Hybrid Alginate–Graphene Composites: Biochemical Features and Biomedical Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of Graphene Oxide–Calcium Alginate Composites and Their Structural Characterisation
2.2. Chemical and Structural Characterisation
2.2.1. Measurement of Calcium Concentration
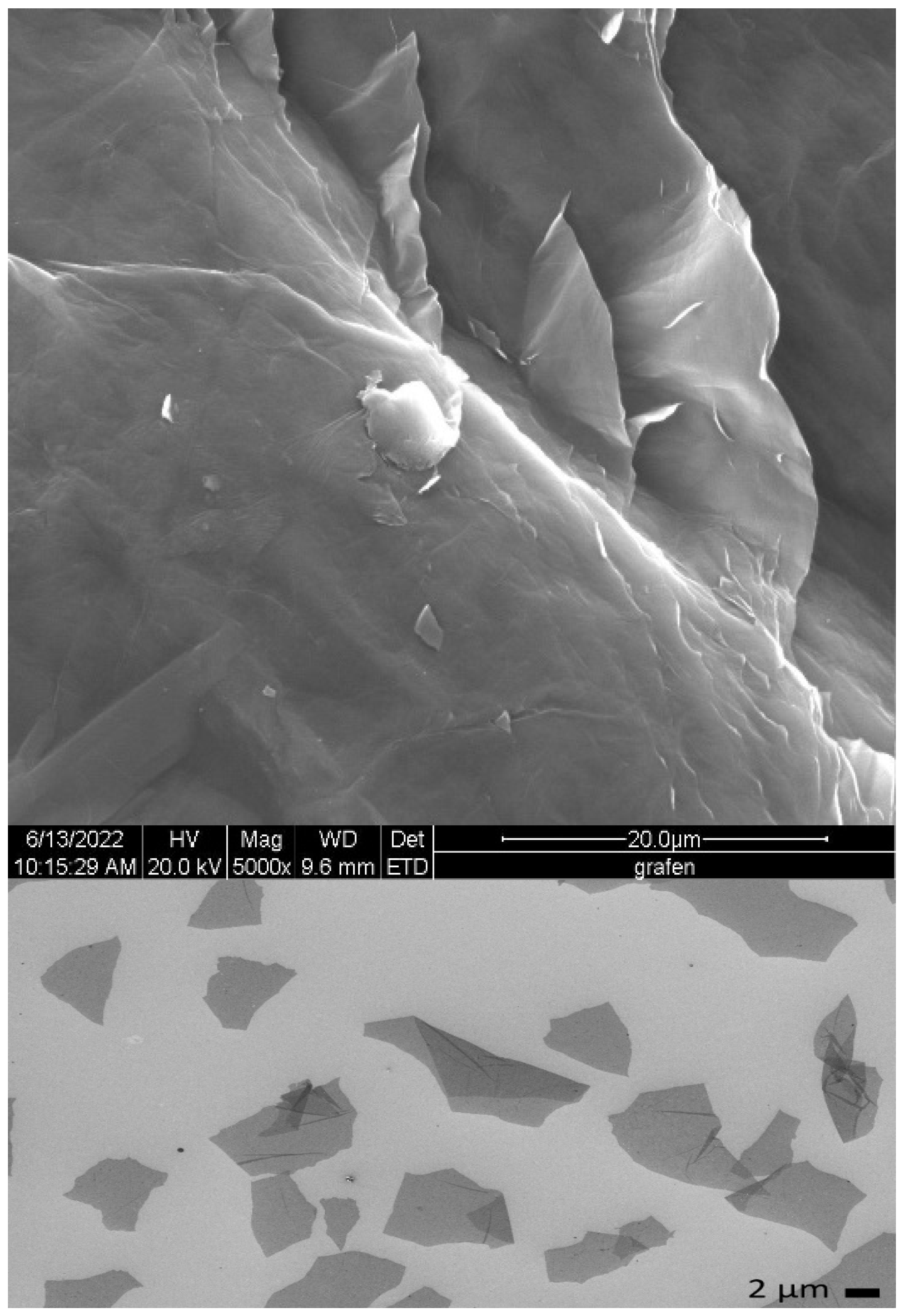
2.2.2. Morphology and Elemental Analysis
2.2.3. Chemical Structure Analysis (FTIR)
2.2.4. Specific Surface Area and Total Pore Volume Analysis
2.3. Biological and Biochemical Properties
2.3.1. Blood Plasma Clotting: aPTT and PT
2.3.2. Effect of Alginate–GO Foams on the Viability of PBM and Hs68 Cells
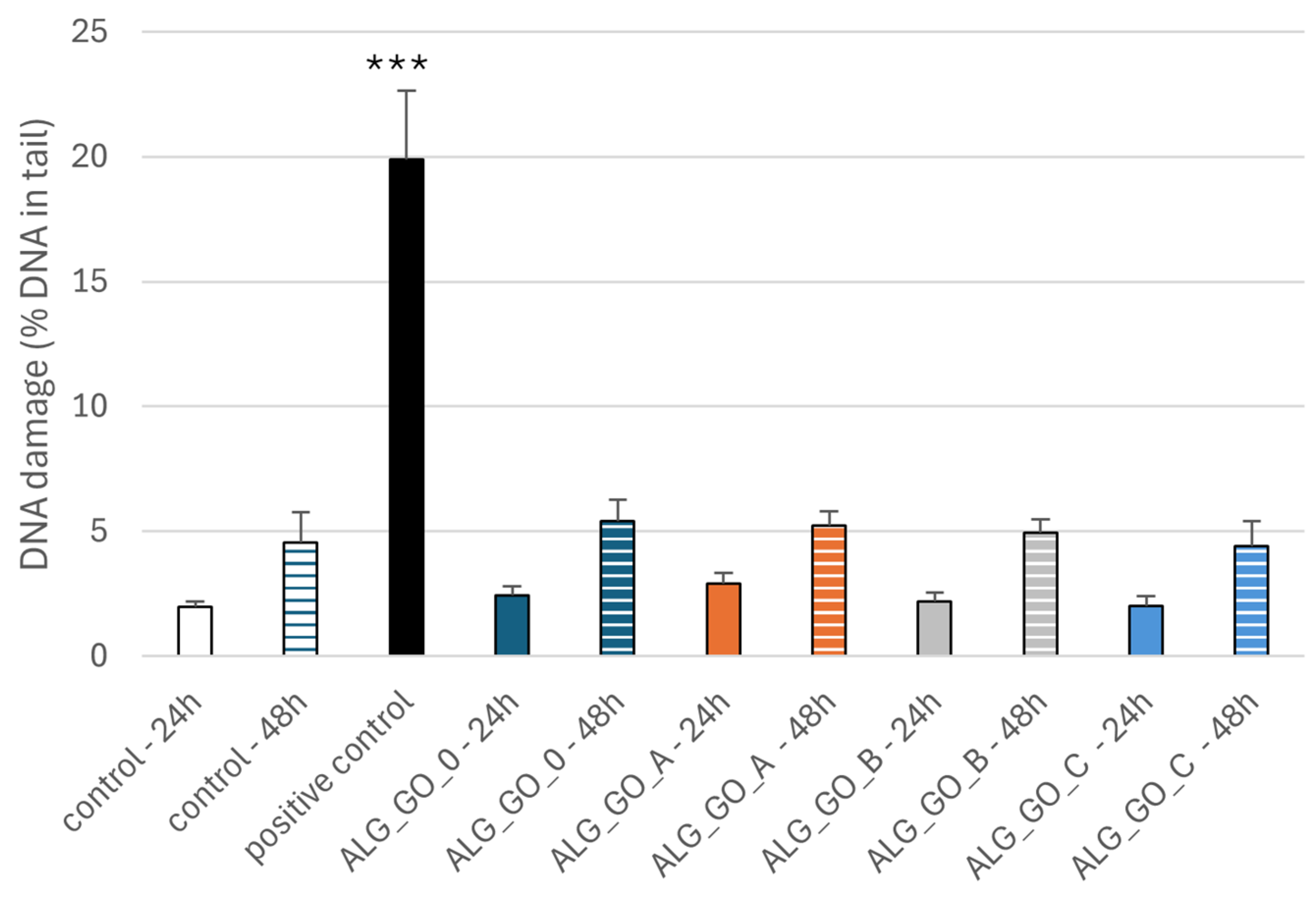
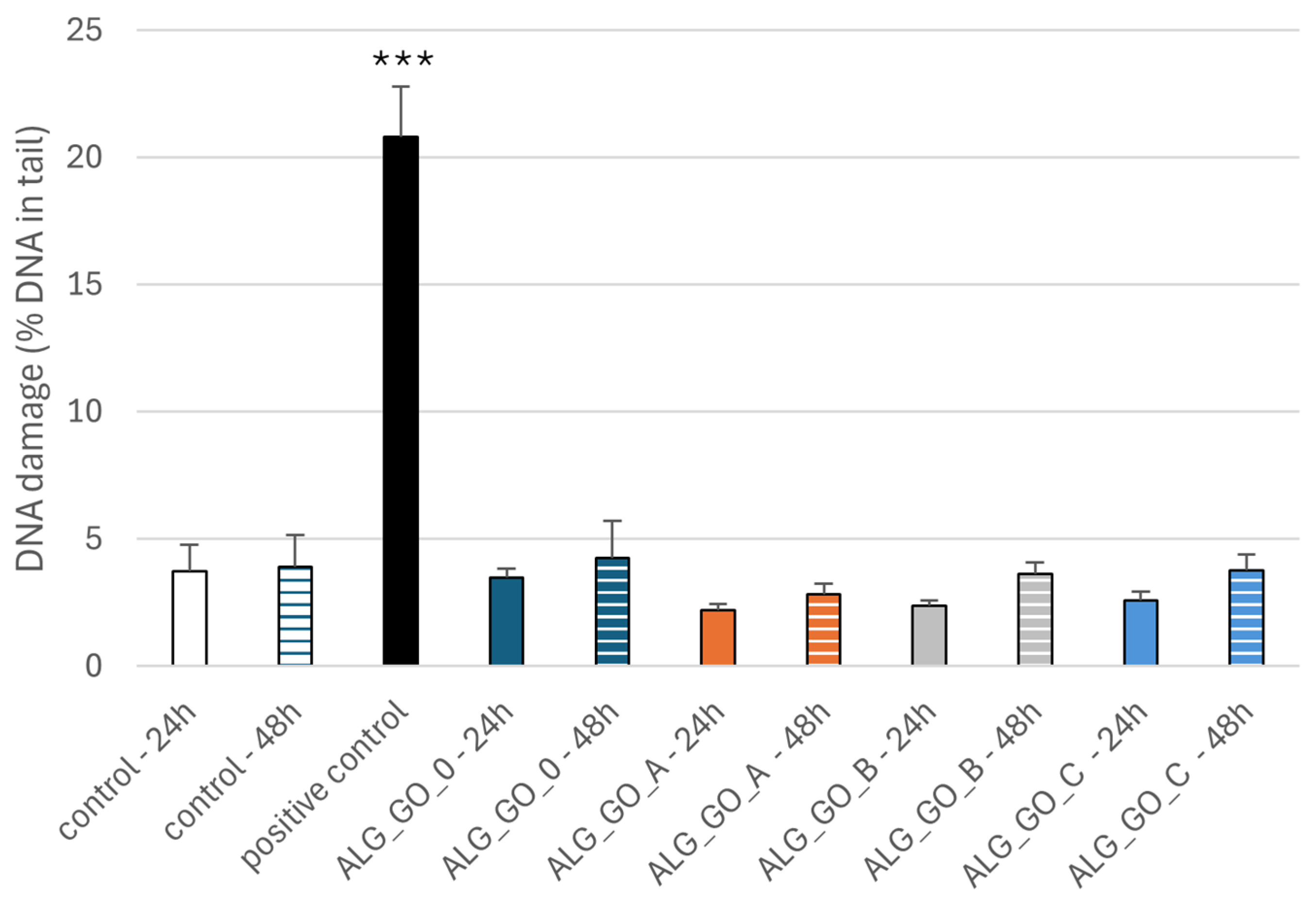
2.3.3. Effect of Alginate–GO Foams on DNA Damage in PBM Cells and Hs68 Cells
3. Materials and Methods
3.1. Preparation of the Composite Material
3.1.1. Materials
- In this study, alginic acid sodium salt from brown algae (sodium alginate) (Merck, Darmstadt, Germany) with viscosity 5.0–40.0 cps for c = 1% in water, 25 °C, was used as a base polymer to prepare porous polymer matrices (foams).
