Catechol Siderophores from a Mangrove-Derived Bacteria Serratia marcescens F2-2 and Their Cytotoxic Activity
Abstract
1. Introduction
2. Results and Discussion
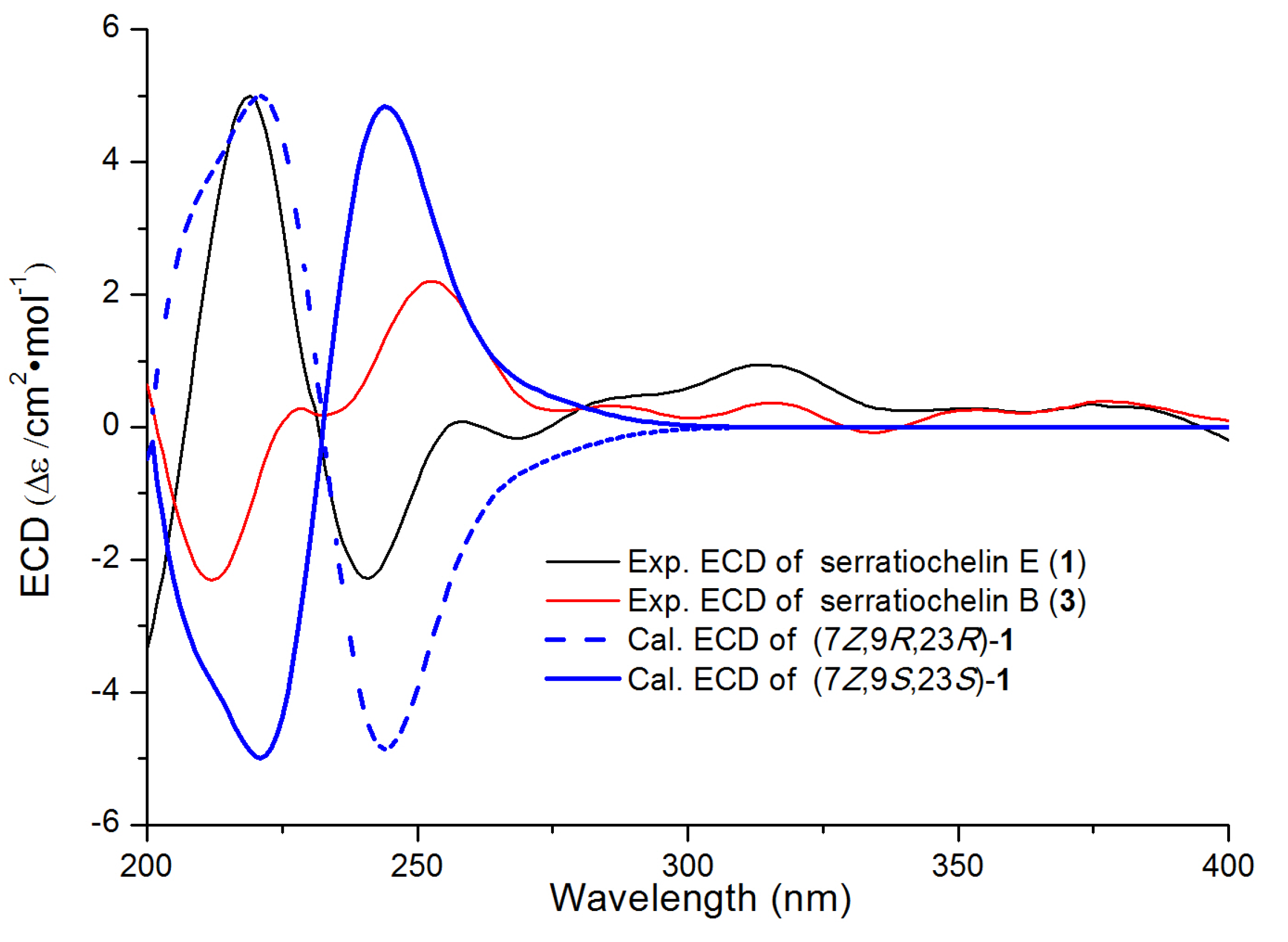
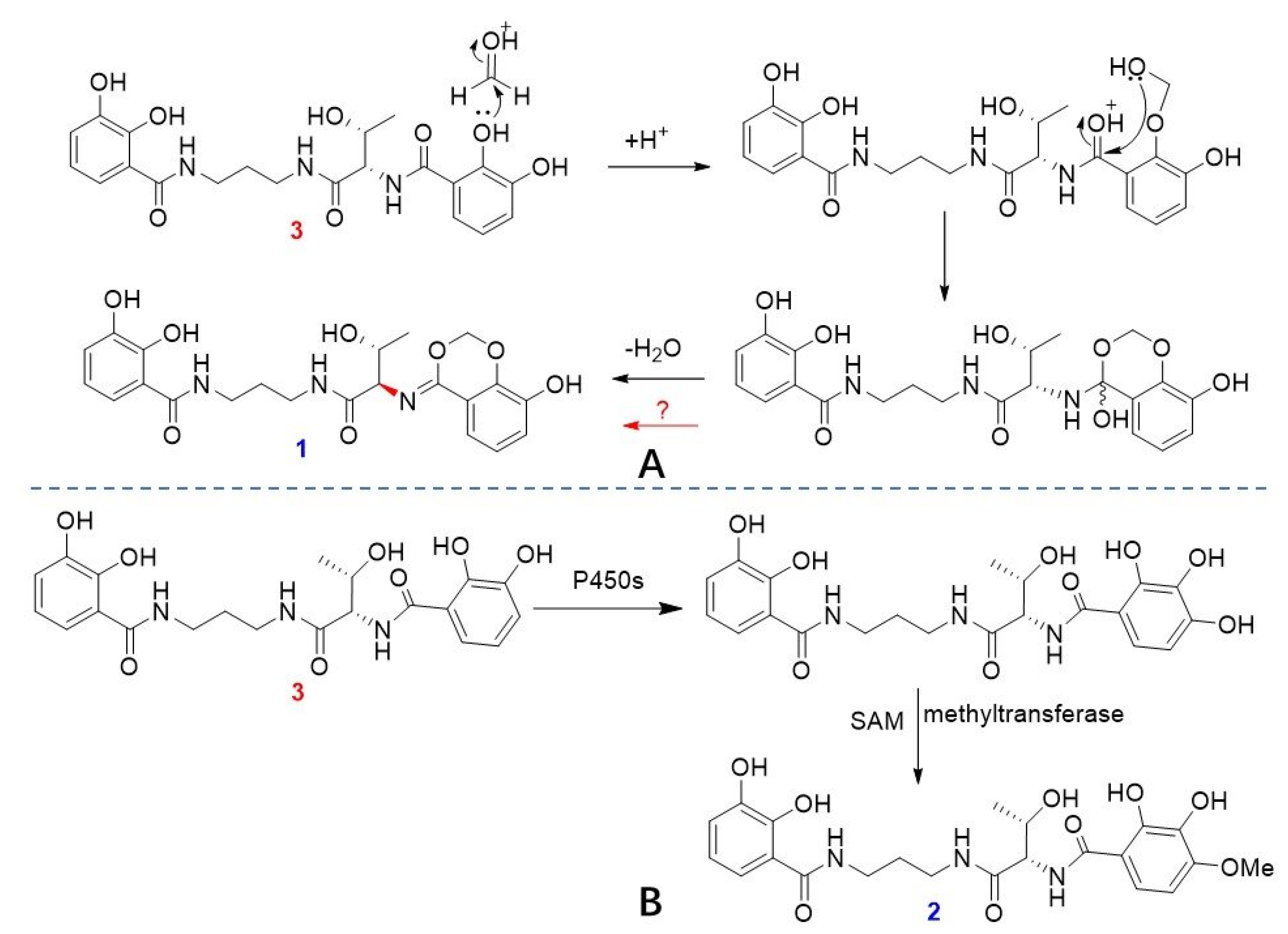
2.1. Structural Elucidation of Serratiochelins E and F
2.2. Cytotoxic Effects of Compounds 1–4
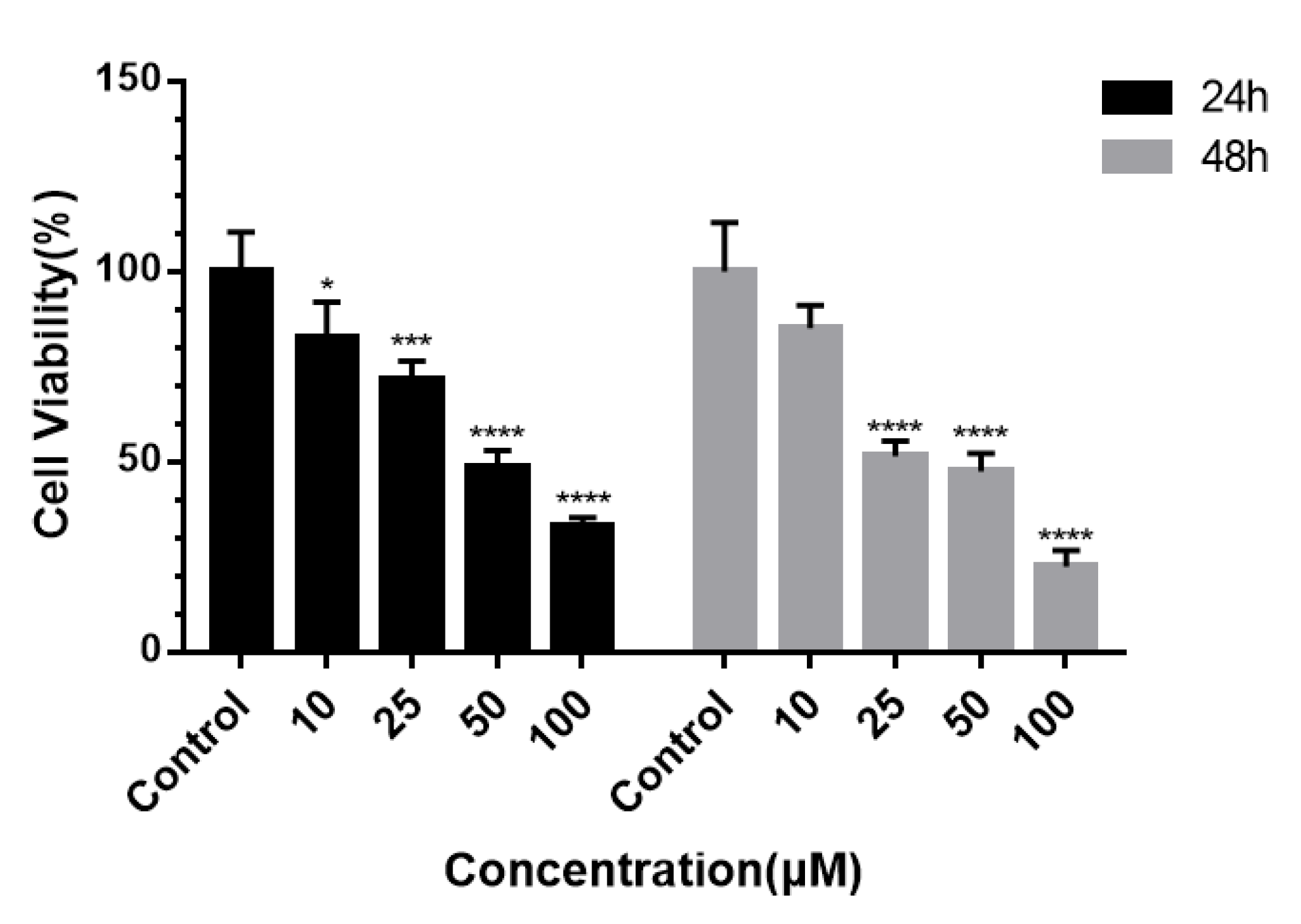
2.2.1. Inhibition and Selectivity of Tumor Cell Proliferation by 3
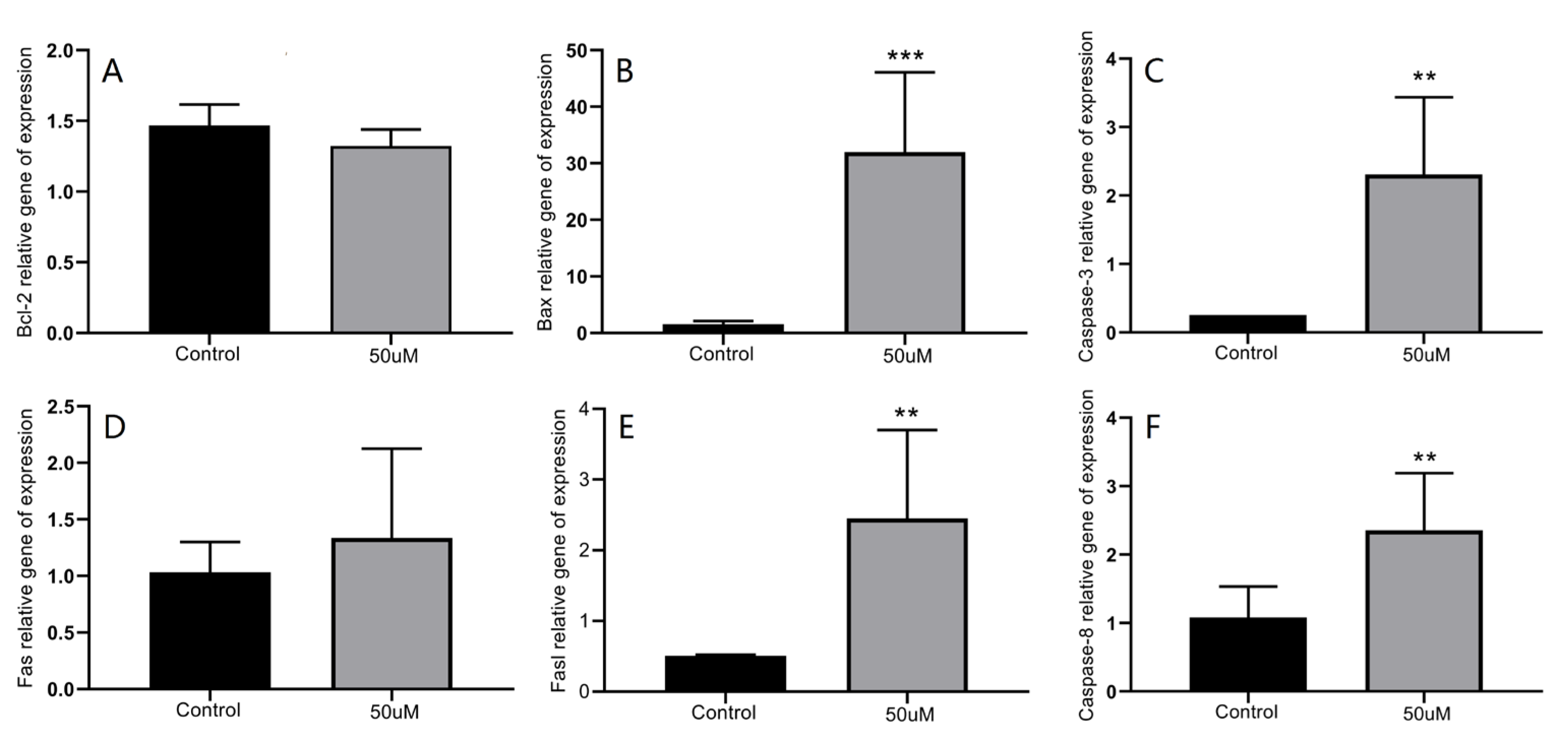
2.2.2. Modulation of Apoptosis-Related Protein Expression by 3
3. Materials and Methods
3.1. Bacterial Materials
3.2. General Experimental Procedures
3.3. Fermentation, Extraction, and Purification
3.4. Determination of the Absolute Configuration of Threonine Residue [29]
3.5. NMR and ECD Calculations
3.6. Toxicity Detection
3.7. Detection of Apoptosis Using AO/PI Double Staining
3.8. RT-qPCR Detection of the Effect of 3 on the Gene Expression of Apoptosis-Related Factors and Signaling Pathway Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eze, P.M.; Simons, V.E.; Frank, M.; van Geelen, L.; Abba, C.C.; Ebada, S.S.; Esimone, C.O.; Okoye, F.B.C.; Proksch, P.; Kalscheuer, R. Serratiochelin D, a new siderophore from Serratia marcescens. Phytochem. Lett. 2023, 57, 22–25. [Google Scholar] [CrossRef]
- Ehlert, G.; Taraz, K.; Budzikiewicz, H. Serratiochelin, a new catecholate siderophore from Serratia marcescens. Z. Naturforsch. 1994, 49, 11–17. [Google Scholar] [CrossRef]
