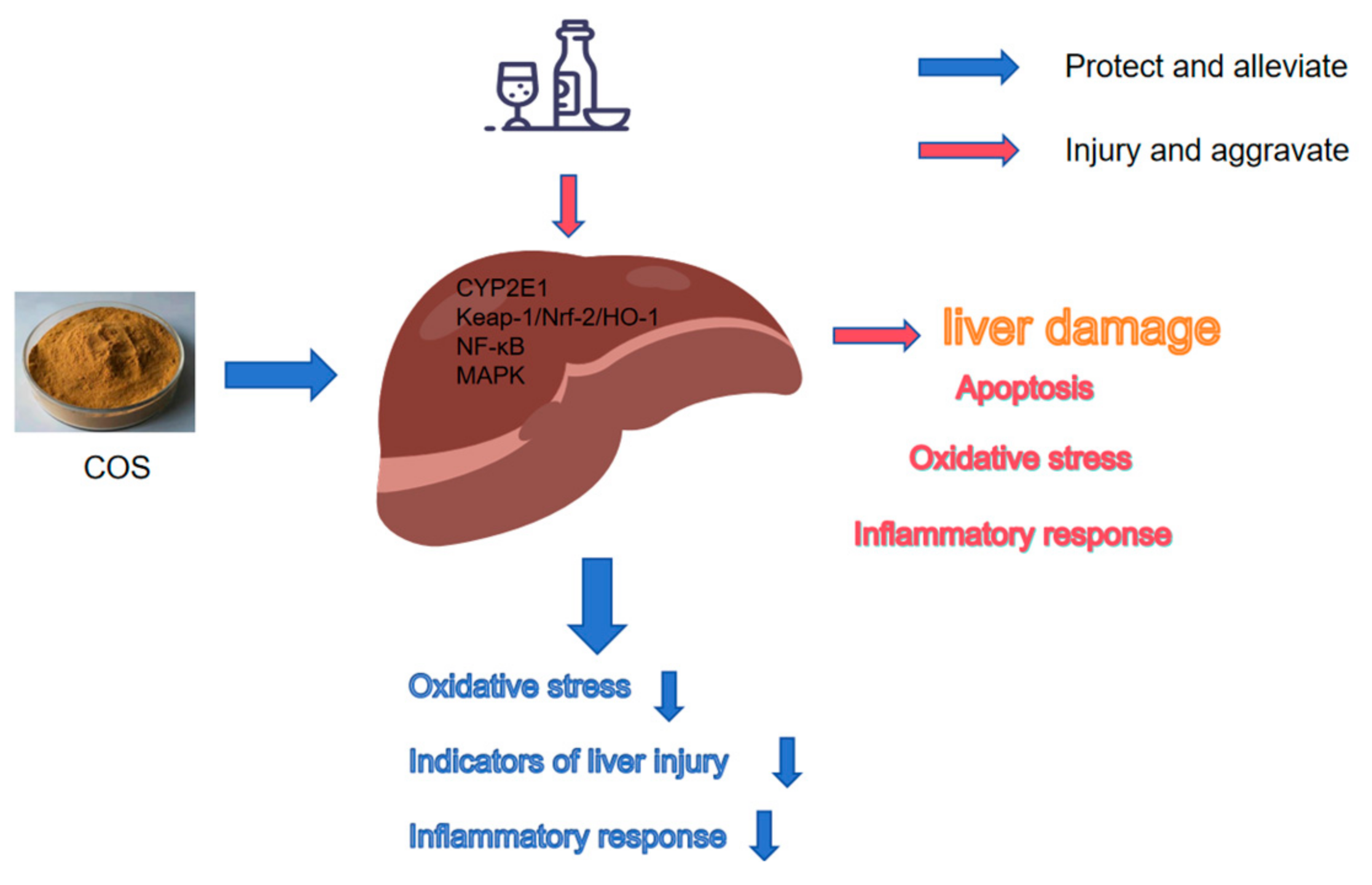
Chitosan Oligosaccharides Prevent Alcohol-Induced Liver Disease by Attenuating Inflammation and Oxidative Stress
Abstract
1. Introduction
2. Results
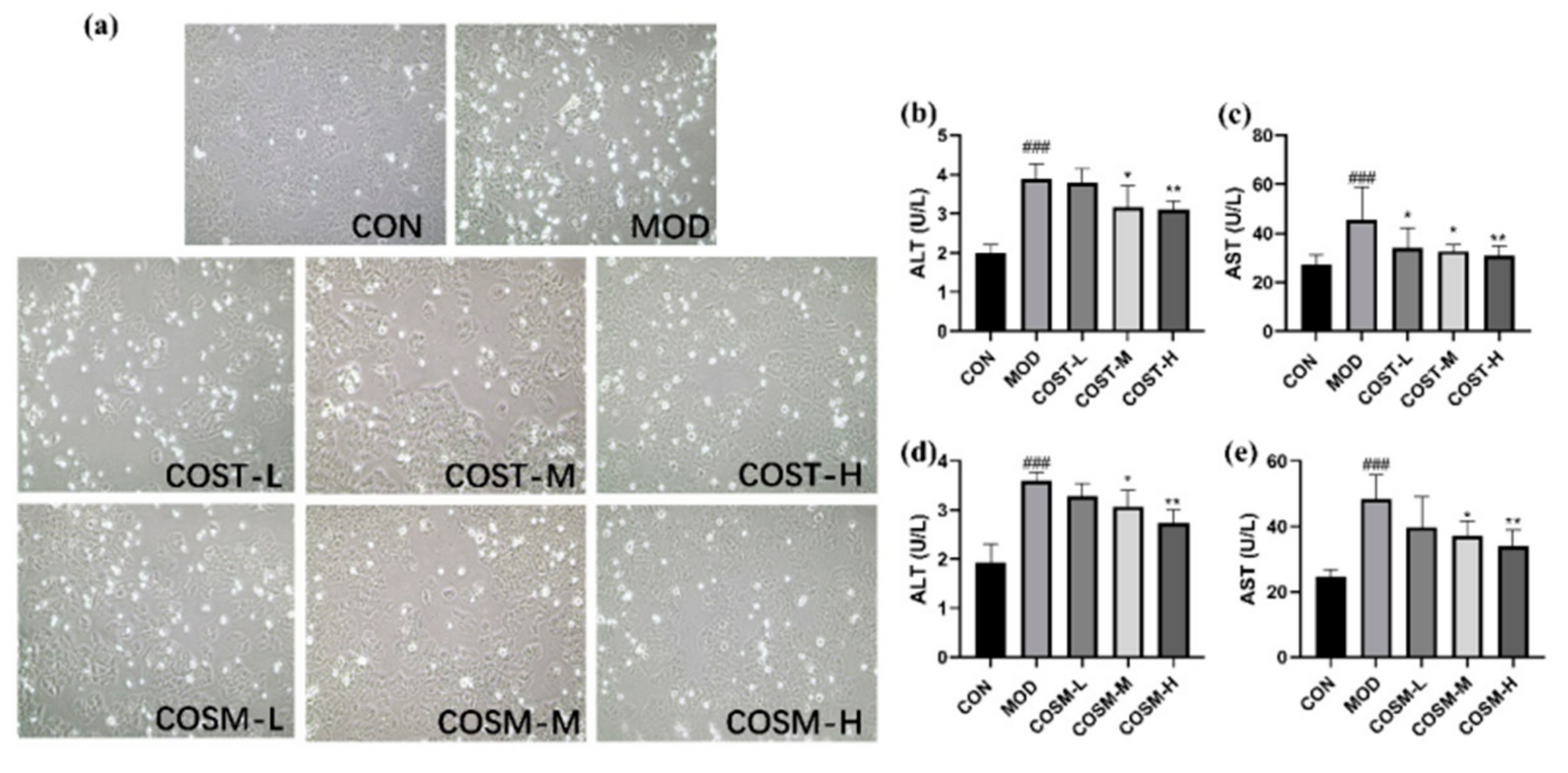
2.1. Effects of COST and COSM on Alcohol-Induced Alt and Ast Contents in L02 Hepatocytes
2.2. Effects of COST and COSM on Oxidative Levels in L02 Hepatocytes
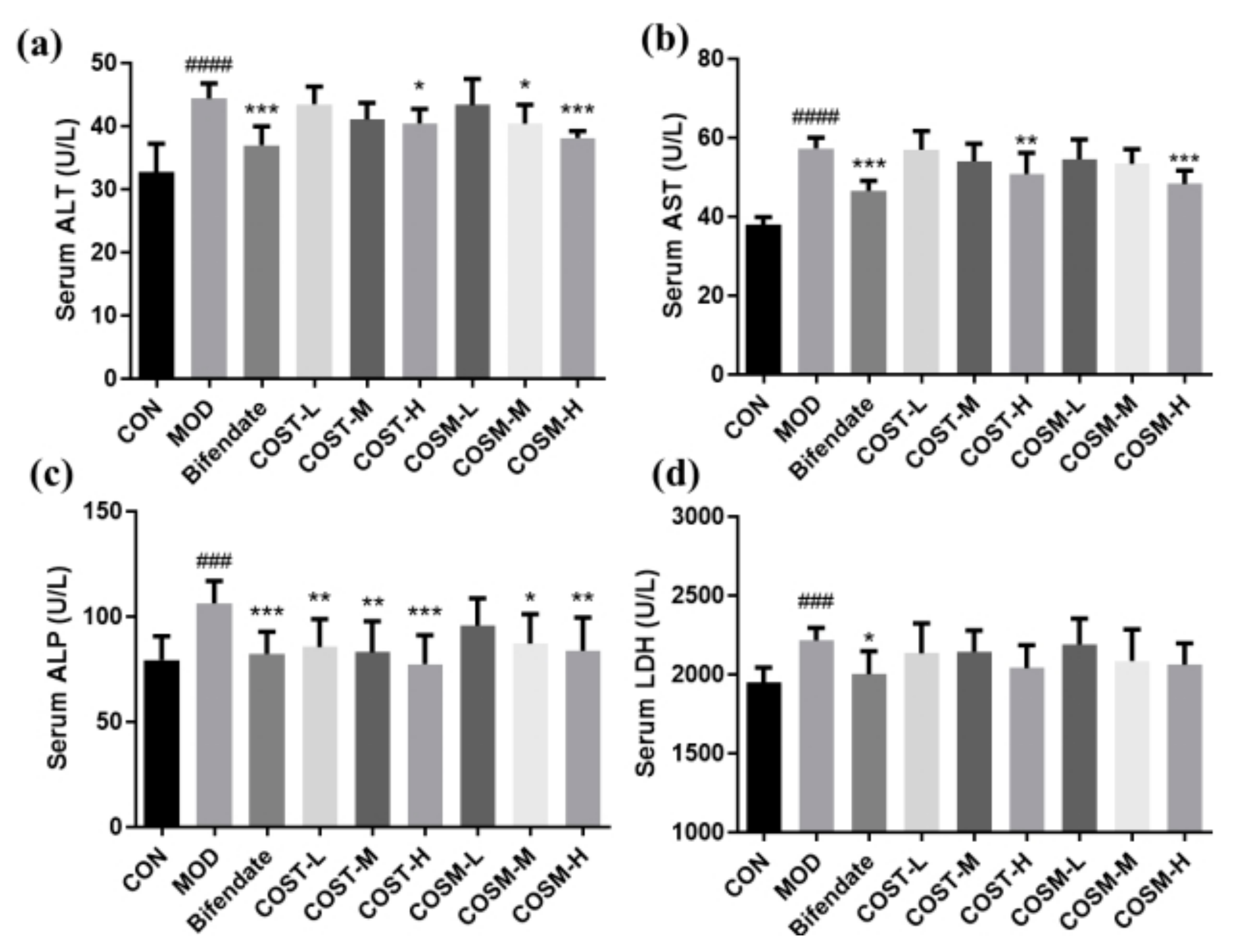
2.3. Effect of COS on Serum Hepatocyte Injury Markers in Mice
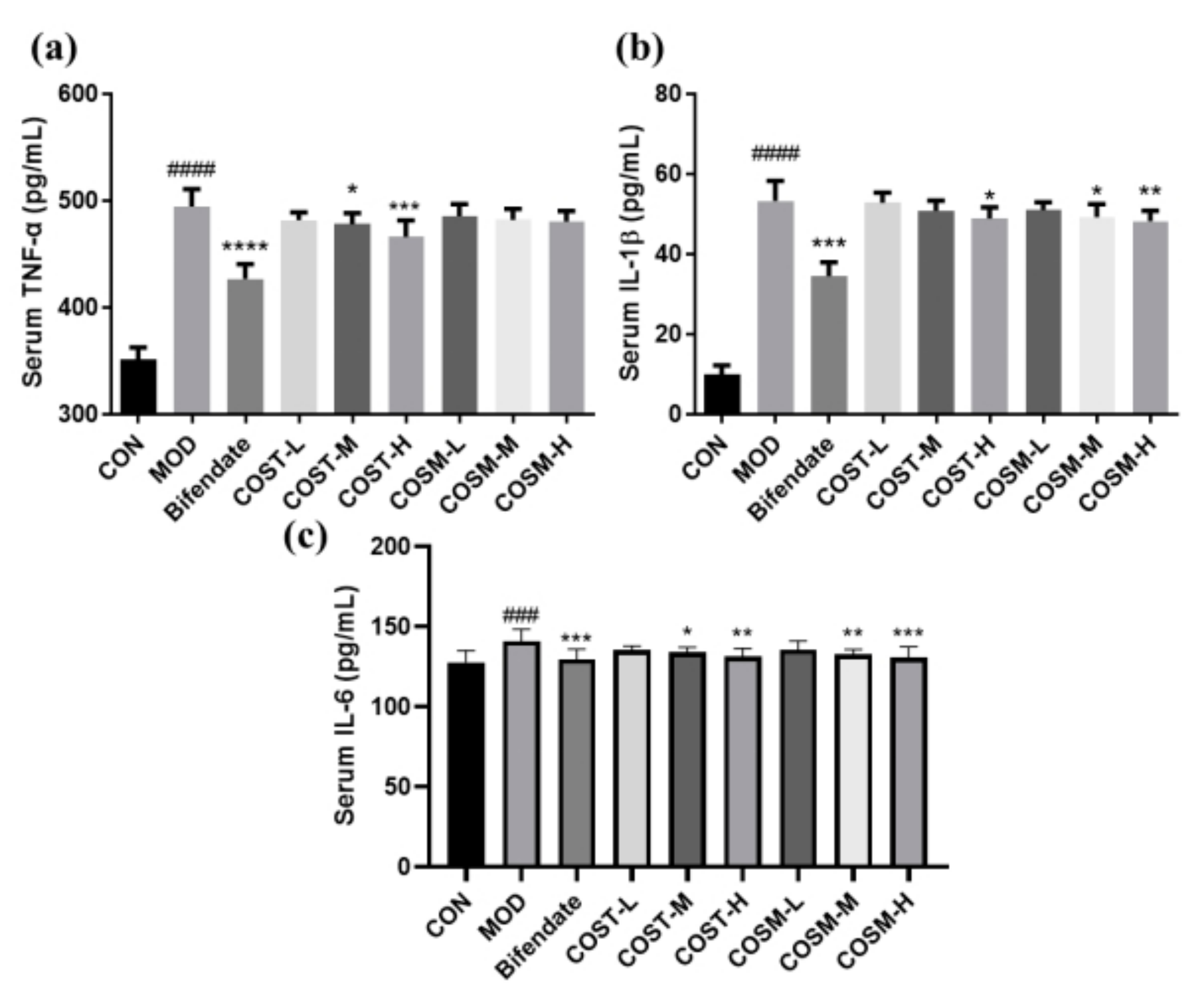
2.4. Effect of COSs on Serum Inflammatory Factors in Mice
2.5. Effect of COS Intervention on Serum Lipid Metabolism in Mice
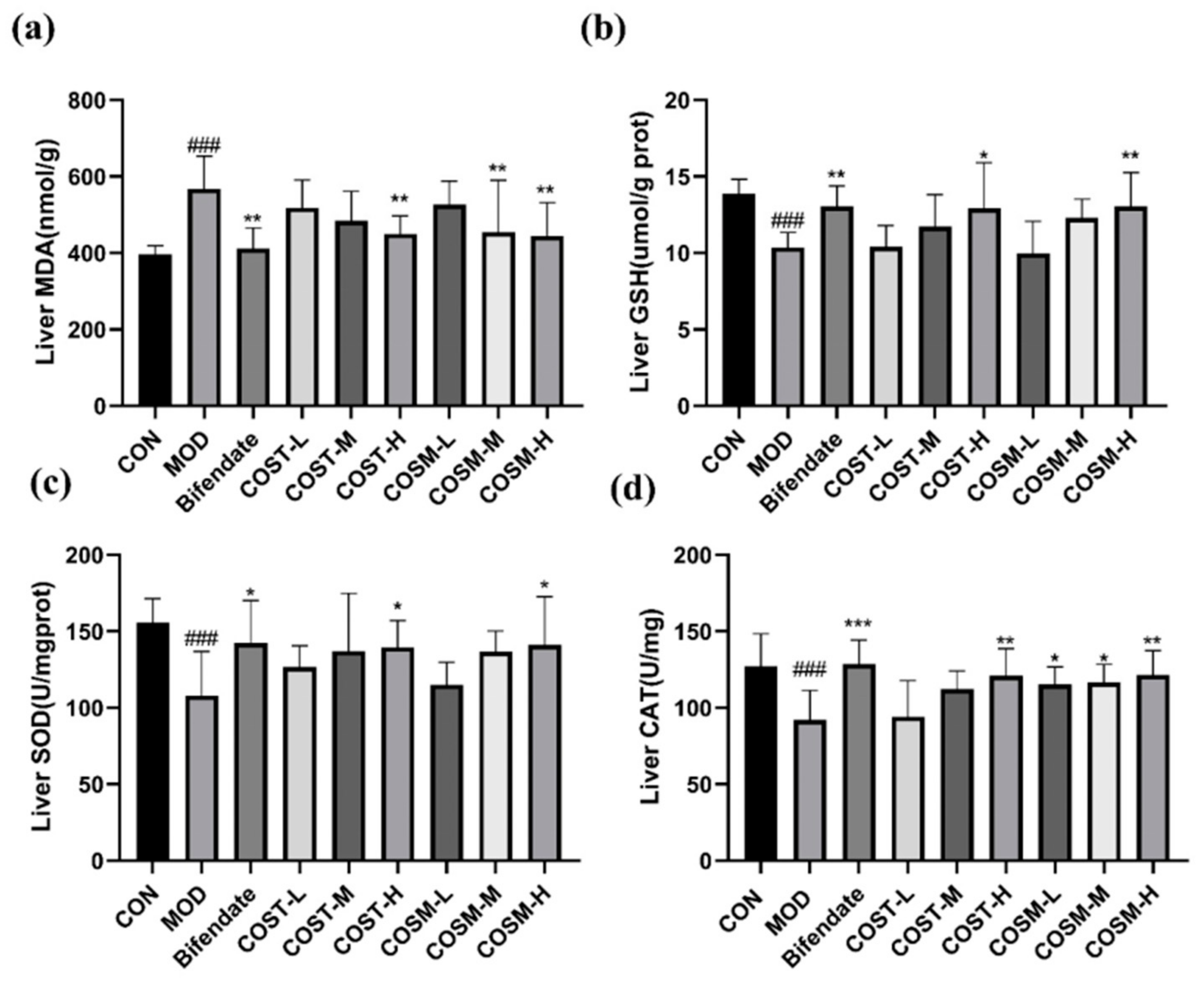
2.6. Effect of COSs on Liver Oxidative Damage Indexes in Mice
2.7. Effect of COST and COSM on Pathological Sections of Mice Liver
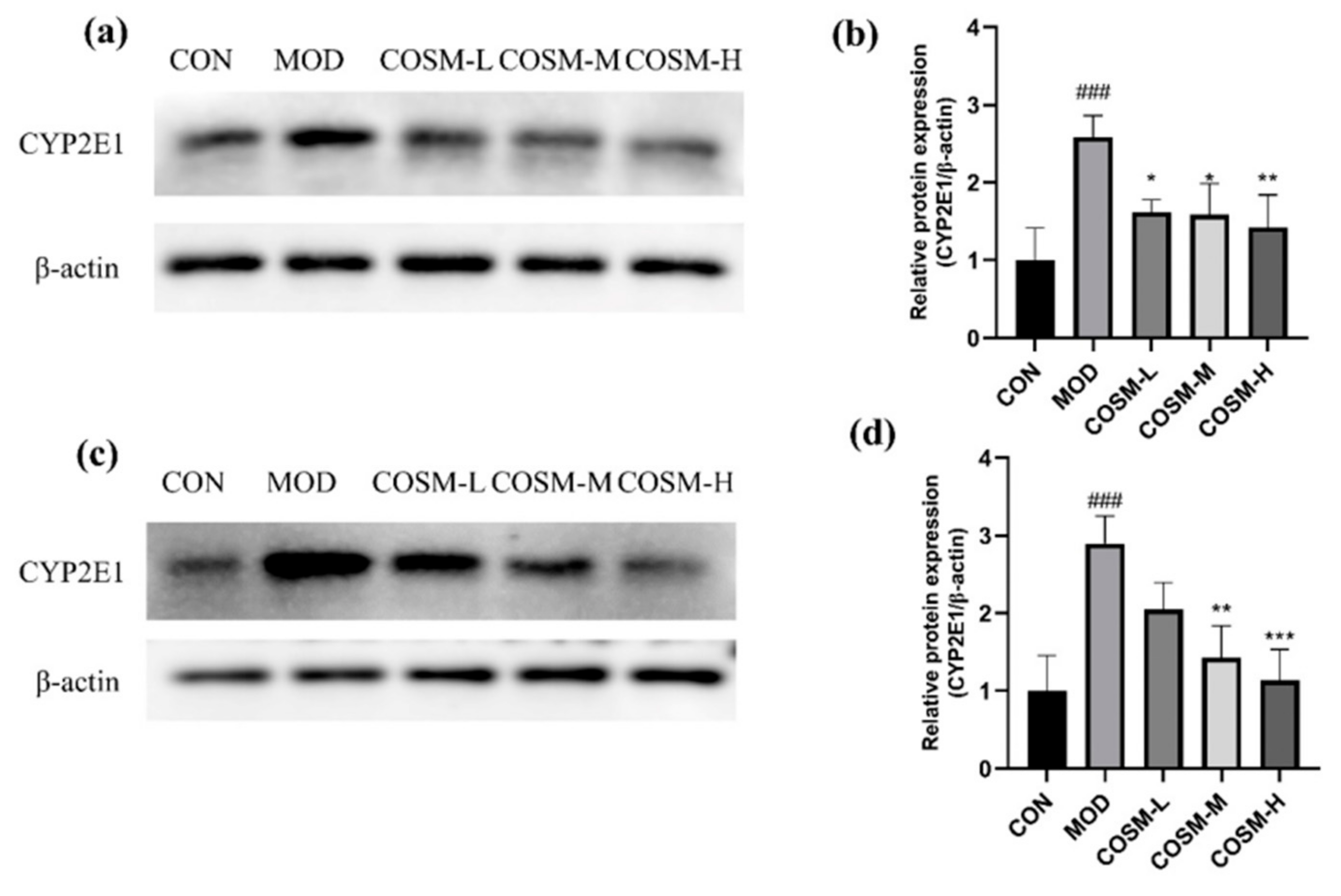
2.8. Effect of COSM on Alcohol Metabolizing Enzyme Cyp2e1 In Vivo and In Vitro Experiments
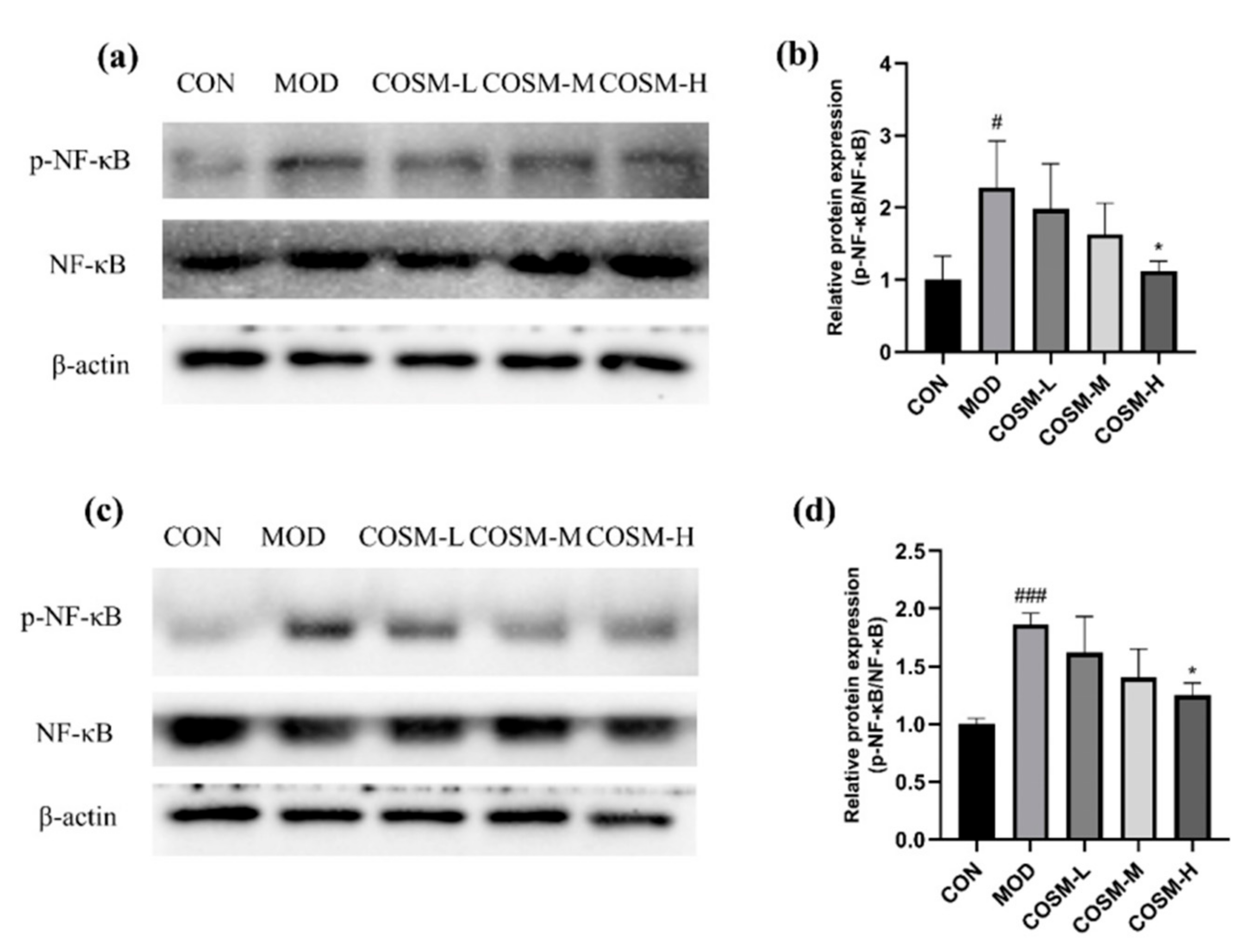
2.9. Effect of COSM on NF-κB Pathway In Vivo and In Vitro Experiments
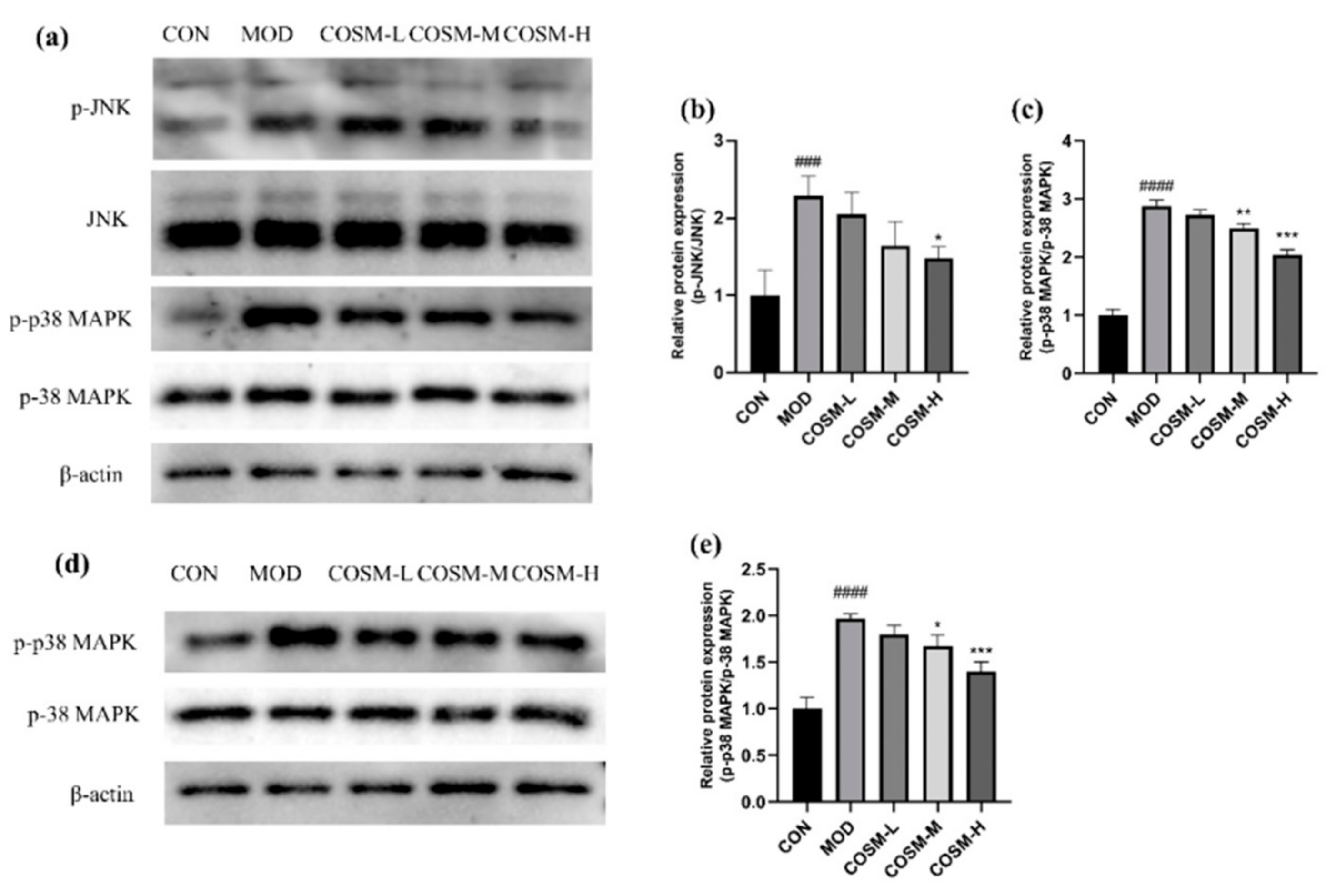
2.10. The Effect of COSM on MAPK Signaling Pathway In Vivo and In Vitro Experiments
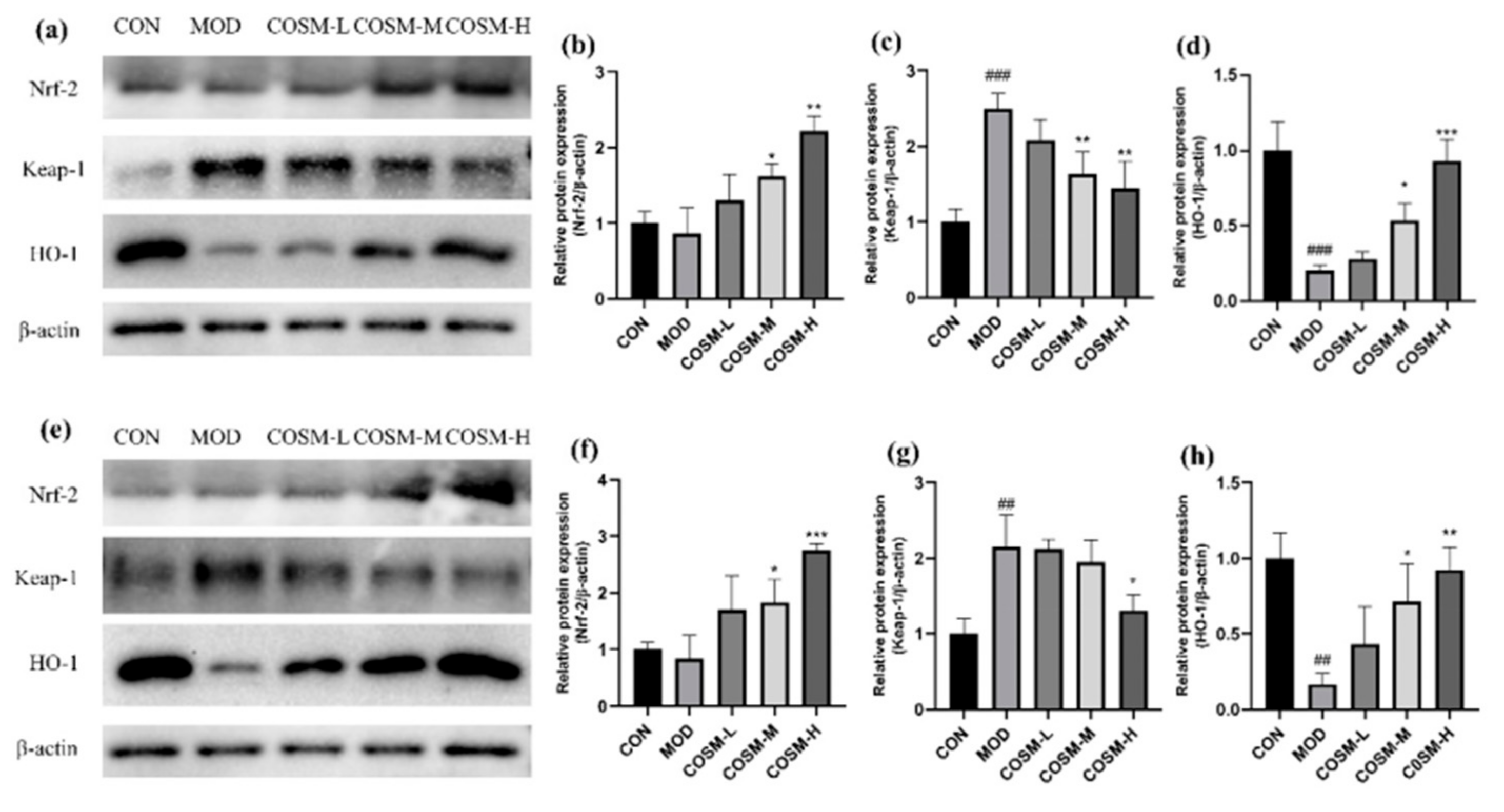
2.11. Effect of COSM on the Antioxidant Pathway Keap-1/Nrf-2/HO-1 In Vivo and In Vitro Experiments
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design In Vitro
4.2.1. Cell Culture
