“Hyphae Intertwined, Biomolecules Co-Born”—New Polyketides Induction by Co-Culture of the Mangrove Endophytic Fungus Phomopsis asparagi DHS-48 and Pestalotiopsis sp. HHL-101 at Both Volatile and Non-Volatile Levels
Abstract
1. Introduction
2. Results and Discussions
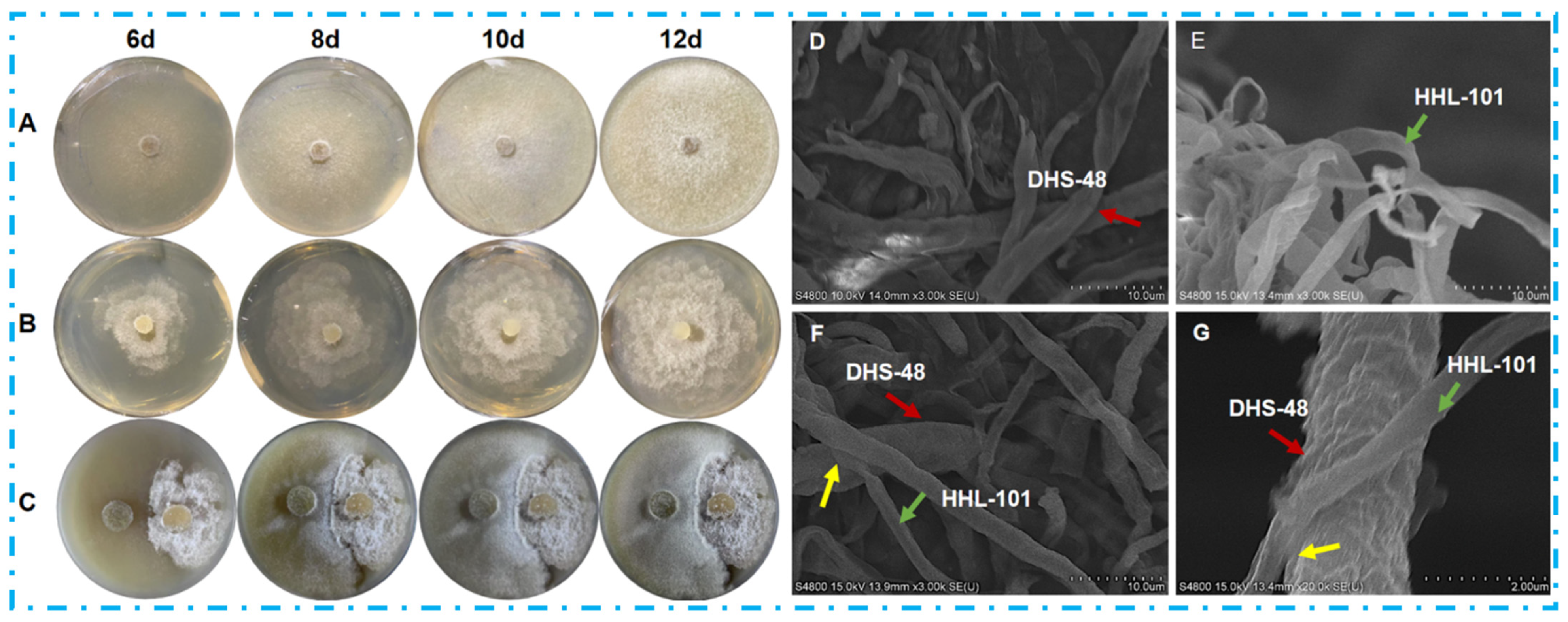
2.1. Morphology of Co-Culture Systems
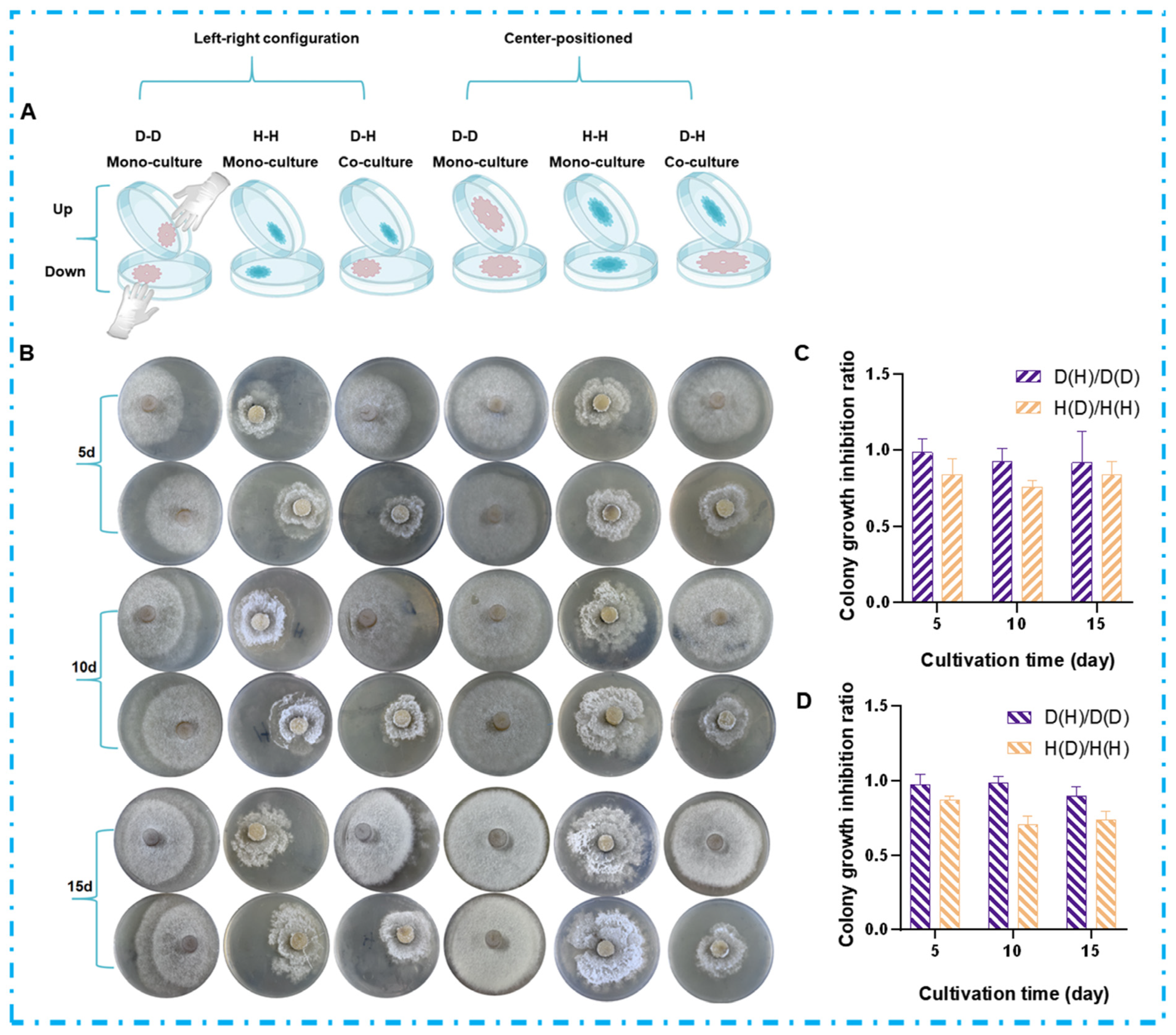
2.2. Chemical Interaction Mediated by VOCs in the Co-Culture Systems
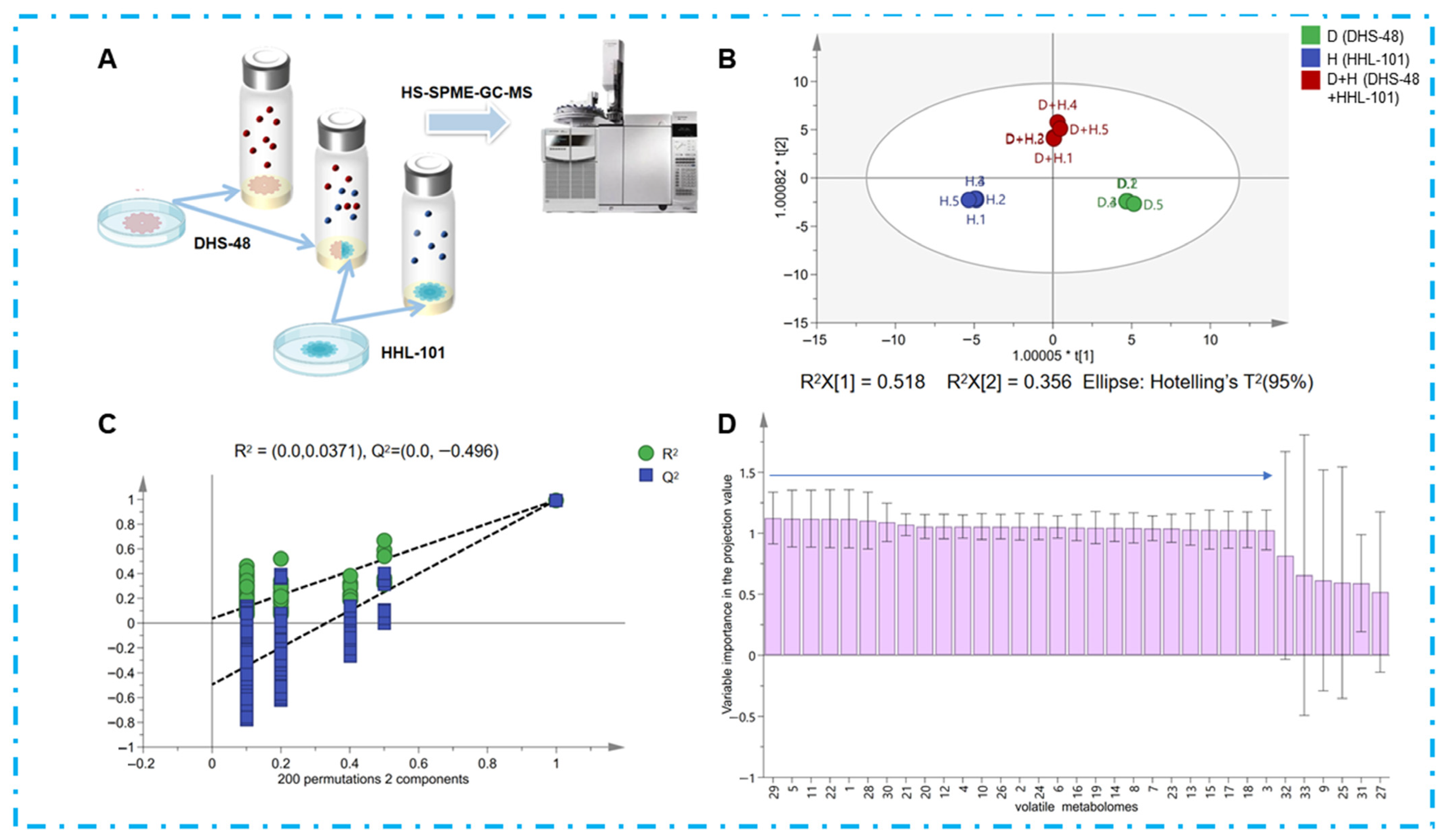
2.3. Volatile Metabolomics Analysis of the Co-Culture Systems
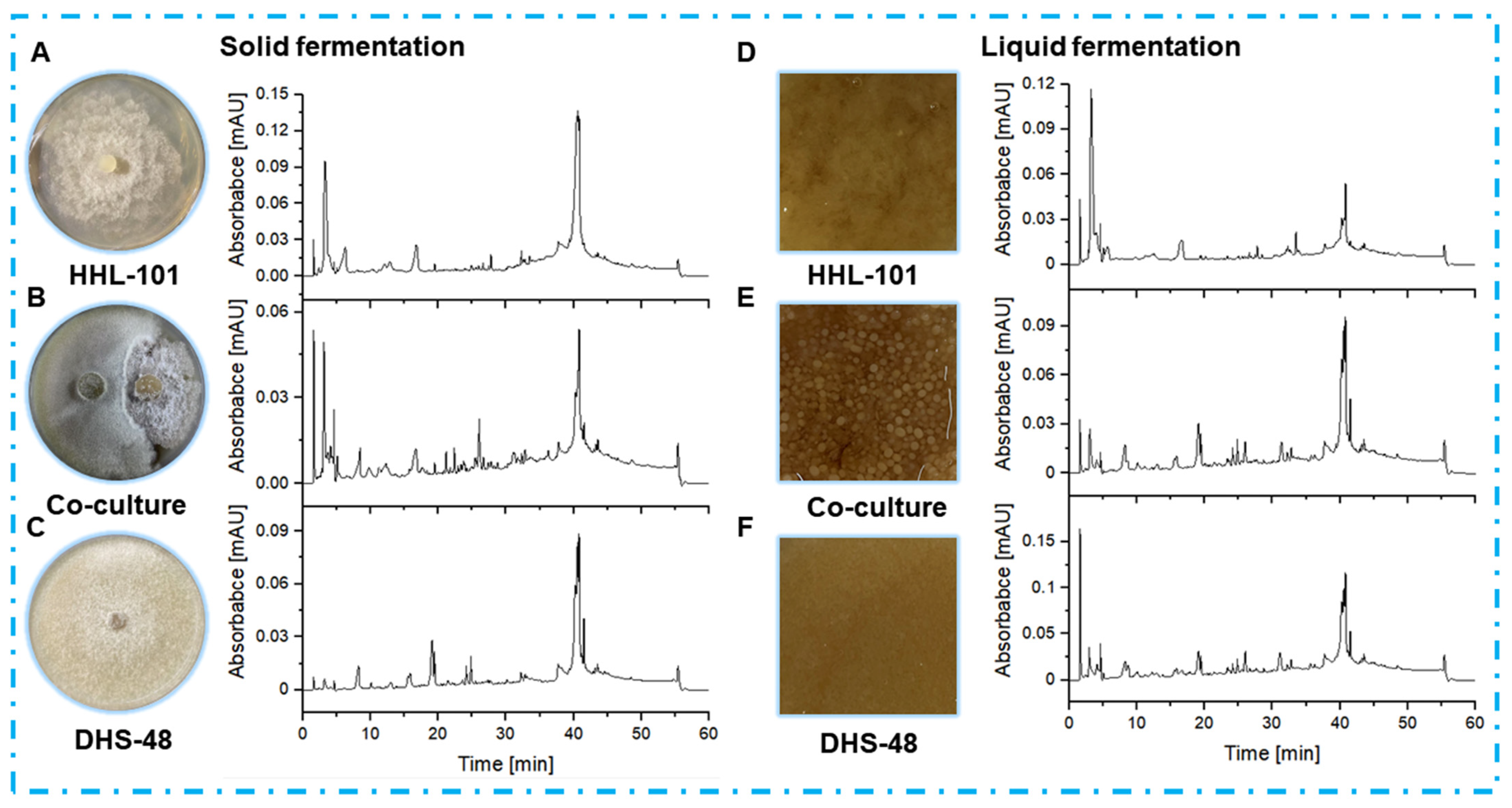
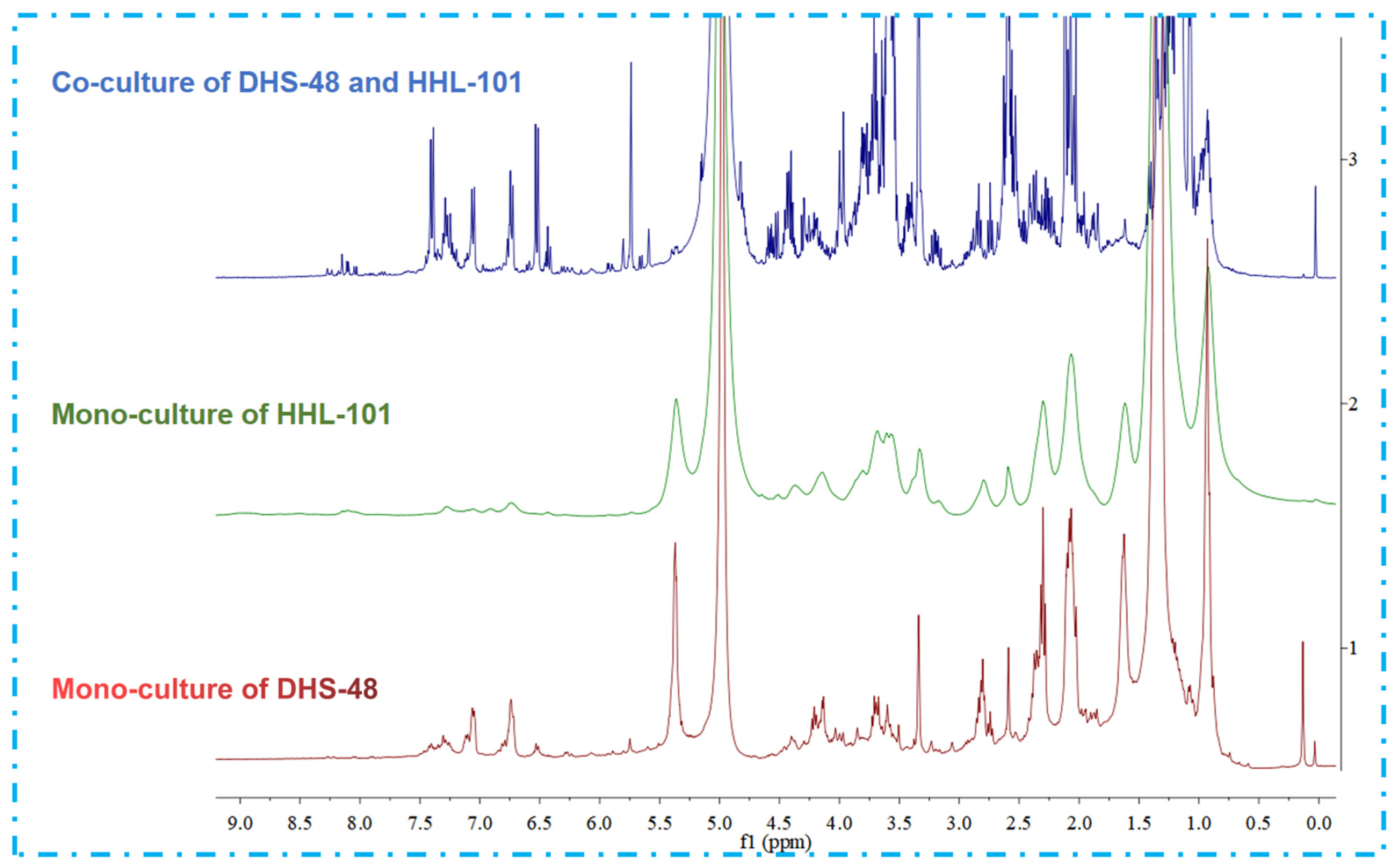
2.4. Non-Volatile Metabolomics Analysis of Small Scale Co-Cultures
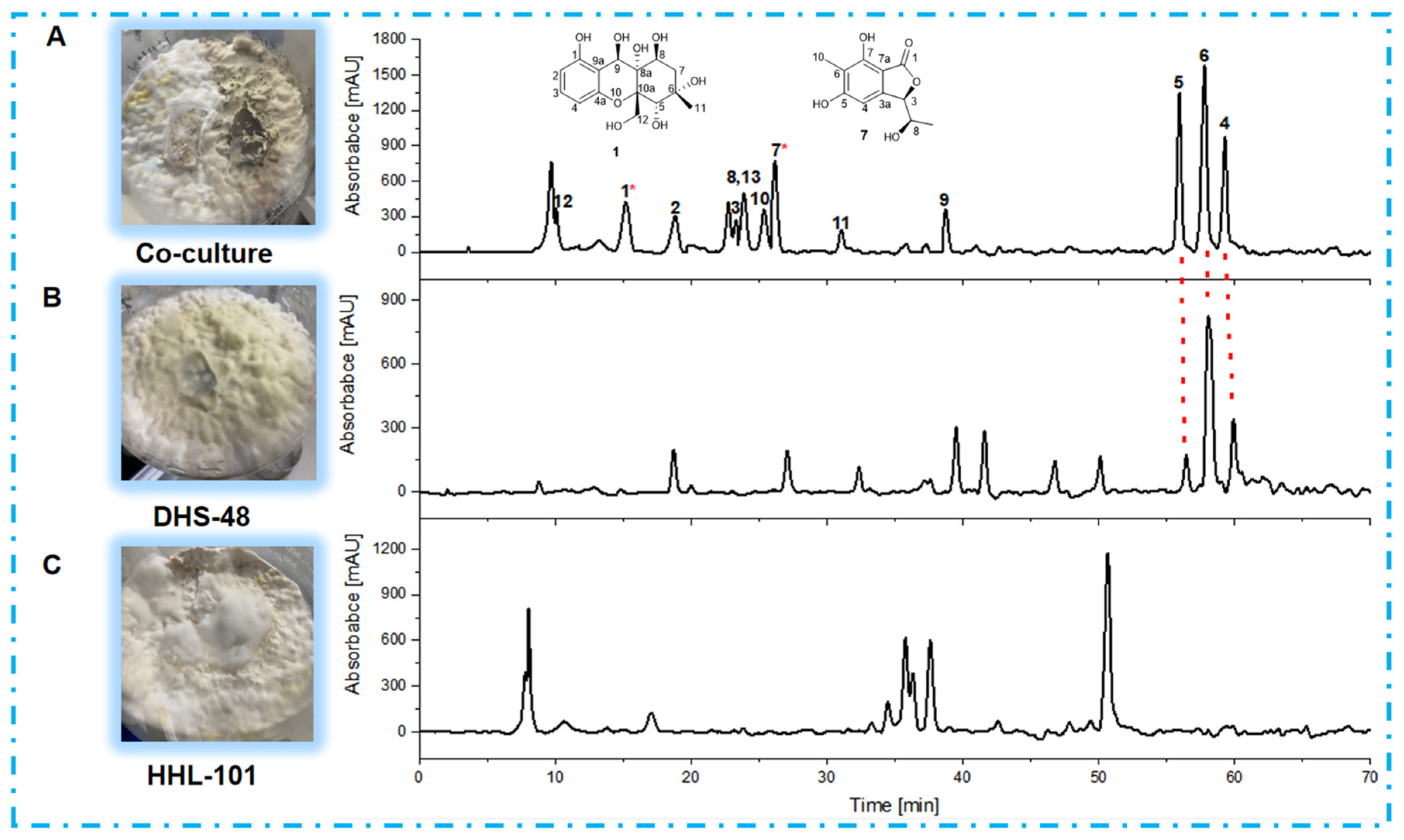
2.5. Non-Volatile Metabolomics Analysis of Large-Scale Co-Cultures
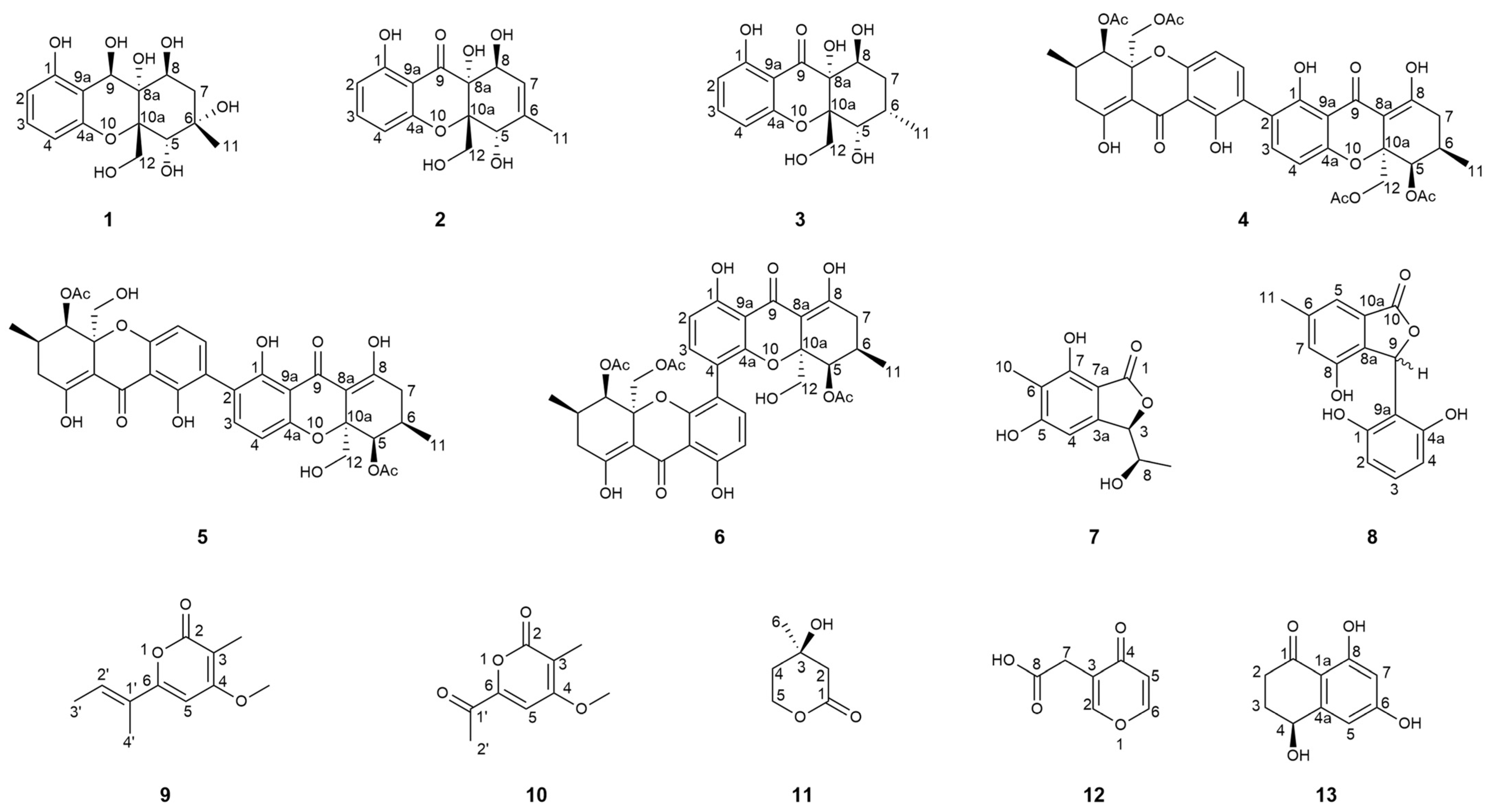
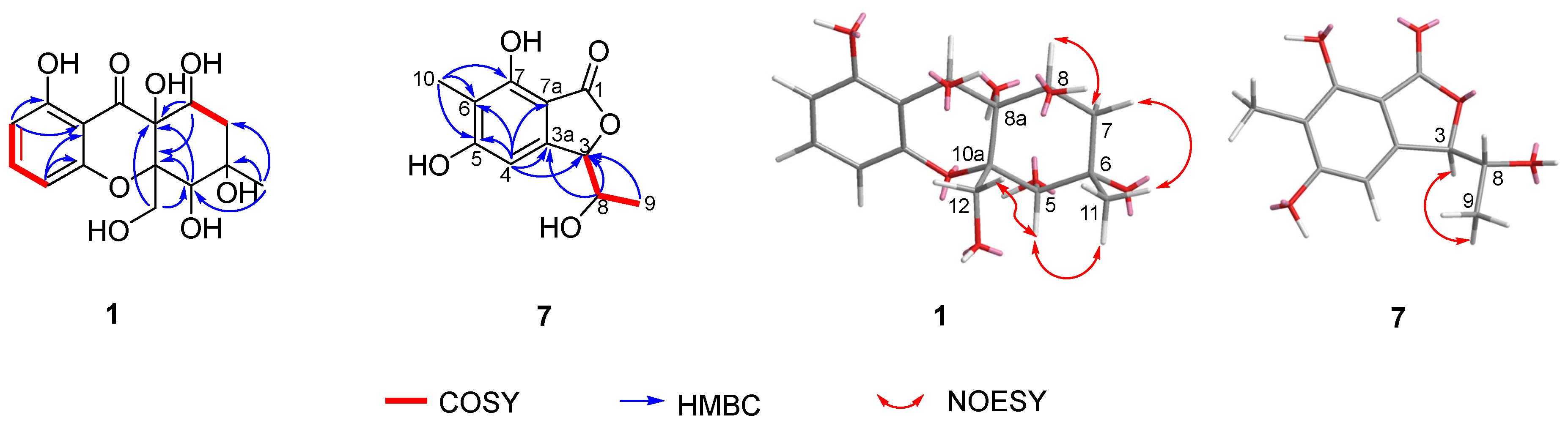
2.6. Structure Elucidation of New Compounds
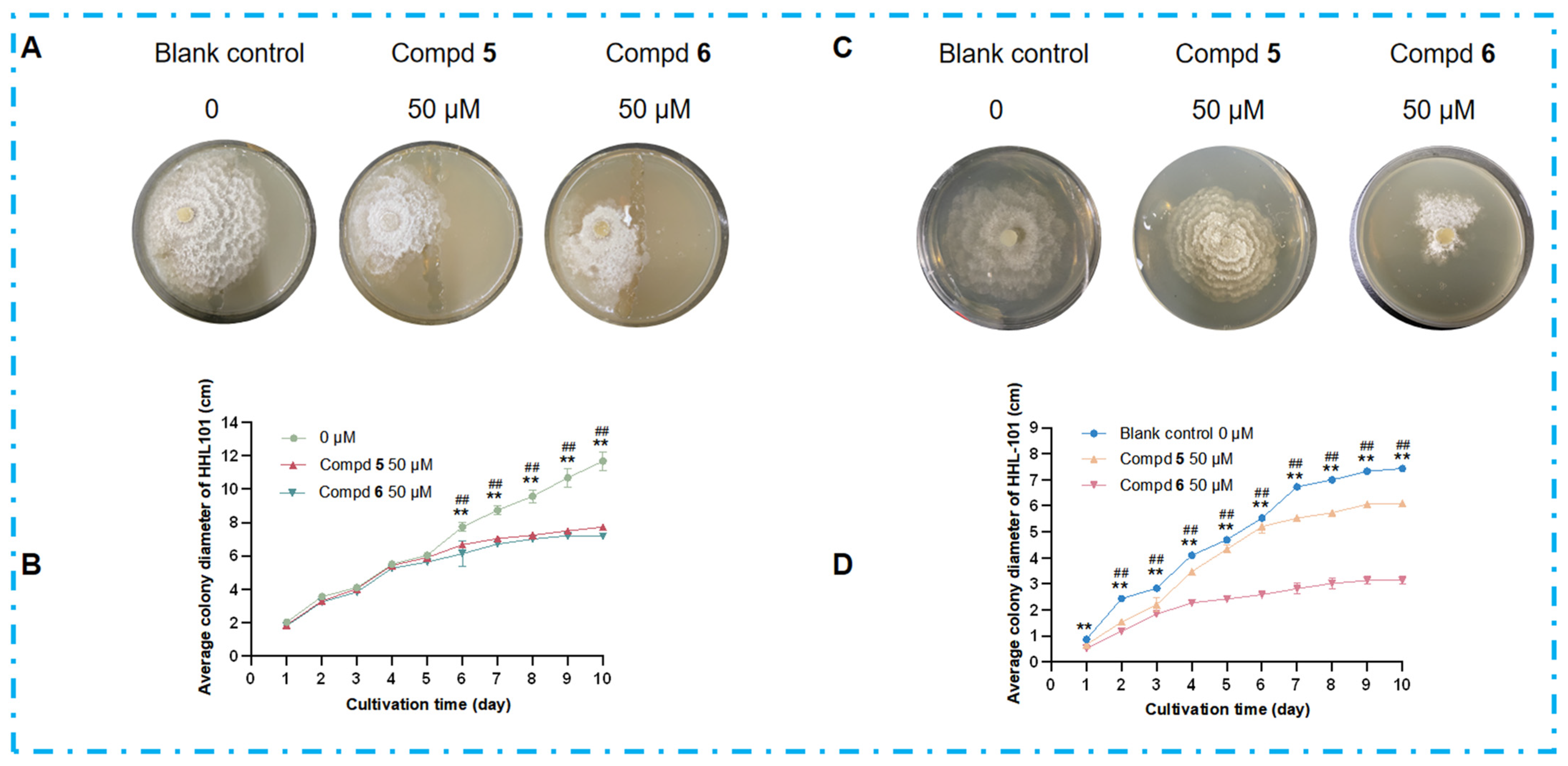
2.7. Chemical Interaction Mediated by Non-Volatile Antimicrobial Metabolites
2.8. Biological Evaluation of Isolated Compounds
3. Materials and Methods
3.1. General Procedures
3.2. Fungal Material
3.3. Preparation of Phomopsis asparagi DHS-48, Pestalotiopsis sp. HHL-101, Co-Cultivation, and Morphological Observation
3.4. Effect of Volatiles on Colony Morphology of Phomopsis asparagi DHS-48, Pestalotiopsis sp. HHL-101
3.5. Experimental Conditions for DHS-48 and HHL-101 Mono- and Co-Cultures Analyzed Using HS-SPME-GC-MS
3.6. HS-SPME-GC-MS for Analyzing Volatile Fractions
3.7. Processing of GC-MS Data and Deconvolution of GC Peaks
3.8. Small-Scale Fermentation of Liquid and Solid Media for DHS-48 and HHL-101 in Mono-Culture and Co-Culture
3.9. Large Fermentation by Solid Fermentation and Isolation of Compounds
3.10. Inhibitory Effects of Compounds (Produced in Large Quantities by DHS-48 After Co-Culture) on HHL-101
3.11. Electron Circular Dichroism Calculation
3.12. Cytotoxicity Assay
3.13. Splenocyte Proliferation Assay
