Photoautotrophic Batch Cultivation of Limnospira (Spirulina) platensis: Optimizing Biomass Productivity and Bioactive Compound Synthesis Through Salinity and pH Modulation
Abstract
1. Introduction
2. Results
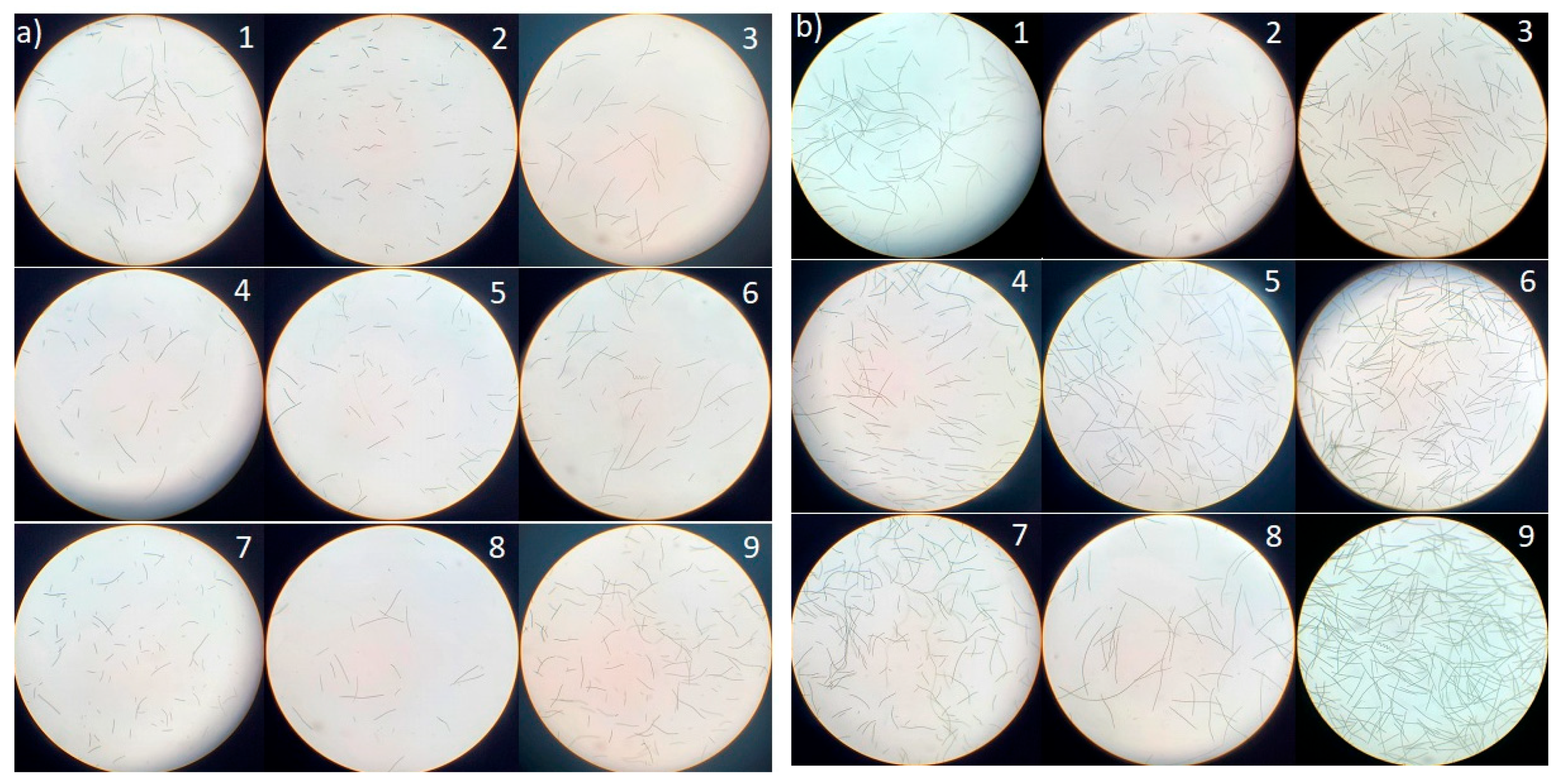
2.1. Growth Profile and Biomass Composition of L. platensis in BWW and SW
Growth Profile of L. platensis Under Salinity and Alkalinity
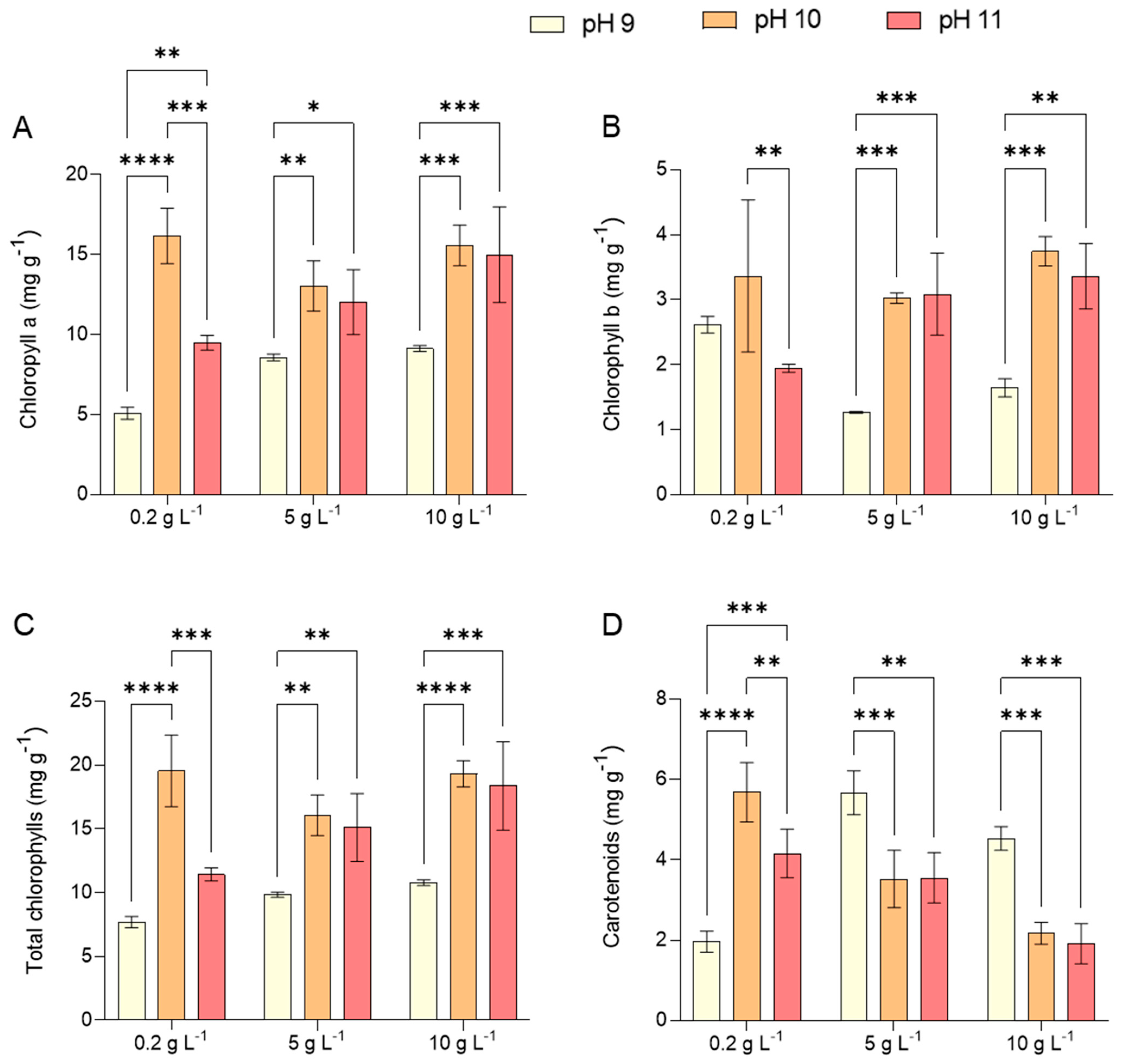
2.2. Pigments and Antioxidants Content of L. platensis Under Salinity and Alkalinity
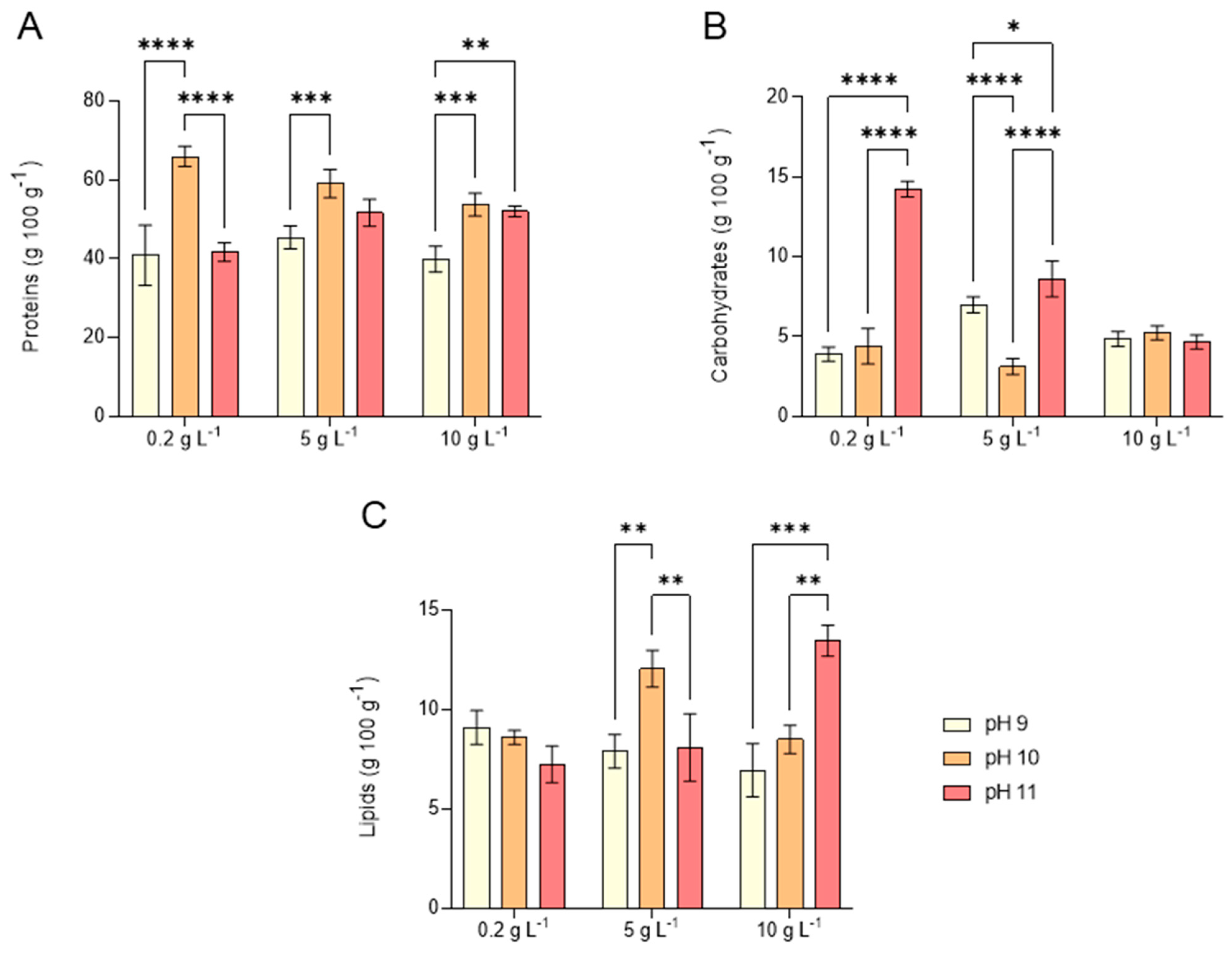
2.3. Proximate Biomass Composition of L. platensis Under Salinity and Alkalinity
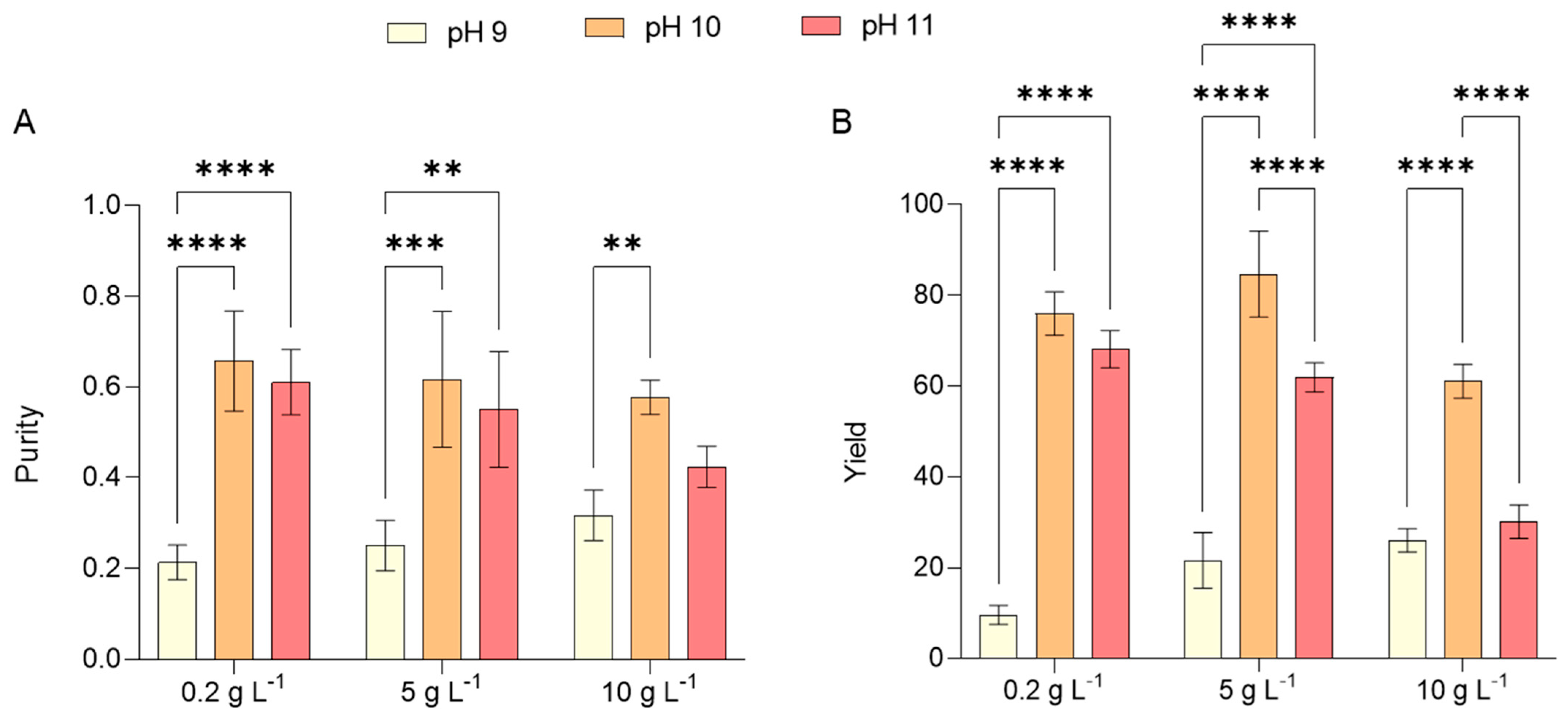
2.4. Phycocyanin Production by L. platensis Under Alkalinity and Salinity
3. Materials and Methods
3.1. Inocula and Culture Media Preparation
3.2. Cultivation Conditions and Experimental Setup
3.3. Cell Growth and Dry
3.4. Analysis of Proximate Composition
3.4.1. Protein Analysis
3.4.2. Lipid Analysis
3.4.3. Carbohydrate Analysis
3.5. Determination of Photosynthetic Pigment Content
3.6. Antioxidant Power Determination
3.7. Total Polyphenols Determination
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbosa, M.J.; Janssen, M.; Südfeld, C.; D’Adamo, S.; Wijffels, R.H. Hypes, Hopes, and the Way Forward for Microalgal Biotechnology. Trends Biotechnol. 2023, 41, 452–471. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. Introductory Chapter: Microalgae—A Versatile and Valuable Resource for Sustainable Development. In Microalgae—Current and Potential Applications; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Usai, L.; Ciurli, A.; Chiellini, C.; Di Caprio, F.; Pagnanelli, F.; Parsaeiemhr, A.; Malina, I.; Malins, K.; Fabbricino, M.; et al. Engineering Strategies of Microalgal Cultivation for Potential Jet Fuel Production: A Critical Review. J. Environ. Chem. Eng. 2024, 12, 113886. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Parsaeimehr, A.; Ozbay, G.; Ciurli, A.; Bacci, L.; Rao, A.R.; Ravishankar, G.A.; Concas, A. Microalgae and Cyanobacteria Role in Sustainable Agriculture: From Wastewater Treatment to Biofertilizer Production. Algae Mediat. Bioremediation Ind. Prospect. 2024, 2, 565–618. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Usai, L.; Ciurli, A.; Chiellini, C.; Di Caprio, F.; Pagnanelli, F.; Parsaeiemhr, A.; Malina, I.; Malins, K.; Fabbricino, M.; et al. Bio Jet-Fuels from Photosynthetic Microorganisms: A Focus on Downstream Processes. Biomass Bioenergy 2025, 198. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Marin, M.A.; Concas, A.; Dunford, N.T. Growing Picochlorum oklahomensis in Hydraulic Fracking Wastewater Supplemented with Animal Wastewater. Water Air Soil Pollut. 2020, 231, 457. [Google Scholar] [CrossRef]
- Rojas-Villalta, D.; Rojas-Rodríguez, D.; Villanueva-Ilama, M.; Guillén-Watson, R.; Murillo-Vega, F.; Gómez-Espinoza, O.; Núñez-Montero, K. Exploring Extremotolerant and Extremophilic Microalgae: New Frontiers in Sustainable Biotechnological Applications. Biology 2024, 13, 712. [Google Scholar] [CrossRef]
- Ashfaq, A.; Syed Salman, A. Harnessing Microalgae: Innovations for Achieving UN Sustainable Development Goals and Climate Resilience. J. Water Process Eng. 2024, 68, 106506. [Google Scholar] [CrossRef]
- Baunach, M.; Guljamow, A.; Miguel-Gordo, M.; Dittmann, E. Harnessing the Potential: Advances in Cyanobacterial Natural Product Research and Biotechnology. Nat. Prod. Rep. 2024, 41, 347–369. [Google Scholar] [CrossRef]
- Citi, V.; Torre, S.; Flori, L.; Usai, L.; Aktay, N.; Dunford, N.T.; Lutzu, G.A.; Nieri, P. Nutraceutical Features of the Phycobiliprotein C-Phycocyanin: Evidence from Arthrospira platensis (Spirulina). Nutrients 2024, 16, 1752. [Google Scholar] [CrossRef]
- Cavallini, A.; Torre, S.; Usai, L.; Casula, M.; Fais, G.; Nieri, P.; Concas, A.; Lutzu, G.A. Effect of Cheese Whey on Phycobiliproteins Production and FAME Profile by Arthrospira platensis (Spirulina): Promoting the Concept of a Circular Bio-Economy. Sustain. Chem. Pharm. 2024, 40, 101625. [Google Scholar] [CrossRef]
- Russo, N.P.; Ballotta, M.; Usai, L.; Torre, S.; Giordano, M.; Casula, M.; Fais, G.; Dessi, D.; Nieri, P.; Damergi, E.; et al. Mixotrophic Cultivation of Arthrospira platensis (Spirulina) under Salt Stress: Effect on Biomass Composition, FAME Profile, and Phycocyanin Content. Mar. Drugs 2024, 22, 381. [Google Scholar] [CrossRef] [PubMed]
