserve. Front. Mar. Sci. 2022, 9, 866906. [Google Scholar] [CrossRef]
- Marchini, A.; Ferrario, J.; Sfriso, A.; Occhipinti-Ambrogi, A. Current status and trends of biological invasions in the Lagoon of Venice, a hotspot of marine NIS introductions in the Mediterranean Sea. Biol. Invasions 2015, 17, 2943–2962. [Google Scholar] [CrossRef]
- Taybi, F.A.; Langeneck, J.; Mabrouki, Y. New Records of Non-Indigenous Fouling serpulid Worms (Polychaeta: Serpulidae) from Morocco (Southern Mediterranean). Thalassas 2025, 41, 156. [Google Scholar] [CrossRef]
- Stabili, L.; Fraschetti, S.; Acquaviva, M.I.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Gerardi, C.; Narracci, M.; Rizzo, L. The potential exploitation of the Mediterranean invasive alga Caulerpa cylindracea: Can the invasion be transformed into a gain? Mar. Drugs 2016, 14, 210. [Google Scholar] [CrossRef]
- Taybi, A.F.; Gourari, K.; Essadek, A.; Mabrouki, Y. Expansion of the Alien Sponge Paraleucilla magna Klautau, Monteiro and Borojevic, 2004 (Porifera: Calcarea) along the Mediterranean Coasts of Morocco. Nat. Croat. 2025, 34, 1–10. [Google Scholar] [CrossRef]
- Taybi, A.F.; Mabrouki, Y.; Piscart, C. Distribution of Freshwater Alien Animal Species in Morocco: Current Knowledge and Management Issues. Diversity 2023, 15, 169. [Google Scholar] [CrossRef]
- Chriqui, A.; Benkhnigue, O.; El Khadir, I.; Mouniane, Y.; Hachimi Alaoui, S.M.; Zidane, L.; Hmouni, D. Floristic diversity and potential of Site of Biological and Ecological Interest Brikcha, Prérif (North-West Morocco). Acta Univ. Agric. Silvic. Mendel. Brun. 2024, 72, 99–128. [Google Scholar] [CrossRef]
- Mabrouki, Y.; Fouzi Taybi, A.; Mabrouki, Y.; Taybi, A.F. Range Expansion of the Alien Polychaete Branchiomma luctuosum (Grube, 1870) (Sabellidae) in Morocco (the Southwestern Mediterranean Sea). Zagreb. Nat. Croat. 2024, 33, 197–202. [Google Scholar] [CrossRef]
- Taybi, A.F.; Mabrouki, Y.; Glöer, P. First Record of the New Zealand Mudsnail Potamopyrgus antipodarum (J.E. Gray, 1843) (Tateidae, Mollusca) in Africa. Graellsia 2021, 77, e140. [Google Scholar] [CrossRef]
- Taybi, A.F.; Mabrouki, Y.; Glöer, P.; Piscart, C. Factors Explaining the Distribution of Physella Acuta (Draparnaud, 1805) in Freshwaters of Morocco. Water 2024, 16, 803. [Google Scholar] [CrossRef]
- Bonanomi, S.; Libralato, S.; Malvarosa, L.; Czechowska, K.M.; Sandalli, G.; Cariani, A.; Ferrari, A.; Azzurro, E.; Scarcella, G. Applying a Regional Certification Scheme for Aquatic Invasive Species Fishery: The Case of Blue Crab (Callinectes sapidus) in the Northern Adriatic Sea. Mar. Policy 2025, 181, 106858. [Google Scholar] [CrossRef]
- Suaria, G.; Pierucci, A.; Zanello, P.; Fanelli, E.; Chiesa, S.; Azzurro, E. Percnon Gibbesi (H. Milne Edwards, 1853) and Callinectes sapidus (Rathbun, 1896) in the Ligurian Sea: Two Additional Invasive Species Detections Made in Collaboration with Local Fishermen. Bioinvasions Rec. 2017, 6, 147–151. [Google Scholar] [CrossRef]
- Garcia, L.; Pinya, S.; Colomar, V.; París, T.; Puig, M.; Rebassa, M.; Mayol, J. The First Recorded Occurrences of the Invasive Crab Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Portunidae) in Coastal Lagoons of the Balearic Islands (Spain). Bioinvasions Rec. 2018, 7, 191–196. [Google Scholar] [CrossRef]
- Durieux, E.D.H.; Volety, A.K.; Donnelly, M.J.; Tolley, S.G. Crassostrea virginica shell as an indicator of estuarine salinity history. Front. Mar. Sci. 2018, 5, 151. [Google Scholar] [CrossRef]
- Gencer, Ö. The Impact of an Abiotic Variable, Temperature, on Larvae of the Blue Crab, Callinectes sapidus Rathbun, 1896 (Brachyura, Portunidae). Crustaceana 2024, 97, 137–150. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Kevrekidis, T.; Chitinroglou, C.C.; Avramoglou, K.; Keisaris, S.; Fryganiotis, K.; Keisaris, S.; Fryganiotis, K.; Apostologamvrou, C.; Roditi, K.; et al. Reproductive Biology of the Invasive Blue Crab Callinectes sapidus in the Thermaikos Gulf (Northwest Aegean Sea, Eastern Mediterranean): Identifying Key Information for an Effective Population Management Policy. J. Mar. Sci. Eng. 2024, 12, 1923. [Google Scholar] [CrossRef]
- Veyssiere, D.; Garrido, M.; Massé, C.; Noël, P.; Romans, P. Etat Des Connaissances Sur Le Bleu, Callinectes sapidus (Rathbun, 1896); Focus sur la Méditerranée française et la Corse; Institut Français de Recherche pour l’Exploitation de la Mer: Plouzané, France, 2022. [Google Scholar]
