Microalgae-Based 3D Bioprinting: Recent Advances, Applications and Perspectives
Abstract
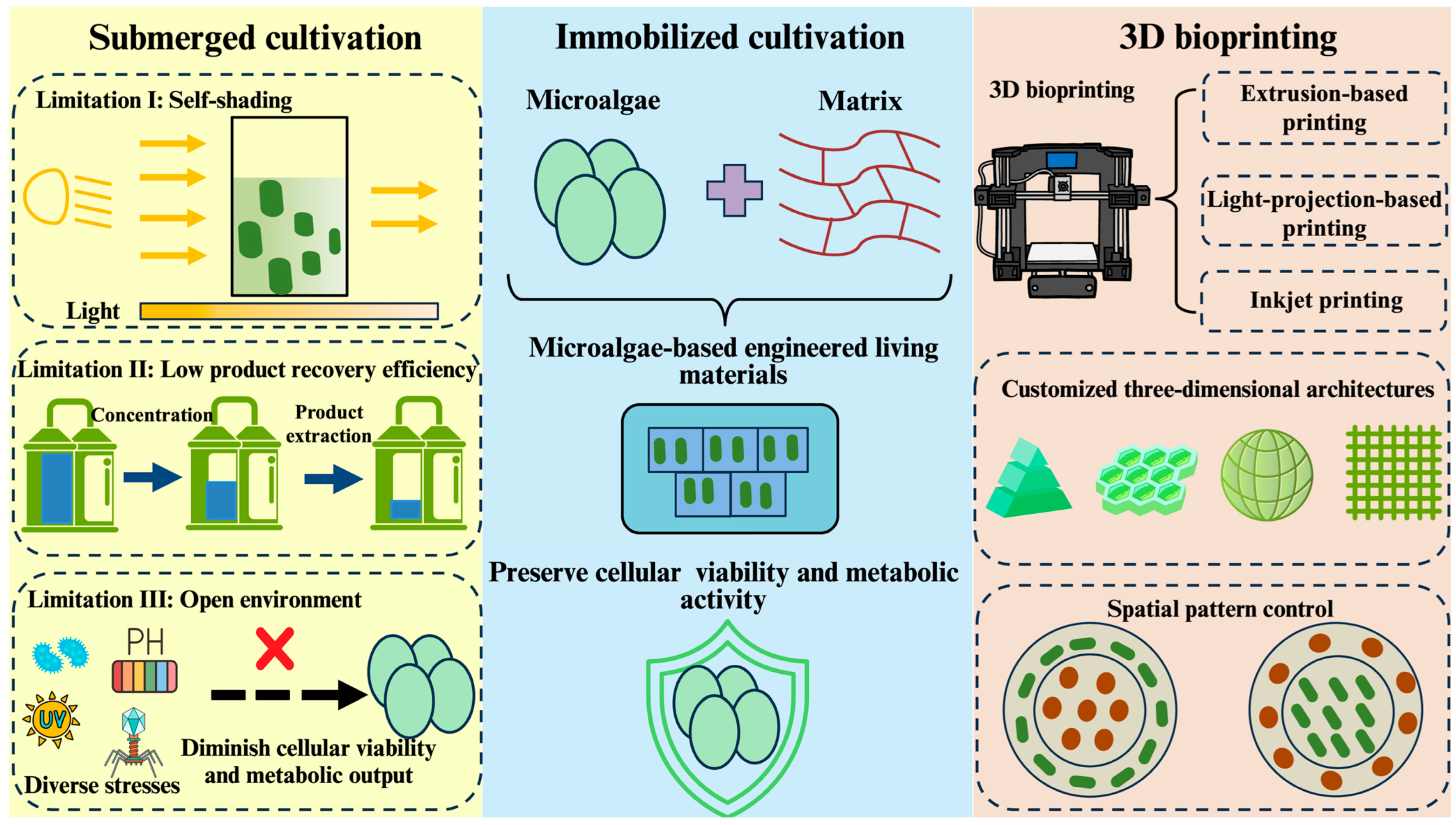
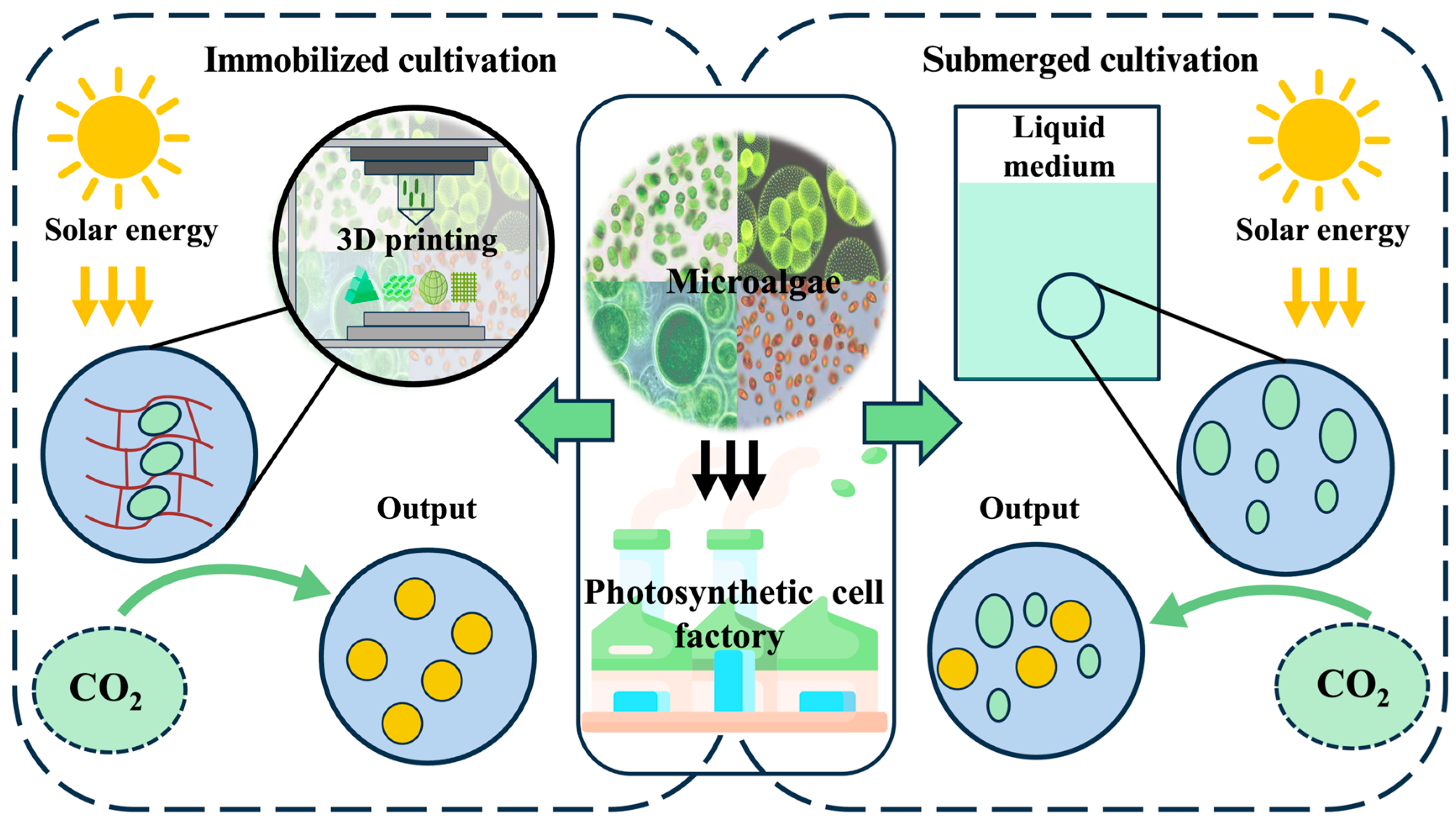
1. Introduction
2. History and Progress of Microalgae-Based 3D Bioprinting
3. Crucial Technical Components of Microalgae-Based 3D Bioprinting
3.1. Matrices of Microalgae-Based 3D Bioprinting
3.2. Methods of Microalgae-Based 3D Bioprinting
3.2.1. Extrusion-Based Bioprinting
3.2.2. Light-Projection-Based Bioprinting
3.2.3. Inkjet-Based Bioprinting
4. Application of Microalgae-Based 3D Bioprinting
4.1. Microalgae-Based 3D Bioprinting in Living Building Materials
4.1.1. Living Building Materials
4.1.2. Three-Dimensional-Bioprinting-Enabled LBMs
4.1.3. Future Perspectives of 3D-Bioprinting-Enabled LBMs
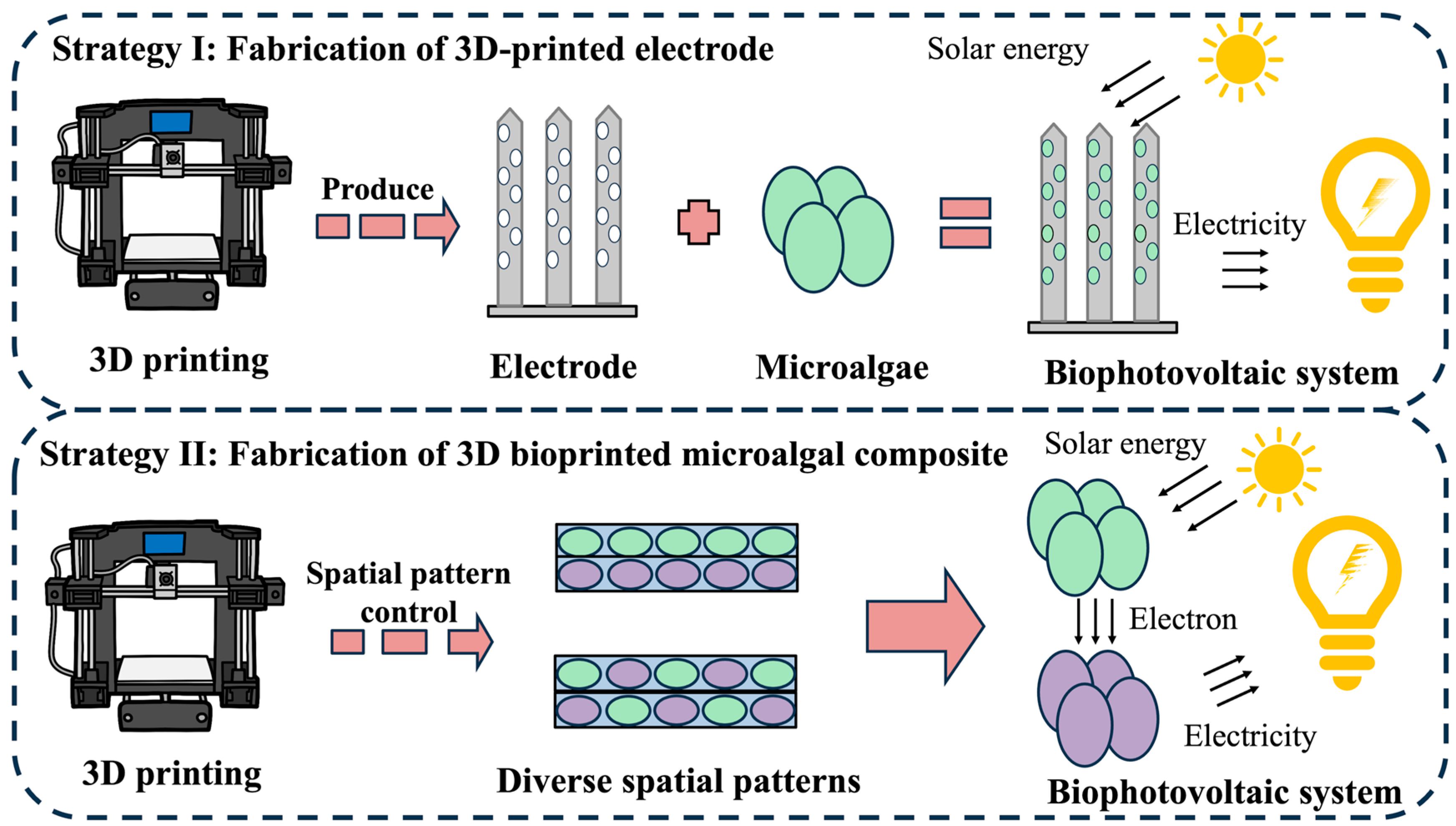
4.2. Microalgae-Based 3D Bioprinting in Biophotovoltaic System
4.2.1. Biophotovoltaic System
4.2.2. Strategies of 3D-Bioprinting-Enabled Biophotovoltaic Systems
Fabrication of 3D-Printed Electrodes
Fabrication of Bioprinted Microalgal Composite
4.2.3. Future Perspectives of 3D-Bioprinting-Enabled BPV System
4.3. Microalgae-Based 3D Bioprinting in Photosynthetic Biosynthesis
4.3.1. Photosynthetic Biosynthesis
4.3.2. Three-Dimensional-Bioprinting-Enabled Photosynthetic Biosynthesis
4.3.3. Future Perspectives of 3D-Bioprinting-Enabled Photosynthetic Biosynthesis
4.4. Microalgae-Based 3D Bioprinting in Bioremediation
4.4.1. Bioremediation
4.4.2. Strategies of 3D-Bioprinting-Enabled Bioremediation
Construction of Synergistic Microbial Consortia
Intelligent Environmental Responsiveness
4.4.3. Future Perspectives of 3D-Bioprinting-Enabled Bioremediation
4.5. Microalgae-Based 3D Bioprinting in Tissue Engineering
4.5.1. Tissue Engineering
4.5.2. Three-Dimensional-Bioprinting-Enabled Tissue Engineering
4.5.3. Future Perspectives of 3D-Bioprinting-Enabled Tissue Engineering
4.6. Microalgae-Based 3D Bioprinting in Food Engineering
4.6.1. Three-Dimensional Printing-Enabled Food Engineering
4.6.2. Three-Dimensional-Bioprinting-Enabled Food Engineering
Living Microalgae System
Microalgal Biomass
4.6.3. Future Perspectives of 3D-Bioprinting-Enabled Food Engineering
5. Prospects
5.1. The Future of 3D Bioprinting Technology
5.2. Microalgae-Based 3D Bioprinting and Synthetic Biology
5.2.1. Printing Microalgal Biomes/Communities
5.2.2. Advancing Microalgae-Based 4D Bioprinting
- (1)
- Intelligent Microalgal Response Systems: Genetic engineering of microalgae could yield chassis strains with tunable environmental sensitivity (e.g., light, humidity, ionic gradients). Coupled with 3D-printed hydrogel matrices, these strains may form adaptive bioreactors capable of structural reconfiguration or gene expression modulation in response to external cues. Such systems could advance applications in precision bioremediation, photosynthetic bioelectronics, and programmable tissue scaffolds.
- (2)
- Spatiotemporally Controlled Microbial Consortia: By leveraging 4D bioprinting to position microalgae as primary producers within synthetic microbial ecosystems, interspecies dynamics can be systematically investigated including metabolic cross-feeding, quorum sensing, and horizontal gene transfer. These engineered communities may serve as self-regulating bioreactors, dynamically adjusting the functional outputs (e.g., metabolite production, carbon sequestration) in response to environmental fluctuations.
- (3)
- Functionalized Bioprinted Edibles: Safety-certified genetically modified microalgae could be embedded into 4D-printed food matrices to enable targeted physiological effects, such as gut microbiome modulation or immunonutritional enhancement. This application necessitates rigorous optimization of algal viability, nutrient retention, and post-ingestion functionality within gastrointestinal environments.
Author Contributions
Funding
Conflicts of Interest
