Effect of Precipitated Extracellular Marennine on Angiogenesis and Tumour Cell Proliferation
Abstract
1. Introduction
2. Results
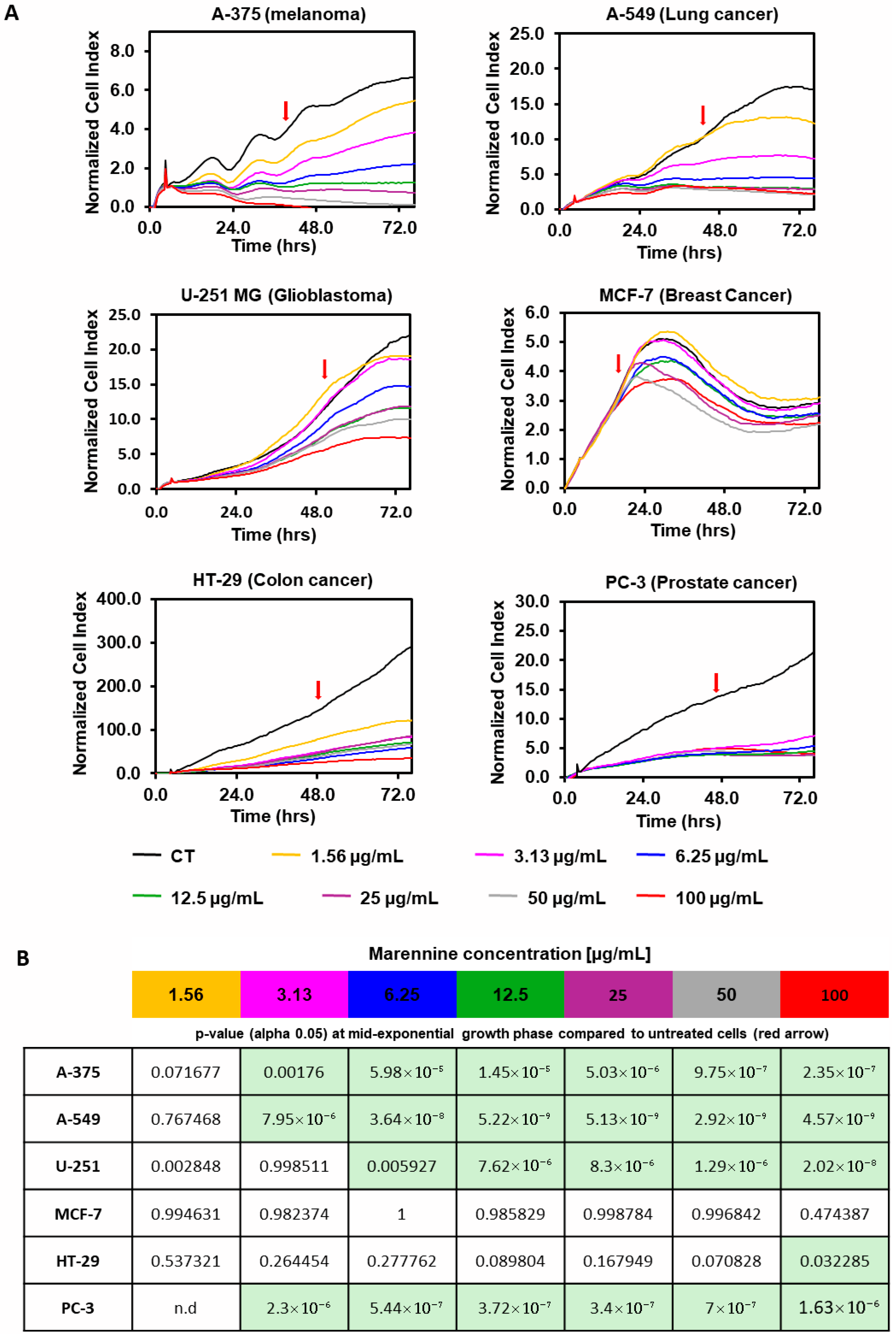
2.1. Effect of PEMn on Tumour Cell Adhesion and Proliferation
2.2. Effect of PEMn on ECFCs Viability and Senescence
2.3. PEMn Induces ECFCs Growth Arrest and Apoptosis
2.4. Effects of PEMn on ECFCs Migration
2.5. Effect of PEMn on ECFCs Cytokine Profiles
3. Discussion
4. Materials and Methods
4.1. Microalgae and Purification of Marennine
4.2. Cell Isolation and ECFCs Culture
4.3. Tumour Cell Lines and Cell Culture
4.4. Real-Time Cell Proliferation Assay
4.5. Real-Time Cell Adhesion Assay
4.6. In Vitro Angiogenesis Assay and Viability
4.7. Senescence
4.8. Cell Cycle Analysis
4.9. Cell Apoptosis Analysis
4.10. Wound Healing Assay
4.11. Cytokine and Growth Factor Multiplex Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Cell Index |
| ECFCs | Endothelial Colony-Forming Cells |
| FBS | Foetal Bovine Serum |
| FGF | Fibroblast Growth Factor |
| IL | Interleukin |
| MMP | Matrix Metalloproteinase |
| n | number of experimental replicates |
| NF-kb | Nuclear Factor kappa B |
| NCI | Matrix Metalloproteinase |
| PEMn | Precipitated Extracellular Marennine |
| RTCA | Real Cell Time Analysis |
| SDF-1 | Stromal Cell-Derived Factor 1 |
| VEGF -R | Vascular Endothelial Growth Factor Receptor |
References
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, F.; Xie, T.; Du, Z.; Zhong, D.; Qi, Y.; Li, Y.; Li, W.; Lu, Z.; Rao, J.; et al. Engineered Algae: A Novel Oxygen-Generating System for Effective Treatment of Hypoxic Cancer. Sci. Adv. 2020, 6, eaba5996. [Google Scholar] [CrossRef]
- Li, G.; Chang, X.; Luo, X.; Zhao, Y.; Wang, W.; Kang, X. Fucoxanthin induces prostate cancer PC-3 cell apoptosis by causing mitochondria dysfunction and oxidative stress. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhong, D.; Ouyang, J.; He, J.; Qi, Y.; Chen, W.; Zhang, X.; Tao, W.; Zhou, M. Microalgae-Based Oral Microcarriers for Gut Microbiota Homeostasis and Intestinal Protection in Cancer Radiotherapy. Nat. Commun. 2022, 13, 1413. [Google Scholar] [CrossRef]
- Falaise, C.; Cormier, P.; Tremblay, R.; Audet, C.; Deschênes, J.-S.; Turcotte, F.; François, C.; Seger, A.; Hallegraeff, G.; Lindquist, N.; et al. Harmful or Harmless: Biological Effects of Marennine on Marine Organisms. Aquat. Toxicol. 2019, 209, 13–25. [Google Scholar] [CrossRef]
- Zebiri, I.; Jacquette, B.; Francezon, N.; Herbaut, M.; Latigui, A.; Bricaud, S.; Tremblay, R.; Pasetto, P.; Mouget, J.-L.; Dittmer, J. The Polysaccharidic Nature of the Skeleton of Marennine as Determined by NMR Spectroscopy. Mar. Drugs 2023, 21, 42. [Google Scholar] [CrossRef]