- Grech, D.; Asciutto, E.; Bakiu, R.; Battaglia, P.; Ben-Grira, C.; Çamlik, Ö.Y.; Cappuccinelli, R.; Carmona, L.; Chebaane, S.; Crocetta, F.; et al. New Records of Rarely Reported Species in the Mediterranean Sea (July 2023). Mediterr. Mar. Sci. 2023, 24, 392–418. [Google Scholar] [CrossRef]
- Chartosia, N.; Anastasiadis, D.; Bazairi, H.; Crocetta, F.; Deidun, A.; Despalatović, M.; Di Martino, V.; Dimitriou, N.; Dragičević, B.; Dulčić, J.; et al. New Mediterranean Biodiversity Records (July 2018). Mediterr. Mar. Sci. 2018, 19, 398. [Google Scholar] [CrossRef]
- Gavioli, A.; Mancinelli, G.; Turolla, E.; Lanzoni, M.; Paesanti, V.; Soana, E.; Eggleston, D.B.; Christian, R.R.; Castaldelli, G. Impacts of the Invasive Blue Crab Callinectes sapidus on Small-Scale Fisheries in a Mediterranean Lagoon Using Fishery Landing Data. Sci. Total Environ. 2025, 974, 179236. [Google Scholar] [CrossRef]
- Taybi, A.F.; Mabrouki, Y. The American Blue Crab Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Portunidae) Is Rapidly Expanding Through the Mediterranean Coast of Morocco. Thalassas 2020, 36, 267–271. [Google Scholar] [CrossRef]
- Gourari, K.; Mabrouki, Y.; Parenteau-Mauffette, É.; Legssyer, B.; Mancinelli, G.; Taybi, A.F. Assessing the Ecological and Socio-Economic Impacts of the Invasive Bleu Crab Callinectes sapidus Using Local Knowledge and Field Surveys in Eastern Morocco. Thalassas 2025, 41, 118. [Google Scholar] [CrossRef]
- Mabrouki, Y.; Gourari, K.; Taybi, A.F. Atlantic Blue Crab Callinectes sapidus Rathbun, 1896 (Portunidae, Malacostraca) Could Lead to Local Extinction of Few Endemic Freshwater Species in the Eastern Morocco. Povolzhskiy J. Ecol. 2024, 1, 91–99. [Google Scholar] [CrossRef]
- Mancinelli, G.; Lago, N.; Scirocco, T.; Lillo, O.A.; De Giorgi, R.; Doria, L.; Mancini, E.; Mancini, F.; Potenza, L.; Cilenti, L. Abundance, Size Structure, and Growth of the Invasive Blue Crab Callinectes sapidus in the Lesina Lagoon, Southern Adriatic Sea. Biology 2024, 13, 1051. [Google Scholar] [CrossRef]
- Mancinelli, G.; Guerra, M.T.; Alujević, K.; Raho, D.; Zotti, M.; Vizzini, S. Trophic Flexibility of the Atlantic Blue Crab Callinectes sapidus in Invaded Coastal Systems of the Apulia Region (SE Italy): A Stable Isotope Analysis. Estuar. Coast. Shelf Sci. 2017, 198, 421–431. [Google Scholar] [CrossRef]
- Marchessaux, G.; Barré, N.; Mauclert, V.; Lombardini, K.; Durieux, E.D.H.; Veyssiere, D.; Filippi, J.J.; Bracconi, J.; Aiello, A.; Garrido, M. Salinity Tolerance of the Invasive Blue Crab Callinectes sapidus: From Global to Local, a New Tool for Implementing Management Strategy. Sci. Total Environ. 2024, 954, 176291. [Google Scholar] [CrossRef]
- Rifi, M.; Basti, L.; Rizzo, L.; Tanduo, V.; Radulovici, A.; Jaziri, S.; Uysal, İ.; Souissi, N.; Mekki, Z.; Crocetta, F. Tackling bioinvasions in commercially exploitable species through interdisciplinary approaches: A case study on blue crabs in Africa’s Mediterranean coast (Bizerte Lagoon, Tunisia). Estuar. Coast. Shelf Sci. 2023, 291, 108419. [Google Scholar] [CrossRef]
- Kleitou, P.; Crocetta, F.; Giakoumi, S.; Giovos, I.; Hall-Spencer, J.M.; Kalogirou, S.; Kletou, D.; Moutopoulos, D.K.; Rees, S. Fishery reforms for the management of non-indigenous species. J. Environ. Manag. 2021, 280, 111690. [Google Scholar] [CrossRef] [PubMed]
- Cauchie, H.M. An Attempt to Estimate Crustacean Chitin Production in the Hydrosphere. In Advances in Chitin Science; Domard, A., Rinaudo, M., Vårum, K.M., Eds.; Jacques André Publisher: Lyon, France, 1997; Volume 2. [Google Scholar]
- Amelia, R.; Sumiwi, S.A.; Saptarini, N.M.; Levita, J. Chitin Extracted from the Shell of Blue Swimming Crabs (Portunus pelagicus Linn.) Inhibits NF-KappaB P65 in Ethanol-Induced Gastric Ulcerative Wistar Rats. Mar. Drugs 2023, 21, 488. [Google Scholar] [CrossRef]
- Alimi, B.A.; Pathania, S.; Wilson, J.; Duffy, B.; Frias, J.M.C. Extraction, Quantification, Characterization, and Application in Food Packaging of Chitin and Chitosan from Mushrooms: A Review. Int. J. Biol. Macromol. 2023, 237, 124195. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P.; Guo, Z. Cationic Chitosan Derivatives as Potential Antifungals: A Review of Structural Optimization and Applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef]
