Synergistic Anticancer Activity of Fucoidan from Lessonia trabeculata Combined with Chemotherapeutic Agents in 4T1 Breast Spheroids
Abstract
1. Introduction
2. Results
2.1. Effect of FuLt on the Viability of Homotypic TNBC 4T1 Spheroids
2.2. Half-Maximal Inhibitory Concentration (IC50)
2.3. Cytotoxic Effect Based on the Chou-Talalay Method
2.4. SI for FuLt and Three Chemotherapeutics Against TNBC 4T1 Spheroids
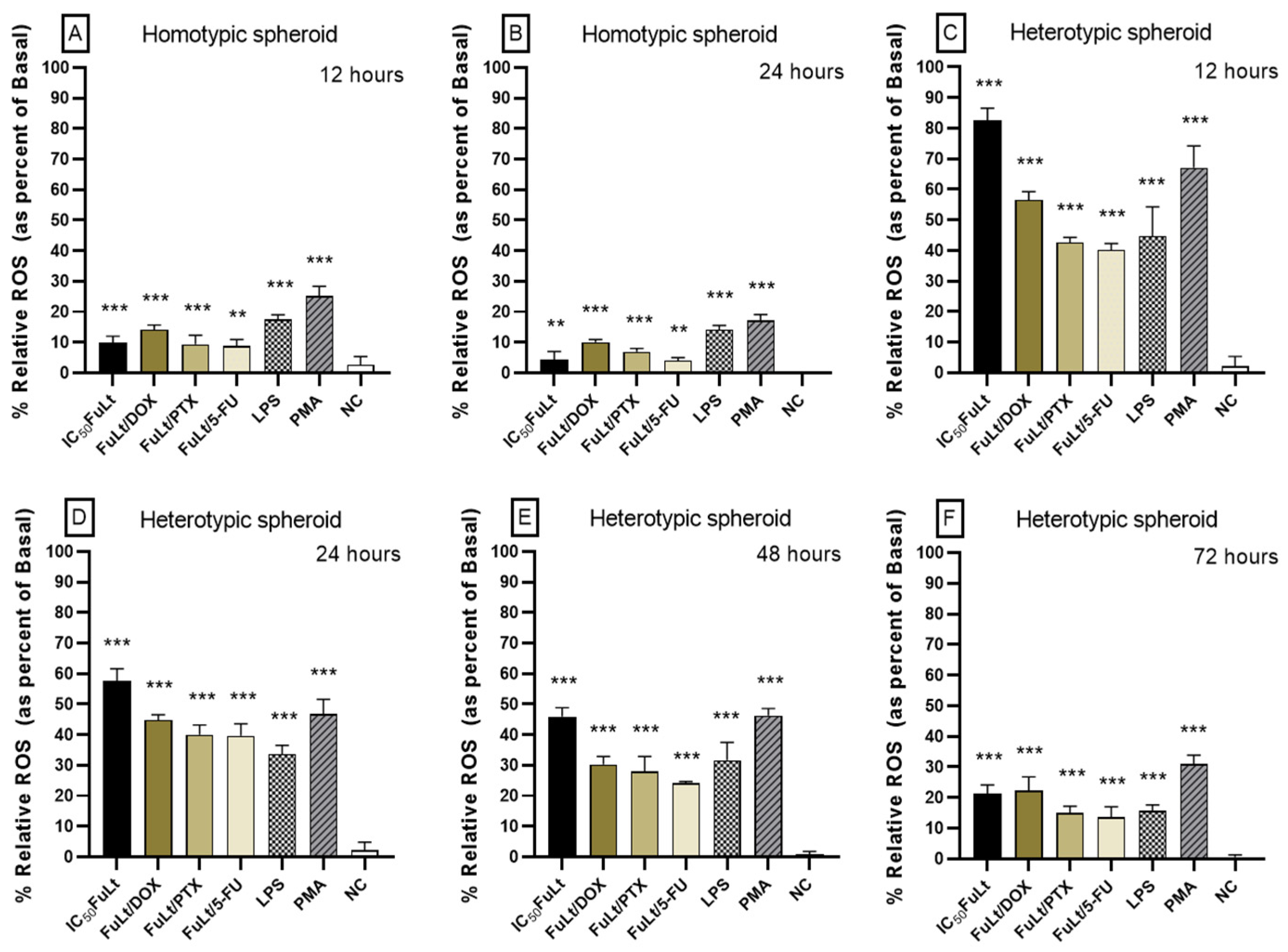
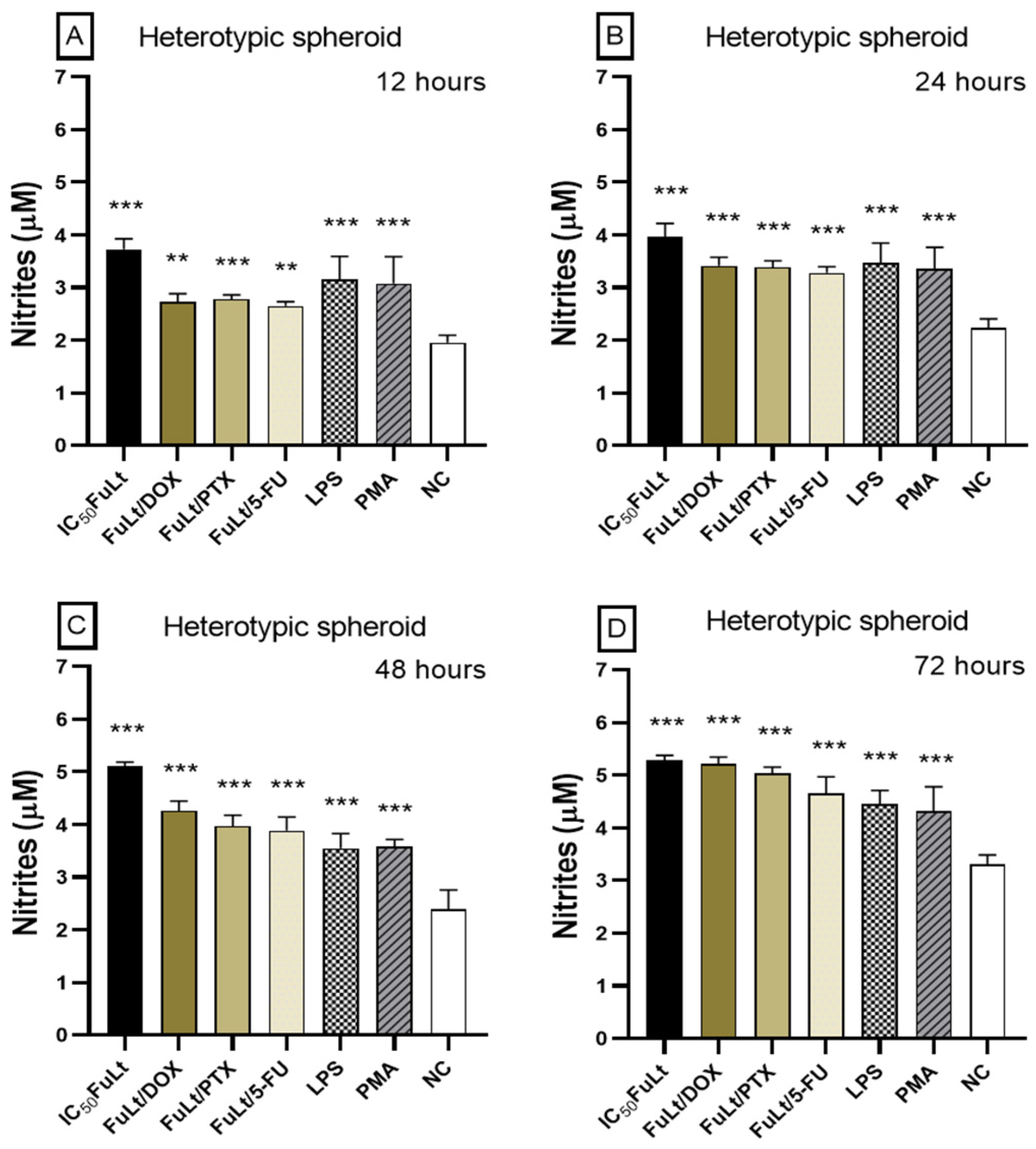
2.5. Oxidative Stress Caused by FuLt, Three Chemotherapeutic Agents and Three SIs in Homotypic and Heterotypic Spheroids
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Cell Culture Conditions
4.4. Formation of 4T1 Homotypic Spheroids
4.5. Formation of the Heterotypic Spheroid
4.6. Viability Assay
4.7. Determination of the Half-Maximal Inhibitory Concentration (IC50)
4.8. Determining the Optimal Binary Combination Using the Chou-Talalay Method
4.9. Oxidative Activity
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024. [Google Scholar]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Qayoom, H.; Wani, N.A.; Alshehri, B.; Mir, M.A. An Insight into the Cancer Stem Cell Survival Pathways Involved in Chemoresistance in Triple-Negative Breast Cancer. Future Oncol. 2021, 17, 4185–4206. [Google Scholar] [CrossRef]
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef]
- Škubník, J.; Svobodová Pavlíčková, V.; Ruml, T.; Rimpelová, S. Autophagy in cancer resistance to paclitaxel: Development of combination strategies. Biomed. Pharmacother. 2023, 161, 114458. [Google Scholar] [CrossRef]
- Valencia-Lazcano, A.A.; Hassan, D.; Pourmadadi, M.; Shamsabadipour, A.; Behzadmehr, R.; Rahdar, A.; Medina, D.I.; Díez-Pascual, A.M. 5-Fluorouracil nano-delivery systems as a cutting-edge for cancer therapy. Eur. J. Med. Chem. 2023, 246, 114995. [Google Scholar] [CrossRef]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Luque-Bolivar, A.; Pérez-Mora, E.; Villegas, V.E.; Rondón-Lagos, M. Resistance and Overcoming Resistance in Breast Cancer. Breast Cancer Targets Ther. 2020, 12, 211–229. [Google Scholar] [CrossRef]
- Wu, L.; Ye, K.; Jiang, S.; Zhou, G. Marine Power on Cancer: Drugs, Lead Compounds, and Mechanisms. Mar. Drugs 2021, 19, 488. [Google Scholar] [CrossRef]
- Shinde, P.; Banerjee, P.; Mandhare, A. Marine natural products as source of new drugs: A patent review (2015–2018). Expert. Opin. Ther. Pat. 2019, 29, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Sanniyasi, E.; Gopal, R.K.; Damodharan, R.; Arumugam, A.; Sampath Kumar, M.; Senthilkumar, N.; Anbalagan, M. In vitro anticancer potential of laminarin and fucoidan from Brown seaweeds. Sci. Rep. 2023, 13, 14452. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Q.; Farooqi, A.A.; Wang, J.; Yue, Y.; Geng, L.; Wu, N. Opportunities and challenges of fucoidan for tumors therapy. Carbohydr. Polym. 2024, 324, 121555. [Google Scholar] [CrossRef]
- Condori-Macuri, R.; Gonzales, L.A.; Cruz-Riquelme, R.; Colona-Vallejos, E.C.V.E.; Chauca-Torres, N. Antitumor and immunomodulatory activity of fucoidan from the brown alga Lessonia trabeculata (Lessoniaceae) on breast cancer spheroids. Rev. Biol. Trop. 2023, 71, e54918. [Google Scholar] [CrossRef]