- Page, M.G.P. The Role of iron and siderophores in infection, and the development of siderophore antibiotics. Clin. Infect. Dis. 2019, 69, S529–S537. [Google Scholar] [CrossRef] [PubMed]
- Weakland, D.R.; Smith, S.N.; Bell, B.; Tripathi, A.; Mobley, H.L. The Serratia marcescens siderophore serratiochelin is necessary for full virulence during bloodstream infection. Infect. Immun. 2020, 88, e00117-20. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R.; Cleto, S.; Carr, G.; Vlamakis, H.; João Vieira, M.; Kolter, R.; Clardy, J. Mixing and matching siderophore clusters: Structure and biosynthesis of serratiochelins from Serratia sp. V4. J. Am. Chem. Soc. 2012, 134, 13550–13553. [Google Scholar] [CrossRef]
- Eze, P.M.; Simons, V.; Seidemann, T.; Wang, L.; Kiffe-Delf, A.-L.; Frank, M.; van Geelen, L.; Abba, C.C.; Esimone, C.O.; Okoye, F.B.C. Serratiochelins A and B from Serratia marcescens show xenosiderophoric characteristics towards Acinetobacter baumannii and Mycobacterium tuberculosis. Trop. J. Pharm. Res. 2021, 20, 2551–2558. [Google Scholar]
- Li, X.; Dong, S.; Pan, Q.; Liu, N.; Zhang, Y. Antibiotic conjugates: Using molecular trojan horses to overcome drug resistance. Biomed. Pharmacother. 2025, 186, 118007. [Google Scholar] [CrossRef]
