Effect of Heterologous Expression of Key Enzymes Involved in Astaxanthin and Lipid Synthesis on Lipid and Carotenoid Production in Aurantiochytrium sp.
Abstract
1. Introduction
2. Results
2.1. Expression Vector Construction and Molecular Characterization of Exogenous Genes Expressed in Aurantiochytrium sp.
2.2. Physiological and Biochemical Characterization of Positive Monocolonal Strains of Aurantiochytrium sp.
2.3. Effect of Exogenous Genes on Carotenoid Content of Aurantiochytrium sp.
2.4. Detection of Fatty Acid Composition in Strain L-S of Aurantiochytrium sp.
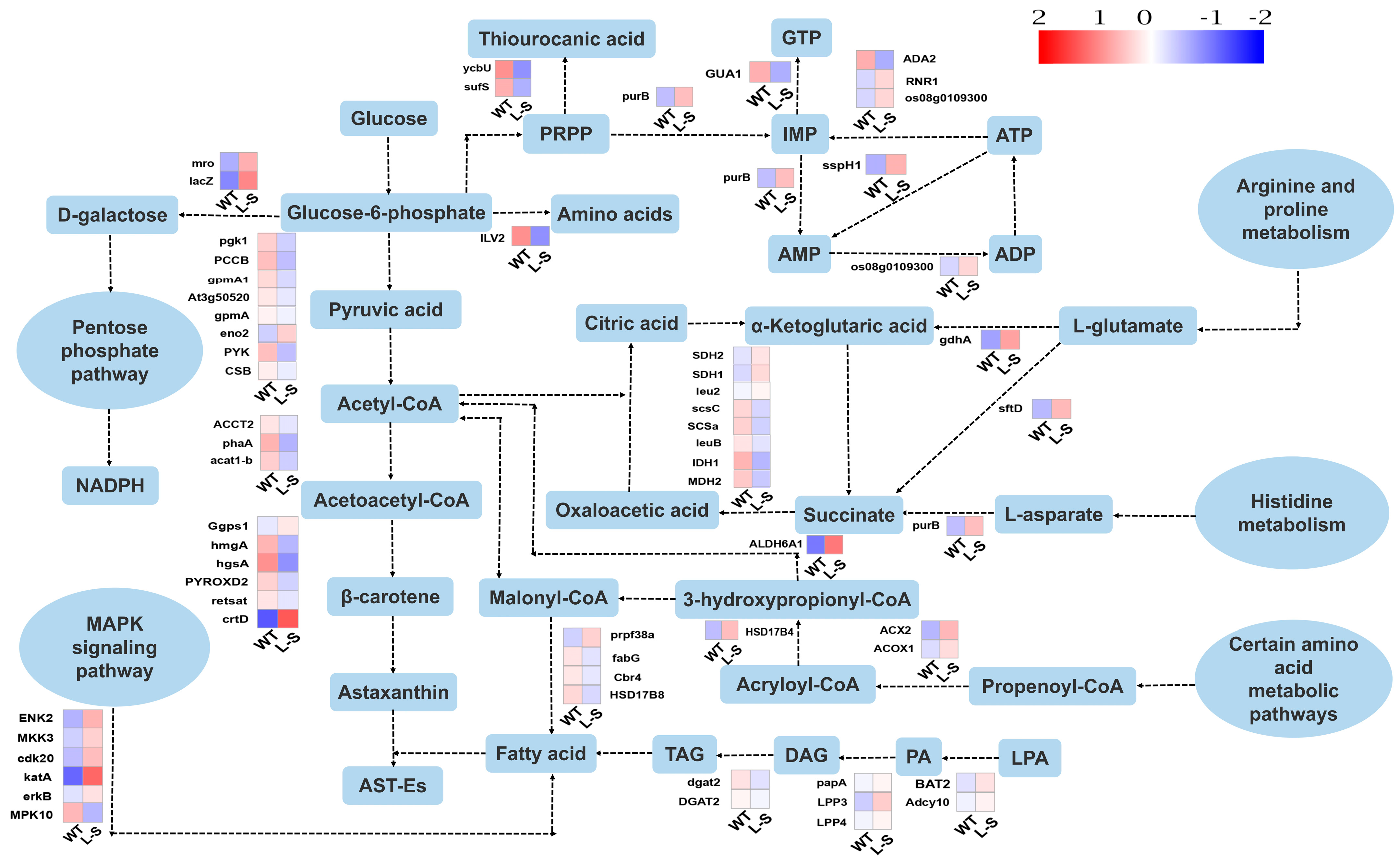
2.5. Transcriptome Analysis of Wild Type and Strain L-S of Aurantiochytrium sp.
3. Discussion
3.1. Effect of Exogenous Genes on Protein and Polysaccharide Accumulation in Aurantiochytrium sp.
3.2. Effect of Exogenous Genes on Biomass and Carotenoid Accumulation in Aurantiochytrium sp.
3.3. Effect of LPAAT on Fatty Acid Composition in Aurantiochytrium sp.
3.4. Differentially Expressed Genes Involved in Carotenoid Synthesis in Aurantiochytrium sp.
3.5. Differentially Expressed Genes Involved in the Production of Unsaturated Fatty Acids and ATP Synthesis in Aurantiochytrium sp.
3.6. Differentially Expressed Genes Involved in the Production of Unsaturated Fatty Acids and Acetyl Coenzyme A Synthesis in Aurantiochytrium sp.