- Njimou, J.R.; Fritsky, J.; Tahmo, N.; Tchameni Martial, J.T.; Elambo, N.G. Development of Chitin Nanogeopolymer Spheres in Mining Wastewater Adsorption Treatment. Health Sci. Dis. 2023, 24. [Google Scholar]
- Tahir, A.; Moeen, R. Comparison of Antibacterial Activity of Water and Ethanol Extracts of Camellia sinensis (L.) Kuntze against Dental Caries and Detection of Antibacterial Components. J. Med. Plant Res. 2011, 5, 4504–4510. [Google Scholar]
- Al-Hmoud, L.; Abu Fara, D.; Rashid, I.; Chowdhry, B.Z.; Badwan, A.A. Influence of Chitin Source and Polymorphism on Powder Compression and Compaction: Application in Drug Delivery. Molecules 2020, 25, 5269. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR Studies of Chitosan Blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Tamburini, E. The Blue Treasure: Comprehensive Biorefinery of Blue Crab (Callinectes sapidus). Foods 2024, 13, 2018. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Vaz, M.; Kurpad, A.V. Protein Intakes in India. Br. J. Nutr. 2012, 108, S50–S58. [Google Scholar] [CrossRef]
- Fredrick, W.S.; Ravichandran, S. Hemolymph Proteins in Marine Crustaceans. Asian Pac. J. Trop. Biomed. 2012, 2, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Aragon, A.A. How Much Protein Can the Body Use in a Single Meal for Muscle-Building? Implications for Daily Protein Distribution. J. Int. Soc. Sports Nutr. 2018, 15, 10. [Google Scholar] [CrossRef]
- Arena, R.; Renda, G.; Ottaviani Aalmo, G.; Debeaufort, F.; Messina, C.M.; Santulli, A. Valorization of the Invasive Blue Crabs (Callinectes sapidus) in the Mediterranean: Nutritional Value, Bioactive Compounds and Sustainable By-Products Utilization. Mar. Drugs 2024, 22, 430. [Google Scholar] [CrossRef]
- Kuley, E.; Özoğul, F.; Özogul, Y.; Olgunoglu, A.I. Comparison of Fatty Acid and Proximate Compositions of the Body and Claw of Male and Female Blue Crabs (Callinectes sapidus) from Different Regions of the Mediterranean Coast. Int. J. Food. Sci. Nutr. 2008, 59, 573–580. [Google Scholar] [CrossRef]
- Fuso, A.; Paris, E.; Orsoni, N.; Bonzanini, F.; Larocca, S.; Caligiani, A. Chemical Composition of Blue Crabs from Adriatic Sea. Ital. J. Food Sci. 2025, 37, 279–285. [Google Scholar] [CrossRef]
- Conti, F.; Pulido-Rodriguez, L.F.; Chemello, G.; Cattaneo, N.; Resente, M.; Parisi, G.; Olivotto, I.; Zarantoniello, M. The Role of Dietary Fatty Acids in Modulating Blue Crab (Callinectes sapidus) Physiology, Reproduction, and Quality Traits in Captivity. Animals 2024, 14, 3304. [Google Scholar] [CrossRef] [PubMed]
- Öndes, F.; Esteso, I.; Guijarro-García, E.; Barcala, E.; Giménez-Casalduero, F.; Ramos-Esplá, A.A.; Barberá, C. Feeding Habits of the Invasive Atlantic Blue Crab Callinectes sapidus in Different Habitats of the SE Iberian Peninsula, Spain (Western Mediterranean). Water 2025, 17, 1615. [Google Scholar] [CrossRef]
- El Hamouti, C.; Castellano-Hinojosa, A.; Mabrouki, Y.; Chaouni, B.; Ghazal, H.; Boukhatem, N.; Chahboune, R.; Bedmar, E.J. Anthropogenic Nitrate Contamination Impacts Nitrous Oxide Emissions and Microbial Communities in the Marchica Lagoon (Morocco). Sustainability 2023, 15, 4077. [Google Scholar] [CrossRef]
- Oujidi, B.; El Bouch, M.; Tahri, M.; Layachi, M.; Boutoumit, S.; Bouchnan, R.; Ouahidi, H.; Bounakhla, M.; El Ouamari, N.; Maanan, M.; et al. Seasonal and Spatial Patterns of Ecotoxicological Indices of Trace Elements in Superficial Sediments of the Marchica Lagoon Following Restoration Actions during the Last Decade. Diversity 2021, 13, 51. [Google Scholar] [CrossRef]
- Taybi, A.F.; Mabrouki, Y.; Legssyer, B.; Berrahou, A. Spatio-Temporal Typology of the Physico-Chemical Parameters of a Large North African River: The Moulouya and Its Main Tributaries (Morocco). Afr. J. Aquat. Sci. 2020, 45, 431–441. [Google Scholar] [CrossRef]
- Ortega-Jiménez, E.; Vilas, C.; de Carvalho-Souza, G.F.; Martinez-Lage, A.; González-Ortegón, E. Isotopic Variability of the Invasive Blue Crab Callinectes sapidus in the Gulf of Cadiz: Impacts and Implications for Coastal Ecosystem Management. J. Environ. Manag. 2025, 374, 124015. [Google Scholar] [CrossRef] [PubMed]
- Herrera, I.; de Carvalho-Souza, G.F.; González-Ortegón, E. Physiological Responses of the Invasive Blue Crabs Callinectes sapidus to Salinity Variations: Implications for Adaptability and Invasive Success. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2024, 297, 111709. [Google Scholar] [CrossRef] [PubMed]
