Research on the Influence of Orthogonal Design Optimized Elicitor Combinations on Fucoxanthin Accumulation in Phaeodactylum tricornutum and Its Expression Regulation
Abstract
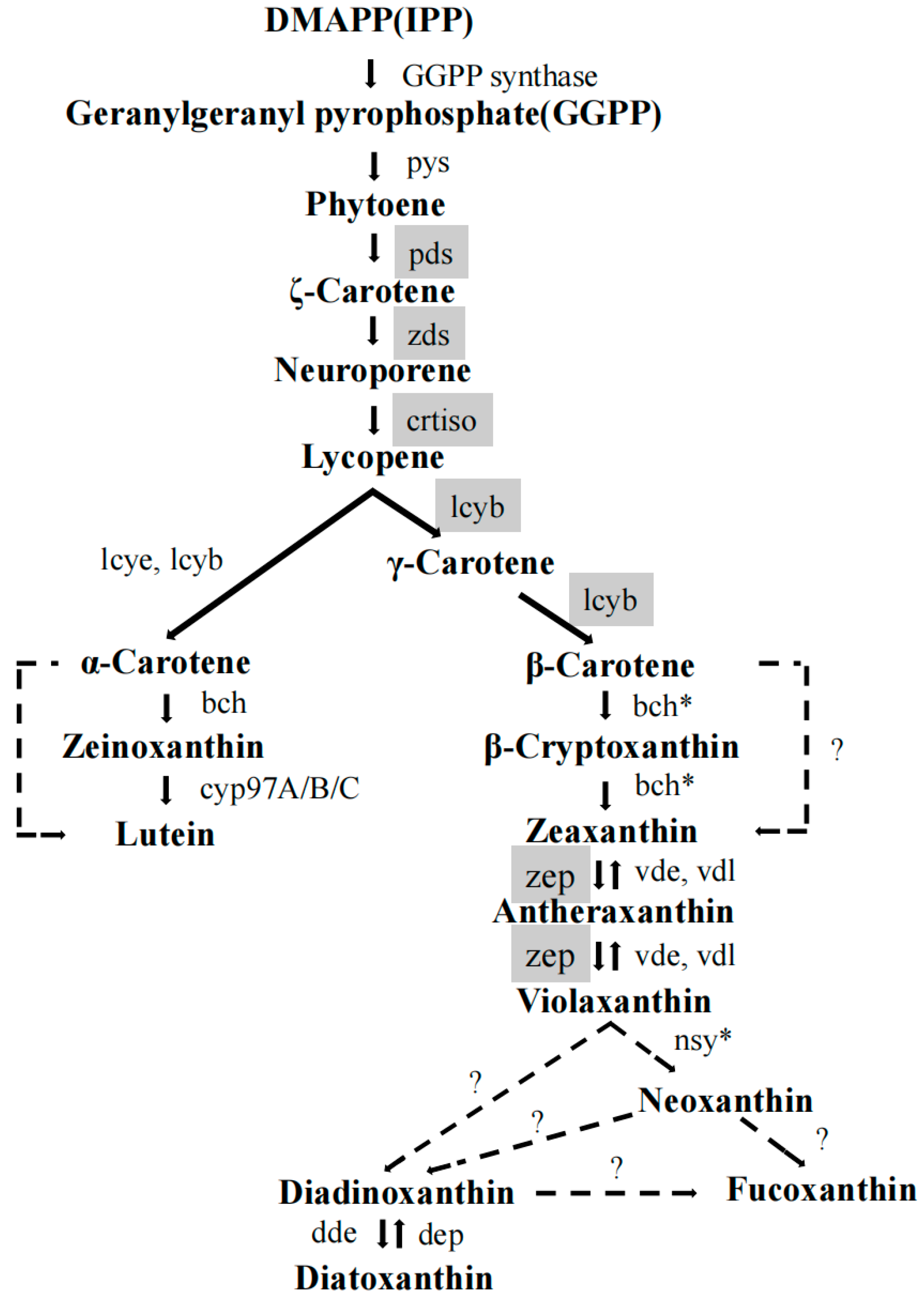
1. Introduction
2. Results
2.1. Effects of Orthogonal Experimental Design on Fucoxanthin Accumulation in P. tricornutum
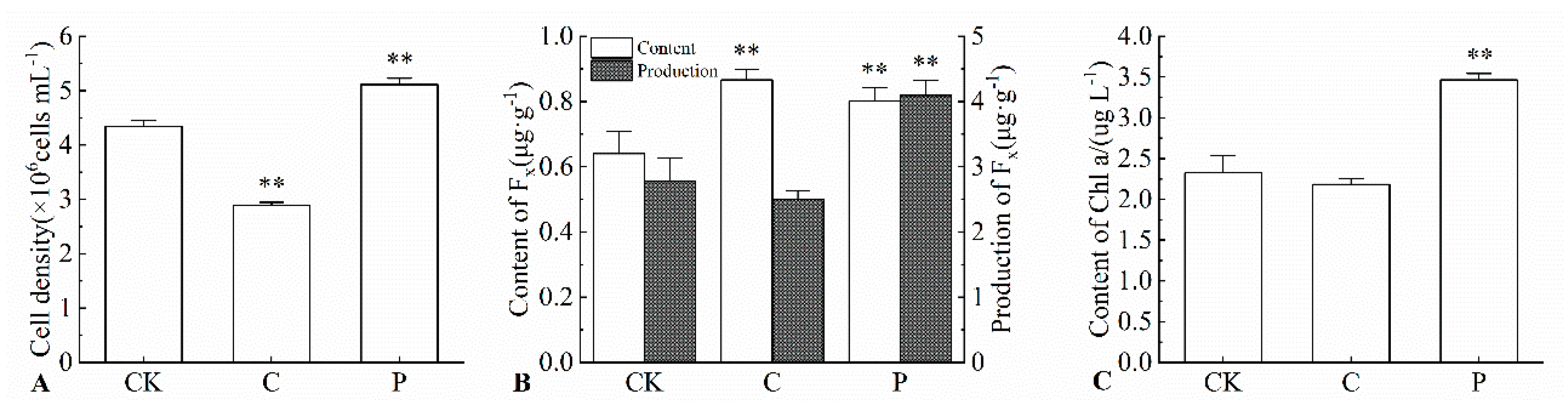
2.2. Effects of the Optimal Inducer Combinations on Cell Density of P. tricornutum
2.3. Effects of the Optimal Inducer Combinations on Fucoxanthin Content and Yield in P. tricornutum
2.4. Effects of the Optimal Inducer Combinations on Chl a Content in P. tricornutum
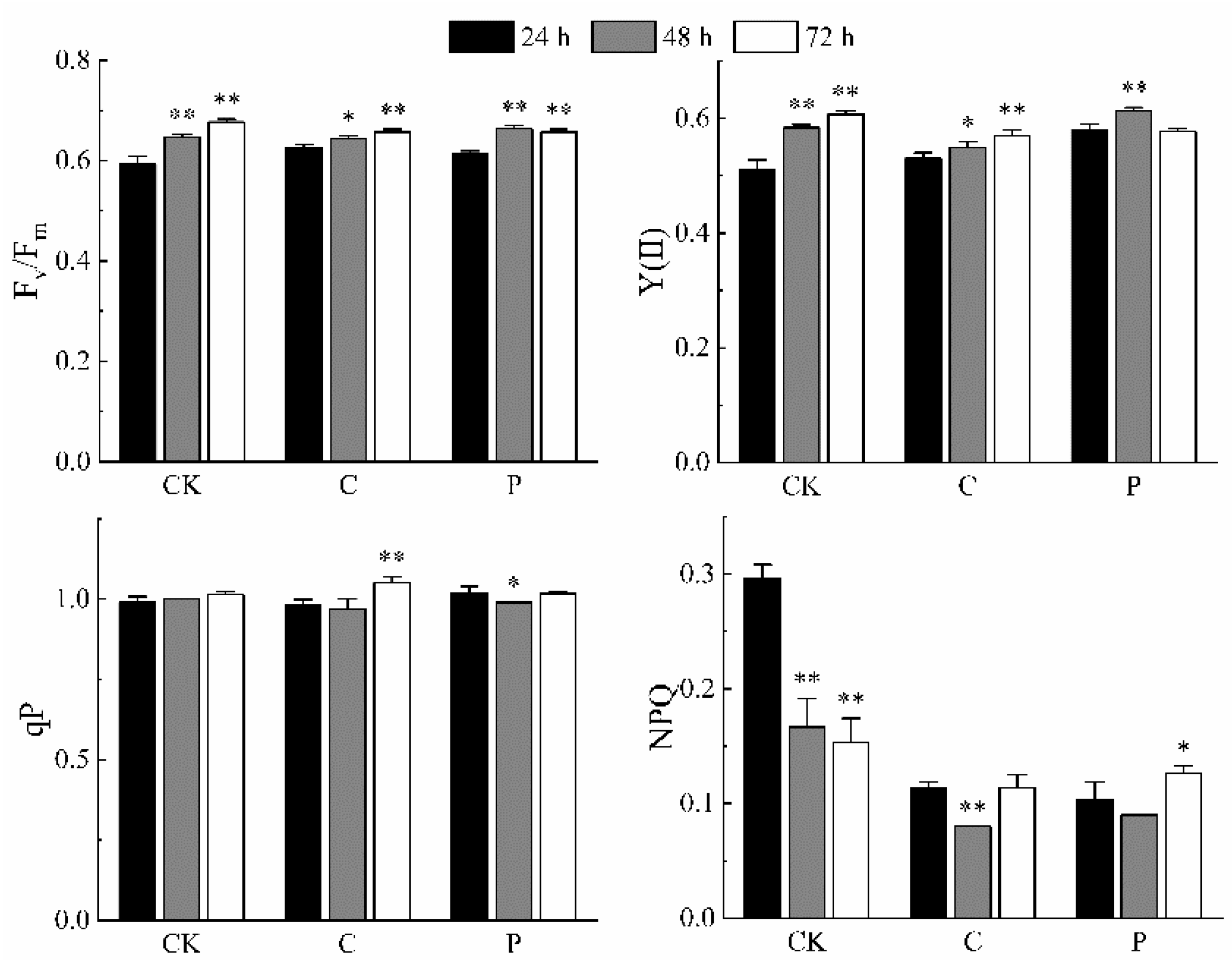
2.5. Effects of the Optimal Inducer Combinations on Chlorophyll Fluorescence Parameters in P. tricornutum
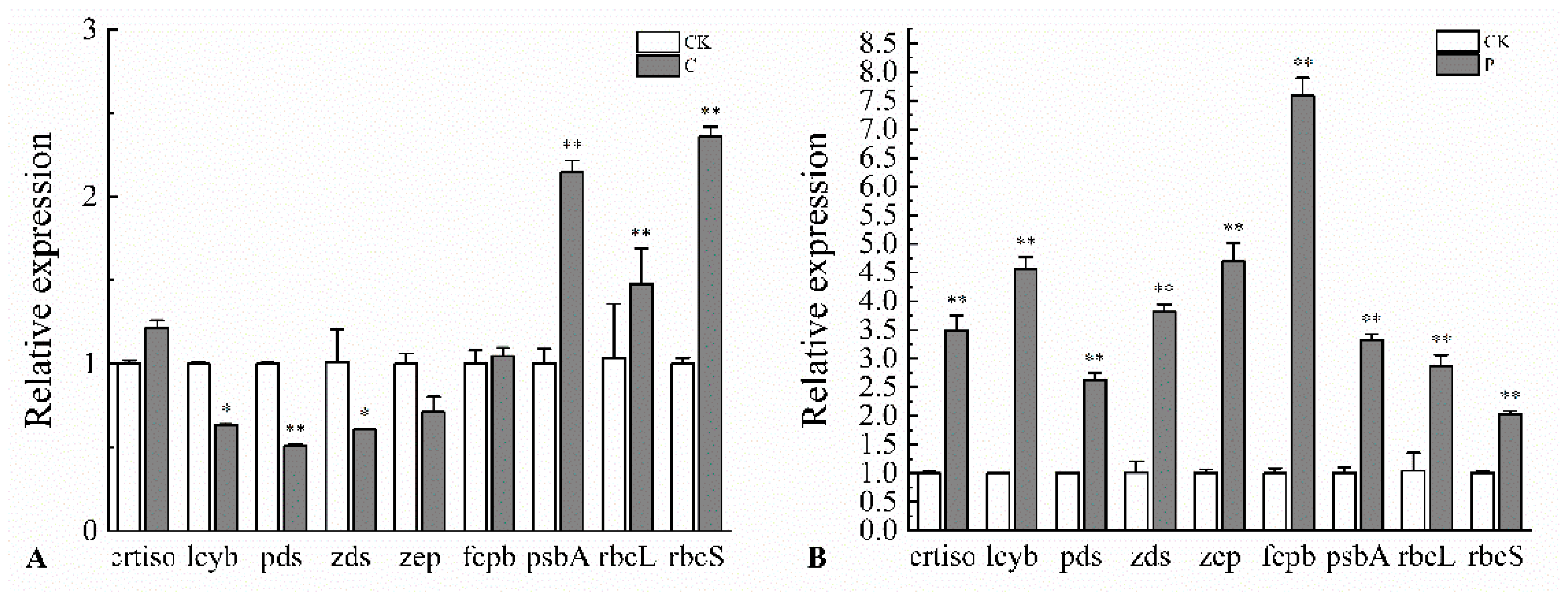
2.6. Effects of the Optimal Inducer Combinations on the Expression of Fucoxanthin-Related Genes in P. tricornutum
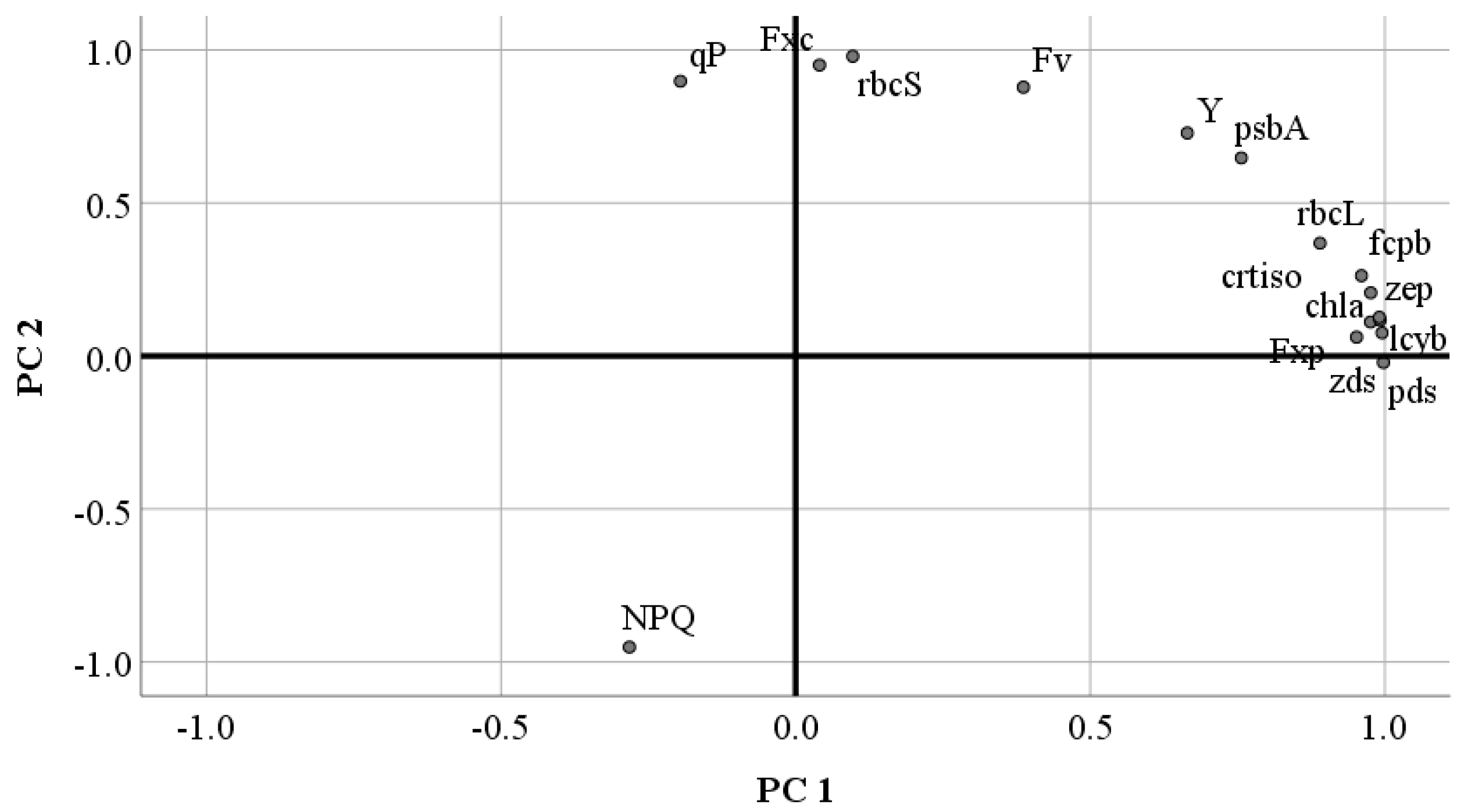
2.7. Correlation and Principal Component Analysis of Fucoxanthin with Related Parameters in P. tricornutum
3. Discussion
4. Materials and Methods
4.1. Cultivation of P. tricornutum
4.2. Orthogonal Experimental Design
4.3. Determination of P. tricornutum Cell Density
4.4. Determination of Fucoxanthin Content and Yield in P. tricornutum
4.5. Data Analysis of Orthogonal Experimental Results
4.6. Validation of Orthogonal Experimental Results
4.7. Determination of Chl a Content, Fucoxanthin Content and Yield Under the Optimal Inducer Combination
4.8. Measurement of Chlorophyll Fluorescence Parameters Under the Optimal Inducer Combination
4.9. Analysis of Fucoxanthin-Related Gene Expression Under the Optimal Inducer Combination