References
- Layani, M.; Wang, X.; Magdassi, S. Novel materials for 3D printing by photopolymerization. Adv. Mater. 2018, 30, e1706344. [Google Scholar] [CrossRef]
- Guillemot, F.; Mironov, V.; Nakamura, M. Bioprinting is coming of age: Report from the International Conference on Bioprinting and Biofabrication in Bordeaux (3B’09). Biofabrication 2010, 2, 010201. [Google Scholar] [CrossRef]
- Mironov, V. The second international workshop on bioprinting, biopatterning and bioassembly. Expert Opin. Biol. Ther. 2005, 5, 1111–1115. [Google Scholar] [CrossRef]
- Ali, H.R.; Collier, P.; Bayston, R. A three-dimensional model of bacterial biofilms and its use in antimicrobial susceptibility testing. Microorganisms 2024, 12, 203. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An introduction to 3D bioprinting: Possibilities, challenges and future aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, Z.; Maniruzzaman, M. Bioprinting: A focus on improving bioink printability and cell performance based on different process parameters. Int. J. Pharm. 2023, 640, 123020. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Khristov, V.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Malda, J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 2011, 3, 021001. [Google Scholar] [CrossRef] [PubMed]
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincón, J.; Zabala, L.L.; Jiao, N.; Karl, D.M.; Li, W.K.W.; Lomas, M.W.; Veneziano, D.; et al. Present and future global distributions of the marine cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Falkowski, P.G. The role of phytoplankton photosynthesis in global biogeochemical cycles. Photosynth. Res. 1994, 39, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Sun, H.; Chen, R.; Sun, J.; Mo, G.; Luan, G.; Lu, X. Multiple routes toward engineering efficient cyanobacterial photosynthetic biomanufacturing technologies. Green Carbon 2023, 1, 210–226. [Google Scholar] [CrossRef]
- Guo, L.P.; Zhang, Y.; Li, W.C. Sustainable microalgae for the simultaneous synthesis of carbon quantum dots for cellular imaging and porous carbon for CO2 capture. J. Colloid Interface Sci. 2017, 493, 257–264. [Google Scholar] [CrossRef]
- Weibel, D.B.; Garstecki, P.; Ryan, D.; DiLuzio, W.R.; Mayer, M.; Seto, J.E.; Whitesides, G.M. Microoxen: Microorganisms to move microscale loads. Proc. Natl. Acad. Sci. USA 2005, 102, 11963–11967. [Google Scholar] [CrossRef]
- Li, W.; Zhong, D.; Hua, S.; Du, Z.; Zhou, M. Biomineralized biohybrid algae for tumor hypoxia modulation and cascade radio-photodynamic therapy. ACS Appl. Mater. Interfaces 2020, 12, 44541–44553. [Google Scholar] [CrossRef]
- Hendrijantini, N.; Sitalaksmi, R.M.; Ari, M.D.A.; Hidayat, T.J.; Putri, P.A.N.; Sukandar, D. The expression of TNF-α, IL-1β, and IL-10 in the diabetes mellitus condition induced by the combination of Spirulina and chitosan. Bali Med. J. 2020, 9, 22–26. [Google Scholar] [CrossRef]
- Kalossaka, L.M.; Sena, G.; Barter, L.M.C.; Myant, C. Review: 3D printing hydrogels for the fabrication of soilless cultivation substrates. Appl. Mater. Today 2021, 24, 101088. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Yang, P.; Långvik, O.; Wang, X.; Zhang, Y.; Cheng, F.; Österberg, M.; Willför, S.; Xu, C. Surface engineered biomimetic inks based on UV cross-linkable wood biopolymers for 3D printing. ACS Appl. Mater. Interfaces 2019, 11, 12389–12400. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Ávila, H.M.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Srubar, W.V. Engineered living materials: Taxonomies and emerging trends. Trends Biotechnol. 2021, 39, 574–583. [Google Scholar] [CrossRef]
- Nguyen, P.Q.; Courchesne, N.M.D.; Duraj-Thatte, A.; Praveschotinunt, P.; Joshi, N.S. Engineered living materials: Prospects and challenges for using biological systems to direct the assembly of smart materials. Adv. Mater. 2018, 30, 1704847. [Google Scholar] [CrossRef]