- Sanchezpaz, A.; Garciacarreno, F.; Muhliaalmazan, A.; Peregrinouriarte, A.; Hernandezlopez, J.; Yepizplascencia, G. Usage of Energy Reserves in Crustaceans during Starvation: Status and Future Directions. Insect Biochem. Mol. Biol. 2006, 36, 241–249. [Google Scholar] [CrossRef]
- Monti, F.; Nibani, H.; Dominici, J.-M.; Idrissi, H.R.; Thévenet, M.; Beaubrun, P.-C.; Duriez, O. The Vulnerable Osprey Breeding Population of the Al Hoceima National Park, Morocco: Present Status and Threats. Ostrich 2013, 84, 199–204. [Google Scholar] [CrossRef]
- El Hadri, S.; Jaoui, A.; Bouziane, H. Le Parc National D’al Hoceima: Panorama Entre Terre Et Mer. Eur. Sci. J. 2019, 15, 311. [Google Scholar] [CrossRef]
- Millikin, M.R.; Biddle, G.N.; Siewicki, T.C.; Fortner, A.R.; Fair, P.H. Effects of Various Levels of Dietary Protein on Survival, Molting Frequency and Growth of Juvenile Blue Crabs (Callinectes sapidus). Aquaculture 1980, 19, 149–161. [Google Scholar] [CrossRef]
- Vittori, M. Structural Diversity of Crustacean Exoskeletons and Its Implications for Biomimetics. Interface Focus. 2024, 14, 20230075. [Google Scholar] [CrossRef] [PubMed]
- Karar, S.; Hazra, S.; Das, S. Assessment of the Heavy Metal Accumulation in the Blue Swimmer Crab (Portunus pelagicus), Northern Bay of Bengal: Role of Salinity. Mar. Pollut. Bull. 2019, 143, 101–108. [Google Scholar] [CrossRef]
- Waqas, W.; Yuan, Y.; Ali, S.; Zhang, M.; Shafiq, M.; Ali, W.; Chen, Y.; Xiang, Z.; Chen, R.; Ikhwanuddin, M.; et al. Toxic Effects of Heavy Metals on Crustaceans and Associated Health Risks in Humans: A Review. Environ. Chem. Lett. 2024, 22, 1391–1411. [Google Scholar] [CrossRef]
- Guerin, J.L.; Stickle, W.B. A Comparative Study of Two Sympatric Species within the Genus Callinectes: Osmoregulation, Long-Term Acclimation to Salinity and the Effects of Salinity on Growth and Moulting. J. Exp. Mar. Biol. Ecol. 1997, 218, 165–186. [Google Scholar] [CrossRef]
- Longmire, K.S. Effects of Acidification and Salinity on Callinectes sapidus, Mercenaria mercenaria, and Their Interactions. Master’s Thesis, College of William & Mary, Williamsburg, VA, USA, 2021. [Google Scholar]
- Londoño-Zuluaga, C.; Jameel, H.; Gonzalez, R.W.; Yang, G.; Lucia, L. The Surprising Role of Endogenous Calcium Carbonate in Crab Shell-Mediated Biosorption of Pb (II). Physchem 2024, 4, 167–180. [Google Scholar] [CrossRef]
- Perry, H.; Trigg, C.; Larsen, K.; Freeman, J.; Erickson, M.; Henry, R. Calcium Concentration in Seawater and Exoskeletal Calcification in the Blue Crab, Callinectes sapidus. Aquaculture 2001, 198, 197–208. [Google Scholar] [CrossRef]
- Jabeen, F.; Younis, T.; Sidra, S.; Muneer, B.; Nasreen, Z.; Saleh, F.; Mumtaz, S.; Saeed, R.F.; Abbas, A.S. Extraction of Chitin from Edible Crab Shells of Callinectes sapidus and Comparison with Market Purchased Chitin. Braz. J. Biol. 2023, 83, e246520. [Google Scholar] [CrossRef]
- Demir, D.; Öfkeli, F.; Ceylan, S.; Bölgen, N. Extraction and Characterization of Chitin and Chitosan from Blue Crab and Synthesis of Chitosan Cryogel Scaffolds. J. Turk. Chem. Soc. A Chem. 2016, 3, 131–144. [Google Scholar] [CrossRef]
- Morgan, K.; Conway, C.; Faherty, S.; Quigley, C. A Comparative Analysis of Conventional and Deep Eutectic Solvent (DES)-Mediated Strategies for the Extraction of Chitin from Marine Crustacean Shells. Molecules 2021, 26, 7603. [Google Scholar] [CrossRef] [PubMed]
- Izadi, H.; Asadi, H.; Bemani, M. Chitin: A Comparison between Its Main Sources. Front. Mater. 2025, 12, 1537067. [Google Scholar] [CrossRef]
- Aberoumand, A.; Hoseinian, M. Extraction of Chitosan from Shells of Crab (Liocarcinus vernalis). Appl. Food Res. 2025, 5, 100964. [Google Scholar] [CrossRef]
- Tissera, W.M.J.C.; Rathnayake, S.I.; Abeyrathne, E.D.N.S.; Nam, K.-C. An Improved Extraction and Purification Method for Obtaining High-Quality Chitin and Chitosan from Blue Swimmer (Portunus pelagicus) Crab Shell Waste. Food Sci. Biotechnol. 2021, 30, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Ilkaeva, M.; Vicente, F.; Vieira, R.; Sardo, M.; Lourenço, M.A.O.; Silvestre, A.; Marin-Montesinos, I.; Mafra, L. Valorization of Crab Shells as Potential Sorbent Materials for Co2 Capture. ACS Omega 2024, 9, 17956–1717965. [Google Scholar] [CrossRef] [PubMed]
