Montagnulans A–D with Anti-Osteoclastogenic Activity from the Marine Fungus Montagnula sp. GXIMD 02514
Abstract
1. Introduction
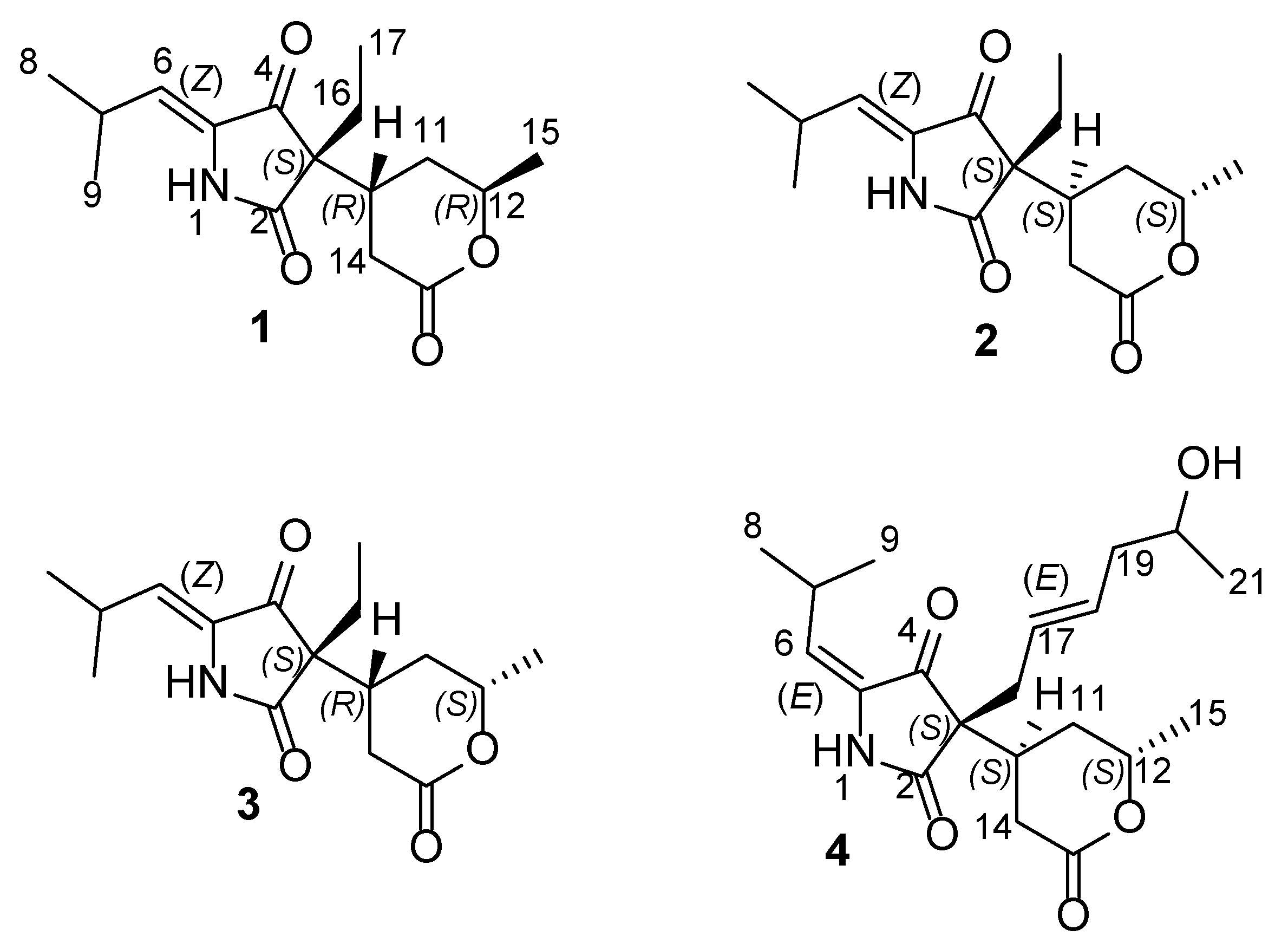
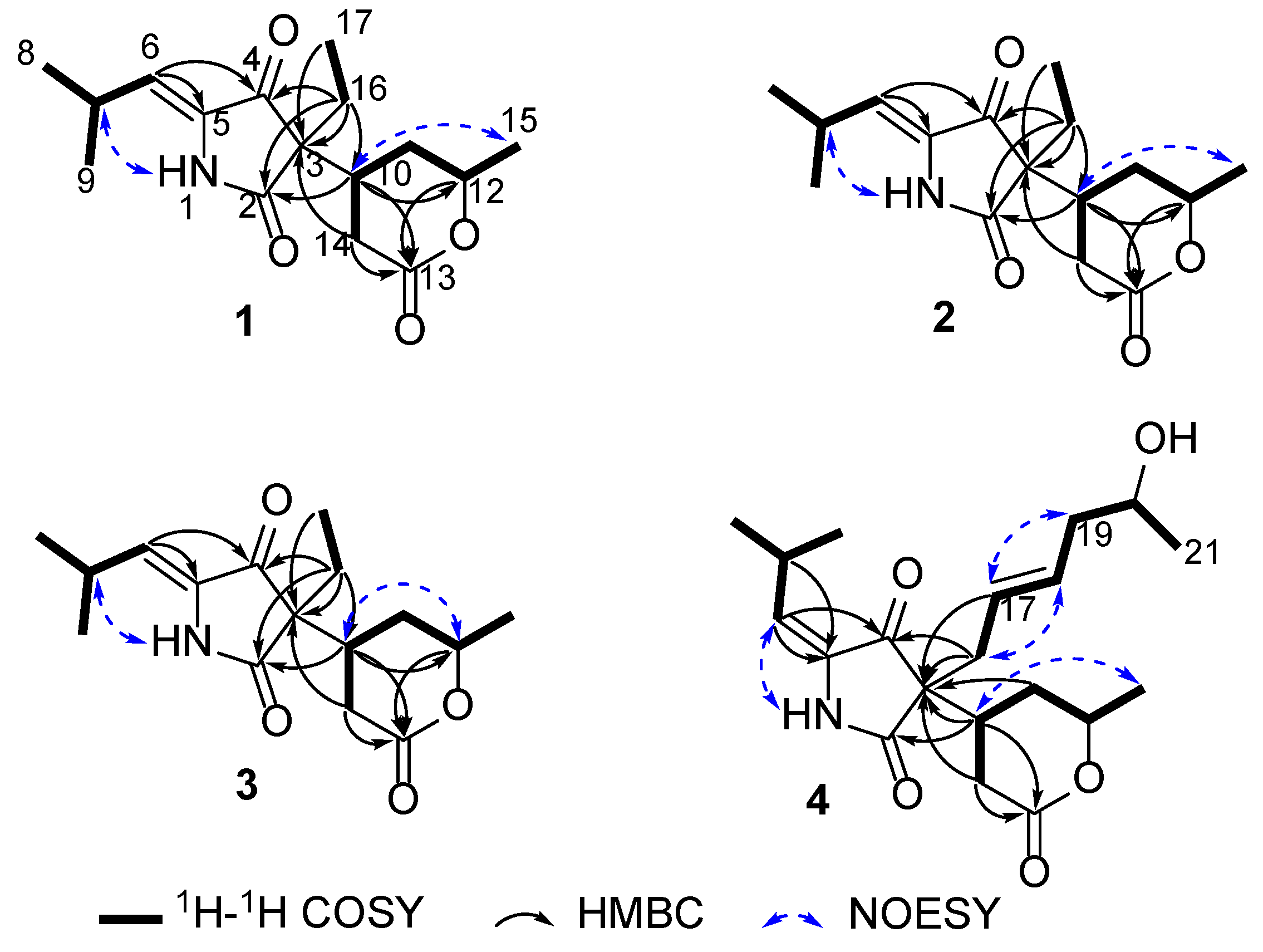
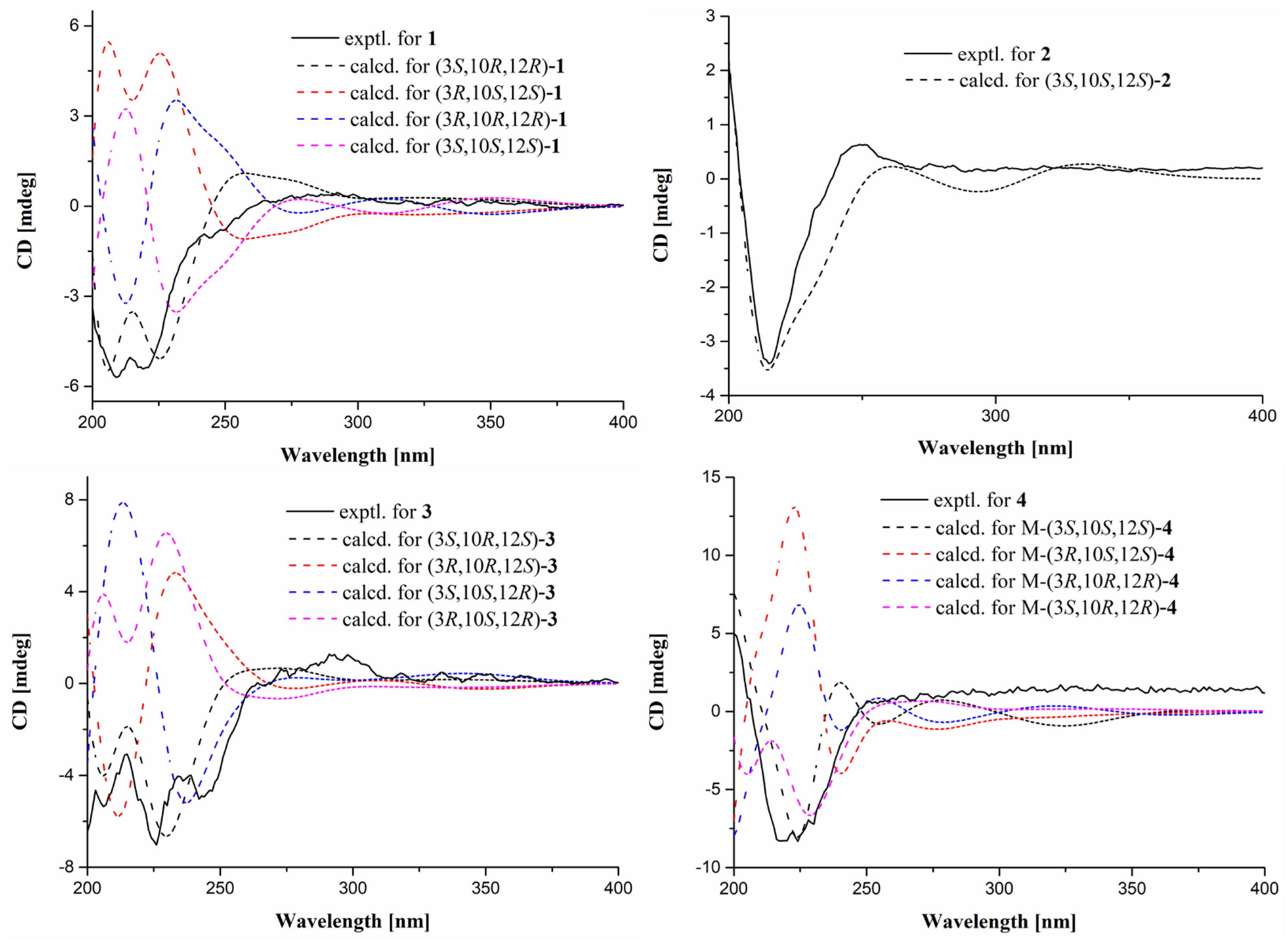
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Strain and Fermentation
3.3. Extraction and Isolation
3.4. ECD Calculations
3.5. Anti-Osteoclastogenic Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qu, Y.R.; Li, S.X.; Luo, H.Y.; Li, J.N.; Wang, T.; Han, X.Z. The crucial role of SPP1 in osteoporosis, osteoarthritis, and cancer. PHSA 2025, 3, 100074. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.H.; Deng, W.D.; Zhang, Y.Y.; Ke, M.H.; Zou, B.H.; Luo, X.W.; Su, J.B.; Wang, Y.Y.; Xu, J.L.; Nandakumar, K.S.; et al. A marine fungus-derived nitrobenzoyl sesquiterpenoid suppresses receptor activator of NF-κB ligand-induced osteoclastogenesis and inflammatory bone destruction. Brit. J. Pharmacol. 2020, 177, 4242–4260. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yamauchi, K.; Mitsunaga, T. A review on osteoclast diseases and osteoclastogenesis inhibitors recently developed from natural resources. Fitoterapia 2020, 142, 104482. [Google Scholar] [CrossRef]
- Jiang, M.H.; Chen, S.H.; Li, J.; Liu, L. The biological and chemical diversity of tetramic acid compounds from marine-derived microorganisms. Mar. Drugs 2020, 18, 114. [Google Scholar] [CrossRef]