- Gastineau, R.; Turcotte, F.; Pouvreau, J.-B.; Morançais, M.; Fleurence, J.; Windarto, E.; Prasetiya, F.; Arsad, S.; Jaouen, P.; Babin, M.; et al. Marennine, Promising Blue Pigments from a Widespread Haslea Diatom Species Complex. Mar. Drugs 2014, 12, 3161–3189. [Google Scholar] [CrossRef]
- Gastineau, R.; Prasetiya, F.S.; Falaise, C.; Cognie, B.; Decottignies, P.; Morançais, M.; Méléder, V.; Davidovich, N.; Turcotte, F.; Tremblay, R.; et al. Marennine-Like Pigments: Blue Diatom or Green Oyster Cult? In Blue Biotechnology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 529–551. ISBN 978-3-527-80171-8. [Google Scholar]
- Yusuf, M.; Baroroh, U.; Nuwarda, R.F.; Prasetiya, F.S.; Ishmayana, S.; Novianti, M.T.; Tohari, T.R.; Hardianto, A.; Subroto, T.; Mouget, J.-L.; et al. Theoretical and Experimental Studies on the Evidence of 1,3-β-Glucan in Marennine of Haslea Ostrearia. Molecules 2023, 28, 5625. [Google Scholar] [CrossRef] [PubMed]
- Young, S.H.; Dong, W.J.; Jacobs, R.R. Observation of a Partially Opened Triple-Helix Conformation in 1-->3-Beta-Glucan by Fluorescence Resonance Energy Transfer Spectroscopy. J. Biol. Chem. 2000, 275, 11874–11879. [Google Scholar] [CrossRef] [PubMed]
- Lehtovaara, B.C.; Gu, F.X. Pharmacological, Structural, and Drug Delivery Properties and Applications of 1,3-β-Glucans. J. Agric. Food Chem. 2011, 59, 6813–6828. [Google Scholar] [CrossRef]
- Bergé, J.-P.; Bourgougnon, N.; Alban, S.; Pojer, F.; Billaudel, S.; Chermann, J.-C.; Robert, J.M.; Franz, G. Antiviral and Anticoagulant Activities of a Water-Soluble Fraction of the Marine Diatom Haslea Ostrearia. Planta Med. 1999, 65, 604–609. [Google Scholar] [CrossRef]
- Carbonnelle, D.; Pondaven, P.; Morancais, M.; Masse, G.; Bosch, S.; Jacquot, C.; Briand, G.; Robert, J.; Roussakis, C. Antitumor and Antiproliferative Effects of an Aqueous Extract from the Marine Diatom Haslea Ostrearia (Simonsen) against Solid Tumors: Lung Carcinoma (NSCLC-N6), Kidney Carcinoma (E39) and Melanoma (M96) Cell Lines. Anticancer Res. 1999, 19, 621–624. [Google Scholar] [PubMed]
- Gastineau, R.; Pouvreau, J.-B.; Hellio, C.; Morançais, M.; Fleurence, J.; Gaudin, P.; Bourgougnon, N.; Mouget, J.-L. Biological Activities of Purified Marennine, the Blue Pigment Responsible for the Greening of Oysters. J. Agric. Food Chem. 2012, 60, 3599–3605. [Google Scholar] [CrossRef]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in Angiogenesis and Lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Pleiotropic Roles of Matrix Metalloproteinases in Tumor Angiogenesis: Contrasting, Overlapping and Compensatory Functions. Biochim. Biophys. Acta 2010, 1803, 103–120. [Google Scholar] [CrossRef]
