Chitosan-Mediated Expression of Caenorhabditis elegans fat-1 and fat-2 in Sparus aurata: Short-Term Effects on the Hepatic Fatty Acid Profile, Intermediary Metabolism, and Proinflammatory Factors
Abstract
1. Introduction
2. Results
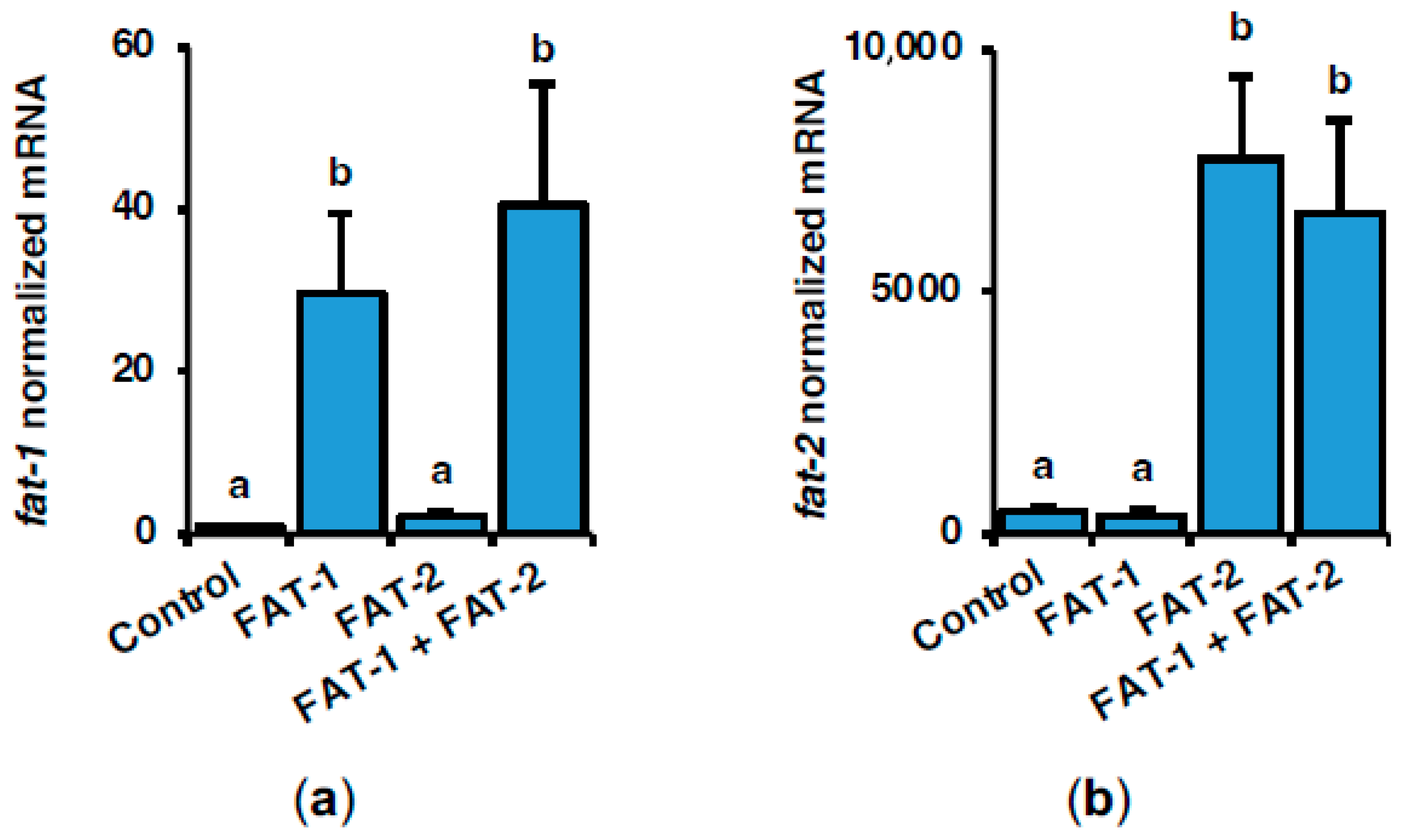
2.1. Effect of Intraperitoneal Administration of Chitosan-TPP Nanoparticles Encapsulating pSG5-FAT-1 and pSG5-FAT-2 on fat-1 and fat-2 mRNA Levels in S. aurata Liver
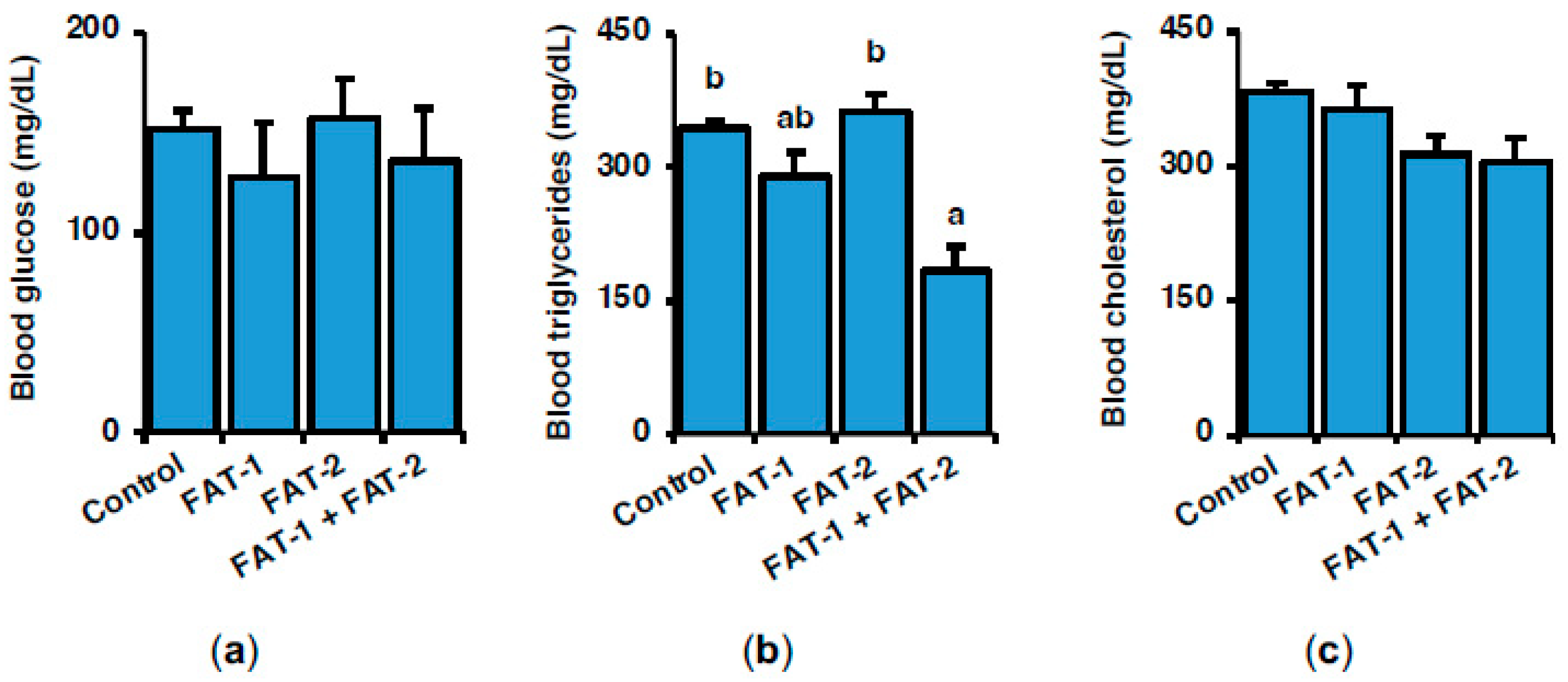
2.2. Effect of Chitosan-Mediated Expression of fat-1 and fat-2 on S. aurata Serum Metabolites and Hepatic Fatty Acid Profile
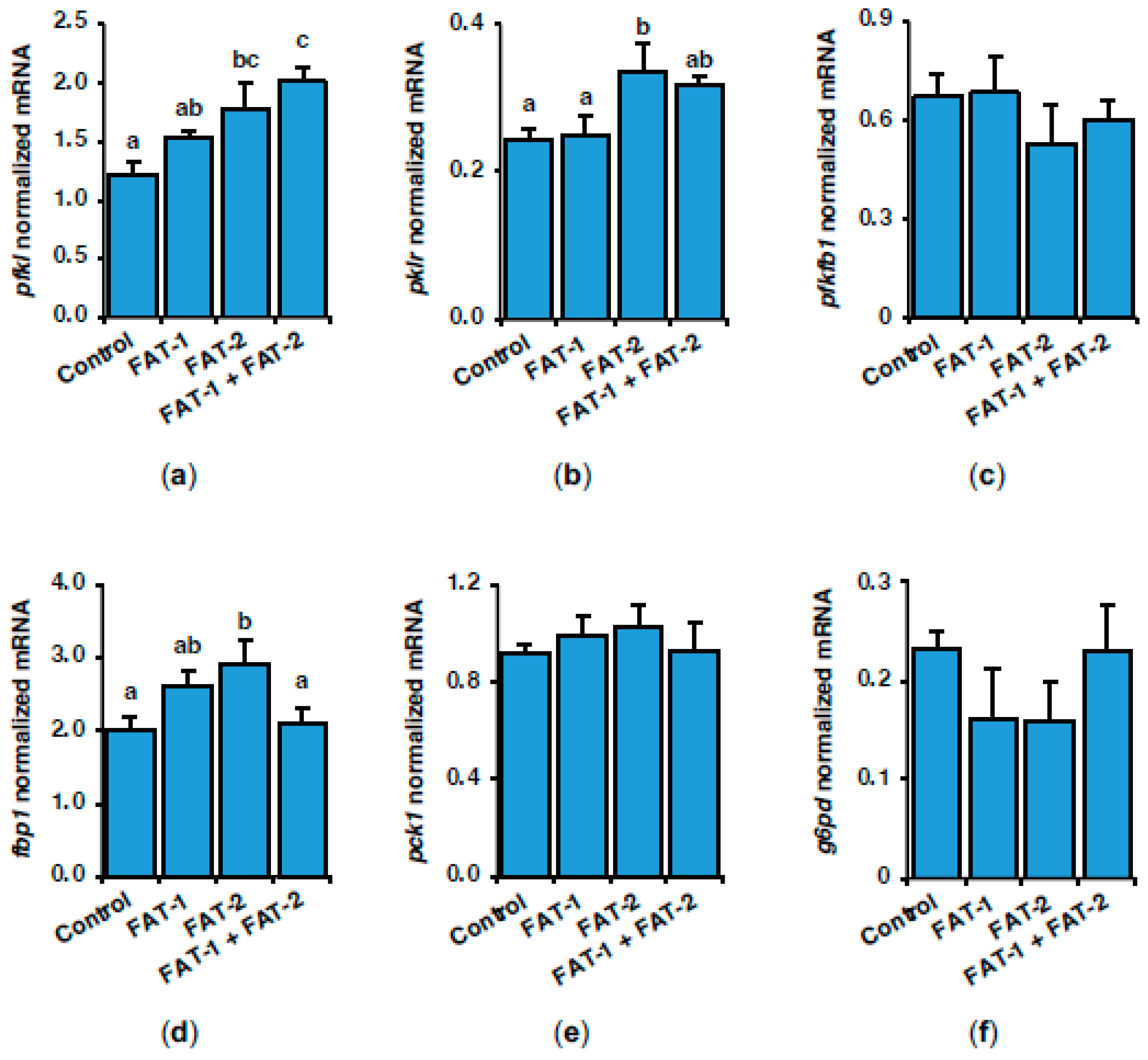
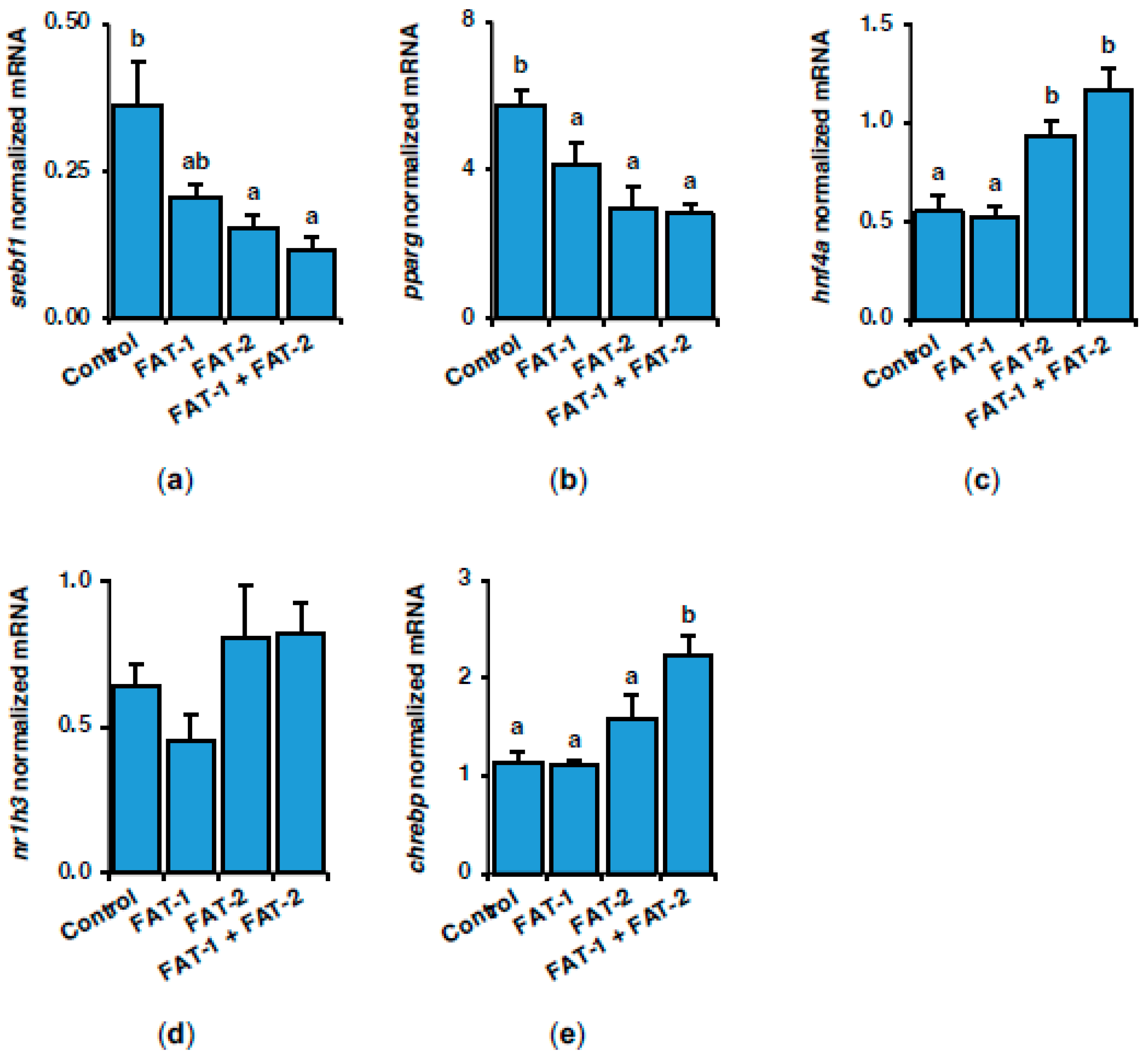
2.3. Effect of Chitosan-Mediated Expression of fat-1 and fat-2 on Key Genes Related to Lipid and Glucose Metabolism
2.4. Effect of Chitosan-Mediated Expression of fat-1 and fat-2 on Genes Related to Inflammation
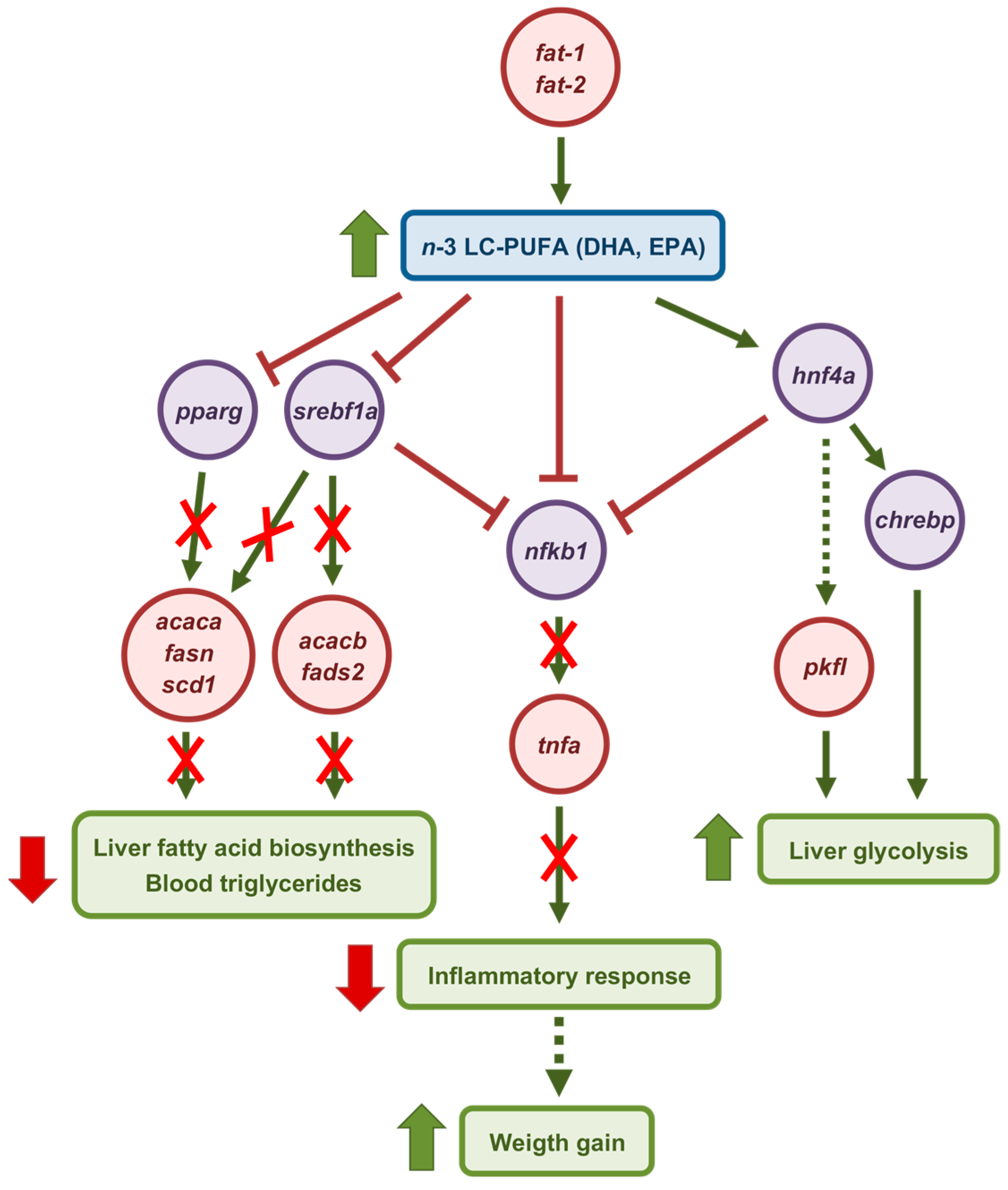
3. Discussion
4. Materials and Methods
4.1. Chitosan-TPP-DNA Nanoparticles
4.2. Animals
4.3. Serum Metabolites
4.4. Reverse Transcriptase-Coupled Quantitative Real-Time PCR (RT-qPCR)
4.5. Fatty Acid Methyl Ester (FAME) Analysis