- Calcium chloride anhydrous (Merck, Darmstadt, Germany) was used as a cross-linking agent with 3 different concentrations: 0.5, 1, and 2%.
- Standard human blood plasma lyophilisates (Dia-CONT I), aPTT reagent (Dia-PTT), PT reagent (Dia-PT), TT reagent (Dia-TT), 0.025 M CaCl2 solution reagent from Dia-PTT (Diagon Kft, Budapest, Hungary), and a coagulometer (K-3002 OPTIC, KSELMED®, Grudziądz, Poland) were used for the aPTT and PT measurements according to the manufacturer’s instructions.
3.1.2. Solutions
- Sodium Alginate 2% wt. % Solution: Sodium alginate (2 G) was added in portions to water (98 G) during slow stirring. Afterwards, the mixture was stirred vigorously and simultaneously heated to a temperature of about 40 °C for about 4–6 h, using a mechanical stirrer.
- Sodium Alginate–Graphene Oxide Solution: GO flake dispersion solution was diluted with water and homogenised using an ultrasonic probe for up to 20 min. Then, an ALG Na solution (2% wt. %) was added in portions, stirring slowly. Afterwards, the solution was stirred vigorously and simultaneously heated to a temperature of about 40 °C for about 4–6 h, using a mechanical stirrer.