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining Endothelial Progenitor Cells via Clonal Analysis and Hematopoietic Stem/Progenitor Cell Principals. Blood 2007, 109, 1801–1809. [Google Scholar] [CrossRef]
- Francezon, N.; Herbaut, M.; Bardeau, J.-F.; Cougnon, C.; Bélanger, W.; Tremblay, R.; Jacquette, B.; Dittmer, J.; Pouvreau, J.-B.; Mouget, J.-L.; et al. Electrochromic Properties and Electrochemical Behavior of Marennine, a Bioactive Blue-Green Pigment Produced by the Marine Diatom Haslea Ostrearia. Mar. Drugs 2021, 19, 231. [Google Scholar] [CrossRef]
- Méresse, S.; Gateau, H.; Tirnan, T.; Larrigaldie, V.; Casse, N.; Pasetto, P.; Mouget, J.-L.; Mortaud, S.; Fodil, M. Haslea Ostrearia Pigment Marennine Affects Key Actors of Neuroinflammation and Decreases Cell Migration in Murine Neuroglial Cell Model. Int. J. Mol. Sci. 2023, 24, 5388. [Google Scholar] [CrossRef]
- Schumacher, M.; Kelkel, M.; Dicato, M.; Diederich, M. Gold from the Sea: Marine Compounds as Inhibitors of the Hallmarks of Cancer. Biotechnol. Adv. 2011, 29, 531–547. [Google Scholar] [CrossRef]
- Janani, G.; Girigoswami, A.; Deepika, B.; Udayakumar, S.; Mercy, D.J.; Girigoswami, K. Dual Mechanism of Amphiroa Anceps: Antiangiogenic and Anticancer Effects in Skin Cancer. Chem. Biodivers. 2025, 22, e202500626. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-K.; Jo, M.-H.; Shin, H.J.; Park, S.J. Anti-Angiogenic Potential of Marine Streptomyces-Derived Lucknolide A on VEGF/VEGFR2 Signaling in Human Endothelial Cells. Molecules 2025, 30, 987. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.E.; Hussein, N.A.; Rashad, M.M.; El-Sikaily, A.M.; Hassanin, A.E.-L.A.; El-Fakharany, E.M. Sea Cucumber Sulfated Polysaccharides Extract Potentiates the Anticancer Effect of 5- Fluorouracil on Hepatocellular Carcinoma Cells. Sci. Rep. 2025, 15, 20255. [Google Scholar] [CrossRef]
- Partida-Martínez, L.P.; Heil, M. The Microbe-Free Plant: Fact or Artifact? Front. Plant Sci. 2011, 2, 100. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature Is the Best Source of Anticancer Drugs: Indexing Natural Products for Their Anticancer Bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Matsubara, K.; Akagi, R.; Mori, M.; Hirata, T. Antiangiogenic Activity of Brown Algae Fucoxanthin and Its Deacetylated Product, Fucoxanthinol. J. Agric. Food Chem. 2006, 54, 9805–9810. [Google Scholar] [CrossRef]
