Secondary Metabolites of the Marine Sponge-Derived Fungus Aspergillus subramanianii 1901NT-1.40.2 and Their Antimicrobial and Anticancer Activities
Abstract
1. Introduction
2. Results
2.1. UPLC-MS Analysis
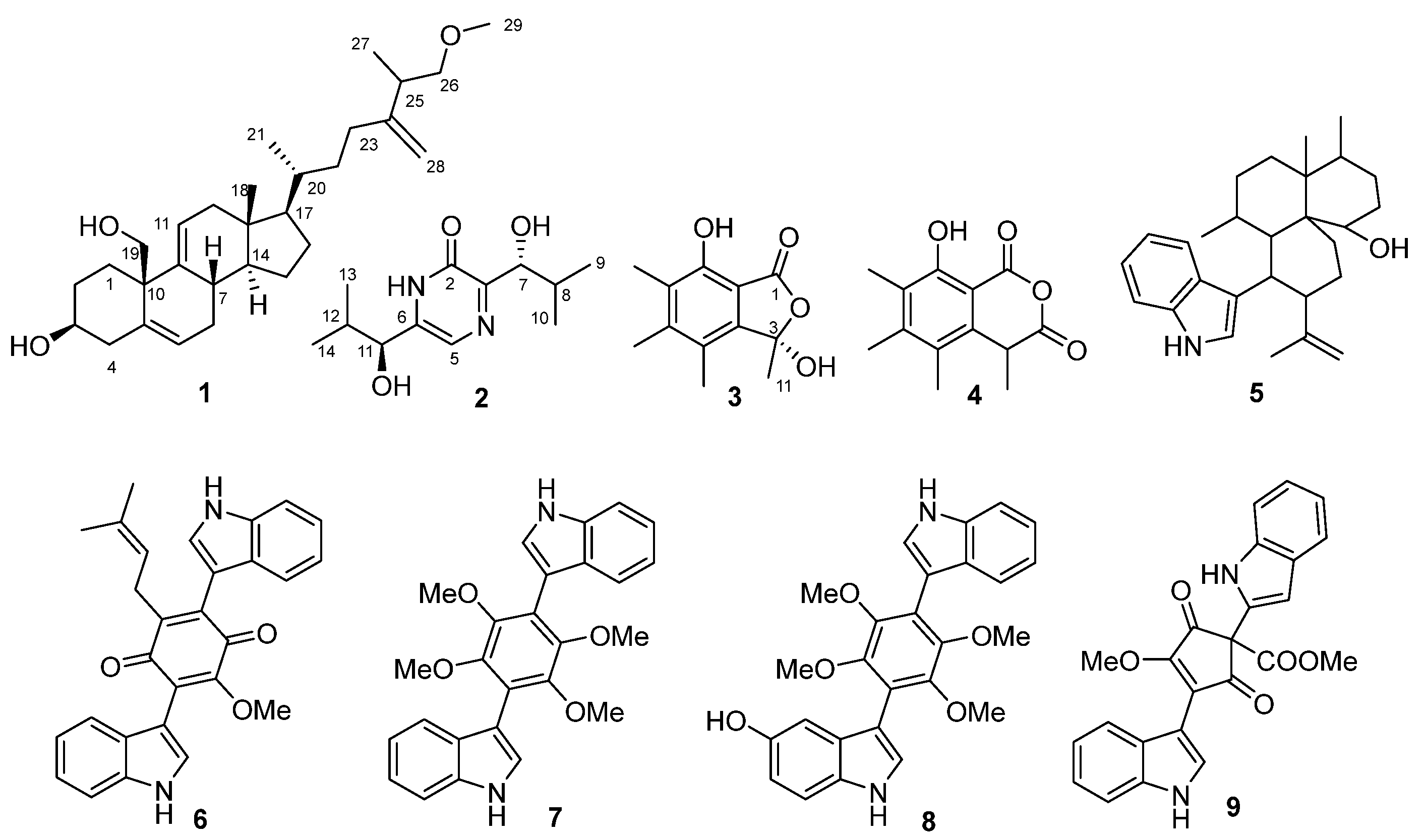
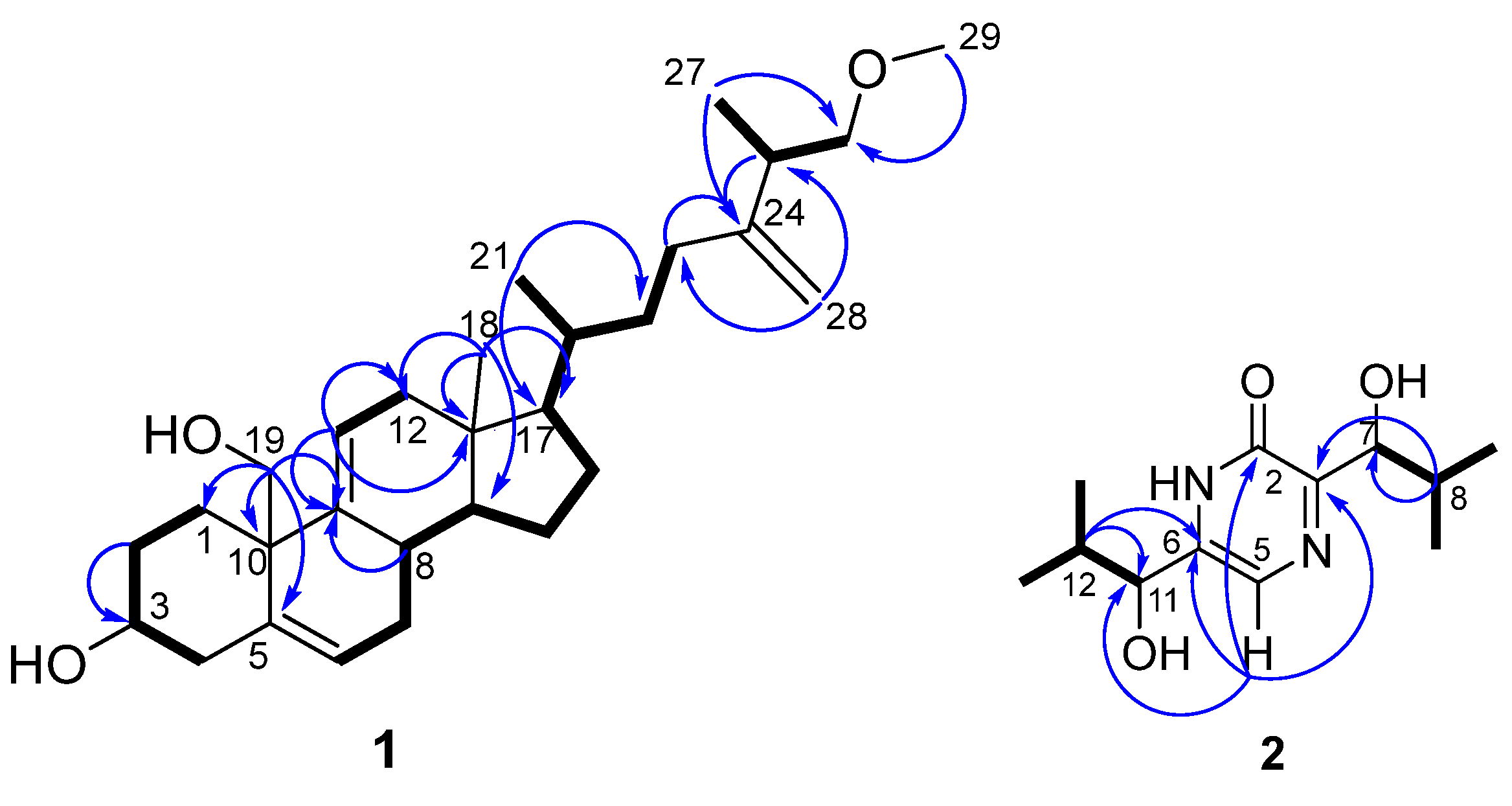
2.2. Isolation and Identification of Compounds 1–9
2.3. Antimicrobial and Antibiofilm Activities of Isolated Compounds
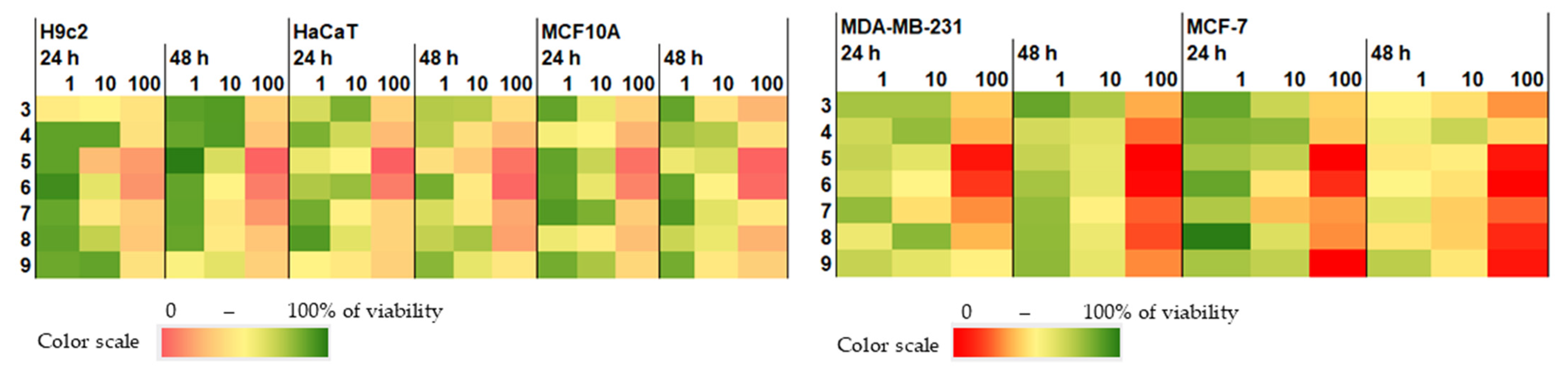
2.4. Cytotoxic Activity of Isolated Compounds 3–9
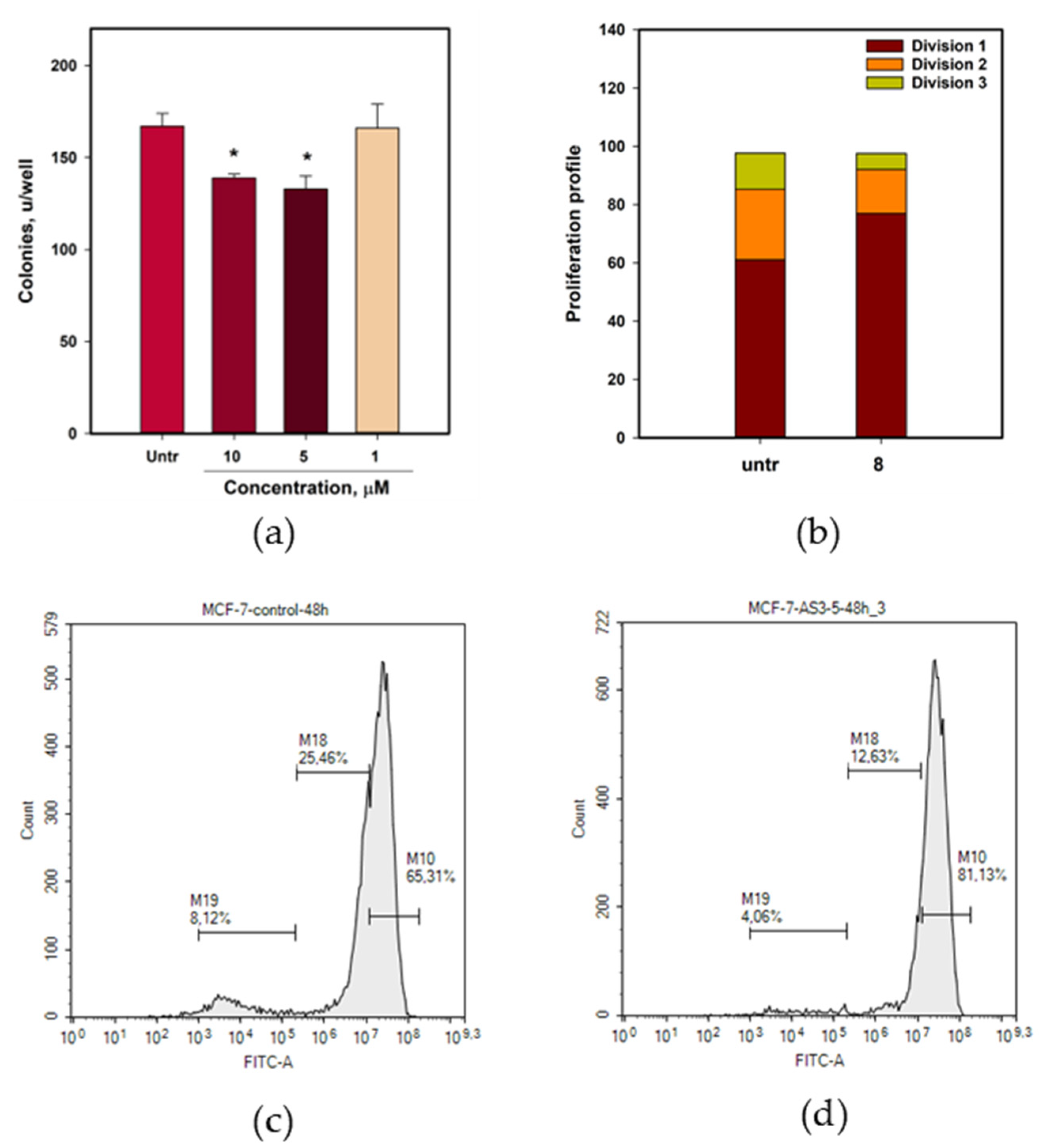
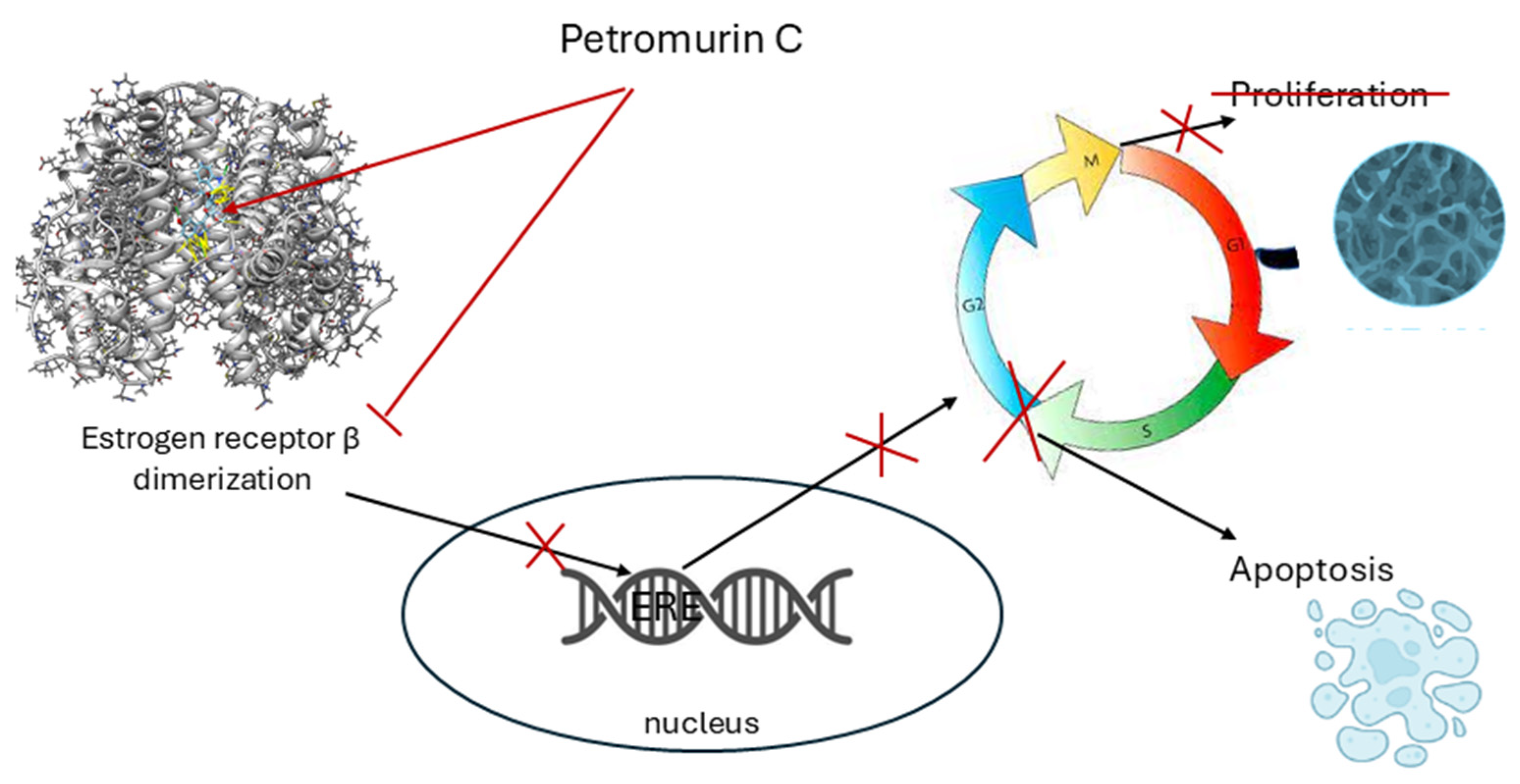
2.5. Petromurin C (8) Activity Against MCF-7 Breast Cancer
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Strain
4.3. Cultivation of Strain 1901NT-1.40.2
4.4. UPLC-MS Analysis of Fungal Extracts
4.5. Extraction and Isolation
4.6. Spectral Data of Isolated Compounds
4.7. Quantum Chemical Modeling
4.8. Bioassays
4.8.1. Bacterial Strains and Antimicrobial Assays
4.8.2. Cell Cultures and Cultivation
4.8.3. Cell Viability Assay
4.8.4. Competitive Assay with 4-Hydroxytamoxifen
4.8.5. Molecular Docking of Petromurin C (8) with Estrogen Receptors
4.8.6. The Breast Cancer MCF-7 Cell Colony Formation Assay
4.8.7. Proliferation Assay
4.8.8. Cell Cycle Investigation
4.8.9. Three-Dimensional Culture of MCF-7 Cells and Treatment with Petromurin C (8)
4.8.10. Statistical Data Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
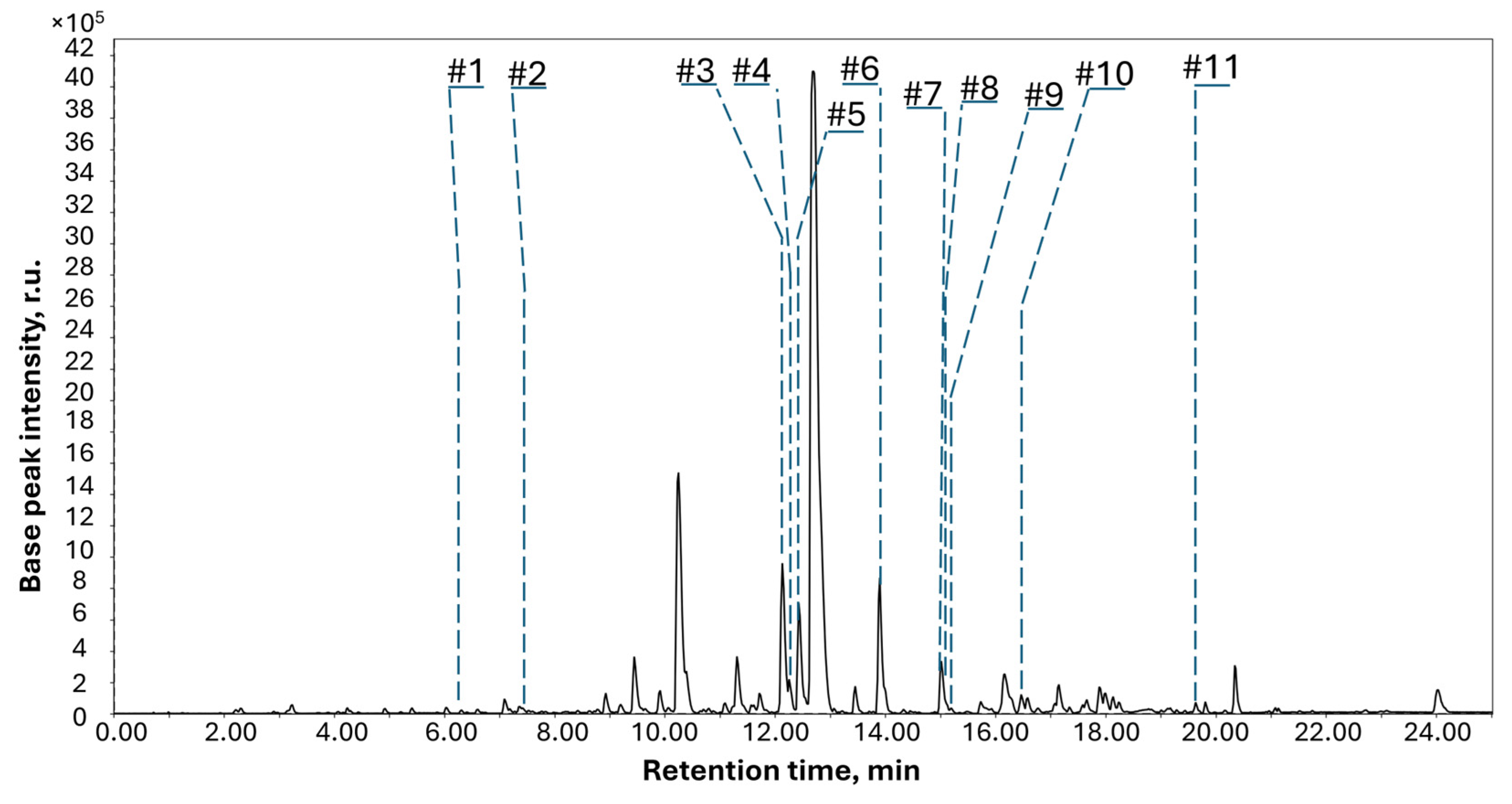
| Peak | RT, min | Measured m/z | MF | Calculated m/z | Error, ppm | Name, Identification Level and (Number of Isolated Compound) | MQScore | Ref | Structure |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 6.42 | 223.0971 | C12H14O4 | 223.097583 [M + H]+ | −2.17 | Sclerolide a (3) | [17] |  | |
| #2 | 7.70 | 235.0970 | C13H14O4 | 235.096485 [M + H]+ | 2.19 | Sclerin a (4) | [17] | 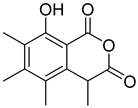 | |
| #3 | 12.18 | 415.1291 | C24H18N2O5 | 415.129945 [M + H]+ | −2.04 | Asterredione a (9) | [20] |  | |
| #4 | 12.50 | 318.2779 | C21H35NO | 318.279141 [M + H]+ | −3.89 | 2-Benzyl-1-methyl-5-nonylpyrrolidin-3-ol b | 0.82 | [13] | 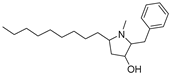 |
| #5 | 12.87 | 445.1742 | C26H24N2O5 | 445.175798 [M + H]+ | −3.58 | Petromurin C a (8) | [19] | 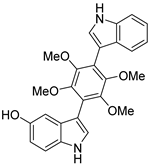 | |
| #6 | 13.90 | 429.1824 | C26H24N2O4 | 429.181981 [M + H]+ | 0.98 | Asterriquinol D dimethyl ether a,b (7) | 0.73 | [19] | 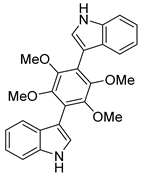 |
| #7 | 15.05 | 427.2452 | C24H36O5 | 427.246593 [M + Na]+ | 3.26 | Lovastatin b | [14] | 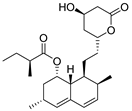 | |
| 405.2634 | 405.2634648 [M + H]+ | 0.16 | |||||||
| 422.2891 | 422.291197 [M + NH4]+ | 4.97 | |||||||
| #8 | 15.01 | 437.1864 | C28H24N2O3 | 437.185969 [M + H]+ | 0.99 | Kumbicin D a (6) | [19] |  | |
| #9 | 15.49 | 1202.8488 | C62H111N11O12 | 1202.848645 [M + H]+ | 0.13 | Cyclosporine b | 0.83 | [15] | 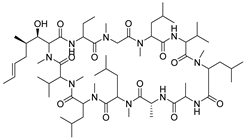 |