- Schneider, Y.; Jenssen, M.; Isaksson, J.; Hansen, K.Ø.; Andersen, J.H.; Hansen, E.H. Bioactivity of serratiochelin A, a siderophore isolated from a co-culture of Serratia sp. and Shewanella sp. Microorganisms 2020, 8, 1042. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Hsu, H.C.; Chen, Y.C.; Liang, T.W.; Wang, S.L. A novel compound with antioxidant activity produced by Serratia ureilytica TKU013. J. Agric. Food Chem 2012, 60, 9043–9047. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Kratzer, T.B.; Bandi, P.; Freedman, N.D.; Smith, R.A.; Travis, W.D.; Jemal, A.; Siegel, R.L. Lung cancer statistics, 2023. Cancer 2024, 130, 1330–1348. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.B.; Lee, D.K.; Cheon, C.; Ribeiro, R.I.M.A.; Kim, B. Natural products for liver cancer treatment: From traditional medicine to modern drug discovery. Nutrients 2022, 14, 4252. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Luo, C.; Yan, M.; Zhao, G.; Ma, L.; Gao, W. Treatment for liver cancer: From sorafenib to natural products. Eur. J. Med. Chem. 2021, 224, 113690. [Google Scholar] [CrossRef] [PubMed]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Li, G.; Li, H.; Zhang, Q.; Yang, M.; Gu, Y.C.; Liang, L.F.; Tang, W.; Guo, Y.W. Rare cembranoids from chinese soft coral Sarcophyton ehrenbergi: Structural and stereochemical studies. J. Org. Chem. 2019, 84, 5091–5098. [Google Scholar] [CrossRef]