- Huang, Z.H.; Nong, X.H.; Liang, X.; Qi, S.H. New tetramic acid derivatives from the deep-sea-derived fungus Cladosporium sp. SCSIO z0025. Tetrahedron 2018, 74, 2620–2626. [Google Scholar] [CrossRef]
- Mo, X.H.; Li, Q.L.; Ju, J.H. Naturally occurring tetramic acid products: Isolation, structure elucidation and biological activity. RSC Adv. 2014, 4, 50566–50593. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, J.W.; Shim, S.H. Biosynthesis, biological activities, and structure-activity relationships of decalin-containing tetramic acid derivatives isolated from fungi. Nat. Prod. Rep. 2024, 41, 1294–1317. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.J.; Liu, Q.; Zhang, J.; Zhang, Q.L.; Yang, M.Y.; Chen, Q.Z.; Qiang, S.; Valverde, B.E.; Chen, S.G. Discovery, herbicidal activity and biosynthesis of a novel natural tetramic acid from Alternaria species. Adv. Sci. 2025, 12, 2416188. [Google Scholar] [CrossRef]
- Xu, X.Y.; Tan, Y.H.; Gao, C.H.; Liu, K.; Tang, Z.Z.; Lu, C.J.; Li, H.Y.; Zhang, X.Y.; Liu, Y.H. New 3-acyl tetramic acid derivatives from the deep-sea-derived fungus Lecanicillium fusisporum. Mar. Drugs 2022, 20, 255. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Li, R.F.; Lin, M.P.; Chen, C.M.; Qi, X.; Zhou, X.F.; Liu, Y.; Tan, Y.H.; Luo, X.W. Antiosteoclastogenic indole alkaloids from the mangrove endophytic fungus Penicillium brefeldianum GXIMD 02511. J. Nat. Prod. 2025, 88, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, M.; Chen, C.M.; Yang, B.; Gao, C.H.; Liu, Y.H.; Luo, X.W.; Tan, Y.H.; Zhou, X.F. Peniditerpenoids A and B: Oxidized indole diterpenoids with osteoclast differentiation inhibitory activity from a mangrove-sediment-derived Penicillium sp. J. Nat. Prod. 2024, 5, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.P.; Tan, Y.H.; Lu, H.M.; Feng, Y.Y.; Li, M.; Gao, C.H.; Liu, Y.H.; Luo, X.W. Azaphilone derivatives with RANKL-induced osteoclastogenesis inhibition from the mangrove endophytic fungus Diaporthe sp. Chin. J. Nat. Med. 2025, 23, 1143–1152. [Google Scholar]
- Chen, C.M.; Xiao, L.X.; Luo, X.W.; Cai, J.; Huang, L.S.; Tao, H.M.; Zhou, X.F.; Tan, Y.H.; Liu, Y.H. Identifying marine-derived tanzawaic acid derivatives as novel inhibitors against osteoclastogenesis and osteoporosis via downregulation of NF-κB and NFATc1 activation. J. Med. Chem. 2024, 67, 2602–2618. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Li, Z.C.; Huang, B.Y.; Liu, K.; Peng, S.; Liu, X.M.; Gao, C.H.; Liu, Y.H.; Tan, Y.H.; Luo, X.W. Anti-osteoclastogenic and antibacterial effects of chlorinated polyketides from the beibu gulf coral-derived fungus Aspergillus unguis GXIMD 02505. Mar. Drugs 2022, 20, 178. [Google Scholar] [CrossRef]