- Toccas-Salas, M.; Alzamora-Gonzales, L.; Colona-Vallejos, E.; Escobar-Guzmán, E.; Chávez, J.A.; Apumayta, E.V. Actividad citotóxica y antiproliferativa de un extracto rico en fucoidan de Lessonia trabeculata sobre células de adenocarcinoma mamario murino 4T1. An. Fac. Med. 2023, 84, 295–301. [Google Scholar] [CrossRef]
- Cruz Riquelme, R.T.; Colona-Vallejos, E.H.; Alzamora-Gonzales, L.; Condori Macuri, R.M. Fucoidan from Lessonia trabeculata Induces Apoptosis through Caspase Dependent and Caspase-Independent Activation in 4T1 Breast Adenocarcinoma In Vitro. Mar. Drugs 2024, 22, 251. [Google Scholar] [CrossRef]
- Yan, C.; Pan, M.; Geng, L.; Zhang, Q.; Hu, Y.; Wang, J.; Ye, S. A novel enzyme-assisted one-pot method for the extraction of fucoidan and alginate oligosaccharides from Lessonia trabeculata and their bioactivities. J. Oceanol. Limnol. 2024, 42, 1998–2012. [Google Scholar] [CrossRef]
- Colona-Vallejos, E.H.; Alzamora-Gonzales, L.; Chávez Pérez, J.; Apumayta Suárez, E.V.; Chang Avila, I. Incremento de la viabilidad, producción de especies reactivas de oxígeno, IL-1 y TNF-α en células mononucleares de sangre periférica humana tratadas con fucoidan de Lessonia trabeculata. Rev. Peru. Biol. 2019, 26, 291–300. [Google Scholar] [CrossRef]
- Huang, C.W.; Chen, Y.C.; Yin, T.C.; Chen, P.J.; Chang, T.K.; Su, W.C.; Ma, C.J.; Li, C.C.; Tsai, H.L.; Wang, J.Y. Low-Molecular-Weight Fucoidan as Complementary Therapy of Fluoropyrimidine-Based Chemotherapy in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 8041. [Google Scholar] [CrossRef] [PubMed]
- Mathew, L.; Burney, M.; Gaikwad, A.; Nyshadham, P.; Nugent, E.K.; Gonzalez, A.; Smith, J.A. Preclinical Evaluation of Safety of Fucoidan Extracts from Undaria pinnatifida and Fucus vesiculosus for Use in Cancer Treatment. Integr. Cancer Ther. 2017, 16, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.K.; Singh, Y.; Sharma, K.; Shrivastav, A.; Sharma, A.; Singh, A.; Meher, J.G.; Singh, P.; Raval, K.; Kumar, A.; et al. Improved chemotherapy against breast cancer through immunotherapeutic activity of fucoidan decorated electrostatically assembled nanoparticles bearing doxorubicin. Int. J. Biol. Macromol. 2019, 122, 1100–1114. [Google Scholar] [CrossRef]
- Zhang, N.; Xue, M.; Wang, Q.; Liang, H.; Yang, J.; Pei, Z.; Qin, K. Inhibition of fucoidan on breast cancer cells and potential enhancement of their sensitivity to chemotherapy by regulating autophagy. Phytother. Res. 2021, 35, 6904–6917. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Lapina, I.M.; Kulminskaya, A.A.; Zhurishkina, E.V.; Shikov, A.N. Comparative Evaluation of Dynamic Maceration and Ultrasonic Assisted Extraction of Fucoidan from Four Arctic Brown Algae on Its Antioxidant and Anticancer Properties. Mar. Drugs 2025, 23, 230. [Google Scholar] [PubMed]
- Ikeguchi, M.; Yamamoto, M.; Arai, Y.; Maeta, Y.; Ashida, K.; Katano, K.; Miki, Y.; Kimura, T. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol. Lett. 2011, 2, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. J. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Mijatović, S.; Savić-Radojević, A.; Plješa-Ercegovac, M.; Simić, T.; Nicoletti, F.; Maksimović-Ivanić, D. The Double-Faced Role of Nitric Oxide and Reactive Oxygen Species in Solid Tumors. Antioxidants 2020, 9, 374. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual Role of Reactive Oxygen Species and their Application in Cancer Therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef]