- Harada, K.-I.; Fujii, K.; Mayumi, T.; Hibino, Y.; Suzuki, M.; Ikai, Y.; Oka, H. A method using LC/MS for determination of absolute configuration of constituent amino acids in peptide-advanced Marfey’s method. Tetrahedron Lett. 1995, 36, 1515–1518. [Google Scholar] [CrossRef]
- Fan, Y.; Shen, J.; Liu, Z.; Xia, K.; Zhu, W.; Fu, P. Methylene-bridged dimeric natural products involving one-carbon unit in biosynthesis. Nat. Prod. Rep. 2022, 39, 1305–1324. [Google Scholar] [CrossRef]
- Greule, A.; Stok, J.E.; De Voss, J.J.; Cryle, M.J. Unrivalled diversity: The many roles and reactions of bacterial cytochromes P450 in secondary metabolism. Nat. Prod. Rep. 2018, 35, 757–791. [Google Scholar] [CrossRef]
- Abdelraheem, E.; Thair, B.; Varela, R.F.; Jockmann, E.; Popadić, D.; Hailes, H.C.; Ward, J.M.; Iribarren, A.M.; Lewkowicz, E.S.; Andexer, J.N.; et al. Methyltransferases: Functions and applications. ChemBioChem 2022, 23, e202200212. [Google Scholar] [CrossRef]
- Li, W.; Ma, G.; Deng, Y.; Wu, Q.; Wang, Z.; Zhou, Q. Artesunate exhibits synergistic anti-cancer effects with cisplatin on lung cancer A549 cells by inhibiting MAPK pathway. Gene 2021, 766, 145134. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Perelmulter, K.; Levín, P.; Romo, A.I.B.; Lemus, L.; Fogolín, M.B.; León, I.E.; Di Virgilio, A.L. Antiproliferative activity of two copper (II) complexes on colorectal cancer cell models: Impact on ROS production, apoptosis induction and NF-κB inhibition. Eur. J. Pharm. Sci. 2022, 169, 106092. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.N.; Davidson, C.; Peslak, S.A.; Kingsley, P.D.; Nakamura, Y.; Palis, J.; Bulger, M. Histone H2A.X phosphorylation and caspase-initiated chromatin condensation in late-stage erythropoiesis. Epigenet. Chromatin 2021, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Hussar, P. Apoptosis regulators Bcl-2 and caspase-3. Encyclopedia 2022, 2, 1624–1636. [Google Scholar] [CrossRef]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.-A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 family: From apoptosis mechanisms to new advances in targeted therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.; Hassanin, K.M.A.; Mahmoud, A.A.; Abdel-Badeea, W.I.E.; Abdel-Razik, A.-R.H.; Attia, E.Z.; Abdelmohsen, U.R.; Abdel Aziz, R.L.; Najda, A.; Alanazi, I.S.; et al. Physiological roles of red carrot methanolic extract and vitamin E to abrogate cadmium-Induced oxidative challenge and apoptosis in rat testes: Involvement of the Bax/Bcl-2 ratio. Antioxidants 2021, 10, 1653. [Google Scholar] [CrossRef]
- Waring, P.; Müllbacher, A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol. Cell Biol. 1999, 77, 312–317. [Google Scholar] [CrossRef]
- Berglund, H.; Olerenshaw, D.; Sankar, A.; Federwisch, M.; McDonald, N.Q.; Driscoll, P.C. The three-dimensional solution structure and dynamic properties of the human FADD death domain. J. Mol. Biol. 2000, 302, 171–188. [Google Scholar] [CrossRef]
- Coe, G.L.; Redd, P.S.; Paschall, A.V.; Lu, C.; Gu, L.; Cai, H.; Albers, T.; Lebedyeva, I.O.; Liu, K. Ceramide mediates FasL-induced caspase 8 activation in colon carcinoma cells to enhance FasL-induced cytotoxicity by tumor-specific cytotoxic T lymphocytes. Sci. Rep. 2016, 6, 30816. [Google Scholar] [CrossRef]
- Takiguchi, S.; Hirota-Takahata, Y.; Nishi, T. Application of the advanced Marfey’s method for the determination of the absolute configuration of ogipeptins. Tetrahedron Lett. 2022, 96, 153760. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Vandermeersch, T.; Flynn, C.J.; Maguire, A.R.; Hutchison, G.R. Confab—Systematic generation of diverse low-energy conformers. J. Cheminform. 2011, 3, 8. [Google Scholar] [CrossRef]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Quinta-Costa, M.; Leite, P.S.; Guimarães, J.E. Critical evaluation of techniques to detect and measure cell death—Study in a model of UV radiation of the leukaemic cell line HL60. Anal. Cell. Pathol. 1999, 19, 176515. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, N.; Li, Q.; Chen, S.; Cheng, H.; Yang, M.; Jiang, T.; Chu, J.; Ma, X.; Yin, D. Lactonic sophorolipid–induced apoptosis in human HepG2 cells through the caspase-3 pathway. Appl. Microbiol. Biot. 2021, 105, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