4.10. Data Processing and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.W.; Ye, Y.M.; Zhou, C.X.; Zhang, J.R. Isolation and Purification of Fucoxanthin from Phaeodactylum tricornutum. Chin. J. Mar. Drugs 2024, 43, 19–27. [Google Scholar]
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum tricornutum, A Diatom Cell Factory. Trends Biotechnol. 2020, 38, 606–622. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.; Yang, G.; Pan, K.; Wang, L.; Hu, Z. A Review on the Progress, Challenges and Prospects in Commercializing Microalgal Fucoxanthin. Biotechnol. Adv. 2021, 53, 107865. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Liu, Y. Anti-Inflammatory and Apoptotic Signaling Effect of Fucoxanthin on Benzo(A)pyrene-Induced Lung Cancer in Mice. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 239–251. [Google Scholar] [CrossRef]
- Yang, H.Y.; Xing, R.E.; Liu, S.; Yu, H.H.; Li, P.C. Role of Fucoxanthin towards Cadmium-Induced Renal Impairment with the Antioxidant and Anti-Lipid Peroxide Activities. Bioengineered 2021, 12, 7235–7247. [Google Scholar] [CrossRef]
- Koo, S.Y.; Hwang, J.H.; Yang, S.H.; Um, J.I.; Hong, K.W.; Kang, K.; Pan, C.H.; Hwang, K.T.; Kin, S.M. Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Mendonca, P.; Elhag, R.; Soliman, K.F.A. Anticancer Effects of Fucoxanthin through Cell Cycle Arrest, Apoptosis Induction, Angiogenesis Inhibition, and Autophagy Modulation. Int. J. Mol. Sci. 2022, 23, 16091. [Google Scholar] [CrossRef]
- Liu, Y.; Zhi, L.C.; Wang, H.R.; Zhao, L.; Ren, D.D.; He, Y.H.; Wang, Q.K. Inhibition of α-Glucosidase Activity and Hypoglycemic Effect by Fucoxanthin Extracted from Seaweed (Sargassum horneri). J. Dalian Ocean. Univ. 2023, 38, 120–128. [Google Scholar]
- Lee, A.H.; Shin, H.Y.; Park, J.H.; Koo, S.Y.; Kim, S.M.; Yang, S.H. Fucoxanthin from Microalgae Phaeodactylum tricornutum Inhibits Pro-Inflammatory Cytokines by Regulating both NF-κB and NLRP3 Inflammasome Activation. Sci. Rep. 2021, 11, 543. [Google Scholar] [CrossRef]