- El-Araby, A.; Janati, W.; Ullah, R.; Ercisli, S.; Errachidi, F. Chitosan, Chitosan Derivatives, and Chitosan-Based Nanocomposites: Eco-Friendly Materials for Advanced Applications (a Review). Front. Chem. 2024, 11, 1327426. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Chitosan-Based Materials: Preparation, Modification and Application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- El-Araby, A.; El Ghadraoui, L.; Errachidi, F. Physicochemical Properties and Functional Characteristics of Ecologically Extracted Shrimp Chitosans with Different Organic Acids during Demineralization Step. Molecules 2022, 27, 8285. [Google Scholar] [CrossRef]
- Ali, D.A.; Ali, R.G. Green Synthesis of Carbonized Chitosan-Fe3O4-SiO2 Nano-Composite for Adsorption of Heavy Metals from Aqueous Solutions. BMC Chem. 2024, 18, 147. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; El Khalfaouy, R.; Laajeb, A.; Addaou, A.; Lahsini, A. Physicochemical Characterization of Chitin and Chitosan Produced from Parapenaeus longirostris Shrimp Shell Wastes. J. Mater. Environ. Sci. 2017, 8, 3648–3653. [Google Scholar]
- Dahmane, E.M.; Taourirte, M.; Eladlani, N.; Rhazi, M. Extraction and Characterization of Chitin and Chitosan from Parapenaeus longirostris from Moroccan Local Sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar] [CrossRef]
- Elouahli, A.; Khallok, H.; Rezzougui, I.; Yahyaoui, M.I.; Jamil, M.; Asehraou, A.; Hatim, Z. Fabrication and Characterization of Chitosan-Loaded Biomimetic Apatite Beads with Their Antimicrobial Activity. J. Inorg. Organomet. Polym. Mater. 2025, 35, 4065–4078. [Google Scholar] [CrossRef]
- Longo, F.; Attanzio, A.; Marretta, L.; Luparello, C.; Indelicato, S.; Bongiorno, D.; Barone, G.; Tesoriere, L.; Giardina, I.C.; Abruscato, G.; et al. Bioactive Molecules from the Invasive Blue Crab Callinectes sapidus Exoskeleton: Evaluation of Reducing, Radical Scavenging, and Antitumor Activities. Mar. Drugs 2025, 23, 45. [Google Scholar] [CrossRef]
- Jabeur, F.; Mechri, S.; Mensi, F.; Gharbi, I.; Ben Naser, Y.; Kriaa, M.; Bejaoui, N.; Bachouche, S.; Badis, A.; Annane, R.; et al. Extraction and Characterization of Chitin, Chitosan, and Protein Hydrolysate from the Invasive Pacific Blue Crab, Portunus segnis (Forskål, 1775) Having Potential Biological Activities. Environ. Sci. Pollut. Res. 2022, 29, 36023–36039. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of Chitin and Chitosan Derived from Hermetia illucens, a Further Step in a Circular Economy Process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef]
- Jang, M.; Kong, B.; Jeong, Y.; Lee, C.H.; Nah, J. Physicochemical Characterization of A-chitin, Β-chitin, and Γ-chitin Separated from Natural Resources. J. Polym. Sci. A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P. Preparation and Characterization of α-Chitin from Cicada Sloughs. Mater. Sci. Eng. C. 2010, 30, 357–363. [Google Scholar] [CrossRef]
- Al-Hmoud, L.; Al-Saida, B.; Sandouqa, A. Olive Mill Wastewater Treatment: A Recent Review. Jordan. J. Eng. Chem. Ind. 2020, 3, 91–106. [Google Scholar]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Luo, X.; Song, X.; Cao, Y.; Song, L.; Bu, X. Investigation of Calcium Carbonate Synthesized by Steamed Ammonia Liquid Waste without Use of Additives. RSC Adv. 2020, 10, 7976–7986. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Oomori, T. Structure, Crystallization and Mineral Composition of Sclerites in the Alcyonarian Coral. J. Cryst. Growth 2008, 310, 3528–3534. [Google Scholar] [CrossRef]
- Hamed, H.; Hale, W.; Stern, B. X-RAY Diffraction to Determine the Mineralogy in Soil Samples in the UK. Int. J. Eng. Appl. Sci. Technol. 2021, 5, 91–98. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, W.-C.; Guo, N.; Zhang, S.; Xue, C.; Mao, X. Two-Step Separation of Chitin from Shrimp Shells Using Citric Acid and Deep Eutectic Solvents with the Assistance of Microwave. Polymers 2019, 11, 409. [Google Scholar] [CrossRef]
- Olaosebikan, A.O.; Kehinde, O.A.; Tolulase, O.A.; Victor, E.B. Extraction and Characterization of Chitin and Chitosan from Callinectes amnicola and Penaeus notialis Shell Wastes. J. Chem. Eng. Mater. Sci. 2021, 12, 1–30. [Google Scholar] [CrossRef]