- Kowshik, J.; Baba, A.B.; Giri, H.; Deepak Reddy, G.; Dixit, M.; Nagini, S. Astaxanthin Inhibits JAK/STAT-3 Signaling to Abrogate Cell Proliferation, Invasion and Angiogenesis in a Hamster Model of Oral Cancer. PLoS ONE 2014, 9, e109114. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin Inhibits Tumour-Related Lymphangiogenesis and Growth of Breast Cancer. J. Cell Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Rokkaku, T.; Kimura, R.; Ishikawa, C.; Yasumoto, T.; Senba, M.; Kanaya, F.; Mori, N. Anticancer Effects of Marine Carotenoids, Fucoxanthin and Its Deacetylated Product, Fucoxanthinol, on Osteosarcoma. Int. J. Oncol. 2013, 43, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Mouget, J.-L.; Gastineau, R.; Davidovich, O.; Gaudin, P.; Davidovich, N.A. Light Is a Key Factor in Triggering Sexual Reproduction in the Pennate Diatom Haslea Ostrearia. FEMS Microbiol. Ecol. 2009, 69, 194–201. [Google Scholar] [CrossRef][Green Version]
- Zemani, F.; Benisvy, D.; Galy-Fauroux, I.; Lokajczyk, A.; Colliec-Jouault, S.; Uzan, G.; Fischer, A.M.; Boisson-Vidal, C. Low-Molecular-Weight Fucoidan Enhances the Proangiogenic Phenotype of Endothelial Progenitor Cells. Biochem. Pharmacol. 2005, 70, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Benslimane-Ahmim, Z.; Heymann, D.; Dizier, B.; Lokajczyk, A.; Brion, R.; Laurendeau, I.; Bièche, I.; Smadja, D.M.; Galy-Fauroux, I.; Colliec-Jouault, S.; et al. Osteoprotegerin, a New Actor in Vasculogenesis, Stimulates Endothelial Colony-Forming Cells Properties. J. Thromb. Haemost. 2011, 9, 834–843. [Google Scholar] [CrossRef] [PubMed]

 ) either alone or in the presence of 100 µg/mL of PEMn (
) either alone or in the presence of 100 µg/mL of PEMn ( , 100 µg/mL) for 24 and 48 h. Senescence was then assessed by senescence-associated-galactosidase (SA-β-gal) staining. SA-β-gal positive cells appear blue. Representative images are shown from one out of three independent experiments (phase contrast micrograph, original 10). (D) Cellular senescence was quantified as the number of SA-CTRL β-gal positive cells. Data are expressed as the mean ± SD from 3 independent experiments by use of one-way ANOVA and Student’s t-test analysis * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. initial number of ECFCs at T0.
, 100 µg/mL) for 24 and 48 h. Senescence was then assessed by senescence-associated-galactosidase (SA-β-gal) staining. SA-β-gal positive cells appear blue. Representative images are shown from one out of three independent experiments (phase contrast micrograph, original 10). (D) Cellular senescence was quantified as the number of SA-CTRL β-gal positive cells. Data are expressed as the mean ± SD from 3 independent experiments by use of one-way ANOVA and Student’s t-test analysis * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. initial number of ECFCs at T0.
 ) either alone or in the presence of 100 µg/mL of PEMn (
