Unraveling of Seaweed Bioactive Substances and Their Nutritional Regulation Functions for Poultry
Abstract
1. Introduction
2. Classification and Biological Functions of Seaweeds and Their Active Substances
2.1. Polysaccharides
2.2. Polyphenols
2.3. Protein and Amino Acids
2.4. Fatty Acids
3. Seaweeds and Their Bioactive Substances in Poultry Production
3.1. Beneficial Effects of Seaweed Bioactive Substances in Broiler Chickens
3.1.1. Improve Growth Performance
3.1.2. Improve Meat Quality
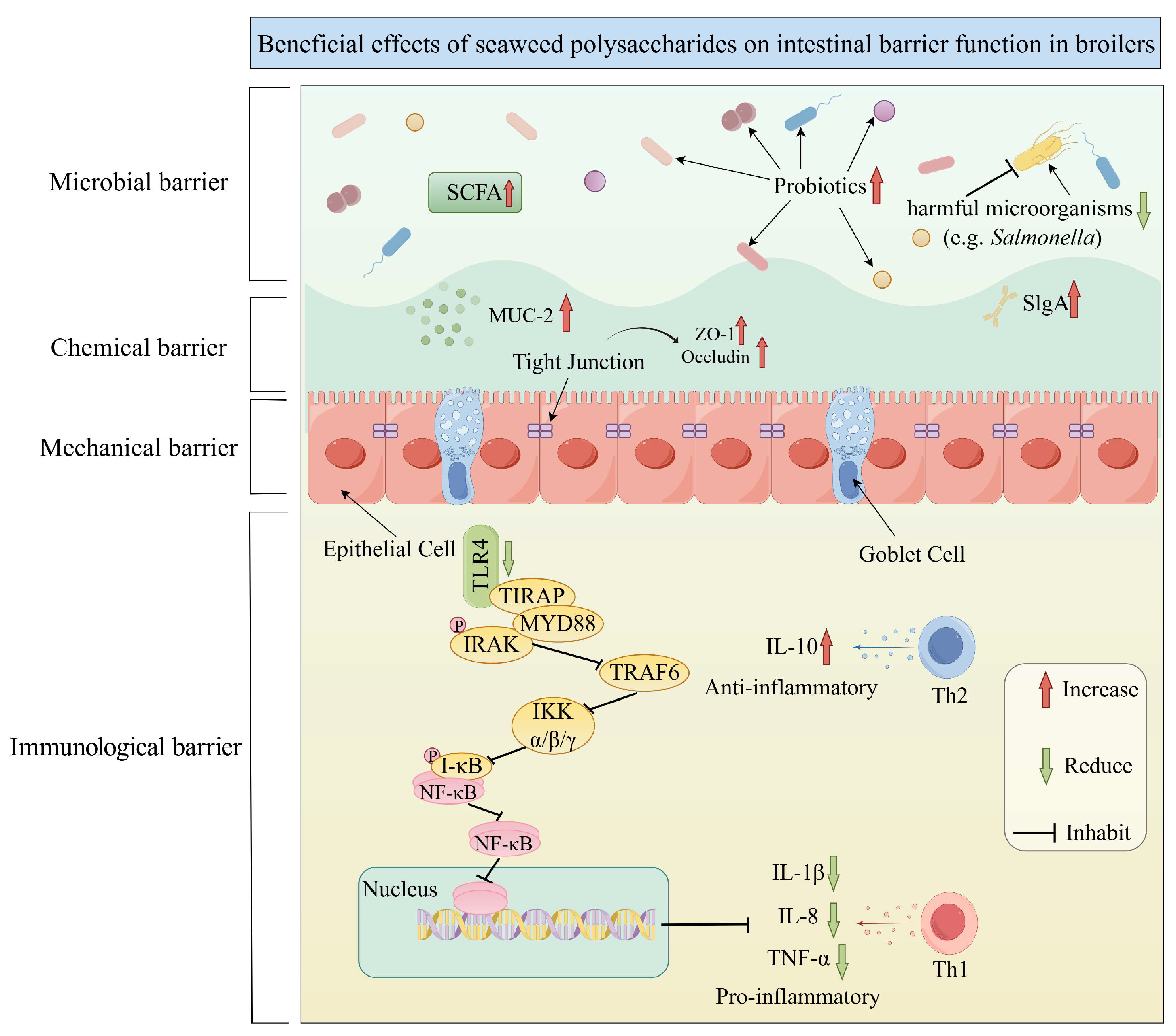
3.1.3. Promote Intestinal Barrier Function
3.2. Beneficial Effects of Seaweed Active Substances in Laying Hens
3.2.1. Improve Egg-Production Performance
3.2.2. Improve Egg Quality
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, J.L.; Van Eerde, A. Pollution by Antibiotics and Antimicrobial Resistance in LiveStock and Poultry Manure in China, and Countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090. [Google Scholar] [CrossRef]
- Oke, O.E.; Akosile, O.A.; Oni, A.I.; Opowoye, I.O.; Ishola, C.A.; Adebiyi, J.O.; Odeyemi, A.J.; Adjei-Mensah, B.; Uyanga, V.A.; Abioja, M.O. Oxidative stress in poultry production. Poult. Sci. 2024, 103, 104003. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Liu, W.C.; Zhuang, D.P.; Zhao, Y.; Balasubramanian, B.; Zhao, Z.H. Seaweed-Derived Polysaccharides Attenuate Heat Stress-Induced Splenic Oxidative Stress and Inflammatory Response via Regulating Nrf2 and NF-κB Signaling Pathways. Mar. Drugs 2022, 20, 358. [Google Scholar] [CrossRef]
- Mandal, A.B.; Biswas, A.; Mir, N.A.; Tyagi, P.K.; Kapil, D.; Biswas, A.K. Effects of dietary supplementation of Kappaphycus alvarezii on productive performance and egg quality traits of laying hens. J. Appl. Phycol. 2018, 31, 2065–2072. [Google Scholar] [CrossRef]
- Wassie, T.; Lu, Z.; Duan, X.; Xie, C.; Gebeyew, K.; Yumei, Z.; Yin, Y.; Wu, X. Dietary Enteromorpha Polysaccharide Enhances Intestinal Immune Response, Integrity, and Caecal Microbial Activity of Broiler Chickens. Front. Nutr. 2021, 8, 783819. [Google Scholar] [CrossRef]
- Dewi, Y.L.; Sofyan, A.; Herdian, H.; Sakti, A.A.; Irawan, A.; Jasmadi, J.; Anggraeni, A.S.; Mardawati, E.; Adriyanto, A.; Mahata, M.E.; et al. Processing technology to improve seaweed nutritional quality as a feed for poultry: A review and its implementation. World’s Poult. Sci. J. 2024, 80, 207–235. [Google Scholar] [CrossRef]
- Michalak, I.; Mahrose, K. Seaweeds, Intact and Processed, as a Valuable Component of Poultry Feeds. J. Mar. Sci. Eng. 2020, 8, 620. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Martins, C.F.; Costa, M.; Coelho, D.; Pestana, J.; Alfaia, C.; Lordelo, M.; De Almeida, A.M.; Freire, J.P.B.; Prates, J.a.M. Quality Traits and Nutritional Value of Pork and Poultry Meat from Animals Fed with Seaweeds. Foods 2021, 10, 2961. [Google Scholar] [CrossRef] [PubMed]
- Haberecht, S.; Wilkinson, S.; Roberts, J.; Wu, S.; Swick, R. Unlocking the potential health and growth benefits of macroscopic algae for poultry. World’s Poult. Sci. J. 2018, 74, 5–20. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Kasimala, M.; Mebrahtu, L.; Magoha, P.; Asgedom, G. A Review on biochemical composition and nutritional aspects of Seaweeds. Caribb. J. Sci. 2015, 3, 789–797. [Google Scholar]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Andri, F.; Dono, N.D.; Sasongko, H.; Zuprizal, Z. The effects of dietary seaweed inclusion on growth performance of broiler chickens: A systematic review and meta-analysis. F1000Research 2020, 9, 1087. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Okab, A.B.; Aljumaah, R.S.; Samara, E.M.; Abdoun, K.A.; Al-Haidary, A.A. Nutritional Value of Green Seaweed (Ulva lactuca) for Broiler Chickens. Ital. J. Anim. Sci. 2016, 12, e28. [Google Scholar] [CrossRef]
- Min, B.R.; Parker, D.; Brauer, D.; Waldrip, H.; Lockard, C.; Hales, K.; Akbay, A.; Augyte, S. The role of seaweed as a potential dietary supplementation for enteric methane mitigation in ruminants: Challenges and opportunities. Anim. Nutr. 2021, 7, 1371–1387. [Google Scholar] [CrossRef]