| #10 | 16.18 | 406.3091 | C28H39NO | 406.311538 [M + H]+ | −3.30 | 10,23-Dihydro-24,25-dehydroaflavinine a (5) | [18] | 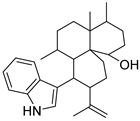 | |
| #11 | 19.83 | 411.3265 | C28H44O3 | 411.3257 [M − H2O + H]+ | −1.94 | Ergosterol peroxide a | [16] |  |
Appendix B
| Compound | Time, h | Concentrations, µM | ||
|---|---|---|---|---|
| 1.0 | 10.0 | 100.0 | ||
| H9c2 | ||||
| 3 | 24 | 81.1 ± 3.2 | 88.7 ± 4.4 | 74.6 ± 8.3 |
| 48 | 102.0 ± 5.5 | 102.7 ± 6.7 | 62.5 ± 4.6 | |
| 4 | 24 | 101.9 ± 5.8 | 101.9 ± 6.1 | 73.5 ± 4.4 |
| 48 | 101.4 ± 5.6 | 102.6 ± 7.4 | 56.2 ± 0.9 | |
| 5 | 24 | 101.9 ± 2.9 | 50.5 ± 1.8 | 33.6 ± 0.3 |
| 48 | 105.6 ± 3.4 | 94.0 ± 3.0 | 10.1 ± 0.2 | |
| 6 | 24 | 104.0 ± 12.9 | 92.9 ± 6.1 | 30.9 ± 2.9 |
| 48 | 99.7 ± 3.7 | 88.6 ± 2.3 | 21.6 ± 2.2 | |
| 7 | 24 | 101.5 ± 1.3 | 76.9 ± 2.8 | 59.0 ± 5.3 |
| 48 | 102.8 ± 7.6 | 76.3 ± 3.9 | 32.8 ± 1.4 | |
| 8 | 24 | 100.9 ± 1.7 | 95.9 ± 2.3 | 57.4 ± 8.5 |
| 48 | 101.6 ± 2.2 | 79.8 ± 4.6 | 55.8 ± 5.5 | |
| 9 | 24 | 101.1 ± 6.6 | 101.7 ± 3.2 | 73.1 ± 5.1 |
| 48 | 90.2 ± 7.1 | 93.0 ± 10.4 | 62.2 ± 10.4 | |
| HaCaT | ||||
| 3 | 24 | 94.4 ± 5.9 | 100.2 ± 3.0 | 63.0 ± 8.6 |
| 48 | 96.8 ± 1.0 | 96.6 ± 4.1 | 69.4 ± 3.5 | |
| 4 | 24 | 100.2 ± 6.6 | 94.7 ± 5.0 | 49.5 ± 6.0 |
| 48 | 96.4 ± 1.4 | 72.1 ± 11.1 | 51.5 ± 2.1 | |
| 5 | 24 | 92.3 ± 5.0 | 88.3 ± 8.6 | 7.8 ± 0.3 |
| 48 | 72.2 ± 4.3 | 56.8 ± 4.8 | 16.8 ± 0.2 | |
| 6 | 24 | 97.2 ± 5.7 | 98.6 ± 3.5 | 21.5 ± 3.3 |
| 48 | 100.5 ± 3.3 | 80.0 ± 0.1 | 11.6 ± 3.5 | |
| 7 | 24 | 100.7 ± 3.8 | 85.6 ± 7.6 | 64.1 ± 3.2 |
| 48 | 94.2 ± 8.7 | 78.1 ± 6.4 | 40.1 ± 2.9 | |
| 8 | 24 | 102.7 ± 7.7 | 93.1 ± 6.9 | 64.3 ± 1.9 |
| 48 | 95.8 ± 1.9 | 97.7 ± 1.4 | 36.8 ± 7.0 | |
| 9 | 24 | 88.7 ± 3.0 | 79.2 ± 2.1 | 62.0 ± 6.9 |
| 48 | 99.5 ± 2.3 | 92.6 ± 11.2 | 77.1 ± 1.5 | |
| MCF10A | ||||
| 3 | 24 | 101.7 ± 1.3 | 92.2 ± 3.5 | 62.5 ± 2.2 |
| 48 | 101.6 ± 5.1 | 74.4 ± 2.4 | 47.5 ± 7.0 | |
| 4 | 24 | 90.8 ± 7.8 | 88.6 ± 5.2 | 47.0 ± 3.2 |
| 48 | 98.0 ± 4.0 | 96.8 ± 2.1 | 73.5 ± 1.1 | |
| 5 | 24 | 101.7 ± 4.4 | 95.4 ± 2.3 | 16.1 ± 8.6 |
| 48 | 92.0 ± 0.9 | 93.9 ± 6.4 | 9.9 ± 0.2 | |
| 6 | 24 | 101.6 ± 3.1 | 92.4 ± 3.6 | 23.9 ± 6.2 |
| 48 | 98.4 ± 4.5 | 87.4 ± 2.7 | 13.7 ± 3.2 | |
| 7 | 24 | 102.7 ± 5.1 | 100.2 ± 3.8 | 59.6 ±2.1 |
| 48 | 97.7 ± 1.4 | 93.1 ± 3.6 | 78.9 ± 5.7 | |
| 8 | 24 | 91.9 ± 9.5 | 81.4 ± 1.0 | 52.0 ± 4.0 |
| 48 | 95.3 ± 3.4 | 92.1 ± 2.1 | 45.7 ± 6.6 | |
| 9 | 24 | 100.7 ± 0.9 | 97.5 ± 3.5 | 66.3 ± 3.6 |
| 48 | 96.7 ± 5.1 | 79.4 ± 4.3 | 61.5 ± 1.9 | |
| MDA-MB-231 | ||||
| 3 | 24 | 99.8 ± 1.3 | 100.6 ± 5.7 | 68.6 ± 8.3 |
| 48 | 107.6 ± 2.8 | 99.0 ± 5.1 | 60.9 ± 3.6 | |
| 4 | 24 | 94.8 ± 4.2 | 102.7 ± 4.9 | 63.1 ±1.6 |
| 48 | 94.3 ± 3.9 | 91.8 ± 7.5 | 47.1 ± 6.2 | |
| 5 | 24 | 96.3 ± 7.5 | 91.3 ± 6.1 | 21.1 ± 0.9 |
| 48 | 103.2 ± 2.4 | 102.3 ± 4.1 | 10.6 ± 0.2 | |
| 6 | 24 | 93.6 ± 4.8 | 84.5 ± 6.7 | 32.7 ± 5.2 |
| 48 | 100.6 ± 2.2 | 90.8 ± 1.2 | 14.7 ± 0.6 | |
| 7 | 24 | 102.7 ± 1.6 | 75.9 ± 4.8 | 53.6 ± 4.2 |
| 48 | 99.8 ± 3.7 | 82.9 ± 6.3 | 43.5 ± 2.5 | |
| 8 | 24 | 89.4 ± 2.4 | 103.6 ± 2.2 | 63.7 ±4.8 |