) either alone or in the presence of 100 µg/mL of PEMn ( , 100 µg/mL) for 24 and 48 h. Senescence was then assessed by senescence-associated-galactosidase (SA-β-gal) staining. SA-β-gal positive cells appear blue. Representative images are shown from one out of three independent experiments (phase contrast micrograph, original 10). (D) Cellular senescence was quantified as the number of SA-CTRL β-gal positive cells. Data are expressed as the mean ± SD from 3 independent experiments by use of one-way ANOVA and Student’s t-test analysis * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. initial number of ECFCs at T0.
, 100 µg/mL) for 24 and 48 h. Senescence was then assessed by senescence-associated-galactosidase (SA-β-gal) staining. SA-β-gal positive cells appear blue. Representative images are shown from one out of three independent experiments (phase contrast micrograph, original 10). (D) Cellular senescence was quantified as the number of SA-CTRL β-gal positive cells. Data are expressed as the mean ± SD from 3 independent experiments by use of one-way ANOVA and Student’s t-test analysis * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. initial number of ECFCs at T0.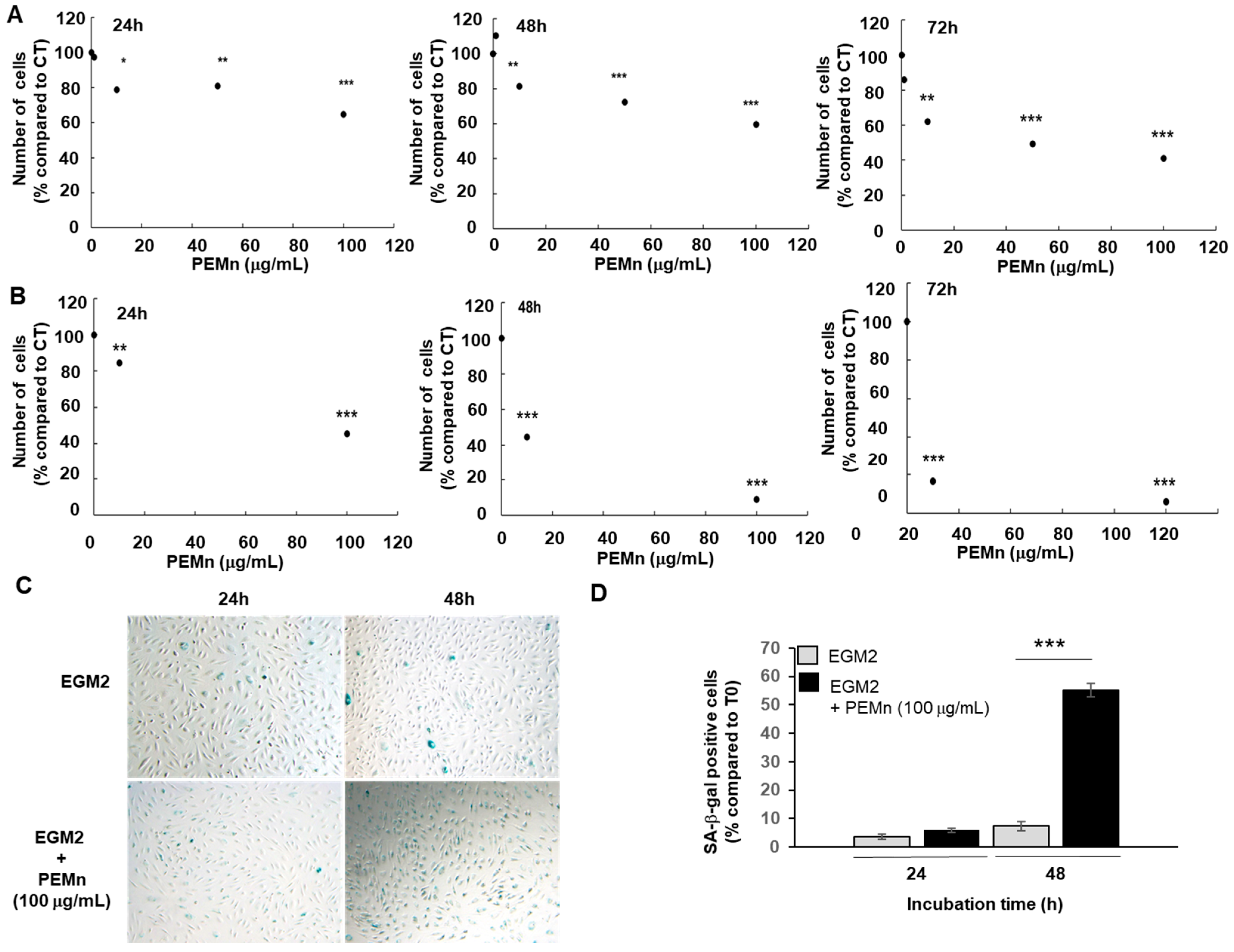
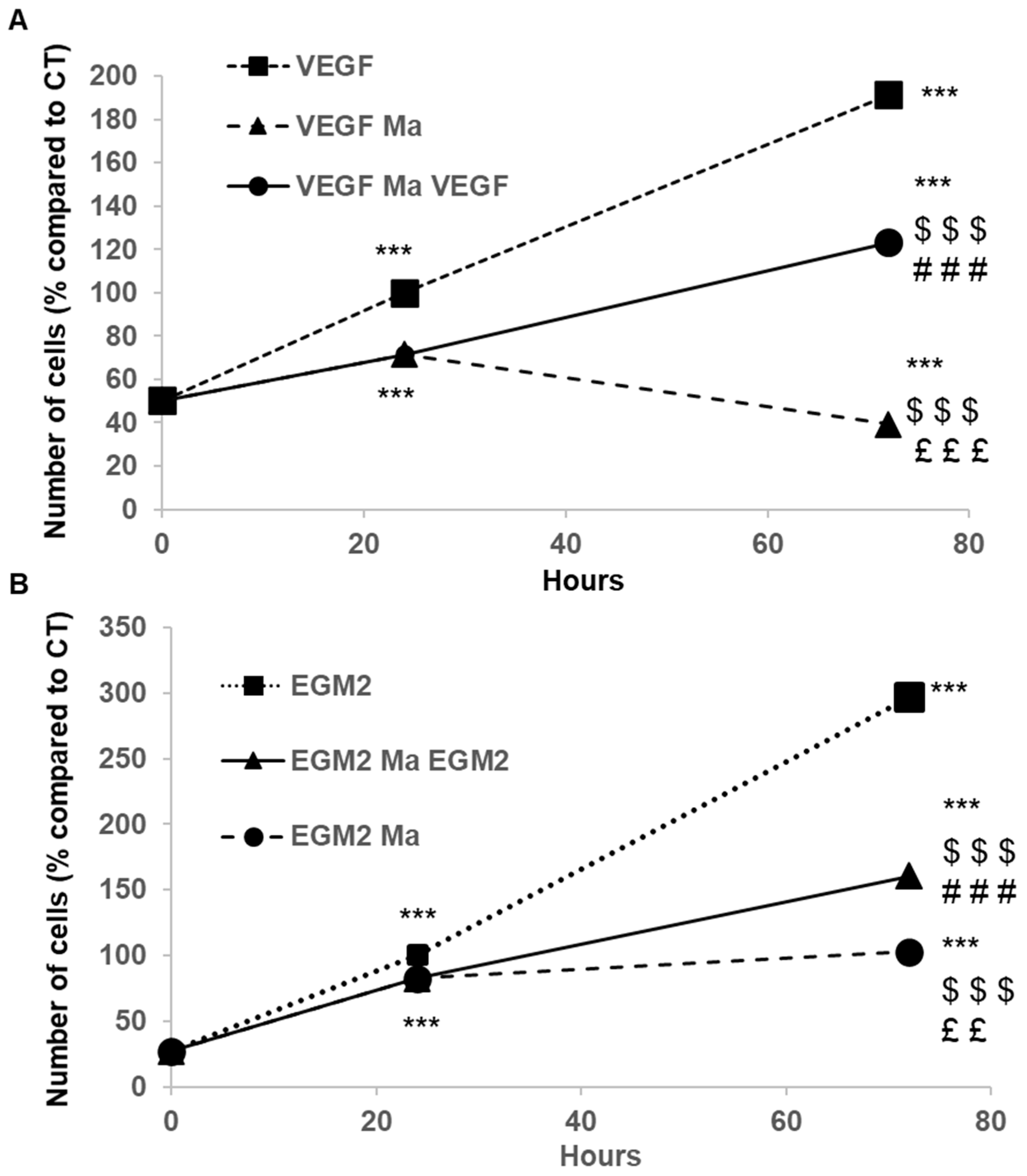
 ) EBM2 5% FBS VEGF supplemented with PEMn 10 µg/mL (
) EBM2 5% FBS VEGF supplemented with PEMn 10 µg/mL ( ) 50 µg/mL (
) 50 µg/mL ( ) and 100 µg/mL (
) and 100 µg/mL ( ). (C) Dose-dependent effect: After 24 h of incubation, the percentage of wound closure was calculated relative to T0 for each condition. (D) Average wound reduction speed (µm/h): Migration speed was determined from the wound reduction kinetics for each condition. The graphs represent the average migration speed from two replicates across four independent experiments. (E) PEMn reduces in vitro tubulogenesis. After synchronisation in EBM2-2% FBS, ECFCs were seeded onto Matrigel® and incubated in EBM2-5% FBS VEGF (40 ng/mL), with or without PEMn (10, 50, 100 µg/mL). Representative images of vascular structure formation in vitro after 16 h of incubation in EGM2-5% VEGF (40 ng/mL), in the absence or presence of PEMn (10, 50, and 100 µg/mL), obtained using light microscopy (10× magnification). Dose–response curve of PEMn’s effect on the average tube length after 16 h of incubation, normalised to the control condition. Tube length was measured using the Histolab® software (Microvision Instruments, Evry, France). Data are expressed as the mean ± SD from 5 independent experiments. 1 one-way or two-way ANOVA, Fisher correction * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. VEGF-treated ECFCs, ££ p < 0.01 and ### p < 0.001 vs. VEGF-PEMn 10 µg/mL or VEGF-PEMn 50 µg/mL -treated ECFCs, respectively.