- Sarah, M.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, A Marine-Derived Carotenoid from Brown Seaweeds and Microalgae, A Promising Bioactive Compound for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9273. [Google Scholar]
- Kim, S.M.; Jung, Y.J.; Kwon, O.N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.H. A Potential Commercial Source of Fucoxanthin Extracted from the Microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Yu, L.J.; Xu, C.Z.; Tomizaki, T.; Zhao, S.H.; Umena, Y.; Chen, X.B.; Qin, X.C.; Xin, Y.Y.; Suga, M.; et al. Structural Basis for Blue-Green Light Harvesting and Energy Dissipation in Diatoms. Science 2019, 363, 598. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, Z.P.; Ge, S.J.; Yang, C.; Qiu, S. Effects of Light Intensity on Growth of Native Microalgal Cellular Components and Performance of Biodiesel. J. Nanjing Univ. Sci. Technol. 2024, 48, 118–126. [Google Scholar]
- Tong, L.H.; Wu, X.Y.; Huang, L.F.; Zeng, J.; Shi, Y.H.; Tang, X.M. Correlation Analysis of Light Intensity and Growth, Photosynthetic Pigment, Color Value of Betaphycus gelatinae. S. China Fish. Sci. 2021, 17, 79–85. [Google Scholar]
- Song, Y.H.; Gong, Y.F.; Liu, B.Y.; Li, L.Q.; Wang, H.Y. Effect of Different Photoperiods on Growth, Fucoxanthin Content, Photosynthesis and Related Gene Expression of Isochrysis zhanjiangensis. J. Nucl. Agric. Sci. 2024, 38, 532–539. [Google Scholar]
- Wang, S.; Verma, S.K.; Inamullah, H.S.; Laurenz, T.; Ullrich, M.S.; Nikolai, K. Changes in the Fucoxanthin Production and Protein Profiles in Cylindrotheca closterium in Response to Blue Light-Emitting Diode Light. Microb. Cell Fact. 2018, 17, 110. [Google Scholar] [CrossRef]