- Jacobs, J.R.; Biesiot, P.M.; Perry, H.M.; Trigg, C. Biochemical Composition Of Embryonic Blue Crabs Callinectes sapidus Rathbun 1896 (Crustacea: Decapoda) from The Gulf Of Mexico. Bull. Mar. Sci. 2003, 72, 311–324. [Google Scholar]
- Rahhou, A.; Layachi, M.; Akodad, M.; El Ouamari, N.; Aknaf, A.; Skalli, A.; Oudra, B.; Kolar, M.; Imperl, J.; Petrova, P.; et al. Analysis and Health Risk Assessment of Heavy Metals in Four Common Seaweeds of Marchica Lagoon (a Restored Lagoon, Moroccan Mediterranean). Arab. J. Chem. 2023, 16, 105281. [Google Scholar] [CrossRef]
- El Mekki, O.; Layachi, M.; Baghour, M.; Petrova, P.; Stankov Jovanovi, V.P.; Benyaich, A.; Rahhou, A.; Zidane, H.; Akodad, M.; Rezzoum, N.; et al. Assessment of Heavy Metal Contamination in the Sediments of Marchica Lagoon: Sources, Ecological Risks, and Mitigation Strategies. E3S Web Conf. 2025, 632, 01019. [Google Scholar] [CrossRef]
- El Ouaty, O.; El M’rini, A.; Nachite, D.; Marrocchino, E.; Rodella, I. Sediment Quality Indices for the Assessment of Heavy Metal Risk in Nador Lagoon Sediments (Morocco) Using Multistatistical Approaches. Sustainability 2024, 16, 1921. [Google Scholar] [CrossRef]
- Alagiri, A.; Parthiban, J.; Ashok, A.; Subramani, T.; Velayutham, M. Copper-Induced Microstructural Alterations in the Chitin-Protein Matrix and Calcium Dissolution in the Exoskeletal Carapace of the Mud Crab Scylla serrata. Mar. Environ. Res. 2025, 208, 107142. [Google Scholar] [CrossRef]
- Ferhat, M.; Kadouche, S.; Drouiche, N.; Messaoudi, K.; Messaoudi, B.; Lounici, H. Competitive Adsorption of Toxic Metals on Bentonite and Use of Chitosan as Flocculent Coagulant to Speed up the Settling of Generated Clay Suspensions. Chemosphere 2016, 165, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Kaya, M.; Dudakli, F.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Baran, T.; Mentes, A.; Erdogan, S. Porous and Nanofiber α-Chitosan Obtained from Blue Crab (Callinectes sapidus) Tested for Antimicrobial and Antioxidant Activities. LWT 2016, 65, 1109–1117. [Google Scholar] [CrossRef]
- Perez, S.; Wertz, J.-L. Chitin and Chitosans in the Bioeconomy; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781003226529. [Google Scholar]
- Sahoo, D.; Sahoo, S.; Mohanty, P.; Sasmal, S.; Nayak, P.L. Chitosan: A New Versatile Bio-Polymer for Various Applications. Des. Monomers Polym. 2009, 12, 377–404. [Google Scholar] [CrossRef]
- Poerio, A.; Petit, C.; Jehl, J.-P.; Arab-Tehrany, E.; Mano, J.F.; Cleymand, F. Extraction and Physicochemical Characterization of Chitin from Cicada orni Sloughs of the South-Eastern French Mediterranean Basin. Molecules 2020, 25, 2543. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kimura, S.; Wada, M.; Kuga, S. Crystal Analysis and High-Resolution Imaging of Microfibrillar α-Chitin from Phaeocystis. J. Struct. Biol. 2010, 171, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Castriota, L.; Falautano, M.; Maggio, T.; Perzia, P. The Blue Swimming Crab Portunus segnis in the Mediterranean Sea: Invasion Paths, Impacts and Management Measures. Biology 2022, 11, 1473. [Google Scholar] [CrossRef]
- Hamdi, M.; Nasri, R.; Dridi, N.; Li, S.; Nasri, M. Development of Novel High-Selective Extraction Approach of Carotenoproteins from Blue Crab (Portunus segnis) Shells, Contribution to the Qualitative Analysis of Bioactive Compounds by HR-ESI-MS. Food Chem. 2020, 302, 125334. [Google Scholar] [CrossRef]
- Galil, B.S. Alien Species in the Mediterranean Sea—Which, When, Where, Why? Hydrobiologia 2008, 606, 105–116.40. [Google Scholar] [CrossRef]
- Williams, A.B. The swimming crabs of the genus Callinectes (Decapoda: Portunidae). Fish. Bull. 1974, 72, 685–798. [Google Scholar]
- Association of Official Analytical Chemists (AOAC) International. Official Method 930.15—Loss on Drying (Moisture) for Feeds (at 135 °C for 2 Hours). In Official Methods of Analysis of AOAC International; Oxford University Press: New York, NY, USA, 2023. [Google Scholar]
- Association of Official Analytical Chemists (AOAC) International. Official Method 954.01—Lead in Foods: Colorimetric Method. In Official Methods of Analysis of AOAC International, 2nd ed.; AOAC International: Rockville, MD, USA, 1996. [Google Scholar]
- Gökoðlu, N.; Yerlikaya, P. Determinaton of Proximate Composition and Mineral Contents of Blue Crab (Callinectes sapidus) and Swim Crab (Portunus pelagicus) Caught off the Gulf of Antalya. Food. Chem. 2003, 80, 495–498. [Google Scholar] [CrossRef]