- Jiang, W.; Dong, W.; Li, M.; Guo, Z.; Wang, Q.; Liu, Y.; Bi, Y.; Zhou, H.; Wang, Y. Nitric Oxide Induces Immunogenic Cell Death and Potentiates Cancer Immunotherapy. ACS Nano 2022, 16, 3881–3894. [Google Scholar] [CrossRef]
- Lee, J. Current Treatment Landscape for Early Triple-Negative Breast Cancer (TNBC). J. Clin. Med. 2023, 12, 1524. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Tarbeeva, D.V.; Ermakova, S.P.; Zvyagintseva, T.N. Structural Characteristics and Biological Activity of Fucoidans from the Brown Algae Alaria sp. and Saccharina japonica of Different Reproductive Status. Chem. Biodivers. 2012, 9, 817–828. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Mak, W.; Wang, S.K.; Liu, T.; Hamid, N.; Li, Y.; Lu, J.; White, W.L. Anti-Proliferation Potential and Content of Fucoidan Extracted from Sporophyll of New Zealand Undaria pinnatifida. Front. Nutr. 2014, 1, 9. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Lin, T.Y.; Hwang, P.A.; Tseng, L.M.; Chen, R.H.; Tsao, S.M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition decreases metastasis by enhancing ubiquitin-dependent TGFβ receptor degradation in breast cancer. J. Carcinog. 2013, 34, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, Y.; Zhang, Y.; Zhang, D. Fucoidan induces cancer cell apoptosis by modulating the endoplasmic reticulum stress cascades. PLoS ONE 2014, 9, e108157. [Google Scholar] [CrossRef]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer Properties Mechanisms of Fucoidan on Mouse Breast Cancer In Vitro In Vivo. PLoS ONE 2012, 7, e43483. [Google Scholar] [CrossRef]
- Xue, M.; Ge, Y.; Zhang, J.; Liu, Y.; Wang, Q.; Hou, L.; Zheng, Z. Fucoidan Inhibited 4T1 Mouse Breast Cancer Cell Growth In Vivo and In Vitro Via Downregulation of Wnt/β-Catenin Signaling. Nutr. Cancer 2013, 65, 460–468. [Google Scholar] [CrossRef]
- Chen, L.M.; Yang, P.P.; Al Haq, A.T.; Hwang, P.A.; Lai, Y.C.; Weng, Y.S.; Chen, M.A.; Hsu, H.L. Oligo-Fucoidan supplementation enhances the effect of Olaparib on preventing metastasis and recurrence of triple-negative breast cancer in mice. J. Biomed. Sci. 2022, 29, 70. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. Less Can Be More: The Hormesis Theory of Stress Adaptation in the Global Biosphere and Its Implications. Biomedicines 2021, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Denis, E.; Papurina, T.; Koliada, A.; Vaiserman, A. Evaluation of the Stimulating and Protective Effects of Fucoxanthin Against Human Skin Fibroblasts: An In Vitro Study. Res. Ther. 2019, 2, 124. [Google Scholar]
- Posadino, A.M.; Giordo, R.; Cossu, A.; Nasrallah, G.K.; Shaito, A.; Abou-Saleh, H.; Eid, A.H.; Pintus, G. Flavin Oxidase-Induced ROS Generation Modulates PKC Biphasic Effect of Resveratrol on Endothelial Cell Survival. Biomolecules 2019, 9, 209. [Google Scholar] [CrossRef]
- Kamat, A.M.; Sethi, G.; Aggarwal, B.B. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Mol. Cancer Ther. 2007, 6, 1022–1030. [Google Scholar] [CrossRef]