- Mandal, A.K.; Parida, S.; Behera, A.K.; Adhikary, S.P.; Lukatkin, A.A.; Lukatkin, A.S.; Jena, M. Seaweed in the Diet as a Source of Bioactive Metabolites and a Potential Natural Immunity Booster: A Comprehensive Review. Pharmaceuticals 2025, 18, 367. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Akhter, M.; Khan, B.S.; Hasan, M.; Bosu, A.; Yasmin, F.; Haque, M.A.; Islam, A.; Mahmud, Y. Seaweed: A prominent source of protein and other nutrients. Sustain. Aquat. Res. 2023, 2, 145–166. [Google Scholar] [CrossRef]
- Fleurence, J. Perspectives on the Use of Algae in Agriculture and Animal Production. Phycology 2021, 1, 79–82. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- Huang, L.; Lee, J.-Y.; Park, Y.-K.; Lee, J. Heavy metals in seaweed: Implications for health benefits, risks, and safety regulations. J. Agric. Food Res. 2025, 21, 101830. [Google Scholar] [CrossRef]
- Xie, C.; Lee, Z.J.; Ye, S.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.a.R. A Review on Seaweeds and Seaweed-Derived Polysaccharides: Nutrition, Chemistry, Bioactivities, and Applications. Food Rev. Int. 2023, 40, 1312–1347. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ganesan, K.; Rao, P.V.S. Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty—An edible seaweed. Food Chem. 2008, 107, 289–295. [Google Scholar] [CrossRef]
- Liu, Z.W.; Sun, X. A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants. Int. J. Mol. Sci. 2020, 21, 7774. [Google Scholar] [CrossRef]
- Michalak, I.; Tiwari, R.; Dhawan, M.; Alagawany, M.; Farag, M.R.; Sharun, K.; Emran, T.B.; Dhama, K. Antioxidant effects of seaweeds and their active compounds on animal health and production—A review. Vet. Q. 2022, 42, 48–67. [Google Scholar] [CrossRef]
- Saeed, M.; Arain, M.A.; Ali Fazlani, S.; Marghazani, I.B.; Umar, M.; Soomro, J.; Bhutto, Z.A.; Soomro, F.; Noreldin, A.E.; Abd El-Hack, M.E.; et al. A comprehensive review on the health benefits and nutritional significance of fucoidan polysaccharide derived from brown seaweeds in human, animals and aquatic organisms. Aquacult. Nutr. 2021, 27, 633–654. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Nagahawatta, D.P.; Fernando, I.P.S.; Kim, Y.-T.; Kim, J.-S.; Kim, W.-S.; Lee, J.S.; Jeon, Y.-J. A Review on Fucoidan Structure, Extraction Techniques, and Its Role as an Immunomodulatory Agent. Mar. Drugs 2022, 20, 755. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qu, H.; Gao, Z.; Zeng, D.; Wang, J.; Baranenko, D.; Li, Y.; Lu, W. Protective effects of Ulva pertusa polysaccharide and polysaccharide-iron (III) complex on cyclophosphamide induced immunosuppression in mice. Int. J. Biol. Macromol. 2019, 133, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Zhou, S.H.; Balasubramanian, B.; Zeng, F.Y.; Sun, C.B.; Pang, H.Y. Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish. Immunol. 2020, 104, 202–212. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Matin, M.; Koszarska, M.; Atanasov, A.G.; Król-Szmajda, K.; Jóźwik, A.; Stelmasiak, A.; Hejna, M. Bioactive Potential of Algae and Algae-Derived Compounds: Focus on Anti-Inflammatory, Antimicrobial, and Antioxidant Effects. Molecules 2024, 29, 4695. [Google Scholar] [CrossRef]
- Rupert, R.; Rodrigues, K.F.; Thien, V.Y.; Yong, W.T.L. Carrageenan from Kappaphycus alvarezii (Rhodophyta, Solieriaceae): Metabolism, structure, production, and application. Front. Plant Sci. 2022, 13, 859635. [Google Scholar] [CrossRef]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Ong Abdullah, J.; Ho, C.-L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.J.; Zhao, R.X. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Du, C.; Mou, H.; Wang, P. Compositional and structural characteristics of sulfated polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2017, 165, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Jin, W.; Guo, Y. Immunomodulatory Effects of a Low-Molecular Weight Polysaccharide from Enteromorpha prolifera on RAW 264.7 Macrophages and Cyclophosphamide- Induced Immunosuppression Mouse Models. Mar. Drugs 2020, 18, 340. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.A.