- Fathy, W.A.; AbdElgawad, H.; Essawy, E.A.; Tawfik, E.; Abdelhameed, M.S.; Hammouda, O.; Korany, S.M.; Elsayed, K.N.M. Glycine Differentially Improved the Growth and Biochemical Composition of Synechocystis sp. PAK13 and Chlorella variabilis DT025. Front. Bioeng. Biotechnol. 2023, 11, 1161911. [Google Scholar] [CrossRef]
- Li, Y.; Cui, L.; Lei, J.M.; Li, Q.; Zhang, X. Effects of Different Concentrations of Organic Affixture on the Growth and Secondary Metabolites Contents in Adventitious Roots of Tripterygium wilfordii. Plant Sci. J. 2014, 32, 174–180. [Google Scholar]
- Zhao, L.X. The Research of Biologic Characteristic of Dunaliella Salina. Master’s Thesis, AAU, Hefei, China, 2005. [Google Scholar]
- Wu, X.; Gong, Y.F.; Li, L.Q.; Gao, X.W.; Lv, J.; Wang, H.Y. Effects of Orthogonal Design Optimized Elicitor Combinations on fucoxanthin Content and Photosynthetic Physiology and Gene Expression of Phaeodactylum Tricornutum. Chin. Pharm. J. 2024, 59, 879–886. [Google Scholar]
- Guo, W.X.; Qiao, F.; Luo, X.F.; Zhou, X.Z.; Zhou, J.J. Effects of Light on the Growth and Physiology of Three Strains of Polygonati rhizoma Seedlings. Chin. J. Trop. Agric. 2024, 44, 57–62. [Google Scholar]
- Zang, W.; Meng, X.Q.; Su, X.H.; Wang, J.Y.; Li, L.H.; Jia, M. Effects of Light Regulation on the Synthesis of Secondary Metabolites in Medicinal Plants. J. Pharm. Pract. Serv. 2024, 42, 50–59. [Google Scholar]
- Fang, H.L.; Qi, X.W.; Li, Y.M.; Yu, X.; Xu, D.B.; Liang, C.Y.; Li, W.L.; Liu, X. De Novo Transcriptomic Analysis of Light-Induced Flavonoid Pathway, Transcription Factors in the Flower Buds of Lonicera japonica. Trees 2020, 34, 267–283. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Zhu, Z.L.; Jia, Z.K.; Ma, L.Y.; Sang, Z.Y.; Wu, N.S.; Xu, M.Y.; Luo, Q.Q.; Deng, Z.W.; Wang, J.W. Effects of Photoperiod on Leaf Nutrition and Photosynthetic Physiology of Magnolia wufengensis “Jiaohong 1”. NWF Res. 2024, 42, 189–200+210. [Google Scholar]