3.7. Potential Limitations and Future Directions of the Work
4. Materials and Methods
4.1. Culture of Aurantiochytrium sp.
4.2. Plasmid Construction
4.3. Transformation of Aurantiochytrium sp.
4.4. Molecular Characterization of Transformant Strains of Aurantiochytrium sp.
4.5. Measurement of Physiological and Biochemical Indices of Aurantiochytrium sp.
4.6. Determination of Carotenoid Content in Aurantiochytrium sp.
4.7. Analysis of Fatty Acid Composition of Aurantiochytrium sp.
4.8. Transcriptome Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Du, H.; Liao, X.; Gao, Z.; Li, Y.; Lei, Y.; Chen, W.; Chen, L.; Fan, X.; Zhang, K.; Chen, S. Effects of methanol on carotenoids as well as biomass and fatty acid biosynthesis in Schizochytrium limacinum B4D1. Appl. Environ. Microbiol. 2019, 85, e01243-19. [Google Scholar] [CrossRef]
- Berzal, G.; García-García, P.; Señoráns, F.J. Integrated Process for Schizochytrium Oil Extraction, Enzymatic Modification of Lipids and Concentration of DHA Fatty Acid Esters Using Alternative Methodologies. Mar. Drugs 2024, 22, 146. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-L.; Zhang, Q.-M.; Du, F.; Yang, W.-Q.; Zhang, Z.-X.; Xu, Y.-S.; Ma, W.; Sun, X.-M.; Huang, H. Identification of lipid synthesis genes in Schizochytrium sp. and their application in improving eicosapentaenoic acid synthesis in Yarrowia lipolytica. Biotechnol. Biofuels Bioprod. 2024, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kwak, M.; Seo, J.; Ju, J.; Heo, S.; Park, S.; Hong, W. Enhanced production of carotenoids using a Thraustochytrid microalgal strain containing high levels of docosahexaenoic acid-rich oil. Bioprocess. Biosyst. Eng. 2018, 41, 1355–1370. [Google Scholar] [CrossRef] [PubMed]
- Burja, A.M.; Radianingtyas, H.; Windust, A.; Barrow, C.J. Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: Screening of strains and optimization of omega-3 production. Appl. Microbiol. Biotechnol. 2006, 72, 1161–1169. [Google Scholar] [CrossRef]
- Siziya, I.N.; Hwang, C.Y.; Seo, M.-J. Antioxidant potential and capacity of microorganism-sourced C30 carotenoids—A review. Antioxidants 2022, 11, 1963. [Google Scholar] [CrossRef]
- Hernández-Almanza, A.; Montanez, J.; Martínez, G.; Aguilar-Jiménez, A.; Contreras-Esquivel, J.C.; Aguilar, C.N. Lycopene: Progress in microbial production. Trends Food Sci. Technol. 2016, 56, 142–148. [Google Scholar] [CrossRef]
- Nishida, Y.; Berg, P.C.; Shakersain, B.; Hecht, K.; Takikawa, A.; Tao, R.; Kakuta, Y.; Uragami, C.; Hashimoto, H.; Misawa, N.; et al. Astaxanthin: Past, Present, and Future. Mar. Drugs 2023, 21, 514. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Nishida, Y.; Yamashita, E.; Miki, W. Quenching activities of common hydrophilic and lipophilic antioxidants against singlet oxygen using chemiluminescence detection system. Carotenoid Sci. 2007, 11, 16–20. [Google Scholar]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar]
- Kim, J.H.; Choi, S.K.; Choi, S.Y.; Kim, H.K.; Chang, H.I. Suppressive effect of astaxanthin isolated from the Xanthophyllomyces dendrorhous mutant on ethanol-induced gastric mucosal injury in rats. Biosci. Biotechnol. Biochem. 2005, 69, 1300–1305. [Google Scholar]
- Zhang, L.; Wang, H. Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar. Drugs 2015, 13, 4310–4330. [Google Scholar] [CrossRef]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar]
- Rebelo, B.A.; Farrona, S.; Ventura, M.R.; Abranches, R. Canthaxanthin, a red-hot carotenoid: Applications, synthesis, and biosynthetic evolution. Plants 2020, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive effect of dietary astaxanthin on UVA-induced skin photoaging in hairless mice. PLoS ONE 2017, 12, e0171178. [Google Scholar]
- Zhou, Q.; Xu, J.; Yang, L.; Gu, C.; Xue, C. Thermal stability and oral absorbability of astaxanthin esters from Haematococcus pluvialis in Balb/c mice. J. Sci. Food Agric. 2019, 99, 3662–3671. [Google Scholar]