| 48 | 102.9 ± 0.5 | 89.5 ± 3.1 | 38.6 ± 1.7 | |
| 9 | 24 | 96.3 ± 3.2 | 106.3 ± 9.9 | 82.5 ± 1.3 |
| 48 | 103.2 ± 0.9 | 90.5 ± 2.7 | 52.9 ± 3.4 | |
| MCF-7 | ||||
| 3 | 24 | 107.2 ± 8.9 | 95.6 ± 2.4 | 70.2 ±5.5 |
| 48 | 83.8 ± 3.1 | 76.0 ± 2.6 | 55.1 ± 1.0 | |
| 4 | 24 | 104.3 ± 4.6 | 103.4 ± 5.4 | 68.3 ± 4.5 |
| 48 | 88.5 ± 4.7 | 95.9 ± 3.5 | 74.1 ± 4.5 | |
| 5 | 24 | 100.5 ± 0.9 | 97.0 ± 2.6 | 10.0 ± 1.0 |
| 48 | 78.5 ± 9.9 | 81.6 ± 3.3 | 21.2 ± 0.1 | |
| 6 | 24 | 107.6 ± 6.5 | 77.7 ± 7.5 | 29.9 ± 0.8 |
| 48 | 84.6 ± 9.4 | 76.8 ± 3.8 | 12.1 ± 2.7 | |
| 7 | 24 | 99.4 ± 4.4 | 65.4 ± 2.9 | 56.0 ± 4.5 |
| 48 | 91.5 ± 6.3 | 70.0 ± 5.2 | 43.9 ± 3.5 | |
| 8 | 24 | 105.4 ± 5.9 | 92.8 ± 3.0 | 53.7 ± 6.8 |
| 48 | 77.7 ± 3.7 | 70.6 ± 8.5 | 28.6 ± 4.3 | |
| 9 | 24 | 100.5 ± 7.0 | 97.0 ± 11.2 | 10.0 ± 8.1 |
| 48 | 97.8 ± 1.1 | 78.6 ± 7.6 | 21.2 ± 6.6 | |
References
- Fisher, J.F.; Mobashery, S. β-Lactams from the Ocean. Mar. Drugs 2023, 21, 86. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.; Kauffman, C.; Mayhead, S.; Faulkner, D.; Sincich, C.; Rao, M.; Kantorowski, E.; West, L.; Strangman, W. New anticancer drugs from cultured and collected marine organisms. Pharm. Biol. 2003, 41, 6–14. [Google Scholar] [CrossRef]
- Mita, M.M.; Spear, M.A.; Yee, L.K.; Mita, A.C.; Heath, E.I.; Papadopoulos, K.P.; Federico, K.C.; Reich, S.D.; Romero, O.; Malburg, L. Phase 1 first-in-human trial of the vascular disrupting agent plinabulin (NPI-2358) in patients with solid tumors or lymphomas. Clin. Cancer Res. 2010, 16, 5892–5899. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Feinstein, T.; Shi, Y.; Chen, G.; Yao, Y.; Hu, C.; Shi, J.; Feng, J.; Wu, H.; Cheng, Y.; et al. Plinabulin plus docetaxel versus docetaxel in patients with non-small-cell lung cancer after disease progression on platinum-based regimen (DUBLIN-3): A phase 3, international, multicentre, single-blind, parallel group, randomised controlled trial. Lancet Respir. Med. 2024, 12, 775–786. [Google Scholar] [CrossRef]
- Wang, P.; Huang, X.; Jiang, C.; Yang, R.; Wu, J.; Liu, Y.; Feng, S.; Wang, T. Antibacterial properties of natural products from marine fungi reported between 2012 and 2023: A review. Arch. Pharm. Res. 2024, 47, 505–537. [Google Scholar] [CrossRef]
- Ganeshkumar, A.; Gonçale, J.C.; Rajaram, R.; Junqueira, J.C. Anti-Candidal Marine Natural Products: A Review. J. Fungi 2023, 9, 800. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Refaey, M.S.; Elias, N.; El-Mallah, M.F.; Albaqami, F.M.K.; Dergaa, I.; Du, M.; Salem, M.F.; Tahir, H.E.; Dagliaa, M.; et al. Marine natural products as a source of novel anticancer drugs: An updated review (2019–2023). Nat. Prod. Bioprospect. 2025, 15, 13. [Google Scholar] [CrossRef]
- Chingizova, E.A.; Yurchenko, E.A.; Chingizov, A.R.; Klimovich, A.A.; Pislyagin, E.A.; Menchinskaya, E.S.; Kuzmich, A.S.; Trinh, P.T.; Ngoc, N.T.; Van, T.T.; et al. The Effects of Marine Fungal Asterripeptides A–C on In Vitro and In Vivo Staphylococcus aureus Skin Infection. Pharmaceuticals 2024, 17, 1345. [Google Scholar] [CrossRef]