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALA | α-Linolenic acid |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| LA | Linoleic acid |
| OA | Oleic acid |
| BW | Body weight |
| TPP | Tripolyphosphate |
| n-3 LC-PUFA | omega-3 long-chain polyunsaturated fatty acids |
References
- Pang, S.-C.; Wang, H.-P.; Li, K.-Y.; Zhu, Z.-Y.; Kang, J.X.; Sun, Y.-H. Double Transgenesis of humanized fat1 and fat2 genes promotes omega-3 polyunsaturated fatty acids synthesis in a zebrafish model. Mar. Biotechnol. 2014, 16, 580–593. [Google Scholar] [CrossRef]
- Wu, Y.; Rashidpour, A.; Metón, I. Bayesian meta-analysis: Impacts of eating habits and habitats on omega-3 long-chain polyunsaturated fatty acid composition and growth in cultured fish. Animals 2024, 14, 2118. [Google Scholar] [CrossRef]
- Rivero-Ramírez, F.; Torrecillas, S.; Betancor, M.B.; Izquierdo, M.S.; Caballero, M.J.; Montero, D. Effects of dietary arachidonic acid in European sea bass (Dicentrarchus labrax) distal intestine lipid classes and gut health. Fish Physiol. Biochem. 2020, 46, 681–697. [Google Scholar] [CrossRef]