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Xu, J. Biomolecules produced by mangrove-associated microbes. Curr. Med. Chem. 2011, 18, 5224–5266. [Google Scholar] [CrossRef] [PubMed]
- Bugni, T.S.; Ireland, C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yi, M.; Ding, L.; He, S. A Review of Anti-Inflammatory Compounds from Marine Fungi, 2000–2018. Mar. Drugs 2019, 17, 636. [Google Scholar] [CrossRef]
- Lin, W.; Li, G.; Xu, J. Bio-Active Products from Mangrove Ecosystems. Mar. Drugs 2023, 21, 239. [Google Scholar] [CrossRef]
- Wu, M.J.; Xu, B.; Guo, Y.W. Unusual Secondary Metabolites from the Mangrove Ecosystems: Structures, Bioactivities, Chemical, and Bio-Syntheses. Mar. Drugs 2022, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Potts, B.C.; Albitar, M.X.; Anderson, K.C.; Baritaki, S.; Berkers, C.; Bonavida, B.; Chandra, J.; Chauhan, D.; Cusack, J.C., Jr.; Fenical, W.; et al. Marizomib, a proteasome inhibitor for all seasons: Preclinical profile and a framework for clinical trials. Curr. Cancer Drug Targets 2011, 11, 254–284. [Google Scholar] [CrossRef]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef]
- Tiwari, P.; Bae, H. Endophytic Fungi: Key Insights, Emerging Prospects, and Challenges in Natural Product Drug Discovery. Microorganisms 2022, 10, 360. [Google Scholar] [CrossRef]
- Rutledge, P.J.; Challis, G.L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760. [Google Scholar] [CrossRef]
- Nai, C.; Meyer, V. From Axenic to Mixed Cultures: Technological Advances Accelerating a Paradigm Shift in Microbiology. Trends Microbiol. 2018, 26, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Xu, X.Y.; Shen, X.T.; Yuan, X.J.; Zhou, Y.M.; Fan, H.; Zhu, L.P.; Du, F.Y.; Sadilek, M.; Yang, J.; Qiao, B.; et al. Metabolomics Investigation of an Association of Induced Features and Corresponding Fungus during the Co-Culture of Trametes versicolor and Ganoderma applanatum. Front. Microbiol. 2018, 8, 2647. [Google Scholar] [CrossRef] [PubMed]