- Lima, C.F.; Pereira-Wilson, C.; Rattan, S.I.S. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: Relevance for anti-aging intervention. Mol. Nutr. Food Res. 2011, 55, 430–442. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Malyarenko, T.V.; Usoltseva, R.V.; Silchenko, A.S.; Kicha, A.A.; Ivanchina, N.V.; Ermakova, S.P. Fucoidan from brown algae Fucus evanescens potentiates the anti-proliferative efficacy of asterosaponins from starfish Asteropsis carinifera in 2D and 3D models of melanoma cells. Int. J. Biol. Macromol. 2021, 185, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nováková, E.; Zima, J.; Špaglová, M.; Labudová, M.; Šupolíková, M. Synergistic Antiproliferative Effects of Chondroitin Sulfate and Fucoidan in Tumor-derived Spheroids: Insights From a 3D Cell Culture Approach. Eur. Pharm. J. 2025, 71, 25–33. [Google Scholar] [CrossRef]
- Jin, Z.J. About the evaluation of drug combination. Acta Pharmacol. Sin. 2004, 25, 146–147. [Google Scholar]
- Zhang, Z.; Teruya, K.; Yoshida, T.; Eto, H.; Shirahata, S. Fucoidan Extract Enhances the Anti-Cancer Activity of Chemotherapeutic Agents in MDA-MB-231 and MCF-7 Breast Cancer Cells. Mar. Drugs 2013, 11, 81–98. [Google Scholar] [CrossRef]
- Banafa, A.M.; Roshan, S.; Liu, Y.Y.; Chen, H.J.; Chen, M.J.; Yang, G.X.; He, G.Y. Fucoidan induces G1 phase arrest and apoptosis through caspases-dependent pathway and ROS induction in human breast cancer MCF-7 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 717–724. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan Extract Induces Apoptosis in MCF-7 Cells via a Mechanism Involving the ROS-Dependent JNK Activation and Mitochondria-Mediated Pathways. PLoS ONE 2011, 6, e27441. [Google Scholar] [CrossRef]
- Bae, H.; Lee, J.Y.; Yang, C.; Song, G.; Lim, W. Fucoidan Derived from Fucus vesiculosus Inhibits the Development of Human Ovarian Cancer via the Disturbance of Calcium Homeostasis, Endoplasmic Reticulum Stress, and Angiogenesis. Mar. Drugs 2020, 18, 45. [Google Scholar] [CrossRef]
- Shiau, J.P.; Chuang, Y.T.; Yang, K.H.; Chang, F.R.; Sheu, J.H.; Hou, M.F.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Brown Algae-Derived Fucoidan Exerts Oxidative Stress-Dependent Antiproliferation on Oral Cancer Cells. Antioxidants 2022, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Xiao, Q.; Kuang, X.; Zhang, T.; Yang, Z.; Wang, L. Fucoidan inhibits proliferation of the SKM-1 acute myeloid leukaemia cell line via the activation of apoptotic pathways and production of reactive oxygen species. Mol. Med. Rep. 2015, 12, 6649. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22. [Google Scholar] [CrossRef]
- Yu, W.; Tu, Y.; Long, Z.; Liu, J.; Kong, D.; Peng, J.; Wu, H.; Zheng, G.; Zhao, J.; Chen, Y.; et al. Reactive Oxygen Species Bridge the Gap between Chronic Inflammation and Tumor Development. Oxidative Med. Cell. Longev. 2022, 2022, 2606928. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Yu, Q. Fucoidan delays apoptosis and induces pro-inflammatory cytokine production in human neutrophils. Int. J. Biol. Macromol. 2015, 73, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Teng, Y.; Zheng, Y.; Zhang, M.; Wang, X.; Cheng, H.; Xu, J.; Chen, X.; Zhao, X.; et al. Enhancement of seaweed polysaccharides (fucoidan and laminarin) on the phagocytosis of macrophages via activation of intelectin in blunt snout bream (Megalobrama amblycephala). Front. Mar. Sci. 2023, 10, 1124880. [Google Scholar] [CrossRef]
