Novel pH-Responsive PSS-Loaded Chitosan Matrix Nanoparticles Ameliorate Pressure Overload-Induced Cardiac Hypertrophy
Abstract
1. Introduction
2. Results and Discussion
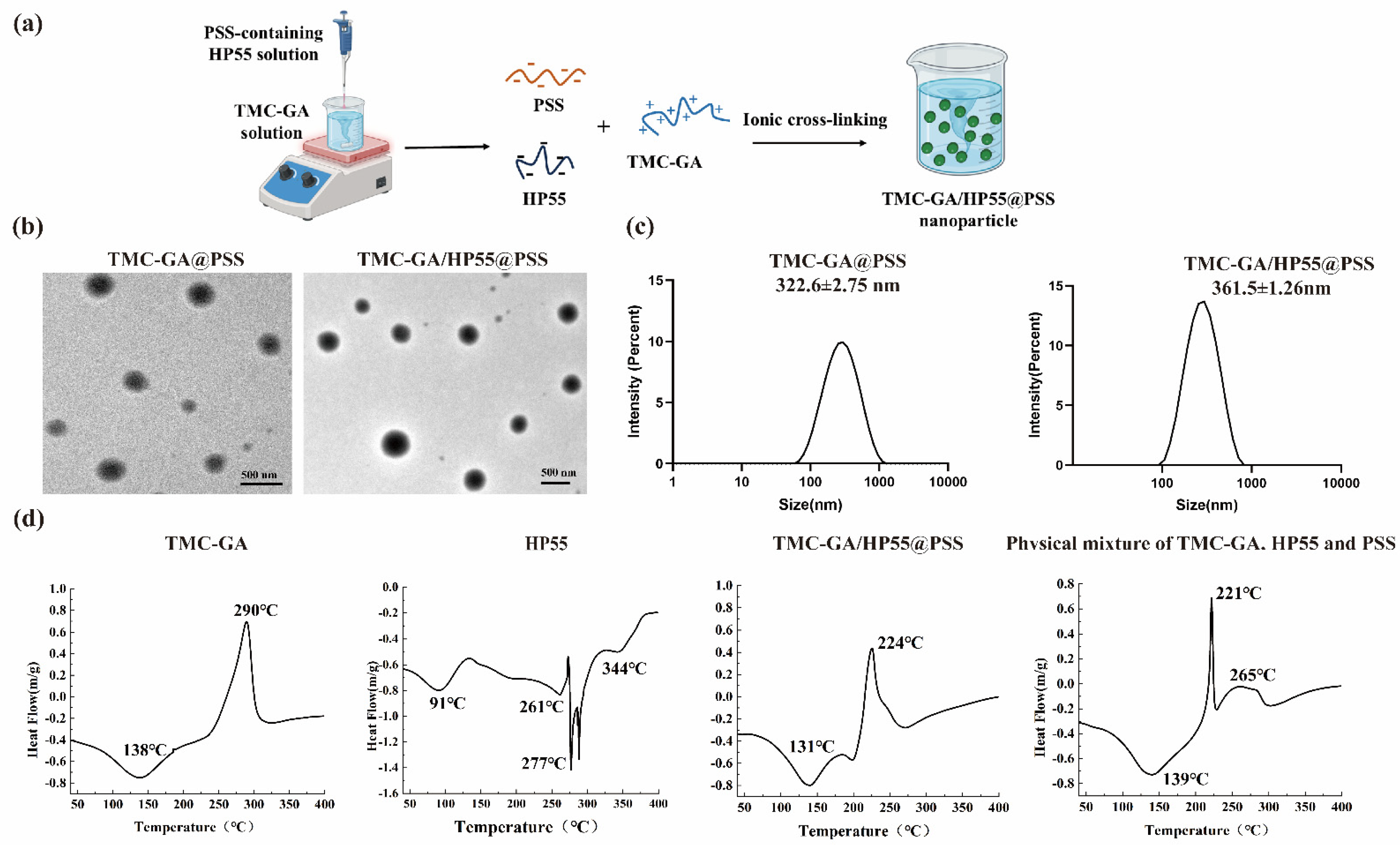
2.1. Preparation and Characterization of PSS Nanoparticles
2.2. Drug Release Study of TMC-GA/HP55@PSS Nanoparticles
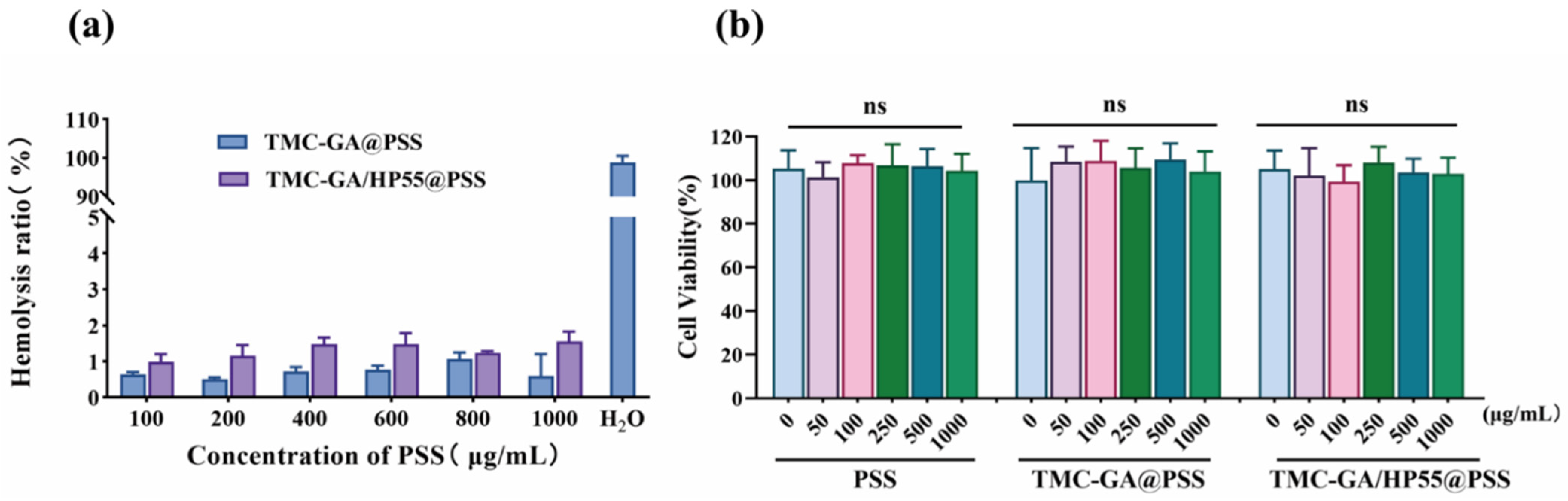
2.3. Biosafety Evaluation of TMC-GA/HP55@PSS Nanoparticles
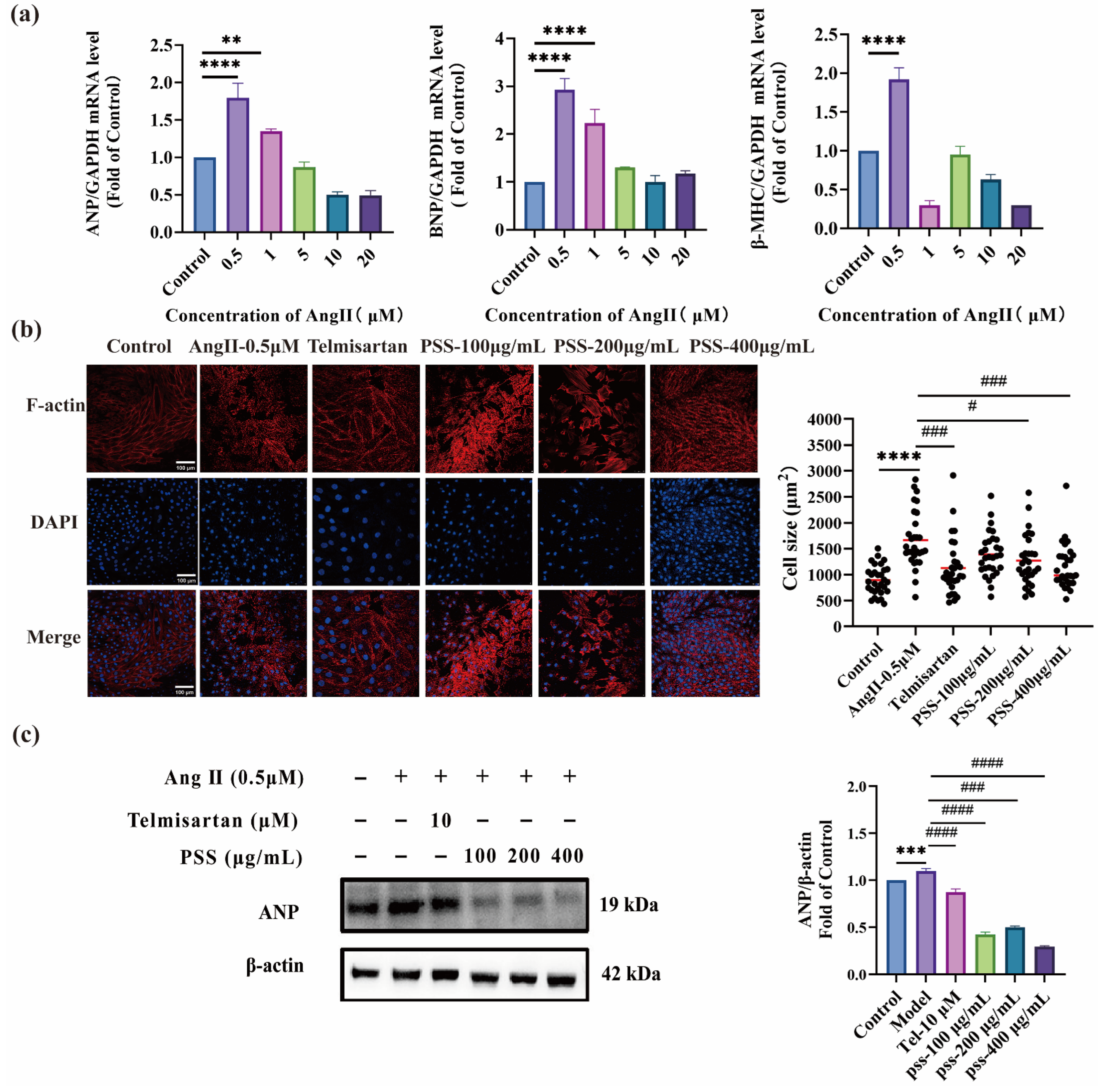
2.4. In Vitro Efficacy of PSS Against Cardiomyocyte Hypertrophy
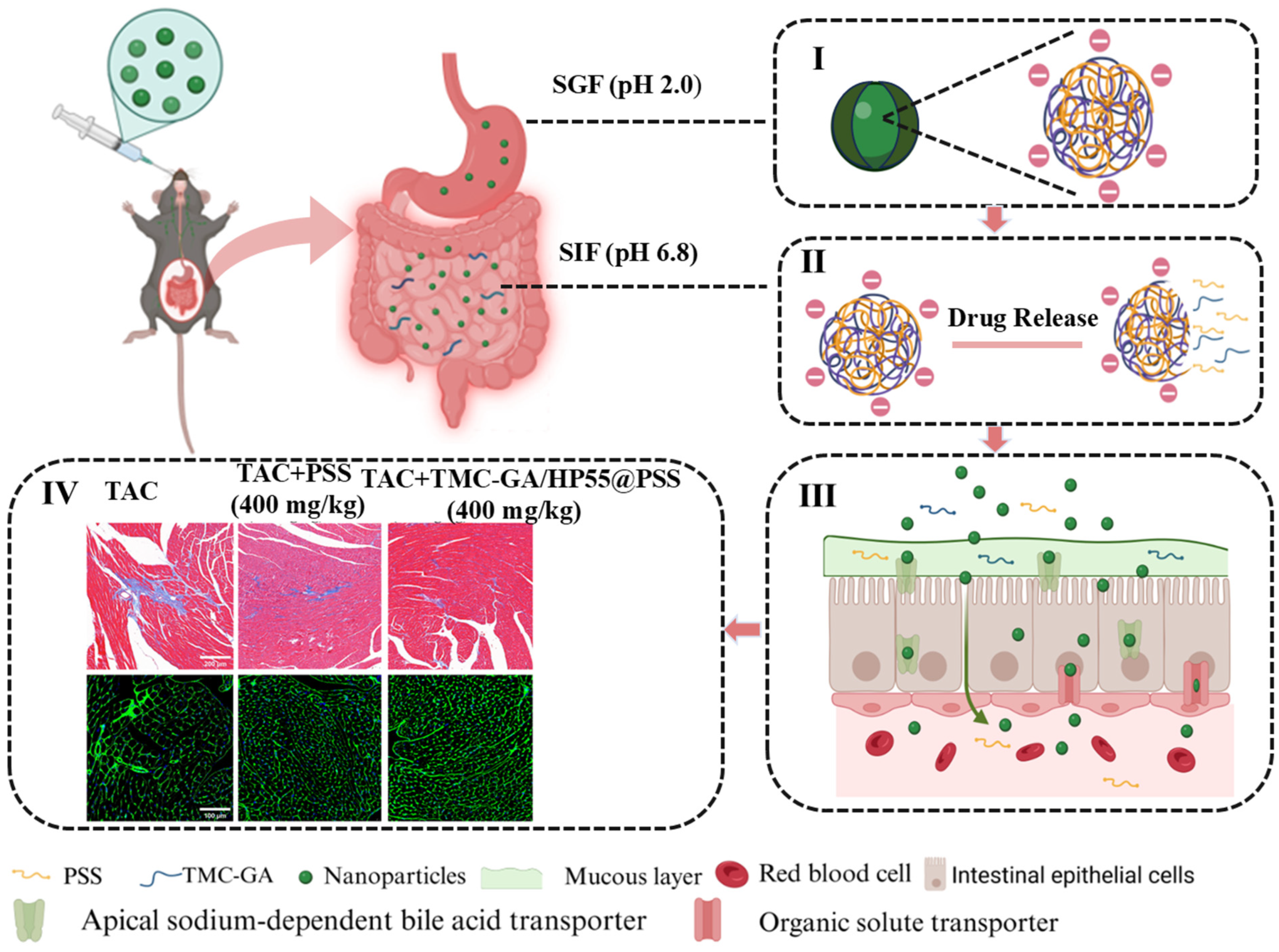
2.5. In Vivo PSS and Its Nanoparticles Ameliorated Cardiac Hypertrophy
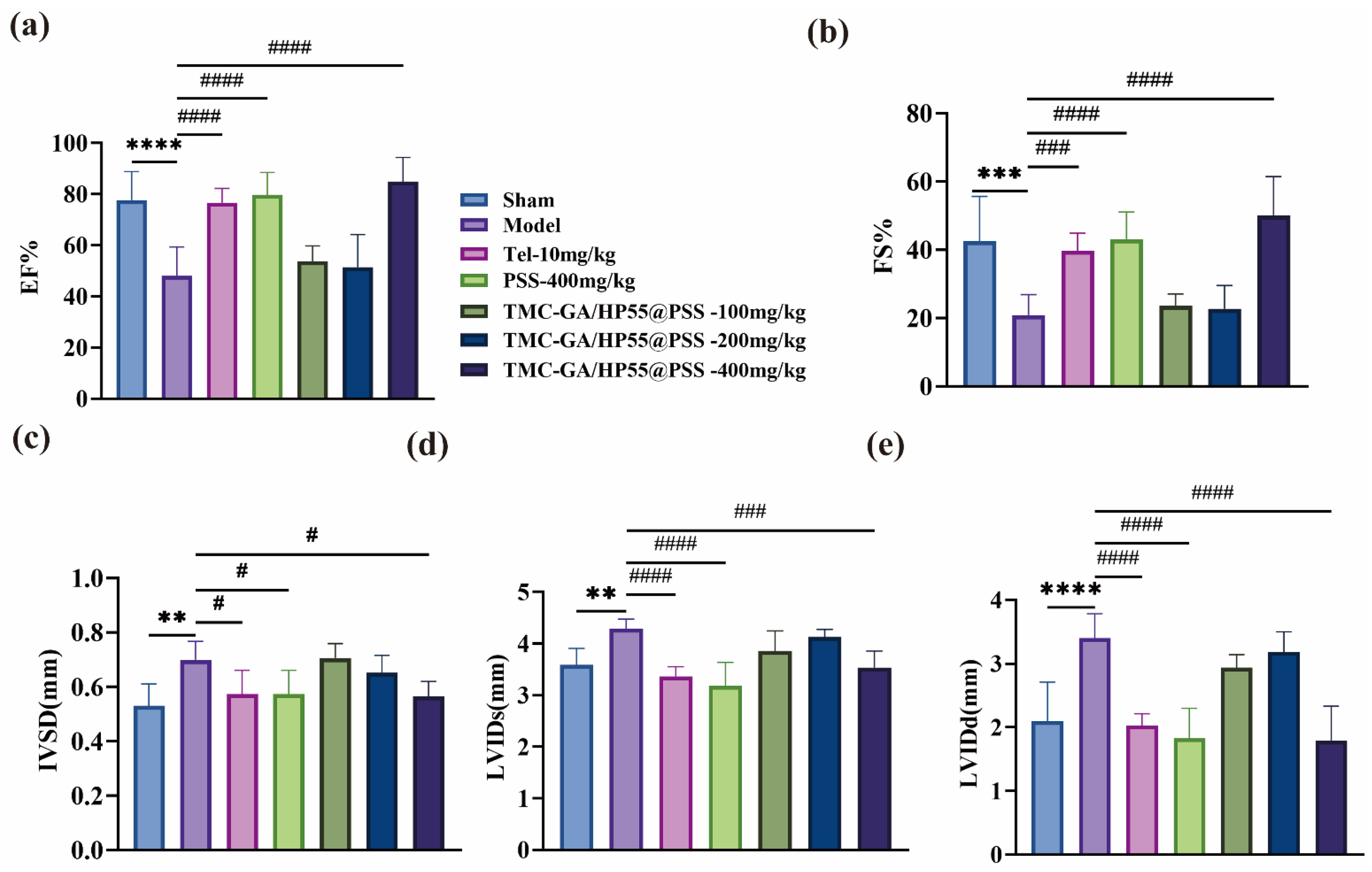
2.5.1. Echocardiography Analysis
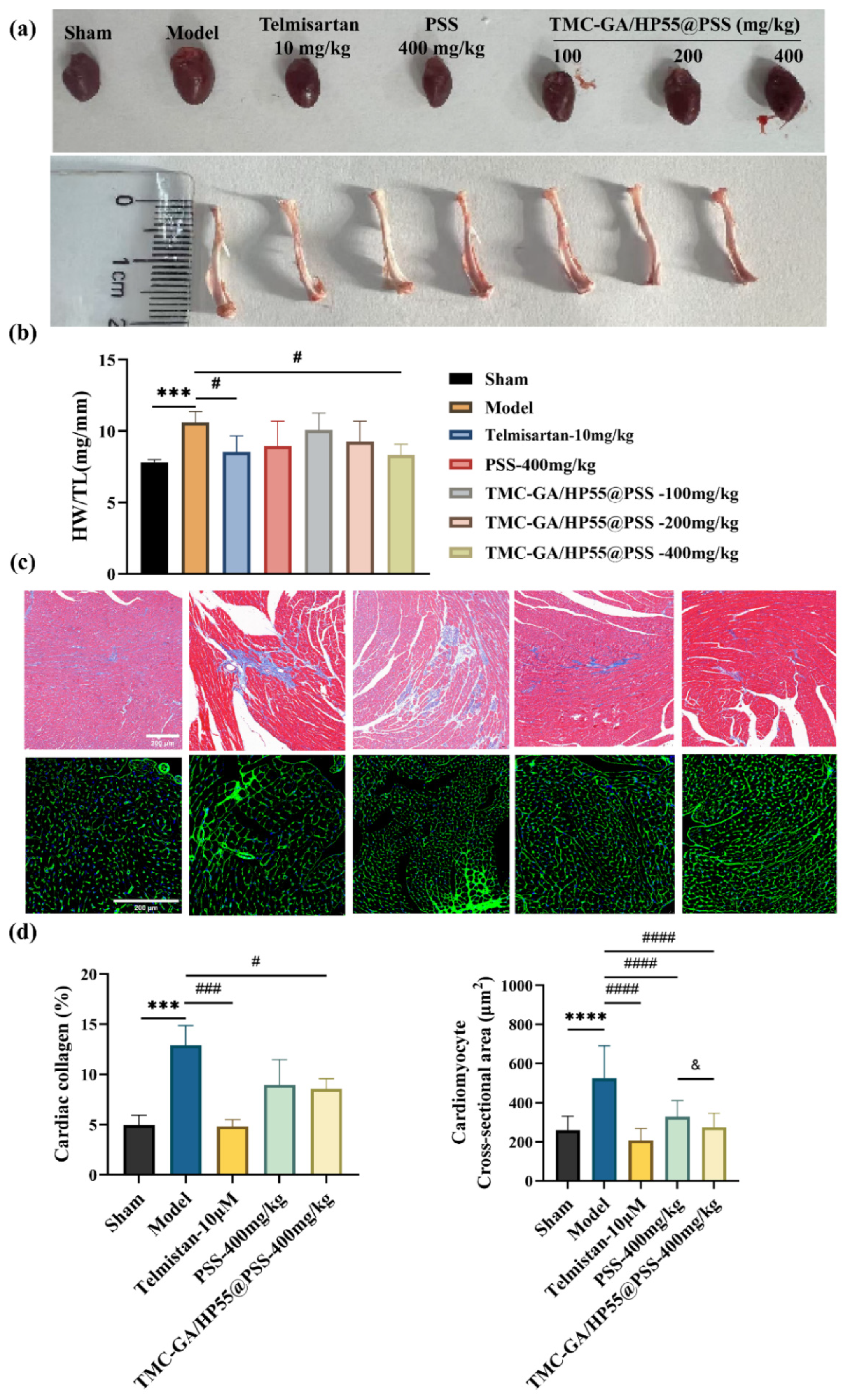
2.5.2. Histopathologic Analysis of Myocardial Tissue
3. Materials and Methods
3.1. Materials and Reagents
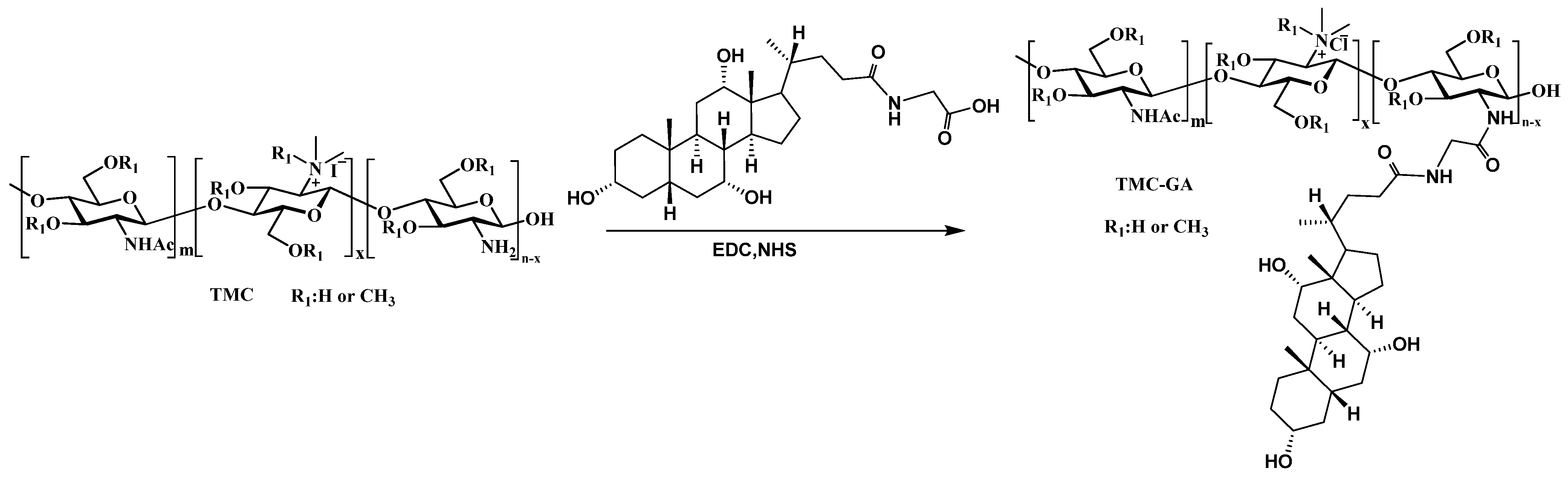
3.2. Synthesis and Characterization of TMC-GA
3.3. Preparation and Characterization of PSS Nanoparticles
3.4. Encapsulation Efficiency of Nanoparticles
3.5. Release of PSS from the Nanoparticles
3.6. Biosafety Evaluations of Nanoparticles
3.6.1. In Vitro Cytotoxicity of Nanoparticles
3.6.2. Hemolytic Test of Nanoparticles
3.7. Cell Culture and Treatments
3.7.1. Real-Time Quantitative PCR
3.7.2. Cell Surface Area
3.7.3. Western Blot Analysis
3.8. Animal Model and Treatment
3.8.1. Echocardiography Analysis
3.8.2. Histopathological Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, M.; Liu, Y.; Zhang, S.; Yuan, Y. A Comprehensive Review of Propylene Glycol Alginate in the Food Industry: Synthesis, Safety, Composite Hydrocolloids and Application. Trends Food Sci. Technol. 2025, 157, 104900. [Google Scholar] [CrossRef]
- Xin, M.; Ren, L.; Sun, Y.; Li, H.; Guan, H.-S.; He, X.-X.; Li, C.-X. Anticoagulant and Antithrombotic Activities of Low-Molecular-Weight Propylene Glycol Alginate Sodium Sulfate (PSS). Eur. J. Med. Chem. 2016, 114, 33–40. [Google Scholar] [CrossRef]