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from Brown Seaweeds as a Potential Antimicrobial Agent in Animal Feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kuznetsova, T.A.; Kryzhanovsky, S.P.; Ermakova, S.P.; Galkina, I.V.; Shchelkanov, M.Y. Molecular Targets of Brown Algae Phlorotannins for the Therapy of Inflammatory Processes of Various Origins. Mar. Drugs 2022, 20, 243. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamad, R.; Baharara, J.; Zafar-Balanejad, S.; Fargahi, F.; Rahman, H.S. Antioxidant, Antiproliferative, and Antiangiogenesis Effects of Polyphenol-Rich Seaweed (Sargassum muticum). BioMed Res. Int. 2013, 2013, 604787. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Tiller, C.; Shen, J.K.; Wang, C.; Girouard, G.S.; Dennis, D.; Barrow, C.J.; Miao, M.; Ewart, H.S. Antidiabetic properties of polysaccharide- and polyphenolic-enriched fractions from the brown seaweed Ascophyllum nodosum. Can. J. Physiol. Pharmacol. 2007, 85, 1116–1123. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.a.C.; Ferreira, A.C.S.; Teixeira, J.A.; Vicente, A.A. Antioxidant Potential of Two Red Seaweeds from the Brazilian Coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; Mcdougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Yuan, Y.M.; Zhao, Z.H.; Yao, Q.H.; Ye, X.Q.; Wang, Y.Y.; Liu, H.M.; Jha, R.; Balasubramanian, B.; Liu, W.C. Phlorotannin Alleviates Liver Injury by Regulating Redox Balance, Apoptosis, and Ferroptosis of Broilers under Heat Stress. Antioxidants 2024, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Q.; Yang, Y.Y.; Yao, Q.H.; Huang, M.Y.; Balasubramanian, B.; Jha, R.; Liu, W.C. The ameliorative role of phlorotannin on aflatoxin B1-induced liver oxidative stress and mitochondrial injury is related to the activation of Nrf2 and Nrf1 signaling pathways in broilers. J. Anim. Sci. Biotechnol. 2025, 16, 75. [Google Scholar] [CrossRef]
- Suwal, S.; Marciniak, A. Technologies for the Extraction, Separation and Purification of polyphenols—A Review. Nepal J. Biotechnol. 2019, 6, 74–91. [Google Scholar] [CrossRef]
- Fleurence, J.; Morançais, M.; Dumay, J. 9—Seaweed proteins. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 245–262. [Google Scholar]
- Alemu, S.; Abrar, B. Utilization of Algae Based-Meal as an Alternative Protein Source in Poultry Nutrition: A Review. Am. J. Zool. 2025, 8, 50–59. [Google Scholar] [CrossRef]
- Angell, A.R.; Angell, S.F.; De Nys, R.; Paul, N.A. Seaweed as a protein source for mono-gastric livestock. Trends Food Sci. Technol. 2016, 54, 74–84. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Robert Waaland, J.; Rabiei, R. Fatty Acids, Amino Acids, Mineral Contents, and Proximate Composition of Some Brown Seaweeds. J. Phycol. 2012, 48, 285–292. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Rohani-Ghadikolaei, K.; Abdulalian, E.; Ng, W.-K. Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J. Food Sci. Technol. 2011, 49, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayana, R.; Vijay, K.; Ambedkar, R.; Ranga Rao, A.; Ravishankar, G.A. Biological Activities and Health Benefits of Seaweed Carotenoids with Special Reference to Fucoxanthin. In Sustainable Global Resources of Seaweeds Volume 2: Food, Pharmaceutical and Health Applications; Ranga Rao, A., Ravishankar, G.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; Volume 2, pp. 539–558. [Google Scholar]
- Zhang, X.; Qiang, W.; Guo, Y.; Gong, J.; Yu, H.; Wu, D.; Tang, P.; Yidan, M.; Zhang, H.; Sun, X. Fucoxanthin alleviates renal aging by regulating the oxidative stress process and the inflammatory response in vitro and in vivo models. Redox Rep. 2025, 30, 2511458. [Google Scholar] [CrossRef] [PubMed]
- Gosch, B.J.; Paul, N.A.; De Nys, R.; Magnusson, M. Seasonal and within-plant variation in fatty acid content and composition in the brown seaweed Spatoglossum macrodontum (Dictyotales, Phaeophyceae). J. Appl. Phycol. 2014, 27, 387–398. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, M. Chemical and nutritional characteristics of brown seaweed lipids: A review. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Costa, M.; Cardoso, C.; Afonso, C.; Bandarra, N.M.; Prates, J.a.M. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: A systematic review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1075–1102. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Hincke, M.T.; Prithiviraj, B.; Critchley, A. A Review of the Varied Uses of Macroalgae as Dietary Supplements in Selected Poultry with Special Reference to Laying Hen and Broiler Chickens. J. Mar. Sci. Eng. 2020, 8, 536. [Google Scholar] [CrossRef]