). (C) Dose-dependent effect: After 24 h of incubation, the percentage of wound closure was calculated relative to T0 for each condition. (D) Average wound reduction speed (µm/h): Migration speed was determined from the wound reduction kinetics for each condition. The graphs represent the average migration speed from two replicates across four independent experiments. (E) PEMn reduces in vitro tubulogenesis. After synchronisation in EBM2-2% FBS, ECFCs were seeded onto Matrigel® and incubated in EBM2-5% FBS VEGF (40 ng/mL), with or without PEMn (10, 50, 100 µg/mL). Representative images of vascular structure formation in vitro after 16 h of incubation in EGM2-5% VEGF (40 ng/mL), in the absence or presence of PEMn (10, 50, and 100 µg/mL), obtained using light microscopy (10× magnification). Dose–response curve of PEMn’s effect on the average tube length after 16 h of incubation, normalised to the control condition. Tube length was measured using the Histolab® software (Microvision Instruments, Evry, France). Data are expressed as the mean ± SD from 5 independent experiments. 1 one-way or two-way ANOVA, Fisher correction * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. VEGF-treated ECFCs, ££ p < 0.01 and ### p < 0.001 vs. VEGF-PEMn 10 µg/mL or VEGF-PEMn 50 µg/mL -treated ECFCs, respectively.
 ) EBM2 5% FBS VEGF supplemented with PEMn 10 µg/mL (
) EBM2 5% FBS VEGF supplemented with PEMn 10 µg/mL ( ) 50 µg/mL (
) 50 µg/mL ( ) and 100 µg/mL (
) and 100 µg/mL ( ). (C) Dose-dependent effect: After 24 h of incubation, the percentage of wound closure was calculated relative to T0 for each condition. (D) Average wound reduction speed (µm/h): Migration speed was determined from the wound reduction kinetics for each condition. The graphs represent the average migration speed from two replicates across four independent experiments. (E) PEMn reduces in vitro tubulogenesis. After synchronisation in EBM2-2% FBS, ECFCs were seeded onto Matrigel® and incubated in EBM2-5% FBS VEGF (40 ng/mL), with or without PEMn (10, 50, 100 µg/mL). Representative images of vascular structure formation in vitro after 16 h of incubation in EGM2-5% VEGF (40 ng/mL), in the absence or presence of PEMn (10, 50, and 100 µg/mL), obtained using light microscopy (10× magnification). Dose–response curve of PEMn’s effect on the average tube length after 16 h of incubation, normalised to the control condition. Tube length was measured using the Histolab® software (Microvision Instruments, Evry, France). Data are expressed as the mean ± SD from 5 independent experiments. 1 one-way or two-way ANOVA, Fisher correction * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. VEGF-treated ECFCs, ££ p < 0.01 and ### p < 0.001 vs. VEGF-PEMn 10 µg/mL or VEGF-PEMn 50 µg/mL -treated ECFCs, respectively.