- Losada, L.; Ajayi, O.; Frisvad, J.C.; Yu, J.; Nierman, W.C. Effect of competition on the production and activity of secondary metabolites in Aspergillus species. Med. Mycol. 2009, 47, S88–S96. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kuang, Y.; Splivallo, R.; Chatterjee, P.; Karlovsky, P. Interactions among filamentous fungi Aspergillus niger, Fusarium verticillioides and Clonostachys rosea: Fungal biomass, diversity of secreted metabolites and fumonisin production. BMC Microbiol. 2016, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Ding, W.J.; Shao, C.L.; She, Z.G.; Lin, Y.C. A new diimide derivative from the co-culture broth of two mangrove fungi (strain no. E33 and K38). J. Asian Nat. Prod. Res. 2010, 12, 809–813. [Google Scholar] [CrossRef]
- Huang, S.; Ding, W.; Li, C.; Cox, D.G. Two new cyclopeptides from the co-culture broth of two marine mangrove fungi and their antifungal activity. Pharmacogn. Mag. 2014, 10, 410–414. [Google Scholar] [CrossRef]
- Peng, X.Y.; Wu, J.T.; Shao, C.L.; Li, Z.Y.; Chen, M.; Wang, C.Y. Co-culture: Stimulate the metabolic potential and explore the molecular diversity of natural products from microorganisms. Mar. Life Sci. Technol. 2021, 3, 363–374. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; David, V.; Hemmler, D.; Schmitt-Kopplin, P.; Alexandre, H. Exploring yeast interactions through metabolic profiling. Sci. Rep. 2020, 10, 6073. [Google Scholar] [CrossRef]
- Adnani, N.; Chevrette, M.G.; Adibhatla, S.N.; Zhang, F.; Yu, Q.; Braun, D.R.; Nelson, J.; Simpkins, S.W.; McDonald, B.R.; Myers, C.L.; et al. Coculture of Marine Invertebrate-Associated Bacteria and Interdisciplinary Technologies Enable Biosynthesis and Discovery of a New Antibiotic, Keyicin. ACS Chem. Biol. 2017, 12, 3093–3102. [Google Scholar] [CrossRef]
- Siddiquee, S. Recent advancements on the role and analysis of Volatile Compounds (VOCs) from Trichoderma. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 139–175. [Google Scholar]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial Volatiles: Small Molecules with an Important Role in Intra- and Inter-Kingdom Interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennett, J.W. Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 2015, 99, 3395–3405. [Google Scholar] [CrossRef]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Wei, C.; Sun, C.; Feng, Z.; Zhang, X.; Xu, J. Four New Chromones from the Endophytic Fungus Phomopsis asparagi DHS-48 Isolated from the Chinese Mangrove Plant Rhizophora mangle. Mar. Drugs 2021, 19, 348. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, X.; Wu, J.; Wei, C.; Feng, T.; Zhou, D.; Wen, Z.; Xu, J. Immunosuppressive Cytochalasins from the Mangrove Endophytic Fungus Phomopsis asparagi DHS-48. Mar. Drugs 2022, 20, 526. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wei, C.; Deng, X.; Chen, D.; Wen, Z.; Xu, J. Epigenetic Manipulation Induced Production of Immunosuppressive Chromones and Cytochalasins from the Mangrove Endophytic Fungus Phomopsis asparagi DHS-48. Mar. Drugs 2022, 20, 616. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, D.; Li, Q.; Feng, T.; Xu, J. Metabolomics-Guided Discovery of New Dimeric Xanthones from Co-Cultures of Mangrove Endophytic Fungi Phomopsis asparagi DHS-48 and Phomopsis sp. DHS-11. Mar. Drugs 2024, 22, 102. [Google Scholar] [CrossRef]
- Wu, J.; Ye, J.; Cen, J.; Chen, Y.; Xu, J. Induction of Three New Secondary Metabolites by the Co-Culture of Endophytic Fungi Phomopsis asparagi DHS-48 and Phomopsis sp. DHS-11 Isolated from the Chinese Mangrove Plant Rhizophora mangle. Mar. Drugs 2024, 22, 332. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Y.; Yan, W.; Gu, P.; Zeng, Z.; Li, Z.; Zhang, G.; Wei, M.; Xue, Y. New Pyranone Derivatives and Sesquiterpenoid Isolated from the Endophytic Fungus Xylaria sp. Z184. Molecules 2024, 29, 1728. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, X.; Li, G.; Feng, Z.; Xu, J. Pestalotiopisorin B, a new isocoumarin derivative from the mangrove endophytic fungus Pestalotiopsis sp. HHL101. Nat. Prod. Res. 2020, 34, 1002–1007. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Z.M.; Chen, Y.C.; Tan, H.B.; Li, S.N.; Li, H.H.; Gao, X.X.; Liu, H.X.; Zhang, W.M. Chromone-Derived Polyketides from the Deep-Sea Fungus Diaporthe phaseolorum FS431. Mar. Drugs 2019, 17, 182. [Google Scholar] [CrossRef]