- Choi, J. Challenges in Poultry Production Systems and Nutritional Interventions. Animals 2025, 15, 530. [Google Scholar] [CrossRef]
- Ochieng, P.E.; Scippo, M.-L.; Kemboi, D.C.; Croubels, S.; Okoth, S.; Kang’ethe, E.K.; Doupovec, B.; Gathumbi, J.K.; Lindahl, J.F.; Antonissen, G. Mycotoxins in Poultry Feed and Feed Ingredients from Sub-Saharan Africa and Their Impact on the Production of Broiler and Layer Chickens: A Review. Toxins 2021, 13, 633. [Google Scholar] [CrossRef]
- Szoke, Z.; Fauszt, P.; Mikolas, M.; David, P.; Szilagyi-Tolnai, E.; Pesti-Asboth, G.; Homoki, J.R.; Kovacs-Forgacs, I.; Gal, F.; Stundl, L.; et al. Comprehensive analysis of antimicrobial resistance dynamics among broiler and duck intensive production systems. Sci. Rep. 2025, 15, 4673. [Google Scholar] [CrossRef]
- Kim, H.R.; Seong, P.; Seol, K.-H.; Park, J.-E.; Kim, H.; Park, W.; Cho, J.H.; Lee, S.D. Effects of heat stress on growth performance, physiological responses, and carcass traits in broilers. J. Therm. Biol. 2025, 127, 103994. [Google Scholar] [CrossRef]
- Liu, W.C.; Liu, H.M.; Wang, Y.Y.; Zhao, Z.X.; Balasubramanian, B.; Jha, R. Effects of Enteromorpha prolifera polysaccharides on growth performance, intestinal barrier function and cecal microbiota in yellow-feathered broilers under heat stress. J. Anim. Sci. Biotechnol. 2023, 14, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Ou, B.H.; Liang, Z.L.; Zhang, R.; Zhao, Z.H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-κB signaling pathway in broilers. Poult. Sci. 2021, 100, 101139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, K.; Mishra, R.; Jha, R. In ovo supplementation of chitooligosaccharide and chlorella polysaccharide affects cecal microbial community, metabolic pathways, and fermentation metabolites in broiler chickens. Poult. Sci. 2020, 99, 4776–4785. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.S.; Ramasamy, K.T.; Rao Vantharam Venkata, H.G.; Rama Rao, S.V.; Lakshmi Narasimha Raju, M.V.; Ramanan, S.; Nori, S.S.; Suryanarayan, S.; Reddy, G.N.; Phani Kumar, P.S.; et al. Evaluation of the potential of extract of seaweed Eucheuma denticulatum as an alternative to antibiotic growth promoter in broiler chickens. Heliyon 2024, 10, e25219. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.a.A.; Williams, A.N.; Salahuddin, M.; Gadekar, S.; Lohakare, J. Algae as an alternative source of protein in poultry diets for sustainable production and disease resistance: Present status and future considerations. Front. Vet. Sci. 2024, 11, 1382163. [Google Scholar] [CrossRef]
- Abu Hafsa, S.H.; Hassan, A.A. The Effect of Sargassum siliquastrum Supplementation on Growth Performance, Cecal Fermentation, Intestine Histomorphology, and Immune Response of Japanese Quails. Animals 2022, 12, 432. [Google Scholar] [CrossRef]
- Mhlongo, G.; Mnisi, C.M. Effect of seaweed (Ecklonia maxima) on apparent nutrient digestibility, growth performance, and physiological and meat quality parameters in Boschveld cockerels. Poult. Sci. 2023, 102, 102361. [Google Scholar] [CrossRef]
- El-Deek, A.A.; Brikaa, M.A. Nutritional and Biological Evaluation of Marine Seaweed as a Feedstuff and as a Pellet Binder in Poultry Diet. Int. J. Poult. Sci. 2009, 8, 875–881. [Google Scholar] [CrossRef]
- Stokvis, L.; Van Krimpen, M.M.; Kwakkel, R.P.; Bikker, P. Evaluation of the nutritional value of seaweed products for broiler chickens’ nutrition. Anim. Feed. Sci. Technol. 2021, 280, 115061. [Google Scholar] [CrossRef]
- Perali, C.; Magnoli, A.P.; Aronovich, M.; Rosa, C.a.D.R.; Cavaglieri, L.R. Lithothamnium calcareum (Pallas) Areschoug seaweed adsorbs aflatoxin B1 in vitro and improves broiler chicken’s performance. Mycotoxin Res. 2020, 36, 371–379. [Google Scholar] [CrossRef]