- Selmane, D.; Christophe, V.; Gholamreza, D. Extraction of Proteins from Slaughterhouse By-Products: Influence of Operating Conditions on Functional Properties. Meat Sci. 2008, 79, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Rolland, G. Choix d’une Méthode d’extraction et de Purification Pour Le Dosage Des Sucres Solubles et de l’amidon Dans Les Tissus Ligneux de La Vigne. Cah. Des Tech. De L’inra 2020, hal-04627100, 39–44. [Google Scholar]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an Analytical Procedure for Extraction of Lipids from Microalgae. J. Am. Oil. Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Ollivier, D.; Artaud, J.; Pinatel, C.; Durbec, J.P.; Guérère, M. Triacylglycerol and Fatty Acid Compositions of French Virgin Olive Oils. Characterization by Chemometrics. J. Agric. Food Chem. 2003, 51, 5723–5731. [Google Scholar] [CrossRef]
- Fehling, H.V. Die quantitative bestimmung von Zucker und Stärkmehl mittelst Kupfervitriol. Liebigs Ann. Chem. 1849, 72, 106–113. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Honoré, T. Elaboration de Nanocomposites et Synthèse de Graphène Dopé; Université de Caen Normandie: Caen, France, 2017. [Google Scholar]
- Kozin, A.V.; Abramova, L.S.; Guseva, E.S.; Derunets, I.V. Establishment of Metrological Parameters of the Method for Measuring the Protein Mass Fraction in Fish Food Products by the Kjeldahl Method. Food Syst. 2022, 4, 239–245. [Google Scholar] [CrossRef]
- Meyer, G.; Streb, P.B.; Siebmanns, C. Alternative SO2 Determination Methods for Food Analysis. Lebensm. Anal. 2022, 5, 10–18. [Google Scholar]
- Smets, R.; Goos, P.; Claes, J.; Van Der Borght, M. Optimisation of the Lipid Extraction of Fresh Black Soldier Fly Larvae (Hermetia illucens) with 2-Methyltetrahydrofuran by Response Surface Methodology. Sep. Purif. Technol. 2021, 258, 118040. [Google Scholar] [CrossRef]
- Watkins, P. Comparing the Use of Chloroform to Petroleum Ether for Soxhlet Extraction of Fat in Meat. Anim. Prod. Sci. 2023, 63, 1445–1449. [Google Scholar] [CrossRef]
- Visnovitz, T.; Osteikoetxea, X.; Sódar, B.W.; Mihály, J.; Lőrincz, P.; Vukman, K.V.; Tóth, E.Á.; Koncz, A.; Székács, I.; Horváth, R.; et al. An Improved 96 Well Plate Format Lipid Quantification Assay for Standardisation of Experiments with Extracellular Vesicles. J. Extracell. Vesicles 2019, 8, 1565263. [Google Scholar] [CrossRef]
- Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. 2023. Available online: https://www.vps.fmvz.usp.br/CRAN/web/packages/agricolae/vignettes/tutorial.pdf (accessed on 12 March 2025).
- Daraghmeh, N.H.; Chowdhry, B.Z.; Leharne, S.A.; Al Omari, M.M.; Badwan, A.A. Chitin. Profiles Drug Subst. Excip. Relat. Methodol. 2011, 36, 35–102. [Google Scholar] [CrossRef]
- Bourachdi, S.E.; Ayub, A.R.; Rakcho, Y.; Amri, A.E.; Moussaoui, F.; Ouadrhiri, F.E.; Lahkimi, A. Optimization of the degree of deacetylation of chitosan beads for efficient anionic dye adsorption: Kinetics, thermodynamics, mechanistic insights via DFT analysis, and regeneration performance. Environ. Sci. Pollut. Res. 2025, 32, 7950–7975. [Google Scholar] [CrossRef]
- Neves, A.C.; Zanette, C.; Grade, S.T. Optimisation of Chitin Production: Properties and Applications—A Short Review. Am. J. Biomed. Sci. Res. 2015, 3, 307–314. [Google Scholar]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial Extraction of Chitin from Seafood Waste Using Sugars Derived from Fruit Waste-Stream. AMB Express 2020, 10, 17. [Google Scholar] [CrossRef]
- Zaghbib, I.; Arafa, S.; Hassouna, M. Biological, Functional and Physico-Chemical Characterization of Chitosan Extracted From Blue Crab (Portunus segnis) Shell Waste by Chemical Method. Am. Acad. Sci. Res. J. Eng. 2022, 85, 2313–4402. [Google Scholar]
- Khoushab, F.; Yamabhai, M. Chitin Research Revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef] [PubMed]
- Perelló, E.; Pinya, S.; Box, A.; Sureda, A.; Compa, M. Assessing Heavy Metal Accumulation in the Invasive Blue Crab (Callinectes sapidus): Environmental and Human Health Implications. Environ. Sci. Pollut. Res. 2025, 32, 12579–12593. [Google Scholar] [CrossRef] [PubMed]
- Basraoui, N.; Ben-tahar, R.; Deliège, J.-F.; El Guerrouj, B.; Chafi, A. Potentially Toxic Elements Contamination and Ecological Risk Assessment in Surface Sediments of Moulouya Estuary (Northeastern, Morocco). Sci. Afr. 2024, 25, e02295. [Google Scholar] [CrossRef]