- Castro, L.F.C.; Tocher, D.R.; Monroig, O. Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of Fads and Elovl gene repertoire. Prog. Lipid Res. 2016, 62, 25–40. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kothapalli, K.S.D.; Brenna, J.T. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 103–110. [Google Scholar] [CrossRef]
- Tu, W.C.; Cook-Johnson, R.J.; James, M.J.; Mühlhäusler, B.S.; Gibson, R.A. Omega-3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 61–68. [Google Scholar] [CrossRef]
- Hattori, T.; Obinata, H.; Ogawa, A.; Kishi, M.; Tatei, K.; Ishikawa, O.; Izumi, T. G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J. Investig. Dermatol. 2008, 128, 1123–1133. [Google Scholar] [CrossRef]
- Tan, P.; Ding, Y.; Li, X.; Dong, X.; Mai, K.; Ai, Q. Nrf2 pathway in vegetable oil-induced inflammation of large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2022, 127, 778–787. [Google Scholar] [CrossRef]
- Yan, X.-B.; Dong, X.-H.; Tan, B.-P.; Zhang, S.; Chi, S.-Y.; Yang, Q.-H.; Liu, H.-Y.; Yang, Y.-Z. Influence of different oil sources on growth, disease resistance, immune response and immune-related gene expression on the hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatu), to Vibrio parahaemolyticus challenge. Fish Shellfish Immunol. 2020, 99, 310–321. [Google Scholar] [CrossRef]