- Storebakken, T.; Sørensen, M.; Bjerkeng, B.; Harris, J. Utilization of astaxanthin from red yeast, Xanthophyllomyces dendrorhous, in rainbow trout, Oncorhynchus mykiss: Effects of enzymatic cell wall disruption and feed extrusion temperature. Aquaculture 2004, 236, 391–403. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Igreja, W.S.; Maia, F.d.A.; Lopes, A.S.; Chisté, R.C. Biotechnological production of carotenoids using low cost-substrates is influenced by cultivation parameters: A review. Int. J. Mol. Sci. 2021, 22, 8819. [Google Scholar] [CrossRef]
- Nogueira, M.; Enfissi, E.M.; Martínez Valenzuela, M.E.; Menard, G.N.; Driller, R.L.; Eastmond, P.J.; Schuch, W.; Sandmann, G.; Fraser, P.D. Engineering of tomato for the sustainable production of ketocarotenoids and its evaluation in aquaculture feed. Proc. Natl. Acad. Sci. USA 2017, 114, 10876–10881. [Google Scholar] [CrossRef]
- Watcharawipas, A.; Runguphan, W. Red yeasts and their carotenogenic enzymes for microbial carotenoid production. FEMS Yeast Res. 2023, 23, foac063. [Google Scholar] [PubMed]
- Umair, M.; Jabbar, S.; Nasiru, M.M.; Lu, Z.; Zhang, J.; Abid, M.; Murtaza, M.A.; Kieliszek, M.; Zhao, L. Ultrasound-assisted extraction of carotenoids from carrot pomace and their optimization through response surface methodology. Molecules 2021, 26, 6763. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Liang, M.; Shi, L.; Shao, J.; Song, C.; Bian, K.; Chen, W.; Yang, Z. Accumulation of carotenoids and expression of carotenogenic genes in peach fruit. Food Chem. 2017, 214, 137–146. [Google Scholar] [CrossRef]
- Barboza, M.; Lima, F.L.d.S.; Ribeiro, R.M.P.; Pereira, C.C.A.; Santos, M.G.d.; Silveira, F.P.d.M.; Nascimento, A.K.Q.d.; Passos, A.B.R.d.J.; Chagas, P.S.F.d.; Ribeiro, J.E.d.S. Determination of Total Carotenoids and β-Carotene in Germplasm of Pumpkin Caboclo (Cucurbita maxima). Agron. J. 2023, 13, 1109. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Ma, W.; Fang, Z.; Jiang, Y.; Jiang, W.; Kong, X.; Xin, F.; Zhang, W.; Jiang, M. Strategies for the efficient biosynthesis of β-carotene through microbial fermentation. World J. Microbiol. Biotechnol. 2024, 40, 160. [Google Scholar]
- Eun, H.; Lee, S.Y. Metabolic engineering and fermentation of microorganisms for carotenoids production. Curr. Opin. Biotechnol. 2024, 87, 103104. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, L.; Xia, Y.; Zhuang, X.; Chu, W. Isolation, identification of carotenoid-producing Rhodotorula sp. from marine environment and optimization for carotenoid production. Mar. Drugs 2019, 17, 161. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, D.; Zhang, X.; Jiang, Y.; Zhang, W.; Xin, F.; Jiang, M. Co-production of lipids and carotenoids by Rhodosporidium toruloides from cane molasses using temperature and pH shifting strategies. Biofuels Bioprod. Biorefining 2023, 17, 873–883. [Google Scholar]
- Ochoa-Viñals, N.; Alonso-Estrada, D.; Pacios-Michelena, S.; García-Cruz, A.; Ramos-González, R.; Faife-Pérez, E.; Michelena-Álvarez, L.G.; Martínez-Hernández, J.L.; Iliná, A. Current Advances in Carotenoid Production by Rhodotorula sp. Fermentation 2024, 10, 190. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Jiang, H.; Mao, X. Biotechnology advances in β-carotene production by microorganisms. Trends Food Sci. Technol. 2021, 111, 322–332. [Google Scholar]
- Li, L.; Tang, X.; Luo, Y.; Hu, X.; Ren, L. Accumulation and conversion of β-carotene and astaxanthin induced by abiotic stresses in Schizochytrium sp. Bioprocess. Biosyst. Eng. 2022, 45, 911–920. [Google Scholar] [PubMed]
- Ding, Y.-W.; Lu, C.-Z.; Zheng, Y.; Ma, H.-Z.; Jin, J.; Jia, B.; Yuan, Y.-J. Directed evolution of the fusion enzyme for improving astaxanthin biosynthesis in Saccharomyces cerevisiae. Synth. Syst. Biotechnol. 2023, 8, 46–53. [Google Scholar] [PubMed]
- Liu, P.-Y.; Wu, J.-J.; Li, G.; Lin, C.-B.; Jiang, S.; Liu, S.; Wan, X. The Biosynthesis of Astaxanthin Esters in Schizochytrium sp. is Mediated by a Bifunctional Diacylglycerol Acyltransferase. J. Agric. Food Chem. 2024, 72, 3584–3595. [Google Scholar]