- Razzak, S.A.; Bahar, K.; KMIslam, K.M.O.; Khaleel Haniffa, A.; Faruque, M.O.; Hossain, S.M.Z.; Hossain, M.M. Microalgae Cultivation in Photobioreactors: Sustainable Solutions for a Greener Future. Green Chem. Eng. 2024, 5, 418–439. [Google Scholar] [CrossRef]
- Uzlasir, T.; Isik, O.; Uslu, L.H.; Selli, S.; Kelebek, H. Impact of Different Salt Concentrations on Growth, Biochemical Composition and Nutrition Quality of Phaeodactylum tricornutum and Spirulina platensis. Food Chem. 2023, 429, 136843. [Google Scholar] [CrossRef]
- Alkhamis, Y.A.; Mathew, R.T.; Nagarajan, G.; Rahman, S.M.; Rahman, M.M. pH Induced Stress Enhances Lipid Accumulation in Microalgae Grown under Mixotrophic and Autotrophic condition. Front. Energy Res. 2022, 10, 1033068. [Google Scholar] [CrossRef]
- Markou, G.; Kougia, E.; Arapoglou, D.; Chentir, I.; Andreou, V.; Tzovenis, I. Production of Arthrospira platensis: Effects on Growth and Biochemical Composition of Long-Term Acclimatization at Different Salinities. Bioengineering 2023, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Yadav, P.; Kumar, A.; Hashem, A.; Avila-Quezada, G.D.; Abd Allah, E.F.; Gupta, R.K. Salinity-Induced Physiochemical Alterations to Enhance Lipid Content in Oleaginous Microalgae Scenedesmus sp. BHU1 via Two-Stage Cultivation for Biodiesel Feedstock. Microorganisms 2023, 11, 2064. [Google Scholar] [CrossRef]
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal lipids: A Review of Lipids Potential and Quantification for 95 Phytoplankton Species. Biomass Bioenergy 2021, 150, 106108. [Google Scholar] [CrossRef]
- Santin, A.; Russo, M.T.; Ferrante, M.I.; Balzano, S.; Orefice, I.; Sardo, A. Highly Valuable Polyunsaturated Fatty Acids from Microalgae: Strategies to Improve Their Yields and Their Potential Exploitation in Aquaculture. Molecules 2021, 26, 7697. [Google Scholar] [CrossRef]
- Macías-de la Rosa, A.; López-Rosales, L.; Contreras-Gómez, A.; Sánchez-Mirón, A.; García-Camacho, F.; Cerón-García, M.d.C. Salinity as an Abiotic Stressor for Eliciting Bioactive Compounds in Marine Microalgae. Toxins 2024, 16, 425. [Google Scholar] [CrossRef]
- Fal, S.; Aasfar, A.; Rabie, R.; Smouni, A.; Arroussi, H.E.L. Salt Induced Oxidative Stress Alters Physiological, Biochemical and Metabolomic Responses of Green Microalga Chlamydomonas reinhardtii. Heliyon 2022, 8, e08811. [Google Scholar] [CrossRef]