4.2.2. Screening of Alcohol Modeling Concentration and Doses of COST and COSM
4.2.3. Determination of the Damage and Oxidative Index in L02 Cells
4.3. Experimental Design In Vivo
4.3.1. Animal Grouping and Administration
4.3.2. Serum Biochemical Analysis
4.3.3. Detection of Liver Oxidation and Antioxidant Indexes
4.3.4. Histopathological Observation of Liver
4.4. Western Blot Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALD | alcoholic liver disease |
| CTS | chitosan |
| COST | chitosan oligosaccharides (MW ≤ 1000 Da) |
| COSM | chitosan oligosaccharides (MW ≤ 3000 Da) |
| DD | degree of deacetylation |
| DP | degree of polymerization |
| MW | molecular weight |
| CYP2E1 | cytochrome P450 proteins 2E1 |
| Keap1 | kelch-like ECH-associated protein 1 |
| Nrf2 | nuclear factor erythroid-2-related factor 2 |
| HO-1 | haem oxygenase-1 |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| MDA | malondialdehyde |
| GSH | glutathione |
| SOD | superoxide dismutase |
| CAT | catalase |
| ALP | alkaline phosphatase |
| LDH | lactate dehydrogenase |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| TC | serum total cholesterol |
| TG | triglyceride |
| VLDL | very low density lipoprotein |
| ROS | reactive oxygen species |
| MAPK | mitogen-activated protein kinase |
| JNK | C-Jun N-terminal kinase |
| NF-κB | nuclear factor kappa-B |
| p38 MAPK | p38 mitogen-activated protein kinase |
References