- Dong, W.; Li, M.M.; Li, Z.A.; Li, S.L.; Zhu, Y.; Hong, X.; Wang, Z.C. Transcriptome Analysis of the Molecular Mechanism of Chrysanthemum Flower Color Change Under Short-Day Photoperiods. Plant Physiol. Biochem. 2020, 146, 315–328. [Google Scholar] [CrossRef]
- Ren, H.M.; Ren, Y.M.; Zhou, Y.C.; Jia, S.J.; Li, W.; Huang, Y. Effects of Glycine Spraying Concentration on the Growth of Spinach. Liaoning Agric. Sci. 2022, 49–53. [Google Scholar]
- Niu, J.J.; Fang, S.M.; Wang, Q.Y.; Liang, X.L. Effects of Different Concentrations of Glycine Mixed in Soil on Growth Characteristics of Rice Seedlings. Chin. Rice. 2024, 30, 47–52. [Google Scholar]
- Qin, J.L.; Yu, M.J.; Li, X.; Xu, N.; Duan, S.S. Effect of Nitrogen Sources on the Growth of Phaeocystis globosa. Ecol. Sci. 2012, 31, 273–277. [Google Scholar]
- Zhang, Z.J.; Wang, P. Optimization of Culture Conditions of Chlorella sp. J. Food Sci. Technol. 2011, 29, 54–58. [Google Scholar]
- Fu, H.H.; He, Y.H.; Yin, Y.Y.; Hu, W.; Yang, Y.; Yue, Z.G. Optimum Conditions for Regulating Content Change of Secondary Metabolites of Gentiana macrophylla by Orthogonal Method. Guihaia 2023, 43, 777–784. [Google Scholar]
- Wang, M.L.; Jiang, Y.L. Effects of Manganese on the Growth and Fluorescence Induction Kinetics of Conticribra weissflogii. Environ. Sci. 2018, 39, 5514–5522. [Google Scholar]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of Photosynthesis During Abiotic Stress-Induced Photoinhibition. Mol. Plant. 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.H.; Hayer, H.M. Complex Chaperone Dependence of Rubisco Biogenesis. Biochemistry 2018, 57, 3210–3216. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K.; Hosokawa, M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int. J. Mol. Sci. 2013, 14, 13763–13781. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.H.; Zhu, J.H.; Jiang, J.G. Carotenoids biosynthesis and cleavage related genes from bacteria to plants. Crit. Rev. Food Sci. Nutr. 2018, 58, 2314–2333. [Google Scholar] [CrossRef]