- Luo, X.W.; Cai, G.D.; Guo, Y.F.; Gao, C.H.; Huang, W.F.; Zhang, Z.H.; Lu, H.M.; Liu, K.; Chen, J.H.; Xiong, X.F.; et al. Exploring marine-derived ascochlorins as novel human dihydroorotate dehydrogenase inhibitors for treatment of triple-negative breast cancer. J. Med. Chem. 2021, 64, 13918–13932. [Google Scholar] [CrossRef]
- Luo, X.W.; Lin, X.P.; Tao, H.M.; Wang, J.F.; Li, J.Y.; Yang, B.; Zhou, X.F.; Liu, Y.H. Isochromophilones A–F, cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef]
- Rischer, M.; Lee, S.R.; Eom, H.J.; Park, H.B.; Vollmers, J.; Kaster, A.K.; Shin, Y.H.; Oh, D.C.; Kim, K.H.; Beemelmanns, C. Spirocyclic cladosporicin A and cladosporiumins I and J from a Hydractinia-associated Cladosporium sphaerospermum SW67. Org. Chem. Front. 2019, 6, 1084–1093. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, C.Y.; Yang, Y.H.; Li, C.; Pei, Y.H. A new tetracyclic triterpenoid from endophytic fungus Fusarium sporotrichioides. Chin. Herb. Med. 2024, 16, 231–234. [Google Scholar] [CrossRef]
- Xiao, D.L.; Li, X.B.; Zhang, X.M.; Jiang, N.; Luo, S.D.Z.; Feng, W.X.; Lu, X.; Feng, B.M. Two new polyketides from Rhodiola tibetica endophytic fungus Penicillium sp. HJT-A-6. Chin. Herb. Med. 2025, 17, 404–408. [Google Scholar] [CrossRef]

| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 8.66, s | 9.56, s | 8.51, s | 8.50, s |
| 6 | 5.61, d (10.0) | 5.60, d (10.0) | 5.62, d (10.0) | 5.31, d (10.2) |
| 7 | 2.45, m | 2.52, m | 2.45, m | 3.56, m |
| 8 | 1.12, d (7.5) | 1.09, d (7.5) | 1.11, d (7.5) | 1.04, d (7.0) |