- Liang, L.; Zheng, X.; Fan, W.; Chen, D.; Huang, Z.; Peng, J.; Zhu, J.; Tang, W.; Chen, Y.; Xue, T. Genome and transcriptome analyses provide insight into the omega-3 long-chain polyunsaturated fatty acids biosynthesis of Schizochytrium limacinum SR21. Front. Microbiol. 2020, 11, 687. [Google Scholar]
- Huang, W.; Ye, J.; Zhang, J.; Lin, Y.; He, M.; Huang, J. Transcriptome analysis of Chlorella zofingiensis to identify genes and their expressions involved in astaxanthin and triacylglycerol biosynthesis. Algal Res. 2016, 17, 236–243. [Google Scholar]
- Leung, D.W. The structure and functions of human lysophosphatidic acid acyltransferases. Front. Biosci. 2001, 6, D944–D953. [Google Scholar]
- Körbes, A.P.; Kulcheski, F.R.; Margis, R.; Margis-Pinheiro, M.; Turchetto-Zolet, A.C. Molecular evolution of the lysophosphatidic acid acyltransferase (LPAAT) gene family. Mol. Phylogenetics Evol. 2016, 96, 55–69. [Google Scholar]
- Cui, H.; Zhao, C.; Xu, W.; Zhang, H.; Hang, W.; Zhu, X.; Ji, C.; Xue, J.; Zhang, C.; Li, R. Characterization of type-2 diacylglycerol acyltransferases in Haematococcus lacustris reveals their functions and engineering potential in triacylglycerol biosynthesis. BMC Plant Biol. 2021, 21, 20. [Google Scholar]
- Inokoshi, J.; Kawamoto, K.; Takagi, Y.; Matsuhama, M.; Ōmura, S.; Tomoda, H. Expression of two human acyl-CoA: Diacylglycerol acyltransferase isozymes in yeast and selectivity of microbial inhibitors toward the isozymes. J. Antibiot. 2009, 62, 51–54. [Google Scholar]
- Chen, G.; Wang, B.; Han, D.; Sommerfeld, M.; Lu, Y.; Chen, F.; Hu, Q. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). Plant J. 2014, 81, 95–107. [Google Scholar]
- van der Vlist, J.; Loos, K. Amylose and amylopectin hybrid materials via enzymatic pathways. Macromol. Symp. 2007, 254, 54–61. [Google Scholar]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef] [PubMed]
- Torabi, S.; Sukumaran, A.; Dhaubhadel, S.; Johnson, S.E.; LaFayette, P.; Parrott, W.A.; Rajcan, I.; Eskandari, M. Effects of type I Diacylglycerol O-acyltransferase (DGAT1) genes on soybean (Glycine max L.) seed composition. Sci. Rep. 2021, 11, 2556. [Google Scholar]
- Götz, T.; Sandmann, G.; Römer, S. Expression of a bacterial carotene hydroxylase gene (crtZ) enhances UV tolerance in tobacco. Plant Mol. Biol. 2002, 50, 127–140. [Google Scholar]
- Huang, K.; Su, Z.; He, M.; Wu, Y.; Wang, M. Simultaneous accumulation of astaxanthin and β-carotene in Chlamydomonas reinhardtii by the introduction of foreign β-carotene hydroxylase gene in response to high light stress. Biotechnol. Lett. 2022, 44, 321–331. [Google Scholar]
- Chungjatupornchai, W.; Areerat, K.; Fa-Aroonsawat, S. Increased triacylglycerol production in oleaginous microalga Neochloris oleoabundans by overexpression of plastidial lysophosphatidic acid acyltransferase. Microb. Cell Factories 2019, 18, 53. [Google Scholar]
- Li, N.; Wu, H.; Ding, Q.; Li, H.; Li, Z.; Ding, J.; Li, Y. The heterologous expression of Arabidopsis PAP2 induces anthocyanin accumulation and inhibits plant growth in tomato. Funct. Integr. Genom. 2018, 18, 341–353. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Gu, C.; Xu, X.; Sun, Y.; Hou, M.; Wang, Y.; Zhang, Y.; Liu, L.; Yuan, H. Heterologous expression of stevia SrERF5 inhibits root growth in Arabidopsis thaliana. Plant Cell Tissue Organ Cult. 2021, 147, 1–8. [Google Scholar]
- Raimundo, A.F.; Ferreira, S.; Farrim, M.I.; Santos, C.N.; Menezes, R. Heterologous expression of immature forms of human islet amyloid polypeptide in yeast triggers intracellular aggregation and cytotoxicity. Front. Microbiol. 2020, 11, 2035. [Google Scholar] [CrossRef]
- Yoshimi, T.; Hashimoto, S.; Kubo, Y.; Takeuchi, M.; Morimoto, D.; Nakagawa, S.; Sawayama, S. Improvement of astaxanthin production in Aurantiochytrium limacinum by overexpression of the beta-carotene hydroxylase gene. Appl. Biochem. Biotechnol. 2023, 195, 1255–1267. [Google Scholar] [PubMed]