- Xu, S.; Mao, Y.; Wu, J.; Feng, J.; Li, J.; Wu, L.; Yu, Q.; Zhou, Y.; Zhang, J.; Chen, J.; et al. TGF-β/Smad and JAK/STAT Pathways Are Involved in the Anti-fibrotic Effects of Propylene Glycol Alginate Sodium Sulphate on Hepatic Fibrosis. J. Cell. Mol. Med. 2020, 24, 5224–5237. [Google Scholar] [CrossRef]
- Mao, Y.; Hu, Y.; Feng, W.; Yu, L.; Li, P.; Cai, B.; Li, C.; Guan, H. Effects and Mechanisms of PSS-Loaded Nanoparticles on Coronary Microcirculation Dysfunction in Streptozotocin-Induced Diabetic Cardiomyopathy Rats. Biomed. Pharmacother. 2020, 121, 109280. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, Y.; Guan, H. Progress of marine drug propylene glycol alginate sodium sulfate (PSS) and inspiration. Chin. Bull. Life Sci. 2012, 24, 1019–1025. [Google Scholar] [CrossRef]
- Ritterhoff, J.; Tian, R. Metabolic Mechanisms in Physiological and Pathological Cardiac Hypertrophy: New Paradigms and Challenges. Nat. Rev. Cardiol. 2023, 20, 812–829. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Mechanisms of Physiological and Pathological Cardiac Hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Di Mattia, R.A.; Mariángelo, J.I.E.; Blanco, P.G.; Jaquenod De Giusti, C.; Portiansky, E.L.; Mundiña-Weilenmann, C.; Aiello, E.A.; Orlowski, A. The Activation of the G Protein-Coupled Estrogen Receptor (GPER) Prevents and Regresses Cardiac Hypertrophy. Life Sci. 2020, 242, 117211. [Google Scholar] [CrossRef]
- Wei, X.; Jin, J.; Wu, J.; He, Y.; Guo, J.; Yang, Z.; Chen, L.; Hu, K.; Li, L.; Jia, M.; et al. Cardiac-Specific BACH1 Ablation Attenuates Pathological Cardiac Hypertrophy by Inhibiting the Ang II Type 1 Receptor Expression and the Ca2+/CaMKII Pathway. Cardiovasc. Res. 2023, 119, 1842–1855. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Hu, C.; Zhao, X.; Zhang, P.; Chang, Y.; Shang, Y.; Pang, Y.; Qian, W.; Qiu, X.; et al. Lingguizhugan Decoction Dynamically Regulates MAPKs and AKT Signaling Pathways to Retrogress the Pathological Progression of Cardiac Hypertrophy to Heart Failure. Phytomedicine 2022, 98, 153951. [Google Scholar] [CrossRef]
- Xu, J.; Li, R.; Zhang, Z.; Yang, C.; Liu, S.; Li, Y.; Chen, M.; Wang, W.; Zhang, G.; Song, G.; et al. Loganin Inhibits Angiotensin II–Induced Cardiac Hypertrophy through the JAK2/STAT3 and NF-κB Signaling Pathways. Front. Pharmacol. 2021, 12, 678886. [Google Scholar] [CrossRef]
- Ye, S.; Luo, W.; Khan, Z.A.; Wu, G.; Xuan, L.; Shan, P.; Lin, K.; Chen, T.; Wang, J.; Hu, X.; et al. Celastrol Attenuates Angiotensin II–Induced Cardiac Remodeling by Targeting STAT3. Circ. Res. 2020, 126, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in Collaboration with the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2012, 14, 803–869. [Google Scholar] [CrossRef]
- Xue, Y.-T.; Li, S.; Jiang, X.-Y.; Xin, M.; Li, H.-H.; Yu, G.-L.; He, X.-X.; Li, C.-X. The Reason and Mechanism of Propylene Glycol Alginate Sodium Sulfate (PSS) Mediated Allergic Side Effect. Int. J. Biol. Macromol. 2023, 241, 124638. [Google Scholar] [CrossRef]
- Xue, Y.-T.; Ren, L.; Li, S.; Wang, L.; He, X.-X.; Zhao, X.; Yu, G.; Guan, H.-S.; Li, C.-X. Study on Quality Control of Sulfated Polysaccharide Drug, Propylene Glycol Alginate Sodium Sulfate (PSS). Carbohydr. Polym. 2016, 144, 330–337. [Google Scholar] [CrossRef]
- Li, P.-L.; Li, C.-X.; Xue, Y.-T.; Li, H.-H.; Liu, H.-B.; He, X.-X.; Yu, G.-L.; Guan, H.-S. An HPLC Method for Microanalysis and Pharmacokinetics of Marine Sulfated Polysaccharide PSS-Loaded Poly Lactic-Co-Glycolic Acid (PLGA) Nanoparticles in Rat Plasma. Mar. Drugs 2013, 11, 1113–1125. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Polyelectrolyte Complexes: Mechanisms, Critical Experimental Aspects, and Applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1615–1625. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. New Nasal Nanocomplex Self-Assembled from Charged Biomacromolecules: N,N,N-Trimethyl Chitosan and Dextran Sulfate. Int. J. Biol. Macromol. 2016, 88, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Bueno, P.V.A.; Souza, P.R.; Follmann, H.D.M.; Pereira, A.G.B.; Martins, A.F.; Rubira, A.F.; Muniz, E.C. N,N-Dimethyl Chitosan/Heparin Polyelectrolyte Complex Vehicle for Efficient Heparin Delivery. Int. J. Biol. Macromol. 2015, 75, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Y.; Meng, L.; Feng, J.; Cheng, M.; Tu, L. Intestinal Transporters and Oral Absorption Enhancing Strategies Based on These Transporters. Chin. Chem. Lett. 2024, 36, 110529. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.; Liu, Y.; Duan, H.; Chen, L.; Zhang, X.; Jin, M.; Cui, M.; Quan, X.; Pan, L.; et al. Versatile Flexible Micelles Integrating Mucosal Penetration and Intestinal Targeting for Effectively Oral Delivery of Paclitaxel. Acta Pharm. Sin. B 2023, 13, 3425–3443. [Google Scholar] [CrossRef]
- Zang, W.; Gao, D.; Yu, M.; Long, M.; Zhang, Z.; Ji, T. Oral Delivery of Gemcitabine-Loaded Glycocholic Acid-Modified Micelles for Cancer Therapy. ACS Nano 2023, 17, 18074–18088. [Google Scholar] [CrossRef]
- Nogami, S.; Uchiyama, H.; Kadota, K.; Tozuka, Y. Design of a pH-Responsive Oral Gel Formulation Based on the Matrix Systems of Gelatin/Hydroxypropyl Methylcellulose Phthalate for Controlled Drug Release. Int. J. Pharm. 2021, 592, 120047. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, W.; Liu, H.; Li, C.; Zhang, Y.; Meng, X.; Tang, T.; Xi, T.; Xing, Y. Oral Helicobacter Pylori Vaccine-Encapsulated Acid-Resistant HP55/PLGA Nanoparticles Promote Immune Protection. Eur. J. Pharm. Biopharm. 