- Seghiri, R.; Kharbach, M.; Essamri, A. Functional Composition, Nutritional Properties, and Biological Activities of Moroccan Spirulina Microalga. J. Food Qual. 2019, 3707219. [Google Scholar] [CrossRef]
- Almendinger, M.; Sascha Rohn, F.S.; Kurth, E.; Springer, M.; Pleissner, D. Characterization of Selected Microalgae and Cyanobacteria as Sources of Compounds with Antioxidant Capacity. Algal Res. 2021, 53, 102168. [Google Scholar] [CrossRef]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, M.M.; El-Ayouty, Y.M.; Piercey-Normore, M. Role of pH on Antioxidants Production by Spirulina (Arthrospira) platensis. Braz. J. Microbiol. 2016, 47, 298–304. [Google Scholar] [CrossRef]
- Babu, M.; Ashok, K.; Senthil, J.; Kalaiyarasu, T. Effect of pH on Arthrospira platensis Production. Alochana Chakra J. 2020, 9, 2297–2305. [Google Scholar]
- Carvallo, J.C.M.; Sato, S.; Moraes, I.D.E.O.; Pelizer, L.H. Spirulina platensis Growth Estimation by pH Determination at Different Cultivation Conditions. Electron. J. Biotechnol. 2022, 5, 251–257. [Google Scholar]
- Kim, C.J.; Jung, Y.H.; Oh, H.M. Factors Indicating Culture Status During Cultivation of Spirulina (Arthospira) platensis. J. Microbiol. 2007, 45, 122–127. [Google Scholar]
- Sharma, G.; Kumar, M.; Ali, M.I.; Jasuja, N.D. Effect of Carbon Content, Salinity and pH on Spirulina platensis for Phycocyanin, Allophycocyanin and Phycoerythrin Accumulation. J. Microb. Biochem. Technol. 2014, 6, 202–206. [Google Scholar] [CrossRef]
- Kurkela, J.; Tyystjärvi, T. Inorganic Carbon Sensing and Signalling in Cyanobacteria. Physiol. Plant. 2024, 176, e14140. [Google Scholar] [CrossRef]
- Zrimec, M.B.; Sforza, E.; Pattaro, L.; Carecci, D.; Ficara, E.; Idà, A.; Ferrer-Ledo, N.; Canziani, S.; Mangini, S.; Lazar, B.; et al. Advances in Spirulina Cultivation: Techniques, Challenges, and Applications. In Insights into Algae—Fundamentals, Culture Techniques and Biotechnological Uses of Microalgae and Cyanobacteria; Severo, I.A., Martinze-Burgos, W.J., Ordonez, J., Eds.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Pisal, D.S.; Lele, S.S. Carotenoid Production from Microalga Dunaliella salina. Indian J. Biotechnol. 2005, 4, 476–483. [Google Scholar]
