Discovery of a Hepatoprotective Trinor-Sesterterpenoid from the Marine Fungus Talaromyces sp. Against Hepatic Ischemia-Reperfusion Injury
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Determination
2.2. Bioactivity Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.4. Spectroscopic Data of Compound 1
3.5. ECD Computation Section
3.6. Cell Culture and Treatment
3.7. Cytotoxic Bioassay
3.8. RNA Sequencing Analysis
3.9. RT-qPCR
3.10. ELISA
3.11. Animal Experiments
3.12. Serum Biochemical Analysis
3.13. Histopathological Examination
3.14. Molecular Docking
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Liu, J.; Luo, R.; Zhang, Y.; Li, X. Current Status and Perspective on Molecular Targets and Therapeutic Intervention Strategy in Hepatic Ischemia-Reperfusion Injury. Clin. Mol. Hepatol. 2024, 30, 585–619. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yao, X.; Zou, Y.; Liu, B.; Zhou, Y.; Guo, Z.; Yao, Q.; Xu, S.; Li, H. Ticlopidine Protects Hepatic Ischemia-Reperfusion Injury via Suppressing Ferroptosis. Biochem. Biophys. Res. Commun. 2024, 733, 150436. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Man, K. Mechanistic Insight and Clinical Implications of Ischemia/Reperfusion Injury Post Liver Transplantation. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Cai, J.; Xie, D.; She, J.; Liu, Y.; Zhou, X.; Tang, L. New Sesquiterpenoids from the Mangrove-Derived Fungus Talaromyces sp. as Modulators of Nuclear Receptors. Mar. Drugs 2024, 22, 403. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X. Natural Products from Mangrove Sediments-Derived Microbes: Structural Diversity, Bioactivities, Biosynthesis, and Total Synthesis. Eur. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef] [PubMed]
- Barzkar, N.; Babich, O.; Das, R.; Sukhikh, S.; Tamadoni Jahromi, S.; Sohail, M. Marine Bacterial Dextranases: Fundamentals and Applications. Molecules 2022, 27, 5533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, X.; Zhang, G.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Inducing Secondary Metabolite Production by Combined Culture of Talaromyces aculeatus and Penicillium variabile. J. Nat. Prod. 2017, 80, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, C.; Lastres-Becker, I.; Demirdöğen, B.C.; Costa, V.M.; Daiber, A.; Foresti, R.; Motterlini, R.; Kalyoncu, S.; Arioz, B.I.; Genc, S.; et