2017, 111, 33–43. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.; Tan, Z.; Zeng, Z.; Yang, H.; Luo, S.; Wang, L.; Xi, T.; Xing, Y. Promoting Immune Efficacy of the Oral Helicobacter Pylori Vaccine by HP55/PBCA Nanoparticles against the Gastrointestinal Environment. Mol. Pharm. 2018, 15, 3177–3186. [Google Scholar] [CrossRef]
- Fan, B.; Liu, L.; Zheng, Y.; Xing, Y.; Shen, W.; Li, Q.; Wang, R.; Liang, G. Novel pH-Responsive and Mucoadhesive Chitosan-Based Nanoparticles for Oral Delivery of Low Molecular Weight Heparin with Enhanced Bioavailability and Anticoagulant Effect. J. Drug Deliv. Sci. Technol. 2022, 78, 103955. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Hu, X.; Zhou, C.; Ma, Y.; Wang, X.; Tang, Y.; Chen, K.; Wang, X.; Liu, Y. Enhanced Oral Absorption and Liver Distribution of Polymeric Nanoparticles through Traveling the Enterohepatic Circulation Pathways of Bile Acid. ACS Appl. Mater. Interfaces 2022, 14, 41712–41725. [Google Scholar] [CrossRef] [PubMed]
- Hattan, C.M.; Kerns, R.J. Variability in the Carbazole Assay for N-Desulfonated/N-Acylated Heparin Derivatives. Carbohydr. Res. 2007, 342, 2664–2669. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, W.; Zhou, Y.; Huang, S.; Yu, J.; Yang, M.; Zhang, Z.; Ma, J.; Luo, J.; Rao, S.; et al. Hypoxia-Activated Cascade Nanovaccine for Synergistic Chemoembolization-Immune Therapy of Hepatocellular Carcinoma. Biomaterials 2024, 306, 122480. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kuo, Y.-H.; Du, C.-X.; Huang, C.-W.; Ku, H.-C. A Novel Caffeic Acid Derivative Prevents Angiotensin II-Induced Cardiac Remodeling. Biomed. Pharmacother. 2023, 162, 114709. [Google Scholar] [CrossRef]