| 9 | 1.10, d (7.5) | 1.09, d (7.5) | 1.11, d (7.5) | 1.02, d (7.0) |
| 10 | 2.55, m | 2.54, m | 2.43, m | 2.54, m |
| 11 | 1.98, ddd (14.0, 6.5, 4.5) 1.75, dt (14.0, 8.5) | 1.94, ddd (14.0, 7.0, 4.5) 1.69, dt (14.0, 8.0) | 1.78, q (7.5) 1.50, q (12.5) | 1.68, dt (14.4, 8.5) 1.84, ddd (14.4, 6.8, 4.3) |
| 12 | 4.46, m | 4.46, m | 4.34, m | 4.47, m |
| 14 | 2.62, m 2.52, m | 2.68, dd (15.5, 12.5) 2.61, dd (15.5, 10.0) | 2.70, m | 2.62, dd (15.6, 5.5) 2.80, dd (15.6, 12.5) |
| 15 | 1.34, d (6.0) | 1.34, d (7.5) | 1.36, d (6.0) | 1.34, d (6.3) |
| 16 | 1.84, q (7.5) | 1.83, q (7.5) | 1.84, q (7.5) | 2.48, m |
| 17 | 0.84, t (7.5) | 0.83, t (7.5) | 0.84, t (7.5) | 5.32, m |
| 18 | 5.55, m | |||
| 19 | 2.16, dt (14.0, 5.0) 2.02, dt (14.0, 8.0) | |||
| 20 | 3.76, m | |||
| 21 | 1.14, (6.2) |
| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 2 | 174.7, C | 175.6, C | 174.5, C | 173.5, C |
| 3 | 57.1, C | 56.9, C | 56.9, C | 57.9, C |
| 4 | 200.0, C | 200.0, C | 199.9, C | 200.4, C |
| 5 | 132.0, C | 132.3, C | 132.1, C | 130.8, C |
| 6 | 119.1, CH | 119.7, CH | 119.0, CH | 126.5, CH |
| 7 | 27.1, CH | 27.0, CH | 27.8, CH | 25.6, CH |
| 8 | 22.2, CH3 | 22.2, CH3 | 22.2, CH3 | 23.2, CH3 |
| 9 | 22.2, CH3 | 22.2, CH3 | 22.2, CH3 | 23.1, CH3 |
| 10 | 33.2, CH | 32.9, CH | 36.4, CH | 32.6, CH |
| 11 | 30.3, CH2 | 30.2, CH2 | 31.9, CH2 | 30.5, CH2 |
| 12 | 73.7, CH | 73.8, CH | 76.6, CH | 73.6, CH |
| 13 | 171.7, C | 171.9, C | 170.0, C | 171.9, C |