- Wagenaar, M.M.; Clardy, J. Dicerandrols, new antibiotic and cytotoxic dimers produced by the fungus Phomopsis longicolla isolated from an endangered mint. J. Nat. Prod. 2001, 64, 1006–1009. [Google Scholar] [CrossRef]
- Shiono, Y.; Sasaki, T.; Shibuya, F.; Yasuda, Y.; Koseki, T.; Supratman, U. Isolation of a Phomoxanthone A derivative, a new metabolite of tetrahydroxanthone, from a Phomopsis sp. isolated from the mangrove, Rhizhopora mucronata. Nat. Prod. Commun. 2013, 8, 1735–1737. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Hu, H.B.; Huang, Z.H.; Yan, R.J.; Tian, L.W.; Wu, J. Phomopsols A and B from the Mangrove Endophytic Fungus Phomopsis sp. xy21: Structures, Neuroprotective Effects, and Biogenetic Relationships. Org. Lett. 2019, 21, 7919–7922. [Google Scholar] [CrossRef] [PubMed]
- Avent, A.G.; Hanson, J.R.; Truneh, A. Two pyrones from Gliocladium vermoesenii. Phytochemistry 1992, 31, 1065–1066. [Google Scholar] [CrossRef]
- Lu, X.; Xu, N.; Dai, H.F.; Mei, W.L.; Yang, Z.X.; Pei, Y.H. Three new compounds from endophytic fungus L10 of Cephalotaxus hainanensis. J. Asian Nat. Prod. Res. 2009, 11, 397–400. [Google Scholar] [CrossRef]
- Ying, Y.M.; Zhang, L.W.; Shan, W.G.; Zhan, Z.J. Secondary Metabolites of Peyronellaea sp. XW-12, an Endophytic Fungus of Huperzia serrata. Chem. Nat. Compd. 2014, 50, 723–725. [Google Scholar] [CrossRef]
- Edwards, R.L.; Maitland, D.J.; Pittayakhajonwut, P.; Whalley, A.J. Metabolites of the higher fungi. Part 33. 1 Grammicin, a novel bicyclic C7H6O4 furanopyranol from the fungus Xylaria grammica (Mont.) Fr. J. Chem. Soc. Perkin Trans. 2001, 1, 1296–1299. [Google Scholar] [CrossRef]
- Dong, J.Y.; Song, H.C.; Li, J.H.; Tang, Y.S.; Sun, R.; Wang, L.; Zhou, Y.P.; Wang, L.M.; Shen, K.Z.; Wang, C.R.; et al. Ymf 1029A-E, preussomerin analogues from the fresh-water-derived fungus YMF 1.01029. J. Nat. Prod. 2008, 71, 952–956. [Google Scholar] [CrossRef]