- Tomita, Y.; Anzai, F.; Misaka, T.; Ogawara, R.; Ichimura, S.; Wada, K.; Kimishima, Y.; Yokokawa, T.; Ishida, T.; Takeishi, Y. Targeting N-Myristoylation through NMT2 Prevents Cardiac Hypertrophy and Heart Failure. JACC Basic Transl. Sci. 2023, 8, 1263–1282. [Google Scholar] [CrossRef]
- Van Heerebeek, L.; Borbély, A.; Niessen, H.W.M.; Bronzwaer, J.G.F.; Van Der Velden, J.; Stienen, G.J.M.; Linke, W.A.; Laarman, G.J.; Paulus, W.J. Myocardial Structure and Function Differ in Systolic and Diastolic Heart Failure. Circulation 2006, 113, 1966–1973. [Google Scholar] [CrossRef]
- Chen, G.; Pan, S.; Shen, C.; Pan, S.; Zhang, X.; He, Q. Puerarin Inhibits Angiotensin II-Induced Cardiac Hypertrophy via the Redox-Sensitive ERK1/2, P38 and NF-κB Pathways. Acta Pharmacol. Sin. 2014, 35, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Ma, S.; Wang, D.; Fan, Z.; Qiu, P.; Wang, S.; Li, C. The development of multifunctional sulfated polyguluronic acid-based polymeric micelles for anticancer drug delivery. Carbohydr. Polym. 2023, 303, 120451. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yin, G.; Huang, C.; Wang, H.; Gao, J.; Luo, J.; Zhang, Z.; Wang, J.; Hong, J.; Chai, X. Peroxiredoxin-1 Ameliorates Pressure Overload-Induced Cardiac Hypertrophy and Fibrosis. Biomed. Pharmacother. 2020, 129, 110357. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Pan, X.; Luo, L.; Zhang, Q.; Huang, X.; Liu, Y.; Wang, K.; Zhang, Y. Advances in Oral Absorption of Polysaccharides: Mechanism, Affecting Factors, and Improvement Strategies. Carbohydr. Polym. 2022, 282, 119110. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, J.; Hu, X.; Ding, K.; Zhang, J.; Liu, B.; Yang, Y.; Wei, Z.; Li, C.; Sun, Q.; et al. Inhibitory Effects of Propylene Glycol Alginate Sodium Sulfate Derivatives on Atrial Fibrosis in Atrial Fibrillation. Arab. J. Chem. 2024, 17, 105792. [Google Scholar] [CrossRef]