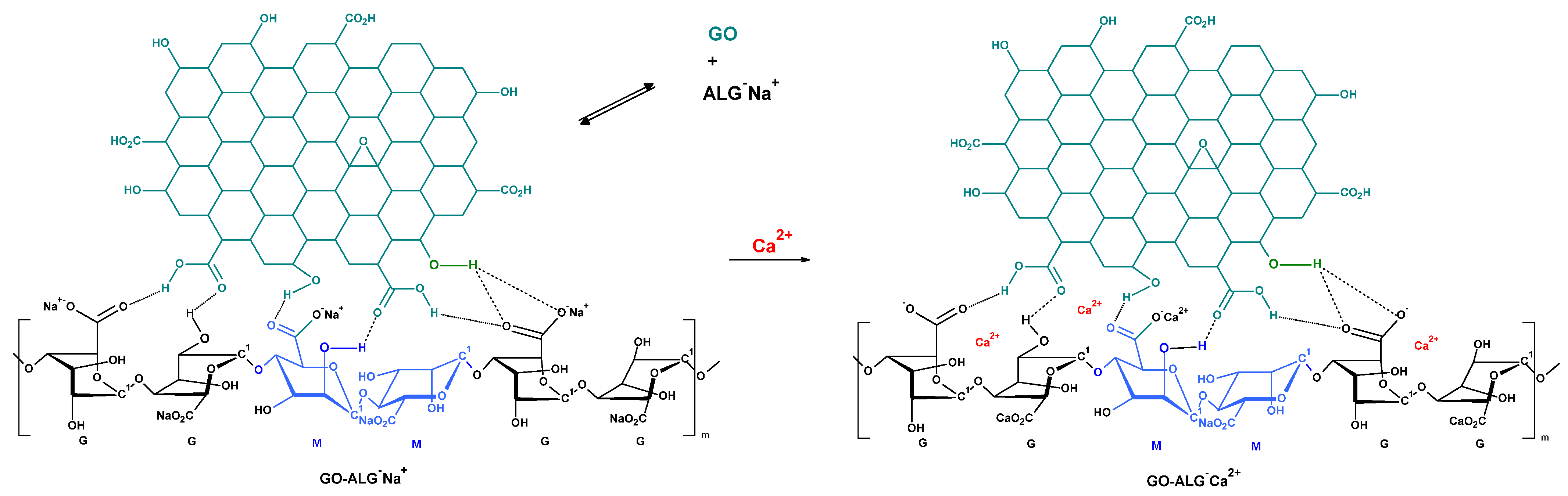
- Calcium Alginate and Graphene Oxide–Calcium Alginate: A highly porous sodium alginate foam was prepared by the freeze-drying technique [89], based on a sodium alginate polymer solution in water (2%). As modifiers, for bulk modification as well as for polymer cross-linking agents, such as G-flakes (24.5 wt. %) (Figure 17) and calcium chloride were used. GO was added to the polymer solutions and stirred vigorously for 5 h. The foams obtained by the method in [89] were then cross-linked using an aqueous solution of calcium chloride. The cross-linked foam samples were rinsed in distilled water until the chlorides were washed out, and then subjected to freezing and freeze-drying.
3.2. Chemical and Structural Characterisation
3.2.1. Inductively Coupled Plasma Mass Spectrometry Method (ICP-MS)
3.2.2. Morphology and Elemental Analysis
3.2.3. Chemical Structure Analysis (FTIR)
3.2.4. Specific Surface Area and Total Pore Volume Analysis
3.3. Biological and Biochemical Properties
3.3.1. Blood Plasma Clotting: aPTT and PT
3.3.2. Preparation of Composites for Assessment of Biological Properties
3.3.3. Cell Culture
3.3.4. Cell Viability Resazurin Assay
3.3.5. DNA Damage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kauvar, D.S.; Lefering, R.; Wade, C.E. Impact of Hemorrhage on Trauma Outcome: An Overview of Epidemiology, Clinical Presentations, and Therapeutic Considerations. J. Trauma-Inj. Infect. Crit. Care 2006, 60, S3–S11. [Google Scholar] [CrossRef]
- Shah, A.; Palmer, A.J.R.; Klein, A.A. Strategies to Minimize Intraoperative Blood Loss during Major Surgery. Br. J. Surg. 2020, 107, e26–e38. [Google Scholar] [CrossRef]
- Malik, A.; Rehman, F.U.; Shah, K.U.; Naz, S.S.; Qaisar, S. Hemostatic Strategies for Uncontrolled Bleeding: A Comprehensive Update. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Lee, D.R.; Chung, D.J. Advances in the Development of Hemostatic Biomaterials for Medical Application. Biomater. Res. 2021, 25, 37. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, J.; Xu, T.; Yao, H.; Yu, L.; Huang, D. Hemostasis Strategies and Recent Advances in Nanomaterials for Hemostasis. Molecules 2023, 28, 5264. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Shander, A.; Ceresoli, M.; Moore, E.; Tian, B.; Parini, D.; Sartelli, M.; Sakakushev, B.; Doklestich, K.; Abu-Zidan, F.; et al. Strategies to Prevent Blood Loss and Reduce Transfusion in Emergency General Surgery, WSES-AAST Consensus Paper. World J. Emerg. Surg. 2024, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, M.; Liu, Q.; Liu, G.; Wang, S.; Li, J. Recent Advances in the Medical Applications of Hemostatic Materials. Theranostics 2023, 13, 161–196. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, C.; Singh, P.; Mukhopadhyay, S.; Gupta, A.; Gupta, B. Alginate Based Biomaterials for Hemostatic Applications: Innovations and Developments. Int. J. Biol. Macromol. 2024, 264, 130771. [Google Scholar] [CrossRef]
- Gacesa, P. Alginates. Carbohydr. Polym. 1988, 8, 161–182. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, Physical and Biological Properties of Alginates and Their Biomedical Implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, Properties and Application of Alginic Acid: A Review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- SC1. Available online: https://www-1scopus-1com-10000145y03aa.han.p.lodz.pl/results/results.uri?sid=4ed83aab7077f9b156f7eba71e34db0d&src=s&sot=b&sdt=b&origin=searchbasic&rr=&sl=41&s=TITLE-ABS-KEY(alginate for drug delivery)&searchterm1=alginate for drug delivery&searchTerms=&co (accessed on 29 July 2025).
- SC2. Available online: https://www-1scopus-1com-10000145y0316.han.p.lodz.pl/results/results.uri?sid=33b3b9f2600cb7bbc5975c67d12053da&src=s&sot=b&sdt=b&origin=searchbasic&rr=&sl=43&s=TITLE-ABS-KEY(alginate for wound dressings)&searchterm1=alginate for wound dressings&searchTerms (accessed on 29 July 2025).
- SC3. Available online: https://www-1scopus-1com-10000145y0303.han.p.lodz.pl/results/results.uri?sid= dbef89d705f695750247b64d1f980f7b&src=s&sot=b&sdt=b&origin=searchbasic&rr=&sl=46&s=TITLE-ABS-KEY(alginate for tissue engineering)&searchterm1= alginate for tissue engineering&s (accessed on 29 July 2025).