- Du, Z.Y.; Alvaro, J.; Hyden, B.; Zienkiewicz, K.; Benning, N.; Zienkiewicz, A.; Bonito, G.; Benning, C. Enhancing oil production and harvest by combining the marine alga Nannochloropsis oceanica and the oleaginous fungus Mortierella elongata. Biotechnol. Biofuels 2018, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Kuang, G.L.; Li, S.; Ning, T.T.; Zhao, G.Z. Differential Volatile Metabolites between Sichuan Baoning Vinegar and Shanxi Aged Vinegar Determined by GC-MS Fingerprint and Multivariate Statistics. Food Sci. 2020, 41, 227–232. [Google Scholar]
- Shi, C.F.; Yin, Z.Q.; Wei, Q.; Jia, R.Y. Bacteriostatic Action and Mechanism of α-terpineol on Escherichia coli. Acta Vet. Zootech. Sin. 2013, 44, 796–801. [Google Scholar]
- Zhang, P.; Wang, P.; Shi, C.F.; Wei, Q.; Du, Y.H.; Zhou, L.J.; Yin, Z.Q.; Jia, R.Y. Antibacterial Activity of Essential Components of Cinnamomum longepaniculatum Oil on Several Typical Pathogenic Bacteria. J. Sichuan Agric. Univ. 2013, 31, 393–397. [Google Scholar]
- Sasikumar, R.; Das, D.; Saravanan, C.; Deka, S.C. GC-HRMS screening of bioactive compounds responsible for antimicrobial and antioxidant activities of blood fruit (Haematocarpus validus Bakh. F. Ex Forman) of North-East India. Arch. Microbiol. 2020, 202, 2643–2654. [Google Scholar] [CrossRef]
- Maheshwari, M.; Hussain, N. Chemical synthesis of substituted naphthalene derivatives: A review. Synthesis 2024, 56, 2145–2182. [Google Scholar]
- Lin, C.A.; Cheng, C.; Chen, L.W.; Chen, C.W.; Duan, K.J. Ethanol production using the whole solid-state fermented sugarcane bagasse cultivated by Trichoderma reesei RUT-C30 supplemented with commercial cellulase. Biocatal. Agric. Biotechnol. 2023, 50, 102667. [Google Scholar] [CrossRef]
- Domingues, F.C.; Queiroz, J.A.; Cabral, J.M.; Fonseca, L.P. The influence of culture conditions on mycelial structure and cellulase production by Trichoderma reesei Rut C-30. Enzyme Microb. Technol. 2000, 26, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Erbert, C.; Lopes, A.A.; Yokoya, N.S.; Furtado, N.A.; Conti, R.; Pupo, M.T.; Debonsi, H.M. Antibacterial compound from the endophytic fungus Phomopsis longicolla isolated from the tropical red seaweed Bostrychia radicans. Bot. Mar. 2012, 55, 435–440. [Google Scholar] [CrossRef]