- Wu, Y.; Yan, P.; Liu, X.; Wang, Z.; Tang, Y.-J.; Chen, T.; Zhao, X. Combinatorial expression of different β-carotene hydroxylases and ketolases in Escherichia coli for increased astaxanthin production. J. Ind. Microbiol. Biotechnol. 2019, 46, 1505–1516. [Google Scholar] [PubMed]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Diaz-MacAdoo, D.; Mata, M.T.; Riquelme, C. Influence of irradiance and wavelength on the antioxidant activity and carotenoids accumulation in Muriellopsis sp. isolated from the antofagasta coastal desert. Molecules 2022, 27, 2412. [Google Scholar] [CrossRef]
- Sekova, V.Y.; Dergacheva, D.I.; Isakova, E.P.; Gessler, N.N.; Tereshina, V.M.; Deryabina, Y.I. Soluble sugar and lipid readjustments in the Yarrowia lipolytica yeast at various temperatures and pH. Metabolites 2019, 9, 307. [Google Scholar] [CrossRef]
- Tran, N.A.T.; Padula, M.P.; Evenhuis, C.R.; Commault, A.S.; Ralph, P.J.; Tamburic, B. Proteomic and biophysical analyses reveal a metabolic shift in nitrogen deprived Nannochloropsis oculata. Algal Res. 2016, 19, 1–11. [Google Scholar]
- Fahs, Z.; Rossez, Y.; Guénin, S.; Gutierrez, L.; Thomasset, B.; Perrin, Y. Cloning and molecular characterization of three lysophosphatidic acid acyltransferases expressed in flax seeds. Plant Sci. 2019, 280, 41–50. [Google Scholar]
- Zhu, Y.; Wang, Y.; Wei, Z.; Zhang, X.; Jiao, B.; Tian, Y.; Yan, F.; Li, J.; Liu, Y.; Yang, X.; et al. Analysis of oil synthesis pathway in Cyperus esculentus tubers and identification of oleosin and caleosin genes. J. Plant Physiol. 2023, 284, 153961. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Feng, Y.; Wan, W.; Zhang, H.; Bai, X.; Cui, Q.; Song, X. Obtaining high-purity docosahexaenoic acid oil in thraustochytrid Aurantiochytrium through a combined metabolic engineering strategy. J. Agric. Food Chem. 2021, 69, 10215–10222. [Google Scholar] [CrossRef]
- Wang, F.; Bi, Y.; Diao, J.; Lv, M.; Cui, J.; Chen, L.; Zhang, W. Metabolic engineering to enhance biosynthesis of both docosahexaenoic acid and odd-chain fatty acids in Schizochytrium sp. S31. Biotechnol. Biofuels 2019, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.; Wang, Y.; Yang, X.; Wang, J.; Chen, H. Over-expression of LPAAT gene in Phaeodactylum tricornutum enhances fatty acid accumulation and increases fatty acid chain length. Indian J. Pharm. Sci. 2020, 82, 19–25. [Google Scholar] [CrossRef]
- Woodfield, H.K.; Fenyk, S.; Wallington, E.; Bates, R.E.; Brown, A.; Guschina, I.A.; Marillia, E.F.; Taylor, D.C.; Fell, D.; Harwood, J.L. Increase in lysophosphatidate acyltransferase activity in oilseed rape (Brassica napus) increases seed triacylglycerol content despite its low intrinsic flux control coefficient. New Phytol. 2019, 224, 700–711. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Lu, J.; Deng, X.; Li, H.; Hu, Z. Effect of overexpression of LPAAT and GPD1 on lipid synthesis and composition in green microalga Chlamydomonas reinhardtii. J. Appl. Phycol. 2018, 30, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, J.; Pei, W.; Wu, M.; Li, H.; Li, X.; Yu, S.; Zhang, J.; Yu, J. A genome-wide analysis of the lysophosphatidate acyltransferase (LPAAT) gene family in cotton: Organization, expression, sequence variation, and association with seed oil content and fiber quality. BMC Genom. 2017, 18, 218. [Google Scholar] [CrossRef]
- Wayne, L.L.; Gachotte, D.J.; Graupner, P.R.; Adelfinskaya, Y.; McCaskill, D.G.; Metz, J.G.; Zirkle, R.; Walsh, T.A. Plant and algal lysophosphatidic acid acyltransferases increase docosahexaenoic acid accumulation at the sn-2 position of triacylglycerol in transgenic Arabidopsis seed oil. PLoS ONE 2021, 16, e0256625. [Google Scholar]
- Starikov, A.Y.; Sidorov, R.A.; Mironov, K.S.; Los, D.A. The Specificities of Lysophosphatidic Acid Acyltransferase and Fatty Acid Desaturase Determine the High Content of Myristic and Myristoleic Acids in Cyanobacterium sp. IPPAS B-1200. Int. J. Mol. Sci. 2024, 25, 774. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, R.; Sun, D.; Bai, Z.; Li, H.; Xue, L.; Zhang, Y.; Niu, L. PrLPAAT4, a putative lysophosphatidic acid acyltransferase from Paeonia rockii, plays an important role in seed fatty acid biosynthesis. Molecules 2017, 22, 1694. [Google Scholar] [CrossRef]