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Brisebois, P.P.; Siaj, M. Harvesting Graphene Oxide–Years 1859 to 2019: A Review of Its Structure, Synthesis, Properties and Exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- Donato, K.Z.; Tan, H.L.; Marangoni, V.S.; Martins, M.V.S.; Ng, P.R.; Costa, M.C.F.; Jain, P.; Lee, S.J.; Koon, G.K.W.; Donato, R.K.; et al. Graphene Oxide Classification and Standardization. Sci. Rep. 2023, 13, 6064. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the Functional Modification of Graphene/Graphene Oxide: A Review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. Biomed Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Graphene Oxide Synthesis, Properties and Characterization Techniques: A Comprehensive Review. ChemEngineering 2021, 5, 64. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef]
- Islam, M.S.; Mitra, S. Development of Nano Structured Graphene Oxide Incorporated Dexamethasone with Enhanced Dissolution. Colloid Interface Sci. Commun. 2022, 47, 100599. [Google Scholar] [CrossRef]
- SAGO. Available online: https://www.sigmaaldrich.com/PL/pl/product/aldrich/794341?utm_source=google&utm_medium=cpc&utm_campaign=21827198313&utm_content=167888511303&gad_source=1&gad_campaignid=21827198313&gbraid=0AAAAAD8kLQSQLl1b4A_yQmI_KEDQB6piK&gclid=CjwKCAjw1ozEBhAdEiwAn9qbzT (accessed on 28 July 2025).
- Wenjing, A.; Du, F.; He, Y.; Wu, B.; Liu, F.; Liu, Y.; Zheng, W.; Li, G.; Wang, X. Graphene Oxide Reinforced Hemostasis of Gelatin Sponge in Noncompressible Hemorrhage via Synergistic Effects. Colloids Surf. B Biointerfaces 2022, 220, 112891. [Google Scholar] [CrossRef]
- Wu, B.; Du, F.; A, W.; Li, G.; Wang, X. Graphene-Based Hemostatic Sponge. Chin. Chem. Lett. 2022, 33, 703–713. [Google Scholar] [CrossRef]
- Purohit, S.D.; Bhaskar, R.; Singh, H.; Yadav, I.; Gupta, M.K.; Mishra, N.C. Development of a Nanocomposite Scaffold of Gelatin–Alginate–Graphene Oxide for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 133, 592–602. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Chen, S.; Huang, Y.; He, J. Adsorption of Lysozyme by Alginate/Graphene Oxide Composite Beads with Enhanced Stability and Mechanical Property. Mater. Sci. Eng. C 2018, 89, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liang, Y.; Xu, C.; Sun, H.; Tao, L.; Wei, Y.; Wang, X. Polydopamine Reinforced Hemostasis of a Graphene Oxide Sponge via Enhanced Platelet Stimulation. Colloids Surf. B Biointerfaces 2019, 174, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Peng, L.; Yuan, C.; Zhao, C.; Tang, W.; Ma, C.; Shen, J.; Yang, W.; Yu, Y.; Min, Y.; et al. Enhanced Properties of Poly(Vinyl Alcohol) Composite Films with Functionalized Graphene. RSC Adv. 2015, 5, 97738–97745. [Google Scholar] [CrossRef]
- Guajardo, S.; Figueroa, T.; Borges, J.; Meléndrez, M.; Fernández, K. Comparative Study of Graphene Oxide-Gelatin Aerogel Synthesis: Chemical Characterization, Morphologies and Functional Properties. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1517–1526. [Google Scholar] [CrossRef]
- Quan, K.; Li, G.; Luan, D.; Yuan, Q.; Tao, L.; Wang, X. Black Hemostatic Sponge Based on Facile Prepared Cross-Linked Graphene. Colloids Surf. B Biointerfaces 2015, 132, 27–33. [Google Scholar] [CrossRef]
- Feng, R.; Yu, Y.; Shen, C.; Jiao, Y.; Zhou, C. Impact of Graphene Oxide on the Structure and Function of Important Multiple Blood Components by a Dose-Dependent Pattern. J. Biomed. Mater. Res.-Part A 2015, 103, 2006–2014. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, M.K.; Nayak, M.K.; Kumari, S.; Shrivastava, S.; Grácio, J.J.A.; Dash, D. Thrombus Inducing Property of Atomically Thin Graphene Oxide Sheets. ACS Nano 2011, 5, 4987–4996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, J.; Wu, J.; Ding, S.; Yang, J.; Zhang, J.; Dong, A.; Deng, L. N-Alkylated Chitosan/Graphene Oxide Porous Sponge for Rapid and Effective Hemostasis in Emergency Situations. Carbohydr. Polym. 2019, 219, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Iskandar, L.; Deb, S. Green Synthetic Routes to Alginate-Graphene Oxide Composite Hydrogels with Enhanced Physical Properties for Bioengineering Applications. Eur. Polym. J. 2018, 103, 198–206. [Google Scholar] [CrossRef]
- Baheiraei, N.; Razavi, M.; Ghahremanzadeh, R. Reduced Graphene Oxide Coated Alginate Scaffolds: Potential for Cardiac Patch Application. Biomater. Res. 2023, 27, 109. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, T.; Jiang, W.; Wang, X.; Ge, R. The Biomass-Derived Graphene-Based Composite Alginate Hydrogel Scaffold for Bone Defect Regeneration. Mater. Today Commun. 2025. [Google Scholar] [CrossRef]
- Frígols, B.; Martí, M.; Salesa, B.; Hernández-Oliver, C.; Aarstad, O.; Ulset, A.S.T.; Sætrom, G.I.; Aachmann, F.L.; Serrano-Aroca, Á. Graphene Oxide in Zinc Alginate Films: Antibacterial Activity, Cytotoxicity, Zinc Release, Water Sorption/Diffusion, Wettability and Opacity. PLoS ONE 2019, 14, e0212819. [Google Scholar] [CrossRef]