- Wong, T.; Dang, K.; Ladhani, S.; Singal, A.K.; Wong, R.J. Prevalence of Alcoholic Fatty Liver Disease Among Adults in the United States, 2001–2016. J. Am. Med. Assoc. 2019, 321, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Lin, Y.; Zhang, L.J. Trends in Alcoholic Fatty Liver Disease. J. Am. Med. Assoc. 2019, 322, 979–980. [Google Scholar] [CrossRef]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic Liver Disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.P.T.; Han, M.; Luu, N.M.; Oh, J.K. Alcoholic liver disease in relation to cancer incidence and mortality: Findings from a large, matched cohort study in South Korea. Cancer Med. 2023, 12, 8754–8766. [Google Scholar] [CrossRef]
- Wen, B.J.; Zhang, C.C.; Zhou, J.W.; Zhang, Z.Y.; Che, Q.S.; Cao, H.; Bai, Y.; Guo, J.; Su, Z.Q. Targeted treatment of alcoholic liver disease based on inflammatory signalling pathways. Pharmacol. Ther. 2021, 222, 18. [Google Scholar] [CrossRef]
- Ohashi, K.; Pimienta, M.; Seki, E. Alcoholic liver disease: A current molecular and clinical perspective. Liver Res. 2018, 2, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Zhang, J.; Sun, J.; Min, T.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Oxidative stress in alcoholic liver disease, focusing on proteins, nucleic acids, and lipids: A review. Int. J. Biol. Macromol. 2024, 278 Pt 3 Pt 3, 134809. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, S.; Tan, Y.; Feng, J.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Biodegradation and Prospect of Polysaccharide from Crustaceans. Mar. Drugs 2022, 20, 310. [Google Scholar] [CrossRef]