). (C) Dose-dependent effect: After 24 h of incubation, the percentage of wound closure was calculated relative to T0 for each condition. (D) Average wound reduction speed (µm/h): Migration speed was determined from the wound reduction kinetics for each condition. The graphs represent the average migration speed from two replicates across four independent experiments. (E) PEMn reduces in vitro tubulogenesis. After synchronisation in EBM2-2% FBS, ECFCs were seeded onto Matrigel® and incubated in EBM2-5% FBS VEGF (40 ng/mL), with or without PEMn (10, 50, 100 µg/mL). Representative images of vascular structure formation in vitro after 16 h of incubation in EGM2-5% VEGF (40 ng/mL), in the absence or presence of PEMn (10, 50, and 100 µg/mL), obtained using light microscopy (10× magnification). Dose–response curve of PEMn’s effect on the average tube length after 16 h of incubation, normalised to the control condition. Tube length was measured using the Histolab® software (Microvision Instruments, Evry, France). Data are expressed as the mean ± SD from 5 independent experiments. 1 one-way or two-way ANOVA, Fisher correction * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. VEGF-treated ECFCs, ££ p < 0.01 and ### p < 0.001 vs. VEGF-PEMn 10 µg/mL or VEGF-PEMn 50 µg/mL -treated ECFCs, respectively.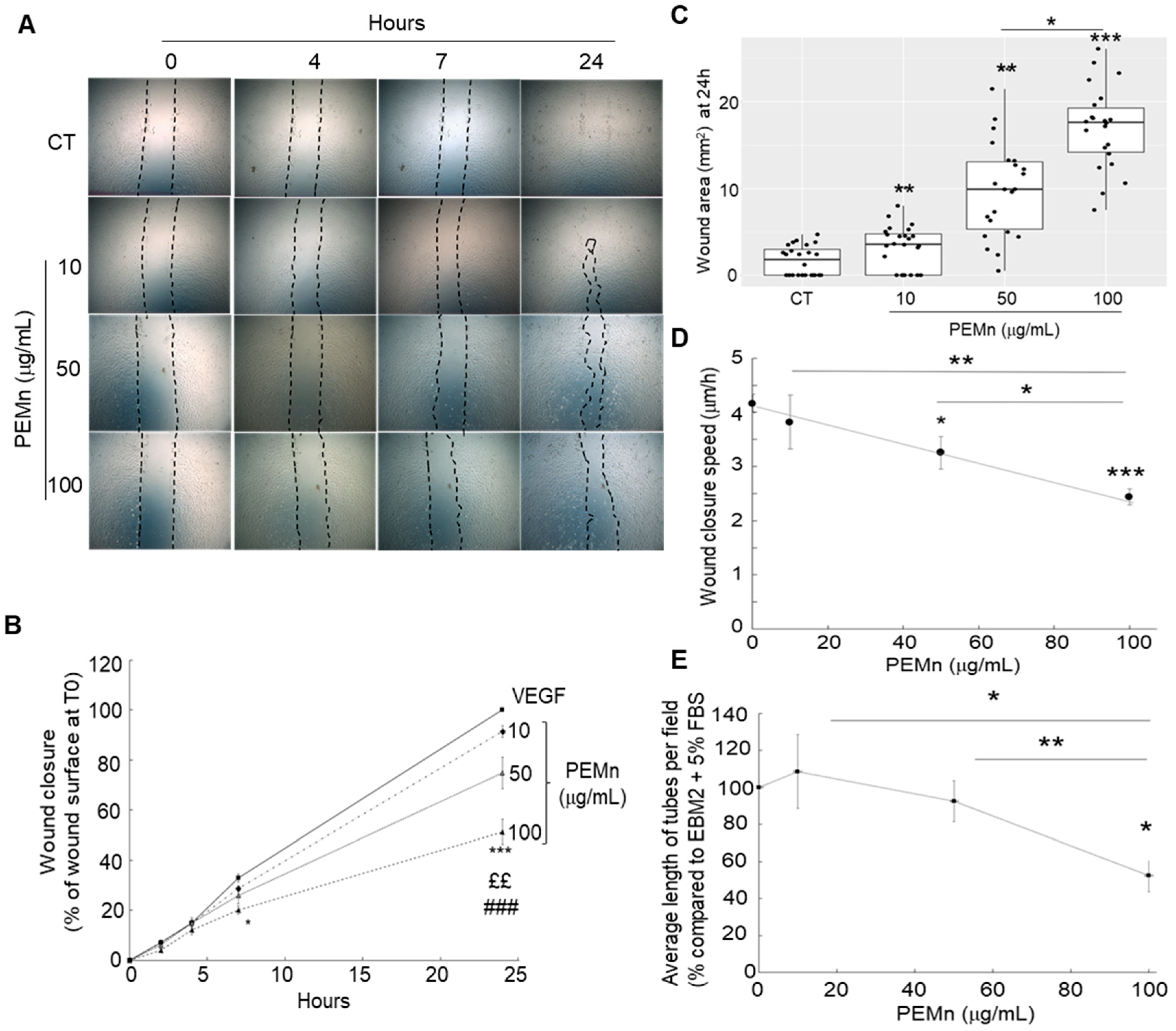

| PEMn in ECFCs (µg/mL) | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|
| 24 h incubation | |||
| 0 | 35.0 ± 6.2 | 22.1 ± 2.4 | 28.7 ± 6.7 |
| 100 | 59.2 ± 5.0 b | 15.0 ± 3.1 | 14.3 ± 5.0 a |
| 72 h incubation | |||
| 0 | 46.4 ± 3.9 | 24.3 ± 4.3 | 20.0 ± 3.6 |
| 100 | 66.9 ± 2.1 c | 17.3 ± 2.8 | 7.8 ± 1.1 a |
| ECFCs | Q4 Live (%) | Q3 Early Apop (%) | Q2 Late Apop (%) | Q1 Dead (%) |
|---|---|---|---|---|
| 24 h incubation | ||||
| Control | 73.6 ± 3.3 | 12.1 ± 4.3 | 8.7 ± 1.0 | 5.7 ± 1.6 |
| PEMn 100 µg/mL | 53.4 ± 2.8 c | 15.2 ± 2.2 | 19.9 ± 2.3 b | 11.5 ± 2.6 |
| 72 h incubation | ||||
| Control | 69.4 ± 5.1 | 10.3 ± 3.3 | 12.1 ± 2.6 | 8.4 ± 1.7 |