- Guan, T.T. A Preliminary Study on the cDNA Cloning and Function of Undaria Pinnatifida Suringar Encoding Phytoene Desaturase. Master’s Thesis, Qingdao University, Qingdao, China, 2020. [Google Scholar]
- Wei, F.J.; Gong, Y.F.; Zhang, L.; Chen, R.S.; Wang, H.Y.; Yang, B.D. Effects of Rapamycin on the Content of Fucoxanthin in Phaeodactylum tricornutum and the Expression of Key Enzyme Genes. J. Biol. 2022, 39, 72. [Google Scholar]
- Chen, R.S.; Zhang, L.; Wei, F.J.; Yuan, L.Y.; Zhao, P.; Wang, H.Y.; Gong, Y.F. Effects of Mn2+ on Neutral Lipid Content, C4 Pathway, and Related Gene Expression in Phaeodactylum tricornutum. SSBM 2021, 33, 2123–2133. [Google Scholar] [CrossRef]
- Li, H.S.; Sun, Q.; Zhao, S.J. Principle and Technology of Plant Physiological and Biochemical Experiments; HEP: Beijing, China, 2000. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| No. | Factors | Fucoxanthin Content (μg g−1) | Fucoxanthin Product (μg L−1) | ||
|---|---|---|---|---|---|
| Gly | Light Intensity | Photoperiod | |||
| 1 | 1 | 1 | 1 | 0.83 ± 0.10 ± 0.07 c | 0.86 ± 0.07 ± 0.05 h |
| 2 | 2 | 2 | 1 | 0.93±0.01 ± 0.01 ab | 3.23 ± 0.07 ± 0.05 a |
| 3 | 3 | 3 | 1 | 0.85 ± 0.03 ± 0.02 bc | 2.72 ± 0.11 ± 0.08 b |
| 4 | 4 | 4 | 1 | 0.82 ± 0.04 ± 0.03 cde | 2.32 ± 0.06 ± 0.04 d |
| 5 | 2 | 1 | 2 | 0.97 ± 0.18 ± 0.13 a | 1.04 ± 0.06 ± 0.04 gh |
| 6 | 1 | 2 | 2 | 0.77 ± 0.03 ± 0.02 cde | 2.63 ± 0.08 ± 0.06 b |
| 7 | 4 | 3 | 2 | 0.80 ± 0.03 ± 0.02 cde | 2.68 ± 0.11 ± 0.08 b |
| 8 | 3 | 4 | 2 | 0.74 ± 0.04 ± 0.03 de | 2.63 ± 0.08 ± 0.06 b |