- Yu, D.; Feng, J.; You, H.; Zhou, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Microstructure, Antibacterial and Antitumor Activities of Chitosan Oligosaccharides and Derivatives. Mar. Drugs 2022, 20, 69. [Google Scholar] [CrossRef]
- Yu, D.W.; Yu, Z.J.; Zhao, W.Y.; Regenstein, J.M.; Xia, W.S. Advances in the application of chitosan as a sustainable bioactive material in food preservation. Crit. Rev. Food Sci. Nutr. 2022, 62, 3782–3797. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Amiri, H.; Moosavi Basri, S.M.; Rastegari, H.; Lam, S.S.; Pan, J.; Gupta, V.K.; Tabatabaei, M. Tuning chitosan’s chemical structure for enhanced biological functions. Trends Biotechnol. 2023, 41, 785–797. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef]
- Sandri, G.; Aguzzi, C.; Rossi, S.; Bonferoni, M.C.; Bruni, G.; Boselli, C.; Cornaglia, A.I.; Riva, F.; Viseras, C.; Caramella, C.; et al. Halloysite and chitosan oligosaccharide nanocomposite for wound healing. Acta Biomater. 2017, 57, 216–224. [Google Scholar] [CrossRef]
- Gopan, G.; Jose, J.; Khot, K.B.; Bandiwadekar, A. The use of cellulose, chitosan and hyaluronic acid in transdermal therapeutic management of obesity: A review. Int. J. Biol. Macromol. 2023, 244, 125374. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, P.; Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009, 50, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Zima, T.; Kalousová, M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol. Clin. Exp. Res. 2005, 29 (Suppl. S11), 110s–115s. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, J.; Xing, F.; Han, T.; Jiao, R.; Liong, E.C.; Fung, M.L.; So, K.F.; Tipoe, G.L. Zeaxanthin dipalmitate therapeutically improves hepatic functions in an alcoholic fatty liver disease model through modulating MAPK pathway. PLoS ONE 2014, 9, e95214. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, J.T.; Tan, L.J.; Sun, C.Y.; Dong, J.N. Synthesis of N-furoyl chitosan and chito-oligosaccharides and evaluation of their antioxidant activity in vitro. Int. J. Biol. Macromol. 2013, 59, 391–395. [Google Scholar] [CrossRef]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Anraku, M. Antioxidant properties of some different molecular weight chitosans. Carbohydr. Res. 2009, 344, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, D.K.; Hu, S.; Mao, J.W.; Mei, L.H. Biochemical activities of low molecular weight chitosans derived from squid pens. Carbohydr. Polym. 2012, 87, 2231–2236. [Google Scholar] [CrossRef]
- Li, M.; Xie, R.S.; Liu, J.; Gan, L.H.; Long, M.N. Preparation of chitooligosaccharide acetate salts with narrow molecular size distribution and the antioxidative activity. Polym. Degrad. Stabil. 2020, 182, 9. [Google Scholar] [CrossRef]