- Ilkhur, A.; Cirik, S.; Goksan, T. Effect of Light Intensity, Salinity and Temperature on Growth in Camalt Strain of Dunaliella viridis and Teo doresco from Turkey. J. Biol. Sci. 2008, 8, 1356–1359. [Google Scholar] [CrossRef][Green Version]
- Chu, W.L.; Lim, Y.W.; Radhakrishnan, A.K.; Lim, P.E. Protective Effect of Aqueous Extract from Spirulina platensis against Cell Death Induced by Free Radicals. BMC Complement. Altern. Med. 2010, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xu, Q.; Yan, C.; Wu, F. Effects of pH on Antioxidant Enzymes and Ultrastructure of Hydrilla verticillata. J. Freshw. Ecol. 2006, 21, 77–80. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Haris, N.; Manan, H.; Jusoh, M.; Khatoon, H.; Katayama, T.; Kasan, N.A. Effect of Different Salinity on the Growth Performance and Proximate Composition of Isolated Indigenous Microalgae Species. Aquac. Rep. 2022, 22, 100925. [Google Scholar] [CrossRef]
- Coelho, R.S.; Vidotti, A.D.S.; Reis, É.M.; Franco, T.T. High Cell Density Cultures of Microalgae under Fed-Batch and Continuous Growth. Chem. Eng. Transact. 2014, 38, 313–318. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, C.Y.; Chang, J.S. Effect of Light Intensity and Nitrogen Starvation on CO2 Fixation and Lipid/Carbohydrate Production of an Indigenous Microalga Scenedesmus obliquus. Bioresour. Technol. 2012, 83, 244–252. [Google Scholar] [CrossRef]
- Shetty, P.; Gitau, M.M.; Maróti, G. Salinity Stress Responses and Adaptation Mechanisms in Eukaryotic Green Microalgae. Cells 2019, 8, 1657. [Google Scholar] [CrossRef]
- Reitan, K.I.; Øie, G.; Jørgensen, H.; Wang, X. Chemical Composition of Selected Marine Microalgae, with Emphasis on Lipid and Carbohydrate Production for Potential Use as Feed Resources. J. Appl. Phycol. 2021, 33, 3831–3842. [Google Scholar] [CrossRef]
- Sujatha, K.; Nagarajan, P. Effect of Salinity on Biomass and Biochemical Constituents of Spirulina platensis (Geitler). Int. J. Plant Prot. 2014, 7, 71–73. [Google Scholar]