- Girich, E.V.; Trinh, P.T.; Nesterenko, L.E.; Popov, R.S.; Kim, N.Y.; Rasin, A.B.; Menchinskaya, E.S.; Kuzmich, A.S.; Chingizova, E.A.; Minin, A.S.; et al. Absolute Stereochemistry and Cytotoxic Effects of Vismione E from Marine Sponge-Derived Fungus Aspergillus sp. 1901NT-1.2.2. Int. J. Mol. Sci. 2023, 24, 8150. [Google Scholar] [CrossRef] [PubMed]
- Khmel, O.O.; Trinh, P.T.H.; Ngoc, N.T.D.; Chingizova, E.A.; Drozdov, K.A.; Popov, R.S.; Van, T.T.T.; Khanh, H.H.N.; Duc Thinh, P.; Yurchenko, E.A.; et al. Mycophenolic Acid Derivatives from Vietnamese Marine Sponge-derived Penicillium sp. 1901NT-2.53.1 Strain and their Antiproliferative Activity. Curr. Bioact. Compd. 2025, 21, e15734072350564. [Google Scholar] [CrossRef]
- Phan Thi, H.T.; Yurchenko, E.A.; Yurchenko, A.N.; Ngo Thi, D.N.; Vo Thi, D.T.; Cao Thi, T.H.; Tran Thi, T.V.; Pham Duc, T.; Huynh Hoang, N.K.; Le Dinh, H.; et al. Evaluation of cytotoxic activity of marine fungi isolated from sponges in Nha Trang bay. Vietnam J. Mar. Sci. Technol. 2022, 22, 51–57. [Google Scholar] [CrossRef]
- Lebar, M.; Mack, B.; Carter-Wientjes, C.; Wei, Q.; Mattison, C.; Cary, J. Small NRPS-like enzymes in Aspergillus sections Flavi and Circumdati selectively form substituted pyrazinone metabolites. Front. Fungal Biol. 2022, 3, 1029195. [Google Scholar] [CrossRef]
- Pares, R.B.; Alves, D.S.; Alves, L.F.A.; Godinho, C.C.; Gobbo Neto, L.; Ferreira, T.T.; Nascimento, M.M.; Ascari, J.; Oliveira, D.F. Acaricidal Activity of Annonaceae Plants for Dermanyssus gallinae (Acari: Dermanyssidae) and Metabolomic Profile by HPLC-MS/MS. Neotrop. Entomol. 2021, 50, 662–672. [Google Scholar] [CrossRef]
- Goswami, S.V.A.; Bhunia, B.; Mandal, T. A review on lovastatin and its production. J. Biochem. Technol. 2012, 4, 581–587. [Google Scholar] [CrossRef]
- Tedesco, D.; Haragsim, L. Cyclosporine: A Review. J. Transplant. 2012, 2012, 230386. [Google Scholar] [CrossRef]
- Borkunov, G.V.; Kirichuk, N.N.; Chausova, V.E.; Popov, R.S.; Zhuravleva, O.I.; Chingizova, E.A.; Yurchenko, E.A.; Isaeva, M.P.; Yurchenko, A.N. Differences in Metabolite Profiles and Bioactivities of Intra-Strain Variants of Marine Fungus Penicillium antarcticum KMM 4668. Metabolites 2025, 15, 77. [Google Scholar] [CrossRef]
- Kubota, T.; Tokoroyama, T.; Kamikawa, T.; Satomura, Y. The structures of sclerin and sclerolide, metabolites of sclerotinia libertiana. Tetrahedron Lett. 1966, 7, 5205–5210. [Google Scholar] [CrossRef]
- TePaske, M.R.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Leporin A: An antiinsectan N-alkoxypyridone from the sclerotia of Aspergillus leporis. Tetrahedron Lett. 1991, 32, 5687–5690. [Google Scholar] [CrossRef]
- Lacey, H.J.; Vuong, D.; Pitt, J.I.; Lacey, E.; Piggott, A.M. Kumbicins A–D: Bis-Indolyl Benzenoids and Benzoquinones from an Australian Soil Fungus, Aspergillus kumbius. Aust. J. Chem. 2016, 69, 152–160. [Google Scholar] [CrossRef]