- Zin, F.A.M.; Noor, A.A.M.; Nasri, W.N.W.M.; Roslan, N.N.; Buniamin, N.A.; Razab, M.K.A.A.; Rasa, M.S.M.; Abdullah, N.H.; Amin, M.F.M.; Wei, L.S.; et al. Synthesis of Sodium Alginate Graphene Oxide-Silver Film for Antibacterial Activity. IOP Conf. Ser. Earth Environ. Sci. 2020, 596, 012041. [Google Scholar] [CrossRef]
- Kolanthai, E.; Sindu, P.A.; Khajuria, D.K.; Veerla, S.C.; Kuppuswamy, D.; Catalani, L.H.; Mahapatra, D.R. Graphene Oxide—A Tool for the Preparation of Chemically Crosslinking Free Alginate-Chitosan-Collagen Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2018, 10, 12441–12452. [Google Scholar] [CrossRef]
- Madaninasab, P.; Mohammadi, M.; Labbaf, S. Electroconductive Gelatin/Alginate/Graphene Hydrogel Based Scaffold for Neural Tissue Repair. Macromol. Mater. Eng. 2025, 310, 2400229. [Google Scholar] [CrossRef]
- Martí, M.; Frígols, B.; Salesa, B.; Serrano-Aroca, Á. Calcium Alginate/Graphene Oxide Films: Reinforced Composites Able to Prevent Staphylococcus Aureus and Methicillin-Resistant Staphylococcus Epidermidis Infections with No Cytotoxicity for Human Keratinocyte HaCaT Cells. Eur. Polym. J. 2019, 110, 14–21. [Google Scholar] [CrossRef]
- McNamara, M.C.; Niaraki-Asli, A.E.; Guo, J.; Okuzono, J.; Montazami, R.; Hashemi, N.N. Enhancing the Conductivity of Cell-Laden Alginate Microfibers with Aqueous Graphene for Neural Applications. Front. Mater. 2020, 7, 500340. [Google Scholar] [CrossRef]
- Mormile, C.; Opriș, O.; Bellucci, S.; Lung, I.; Kacso, I.; Turza, A.; Stegarescu, A.; Tripon, S.; Soran, M.L.; Bâldea, I. Natrium Alginate and Graphene Nanoplatelets-Based Efficient Material for Resveratrol Delivery. Gels 2024, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Luo, H.; Zuo, G.; Ren, K.; Wan, Y. Novel Porous Graphene Oxide and Hydroxyapatite Nanosheets-Reinforced Sodium Alginate Hybrid Nanocomposites for Medical Applications. Mater. Charact. 2015, 107, 419–425. [Google Scholar] [CrossRef]
- Ionita, M.; Pandele, M.A.; Iovu, H. Sodium Alginate/Graphene Oxide Composite Films with Enhanced Thermal and Mechanical Properties. Carbohydr. Polym. 2013, 94, 339–344. [Google Scholar] [CrossRef]
- Sabater i Serra, R.; Molina-Mateo, J.; Torregrosa-Cabanilles, C.; Andrio-Balado, A.; Dueñas, J.M.M.; Serrano-Aroca, Á. Bio-Nanocomposite Hydrogel Based on Zinc Alginate/Graphene Oxide: Morphology, Structural Conformation, Thermal Behavior/Degradation, and Dielectric Properties. Polymers 2020, 12, 702. [Google Scholar] [CrossRef] [PubMed]
- Mahmudunnabi, D.M.; Alam, M.Z.; Choudhury, T.R.; Nurnabi, M. Preparation of Calcium Alginate-Graphene Oxide Composite and Its Application for the Removal of Maxilon Blue. Desalination Water Treat. 2021, 242, 293–303. [Google Scholar] [CrossRef]
- Świerczyńska, M.; Król, P.; Hernández Vázquez, C.I.; Piekarska, K.; Woźniak, K.; Juszczak, M.; Mrozińska, Z.; Kudzin, M.H. Blood Coagulation Activities and Influence on DNA Condition of Alginate—Calcium Composites Prepared by Freeze-Drying Technique. Mar. Drugs 2024, 22, 415. [Google Scholar] [CrossRef]
- Rostami-Tapeh-esmaeil, E.; Vahidifar, A.; Esmizadeh, E.; Rodrigue, D. Chemistry, Processing, Properties, and Applications of Rubber Foams. Polymers 2021, 13, 1565. [Google Scholar] [CrossRef]
- Lee, L.J.; Zeng, C.; Cao, X.; Han, X.; Shen, J.; Xu, G. Polymer Nanocomposite Foams. Compos. Sci. Technol. 2005, 65, 2344–2363. [Google Scholar] [CrossRef]
- Caroline, C.; Raya, B.; Daniel, C.; Benjamin, D.; Christophe, T.; Philippe, B.; Angelo, P.; Brigitte, S.; Sophie, G.F. Elaboration and Evaluation of Alginate Foam Scaffolds for Soft Tissue Engineering. Int. J. Pharm. 2017, 524, 433–442. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2009, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Belattmania, Z.; Kaidi, S.; Atouani, S.; El Katif, C.; Bentiss, F.; Jama, C.; Reani, A.; Sabour, B.; Vasconcelos, V. Isolation and FTIR-ATR and 1H NMR Characterization of Alginates from the Main Alginophyte Species of the Atlantic Coast of Morocco. Molecules 2020, 25, 4335. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, A. FTIR Spectra of Graphene Oxide; Instituto Nacional de Pesquisas Espaciais: São José dos Campos, São Paulo, Brazil, 2019; Version 1. [Google Scholar] [CrossRef]
- Pragya, A.; Mutalik, S.; Younas, M.W.; Pang, S.K.; So, P.K.; Wang, F.; Zheng, Z.; Noor, N. Dynamic Cross-Linking of an Alginate–Acrylamide Tough Hydrogel System: Time-Resolved in Situ Mapping of Gel Self-Assembly. RSC Adv. 2021, 11, 10710–10726. [Google Scholar] [CrossRef] [PubMed]
- Pettignano, A.; Tanchoux, N.; Cacciaguerra, T.; Vincent, T.; Bernardi, L.; Guibal, E.; Quignard, F. Sodium and Acidic Alginate Foams with Hierarchical Porosity: Preparation, Characterization and Efficiency as a Dye Adsorbent. Carbohydr. Polym. 2017, 178, 78–85. [Google Scholar] [CrossRef]