- Huang, J.; Liu, S.; Zhang, C.; Wang, X.; Pu, J.; Ba, F.; Xue, S.; Ye, H.; Zhao, T.; Li, K.; et al. Programmable and printable Bacillus subtilis biofilms as engineered living materials. Nat. Chem. Biol. 2019, 15, 34–41. [Google Scholar] [CrossRef]
- González-Camejo, J.; Viruela, A.; Ruano, M.V.; Barat, R.; Seco, A.; Ferrer, J. Effect of light intensity, light duration and photoperiods in the performance of an outdoor photobioreactor for urban wastewater treatment. Algal Res. 2019, 40, 101511. [Google Scholar] [CrossRef]
- Ooms, M.D.; Dinh, C.T.; Sargent, E.H.; Sinton, D. Photon management for augmented photosynthesis. Nat. Commun. 2016, 7, 12699. [Google Scholar] [CrossRef]
- Daly, A.C.; Prendergast, M.E.; Hughes, A.J.; Burdick, J.A. Bioprinting for the biologist. Cell 2021, 184, 18–32. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Haghiashtiani, G.; Hübscher, T.; Kelly, D.J.; Lee, J.M.; Lutolf, M.; McAlpine, M.C.; Yeong, W.Y.; Zenobi-Wong, M.; Malda, J. 3D extrusion bioprinting. Nat. Rev. Methods Primers 2021, 1, 75. [Google Scholar] [CrossRef]
- Kumar, V.; Vlaskin, M.S.; Grigorenko, A.V. 3D bioprinting to fabricate living microalgal materials. Trends Biotechnol. 2021, 39, 1243–1244. [Google Scholar] [CrossRef] [PubMed]
- Lode, A.; Krujatz, F.; Brüggemeier, S.; Quade, M.; Schütz, K.; Knaack, S.; Weber, J.; Bley, T.; Gelinsky, M. Green bioprinting: Fabrication of photosynthetic algae-laden hydrogel scaffolds for biotechnological and medical applications. Eng. Life Sci. 2015, 15, 177–183. [Google Scholar] [CrossRef]
- Krujatz, F.; Lode, A.; Brüggemeier, S.; Schütz, K.; Kramer, J.; Bley, T.; Gelinsky, M.; Weber, J. Green bioprinting: Viability and growth analysis of microalgae immobilized in 3D-plotted hydrogels versus suspension cultures. Eng. Life Sci. 2015, 15, 678–688. [Google Scholar] [CrossRef]
- Windisch, J.; Reinhardt, O.; Duin, S.; Schütz, K.; Rodriguez, N.J.N.; Liu, S.; Lode, A.; Gelinsky, M. Bioinks for space missions: The influence of long-term storage of alginate-methylcellulose-based bioinks on printability as well as cell viability and function. Adv. Healthc. Mater. 2023, 12, 2300436. [Google Scholar] [CrossRef]
- Alasibi, S.; Kazir, M.; Israel, Á.; Livney, Y.D. Algal protein-based 3D-printed fish-analogs as a new approach for sustainable seafood. Curr. Res. Food Sci. 2024, 9, 100905. [Google Scholar] [CrossRef] [PubMed]
- Fredricks, J.L.; Iyer, H.; McDonald, R.; Hsu, J.; Jimenez, A.M.; Roumeli, E. Spirulina-based composites for 3D-printing. J. Polym. Sci. 2021, 59, 2878–2894. [Google Scholar] [CrossRef]
- Xia, X.; Xu, X.; Lin, C.; Yang, Y.; Zeng, L.; Zheng, Y.; Wu, X.; Li, W.; Xiao, L.; Qian, Q.; et al. Microalgal-immobilized biocomposite scaffold fabricated by fused deposition modeling 3D printing technology for dyes removal. ES Mater. Manuf. 2020, 7, 40–50. [Google Scholar] [CrossRef]
- Kwak, C.; Ryu, S.Y.; Park, H.; Lim, S.; Yang, J.; Kim, J.; Kim, J.H.; Lee, J. A Pickering emulsion stabilized by Chlorella microalgae as an eco-friendly extrusion-based 3D printing ink processable under ambient conditions. J. Colloid Interface Sci. 2021, 582, 81–89. [Google Scholar] [CrossRef]
- Wang, M.; Lu, X.; Zheng, X.; Li, W.; Wang, L.; Qian, Y.; Zeng, M. Rheological and physicochemical properties of Spirulina platensis residues-based inks for extrusion 3D food printing. Food Res. Int. 2023, 169, 112823. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Igual, M.; Reino-Moyón, J.; García-Segovia, P.; Martínez-Monzó, J. Effect of microalgae (Arthrospira platensis and Chlorella vulgaris) addition on 3D printed cookies. Food Biophys. 2021, 16, 27–39. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Zhang, L.; Noort, M.W.J.; Schutyser, M.A.I.; García-Segovia, P.; Martínez-Monzó, J. Printability and physicochemical properties of microalgae-enriched 3D-printed snacks. Food Bioprocess Technol. 2020, 13, 2029–2042. [Google Scholar] [CrossRef]