- Wijeratne, E.M.; Turbyville, T.J.; Zhang, Z.; Bigelow, D.; Pierson, L.S., 3rd; VanEtten, H.D.; Whitesell, L.; Canfield, L.M.; Gunatilaka, A.A. Cytotoxic constituents of Aspergillus terreus from the rhizosphere of Opuntia versicolor of the Sonoran Desert. J Nat Prod 2003, 66, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Nkinin, S.W.; Stringer, J.R.; Keely, S.P.; Setchell, K.D.R.; Giner, J.-L.; Kaneshiro, E.S. Pneumocystis carinii Sterol 14α-Demethylase Activity in Saccharomyces cerevisiae erg11 Knockout Mutant: Sterol Biochemistry. J. Eukaryot. Microbiol. 2011, 58, 383–392. [Google Scholar] [CrossRef]
- Whuang, T.-Y.; Tsai, H.-C.; Su, Y.-D.; Hwang, T.-L.; Sung, P.-J. Sterols from the Octocoral Nephthea columnaris. Mar. Drugs 2017, 15, 212. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Y.; Zhu, T.; Zhu, W. Pyrazinone derivatives from the coral-derived Aspergillus ochraceus LCJ11-102 under high iodide salt. Arch. Pharmacal. Res. 2018, 41, 184–191. [Google Scholar] [CrossRef]
- Wang, H.J.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Aflavinines and other antiinsectan metabolites from the ascostromata of Eupenicillium crustaceum and related species. Appl. Environ. Microbiol. 1995, 61, 4429–4435. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Meng, L.-H.; Li, X.; Yang, S.-Q.; Li, X.-M.; Wang, B.-G. Three New Indole Diterpenoids from the Sea-Anemone-Derived Fungus Penicillium sp. AS-79. Mar. Drugs 2017, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Buttachon, S.; Ramos, A.A.; Inácio, Â.; Dethoup, T.; Gales, L.; Lee, M.; Costa, P.M.; Silva, A.M.; Sekeroglu, N.; Rocha, E. Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA0062. Mar. Drugs 2018, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Pressete, C.G.; Giannini, L.S.V.; de Paula, D.A.C.; do Carmo, M.A.V.; Assis, D.M.; Santos, M.F.C.; Machado, J.d.C.; Marques, M.J.; Soares, M.G.; Azevedo, L. Sclerotinia sclerotiorum (white mold): Cytotoxic, mutagenic, and antimalarial effects in vivo and in vitro. J. Food Sci. 2019, 84, 3866–3875. [Google Scholar] [CrossRef]
- Ha, Y.N.; Song, S.; Orlikova-Boyer, B.; Cerella, C.; Christov, C.; Kijjoa, A.; Diederich, M. Petromurin C Induces Protective Autophagy and Apoptosis in FLT3-ITD-Positive AML: Synergy with Gilteritinib. Mar. Drugs 2020, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, A.M.; Pike, A.C.; Dauter, Z.; Hubbard, R.E.; Bonn, T.; Engström, O.; Öhman, L.; Greene, G.L.; Gustafsson, J.-Å.; Carlquist, M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997, 389, 753–758. [Google Scholar] [CrossRef]
- Pike, A.C.; Brzozowski, A.M.; Hubbard, R.E.; Bonn, T.; Thorsell, A.G.; Engström, O.; Ljunggren, J.; Gustafsson, J.Å.; Carlquist, M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999, 18, 4608–4618. [Google Scholar] [CrossRef]
- Minin, A.; Semerikova, T.; Belousova, A.V.; Karavashkova, O.; Pozdina, V.; Tomilina, M.; Zubarev, I. MSLASpheroidStamp: 3d cell spheroids for everyone. Bioprinting 2025, 49, e00416. [Google Scholar] [CrossRef]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef]