- Bahrami, N.; Farzin, A.; Bayat, F.; Goodarzi, A.; Salehi, M.; Karimi, R.; Mohamadnia, A.; Parhiz, A.; Ai, J.; Bahrami, N.; et al. Optimization of 3D Alginate Scaffold Properties with Interconnected Porosity Using Freeze-Drying Method for Cartilage Tissue Engineering Application. Arch. Neurosci. 2019, 6, 85122. [Google Scholar] [CrossRef]
- Rassis, D.K.; Saguy, I.S.; Nussinovitch, A. Collapse, Shrinkage and Structural Changes in Dried Alginate Gels Containing Fillers. Food Hydrocoll. 2002, 16, 139–151. [Google Scholar] [CrossRef]
- Airaksinen, S. Role of Excipients in Moisture Sorption and Physical Stability of Solid Pharmaceutical Formulations. Academic Dissertation, University of Helsinky, Helsinki, Finland, October 2005. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore Characterization of Different Types of Coal from Coal and Gas Outburst Disaster Sites Using Low Temperature Nitrogen Adsorption Approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Xie, F.; Zou, L.; Xu, Z.; Ou, X.; Guo, W.; Gao, Y.; Gao, G. Alginate Foam Gel Modified by Graphene Oxide for Wound Dressing. Int. J. Biol. Macromol. 2022, 223, 391–403. [Google Scholar] [CrossRef]
- Chaudhry, R.; Killeen, R.B.; Babiker, H.M. Physiology, Coagulation Pathways; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- López-Santago, N.; Raber, M.N. Coagulation Tests. In Acta Pediatrica de Mexico; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Instituto Nacional de Pediatria: Boston, MA, USA, 1990; Volume 37, pp. 241–245. ISBN 0-409-90077-X. [Google Scholar]
- Barmore, W.; Bajwa, T.; Burns, B. Biochemistry, Clotting Factors; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cai, B.; Hu, K.; Li, C.; Jin, J.; Hu, Y. Bovine Serum Albumin Bioconjugated Graphene Oxide: Red Blood Cell Adhesion and Hemolysis Studied by QCM-D. Appl. Surf. Sci. 2015, 356, 844–851. [Google Scholar] [CrossRef]
- Kenry. Understanding the Hemotoxicity of Graphene Nanomaterials through Their Interactions with Blood Proteins and Cells. J. Mater. Res. 2018, 33, 44–57. [Google Scholar] [CrossRef]
- Wilczek, P.; Major, R.; Lipinska, L.; Lackner, J.; Mzyk, A. Thrombogenicity and Biocompatibility Studies of Reduced Graphene Oxide Modified Acellular Pulmonary Valve Tissue. Mater. Sci. Eng. C 2015, 53, 310–321. [Google Scholar] [CrossRef]
- Ambrusa, A.; Bányaib, I.; Weissc, M.S.; Hilgenfeldc, R.; Keresztessya, Z.; Muszbekd, L.; Fésüsa, L. Calcium Binding of Transglutaminases: A 43Ca NMR Study Combined with Surface Polarity Analysis. J. Biomol. Struct. Dyn. 2001, 19, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Varga-Szabo, D.; Braun, A.; Nieswandt, B. Calcium Signaling in Platelets. J. Thromb. Haemost. 2009, 7, 1057–1066. [Google Scholar] [CrossRef]
- Singh, S.; Dodt, J.; Volkers, P.; Hethershaw, E.; Philippou, H.; Ivaskevicius, V.; Imhof, D.; Oldenburg, J.; Biswas, A. Structure Functional Insights into Calcium Binding during the Activation of Coagulation Factor XIII A. Sci. Rep. 2019, 9, 11324. [Google Scholar] [CrossRef]
- Borgogna, M.; Skjåk-Bræk, G.; Paoletti, S.; Donati, I. On the Initial Binding of Alginate by Calcium Ions. the Tilted Egg-Box Hypothesis. J. Phys. Chem. B 2013, 117, 7277–7282. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A.; Li, A. Multiple Steps and Critical Behaviors of the Binding of Calcium to Alginate. J. Phys. Chem. B 2007, 111, 2456–2462. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, B.; Zhu, X.; Xing, B. Aggregation, Adsorption, and Morphological Transformation of Graphene Oxide in Aqueous Solutions Containing Different Metal Cations. Environ. Sci. Technol. 2016, 50, 11066–11075. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.S.; Bozoklu, G.; Cai, W.; Nguyen, S.B.T.; Ruoff, R.S. Graphene Oxide Papers Modified by Divalent Ions—Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Jin, H.; Lai, N.; Jiang, C.; Wang, M.; Yao, W.; Han, Y.; Song, W. Potential Health Risks of Exposure to Graphene and Its Derivatives: A Review. Processes 2025, 13, 209. [Google Scholar] [CrossRef]
- Ghulam, A.N.; Dos Santos, O.A.L.; Hazeem, L.; Backx, B.P.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Liao, K.H.; Lin, Y.S.; MacOsko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Jachak, A.C.; Creighton, M.; Qiu, Y.; Kane, A.B.; Hurt, R.H. Biological Interactions and Safety of Graphene Materials. MRS Bull. 2012, 37, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Chlanda, A.; Kowiorski, K.; Małek, M.; Kijeńska-Gawrońska, E.; Bil, M.; Djas, M.; Strachowski, T.; Swieszkowski, W.; Lipińska, L. Morphology and Chemical Purity Ofwater Suspension of Graphene Oxide Flakes Aged for 14 Months in Ambient Conditions. a Preliminary Study. Materials 2021, 14, 4108. [Google Scholar] [CrossRef] [PubMed]
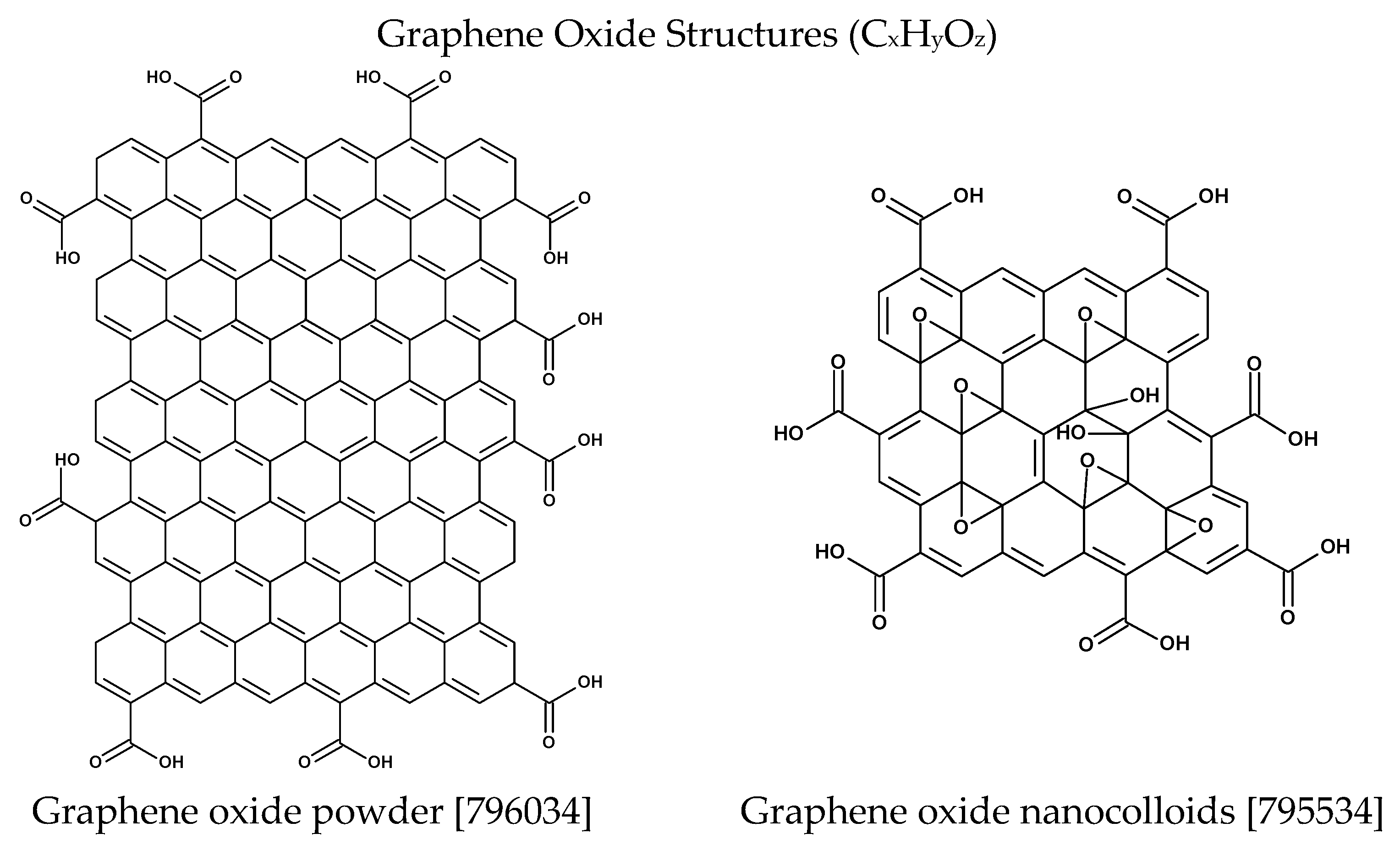
- G-Flake® Graphene Oxide [Publication in CARBON]. Available online: https://imif.lukasiewicz.gov.pl/en/gflake-carbon/ (accessed on 29 July 2025).