- Vieira, M.V.; Oliveira, S.M.; Amado, I.R.; Fasolin, L.H.; Vicente, A.A.; Pastrana, L.M.; Fuciños, P. 3D printed functional cookies fortified with Arthrospira platensis: Evaluation of its antioxidant potential and physical-chemical characterization. Food Hydrocoll. 2020, 107, 105893. [Google Scholar] [CrossRef]
- Malik, S.; Hagopian, J.; Mohite, S.; Lintong, C.; Stoffels, L.; Giannakopoulos, S.; Beckett, R.; Leung, C.; Ruiz, J.; Cruz, M.; et al. Robotic extrusion of algae-laden hydrogels for large-scale applications. Glob. Chall. 2020, 4, 1900064. [Google Scholar] [CrossRef]
- Munaz, A.; Vadivelu, R.K.; St John, J.; Barton, M.; Kamble, H.; Nguyen, N.T. Three-dimensional printing of biological matters. J. Sci. Adv. Mater. Devices 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Yu, K.; Meyer, A.S.; Karana, E.; Aubin-Tam, M.E. Bioprinting of regenerative photosynthetic living materials. Adv. Funct. Mater. 2021, 31, 2011162. [Google Scholar] [CrossRef]
- Léon, R.; Galván, F. Glycerol photoproduction by free and Ca-alginate entrapped cells of Chlamydomonas reinhardtii. J. Biotechnol. 1995, 42, 61–67. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Tripathi, U.; Ravishankar, G.A. Biotransformation of codeine to morphine in freely suspended cells and immobilized cultures of Spirulina platensis. World J. Microbiol. Biotechnol. 1999, 15, 465–469. [Google Scholar] [CrossRef]
- Aguilar-May, B.; Sánchez-Saavedra, M.P.; Lizardi, J.; Voltolina, D. Growth of Synechococcus sp. immobilized in chitosan with different times of contact with NaOH. J. Appl. Phycol. 2007, 19, 181–183. [Google Scholar] [CrossRef]
- Stowers, R.S.; Allen, S.C.; Suggs, L.J. Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 1953. [Google Scholar] [CrossRef]
- Tóth, G.S.; Backman, O.; Siivola, T.; Xu, W.; Kosourov, S.; Siitonen, V.; Xu, C.; Allahverdiyeva, Y. Employing photocurable biopolymers to engineer photosynthetic 3D-printed living materials for production of chemicals. Green Chem. 2024, 26, 4032–4042. [Google Scholar] [CrossRef]
- Dranseike, D.; Cui, Y.; Ling, A.S.; Donat, F.; Bernhard, S.; Bernero, M.; Areeckal, A.; Qin, X.H.; Oakey, J.S.; Dillenburger, B.; et al. Dual carbon sequestration with photosynthetic living materials. Nat Commun. 2025, 16, 3832. [Google Scholar] [CrossRef]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef]
- Vazquez-Martel, C.; Martins, L.F.; Genthner, E.; Almeida, C.; Quintana, A.M.; Bastmeyer, M.; Gómez Pinchetti, J.L.; Blasco, E. Printing green: Microalgae-based materials for 3D printing with light. Adv. Mater. 2024, 36, 2402786. [Google Scholar] [CrossRef]
- Pozzobon, V.; Otaola, F.; Arnoudts, C.; Lagirarde, J. Impact of 3D printing materials on microalga Chlorella vulgaris. Bioresour. Technol. 2023, 389, 129807. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Guo, C.; Kumarasena, A.; Omenetto, F.G.; Kaplan, D.L. 3D printing of functional microalgal silk structures for environmental applications. ACS Biomater. Sci. Eng. 2019, 5, 4808–4816. [Google Scholar] [CrossRef]
- He, F.; Ou, Y.; Liu, J.; Huang, Q.; Tang, B.; Xin, F.; Zhang, J.; Jiang, M.; Chen, S.; Yu, Z. 3D printed biocatalytic living materials with dual-network reinforced bioinks. Small 2022, 18, 2104820. [Google Scholar] [CrossRef]
- Wangpraseurt, D.; You, S.; Sun, Y.; Chen, S. Biomimetic 3D living materials powered by microorganisms. Trends Biotechnol. 2022, 40, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric bioprinting of complex living-tissue constructs within seconds. Adv. Mater. 2019, 31, 1904209. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef]
- Merceron, T.K.; Burt, M.; Seol, Y.J.; Kang, H.W.; Lee, S.J.; Yoo, J.J.; Atala, A. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication 2015, 7, 035003. [Google Scholar] [CrossRef]