- Yuan, Y.; Liang, Y.; Gao, L.; Sun, R.; Zheng, Y.; Li, D. Functional heterologous expression of a lysophosphatidic acid acyltransferase from coconut (Cocos nucifera L.) endosperm in Saccharomyces cerevisiae and Nicotiana tabacum. Sci. Hortic. 2015, 192, 224–230. [Google Scholar] [CrossRef]
- Saito, M.; Endo, K.; Kobayashi, K.; Watanabe, M.; Ikeuchi, M.; Murakami, A.; Murata, N.; Wada, H. High myristic acid content in the cyanobacterium Cyanothece sp. PCC 8801 results from substrate specificity of lysophosphatidic acid acyltransferase. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 939–947. [Google Scholar] [CrossRef]
- Ogawa, T.; Kuboshima, M.; Suwanawat, N.; Kawamoto, J.; Kurihara, T. Division of the role and physiological impact of multiple lysophosphatidic acid acyltransferase paralogs. BMC Microbiol. 2022, 22, 241. [Google Scholar] [CrossRef] [PubMed]
- Shui, G.; Guan, X.L.; Gopalakrishnan, P.; Xue, Y.; Goh, J.S.Y.; Yang, H.; Wenk, M.R. Characterization of substrate preference for Slc1p and Cst26p in Saccharomyces cerevisiae using lipidomic approaches and an LPAAT activity assay. PLoS ONE 2010, 5, e11956. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.; West, J.A.; Koulman, A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) in health and disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef]
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. The structure and function of acylglycerophosphate acyltransferase 4/lysophosphatidic acid acyltransferase delta (AGPAT4/LPAATδ). Front. Cell Dev. Biol. 2019, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.M.; Duncan, R.E. The lysophosphatidic acid acyltransferases (acylglycerophosphate acyltransferases) family: One reaction, five enzymes, many roles. Curr. Opin. Lipidol. 2018, 29, 110–115. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Li, Z.; Cheng, P.; Yu, G. Transcriptomic and metabolomic analysis reveals the potential mechanisms underlying the improvement of β-carotene and torulene production in Rhodosporidiobolus colostri under low temperature treatment. Food Res. Int. 2022, 156, 111158. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Xu, L.W.; Xu, Y.S.; Li, J.; Ma, W.; Sun, X.M.; Huang, H. Integration of genetic engineering and multi-factor fermentation optimization for co-production of carotenoid and DHA in Schizochytrium sp. Bioresour. Technol. 2024, 394, 130250. [Google Scholar] [CrossRef]
- Boyer-Diaz, Z.; Morata, P.; Aristu-Zabalza, P.; Gibert-Ramos, A.; Bosch, J.; Gracia-Sancho, J. Oxidative stress in chronic liver disease and portal hypertension: Potential of DHA as nutraceutical. Nutrients 2020, 12, 2627. [Google Scholar] [CrossRef]
- Patel, A.K.; Tambat, V.S.; Chen, C.-W.; Chauhan, A.S.; Kumar, P.; Vadrale, A.P.; Huang, C.-Y.; Dong, C.-D.; Singhania, R.R. Recent advancements in astaxanthin production from microalgae: A review. Bioresour. Technol. 2022, 364, 128030. [Google Scholar]
- Kloska, S.M.; Paczyński, K.; Marciniak, T.; Talaka, T.; Wysocki, B.J.; Davis, P.; Wysocki, T.A. Integrating glycolysis, citric acid cycle, pentose phosphate pathway, and fatty acid beta-oxidation into a single computational model. Sci. Rep. 2023, 13, 14484. [Google Scholar]
- Yoshino, M.; Murakami, K. AMP deaminase as a control system of glycolysis in yeast. Mechanism of the inhibition of glycolysis by fatty acid and citrate. J. Biol. Chem. 1982, 257, 10644. [Google Scholar]
- Weber, G.; Convery, H.J.H.; Lea, M.A.; Stamm, N.B. Feedback inhibition of key glycolytic enzymes in liver: Action of free fatty acids. Science 1966, 154, 1357–1360. [Google Scholar] [PubMed]
- Farré, G.; Perez-Fons, L.; Decourcelle, M.; Breitenbach, J.; Hem, S.; Zhu, C.; Capell, T.; Christou, P.; Fraser, P.D.; Sandmann, G. Metabolic engineering of astaxanthin biosynthesis in maize endosperm and characterization of a prototype high oil hybrid. Transgenic Res. 2016, 25, 477–489. [Google Scholar]