al. Biomarkers of NRF2 Signalling: Current Status and Future Challenges. Redox Biol. 2024, 72, 103134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, M.; Wang, W.; Zhu, L.; Wang, X.; Jin, H.; Feng, L. The Regulation and Function of Nrf2 Signaling in Ferroptosis-Activated Cancer Therapy. Acta Pharmacol. Sin. 2024, 45, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Weng, X.; Bao, X.; Bai, X.; Lv, Y.; Zhang, S.; Chen, Y.; Zhao, C.; Zeng, M.; Huang, J.; et al. A Novel Anti-Atherosclerotic Mechanism of Quercetin: Competitive Binding to KEAP1 via Arg483 to Inhibit Macrophage Pyroptosis. Redox Biol. 2022, 57, 102511. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Gu, T.; Rao, Y.; Liang, W.; Zhang, X.; Jiang, H.; Lu, J.; She, J.; Guo, J.; Yang, W.; et al. A Novel Marine-Derived Anti-Acute Kidney Injury Agent Targeting Peroxiredoxin 1 and Its Nanodelivery Strategy Based on ADME Optimization. Acta Pharm. Sin. B 2024, 14, 3232–3250. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Lin, X.; Tao, H.; Wang, J.; Li, J.; Yang, B.; Zhou, X.; Liu, Y. Isochromophilones A–F, Cytotoxic Chloroazaphilones from the Marine Mangrove Endophytic Fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef] [PubMed]