- Kar, S.; Sharma, G.; Das, P.K. Fucoidan cures infection with both antimony-susceptible and -resistant strains of Leishmania donovani through Th1 response and macrophage-derived oxidants. J. Antimicrob. Chemother. 2011, 66, 618–625. [Google Scholar] [CrossRef]
- Jin, J.O.; Song, M.G.; Kim, Y.N.; Park, J.I.; Kwak, J.Y. The mechanism of fucoidan-induced apoptosis in leukemic cells: Involvement of ERK1/2, JNK, glutathione, and nitric oxide. Mol. Carcinog. 2010, 49, 771–782. [Google Scholar] [CrossRef]
- Jang, J.Y.; Moon, S.Y.; Joo, H.G. Differential effects of fucoidans with low and high molecular weight on the viability and function of spleen cells. Food Chem. Toxicol. 2014, 68, 234–238. [Google Scholar] [CrossRef]
- Choi, E.; Kim, A.; Kim, Y.; Hwang, J. Immunomodulating activity of arabinogalactan and fucoidan in vitro. J. Med. Food 2005, 8, 446–453. [Google Scholar] [CrossRef]
- Rajbhandary, S.; Dhakal, H.; Shrestha, S. Tumor immune microenvironment (TIME) to enhance antitumor immunity. Eur. J. Med. Res. 2023, 28, 169. [Google Scholar] [CrossRef]
- Morris, R.M.; Mortimer, T.O.; O’Neill, K.L. Cytokines: Can Cancer Get the Message? Cancers 2022, 14, 2178. [Google Scholar] [CrossRef]
- Nilofar Danishmalik, S.; Lee, S.H.; Sin, J.I. Tumor regression is mediated via the induction of HER263-71- specific CD8+ CTL activity in a 4T1.2/HER2 tumor model: No involvement of CD80 in tumor control. Oncotarget 2017, 8, 26771–26788. [Google Scholar] [CrossRef] [PubMed]
- Blockhuys, S.; Vanhoecke, B.; Smet, J.; De Paepe, B.; Van Coster, R.; Bracke, M.; De Wagter, C. Unraveling the mechanisms behind the enhanced MTT conversion by irradiated breast cancer cells. Radiat. Res. 2013, 179, 433–443. [Google Scholar] [CrossRef] [PubMed]
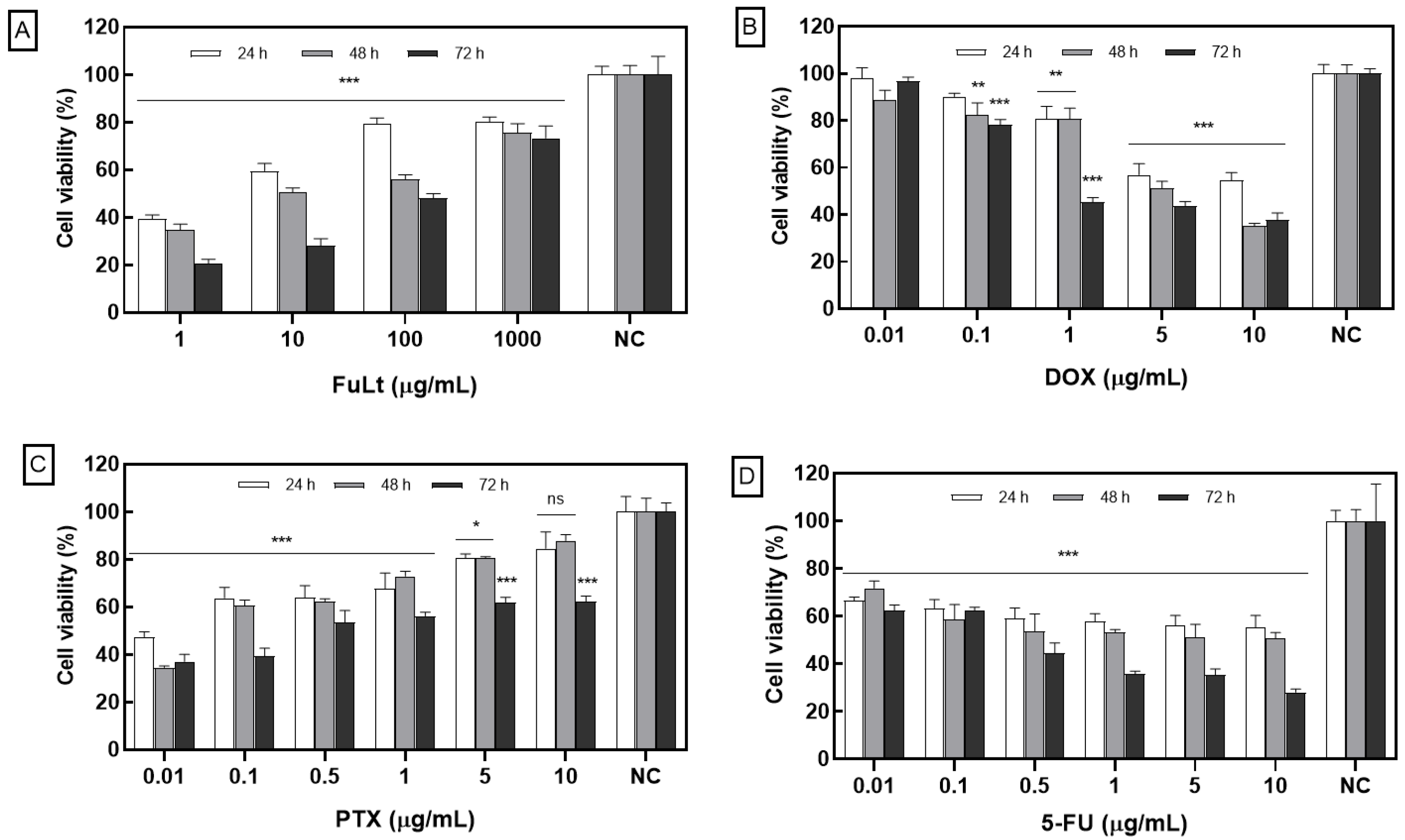
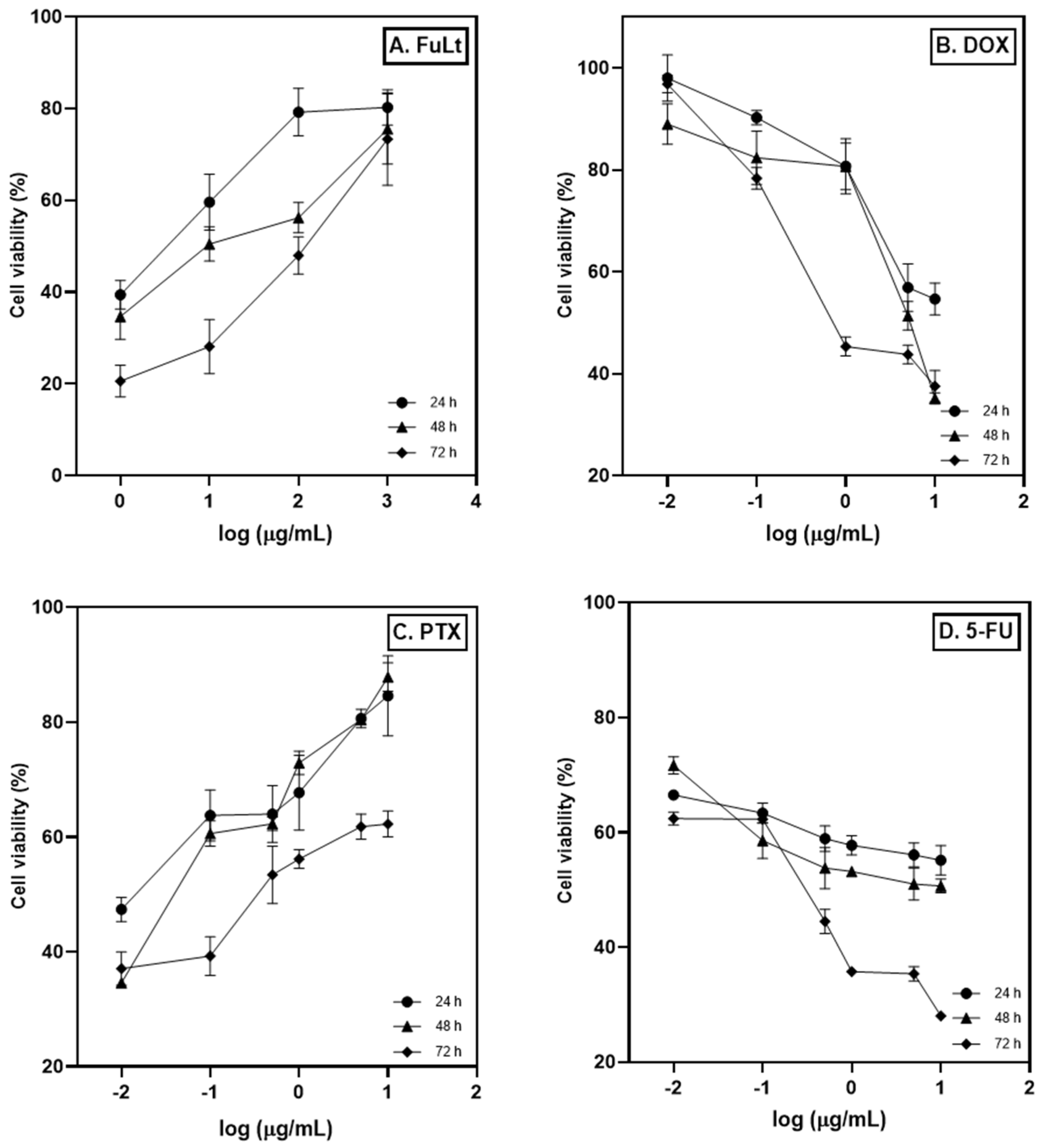
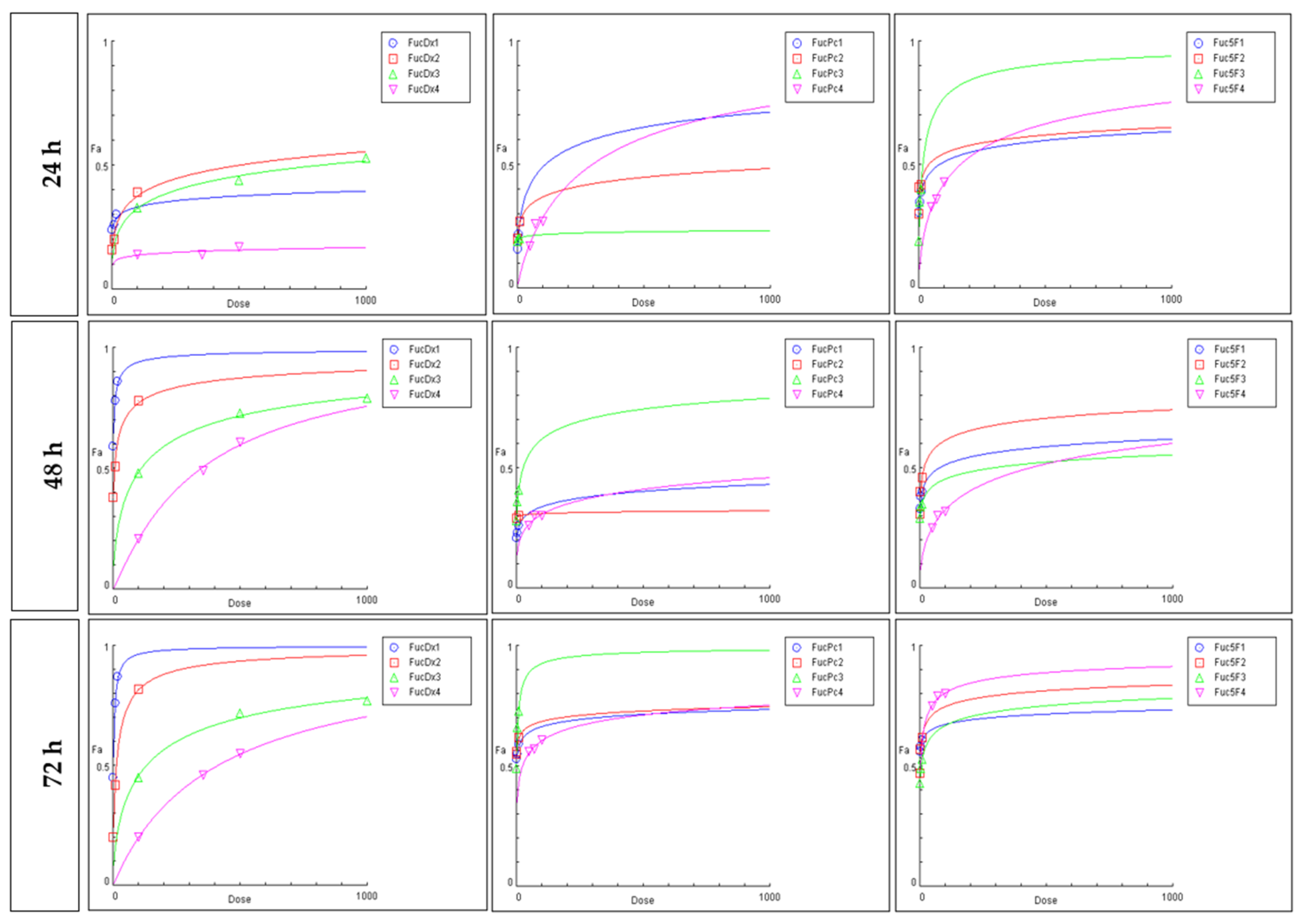
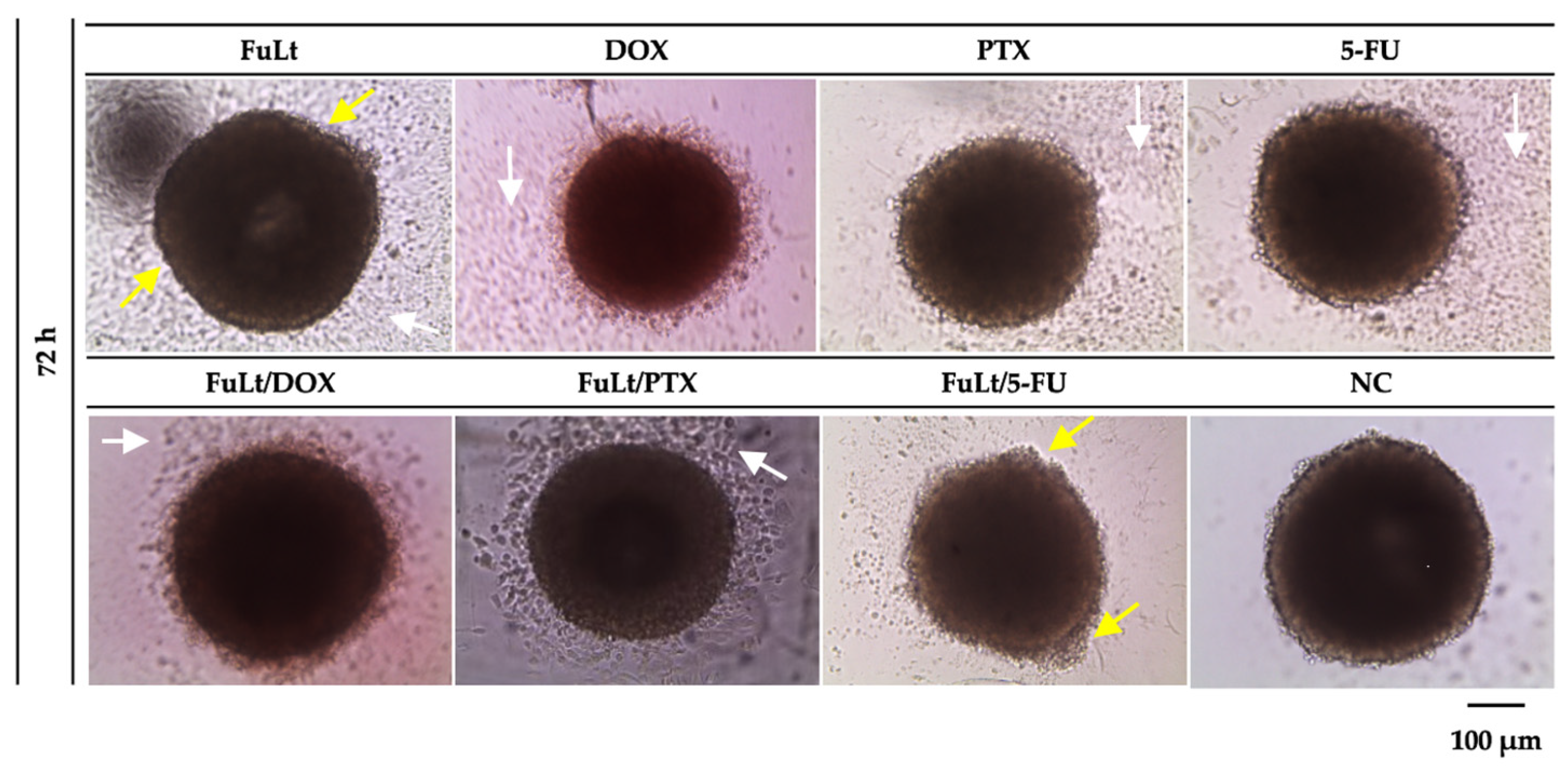
| IC50 (μg/mL) | ||||
|---|---|---|---|---|
| Time (h) | FuLt | DOX | PTX | 5-FU |
| 24 | 3 | 12 | 0.02 | 5 |
| 48 | 15 | 5 | 0.05 | 2 |
| 72 | 100 | 2 | 0.5 | 0.5 |
| Treatments | Parameters | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FuLt. | DOX | PTX | 5-FU | fa | m | Dm | r | m | Dm | r | m | Dm | r | ||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||||||||||
| 1 | 0.61 | 0.65 | 0.79 | 0.28 | 2.73 | 0.95 | 0.24 | 13.5 | 0.97 | 0.34 | 85.69 | 0.98 | |||
| 10 | 0.39 | 0.49 | 0.72 | ||||||||||||
| 100 | 0.21 | 0.44 | 0.52 | ||||||||||||
| 1000 | 0.2 | 0.24 | 0.27 | ||||||||||||
| 0.01 | 0.02 | 0.11 | 0.03 | 0.53 | 11.4 | 0.99 | 0.36 | 6.5 | 0.91 | 0.56 | 2.16 | 0.95 | |||
| 0.1 | 0.1 | 0.18 | 0.22 | ||||||||||||
| 1.0 | 0.19 | 0.19 | 0.55 | ||||||||||||
| 5.0 | 0.43 | 0.49 | 0.56 | ||||||||||||
| 10.0 | 0.45 | 0.65 | 0.62 | ||||||||||||
| 0.01 | 0.53 | 0.66 | 0.63 | 0.25 | 0.02 | 0.96 | 0.35 | 0.06 | 0.98 | 0.17 | 0.38 | 0.96 | |||
| 0.1 | 0.36 | 0.39 | 0.61 | ||||||||||||
| 0.5 | 0.36 | 0.38 | 0.47 | ||||||||||||
| 1.0 | 0.32 | 0.27 | 0.44 | ||||||||||||
| 5.0 | 0.19 | 0.2 | 0.38 | ||||||||||||
| 10.0 | 0.15 | 0.12 | 0.38 | ||||||||||||
| 0.01 | 0.33 | 0.06 | 0.38 | 0.07 | 10.8 | 0.99 | 0.4 | 4.73 | 0.93 | 0.22 | 0.17 | 0.95 | |||