- Wu, K.; Zhou, Q.; Ouyang, S. Direct and Indirect Genotoxicity of Graphene Family Nanomaterials on DNA—A Review. Nanomaterials 2021, 11, 2889. [Google Scholar] [CrossRef] [PubMed]
- Arya, B.D.; Mittal, S.; Joshi, P.; Pandey, A.K.; Ramirez-Vick, J.E.; Gupta, G.; Singh, S.P. Graphene Oxide–Chloroquine Conjugate Induces DNA Damage in A549 Lung Cancer Cells through Autophagy Modulation. Beilstein J. Nanotechnol. 2025, 16, 316–332. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Piastowska-Ciesielska, A.W.; Kaarniranta, K.; Blasiak, J. All-Trans Retinoic Acid Modulates DNA Damage Response and the Expression of the VEGF-A and MKI67 Genes in ARPE-19 Cells Subjected to Oxidative Stress. Int. J. Mol. Sci. 2016, 17, 898. [Google Scholar] [CrossRef] [PubMed]
- Kluska, M.; Juszczak, M.; Wysokiński, D.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Kaempferol Derivatives Isolated from Lens Culinaris Medik. Reduce DNA Damage Induced by Etoposide in Peripheral Blood Mononuclear Cells. Toxicol. Res. 2019, 8, 896–907. [Google Scholar] [CrossRef] [PubMed]










| Composite | Biomedical Application | Lit. |
|---|---|---|
| GO-ALG-Ca2+ | Bioengineering applications | [40] |
| rGO-ALG-Ca2+ | Potential for cardiac patch application | [41] |
| GO-DFO-ALG-Mg2+ | Bone defect regeneration | [42] |
| GO-ALG-Zn2+ | Antibacterial activity and cytotoxicity | [43] |
| GO-Ag-ALG-Na+ | Antibacterial activity | [44] |
| ALG-Na+-CTS-Col-GO; ALG-Ca2+-CTS-Col-GO | Scaffolds for bone tissue engineering | [45] |
| Gel/ALG/GO * | Scaffold for neural tissue repair | [46] |
| GO-ALG-Zn2+ | Prevents Staphylococcus aureus and MRSE | [47] |
| G-ALG-Ca2+ | Neural applications | [48] |
| G-ALG-Na+ | Resveratrol delivery | [49] |
| G-HP-ALG-Na+ | For medical applications | [50] |
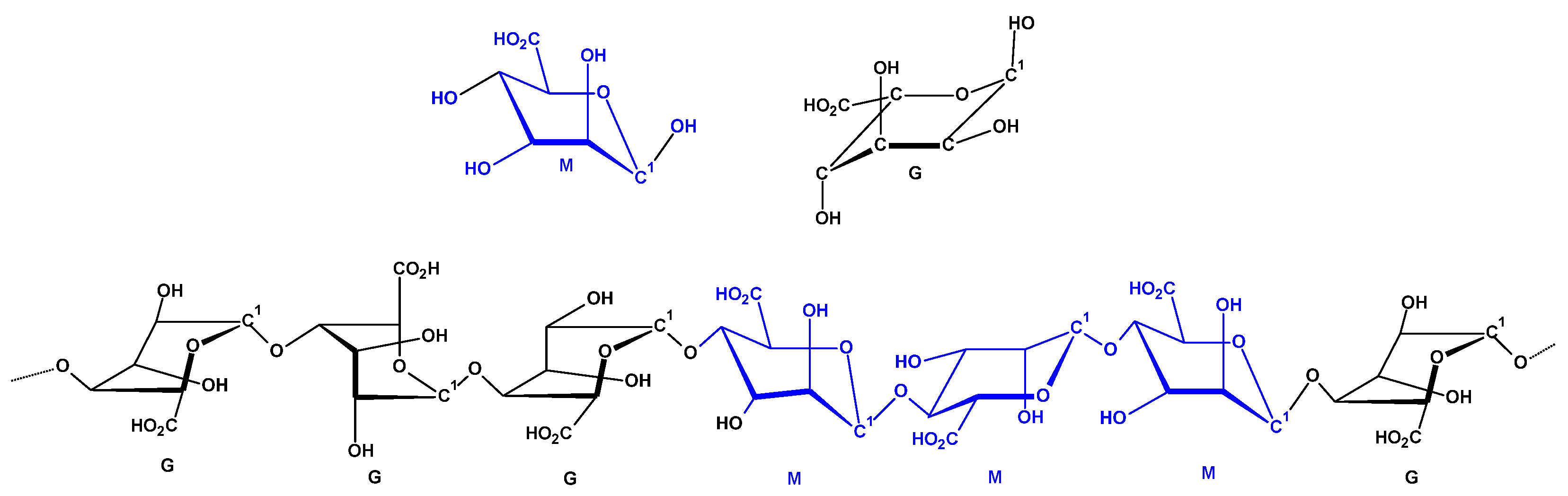
| ALGNa → GO-ALGNa→ GO-ALGNa → GO-ALGCa → GO-ALGCa(gel) | ||||
| ALGNa → GO-ALGNa | GO-ALGNa → GO-ALGCa → GO-ALGCa(gel) | |||
| Coordination of GO by ALG | Cross-linking reaction | |||
| ALG:GO | Ca2+ concentr. | Reaction temp. | Reaction time | |
| 0.5%, 1%, and 2% | 4 °C, 40 °C | 30, 60, 120, and 300 | ||
| Sample Symbol | Cross-Linking Agent Conc. | Calcium Content in Polymer | GO-ALG-Ca (mM *) /a | |
|---|---|---|---|---|
| [%] | [mg/kg] | Mmol/kg (mM *) | ||
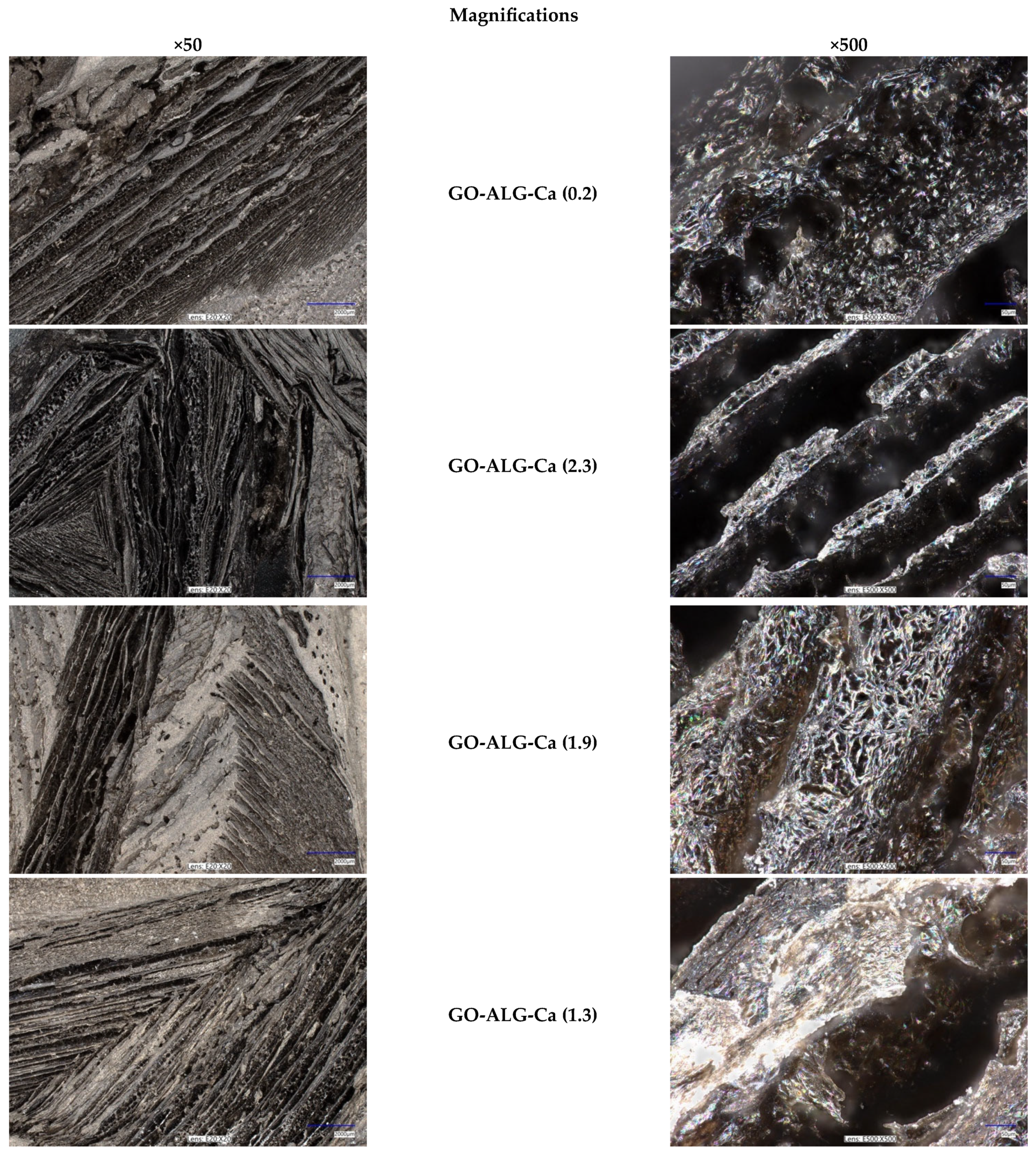
| ALG_GO_0 | no agent (0% CaCl2) | 8.562 | 0.21 | GO-ALG-Ca (0.2) |