- Qadri, S.S.N.; Biswas, A.; Mandal, A.B.; Kumawat, M.; Saxena, R.; Nasir, A.M. Production performance, immune response and carcass traits of broiler chickens fed diet incorporated with Kappaphycus alvarezii. J. Appl. Phycol. 2018, 31, 753–760. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Shanmugam, S.; Park, S.; Recharla, N.; Koo, J.S.; Andretta, I.; Kim, I.H. Supplemental Impact of Marine Red Seaweed (Halymenia palmata) on the Growth Performance, Total Tract Nutrient Digestibility, Blood Profiles, Intestine Histomorphology, Meat Quality, Fecal Gas Emission, and Microbial Counts in Broilers. Animals 2021, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Y.; Ayala, L.; Hurtado, C.; Más, D.; Rodríguez, R. Effects of Dietary Supplementation with Red Algae Powder (Chondrus crispus) on Growth Performance, Carcass Traits, Lymphoid Organ Weights and Intestinal pH in Broilers. Braz. J. Poult. Sci. 2019, 21, eRBCA-2019-1015. [Google Scholar] [CrossRef]
- Olowe, O.S.; Johnson, T.; Adeola, O. Red seaweed Chondracanthus chamissoi supplementation improved the growth performance of broiler chickens partially through effects on intestinal morphology and cecal microbiota. Poult. Sci. 2025, 104, 105402. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, S.R.; Oh, J.W. Effects of dietary fermented seaweed and seaweed fusiforme on growth performance, carcass parameters and immunoglobulin concentration in broiler chicks. Asian-Australas. J. Anim. Sci. 2014, 27, 862–870. [Google Scholar] [CrossRef]
- Akinyemi, F.; Adewole, D. Effects of brown seaweed products on growth performance, plasma biochemistry, immune response, and antioxidant capacity of broiler chickens challenged with heat stress. Poult. Sci. 2022, 101, 102215. [Google Scholar] [CrossRef]
- Liu, W.C.; Yang, Y.Y.; Pushparaj, K.; Balasubramanian, B. Evaluation of Hepatic Detoxification Effects of Enteromorpha prolifera Polysaccharides against Aflatoxin B1 in Broiler Chickens. Antioxidants 2022, 11, 1757. [Google Scholar] [CrossRef]
- Guo, Y.; Balasubramanian, B.; Zhao, Z.H.; Liu, W.C. Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers. Poult. Sci. 2021, 100, 844–857. [Google Scholar] [CrossRef]
- Zhao, Y.; Balasubramanian, B.; Guo, Y.; Qiu, S.J.; Jha, R.; Liu, W.C. Dietary Enteromorpha polysaccharides supplementation improves breast muscle yield and is associated with modification of mRNA transcriptome in broiler chickens. Front. Vet. Sci. 2021, 8, 663988. [Google Scholar] [CrossRef]
- Matshogo, T.B.; Mlambo, V.; Mnisi, C.M.; Manyeula, F. Effect of pre-treating dietary green seaweed with fibrolytic enzymes on growth performance, blood indices, and meat quality parameters of Cobb 500 broiler chickens. Livest. Sci. 2021, 251, 104652. [Google Scholar] [CrossRef]
- Coudert, E.; Baéza, E.; Berri, C. Use of algae in poultry production: A review. World’s Poult. Sci. J. 2020, 76, 767–786. [Google Scholar] [CrossRef]
- Purnamasari, L.; Carreon, J.M.; Dela Cruz, J.F. Benefits of Green Seaweed as Protein Source for Broiler: A Review. J. Livest. Sci. Prod. 2022, 6, 381–400. [Google Scholar] [CrossRef]
- Matshogo, T.B.; Mnisi, C.M.; Mlambo, V. Dietary Green Seaweed Compromises Overall Feed Conversion Efficiency but not Blood Parameters and Meat Quality and Stability in Broiler Chickens. Agriculture 2020, 10, 547. [Google Scholar] [CrossRef]
- Liu, W.C.; Pan, Z.Y.; Zhao, Y.; Guo, Y.; Qiu, S.J.; Balasubramanian, B.; Jha, R. Effects of heat stress on production performance, redox status, intestinal morphology and barrier-related gene expression, cecal microbiome, and metabolome in indigenous broiler chickens. Front. Physiol. 2022, 13, 890520. [Google Scholar] [CrossRef]
- Jiang, S.W.; Yang, C.Y.; Xiao, Y.T.; Zheng, S.Z.; Jiang, Q.; Chen, J.S. Effects of Polysaccharides-Rich Extract from Gracilaria lemaneiformis on Growth Performance, Antioxidant Capacity, Immune Function, and Meat Quality in Broiler Chickens. J. Poult. Sci. 2023, 60, 2023018. [Google Scholar] [CrossRef]
- Yan, G.L.; Guo, Y.M.; Yuan, J.M.; Liu, D.; Zhang, B.K. Sodium alginate oligosaccharides from brown algae inhibit Salmonella Enteritidis colonization in broiler chickens. Poult. Sci. 2011, 90, 1441–1448. [Google Scholar] [CrossRef]