| 0.1 | 0.37 | 0.15 | 0.4 | ||||||||||||
| 0.5 | 0.41 | 0.43 | 0.56 | ||||||||||||
| 1.0 | 0.42 | 0.44 | 0.64 | ||||||||||||
| 5.0 | 0.44 | 0.47 | 0.65 | ||||||||||||
| 10.0 | 0.45 | 0.47 | 0.72 | ||||||||||||
| FuLt + DOX (μg/mL) | Parameters | DRI | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hours | Ratio | Total Concentration | fa | CI Value | m | Dm | r | FuLt | DOX | |
| 24 | 1:1 | 2 | 0.24 | 0.78 | 1.76 × 102 | 1.29 | synergism 1 | |||
| 10 | 0.26 | 3.21 | 0.12 | 3.26 × 104 | 0.92 | 2.39 × 101 | 0.32 | antagonism | ||
| 20 | 0.3 | 4.53 | 5.83 | 0.23 | antagonism | |||||
| 10:1 | 2 | 0.16 | 0.37 | 5.99 × 102 | 2.73 | synergism 2 | ||||
| 10 | 0.2 | 1.12 | 0.32 | 4.99 × 102 | 0.98 | 4.49 × 101 | 0.91 | additive | ||
| 100 | 0.39 | 8.47 | 0.15 | 0.54 | antagonism | |||||
| 100:1 | 100 | 0.33 | 3.14 | 0.36 | 3.02 | antagonism | ||||
| 500 | 0.44 | 7.64 × 101 | 0.35 | 8.16 × 102 | 0.99 | 0.01 | 1.46 | antagonism | ||
| 1000 | 0.53 | 5.59 × 102 | 0.00 | 1.44 | antagonism | |||||
| 175:1 | 100 | 0.14 | 1.57 | 1.93 × 101 | 0.66 | antagonism | ||||
| 175 | 0.14 | 5.58 | 0.10 | 4.80 | 0.66 | 5.44 | 0.19 | antagonism | ||
| 500 | 0.17 | 5.52 | 1.69 | 0.20 | antagonism | |||||
| 48 | 1:1 | 2 | 0.59 | 0.39 | 2.96 | 1.79 × 101 | synergism 3 | |||
| 10 | 0.78 | 7.29 × 101 | 0.62 | 1.15 | 1.00 | 0.01 | 4.32 × 101 | antagonism | ||
| 20 | 0.86 | 1.45 × 103 | 0.00 | 9.86 × 101 | antagonism | |||||
| 10:1 | 2 | 0.38 | 0.13 | 5.74 × 101 | 9.28 | synergism 4 | ||||
| 10 | 0.51 | 0.92 | 0.45 | 6.92 | 0.99 | 1.26 | 8.02 | additive | ||
| 100 | 0.78 | 1.33 × 103 | 0.00 | 23.74 | antagonism | |||||
| 100:1 | 100 | 0.48 | 5.44 | 0.19 | 5.29 | antagonism | ||||
| 500 | 0.73 | 2.33 × 103 | 0.62 | 1.11 × 102 | 1.00 | 0.00 | 2.06 × 101 | antagonism | ||
| 1000 | 0.79 | 1.85 × 104 | 0.00 | 2.57 × 101 | antagonism | |||||
| 175:1 | 100 | 0.21 | 3.37 | 3.43 × 101 | 0.30 | antagonism | ||||
| 175 | 0.49 | 2.24 × 101 | 1.08 × 105 | 3.46 × 102 | 1.00 | 0.05 | 2.94 | antagonism | ||
| 500 | 0.61 | 2.38 × 102 | 0.00 | 8.02 | antagonism | |||||
| 72 | 1:1 | 2 | 0.45 | 0.67 | 1.55 × 102 | 1.51 | synergism 5 | |||
| 10 | 0.76 | 2.03 | 0.90 | 2.56 | 1.00 | 0.58 | 3.42 | antagonism | ||
| 20 | 0.87 | 3.16 × 101 | 0.03 | 6.56 | antagonism | |||||
| 10:1 | 2 | 0.2 | 1.02 | 2.79 × 103 | 0.98 | additive | ||||
| 10 | 0.42 | 0.79 | 0.75 | 1.37 × 101 | 1.00 | 2.44 × 101 | 1.33 | synergism 6 | ||
| 100 | 0.82 | 9.23 × 101 | 0.01 | 3.62 | antagonism | |||||
| 100:1 | 100 | 0.45 | 1.30 | 1.56 | 1.52 | antagonism | ||||
| 500 | 0.72 | 9.35 × 101 | 0.63 | 1.31 × 102 | 0.99 | 0.01 | 2.38 | antagonism | ||
| 1000 | 0.77 | 4.05 × 102 | 0.00 | 1.91 | antagonism | |||||
| 175:1 | 100 | 0.2 | 3.15 | 5.10 × 101 | 0.32 | antagonism | ||||
| 175 | 0.46 | 3.80 | 0.98 | 4.12 × 102 | 1.00 | 0.39 | 0.81 | antagonism | ||
| 500 | 0.55 | 1.14 × 101 | 0.10 | 1.11 | antagonism | |||||
| FuLt + PTX (μg/mL) | Parameters | DRI | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hours | Ratio | Total Dose | fa | CI Value | m | Dm | r | FuLt | PTX | |
| 24 | 2:1 | 2 | 0.16 | 0.05 | 8.29 × 102 | 2.19 × 101 | synergism 7 | |||
| 5 | 0.22 | 0.55 | 0.41 | 1.10 × 102 | 1.00 | 8.11 × 101 | 1.84 | synergism 8 | ||