| 9 | 3 | 1 | 3 | 0.84 ± 0.03 ± 0.02 c | 1.28 ± 0.14 ± 0.10 fg |
| 10 | 4 | 2 | 3 | 0.80 ± 0.08 ± 0.06 cde | 2.61 ± 0.37 ± 0.26 bc |
| 11 | 1 | 3 | 3 | 0.73 ± 0.01 ± 0.01 e | 2.38 ± 0.14 ± 0.10 cd |
| 12 | 2 | 4 | 3 | 0.77 ± 0.04 ± 0.03 cde | 3.04 ± 0.13 ± 0.09 a |
| 13 | 4 | 1 | 4 | 0.85 ± 0.03 ± 0.02 bc | 1.46 ± 0.00 ± 0.00 f |
| 14 | 3 | 2 | 4 | 0.83 ± 0.01 ± 0.01 cd | 3.08 ± 0.08 ± 0.06 a |
| 15 | 2 | 3 | 4 | 0.79 ± 0.06 ± 0.04 cde | 3.15 ± 0.34 ± 0.24 a |
| 16 | 1 | 4 | 4 | 0.61 ± 0.08 ± 0.06 f | 1.73 ± 0.28 ± 0.20 e |
| kC1 | 0.73 | 0.87 | 0.86 | - | - |
| kC2 | 0.86 | 0.83 | 0.82 | - | - |
| kC3 | 0.81 | 0.79 | 0.78 | - | - |
| kC4 | 0.82 | 0.73 | 0.77 | - | - |
| kP1 | 1.90 | 1.16 | 2.28 | - | - |
| kP2 | 2.62 | 2.89 | 2.25 | - | - |
| kP3 | 2.43 | 2.73 | 2.33 | - | - |
| kP4 | 2.27 | 2.43 | 2.36 | - | - |
| RC | 0.13 | 0.14 | 0.09 | - | - |
| RP | 0.72 | 1.73 | 0.11 | - | - |
| Source of Variation | Square Sum | Grees of Freedom | Mean Square | F | p | Significance |
|---|---|---|---|---|---|---|
| Gly | 0.04 | 3 | 0.01 | 12.44 | 0.01 | ** |
| Light intensity | 0.04 | 3 | 0.01 | 14.69 | 0.00 | ** |
| Photoperiod | 0.02 | 3 | 0.01 | 7.02 | 0.02 | * |
| Error | 0.01 | 6 | 0.00 |
| Source of Variation | Square Sum | Degrees of Freedom | Mean Square | F | p | Significance |
|---|---|---|---|---|---|---|
| Gly | 1.11 | 3 | 0.37 | 3.76 | 0.08 | ** |
| Light intensity | 7.39 | 3 | 2.46 | 24.98 | 0.00 | ** |
| Photoperiod | 0.03 | 3 | 0.01 | 0.10 | 0.96 | |
| Error | 0.59 | 6 | 0.10 |
| Items | Indicators | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chla | Fv/Fm | Y(II) | qP | NPQ | crtiso | lcyb | pds | zds | zep | fcpb | psbA | rbcL | rbcS | ||
| C | Correlation coefficient | 0.18 | 0.78 | 0.75 | 0.85 | −0.90 | 0.28 | 0.15 | 0.02 | 0.10 | 0.15 | 0.23 | 0.64 | 0.33 | 0.93 |