- Deo, K.A.; Singh, K.A.; Peak, C.W.; Alge, D.L.; Gaharwar, A.K. Bioprinting 101: Design, fabrication, and evaluation of cell-laden 3D bioprinted scaffolds. Tissue Eng. Part. A. 2020, 26, 318–338. [Google Scholar] [CrossRef]
- Lim, K.S.; Levato, R.; Costa, P.F.; Castilho, M.D.; Alcala-Orozco, C.R.; van Dorenmalen, K.M.A.; Melchels, F.P.W.; Gawlitta, D.; Hooper, G.J.; Malda, J.; et al. Bio-resin for high resolution lithography-based biofabrication of complex cell-laden constructs. Biofabrication 2018, 10, 034101. [Google Scholar] [CrossRef]
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.W.; Lee, K.X.A.; Yeong, W.Y.; Shen, Y.F. Vat polymerization-based bioprinting-process, materials, applications and regulatory challenges. Biofabrication 2020, 12, 022001. [Google Scholar] [CrossRef]
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2012, 5, 015001. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Song, S.J.; Lee, J.Y.; Jang, H.; Choi, J.; Sun, K.; Park, Y. Cellular behavior in micropatterned hydrogels by bioprinting system depended on the cell types and cellular interaction. J. Biosci. Bioeng. 2013, 116, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Khairullah, N.F.; How, Y.P.; Sajab, M.S.; Kaco, H. 3D printed laminated CaCO3-nanocellulose films as controlled-release 5-fluorouracil. Polymers 2020, 12, 986. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Oh, J.J.; Ammu, S.; Vriend, V.D.; Kieffer, R.; Kleiner, F.H.; Balasubramanian, S.; Karana, E.; Masania, K.; Aubin-Tam, M.E. Growth, distribution, and photosynthesis of Chlamydomonas reinhardtii in 3D hydrogels. Adv. Mater. 2024, 36, e2305505. [Google Scholar] [CrossRef]
- Wangpraseurt, D.; You, S.; Azam, F.; Jacucci, G.; Gaidarenko, O.; Hildebrand, M.; Kühl, M.; Smith, A.G.; Davey, M.P.; Smith, A.; et al. Bionic 3D printed corals. Nat. Commun. 2020, 11, 1748. [Google Scholar] [CrossRef]
- Wangpraseurt, D.; Sun, Y.; You, S.; Chua, S.; Noel, S.K.; Willard, H.F.; Berry, D.B.; Clifford, A.M.; Plummer, S.; Xiang, Y.; et al. Bioprinted living coral microenvironments mimicking coral-algal symbiosis. Adv. Funct. Mater. 2022, 32, 2202273. [Google Scholar] [CrossRef]
- Gong, Y.; Bi, Z.; Bian, X.; Ge, A.; He, J.; Li, W.; Shao, H.; Chen, G.; Zhang, X. Study on linear bio-structure print process based on alginate bio-ink in 3D bio-fabrication. Bio-Des. Manuf. 2020, 3, 109–121. [Google Scholar] [CrossRef]
- Sawa, M.; Fantuzzi, A.; Bombelli, P.; Howe, C.J.; Hellgardt, K.; Nixon, P.J. Electricity generation from digitally printed cyanobacteria. Nat. Commun. 2017, 8, 1327. [Google Scholar] [CrossRef]
- Krujatz, F.; Dani, S.; Windisch, J.; Emmermacher, J.; Hahn, F.; Mosshammer, M.; Murthy, S.; Steingröwer, J.; Walther, T.; Kühl, M.; et al. Think outside the box: 3D bioprinting concepts for biotechnological applications—Recent developments and future perspectives. Biotechnol. Adv. 2022, 58, 107930. [Google Scholar] [CrossRef]
- Ma, Z.; Cheah, W.Y.; Ng, I.S.; Chang, J.S.; Zhao, M.; Show, P.L. Microalgae-based biotechnological sequestration of carbon dioxide for net zero emissions. Trends Biotechnol. 2022, 40, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Concrete needs to lose its colossal carbon footprint. Nature 2021, 597, 593–594. [CrossRef] [PubMed]
- Qiu, J.; Artier, J.; Cook, S.; Srubar, W.V.; Cameron, J.C.; Hubler, M.H. Engineering living building materials for enhanced bacterial viability and mechanical properties. iScience 2021, 24, 102083. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Jiao, K.; Tonggu, L.; Wang, L.G.; Zhang, S.L.; Yang, Y.D.; Zhang, L.; Bian, J.H.; Hao, D.X.; Wang, C.Y.; et al. Contribution of biomimetic collagen-ligand interaction to intrafibrillar mineralization. Sci Adv. 2019, 5, eaav9075. [Google Scholar] [CrossRef]
- Goodchild-Michelman, I.M.; Church, G.M.; Schubert, M.G.; Tang, T.C. Light and carbon: Synthetic biology toward new cyanobacteria-based living biomaterials. Mater. Today Bio 2023, 19, 100583. [Google Scholar] [CrossRef]