- Oretomiloye, F.; Adewole, D. Exploring the modulatory effects of brown seaweed meal and extracts on intestinal microbiota and morphology of broiler chickens challenged with heat stress. Poult. Sci. 2024, 103, 103562. [Google Scholar] [CrossRef]
- Liu, W.C.; Guo, Y.; Zhao, Z.H.; Jha, R.; Balasubramanian, B. Algae-Derived Polysaccharides Promote Growth Performance by Improving Antioxidant Capacity and Intestinal Barrier Function in Broiler Chickens. Front. Vet. Sci. 2020, 7, 601336. [Google Scholar] [CrossRef]
- Liu, W.C.; Zhu, Y.R.; Zhao, Z.H.; Jiang, P.; Yin, F.Q. Effects of Dietary Supplementation of Algae-Derived Polysaccharides on Morphology, Tight Junctions, Antioxidant Capacity and Immune Response of Duodenum in Broilers under Heat Stress. Animals 2021, 11, 2279. [Google Scholar] [CrossRef]
- Sun, J.F.; Song, H.L.; Zhao, J.; Xiao, Y.; Qi, R.; Lin, Y.T. Effects of Different Dietary Levels of Enteromorpha prolifera on Nutrient Availability and Digestive Enzyme Activities of Broiler Chickens. Chin. J. Anim. Nutr. 2010, 22, 1658–1664. [Google Scholar]
- Hang, H.Y. Seaweed can Enhance the Integrity of the Chick’s Intestine. Anim. Sci. Abroad (Pigs Poult.) 2021, 41, 42–45. [Google Scholar]
- Guo, Y.; Zhao, Z.H.; Pan, Z.Y.; An, L.L.; Balasubramanian, B.; Liu, W.C. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult. Sci. 2020, 99, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Azevedo, G.Z.; De Souza Dutra, F.; Dos Santos, B.R.; Schneider, A.R.; Oliveira, E.R.; Moura, S.; Vianello, F.; Maraschin, M.; Lima, G.P.P. Uses and applications of the red seaweed Kappaphycus alvarezii: A systematic review. J. Appl. Phycol. 2024, 36, 3409–3450. [Google Scholar] [CrossRef]
- Borzouie, S.; Rathgeber, B.M.; Stupart, C.M.; MacIsaac, J.; MacLaren, L.A. Effects of Dietary Inclusion of Seaweed, Heat Stress and Genetic Strain on Performance, Plasma Biochemical and Hematological Parameters in Laying Hens. Animals 2020, 10, 1570. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Rathgeber, B.; Macisaac, J.; Boulianne, M.; Brigitte, L.; Stratton, G.; Thomas, N.A.; Critchley, A.T.; Hafting, J.; Prithiviraj, B. Feed Supplementation with Red Seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, Reduce Salmonella Enteritidis in Laying Hens. Front. Microbiol. 2017, 8, 00567. [Google Scholar] [CrossRef]
- Sulistiawati, D.; Hafsah; Damayanti, A.P.; Rizal, A. Response of sea grapes (Caulerpa sp.) in diets on laying hens performance and egg quality. IOP Conf. Ser. Earth Environ. Sci. 2024, 1341, 012081. [Google Scholar] [CrossRef]
- Li, Q.Q.; Luo, J.; Wang, C.M.; Tai, W.J.; Wang, H.H.; Zhang, X.; Liu, K.S.; Jia, Y.X.; Lyv, X.Z.; Wang, L.; et al. Ulvan extracted from green seaweeds as new natural additives in diets for laying hens. J. Appl. Phycol. 2018, 30, 2017–2027. [Google Scholar] [CrossRef]
- Dewi, Y.L.; Yuniza, A.; Sayuti, K.; Nuraini; Mahata, M.E. Effects of Different Dietary Concentration of Fermented Brown Algae Sargassum Binderi on Plasma Lipid Profiles, Yolk Lipid, and Cholesterol Total of Laying Hens. J. Anim. Plant Sci. 2023, 33, 1–10. [Google Scholar] [CrossRef]
- Selim, S.; Hussein, E.; Abou-Elkhair, R. Effect of Spirulina platensis as a feed additive on laying performance, egg quality and hepatoprotective activity of laying hens. Eur. Poult. Sci. 2018, 82, 1–13. [Google Scholar] [CrossRef]
- García Jacome, X.N.; González Ramírez, P.; Piñón Gimate, A.; Casas Valdéz, M. Sargassum en las dietas de gallinas Rhode Island mejora la calidad del huevo y funcionalidad por enriquecimiento con iodo. CICIMAR Oceánides 2023, 37, 1–12. [Google Scholar] [CrossRef]
- Abo El-Maaty, H.A.; El-Khateeb, A.Y.; Al-Khalaifah, H.; El.Hamed, E.-S.A.; Hamed, S.; El-Said, E.A.; Mahrose, K.M.; Metwally, K.; Mansour, A.M. Effects of ecofriendly synthesized calcium nanoparticles with biocompatible Sargassum latifolium algae extract supplementation on egg quality and scanning electron microscopy images of the eggshell of aged laying hens. Poult. Sci. 2021, 100, 675–684. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Rathgeber, B.; Stratton, G.; Thomas, N.; Evans, F.; Critchley, A.; Hafting, J.; Prithiviraj, B. Feed supplementation with red seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens. Poult. Sci. 2014, 93, 2991–3001. [Google Scholar] [CrossRef]