- Lingappa, B.T.; Prasad, M.; Lingappa, Y.; Hunt, D.F.; Biemann, K. Phenethyl Alcohol and Tryptophol: Autoantibiotics Produced by the Fungus Candida albicans. Science 1969, 163, 192–194. [Google Scholar] [CrossRef]
- Alem Mohammed, A.S.; Oteef Mohammed, D.Y.; Flowers, T.H.; Douglas, L.J. Production of Tyrosol by Candida albicans Biofilms and Its Role in Quorum Sensing and Biofilm Development. Eukaryot. Cell 2006, 5, 1770–1779. [Google Scholar] [CrossRef]
- Ahn, C.-H.; Lee, S.; Cho, E.; Kim, H.; Chung, B.; Park, W.; Shin, J.; Oh, K.-B. A farnesoic acid-responsive transcription factor, Hot1, regulates yeast-hypha morphogenesis in Candida albicans. FEBS Lett. 2017, 591, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- do Rosário Esteves Guimarães, C.; de Freitas, H.F.; Barros, T.F. Candida albicans antibiofilm molecules: Analysis based on inhibition and eradication studies. Braz. J. Microbiol. 2023, 54, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Lopez-Ribot Jose, L. Screening the Pathogen Box for Identification of Candida albicans Biofilm Inhibitors. Antimicrob. Agents Chemother. 2016, 61, e02006. [Google Scholar] [CrossRef]
- Wang, J.; Hu, F.; Luo, Y.; Luo, H.; Huang, N.; Cheng, F.; Deng, Z.; Deng, W.; Zou, K. Estrogenic and anti-estrogenic activities of hispolon from Phellinus lonicerinus (Bond.) Bond. et sing. Fitoterapia 2014, 95, 93–101. [Google Scholar] [CrossRef]
- Lim, W.; Park, J.; Lee, Y.H.; Hong, J.; Lee, Y. Subglutinol A, an immunosuppressive α-pyrone diterpenoid from Fusarium subglutinans, acts as an estrogen receptor antagonist. Biochem. Biophys. Res. Commun. 2015, 461, 507–512. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Nesterenko, L.E.; Popov, R.S.; Zhuravleva, O.I.; Kirichuk, N.N.; Chausova, V.E.; Krasnov, K.S.; Pivkin, M.V.; Yurchenko, E.A.; Isaeva, M.P.; Yurchenko, A.N. A Study of the Metabolic Profiles of Penicillium dimorphosporum KMM 4689 Which Led to Its Re-Identification as Penicillium hispanicum. Fermentation 2023, 9, 337. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Campbell, J. High-throughput assessment of bacterial growth inhibition by optical density measurements. Curr. Protoc. Chem. Biol. 2011, 3, 100115. [Google Scholar] [CrossRef] [PubMed]
- Kifer, D.; Mužinić, V.; Klarić, M.Š. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. 2016, 69, 689–696. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks Iii, C.L.; Mackerell Jr, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. Fast docking using the CHARMM force field with EADock DSS. J. Comput. Chem. 2011, 32, 21797. [Google Scholar] [CrossRef]