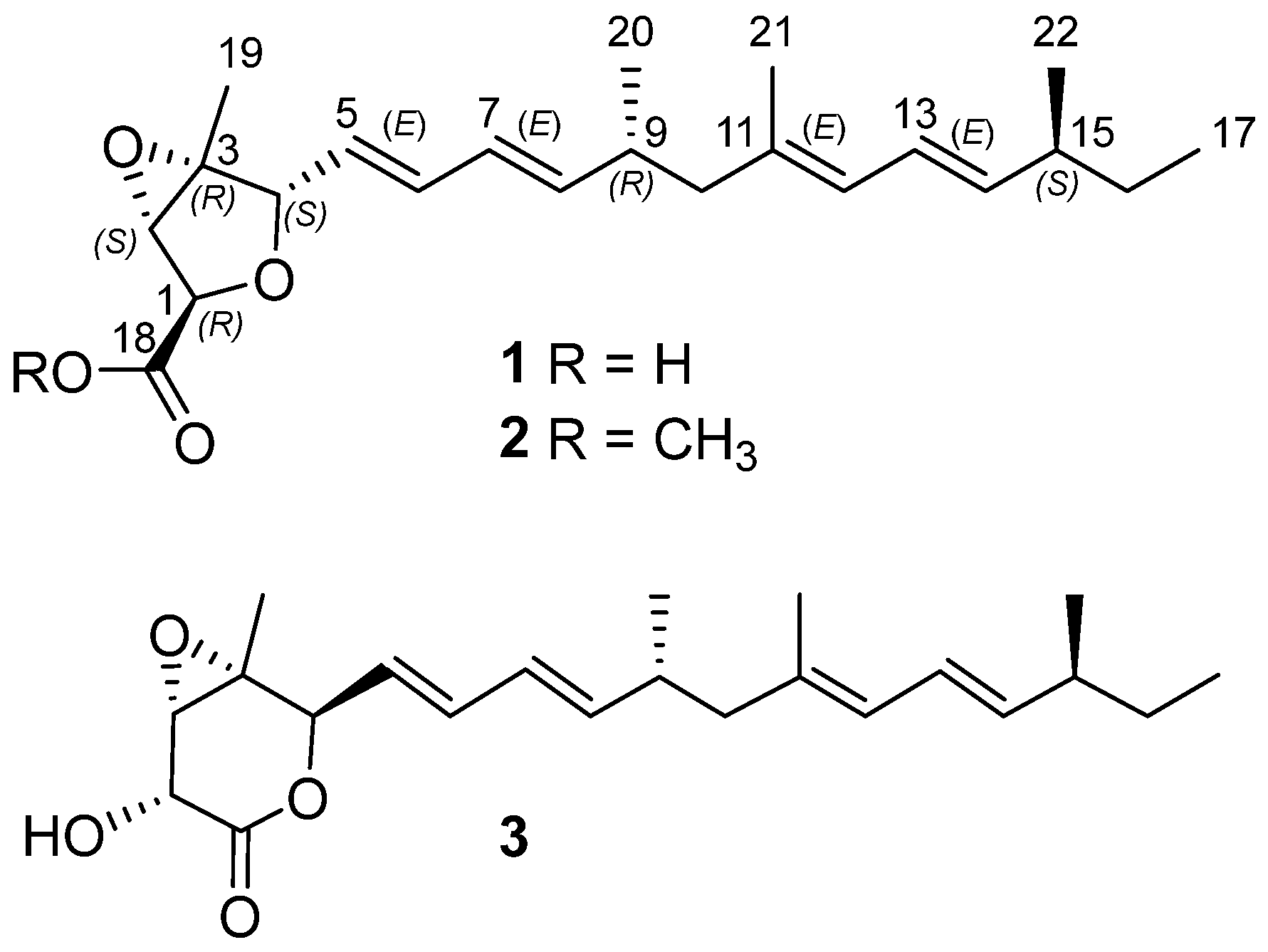
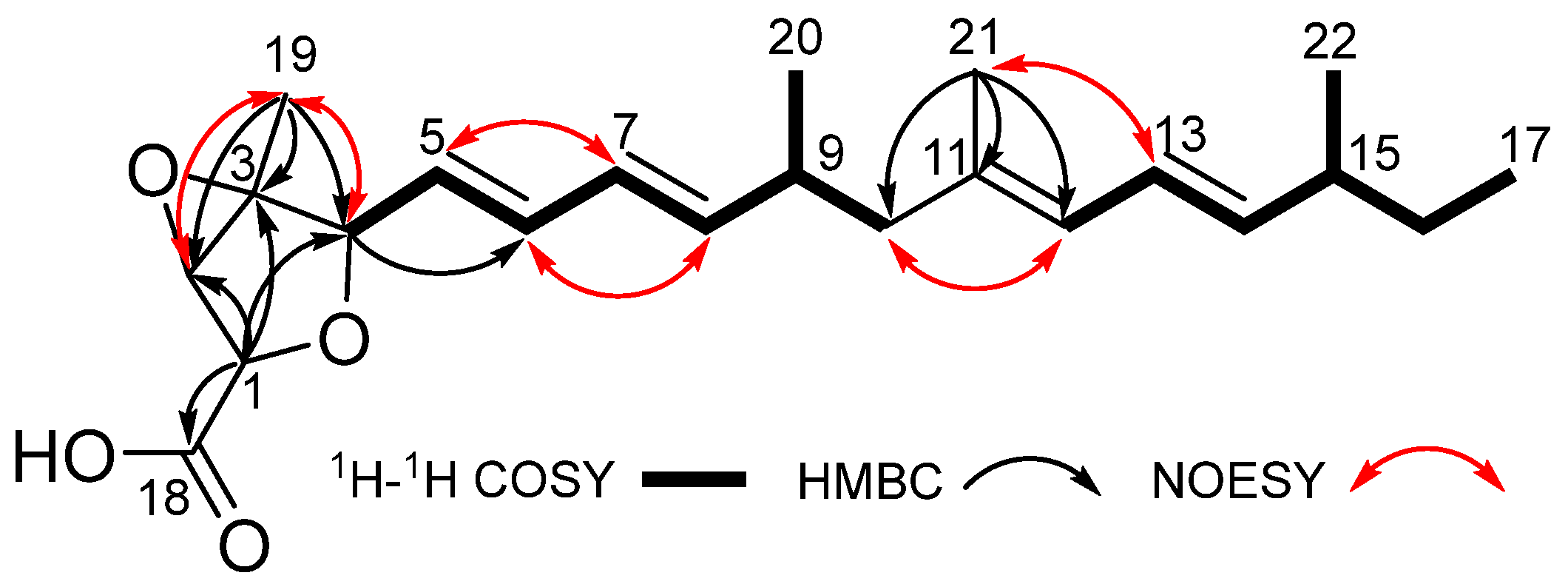
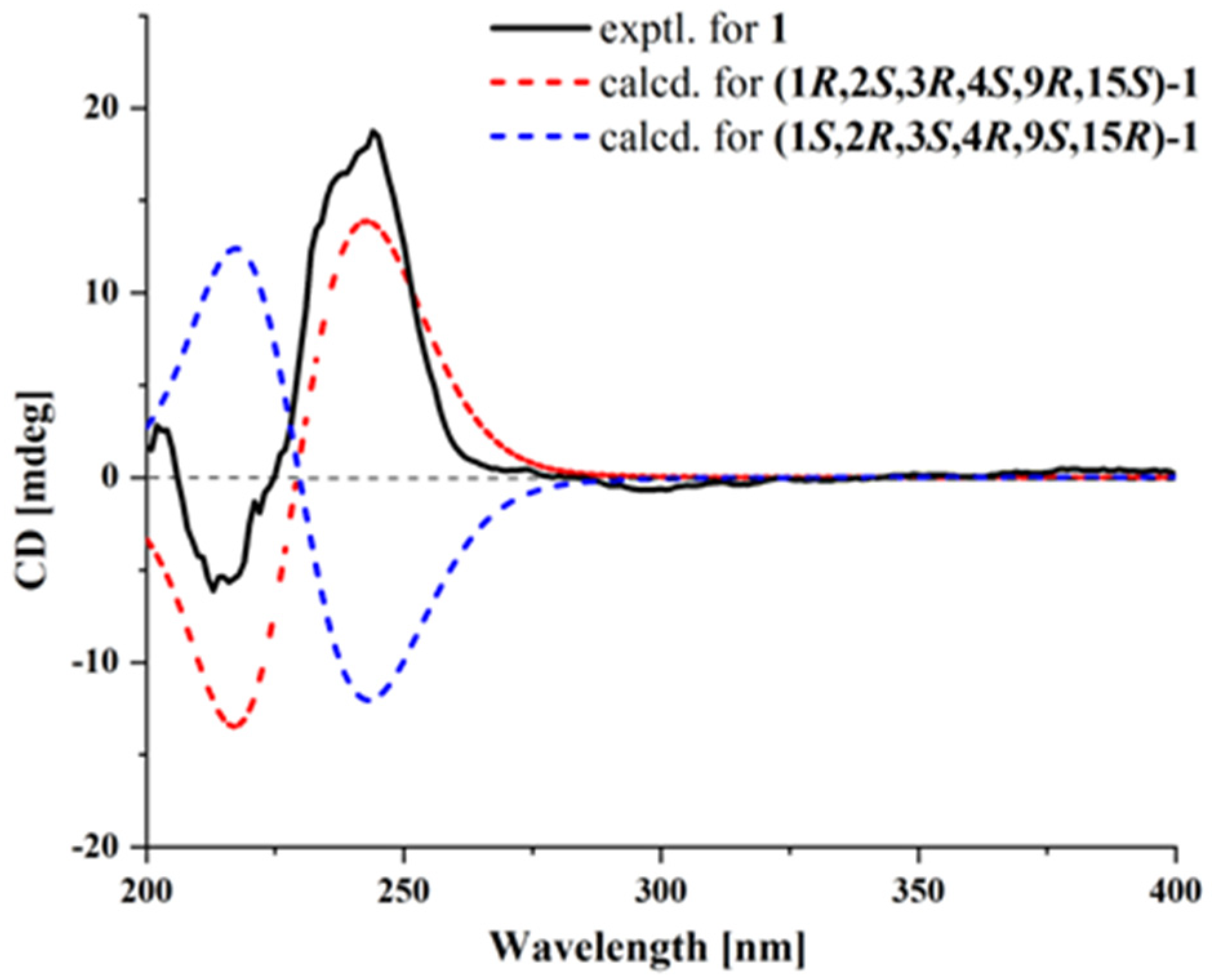
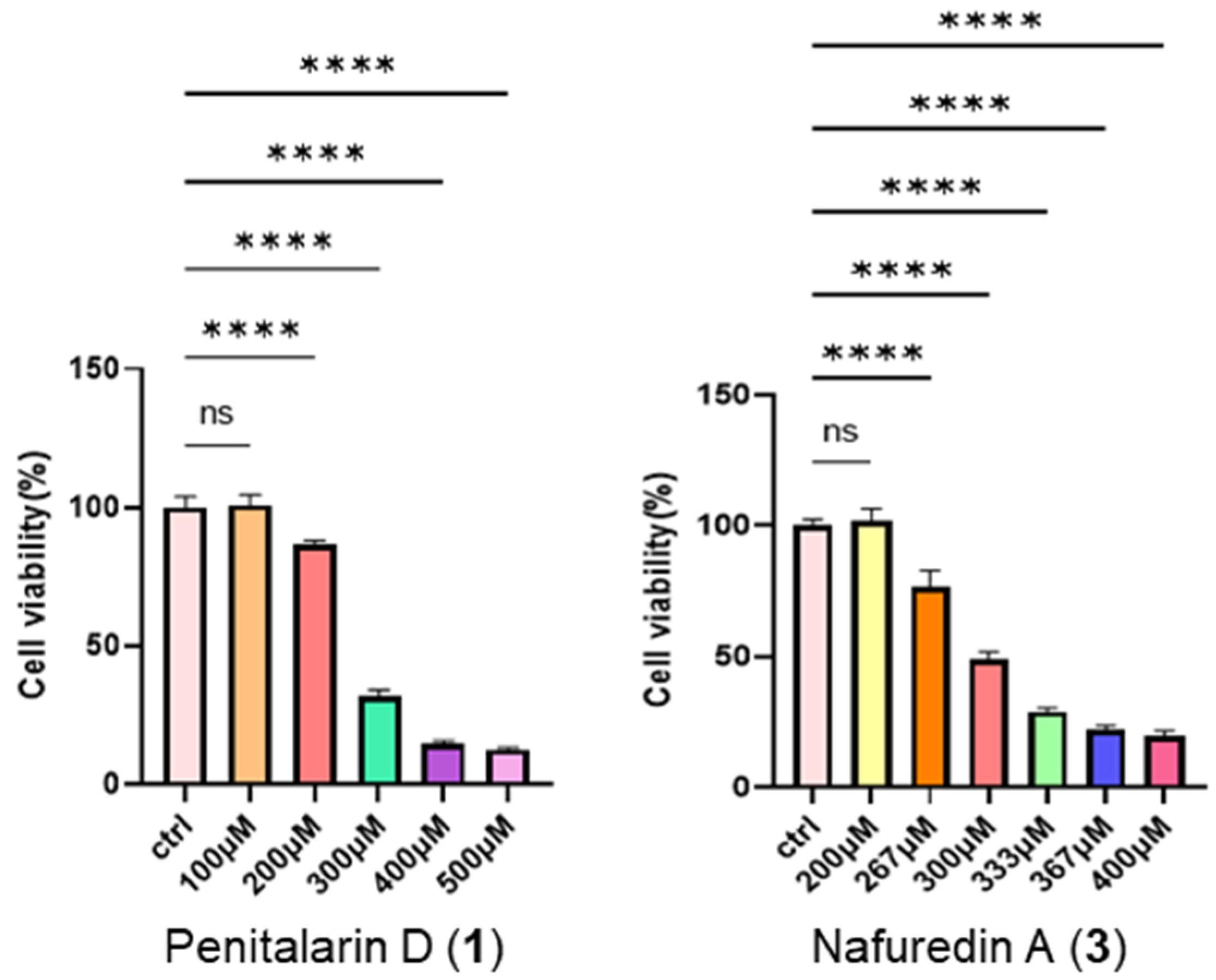


| Pos. | δC Type | δH (J in Hz) |
|---|---|---|
| 1 | 76.7, CH | 4.36, overlapped |
| 2 | 63.7, CH | 3.92, s |
| 3 | 65.3, C | |
| 4 | 82.6, CH | 4.37, d (8.1) |
| 5 | 128.6, CH | 5.65, overlapped |
| 6 | 133.7, CH | 6.25, dd (15.3, 10.5) |
| 7 | 127.3, CH | 6.02, dd (15.3, 10.4) |
| 8 | 141.3, CH | 5.65, overlapped |
| 9 | 34.2, CH | 2.41, m |
| 10 | 46.9, CH2 | 2.06, overlapped 1.94, m |
| 11 | 134.1, C | |
| 12 | 126.4, CH | 5.75, d (10.8) |
| 13 | 124.8, CH | 6.18, dd (15.1, 10.8) |
| 14 | 138.0, CH | 5.43, dd (15.1, 7.8) |
| 15 | 37.9, CH | 2.06, overlapped |
| 16 | 29.3, CH2 | 1.28, m |
| 17 | 11.6, CH3 | 0.81, t (7.4) |
| 18 | 171.7, C | |
| 19 | 13.7, CH3 | 1.32, s |
| 20 | 19.6, CH3 | 0.92, d (6.7) |
| 21 | 16.3, CH3 | 1.67, s |