- Sun, S.; Castro, F.; Monroig, Ó.; Cao, X.; Gao, J. Fat-1 transgenic zebrafish are protected from abnormal lipid deposition induced by high-vegetable oil feeding. Appl. Microbiol. Biotechnol. 2020, 104, 7355–7365. [Google Scholar] [CrossRef]
- Zhang, X.; Pang, S.; Liu, C.; Wang, H.; Ye, D.; Zhu, Z.; Sun, Y. A Novel Dietary Source of EPA and DHA: Metabolic engineering of an important freshwater species-common carp by fat1-transgenesis. Mar. Biotechnol. 2018, 21, 171–185. [Google Scholar] [CrossRef]
- Tang, F.; Yang, X.; Liu, D.; Zhang, X.; Huang, X.; He, X.; Shi, J.; Li, Z.; Wu, Z. Co-expression of fat1 and fat2 in transgenic pigs promotes synthesis of polyunsaturated fatty acids. Transgenic Res. 2019, 28, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-based drug delivery system: Applications in fish biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rashidpour, A.; Fàbregas, A.; Almajano, M.P.; Metón, I. Chitosan-based delivery of fish codon-optimised Caenorhabditis elegans FAT-1 and FAT-2 boosts EPA and DHA biosynthesis in Sparus aurata. Rev. Fish Biol. Fish. 2024, 34, 995–1016. [Google Scholar] [CrossRef]