- Ye, J.; Liu, M.; He, M.; Ye, Y.; Huang, J. Illustrating and enhancing the biosynthesis of astaxanthin and docosahexaenoic acid in Aurantiochytrium sp. SK4. Mar. Drugs 2019, 17, 45. [Google Scholar] [CrossRef]
- Izzo, L.; Trefely, S.; Demetriadou, C.; Drummond, J.M.; Mizukami, T.; Kuprasertkul, N.; Farria, A.T.; Nguyen, P.T.T.; Murali, N.; Reich, L. Acetylcarnitine shuttling links mitochondrial metabolism to histone acetylation and lipogenesis. Sci. Adv. 2023, 9, eadf0115. [Google Scholar]
- Bi, Z.-Q.; Ren, L.-J.; Hu, X.-C.; Sun, X.-M.; Zhu, S.-Y.; Ji, X.-J.; Huang, H. Transcriptome and gene expression analysis of docosahexaenoic acid producer Schizochytrium sp. under different oxygen supply conditions. Biotechnol. Biofuels 2018, 11, 249. [Google Scholar]
- Mu, X.; Evans, T.D.; Zhang, F. ATP biosensor reveals microbial energetic dynamics and facilitates bioproduction. Nat. Commun. 2024, 15, 5299. [Google Scholar]
- Geelen, M.J.H.; Harris, R.A.; Bergh, S.G.V.D. Enigmatic effect of cellular ATP on fatty acid biosynthesis. Stimulation by moderate decrease and inhibition by increase of cellular ATP. FEBS Lett. 2008, 582, 2242–2246. [Google Scholar]
- Zhou, Y.; Lv, R.; Richard, D.Y.; Ren, R.; Yu, L. The 3-hydroxyacyl-CoA dehydratase 1/2 form complex with trans-2-enoyl-CoA reductase involved in substrates transfer in very long chain fatty acid elongation. Biochem. Biophys. Res. Commun. BBRC 2024, 704, 149588. [Google Scholar]
- Inoki, K.; Guan, K.-L. Rag GTPases regulate cellular amino acid homeostasis. Proc. Natl. Acad. Sci. USA 2022, 119, e2200788119. [Google Scholar] [PubMed]
- Höfler, S.; Lorenz, C.; Busch, T.; Brinkkötter, M.; Tohge, T.; Fernie, A.R.; Braun, H.P.; Hildebrandt, T.M. Dealing with the sulfur part of cysteine: Four enzymatic steps degrade l-cysteine to pyruvate and thiosulfate in Arabidopsis mitochondria. Physiol. Plant 2016, 157, 352–366. [Google Scholar] [PubMed]
- Petillo, A.; Abruzzese, V.; Koshal, P.; Ostuni, A.; Bisaccia, F. Extracellular citrate is a trojan horse for cancer cells. Front. Mol. Biosci. 2020, 7, 593866. [Google Scholar]
- Rangasamy, D.; Ratledge, C. Genetic enhancement of fatty acid synthesis by targeting rat liver ATP: Citrate lyase into plastids of tobacco. Plant Physiol. 2000, 122, 1231–1238. [Google Scholar]
- Chen, W.; Zhou, P.P.; Zhang, M.; Zhu, Y.M.; Wang, X.P.; Luo, X.A.; Bao, Z.D.; Yu, L.J. Transcriptome analysis reveals that up-regulation of the fatty acid synthase gene promotes the accumulation of docosahexaenoic acid in Schizochytrium sp. S056 when glycerol is used. Algal Res. 2016, 15, 83–92. [Google Scholar]
- Pasham, S.; Fakas, S. Citric acid cycle metabolites regulate phosphatidate phosphatase activity from the oleaginous yeast Yarrowia lipolytica. FASEB J. 2022, 36, 102868. [Google Scholar]
- Liu, L.; Fu, C.; Liu, Y.; Li, F. Acetate stimulates lipogenesis via AMPKα signaling in rabbit adipose-derived stem cells. Gen. Comp. Endocrinol. 2021, 303, 113715. [Google Scholar]
- Ryu, Y.K.; Lee, W.K.; Choi, W.Y.; Kim, T.; Lee, Y.J.; Park, A.; Kim, T.; Oh, C.; Heo, S.J.; Kim, J.H.; et al. A novel drying film culture method applying a natural phenomenon: Increased carotenoid production by Haematococcus sp. Bioresour. Technol. 2023, 390, 129827. [Google Scholar]
- Park, Y.K.; Dulermo, T.; Ledesma-Amaro, R.; Nicaud, J.M. Optimization of odd chain fatty acid production by Yarrowia lipolytica. Biotechnol. Biofuels 2018, 11, 158. [Google Scholar]
- Keasling, J.D. Manufacturing molecules through metabolic engineering. Science 2010, 330, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, F.; Boo, A.; Furini, S.; Gorochowski, T.E.; Borkowski, O.; Ladak, Y.N.; Awan, A.R.; Gilbert, C.; Stan, G.-B.; Ellis, T. Burden-driven feedback control of gene expression. Nat. Methods 2018, 15, 387–393. [Google Scholar]
- Xu, P.; Rizzoni, E.A.; Sul, S.Y.; Stephanopoulos, G. Improving metabolic pathway efficiency by statistical model-based multivariate regulatory metabolic engineering. ACS Synth. Biol. 2017, 6, 148–158. [Google Scholar] [CrossRef]