| 14 | 30.5, CH2 | 30.5, CH2 | 30.8, CH2 | 30.3, CH2 |
| 15 | 20.8, CH3 | 20.9, CH3 | 21.9, CH3 | 20.9, CH3 |
| 16 | 26.0, CH2 | 25.9, CH2 | 25.5, CH2 | 36.0, CH2 |
| 17 | 8.7, CH3 | 8.7, CH3 | 8.7, CH3 | 125.2, CH |
| 18 | 133.2, CH | |||
| 19 | 42.5, CH2 | |||
| 20 | 67.0, CH | |||
| 21 | 22.8, CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Lu, H.; Wang, J.; Qin, H.; Xu, X.; Gao, C.; Liu, Y.; Tan, Y.; Luo, X. Montagnulans A–D with Anti-Osteoclastogenic Activity from the Marine Fungus Montagnula sp. GXIMD 02514. Mar. Drugs 2025, 23, 416. https://doi.org/10.3390/md23110416
Lin M, Lu H, Wang J, Qin H, Xu X, Gao C, Liu Y, Tan Y, Luo X. Montagnulans A–D with Anti-Osteoclastogenic Activity from the Marine Fungus Montagnula sp. GXIMD 02514. Marine Drugs. 2025; 23(11):416. https://doi.org/10.3390/md23110416
Chicago/Turabian StyleLin, Miaoping, Humu Lu, Jiaxi Wang, Huangxue Qin, Xinya Xu, Chenghai Gao, Yonghong Liu, Yanhui Tan, and Xiaowei Luo. 2025. "Montagnulans A–D with Anti-Osteoclastogenic Activity from the Marine Fungus Montagnula sp. GXIMD 02514" Marine Drugs 23, no. 11: 416. https://doi.org/10.3390/md23110416
APA StyleLin, M., Lu, H., Wang, J., Qin, H., Xu, X., Gao, C., Liu, Y., Tan, Y., & Luo, X. (2025). Montagnulans A–D with Anti-Osteoclastogenic Activity from the Marine Fungus Montagnula sp. GXIMD 02514. Marine Drugs, 23(11), 416. https://doi.org/10.3390/md23110416