- Mlambo, V.; Mnisi, C.M.; Matshogo, T.B.; Mhlongo, G. Prospects of dietary seaweeds and their bioactive compounds in sustainable poultry production systems: A symphony of good things? Front. Anim. Sci. 2022, 3, 998042. [Google Scholar] [CrossRef]

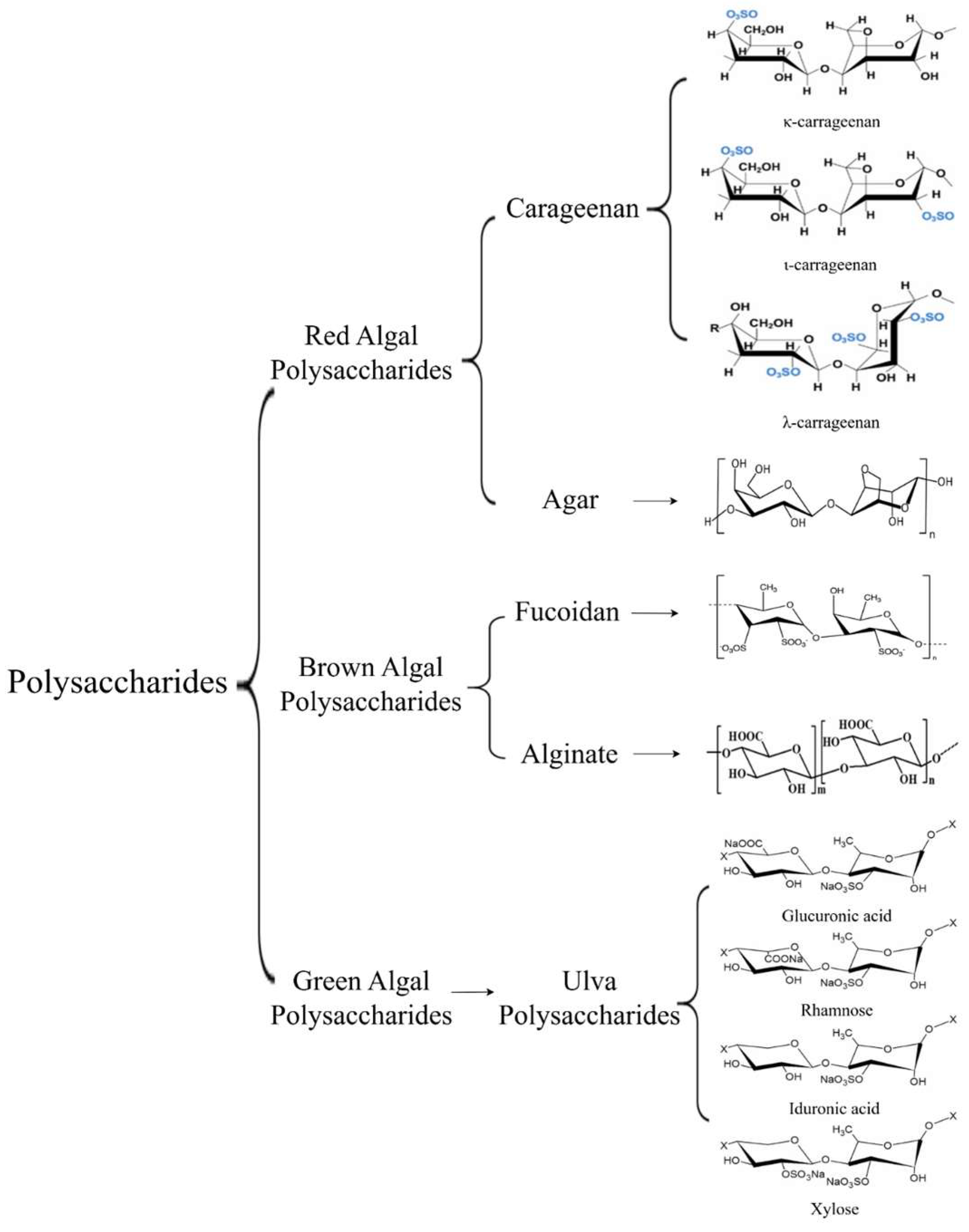
| Items | Lipid Content (%DW) | Carbohydrate Content (%DW) | Protein Content (%DW) | Dietary Fiber (%DW) | References |
|---|---|---|---|---|---|
| Brown algae | 0.1–11.5 | 12.8–81 | 3.1–42.1 | 2.9–75 | [27] |
| Green algae | 0.2–15 | 4–79.9 | 0.4–32.1 | 1.1–64.9 | |
| Red algae | 0.4–12 | 8.3–68.2 | 3.5–47 | 3.3–65.6 |
| Seaweed Species | Main Polysaccharides | Source of Polysaccharides | Composition Structure | Biological Activity | References |
|---|---|---|---|---|---|
| Red algae | Carrageenan | Kappaphycus alvarezii (Rhodophyta, Solieriaceae), etc. | A linear sulfated polysaccharide composed of D-galactopyranose linked by β-(1,3)- and α-(1,4)-glycosidic bonds. | Antiviral, anti-inflammatory, anticoagulant, lipid metabolism regulation, and hypoglycemic effects, etc. | [38] |
| Agar | Gracilariaceae, Gelidiaceae, Pterocladiaceae, etc. | A long-chain neutral polysaccharide formed by the alternating repetition of β-D-Gal linked by 1,3-glycosidic bonds and 3,6-anhydro-α-Gal linked by 1,4-glycosidic bonds. | [39] | ||
| Brown algae | Fucoidan | Fucus vesiculosus (Ochrophyta, Fucaceae), etc. | A linear core (formed by (1→6)-β-d-Gal and/or (1→2)-β-d-Man units) with branched chains of “fucan” (formed by (1→3) and/or (1→4)-α-l-Fuc, (1→4)-α-d-GlcA, teminal β-d-Xyl and, sometimes, (1→4)-α-d-Glu). | Antithrombotic, antioxidant, hypolipidemic, and immunomodulatory effects, etc. | [33,40] |
| Alginate | Macrocystis pyrifera (Ochrophyta, Laminariaceae), Laminaria hyperborea (Ochrophyta, Laminariaceae), Ascophyllum nodosum (Ochrophyta, Fucaceae), etc. | A linear polysaccharide composed of three forms: β-D-mannuronic acid (M residues), α-L-guluronic acid (G residues), or alternating M and G residues. | [28,41,42] | ||
| Green algae | Ulvan Polysaccharide | Enteromorpha prolifera (Chlorophyta, Ulvaceae), etc. | A water-soluble sulfated polysaccharide mainly composed of glucuronic acid, xylose, rhamnose, and galactose. | Immunomodulation, anti-inflammation, anti-tumor effects, etc. | [43,44] |
| Seaweed Species | Seaweed | The Optimal Addition Amount of Seaweed (in the Basal Diet) | Mechanism of Action | Application Effectiveness | References |
|---|---|---|---|---|---|
| Red algae | Lithothamnium calcareum (Rhodophyta, Corallinaceae) | 1018 μg/kg AFB1 + 0.2% L. calcareum | Binding of surface functional groups (such as carboxyl and hydroxyl) of L. calcareum to AFB1 for AFB1 adsorption. | Alleviation of AFB1-induced hepatic injury in broilers. | [83] |
| Kappaphycus alvarezii (Rhodophyta, Solieriaceae) | 1.0 g/kg seaweed extract PBD1 | Intestinal gene expression controlling gut permeability and mucosal immunity. | Growth performance and immune response in broilers ↑. | [84] | |
| Halymenia palmata (Rhodophyta, Halymeniaceae) | 0.25% | — | Linear growth trend of body weight gain and feed conversion rate. | [85] | |
| Eucheuma denticulatum (Rhodophyta, Solieriaceae) | 1.0 g/kg seaweed extract PBD5 | Enhancement of intestinal immunity and growth hormone-receptor gene expression. | Feed efficiency ↑, intestinal pathogen load↓, body growth ↑. | [77] | |
| Chondrus crispus (Rhodophyta, Gigartinaceae) | 0.30% | — | Carcass and breast meat yield ↑, abdominal fat yield ↓ | [86] | |
| 0.40% | — | Relative weights of bursa and thymus ↑, and maintenance of intestinal pH stability. | |||
| Chondracanthus chamissoi (Rhodophyta, Gigartinaceae) | 1.5% | Improvement in villus height (VH) and the ratio of villus height to crypt depth (VH:CD), and increase in the abundance of beneficial bacterial genera. | Intestinal morphology ↑, and growth performance ↑. | [87] | |
| Brown algae | Undaria pinnatifida (Ochrophyta, Alariaceae) | 0.5% | — | Weight ↑, mortality ↓. | [88] |
| Hizikia fusiformis (Ochrophyta, Sargassaceae) | 0.5% | ||||