- Liu, D.; Chi, S.; Tan, B.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S.; Han, F.; He, Y. Effects of fish oil with difference oxidation degree on growth performance and expression abundance of antioxidant and fat metabolism genes in orange spotted grouper, Epinephelus coioides. Aquac. Res. 2019, 50, 188–197. [Google Scholar] [CrossRef]
- Song, C.; Liu, B.; Xu, P.; Xie, J.; Ge, X.; Zhou, Q.; Sun, C.; Zhang, H.; Shan, F.; Yang, Z. Oxidized fish oil injury stress in Megalobrama amblycephala: Evaluated by growth, intestinal physiology, and transcriptome-based PI3K-Akt/NF-κB/TCR inflammatory signaling. Fish Shellfish Immunol. 2018, 81, 446–455. [Google Scholar] [CrossRef]
- Shearer, G.C.; Savinova, O.V.; Harris, W.S. Fish oil—How does it reduce plasma triglycerides? Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2012, 1821, 843–851. [Google Scholar] [CrossRef]
- Romanatto, T.; Fiamoncini, J.; Wang, B.; Curi, R.; Kang, J.X. Elevated tissue omega-3 fatty acid status prevents age-related glucose intolerance in fat-1 transgenic mice. Biochim. Biophys. Acta—Mol. Basis Dis. 2014, 1842, 186–191. [Google Scholar] [CrossRef]