- Heveran, C.M.; Williams, S.L.; Qiu, J.; Artier, J.; Hubler, M.H.; Cook, S.M.; Cameron, J.C.; Srubar, W.V. Biomineralization and successive regeneration of engineered living building materials. Matter 2020, 2, 481–494. [Google Scholar] [CrossRef]
- Zhu, T.; Paulo, C.; Merroun, M.L.; Dittrich, M. Potential application of biomineralization by Synechococcus PCC8806 for concrete restoration. Ecol. Eng. 2015, 82, 459–468. [Google Scholar] [CrossRef]
- Reinhardt, O.; Ihmann, S.; Ahlhelm, M.; Gelinsky, M. 3D bioprinting of mineralizing cyanobacteria as novel approach for the fabrication of living building materials. Front. Bioeng. Biotechnol. 2023, 11, 1145177. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef]
- McCormick, A.J.; Bombelli, P.; Bradley, R.W.; Thorne, R.; Wenzel, T.; Howe, C.J. Biophotovoltaics: Oxygenic photosynthetic organisms in the world of bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 1092–1109, Erratum in Energy Environ. Sci. 2015, 8, 1627. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Zhang, Y.; Li, Y. Biophotovoltaics: Recent advances and perspectives. Biotechnol. Adv. 2023, 64, 108101. [Google Scholar] [CrossRef]
- Wang, H.Y.; Bernarda, A.; Huang, C.Y.; Lee, D.J.; Chang, J.S. Micro-sized microbial fuel cell: A mini-review. Bioresour. Technol. 2011, 102, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; He, Z.; Angenent, L.T. Light energy to bioelectricity: Photosynthetic microbial fuel cells. Curr. Opin. Biotechnol. 2010, 21, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lawrence, J.M.; Wey, L.T.; Schertel, L.; Jing, Q.; Vignolini, S.; Howe, C.J.; Kar-Narayan, S.; Zhang, J.Z. 3D-printed hierarchical pillar array electrodes for high-performance semi-artificial photosynthesis. Nat. Mater. 2022, 21, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Cook, E.; Mannoor, M.S. Bacterial nanobionics via 3D printing. Nano Lett. 2018, 18, 7448–7456. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Y.; Lee, S.; Choi, S. 3D bioprinting of cyanobacteria for solar-driven bioelectricity generation in resource-limited environments. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5329–5332. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, M.; Soni, T.; Vivekanand, V.; Pareek, N. Insights into the genetic and metabolic engineering approaches to enhance the competence of microalgae as biofuel resource: A review. Bioresour. Technol. 2021, 339, 125597. [Google Scholar] [CrossRef]
- Cao, K.; Cui, Y.; Sun, F.; Zhang, H.; Fan, J.; Ge, B.; Cao, Y.; Wang, X.; Zhu, X.; Wei, Z.; et al. Metabolic engineering and synthetic biology strategies for producing high-value natural pigments in microalgae. Biotechnol. Adv. 2023, 68, 108236. [Google Scholar] [CrossRef]
- Giraldo Calderón, N.D.; Díaz Bayona, K.C.; Atehortúa Garcés, L. Immobilization of the green microalga Botryococcus braunii in polyester wadding: Effect on biomass, fatty acids, and exopolysaccharide production. Biocatal. Agric. Biotechnol. 2018, 14, 80–87. [Google Scholar] [CrossRef]
- Moreno-Garrido, I. Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef]
- Dawiec-Liśniewska, A.; Podstawczyk, D.; Bastrzyk, A.; Czuba, K.; Pacyna-Iwanicka, K.; Okoro, O.V.; Shavandi, A. New trends in biotechnological applications of photosynthetic microorganisms. Biotechnol. Adv. 2022, 59, 107988. [Google Scholar] [CrossRef]
- Caldwell, G.S.; In-na, P.; Hart, R.; Sharp, E.; Stefanova, A.; Pickersgill, M.; Walker, M.; Unthank, M.; Perry, J.; Lee, J.G.M. Immobilising microalgae and cyanobacteria as biocomposites: New opportunities to intensify algae biotechnology and bioprocessing. Energies 2021, 14, 2566. [Google Scholar] [CrossRef]