- Lim, C.; Kim, J.; Choi, J.N.; Ponnusamy, K.; Jeon, Y.; Kim, S.U.; Kim, J.G.; Lee, C. Identification, fermentation, and bioactivity against Xanthomonas oryzae of antimicrobial metabolites isolated from Phomopsis longicolla S1B4. J. Microbiol. Biotechnol. 2010, 20, 494–500. [Google Scholar]
- Ding, G.; Liu, S.; Guo, L.; Zhou, Y.; Che, Y. Antifungal metabolites from the plant endophytic fungus Pestalotiopsis foedan. J. Nat. Prod. 2008, 71, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Wisecaver, J.H.; Slot, J.C.; Rokas, A. The evolution of fungal metabolic pathways. PLoS Genet. 2014, 10, e1004816. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Diao, X.; Wang, T.; Chen, G.; Lin, Q.; Yang, X.; Xu, J. Phylogenetic diversity and antioxidant activities of culturable fungal endophytes associated with the mangrove species Rhizophora stylosa and R. mucronate in the South China Sea. PLoS ONE 2018, 13, e0197359. [Google Scholar]
- Zhou, J.; Li, G.; Deng, Q.; Zheng, D.; Yang, X.; Xu, J. Cytotoxic constituents from the mangrove endophytic Pestalotiopsis sp. induce G0/G1 cell cycle arrest and apoptosis in human cancer cells. Nat. Prod. Res. 2018, 32, 2968–2972. [Google Scholar] [CrossRef]
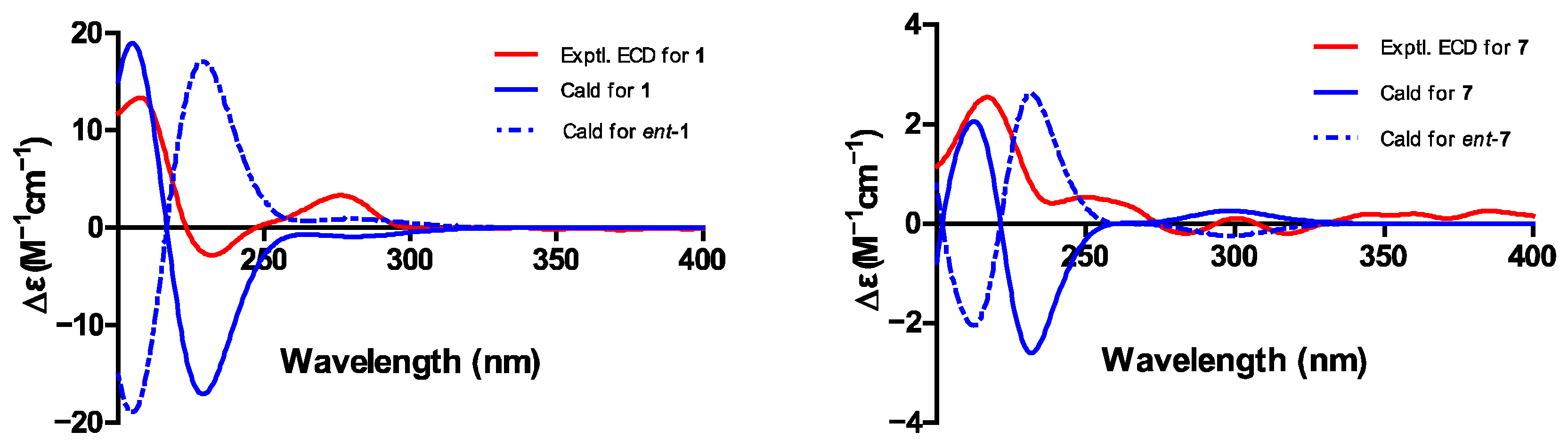
| Position | 1 | 7 | ||
|---|---|---|---|---|
| δC Type | δH (J in Hz) | δC Type | δH (J in Hz) | |
| 1 | 159.3, C | 173.5, C | ||
| 2 | 109.1, CH | 6.37, d, 8.0 | ||
| 3 | 129.9, CH | 6.97, t, 8.2 | 83.3, CH | 5.24, d,3.7 |
| 3a | 151.3, C | |||
| 4 | 108.8, CH | 6.40, d, 8.2 | 102.1, CH | 6.51, s |
| 4a | 154.5, C | |||
| 5 | 76.8, CH | 4.21, s | 163.9, C | |
| 6 | 85.4, C | 112.8, C | ||
| 7 | 42.9, CH2 | Ha 2.15, dd, 15.0, 5.4 | 158.3, C | |
| Hb 1.92, d, 15.0 | ||||
| 7a | 105.0, C | |||
| 8 | 70.5, CH | 3.90, d, 4.9 | 68.8, CH | 4.10, m |
| 8a | 74.5, C | |||
| 9 | 64.7, CH | 5.67, s | 20.4, CH3 | 1.20, d, 6.5 |