| PEMn 100 µg/mL | 44.1 ± 7.1 c | 10.2 ± 1.3 | 27.8 ± 6.0 b | 17.8 ± 2.0 |
| 24 h | 48 h | 72 h | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables (pg/mL) | EGM2 | PEMn | EGM2 | PEMN | p-Value | EGM2 | PEMn | p-Value |
| VEGF-R1 | 2.70 ± 0.26 | 1.31 ± 0.15 | 6.12 ± 0.06 | 2.20 ± 0.02 | <0.001 | 6.04 ± 0.11 | 3.47 ± 0.48 | <0.001 |
| IL-6 | 47.00 ± 1.75 | 55.64 ± 4.53 | 86.68 ± 1.79 | 96.63 ± 2.34 | 0.8030 | 88.30 ± 1.04 | 453.04 ± 60.95 | <0.001 |
| IL-1β | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.9792 | 0.05 ± 0.00 | 1.47 ± 0.44 | <0.001 |
| MMP-9 | 0.17 ± 0.01 | 0.27 ± 0.1 | 0.27 ± 0.05 | 0.16 ± 0.01 | 0.9084 | 0.21 ± 0.01 | 6.3 ± 2.10 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fodil, M.; Muñoz-Garcia, J.; Benali, A.-K.; Rogozarski, J.; Mignon, V.; Labrana, H.; Lokajczyk, A.; Pasetto, P.; Mouget, J.-L.; Boisson-Vidal, C.; et al. Effect of Precipitated Extracellular Marennine on Angiogenesis and Tumour Cell Proliferation. Mar. Drugs 2025, 23, 364. https://doi.org/10.3390/md23090364
Fodil M, Muñoz-Garcia J, Benali A-K, Rogozarski J, Mignon V, Labrana H, Lokajczyk A, Pasetto P, Mouget J-L, Boisson-Vidal C, et al. Effect of Precipitated Extracellular Marennine on Angiogenesis and Tumour Cell Proliferation. Marine Drugs. 2025; 23(9):364. https://doi.org/10.3390/md23090364
Chicago/Turabian StyleFodil, Mostefa, Javier Muñoz-Garcia, Amel-Khitem Benali, Jasmina Rogozarski, Virginie Mignon, Honora Labrana, Anna Lokajczyk, Pamela Pasetto, Jean-Luc Mouget, Catherine Boisson-Vidal, and et al. 2025. "Effect of Precipitated Extracellular Marennine on Angiogenesis and Tumour Cell Proliferation" Marine Drugs 23, no. 9: 364. https://doi.org/10.3390/md23090364
APA StyleFodil, M., Muñoz-Garcia, J., Benali, A.-K., Rogozarski, J., Mignon, V., Labrana, H., Lokajczyk, A., Pasetto, P., Mouget, J.-L., Boisson-Vidal, C., & Heymann, D. (2025). Effect of Precipitated Extracellular Marennine on Angiogenesis and Tumour Cell Proliferation. Marine Drugs, 23(9), 364. https://doi.org/10.3390/md23090364