- Park, P.J.; Je, J.Y.; Kim, S.K. Free radical scavenging activity of chitooligosaccharides by electron spin resonance spectrometry. J. Agric. Food Chem. 2003, 51, 4624–4627. [Google Scholar] [CrossRef]
- Feng, J.; Liu, Y.; Chen, J.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Marine Chitooligosaccharide Alters Intestinal Flora Structure and Regulates Hepatic Inflammatory Response to Influence Nonalcoholic Fatty Liver Disease. Mar. Drugs 2022, 20, 383. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, J.; Xie, H.; Liu, Y.; Bai, Y.; Che, Q.; Cao, H.; Huang, G.; Guo, J.; Su, Z. Protective effect and mechanism of chitooligosaccharides on acetaminophen-induced liver injury. Food Funct. 2021, 12, 9979–9993. [Google Scholar] [CrossRef]
- Li, K.C.; Xing, R.E.; Liu, S.; Qin, Y.K.; Li, B.; Wang, X.Q.; Li, P.C. Separation and scavenging superoxide radical activity of chitooligomers with degree of polymerization 6–16. Int. J. Biol. Macromol. 2012, 51, 826–830. [Google Scholar] [CrossRef]
- Zhou, J.W.; Wen, B.J.; Xie, H.Y.; Zhang, C.C.; Bai, Y.; Cao, H.; Che, Q.S.; Guo, J.; Su, Z.Q. Advances in the preparation and assessment of the biological activities of chitosan oligosaccharides with different structural characteristics. Food Funct. 2021, 12, 926–951. [Google Scholar] [CrossRef]