| 9a | 109.9, C | |||
| 10 | 7.84, CH3 | 2.05, s | ||
| 10a | 86.4, C | |||
| 11 | 20.5, CH3 | 1.30, s | ||
| 12 | 69.3, CH2 | Ha 4.07, d, 7.7 | ||
| Hb 3.81, d, 7.7 | ||||
| Compound | IC50 (µM) a | |
|---|---|---|
| HepG2 | Hela | |
| 1–3 | \ | \ |
| 4 | 14.03 ± 0.56 | 24.17 ± 2.35 |
| 5 | 4.94 ± 0.33 | 19.26 ± 0.78 |
| 6 | 13.11 ± 0.43 | 21.06 ± 0.87 |
| 7–13 | \ | \ |
| Adriamycin b | \ | 0.92 ± 0.56 |
| Fluorouracil c | 178.12 ± 28.82 | \ |
| Compound | IC50 (µM) a | |
|---|---|---|
| ConA-Induced T-Cell Proliferation | LPS-Induced B-Cell Proliferation | |
| 1 | \ | \ |
| 2 | 42.35 ± 2.49 | 88.19 ± 2.59 |
| 3–13 | \ | \ |
| cyclosporin A b | 4.39 ± 0.02 | 25.11 ± 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Li, X.; Liang, Z.; Xu, J. “Hyphae Intertwined, Biomolecules Co-Born”—New Polyketides Induction by Co-Culture of the Mangrove Endophytic Fungus Phomopsis asparagi DHS-48 and Pestalotiopsis sp. HHL-101 at Both Volatile and Non-Volatile Levels. Mar. Drugs 2025, 23, 452. https://doi.org/10.3390/md23120452
Feng T, Li X, Liang Z, Xu J. “Hyphae Intertwined, Biomolecules Co-Born”—New Polyketides Induction by Co-Culture of the Mangrove Endophytic Fungus Phomopsis asparagi DHS-48 and Pestalotiopsis sp. HHL-101 at Both Volatile and Non-Volatile Levels. Marine Drugs. 2025; 23(12):452. https://doi.org/10.3390/md23120452
Chicago/Turabian StyleFeng, Ting, Xiaojing Li, Zhenyi Liang, and Jing Xu. 2025. "“Hyphae Intertwined, Biomolecules Co-Born”—New Polyketides Induction by Co-Culture of the Mangrove Endophytic Fungus Phomopsis asparagi DHS-48 and Pestalotiopsis sp. HHL-101 at Both Volatile and Non-Volatile Levels" Marine Drugs 23, no. 12: 452. https://doi.org/10.3390/md23120452
APA StyleFeng, T., Li, X., Liang, Z., & Xu, J. (2025). “Hyphae Intertwined, Biomolecules Co-Born”—New Polyketides Induction by Co-Culture of the Mangrove Endophytic Fungus Phomopsis asparagi DHS-48 and Pestalotiopsis sp. HHL-101 at Both Volatile and Non-Volatile Levels. Marine Drugs, 23(12), 452. https://doi.org/10.3390/md23120452