| Sig. (both sides) | 0.64 | 0.01 | 0.02 | 0.00 | 0.00 | 0.47 | 0.71 | 0.97 | 0.80 | 0.70 | 0.55 | 0.07 | 0.38 | 0.00 | |
| P | Correlation coefficient | 0.98 | 0.35 | 0.71 | −0.07 | −0.30 | 0.91 | 0.95 | 0.95 | 0.94 | 0.94 | 0.95 | 0.74 | 0.80 | 0.12 |
| Sig. (both side) | 0.00 | 0.36 | 0.03 | 0.86 | 0.43 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | 0.75 | |
| No. | Gly (g L−1) | Light Intensity (μmol Photons·m−2·s−1) | Photoperiod (h Light/h Dark) |
|---|---|---|---|
| 1 | 0 | 18 | 12/12 |
| 2 | 0.5 | 36 | 16/8 |
| 3 | 1.0 | 54 | 20/4 |
| 4 | 2.0 | 72 | 24/0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Gong, Y.; Liu, B.; Chen, Y.; Qin, H.; Wang, H.; Liu, H. Research on the Influence of Orthogonal Design Optimized Elicitor Combinations on Fucoxanthin Accumulation in Phaeodactylum tricornutum and Its Expression Regulation. Mar. Drugs 2025, 23, 244. https://doi.org/10.3390/md23060244
Yang H, Gong Y, Liu B, Chen Y, Qin H, Wang H, Liu H. Research on the Influence of Orthogonal Design Optimized Elicitor Combinations on Fucoxanthin Accumulation in Phaeodactylum tricornutum and Its Expression Regulation. Marine Drugs. 2025; 23(6):244. https://doi.org/10.3390/md23060244
Chicago/Turabian StyleYang, Han, Yifu Gong, Boyue Liu, Yuru Chen, Huan Qin, Heyu Wang, and Hao Liu. 2025. "Research on the Influence of Orthogonal Design Optimized Elicitor Combinations on Fucoxanthin Accumulation in Phaeodactylum tricornutum and Its Expression Regulation" Marine Drugs 23, no. 6: 244. https://doi.org/10.3390/md23060244
APA StyleYang, H., Gong, Y., Liu, B., Chen, Y., Qin, H., Wang, H., & Liu, H. (2025). Research on the Influence of Orthogonal Design Optimized Elicitor Combinations on Fucoxanthin Accumulation in Phaeodactylum tricornutum and Its Expression Regulation. Marine Drugs, 23(6), 244. https://doi.org/10.3390/md23060244