- Ata, A.; Nalcaci, O.O.; Ovez, B. Macro algae Gracilaria verrucosa as a biosorbent: A study of sorption mechanisms. Algal Res. 2012, 1, 194–204. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Jbari, N.; Trabucco, F.; Martínez, M.E.; Sánchez, S.; Toro, L. Biosorption-mediated reduction of Cr(VI) using heterotrophically-grown Chlorella vulgaris: Active sites and ionic strength effect. Chem. Eng. J. 2013, 231, 94–102. [Google Scholar] [CrossRef]
- Kariyawasam, T.; Petkovich, M.; Vriens, B. Diclofenac degradation by immobilized Chlamydomonas reinhardtii and Scenedesmus obliquus. MicrobiologyOpen 2024, 13, e70013. [Google Scholar] [CrossRef]
- Jiang, M.; Zheng, J.; Tang, Y.; Liu, H.; Yao, Y.; Zhou, J.; Lin, W.; Ma, Y.; Liu, J.; Zhou, J. Retrievable hydrogel networks with confined microalgae for efficient antibiotic degradation and enhanced stress tolerance. Nat. Commun. 2025, 16, 3160. [Google Scholar] [CrossRef]
- Ou, Y.; Cao, S.; Zhang, Y.; Zhu, H.; Guo, C.; Yan, W.; Xin, F.; Dong, W.; Zhang, Y.; Narita, M.; et al. Bioprinting microporous functional living materials from protein-based core-shell microgels. Nat. Commun. 2023, 14, 322. [Google Scholar] [CrossRef]
- Sun, Z.; Wen, H.; Di, Z.; Zhang, Y.; Zhang, S.; Zhang, Z.; Zhang, J.; Yu, Z. Photosynthetic living fiber fabrication from algal-bacterial consortia with controlled spatial distribution. ACS Biomater. Sci. Eng. 2023, 9, 6481–6489. [Google Scholar] [CrossRef]
- Datta, D.; Weiss, E.L.; Wangpraseurt, D.; Hild, E.; Chen, S.; Golden, J.W.; Golden, S.S.; Pokorski, J.K. Phenotypically complex living materials containing engineered cyanobacteria. Nat. Commun. 2023, 14, 4742. [Google Scholar] [CrossRef]
- Rademakers, T.; Horvath, J.M.; Blitterswijk, C.A.; LaPointe, V.L.S. Oxygen and nutrient delivery in tissue engineering: Approaches to graft vascularization. J. Tissue Eng. Regen. Med. 2019, 13, 1815–1829. [Google Scholar] [CrossRef]
- Centeno-Cerdas, C.; Jarquín-Cordero, M.; Chávez, M.N.; Hopfner, U.; Holmes, C.; Schmauss, D.; Machens, H.G.; Nickelsen, J.; Egaña, J.T. Development of photosynthetic sutures for the local delivery of oxygen and recombinant growth factors in wounds. Acta Biomater. 2018, 81, 184–194. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, F.; Xie, T.; Du, Z.; Zhong, D.; Qi, Y.; Li, Y.; Li, W.; Lu, Z.; Rao, J.; et al. Engineered algae: A novel oxygen-generating system for effective treatment of hypoxic cancer. Sci Adv. 2020, 6, eaba5996. [Google Scholar] [CrossRef]
- Cohen, J.E.; Goldstone, A.B.; Paulsen, M.J.; Shudo, Y.; Steele, A.N.; Edwards, B.B.; Patel, J.B.; MacArthur, J.W.; Hopkins, M.S.; Burnett, C.E.; et al. An innovative biologic system for photon-powered myocardium in the ischemic heart. Sci. Adv. 2017, 3, e1603078. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, Y.; Tian, J.; Yang, P.; Zhang, X.; Chen, Y.; Hu, Y.; Wu, J. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci Adv. 2020, 6, eaba4311. [Google Scholar] [CrossRef] [PubMed]
- Dani, S.; Windisch, J.; Guerrero, X.M.V.; Bernhardt, A.; Gelinsky, M.; Krujatz, F.; Lode, A. Selection of a suitable photosynthetically active microalgae strain for the co-cultivation with mammalian cells. Front. Bioeng. Biotechnol. 2022, 10, 994134. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, S.; Alva, J.; Cámara, C.; Rubio, A.G.; Hernández, D.; Delavaux, C.; Correa, E.; Romo, M.D.; Bonilla, D.; Santiago, M.L.; et al. Symbiotic photosynthetic oxygenation within 3D-bioprinted vascularized tissues. Matter 2021, 4, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, C.; Yu, Y.; Zhao, Y. In situ 3D bioprinting living photosynthetic scaffolds for autotrophic wound healing. Research 2022, 2022, 9794745. [Google Scholar] [CrossRef]
- Liu, H.; Mei, H.; Jiang, H.; Jiang, L.; Lin, K.; Jiang, M.; Ding, N.; Li, X.; Gao, Z.; Liu, B.; et al. Bioprinted symbiotic dressings: A lichen-inspired approach to diabetic wound healing with enhanced bioactivity and structural integrity. Small 2025, 21, 2407105. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Kagawa, Y.; Sakaguchi, K.; Matsuura, K.; Shimizu, T.; Okano, T. Thicker three-dimensional tissue from a “symbiotic recycling system” combining mammalian cells and algae. Sci. Rep. 2017, 7, 41594. [Google Scholar] [CrossRef]