- Castillo, T.; Ramos, D.; García-Beltrán, T.; Brito-Bazan, M.; Galindo, E. Mixotrophic Cultivation of Microalgae: An Alternative to Produce High-Value Metabolites. Biochem. Eng. J. 2021, 176, 108183. [Google Scholar] [CrossRef]
- Yao, T.; Huang, J.; Su, B.; Wei, L.; Zhang, A.-H.; Zhang, D.-F.; Zhou, Y.; Ma, G. Enhanced Phycocyanin Production of Arthrospira maxima by Addition of Mineral Elements and Polypeptides using Response Surface Methodology. Front. Mar. Sci. 2022, 9, 1057201. [Google Scholar] [CrossRef]
- Deshmukh, D.V.; Puranik, P.R. Statistical Evaluation of Nutritional Components Impacting Phycocyanin Production in Synechocystis sp. Braz. J. Microbiol. 2012, 43, 348–355. [Google Scholar] [CrossRef]
- Poza-Carrión, C.; Fernandez-Valiente, E.; Pinas, F.F.; Fernadez-Valiente, F.L. Acclimation of Photosynthetic Pigments and Photosynthesis of the Cyanobacterium Nostoc sp. Strain UAM 206 to Combined Fuctuations of Irradiance, pH and Inorganic Carbon Availability. J. Plant Physiol. 2001, 158, 1455–1461. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El Baz, F.K.; El-Baroty, G.S. Spirulina Species as a Source of Carotenoids and a-Tocopherol and its Anticarcinoma Factors. Biotechnology 2003, 2, 222–240. [Google Scholar] [CrossRef]
- Liu, L.-N.; Chen, X.-L.; Zhang, Y.-Z.; Zhou, B.-C. Characterization, Structure and Function of Linker Polypeptides in Phycobilisomes of Cyanobacteria and Red Algae: An Overview. Biochim. Biophys. Acta—Bioenerg. 2005, 1708, 133–142. [Google Scholar] [CrossRef]
- Garab, G. Hierarchical Organization and Structural Flexibility of Thylakoid Membranes. Biochim. Biophys. Acta—Bioenerg. 2014. 1837. [CrossRef]
- Mountourakis, F.; Papazi, A.; Maragkoudakis, A.; Stamatis, N.; Kotzabasis, K. Evidence of Physiological Adaptation of Chlorella vulgaris under Extreme Salinity—New Insights into a Potential Halotolerance Strategy. Environ. Exp. Bot. 2023, 216, 105543. [Google Scholar] [CrossRef]
- Hemlata; Fatma, T. Screening of Cyanobacteria for Phycobiliproteins and Effect of Different Environmental Stress on its Yield. Bull. Environ. Contam. Toxicol. 2009, 83, 509–515. [Google Scholar] [CrossRef]
- Chu, W.L.; Alwi, A.; Phang, S.M. Phycoerythrin Production by a Marine Oscillatoria (Cyanophyta). Mal. J. Sci. 2002, 21, 67–73. [Google Scholar]