| 10 | 0.27 | 3.25 | 1.53 × 101 | 0.31 | antagonism | |||||
| 1:1 | 1 | 0.19 | 0.08 | 1.05 × 103 | 1.28 × 101 | synergism 9 | ||||
| 2 | 0.2 | 0.20 | 0.21 | 1.34 × 103 | 0.99 | 4.17 × 102 | 4.96 | synergism 10 | ||
| 10 | 0.27 | 4.82 | 2.05 × 101 | 0.21 | antagonism | |||||
| 10:1 | 2 | 0.19 | 0.03 | 2.88 × 102 | 3.51 × 101 | synergism 11 | ||||
| 5 | 0.2 | 0.10 | 0.04 | 3.31 × 1015 | 0.90 | 9.17 × 101 | 1.09 × 101 | synergism 12 | ||
| 10 | 0.2 | 0.21 | 4.58 × 101 | 5.46 | synergism 13 | |||||
| 100:1 | 50 | 0.17 | 0.10 | 1.72 × 101 | 2.21 × 101 | synergism 14 | ||||
| 70 | 0.26 | 1.10 | 0.85 | 2.94 × 102 | 0.90 | 1.77 | 1.85 | additive | ||
| 100 | 0.27 | 1.91 | 1.03 | 1.06 | antagonism | |||||
| 48 | 2:1 | 2 | 0.21 | 0.25 | 2.56 × 103 | 3.94 | synergism 15 | |||
| 5 | 0.23 | 0.89 | 0.17 | 4.99 × 103 | 0.98 | 6.28 × 102 | 1.13 | synergism 16 | ||
| 10 | 0.26 | 2.83 | 1.60 × 102 | 0.35 | antagonism | |||||
| 1:1 | 1 | 0.29 | 0.65 | 1.13 × 103 | 1.53 | synergism 17 | ||||
| 2 | 0.29 | 1.31 | 0.02 | 2.70 × 1017 | 0.96 | 5.67 × 102 | 0.77 | antagonism | ||
| 10 | 0.3 | 7.51 | 9.28 × 101 | 0.13 | antagonism | |||||
| 10:1 | 2 | 0.28 | 0.21 | 3.83 × 102 | 4.84 | synergism 18 | ||||
| 5 | 0.36 | 1.52 | 0.36 | 2.62 × 101 | 1.00 | 3.28 × 101 | 0.67 | antagonism | ||
| 10 | 0.41 | 5.61 | 6.79 | 0.18 | antagonism | |||||
| 100:1 | 50 | 0.26 | 0.47 | 2.15 × 101 | 2.38 | synergism 19 | ||||
| 70 | 0.3 | 1.19 | 0.28 | 1.76 × 103 | 0.86 | 6.70 | 0.96 | antagonism | ||
| 100 | 0.3 | 1.70 | 4.69 | 0.67 | antagonism | |||||
| 72 | 2:1 | 2 | 0.53 | 3.59 | 4.51 × 101 | 0.28 | antagonism | |||
| 5 | 0.55 | 1.44 × 101 | 0.15 | 0.99 | 0.96 | 1.42 × 101 | 0.07 | antagonism | ||
| 10 | 0.59 | 7.53 × 101 | 4.40 | 0.01 | antagonism | |||||
| 1:1 | 1 | 0.55 | 4.31 | 9.49 × 101 | 0.23 | antagonism | ||||
| 2 | 0.56 | 1.09 × 101 | 0.13 | 0.25 | 0.99 | 4.21 × 101 | 0.09 | antagonism | ||
| 10 | 0.62 | 2.36 × 102 | 4.06 | 0.00 | antagonism | |||||
| 10:1 | 2 | 0.50 | 0.40 | 5.30 × 101 | 2.64 | synergism 20 | ||||
| 5 | 0.66 | 6.01 × 101 | 0.65 | 2.02 | 0.99 | 2.68 | 0.02 | antagonism | ||
| 10 | 0.73 | 8.43 × 102 | 0.50 | 0.00 | antagonism | |||||
| 100:1 | 50 | 0.56 | 6.58 | 0.85 | 0.19 | antagonism | ||||
| 70 | 0.57 | 1.15 × 101 | 0.30 | 2.39 × 101 | 0.95 | 0.54 | 0.10 | antagonism | ||
| 100 | 0.61 | 4.07 × 101 | 0.23 | 0.03 | antagonism | |||||
| FuLt + 5-FU (μg/mL) | Parameters | DRI | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hours | Ratio | Total Dose | fa | CI Value | m | Dm | r | FuLt | 5-FU | |
| 24 | 2:1 | 2 | 0.31 | 2.82 × 102 | 3.82 × 101 | 0.00 | antagonism | |||
| 5 | 0.35 | 6.24 × 101 | 0.22 | 8.05 × 101 | 1.00 | 7.98 | 0.02 | antagonism | ||
| 10 | 0.39 | 1.29 × 101 | 2.15 × 105 | 0.08 | antagonism | |||||
| 1:1 | 1 | 0.3 | 3.99 × 102 | 1.21 × 102 | 0.00 | antagonism | ||||
| 2 | 0.41 | 1.32 | 0.19 | 3.88 × 101 | 0.78 | 1.07 × 101 | 0.82 | antagonism | ||
| 10 | 0.42 | 4.06 | 1.84 | 0.28 | antagonism | |||||
| 10:1 | 2 | 0.19 | 4.69 × 105 | 2.88 × 102 | 0.00 | antagonism | ||||
| 5 | 0.35 | 1.72 × 101 | 0.66 | 1.61 × 101 | 0.97 | 5.85 | 0.06 | antagonism | ||
| 10 | 0.4 | 2.67 | 1.36 | 0.52 | antagonism | |||||
| 100:1 | 50 | 0.33 | 6.25 × 101 | 0.74 | 0.02 | antagonism | ||||
| 70 | 0.36 | 1.75 × 101 | 0.62 | 1.64 × 102 | 0.98 | 0.33 | 0.07 | antagonism | ||