- Haberthür, U.; Caflisch, A. FACTS: Fast analytical continuum treatment of solvation. J. Comput. Chem. 2008, 29, 701–715. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Khmel, O.O.; Nesterenko, L.E.; Aminin, D.L. The Kelch/Nrf2 antioxidant system as a target for some marine fungal metabolites. Oxygen 2023, 3, 24. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, N.; Sheng, D.; Hou, J.; Hao, C.; Yang, X.; Zhu, B.; Zhang, S.; Han, Z.; Wei, L. Inhibition of growth and metastasis of colon cancer by delivering 5-fluorouracil-loaded pluronic P85 copolymer micelles. Sci. Rep. 2016, 6, 20896. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in speed, utility and usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef] [PubMed]







| Position | 2 | 1 | ||
|---|---|---|---|---|
| δС, mult | δH (J in Hz) | δС, mult | δH (J in Hz) | |
| 1 | 28.3, CH2 | a: 2.33 dt, (3.5, 13.6) b:1.14, m | ||
| 2 | 158.1, C | 37.9, CH2 | a: 1.82, m b:1.38, m | |
| 3 | 155.5, C | 70.9, CH | 3.67, m | |
| 4 | 31.5, CH2 | a: 1.51, m b: 1.89, m | ||
| 5 | 120.9, CH | 7.25, s | 139.1, C | |
| 6 | 139.3, C | 122.2, CH | 5.46 d, (6.1) | |
| 7 | 73.8, CH | 4.39, d (6.0) | 29.8, CH2 | a: 2.00, m b: 1.91, m |
| 8 | 31.9, CH | 2.29, m | 38.8, CH | 1.74, m |
| 9 | 20.1, CH3 | 1.08, d (6.8) | 136.1, C | |
| 10 | 15.8, CH3 | 0.76, d (6.7) | 41.2, C | |
| 11 | 75.1, CH | 4.70, (3.7) | 121.8, CH | 5.51 d, (6.1) |
| 12 | 34.6, CH | 2.00, m | 42.6, CH2 | a: 2.38, m b: 2.16, m |
| 13 | 18.8, CH3 | 0.95, d (6.8) | 42.1, C | |
| 14 | 17.3, CH3 | 0.99, d (6.7) | 51.8, CH | 2.20, m |
| 15 | 23.4, CH2 | a: 1.80, m b: 1.38, m | ||
| 16 | 28.5, CH2 | a: 2.00, m b: 1.36, m | ||
| 17 | 56.4, CH | 1.32, m | ||
| 18 | 11.9, CH3 | 0.53, s | ||
| 19 | 59.4, CH2 | a: 3.40, m b: 3.74, d (10.8) | ||
| 20 | 36.1, CH | 1.45, m | ||
| 21 | 18.6, CH3 | 0.95, d (6.5) | ||
| 22 | 34.6, CH2 | a: 1.62 m b: 1.18 m | ||
| 23 | 31.4, CH2 | a: 2.13 m b: 1.89 m | ||
| 24 | 152.8, C | |||
| 25 | 40.0, CH | 2.38, m | ||
| 26 | 77.1 CH2 | a: 3.22, m b: 3.39, m | ||
| 27 | 17.4, CH3 | 1.06, d (6.7) | ||
| 28 | 108.5, CH2 | 4.77, m | ||
| 29 | 58.9, CH3 | 3.34, s | ||
| Compound | S. aureus | E. coli | C. albicans |
|---|---|---|---|
| Growth inhibition, MIC50, µM | |||
| 3 | 20.34 ± 2.58 | 21.07 ± 2.11 | >100 |
| 4 | >100 | >100 | >100 |
| 5 | 3.12 ± 0.30 | 4.92 ± 0.45 | 7.01 ± 0.54 |
| 6 | 9.38 ± 0.77 | 11.42 ± 1.02 | 10.44 ± 1.07 |
| 7 | >100 | >100 | >100 |
| 8 | 26.74 ± 1.98 | 52.14 ± 2.01 | 34.14 ± 2.1 |
| 9 | >100 | >100 | >100 |
| Gentamycin | 4.52 ± 0.98 | 5.03 ± 0.77 | - |
| Amphotericin B | - | - | 1.18 ± 0.15 |
| Biofilm formation, MIC50, µM | |||
| 3 | 31.14 ± 2.34 | 43.51 ± 4.03 | >100 |
| 4 | >100 | >100 | >100 |
| 5 | 3.08 ± 0.30 | 11.04 ± 0.95 | 10.01 ± 1.00 |
| 6 | 7.98 ± 0.57 | 10.34 ± 0.98 | 8.36 ± 0.95 |
| 7 | >100 | >100 | >100 |
| 8 | >100 | 83.01 ± 6.42 | 24.12 ± 3.07 |
| 9 | >100 | >100 | >100 |
| Gentamycin | 4.34 ± 0.32 | 5.34 ± 0.50 | - |
| Amphotericin B | - | - | 1.38 ± 0.14 |
| Cell Line | Time, h | Compound | |||||
|---|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 8 | 9 | ||
| H9c2 | 24 | >100 | 9.8 ± 1.3 | 72.2 ± 4.1 | >100 | >100 | >100 |
| 48 | 57.2 ± 2.4 | 57.2 ± 3.0 | 65.4 ± 2.1 | 69.9 ± 2.3 | >100 | >100 | |
| HaCaT | 24 | ≥ 100 | 52.8 ± 1.1 | >100 | >100 | >100 | >100 |
| 48 | ≥100 | 25.3 ± 0.8 | 49.4 ± 1.2 | 80.5 ± 1.8 | 80.5 ± 1.8 | >100 | |