- Rohini, A.; Agrawal, N.; Koyani, C.N.; Singh, R. Molecular Targets and Regulators of Cardiac Hypertrophy. Pharmacol. Res. 2010, 61, 269–280. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Jiang, Y.-N.; Guan, X.; Ren, F.-F.; Wu, S.-J.; Chu, M.-P.; Wu, L.-P.; Lai, T.-F.; Li, L. Aerobic Exercise Attenuates Pressure Overload–Induced Myocardial Remodeling and Myocardial Inflammation via Upregulating miR-574-3p in Mice. Circ. Heart Fail. 2024, 17, e010569. [Google Scholar] [CrossRef]
- Lin, C.-Z.; Guan, H.-S.; Li, H.-H.; Yu, G.-L.; Gu, C.-X.; Li, G.-Q. The Influence of Molecular Mass of Sulfated Propylene Glycol Ester of Low-Molecular-Weight Alginate on Anticoagulant Activities. Eur. Polym. J. 2007, 43, 3009–3015. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, H.; Liu, Z.; Yao, P. Effective Enhancement of Hypoglycemic Effect of Insulin by Liver-Targeted Nanoparticles Containing Cholic Acid-Modified Chitosan Derivative. Mol. Pharm. 2016, 13, 2433–2442. [Google Scholar] [CrossRef]
- Cheng, Y.; Cai, H.; Yin, B.; Yao, P. Cholic Acid Modified N-(2-Hydroxy)-Propyl-3-Trimethylammonium Chitosan Chloride for Superoxide Dismutase Delivery. Int. J. Pharm. 2013, 454, 425–434. [Google Scholar] [CrossRef]
- Jing, C.; Li, B.; Tan, H.; Zhang, C.; Liang, H.; Na, H.; Chen, S.; Liu, C.; Zhao, L. Alendronate-Decorated Nanoparticles as Bone-Targeted Alendronate Carriers for Potential Osteoporosis Treatment. ACS Appl. Bio Mater. 2021, 4, 4907–4916. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zheng, R.; Ouyang, M.; Zhu, Y.; Lu, H.; Liao, K.; Dong, Y.; Su, B.; Huang, J.; Zhong, T.; et al. The Water Extract of Amydrium sinense (Engl.) H. Li Ameliorates Isoproterenol-Induced Cardiac Hypertrophy through Inhibiting the NF-κB Signaling Pathway. Biomed. Pharmacother. 2024, 172, 116241. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, Y.; Zhu, H.; Wang, Q.; Chen, Z.; Chen, L.; Zhu, Y.; Zheng, C.; Wang, Y.; Liao, W.; et al. Lansoprazole Alleviates Pressure Overload-Induced Cardiac Hypertrophy and Heart Failure in Mice by Blocking the Activation of β-Catenin. Cardiovasc. Res. 2020, 116, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-J.; Xu, J.-J.; Zhang, Z.-H.; Chen, M.-W.; Liu, S.-X.; Yang, C.; Li, Y.-L.; Luo, P.; Liu, Y.-J.; Tang, R.; et al. Rhein Ameliorates Transverse Aortic Constriction-Induced Cardiac Hypertrophy via Regulating STAT3 and P38 MAPK Signaling Pathways. Front. Pharmacol. 2022, 13, 940574. [Google Scholar] [CrossRef]

| Gene (Rat) | Forward Primer | Reverse Primer |
|---|---|---|
| ANP | CAGCACAATAGAGCCGCTGA | GGGCAGGAGCTTGAACACG |
| BNP | GCAGAAGCTGCTGGAGCTGA | ATCCGGAAGGCGCTGTCTTG |
| β-MHC | TTCGGGCGAGTCAAAGATGC | CCTTGTTCTCTGTTGCGTGC |
| GAPDH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Fan, Z.; Wang, D.; Li, D.; Zou, H.; Xue, Y.; Wang, S.; Li, C. Novel pH-Responsive PSS-Loaded Chitosan Matrix Nanoparticles Ameliorate Pressure Overload-Induced Cardiac Hypertrophy. Mar. Drugs 2025, 23, 365. https://doi.org/10.3390/md23090365
Xu M, Fan Z, Wang D, Li D, Zou H, Xue Y, Wang S, Li C. Novel pH-Responsive PSS-Loaded Chitosan Matrix Nanoparticles Ameliorate Pressure Overload-Induced Cardiac Hypertrophy. Marine Drugs. 2025; 23(9):365. https://doi.org/10.3390/md23090365
Chicago/Turabian StyleXu, Meijie, Zhen Fan, Dingfu Wang, Dan Li, Haimiao Zou, Yiting Xue, Shixin Wang, and Chunxia Li. 2025. "Novel pH-Responsive PSS-Loaded Chitosan Matrix Nanoparticles Ameliorate Pressure Overload-Induced Cardiac Hypertrophy" Marine Drugs 23, no. 9: 365. https://doi.org/10.3390/md23090365
APA StyleXu, M., Fan, Z., Wang, D., Li, D., Zou, H., Xue, Y., Wang, S., & Li, C. (2025). Novel pH-Responsive PSS-Loaded Chitosan Matrix Nanoparticles Ameliorate Pressure Overload-Induced Cardiac Hypertrophy. Marine Drugs, 23(9), 365. https://doi.org/10.3390/md23090365