| 100 | 0.43 | 1.29 × 101 | 0.08 | 2.48 | antagonism | |||||
| 48 | 2:1 | 2 | 0.33 | 0.82 | 1.95 × 102 | 1.23 | synergism 21 | |||
| 5 | 0.38 | 1.22 | 0.19 | 7.65 × 101 | 0.99 | 3.13 × 101 | 0.84 | antagonism | ||
| 10 | 0.4 | 2.02 | 1.10 × 101 | 0.52 | antagonism | |||||
| 1:1 | 1 | 0.31 | 0.77 | 7.62 × 102 | 1.30 | synergism 22 | ||||
| 2 | 0.4 | 0.59 | 0.26 | 1.61 × 101 | 0.94 | 7.34 × 101 | 1.73 | synergism 23 | ||
| 10 | 0.46 | 1.76 | 5.28 | 0.64 | antagonism | |||||
| 10:1 | 2 | 0.29 | 0.36 | 3.12 × 102 | 2.83 | synergism 24 | ||||
| 5 | 0.34 | 0.52 | 0.18 | 2.87 × 102 | 0.96 | 4.74 × 101 | 2.01 | synergism 25 | ||
| 10 | 0.35 | 0.94 | 1.97 × 101 | 1.12 | additive | |||||
| 100:1 | 50 | 0.25 | 1.63 | 2.68 × 101 | 0.63 | antagonism | ||||
| 70 | 0.3 | 1.35 | 0.50 | 4.34 × 102 | 0.96 | 6.70 | 0.84 | antagonism | ||
| 100 | 0.32 | 1.67 | 3.17 | 0.74 | antagonism | |||||
| 72 | 2:1 | 2 | 0.56 | 1.30 | 3.16 × 101 | 0.79 | antagonism | |||
| 5 | 0.58 | 2.28 | 0.13 | 0.32 | 0.98 | 9.94 | 0.46 | antagonism | ||
| 10 | 0.61 | 2.75 | 3.45 | 0.41 | antagonism | |||||
| 1:1 | 1 | 0.47 | 5.00 | 2.44 × 102 | 0.20 | antagonism | ||||
| 2 | 0.57 | 1.61 | 0.24 | 1.10 | 0.92 | 3.74 × 101 | 0.63 | antagonism | ||
| 10 | 0.62 | 3.29 | 4.06 | 0.33 | antagonism | |||||
| 10:1 | 2 | 0.43 | 3.83 | 1.08 × 102 | 0.26 | antagonism | ||||
| 5 | 0.49 | 3.19 | 0.25 | 6.07 | 1.00 | 2.12 × 101 | 0.32 | antagonism | ||
| 10 | 0.53 | 3.17 | 6.62 | 0.33 | antagonism | |||||
| 100:1 | 50 | 0.75 | 1.47 × 101 | 0.07 | 5.43 × 101 | antagonism | ||||
| 70 | 0.79 | 4.00 × 101 | 0.41 | 3.25 | 0.94 | 0.03 | 1.10 × 102 | antagonism | ||
| 100 | 0.8 | 6.84 × 101 | 0.01 | 1.02 × 102 | antagonism | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condori Macuri, R.M.; Alzamora-Gonzales, L.; Colona-Vallejos, E.H.; Cruz Riquelme, R.T.; Pecho Chávez, L.I.; Cisneros Gutierrez, J.O.; Montejo Anlas, V.A. Synergistic Anticancer Activity of Fucoidan from Lessonia trabeculata Combined with Chemotherapeutic Agents in 4T1 Breast Spheroids. Mar. Drugs 2025, 23, 451. https://doi.org/10.3390/md23120451
Condori Macuri RM, Alzamora-Gonzales L, Colona-Vallejos EH, Cruz Riquelme RT, Pecho Chávez LI, Cisneros Gutierrez JO, Montejo Anlas VA. Synergistic Anticancer Activity of Fucoidan from Lessonia trabeculata Combined with Chemotherapeutic Agents in 4T1 Breast Spheroids. Marine Drugs. 2025; 23(12):451. https://doi.org/10.3390/md23120451
Chicago/Turabian StyleCondori Macuri, Rosa María, Libertad Alzamora-Gonzales, Erasmo Honorio Colona-Vallejos, Raisa Teresa Cruz Riquelme, Laura Inés Pecho Chávez, Jherson Oscar Cisneros Gutierrez, and Victor Alonso Montejo Anlas. 2025. "Synergistic Anticancer Activity of Fucoidan from Lessonia trabeculata Combined with Chemotherapeutic Agents in 4T1 Breast Spheroids" Marine Drugs 23, no. 12: 451. https://doi.org/10.3390/md23120451
APA StyleCondori Macuri, R. M., Alzamora-Gonzales, L., Colona-Vallejos, E. H., Cruz Riquelme, R. T., Pecho Chávez, L. I., Cisneros Gutierrez, J. O., & Montejo Anlas, V. A. (2025). Synergistic Anticancer Activity of Fucoidan from Lessonia trabeculata Combined with Chemotherapeutic Agents in 4T1 Breast Spheroids. Marine Drugs, 23(12), 451. https://doi.org/10.3390/md23120451