- Li, K.C.; Xing, R.E.; Liu, S.; Li, P.C. Advances in preparation, analysis and biological activities of single chitooligosaccharides. Carbohydr. Polym. 2016, 139, 178–190, Erratum in Carbohydr. Polym. 2017, 167, 365. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Kong, J.; Hu, X.M.; Cai, W.W.; Wang, Y.M.; Chi, C.F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs (II): Protective Function on UVB-Irradiated HaCaT Cells through Antioxidant and Anti-Apoptotic Mechanisms. Mar. Drugs 2023, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Y.; Xu, Z.; Li, F.; Yang, M.; Shi, F.; Lin, L.; Qin, Z. Characterization of effects of chitooligosaccharide monomer addition on immunomodulatory activity in macrophages. Food Res. Int. 2023, 163, 112268. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef]
- You, H.; Deng, X.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Ameliorative Effect of COST on Diet-Induced Lipid Metabolism Disorders by Regulating Intestinal Microbiota. Mar. Drugs 2022, 20, 444. [Google Scholar] [CrossRef]
- Tao, W.; Wang, G.; Wei, J. The Role of Chitosan Oligosaccharide in Metabolic Syndrome: A Review of Possible Mechanisms. Mar. Drugs 2021, 19, 501. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, L.; Yan, H.; Wu, M.; Hao, X.; Liu, H. Nutritional values, bioactive compounds and health benefits of purslane (Portulaca oleracea L.): A comprehensive review. Food Sci. Hum. Wellness 2024, 13, 2480–2501. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, J.; Bai, Y.; Che, Q.; Cao, H.; Guo, J.; Su, Z. Ameliorating the effect and mechanism of chitosan oligosaccharide on nonalcoholic fatty liver disease in mice. Food Funct. 2023, 14, 10459–10474. [Google Scholar] [CrossRef]