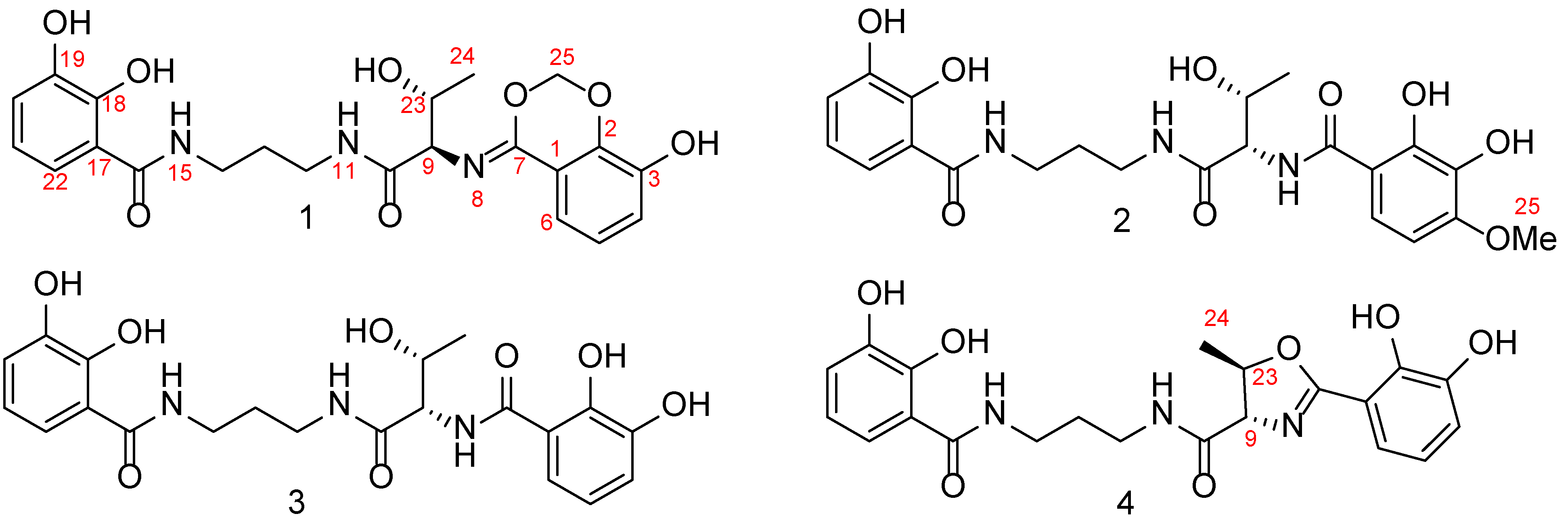
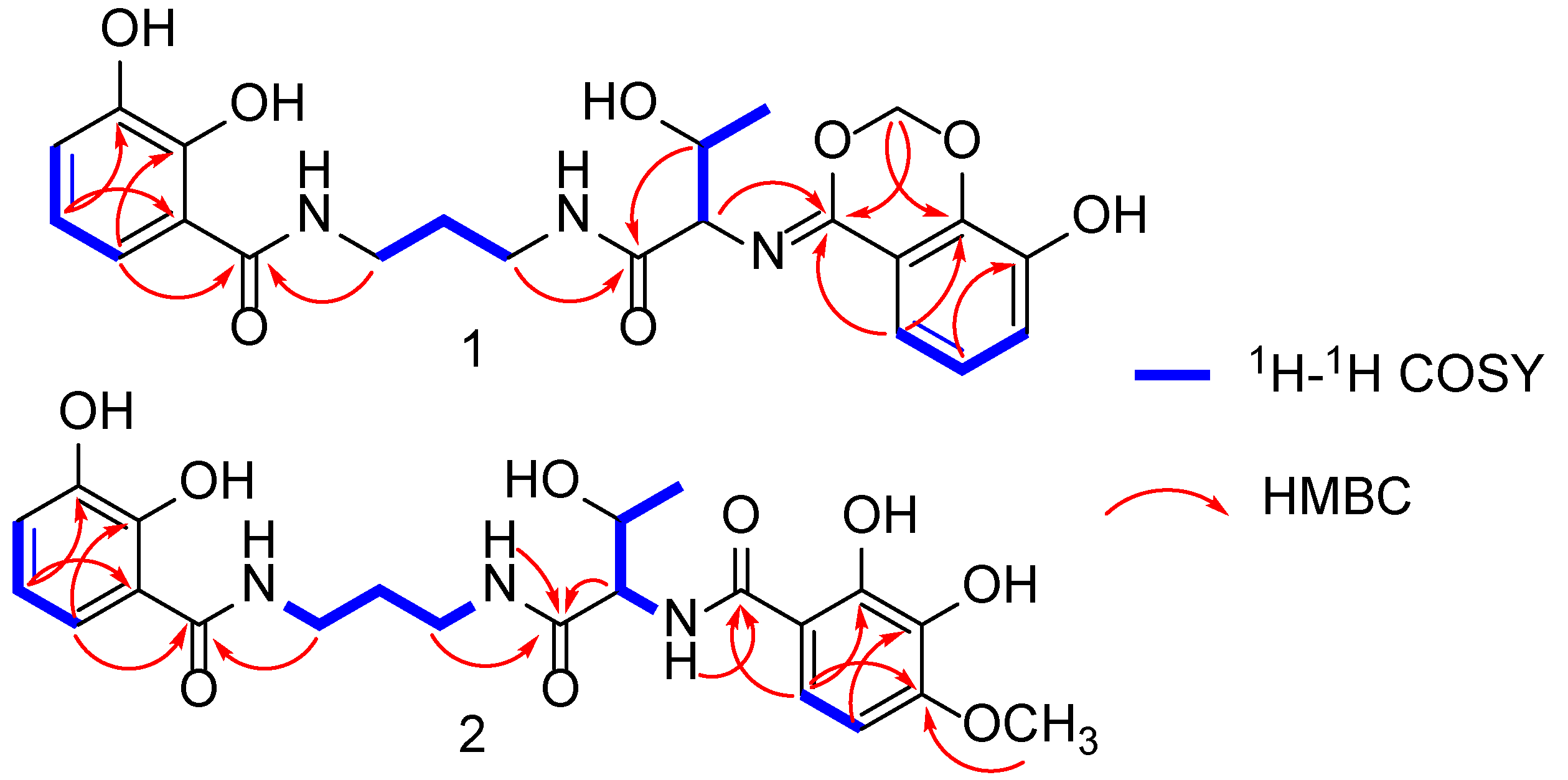
| No. | 1 a | 2 b | ||
|---|---|---|---|---|
| δC, Type | δH (Mult., J in Hz) | δC, Type | δH (Mult., J in Hz) | |
| 1 | 120.6 s | 104.0 s | ||
| 2 | 148.2 s | 151.7 s | ||
| 3 | 146.8 s | 140.4 s | ||
| 4 | 121.8 d | 7.05 (d, J = 7.5 Hz, 1H) | 151.0 s | |
| 5 | 123.6 d | 6.97 (t, J = 7.5 Hz, 1H) | 118.4 d | 6.43 (d, overlapped, 1H) |
| 6 | 119.6 d | 7.38 (d, J = 7.5 Hz, 1H) | 100.8 d | 6.42 (d, J = 8.9 Hz, 1H) |
| 7 | 165.3 s | 169.8 s | ||
| 8 | 9.18 (d, J = 7.4 Hz, 1H) | |||
| 9 | 62.2 d | 5.08 (d, J = 5.0 Hz, 1H) | 59.2 d | 4.29 (dd, J = 7.4, 3.3 Hz, 1H) |
| 10 | 171.7 s | 169.5 s | ||
| 11 | 8.07 (t, J = 5.3 Hz, 1H) | |||
| 12 | 37.7 t | 3.31 (m, overlapped, 2H) | 36.5 t | 3.16 (m, 2H) |
| 13 | 30.2 t | 1.84 (m, 2H) | 29.0 t | 1.84 (m, 2H) |
| 14 | 37.8 t | 3.44 (t, J = 6.4 Hz, 2H) | 36.6 t | 3.30 (m, overlapped, 2H) |
| 15 | 8.86 (br s, 1H) | |||
| 16 | 171.7 s | 169.8 s | ||
| 17 | 116.7 s | 114.9 s | ||
| 18 | 150.3 s | 149.9 s | ||
| 19 | 147.4 s | 146.3 s | ||
| 20 | 119.7 d | 6.98 (d, J = 7.8 Hz, 1H) | 118.6 d | 6.89 (d, J = 8.3 Hz, 1H) |
| 21 | 119.6 d | 6.71 (t, overlapped, 1H) | 117.7 d | 6.65 (t, J = 7.9 Hz, 1H) |
| 22 | 118.6 d | 7.22 (d, J = 7.9 Hz, 1H) | 117.1 d | 7.24 (d, J = 8.0 Hz, 1H) |