| MCF10A | 24 | 93.4 ± 3.5 | 61.5 ± 1.3 | 65.7 ± 1.9 | >100 | ≥100 | >100 |
| 48 | >100 | 57.1 ± 2.8 | 55.6 ± 2.3 | >100 | 91.0 ± 4.1 | >100 | |
| MDA-MB-231 | 24 | >100 | 62.9 ± 3.1 | 74.4 ± 0.6 | >100 | >100 | >100 |
| 48 | 94.1 ± 3.2 | 61.3 ± 2.1 | 58.2 ± 2.0 | 85.1 ± 1.5 | 79.9 ± 1.2 | ≥100 | |
| MCF-7 | 24 | >100 | 58.6 ± 4.2 | 62.2 ± 2.2 | >100 | >100 | 51.3 ± 2.6 |
| 48 | >100 | 53.5 ± 1.1 | 47.3 ± 1.7 | 79.1 ± 2.4 | 61.5 ± 1.2 | 55.1 ± 3.9 | |
| Complex | ∆G, kcal/mol | H-Bond | Hydrophobic Interactions |
|---|---|---|---|
| ERα (PDB ID 1A52) | −7.21 | Asn348, Lys529 | Val533, Gly344, and Lys529 |
| −6.788 | Leu320, Trp393 | Ile326, Trp393, Leu320 | |
| ERβ (PDB ID 5TOA) | −8.54 | Glu389, Met410 | Ala427, Leu430, Leu426, Met410, Tyr411, Ala382 |
| −8.26 | Glu389, Met410, Asn431 | Ala427, Ser423, Leu430, Leu426, Tyr411, Ala382 |
| Time, h | Treatment | Cell Disturbance, % | ||
|---|---|---|---|---|
| G1 Phase | S Phase | G2/M Phase | ||
| 1 | Untreated | 24.31 ± 1.27 | 47.91 ± 2.06 | 27.80 ± 0.79 |
| 8 at 5 µM | 22.69 ± 0.42 | 45.52 ± 1.05 | 29.91 ± 1.95 | |
| 8 at 10 µM | 21.37 ± 2.18 | 53.15 ± 1.53 # | 25.96 ± 0.62 # | |
| 3 | Untreated | 31.72 ± 0.30 | 47.49 ± 1.44 | 20.80 ± 1.37 |
| 8 at 5 µM | 26.20 ± 1.17 * | 56.60 ± 1.25 * | 17.20 ± 1.23 * | |
| 8 at 10 µM | 33.82 ± 0.98 | 51.79 ± 0.86 * | 14.95 ± 1.08 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khmel, O.O.; Yurchenko, A.N.; Trinh, P.T.H.; Ngoc, N.T.D.; Trang, V.T.D.; Khanh, H.H.N.; Antonov, A.S.; Drozdov, K.A.; Popov, R.S.; Kim, N.Y.; et al. Secondary Metabolites of the Marine Sponge-Derived Fungus Aspergillus subramanianii 1901NT-1.40.2 and Their Antimicrobial and Anticancer Activities. Mar. Drugs 2025, 23, 353. https://doi.org/10.3390/md23090353
Khmel OO, Yurchenko AN, Trinh PTH, Ngoc NTD, Trang VTD, Khanh HHN, Antonov AS, Drozdov KA, Popov RS, Kim NY, et al. Secondary Metabolites of the Marine Sponge-Derived Fungus Aspergillus subramanianii 1901NT-1.40.2 and Their Antimicrobial and Anticancer Activities. Marine Drugs. 2025; 23(9):353. https://doi.org/10.3390/md23090353
Chicago/Turabian StyleKhmel, Olga O., Anton N. Yurchenko, Phan Thi Hoai Trinh, Ngo Thi Duy Ngoc, Vo Thi Dieu Trang, Huynh Hoang Nhu Khanh, Alexandr S. Antonov, Konstantin A. Drozdov, Roman S. Popov, Natalya Y. Kim, and et al. 2025. "Secondary Metabolites of the Marine Sponge-Derived Fungus Aspergillus subramanianii 1901NT-1.40.2 and Their Antimicrobial and Anticancer Activities" Marine Drugs 23, no. 9: 353. https://doi.org/10.3390/md23090353
APA StyleKhmel, O. O., Yurchenko, A. N., Trinh, P. T. H., Ngoc, N. T. D., Trang, V. T. D., Khanh, H. H. N., Antonov, A. S., Drozdov, K. A., Popov, R. S., Kim, N. Y., Berdyshev, D. V., Chingizova, E. A., Menchinskaya, E. S., & Yurchenko, E. A. (2025). Secondary Metabolites of the Marine Sponge-Derived Fungus Aspergillus subramanianii 1901NT-1.40.2 and Their Antimicrobial and Anticancer Activities. Marine Drugs, 23(9), 353. https://doi.org/10.3390/md23090353