- Lai, W.; Zhou, S.; Bai, Y.; Che, Q.; Cao, H.; Guo, J.; Su, Z. Glucosamine attenuates alcohol-induced acute liver injury via inhibiting oxidative stress and inflammation. Curr. Res. Food Sci. 2024, 8, 100699. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Sun, J.; Yan, Q.; Wen, B.; Bai, Y.; Che, Q.; Cao, H.; Guo, J.; Su, Z. Chitosan Oligosaccharides Prevent Alcohol-Induced Liver Disease by Attenuating Inflammation and Oxidative Stress. Mar. Drugs 2025, 23, 134. https://doi.org/10.3390/md23030134
Liu Y, Sun J, Yan Q, Wen B, Bai Y, Che Q, Cao H, Guo J, Su Z. Chitosan Oligosaccharides Prevent Alcohol-Induced Liver Disease by Attenuating Inflammation and Oxidative Stress. Marine Drugs. 2025; 23(3):134. https://doi.org/10.3390/md23030134
Chicago/Turabian StyleLiu, Yanglong, Jiawei Sun, Qihao Yan, Bingjian Wen, Yan Bai, Qishi Che, Hua Cao, Jiao Guo, and Zhengquan Su. 2025. "Chitosan Oligosaccharides Prevent Alcohol-Induced Liver Disease by Attenuating Inflammation and Oxidative Stress" Marine Drugs 23, no. 3: 134. https://doi.org/10.3390/md23030134
APA StyleLiu, Y., Sun, J., Yan, Q., Wen, B., Bai, Y., Che, Q., Cao, H., Guo, J., & Su, Z. (2025). Chitosan Oligosaccharides Prevent Alcohol-Induced Liver Disease by Attenuating Inflammation and Oxidative Stress. Marine Drugs, 23(3), 134. https://doi.org/10.3390/md23030134