| 23 | 67.8 d | 4.44 (m, 1H) | 66.3 d | 4.13 (m, 1H) |
| 24 | 20.7 q | 1.27 (d, J = 6.1Hz, 3H) | 20.4 q | 1.10 (d, J = 6.3 Hz, 3H) |
| 25 | 78.3 t | 5.64 (d, J = 9.0 Hz, 1H) 5.55 (d, J = 9.0 Hz, 1H) | 56.6 q | 3.85 (s, 1H) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, X.; Zhang, X.; Ye, L.; Ke, L.; Fan, S.; Hong, X.; Li, G.; Yang, B.; Luo, L. Catechol Siderophores from a Mangrove-Derived Bacteria Serratia marcescens F2-2 and Their Cytotoxic Activity. Mar. Drugs 2025, 23, 241. https://doi.org/10.3390/md23060241
Zhang G, Wang X, Zhang X, Ye L, Ke L, Fan S, Hong X, Li G, Yang B, Luo L. Catechol Siderophores from a Mangrove-Derived Bacteria Serratia marcescens F2-2 and Their Cytotoxic Activity. Marine Drugs. 2025; 23(6):241. https://doi.org/10.3390/md23060241
Chicago/Turabian StyleZhang, Gang, Xunming Wang, Xingwang Zhang, Lin Ye, Longyang Ke, Shimin Fan, Xuan Hong, Guoqiang Li, Bingye Yang, and Lianzhong Luo. 2025. "Catechol Siderophores from a Mangrove-Derived Bacteria Serratia marcescens F2-2 and Their Cytotoxic Activity" Marine Drugs 23, no. 6: 241. https://doi.org/10.3390/md23060241
APA StyleZhang, G., Wang, X., Zhang, X., Ye, L., Ke, L., Fan, S., Hong, X., Li, G., Yang, B., & Luo, L. (2025). Catechol Siderophores from a Mangrove-Derived Bacteria Serratia marcescens F2-2 and Their Cytotoxic Activity. Marine Drugs, 23(6), 241. https://doi.org/10.3390/md23060241