- Katari, J.K.; Uz Zama Khan, M.R.; Trivedi, V.; Das, D. Extraction, Purification, Characterization and Bioactivity Evaluation of High Purity C-Phycocyanin from Spirulina Sp. NCIM 5143. Process Biochem. 2023, 130, 322–333. [Google Scholar] [CrossRef]
- Käferböck, A.; Smetana, S.; de Vos, R.; Schwarz, C.; Toepfl, S.; Parniakov, O. Sustainable Extraction of Valuable Components from Spirulina Assisted by Pulsed Electric Fields Technology. Algal Res. 2020, 48, 101914. [Google Scholar] [CrossRef]
- Fratelli, C.; Bürck, M.; Silva-Neto, A.F.; Oyama, L.M.; De Rosso, V.V.; Braga, A.R.C. Green Extraction Process of Food Grade C-Phycocyanin: Biological Effects and Metabolic Study in Mice. Processes 2022, 10, 1793. [Google Scholar] [CrossRef]
- da Silva Figueira, F.; Moraes, C.C.; Kalil, S.J. C-Phycocyanin Purification: Multiple Processes for Different Applications. Braz. J. Chem. Eng. 2018, 35, 1117–1128. [Google Scholar] [CrossRef]
- Meticulous Research. Phycocyanin Market to Be Worth $279.6 Million by 2030. Available online: https://www.meticulousresearch.com/pressrelease/30/phycocyanin-market-2030 (accessed on 2 May 2025).
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bligh, E.; Dyer, W. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.A.J.N. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic/Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]

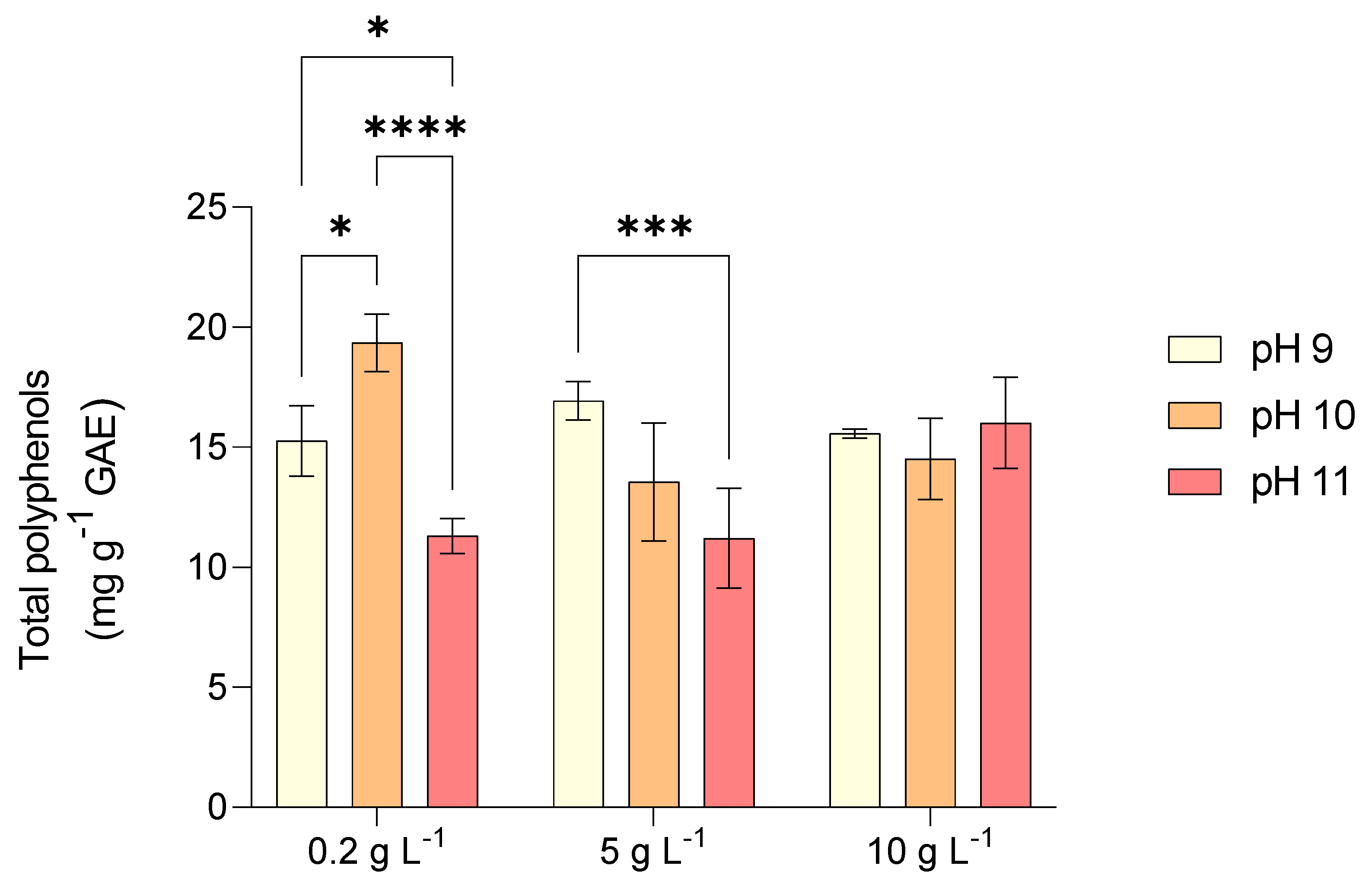
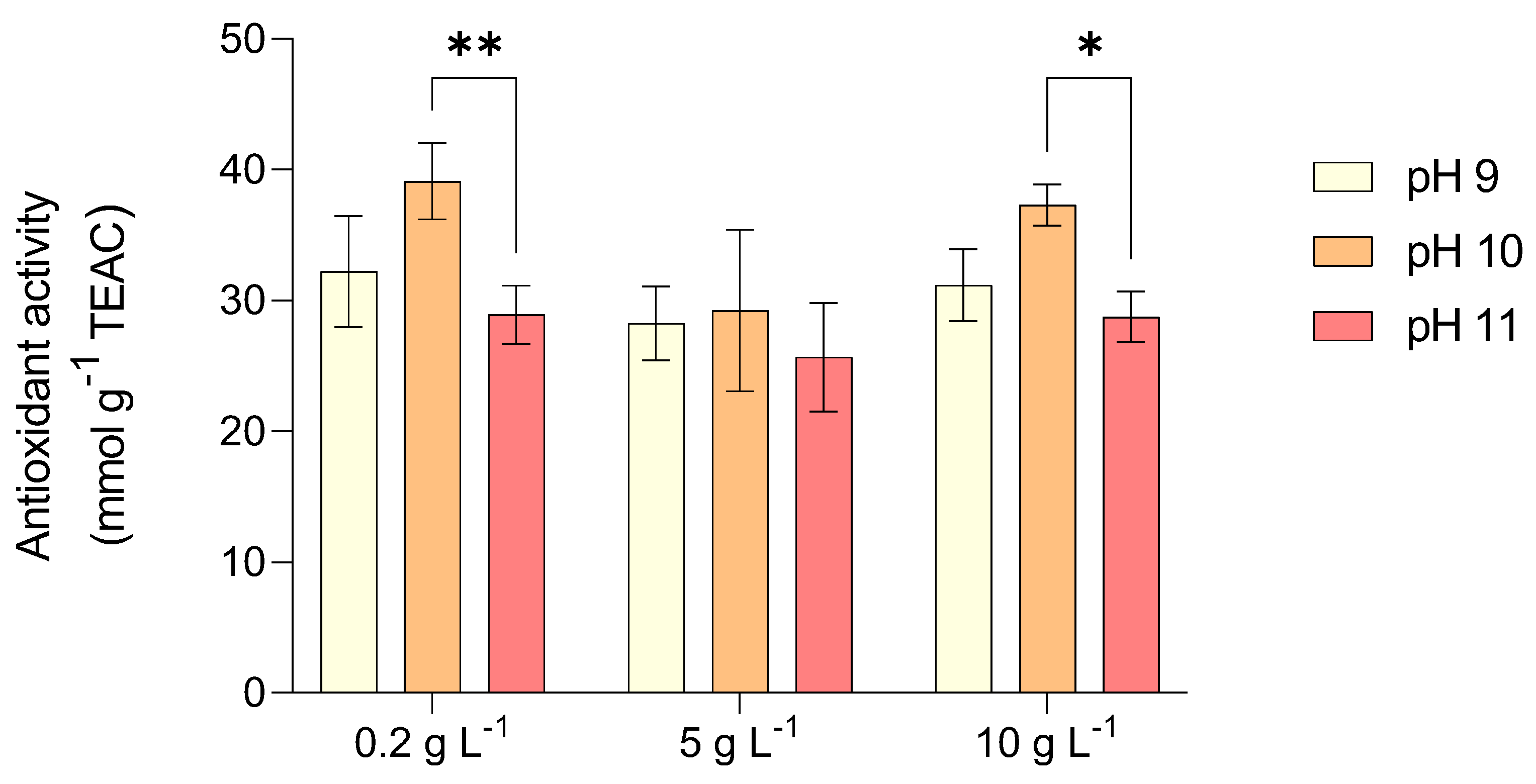
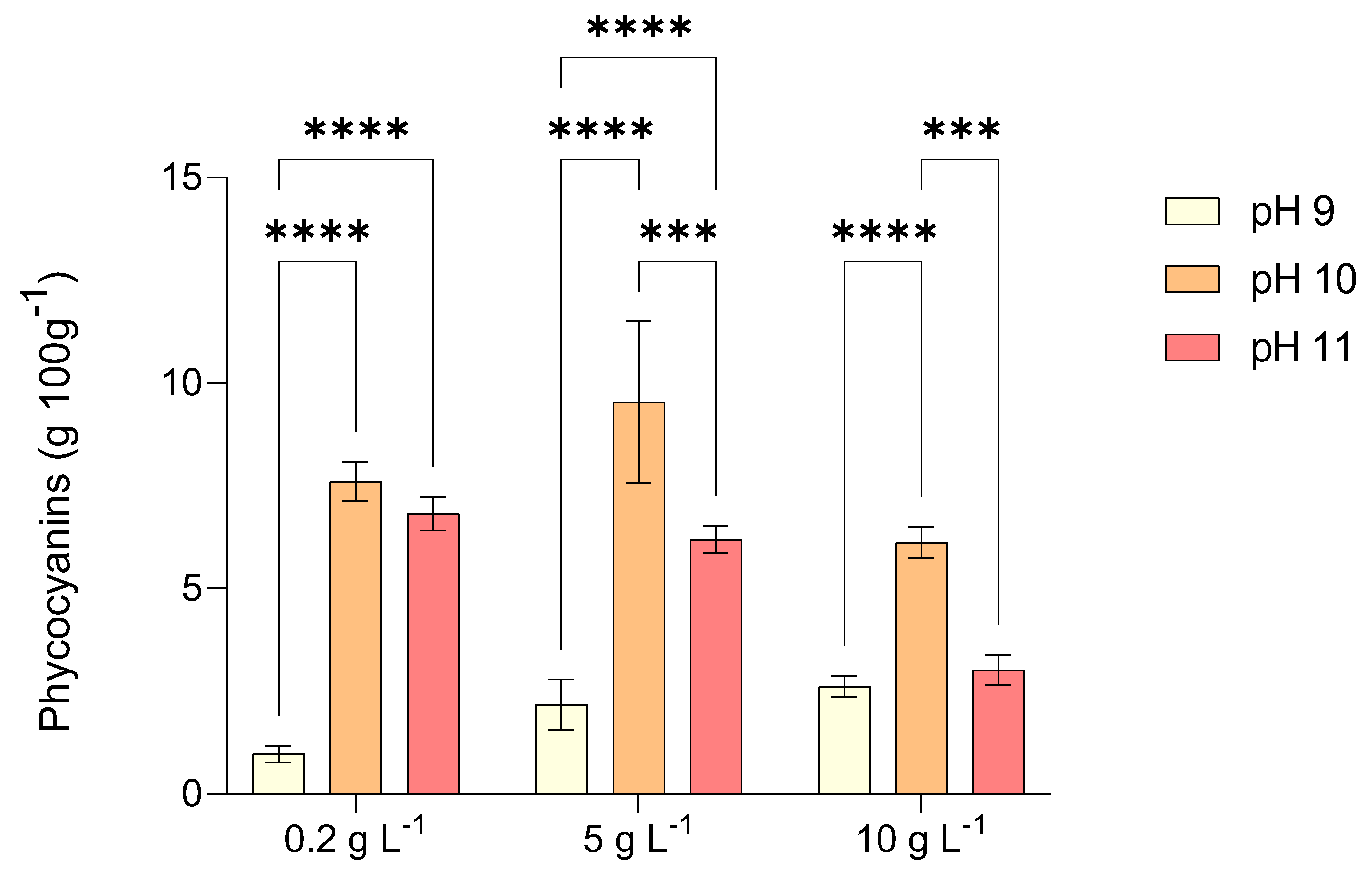
| pHfinal / | Xf g L−1 | Qx mg L−1 day−1 | ΔX g L−1 | µav day−1 | |
|---|---|---|---|---|---|
| Test 1 | 10.43 ± 0.10 | 1.299 ± 0.277 | 135 ± 0.024 | 0.676 ± 0.120 | 0.152 ± 0.035 |
| Test 2 | 10.32 ± 0.12 | 1.058 ± 0.196 | 123 ± 0.040 | 0.613 ± 0.200 | 0.172 ± 0.045 |
| Test 3 | 10.20 ± 0.20 | 1.255 ± 0.216 | 107 ± 0.029 | 0.534 ± 0.144 | 0.123 ± 0.057 |
| Test 4 | 10.34 ± 0.08 | 1.225 ± 0.246 | 83 ± 0.037 | 0.417 ± 0.184 | 0.081 ± 0.024 |
| Test 5 | 10.20 ± 0.10 | 1.217 ± 0.104 | 128 ± 0.026 | 0.642 ± 0.128 | 0.082 ± 0.014 * |
| Test 6 | 10.24 ± 0.08 | 1.392 ± 0.101 | 144 ± 0.041 | 0.721 ± 0.205 | 0.109 ± 0.012 |
| Test 7 | 10.63 ± 0.15 | 1.215 ± 0.175 | 87 ± 0.064 | 0.435 ± 0.019 | 0.090 ± 0.069 |
| Test 8 | 10.47 ± 0.25 | 1.142 ± 0.188 | 82 ± 0.036 | 0.408 ± 0.181 | 0.077 ± 0.009 * |