| ALG_GO_C | 0.5% CaCl2 | 51.820 | 1.3 | GO-ALG-Ca (1.3) |
| ALG_GO_B | 1% CaCl2 | 75.278 | 1.88 | GO-ALG-Ca (1.9) |
| ALG_GO_A | 2% CaCl2 | 93.776 | 2.34 | GO-ALG-Ca (2.3) |
| Samples | Element Contents (Atomic Conc.) | ||||
|---|---|---|---|---|---|
| C | O | Na | Ca | Cl | |
| GO-ALG-Ca (0.2) | 48.651 | 40.089 | 1.348 | 4.113 | 5.799 |
| GO-ALG-Ca (1.3) | 53.053 | 36.851 | 2.436 | 4.283 | 3.397 |
| GO-ALG-Ca (1.9) | 46.162 | 39.926 | 4.389 | 3.923 | 5.600 |
| GO-ALG-Ca (2.3) | 50.918 | 41.243 | 0.746 | 3.970 | 3.124 |
| Samples | Element Contents (weight Conc.) | ||||
| C | O | Na | Ca | Cl | |
| GO-ALG-Ca (0.2) | 35.908 | 39.418 | 1.906 | 10.130 | 12.638 |
| GO-ALG-Ca (1.3) | 40.200 | 37.200 | 3.600 | 11.400 | 7.600 |
| GO-ALG-Ca (1.9) | 33.601 | 38.716 | 6.118 | 9.529 | 12.036 |
| GO-ALG-Ca (2.3) | 39.239 | 42.342 | 1.101 | 10.210 | 7.107 |
| Sample Name | Specific Surface Area (SSA) | Total Pore Volume (TPV) | Average Pore Diameter (APD) |
|---|---|---|---|
| m2/g | cm3/g | nm | |
| GO-ALG-Ca (0.2) | 1.2920 | 3.453 × 10−3 | 12.6 |
| GO-ALG-Ca (2.3) | 1.0222 | 3.798 × 10−2 | 15.2 |
| GO-ALG-Ca (1.9) | 1.0332 | 4.322 × 10−3 | 20.1 |
| GO-ALG-Ca (1.3) | 0.9401 | 3.948 × 10−3 | 21.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudzin, M.H.; Kaczmarek, A.; Mrozińska, Z.; Hernandez, C.; Piekarska, K.; Woźniak, K.; Juszczak, M.; Król, P. Hybrid Alginate–Graphene Composites: Biochemical Features and Biomedical Potential. Mar. Drugs 2025, 23, 323. https://doi.org/10.3390/md23080323
Kudzin MH, Kaczmarek A, Mrozińska Z, Hernandez C, Piekarska K, Woźniak K, Juszczak M, Król P. Hybrid Alginate–Graphene Composites: Biochemical Features and Biomedical Potential. Marine Drugs. 2025; 23(8):323. https://doi.org/10.3390/md23080323
Chicago/Turabian StyleKudzin, Marcin H., Anna Kaczmarek, Zdzisława Mrozińska, Cesar Hernandez, Klaudia Piekarska, Katarzyna Woźniak, Michał Juszczak, and Paulina Król. 2025. "Hybrid Alginate–Graphene Composites: Biochemical Features and Biomedical Potential" Marine Drugs 23, no. 8: 323. https://doi.org/10.3390/md23080323
APA StyleKudzin, M. H., Kaczmarek, A., Mrozińska, Z., Hernandez, C., Piekarska, K., Woźniak, K., Juszczak, M., & Król, P. (2025). Hybrid Alginate–Graphene Composites: Biochemical Features and Biomedical Potential. Marine Drugs, 23(8), 323. https://doi.org/10.3390/md23080323