| 22 | 20.0, CH3 | 0.95, d (6.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, W.; Cai, J.; Yan, L.; Wu, X.; Zhang, L.; Chen, C.; Liu, Z.; Zhou, X.; Tang, L. Discovery of a Hepatoprotective Trinor-Sesterterpenoid from the Marine Fungus Talaromyces sp. Against Hepatic Ischemia-Reperfusion Injury. Mar. Drugs 2025, 23, 329. https://doi.org/10.3390/md23080329
Lan W, Cai J, Yan L, Wu X, Zhang L, Chen C, Liu Z, Zhou X, Tang L. Discovery of a Hepatoprotective Trinor-Sesterterpenoid from the Marine Fungus Talaromyces sp. Against Hepatic Ischemia-Reperfusion Injury. Marine Drugs. 2025; 23(8):329. https://doi.org/10.3390/md23080329
Chicago/Turabian StyleLan, Wenxun, Jian Cai, Liyan Yan, Xinyi Wu, Lisha Zhang, Chunmei Chen, Zhongqiu Liu, Xuefeng Zhou, and Lan Tang. 2025. "Discovery of a Hepatoprotective Trinor-Sesterterpenoid from the Marine Fungus Talaromyces sp. Against Hepatic Ischemia-Reperfusion Injury" Marine Drugs 23, no. 8: 329. https://doi.org/10.3390/md23080329
APA StyleLan, W., Cai, J., Yan, L., Wu, X., Zhang, L., Chen, C., Liu, Z., Zhou, X., & Tang, L. (2025). Discovery of a Hepatoprotective Trinor-Sesterterpenoid from the Marine Fungus Talaromyces sp. Against Hepatic Ischemia-Reperfusion Injury. Marine Drugs, 23(8), 329. https://doi.org/10.3390/md23080329