- Nielsen, J.; Keasling, J.D. Engineering cellular metabolism. Cell 2016, 164, 1185–1197. [Google Scholar]
- Sandberg, T.E.; Salazar, M.J.; Weng, L.L.; Palsson, B.O.; Feist, A.M. The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology. Metab. Eng. 2019, 56, 1–16. [Google Scholar]
- Portnoy, V.A.; Bezdan, D.; Zengler, K. Adaptive laboratory evolution—Harnessing the power of biology for metabolic engineering. COBIOT Curr. Opin. Biotechnol. 2011, 22, 590–594. [Google Scholar] [PubMed]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lü, X.; Liu, M. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front. Nutr. 2022, 9, 963318. [Google Scholar]
- Bittencourt, A.S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics Group: Cambridge, UK, 2010; Available online: https://www.scienceopen.com/document?vid=de674375-ab83-4595-afa9-4c8aa9e4e736 (accessed on 19 January 2025).
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar]
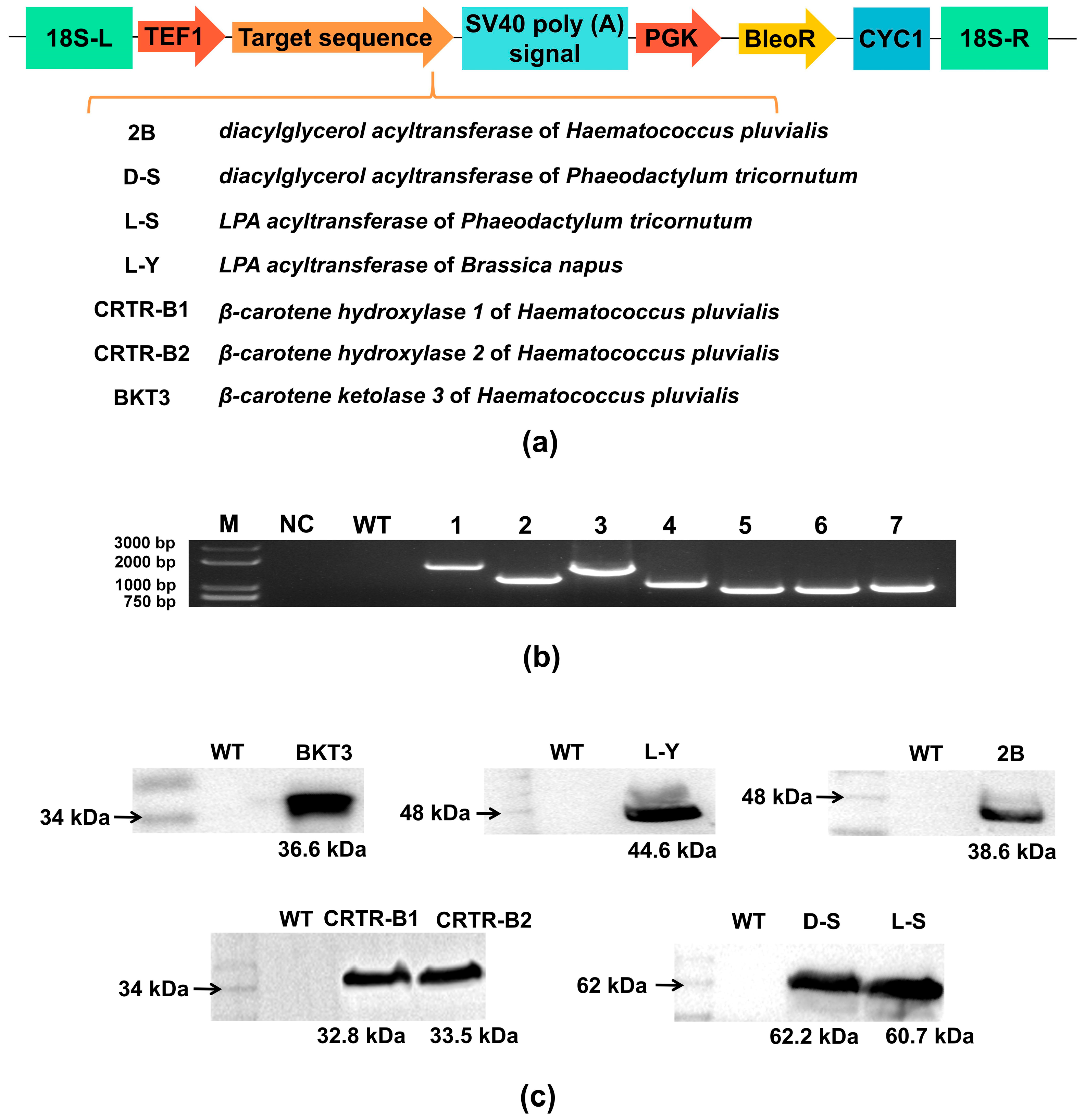

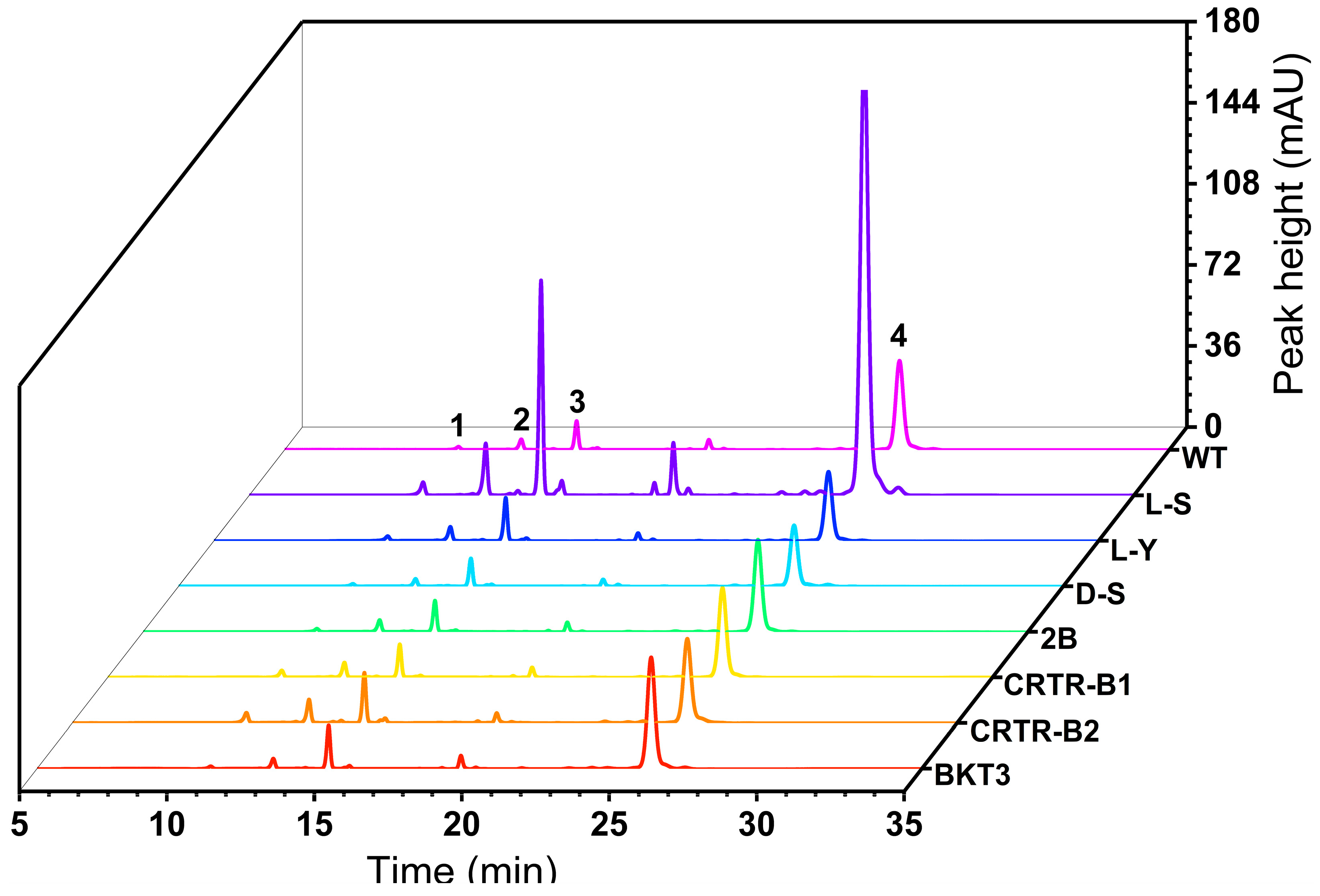
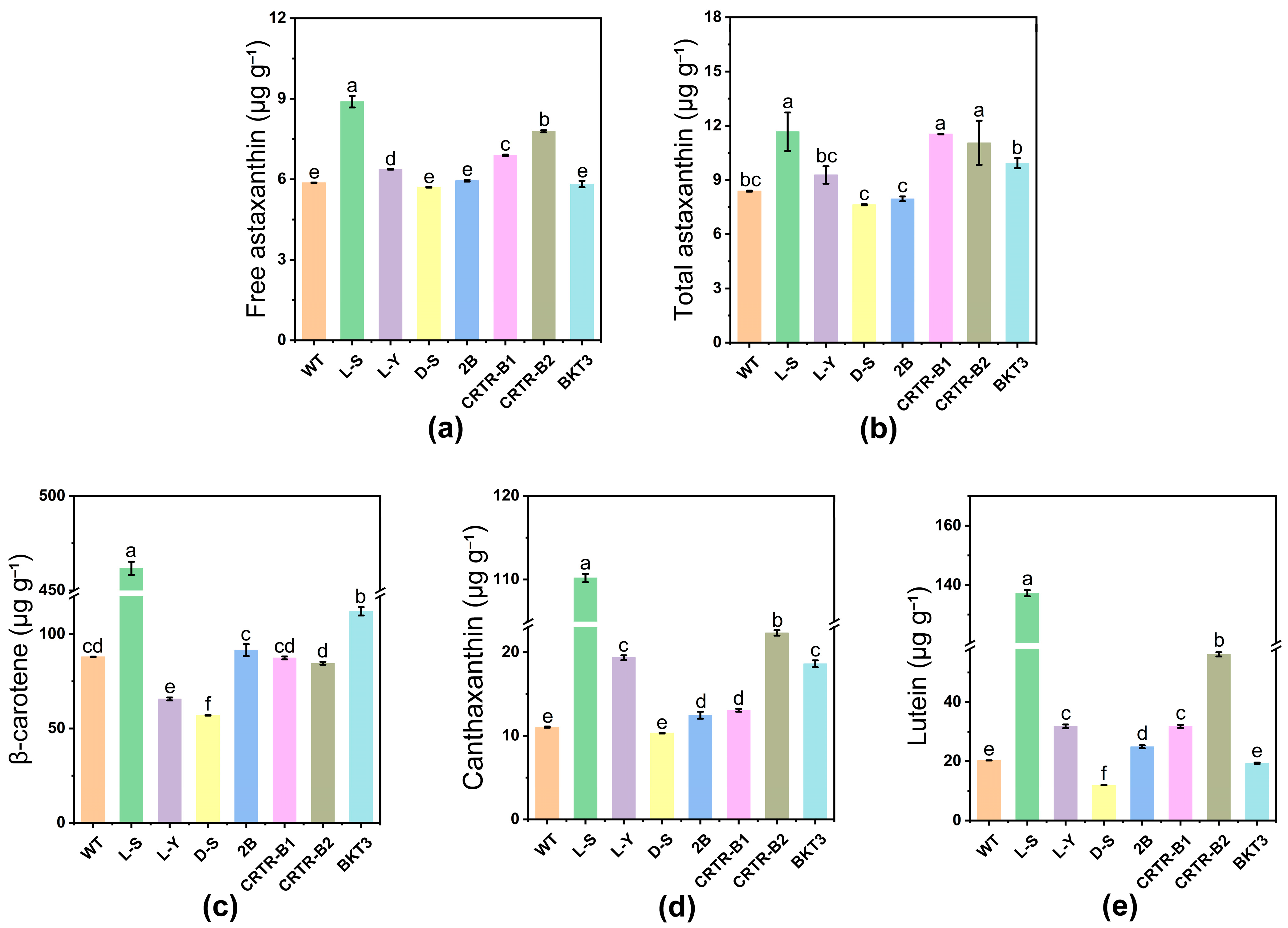


| Strain | Astaxanthin Ester | Free Astaxanthin | Total Astaxanthin | |||
|---|---|---|---|---|---|---|
| Content (μg g−1) | Increase from WT (%) (p < 0.05) | Content (μg g−1) | Increase from WT (%) (p < 0.05) | Content (μg g−1) | Increase from WT (%) (p < 0.05) | |
| WT | 2.5 ± 0.0 | 5.9 ± 0.0 | 8.4 ± 0.0 | |||
| L-S | 2.8 ± 0.9 | – | 8.9 ± 0.2 | 50.8% | 11.7 ± 1.1 | 39.3% |
| L-Y | 2.9 ± 0.5 | – | 6.4 ± 0.0 | 8.5% | 9.3 ± 0.5 | – |
| D-S | 1.9 ± 0.0 | – | 5.7 ± 0.0 | – | 7.6 ± 0.0 | – |
| 2B | 2.0 ± 0.2 | – | 6.0 ± 0.0 | – | 8.0 ± 0.1 | – |
| CRTR-B1 | 4.7 ± 0.0 | 88.0% | 6.9 ± 0.0 | 16.9% | 11.5 ± 0.0 | 36.9% |
| CRTR-B2 | 3.3 ± 1.2 | – | 7.8 ± 0.0 | 32.2% | 11.1 ± 1.2 | 32.1% |
| BKT3 | 4.1 ± 0.2 | 64.0% | 5.8 ± 0.1 | – | 9.8 ± 0.3 | – |
| Strain | β-Carotene | Canthaxanthin | Lutein | |||
|---|---|---|---|---|---|---|
| Content (μg g−1) | Increase from WT (%) (p < 0.05) | Content (μg g−1) | Increase from WT (%) (p < 0.05) | Content (μg g−1) | Increase from WT (%) (p < 0.05) | |
| WT | 88.0 ± 0.1 | 11.0 ± 0.1 | 20.3 ± 0.1 | |||
| L-S | 461.7 ± 3.5 | 424.7% | 110.2 ± 0.5 | 901.8% | 137.2 ± 1.0 | 575.9% |
| L-Y | 65.7 ± 0.8 | – | 19.4 ± 0.3 | 76.4% | 31.8 ± 0.6 | 56.7% |
| D-S | 57.0 ± 0.1 | – | 10.3 ± 0.1 | – | 12.0 ± 0.0 | – |
| 2B | 91.6 ± 3.2 | – | 12.5 ± 0.4 | 13.6% | 24.9 ± 0.5 | 22.7% |
| CRTR-B1 | 87.4 ± 0.8 | – | 13.1 ± 0.2 | 19.1% | 31.8 ± 0.5 | 56.7% |
| CRTR-B2 | 84.5 ± 0.8 | – | 22.3 ± 0.3 | 102.7% | 56.1 ± 0.7 | 176.4% |
| BKT3 | 112.0 ± 2.2 | 27.3% | 18.6 ± 0.4 | 69.1% | 19.3 ± 0.2 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Gao, Z.; Sun, F.; Cui, Y.; Zou, X.; Ma, J.; Wang, Q.; Zhang, H.; Wu, Y.; Meng, C. Effect of Heterologous Expression of Key Enzymes Involved in Astaxanthin and Lipid Synthesis on Lipid and Carotenoid Production in Aurantiochytrium sp. Mar. Drugs 2025, 23, 164. https://doi.org/10.3390/md23040164
Shao Y, Gao Z, Sun F, Cui Y, Zou X, Ma J, Wang Q, Zhang H, Wu Y, Meng C. Effect of Heterologous Expression of Key Enzymes Involved in Astaxanthin and Lipid Synthesis on Lipid and Carotenoid Production in Aurantiochytrium sp. Marine Drugs. 2025; 23(4):164. https://doi.org/10.3390/md23040164
Chicago/Turabian StyleShao, Yaping, Zhengquan Gao, Fengjie Sun, Yulin Cui, Xinyu Zou, Jinju Ma, Qiaolei Wang, Hao Zhang, Yuyong Wu, and Chunxiao Meng. 2025. "Effect of Heterologous Expression of Key Enzymes Involved in Astaxanthin and Lipid Synthesis on Lipid and Carotenoid Production in Aurantiochytrium sp." Marine Drugs 23, no. 4: 164. https://doi.org/10.3390/md23040164
APA StyleShao, Y., Gao, Z., Sun, F., Cui, Y., Zou, X., Ma, J., Wang, Q., Zhang, H., Wu, Y., & Meng, C. (2025). Effect of Heterologous Expression of Key Enzymes Involved in Astaxanthin and Lipid Synthesis on Lipid and Carotenoid Production in Aurantiochytrium sp. Marine Drugs, 23(4), 164. https://doi.org/10.3390/md23040164