| Test 9 | 10.38 ± 0.14 | 1.596 ± 0.175 | 94 ± 0.002 | 0.470 ± 0.012 | 0.145 ± 0.062 |
| Test Number | Initial pH | NaCl Concentration (g L−1) | Condition Description |
|---|---|---|---|
| Test 1 | 9 | 0.2 | Low salinity, baseline alkalinity |
| Test 2 | 9 | 5 | Moderate salinity, baseline alkalinity |
| Test 3 | 9 | 10 | High salinity, baseline alkalinity |
| Test 4 | 10 | 0.2 | Low salinity, baseline alkalinity |
| Test 5 | 10 | 5 | Moderate salinity, baseline alkalinity |
| Test 6 | 10 | 10 | High salinity, baseline alkalinity |
| Test 7 | 11 | 0.2 | Low salinity, baseline alkalinity |
| Test 8 | 11 | 5 | Moderate salinity, baseline alkalinity |
| Test 9 | 11 | 10 | High salinity, baseline alkalinity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzoli, M.; Lutzu, G.A.; Usai, L.; Fais, G.; Dessì, D.; Soto-Ramirez, R.; Cosenza, B.; Concas, A. Photoautotrophic Batch Cultivation of Limnospira (Spirulina) platensis: Optimizing Biomass Productivity and Bioactive Compound Synthesis Through Salinity and pH Modulation. Mar. Drugs 2025, 23, 281. https://doi.org/10.3390/md23070281
Rizzoli M, Lutzu GA, Usai L, Fais G, Dessì D, Soto-Ramirez R, Cosenza B, Concas A. Photoautotrophic Batch Cultivation of Limnospira (Spirulina) platensis: Optimizing Biomass Productivity and Bioactive Compound Synthesis Through Salinity and pH Modulation. Marine Drugs. 2025; 23(7):281. https://doi.org/10.3390/md23070281
Chicago/Turabian StyleRizzoli, Matteo, Giovanni Antonio Lutzu, Luca Usai, Giacomo Fais, Debora Dessì, Robinson Soto-Ramirez, Bartolomeo Cosenza, and Alessandro Concas. 2025. "Photoautotrophic Batch Cultivation of Limnospira (Spirulina) platensis: Optimizing Biomass Productivity and Bioactive Compound Synthesis Through Salinity and pH Modulation" Marine Drugs 23, no. 7: 281. https://doi.org/10.3390/md23070281
APA StyleRizzoli, M., Lutzu, G. A., Usai, L., Fais, G., Dessì, D., Soto-Ramirez, R., Cosenza, B., & Concas, A. (2025). Photoautotrophic Batch Cultivation of Limnospira (Spirulina) platensis: Optimizing Biomass Productivity and Bioactive Compound Synthesis Through Salinity and pH Modulation. Marine Drugs, 23(7), 281. https://doi.org/10.3390/md23070281