| Meat | Exoskeleton | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry Matter | Organic Matter | Proteins | Lipids | Reducing Sugars | Ash | OM/MM | Dry Matter | Organic Matter | Ash | OM/MM | ||||
| (%WW) | (%DM) | (%WW) | (%DM) | (%WW) | (%DM) | |||||||||
| Marchica (S1) | 15.38 ±0.49 | 12.75 ±0.45 | 3.36 ±0.21 | 21.87 ±1.15 | 0.30 ±0.01 | 1.98 ±0.05 | 0.09 ±0.02 | 0.56 ±0.15 | 2.89 ±0.21 | 4.41 ±0.36 | 87.82 ±1.35 | 37.43 ±1.12 | 48.52 ±2.05 | 0.77 ±0.048 |
| Moulouya (S2) | 15.64 ±2.85 | 13.71 ±1.70 | 2.89 ± 0.55 | 18.46 ±1.07 | 0.27 ±0.05 | 1.73 ±0.09 | 0.14 ±0.03 | 0.91 ±0.1 | 1.67 ±0.58 | 8.21 ±1.03 | 75.41 ±0.96 | 26.89 ±0.29 | 50.39 ±0.21 | 0.53 ±0.007 |
| Al Hoceima (S3) | 13.49 ±0.70 | 12.17 ±0.12 | 2.31 ±0.20 | 17.11 ±1.17 | 0.21 ±0.01 | 1.56 ±0.04 | 0.06 ±0.01 | 0.41 ±0.09 | 1.32 ±0.06 | 9.22 ±0.43 | 93.91 ±0.29 | 30.74 ±0.47 | 62.17 ±0.15 | 0.49 ±0.008 |
| Chitin Yield (% of Dry Exoskeleton Mass) 1 | Chitosan Yield (% of Extracted Chitin) 2 | Chitosan Yield (% of Total Crab Mass) 3 | |
|---|---|---|---|
| Marchica | 17.28% | 80.45% | 15.67% |
| Moulouya | 12.23% | 84.12% | 13.59% |
| Al Hoceima | 10.30% | 78.75% | 14.50% |
| T (Control) | N/A | ≈80% | N/A |
| Mean ± SD | 13.27 ± 2.54% | 81.11 ± 2.75% 4 | 14.59 ± 1.04% |
| Crystallinity Index (CrI) | Crystallite Size (D) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (20) | (110) | (120) | (130) | Miller Plane | |||||
| 2θ [°] | I19.6° | Iamorphic | CrI % * | 9.4 | 19.3 | 20.7 | 23.3 | Nominal 2θ (°) | |
| Lowest | Moderate | Highest | High | Packing Density | |||||
| S1 | 19.38 | 1130 | 176 | 84.42 | 8.9 | 5.4 | 6.6 | 3.5 | Dvalue (nm) |
| S2 | 19.66 | 1000 | 158 | 84.20 | 7.4 | 5.6 | 7.1 | 5.7 | |
| S3 | 19.66 | 882 | 168 | 80.95 | 8.7 | 6.3 | 5.7 | 3.5 | |
| T | 19.36 | 556 | 144 | 74.10 | 6.7 | 4.6 | 4.0 | 3.5 | |
| % Transmittance of Amide Groups (1619–1620) | % Transmittance of Hydroxyl Groups -OH (3443–3245) | Absorbance of 1619–1620 Amide Groups | Absorbance of Hydroxyl Groups -OH (3443–3245) | DA | |
|---|---|---|---|---|---|
| -N-C=O | -N-C=O | ||||
| S1 | 48.551 | 42.995 | 0.361 | 0.351 | 77.36 |
| S2 | 63.261 | 54.520 | 0.257 | 0.226 | 85.52 |
| S3 | 61.982 | 51.384 | 0.276 | 0.291 | 71.21 |
| T | 86.301 | 86.605 | 0.064 | 0.070 | 68.41 |
| Absorbance of 1620 Amide Groups | Absorbance of Hydroxyl Groups -OH (3441) | DDA | |
|---|---|---|---|
| -N-C=O | |||
| S1 | 0.00811 | 0.02960 | 79.4 |
| S2 | 0.00979 | 0.03437 | 78.5 |
| S3 | 0.01524 | 0.04527 | 74.6 |
| T | 0.02909 | 0.05650 | 61.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gourari, K.; Mabrouki, Y.; Taybi, A.F.; Essadek, A.; Tanduo, V.; Crocetta, F.; Rahhou, I.; Belbachir, C.; Rizzo, L.; Legssyer, B. Meat Nutritional Value and Exoskeleton Valorisation of Callinectes sapidus from Three Sites of Biological and Ecological Interest in Morocco: Scientific Insights Toward a Management Strategy in the Mediterranean Sea. Mar. Drugs 2025, 23, 367. https://doi.org/10.3390/md23090367
Gourari K, Mabrouki Y, Taybi AF, Essadek A, Tanduo V, Crocetta F, Rahhou I, Belbachir C, Rizzo L, Legssyer B. Meat Nutritional Value and Exoskeleton Valorisation of Callinectes sapidus from Three Sites of Biological and Ecological Interest in Morocco: Scientific Insights Toward a Management Strategy in the Mediterranean Sea. Marine Drugs. 2025; 23(9):367. https://doi.org/10.3390/md23090367
Chicago/Turabian StyleGourari, Kamal, Youness Mabrouki, Abdelkhaleq Fouzi Taybi, Abdessadek Essadek, Valentina Tanduo, Fabio Crocetta, Ilyesse Rahhou, Chaouki Belbachir, Lucia Rizzo, and Bouchra Legssyer. 2025. "Meat Nutritional Value and Exoskeleton Valorisation of Callinectes sapidus from Three Sites of Biological and Ecological Interest in Morocco: Scientific Insights Toward a Management Strategy in the Mediterranean Sea" Marine Drugs 23, no. 9: 367. https://doi.org/10.3390/md23090367
APA StyleGourari, K., Mabrouki, Y., Taybi, A. F., Essadek, A., Tanduo, V., Crocetta, F., Rahhou, I., Belbachir, C., Rizzo, L., & Legssyer, B. (2025). Meat Nutritional Value and Exoskeleton Valorisation of Callinectes sapidus from Three Sites of Biological and Ecological Interest in Morocco: Scientific Insights Toward a Management Strategy in the Mediterranean Sea. Marine Drugs, 23(9), 367. https://doi.org/10.3390/md23090367