| Ascophyllum nodosum (Ochrophyta, Fucaceae) | 2% | Reduction in plasma alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) activities in broiler chickens. | Heat stress alleviation, and growth performance ↑. | [89] | |
| Green algae | Enteromorpha prolifera (Chlorophyta, Ulvaceae) | 0.1 mg/kg AFB1 + 0.25% Enteromorpha prolifera polysaccharides | Inhibition of phase I detoxification enzymes and up-regulation of the p38MAPK/NRF2–mediated phase II detoxification enzyme pathway. | Alleviation of AFB1-induced hepatic injury in broilers. | [90] |
| 100 mg/kg AFB1 + 2500 mg/kg Enteromorpha prolifera polysaccharides | Regulation of NRF2-mediated redox and mitochondrial apoptosis signaling pa.thways in broilers | Alleviation of AFB1-induced bursal injury in broilers. | [91] | ||
| 7000 mg/kg | Up-regulation of Lipin1 and SESN1 gene expression, down-regulation of FGFR2 gene expression, promotion of protein synthesis and reduction in fat deposition. | Pectoral muscle yield ↑. | [92] | ||
| Ulva lactuca (Chlorophyta, Ulvaceae) | Replacement of corn in the basal diet with 3.0% U. lactuca | — | Slaughter rate and pectoral muscle rate in broilers ↑. | [20] |
| Seaweed Species | Seaweed | The Optimal Amount of Seaweed to Add (in the Basal Diet) | Mechanism of Action | Application Effectiveness | References |
|---|---|---|---|---|---|
| Red algae | Kappaphycus alvarezii (Rhodophyta, Solieriaceae) | 1.5% | Reduction of lipid oxidation and cholesterol content of egg yolks. | Improvement in age at sexual maturity, egg production, egg quality and immune response. | [9,106] |
| Chondrus crispus (Rhodophyta, Gigartinaceae) | 3% | Stimulation of the growth of beneficial intestinal bacteria and inhibition of Salmonella colonization. | Weight ↑, feed conversion ratio (FCR) for egg production ↓, egg production rate ↑. | [107] | |
| Chondrus crispus (Rhodophyta, Gigartinaceae) | 4% | Increased concentration of SCFAs in cecum contents and decreased Salmonella colonization. | Immune response ↑, improvement in intestinal microbial community structure, and growth performance and egg quality ↑. | [108] | |
| Sarcodiotheca gaudichaudii (Rhodophyta, Solieriaceae) | 4% | ||||
| Green algae | Caulerpa lentillifera (Chlorophyta, Caulerpaceae) | 1.5% | Increased protein intake, multiple antioxidant ingredients to scavenge free radicals in the body and reduce oxidative stress. | Maintenance of liver health, egg production rate ↑, and antioxidant activity in egg yolk ↑. | [109] |
| Ulva sp. (Chlorophyta, Ulvaceae) | 0.5~1.0% Ulvan | Up-regulation of intestinal FXR and hepatic CYP7A1 gene expression, reduction of cholesterol levels, and improvement in intestinal function. | Immune function ↑, antioxidant capacity ↑, improvement in laying performance and egg quality. | [110] | |
| Brown algae | Sargassum binderi (Ochrophyta, Sargassaceae) | 16% | Reduction of cholesterol content and low-density lipoprotein (LDL) in hen plasma and egg yolk. | Improvement in lipid metabolism in hens and egg quality ↑. | [111] |
| Cyanoba-cteria | Spirulina platensis (Cyanobacteria, Oscillatoriaceae) | 3 kg/t | Reduction of cholesterol levels in serum and egg yolk. | Egg-laying performance ↑, egg quality ↑ and FCR ↑. | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-B.; Yao, Q.-H.; Ye, X.-Q.; Balasubramanian, B.; Liu, W.-C. Unraveling of Seaweed Bioactive Substances and Their Nutritional Regulation Functions for Poultry. Mar. Drugs 2025, 23, 324. https://doi.org/10.3390/md23080324
Li S-B, Yao Q-H, Ye X-Q, Balasubramanian B, Liu W-C. Unraveling of Seaweed Bioactive Substances and Their Nutritional Regulation Functions for Poultry. Marine Drugs. 2025; 23(8):324. https://doi.org/10.3390/md23080324
Chicago/Turabian StyleLi, Si-Bing, Qing-Hua Yao, Xue-Qing Ye, Balamuralikrishnan Balasubramanian, and Wen-Chao Liu. 2025. "Unraveling of Seaweed Bioactive Substances and Their Nutritional Regulation Functions for Poultry" Marine Drugs 23, no. 8: 324. https://doi.org/10.3390/md23080324
APA StyleLi, S.-B., Yao, Q.-H., Ye, X.-Q., Balasubramanian, B., & Liu, W.-C. (2025). Unraveling of Seaweed Bioactive Substances and Their Nutritional Regulation Functions for Poultry. Marine Drugs, 23(8), 324. https://doi.org/10.3390/md23080324