- Trampe, E.; Koren, K.; Akkineni, A.R.; Senwitz, C.; Krujatz, F.; Lode, A.; Gelinsky, M.; Kühl, M. Functionalized bioink with optical sensor nanoparticles for O~2~ imaging in 3D-bioprinted constructs. Adv. Funct. Mater. 2018, 28, 1804411. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, T.; Chen, S.H.Y.; Liu, B.; Sun, P.; Sun, H.; Chen, F. The potentials and challenges of using microalgae as an ingredient to produce meat analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Papa, C.; Pollio, A.; Ingenito, A.; Sangianantoni, G.; Cantile, T. Cyanobacteria and microalgae as sources of functional foods to improve human general and oral health. Molecules 2020, 25, 5164. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Mantihal, S.; Kobun, R.; Lee, B.B. 3D food printing of as the new way of preparing food: A review. Int. J. Gastron. Food Sci. 2020, 22, 100260. [Google Scholar] [CrossRef]
- Liu, H.; Yu, S.; Liu, B.; Xiang, S.; Jiang, M.; Yang, F.; Tan, W.; Zhou, J.; Xiao, M.; Li, X.; et al. Space-efficient 3D microalgae farming with optimized resource utilization for regenerative food. Adv. Mater. 2024, 36, 2401172. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, C.; Dai, C.; Shi, Q.; Fang, G.; Xie, D.; Zhao, X.; Liu, Y.J.; Wang, C.C.L.; Wang, X.J. A multi-axis robot-based bioprinting system supporting natural cell function preservation and cardiac tissue fabrication. Bioact. Mater. 2022, 18, 138–150. [Google Scholar] [CrossRef]
- Global Bioprinting Market Research and Growth Forecast Analysis; BCC Research: Boston, MA, USA, 2025; Available online: https://www.bccresearch.com/market-research/biotechnology/bioprinting-markets-technologies-report.html (accessed on 17 August 2025).
- Tong, A.; Pham, Q.L.; Abatemarco, P.; Mathew, A.; Gupta, D.; Iyer, S.; Voronov, R. Review of low-cost 3D bioprinters: State of the market and observed future trends. SLAS Technol. 2021, 26, 333–366. [Google Scholar] [CrossRef]
- Lee, H.; Shin, D.; Choi, J.; Ki, C.S.; Hyun, J. Mimicry of the plant leaf with a living hydrogel sheet of cellulose nanofibers. Carbohydr. Polym. 2022, 290, 119485. [Google Scholar] [CrossRef]
- Taylor, S.; Mueller, E.; Jones, L.R.; Makela, A.V.; Ashammakhi, N. Translational aspects of 3D and 4D printing and bioprinting. Adv. Healthc. Mater. 2024, 13, 2400463. [Google Scholar] [CrossRef]
- Zhang, H.; Hua, S.; He, C.; Yin, M.; Qin, J.; Liu, H.; Zhou, H.; Wu, S.; Yu, X.; Jiang, H.; et al. Application of 4D-printed magnetoresponsive FOGS hydrogel scaffolds in auricular cartilage regeneration. Adv. Healthc. Mater. 2025, 14, 2404488. [Google Scholar] [CrossRef]
- Ding, A.; Lee, S.J.; Tang, R.; Gasvoda, K.L.; He, F.; Alsberg, E. 4D cell-condensate bioprinting. Small 2022, 18, 2202196. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Sun, J.; Cui, J.; Yuan, X.; Luan, G.; Lu, X. Microalgae-Based 3D Bioprinting: Recent Advances, Applications and Perspectives. Mar. Drugs 2025, 23, 342. https://doi.org/10.3390/md23090342
Tang J, Sun J, Cui J, Yuan X, Luan G, Lu X. Microalgae-Based 3D Bioprinting: Recent Advances, Applications and Perspectives. Marine Drugs. 2025; 23(9):342. https://doi.org/10.3390/md23090342
Chicago/Turabian StyleTang, Jinhui, Jiahui Sun, Jinyu Cui, Xiangyi Yuan, Guodong Luan, and Xuefeng Lu. 2025. "Microalgae-Based 3D Bioprinting: Recent Advances, Applications and Perspectives" Marine Drugs 23, no. 9: 342. https://doi.org/10.3390/md23090342
APA StyleTang, J., Sun, J., Cui, J., Yuan, X., Luan, G., & Lu, X. (2025). Microalgae-Based 3D Bioprinting: Recent Advances, Applications and Perspectives. Marine Drugs, 23(9), 342. https://doi.org/10.3390/md23090342