- Liu, X.; Pang, D.; Yuan, T.; Li, Z.; Li, Z.; Zhang, M.; Ren, W.; Ouyang, H.; Tang, X. N-3 polyunsaturated fatty acids attenuates triglyceride and inflammatory factors level in hfat-1 transgenic pigs. Lipids Health Dis. 2016, 15, 89. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Z.; Bai, C.; Ding, X.; Li, X.; Su, G.; Cheng, L.; Zhang, L.; Guo, H.; Li, G. Insights into the function of n-3 PUFAs in fat-1 transgenic cattle. J. Lipid Res. 2017, 58, 1524–1535. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef]
- He, L.; Qin, Y.; Wang, Y.; Li, D.; Chen, W.; Ye, J. Effects of dietary replacement of fish oil with soybean oil on the growth performance, plasma components, fatty acid composition and lipid metabolism of groupers Epinephelus coioides. Aquac. Nutr. 2021, 27, 1494–1511. [Google Scholar] [CrossRef]
- Gu, Z.; Mu, H.; Shen, H.; Deng, K.; Liu, D.; Yang, M.; Zhang, Y.; Zhang, W.; Mai, K. High level of dietary soybean oil affects the glucose and lipid metabolism in large yellow croaker Larimichthys crocea through the insulin-mediated PI3K/AKT signaling pathway. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 231, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.L.; Browse, J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 5854–5859. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.R.; Green, A.G.; Singh, S.P. Caenorhabditis elegans Δ12-desaturase FAT-2 is a bifunctional desaturase able to desaturate a diverse range of fatty acid substrates at the Δ12 and Δ15 positions. J. Biol. Chem. 2011, 286, 43644–43650. [Google Scholar] [CrossRef]
- Lai, L.; Kang, J.X.; Li, R.; Wang, J.; Witt, W.T.; Yong, H.Y.; Hao, Y.; Wax, D.M.; Murphy, C.N.; Rieke, A.; et al. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat. Biotechnol. 2006, 24, 435–436. [Google Scholar] [CrossRef]
- Carmona-Antoñanzas, G.; Tocher, D.R.; Martinez-Rubio, L.; Leaver, M.J. Conservation of lipid metabolic gene transcriptional regulatory networks in fish and mammals. Gene 2014, 534, 1–9. [Google Scholar] [CrossRef]
- Rashidpour, A.; Wu, Y.; Almajano, M.P.; Fàbregas, A.; Metón, I. Chitosan-based sustained expression of sterol regulatory element-binding protein 1a stimulates hepatic glucose oxidation and growth in Sparus aurata. Mar. Drugs 2023, 21, 562. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr. Opin. Lipidol. 2008, 19, 242–247. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as metabolic regulators in the liver: Lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef]
- Tian, J.; Ji, H.; Oku, H.; Zhou, J. Effects of dietary arachidonic acid (ARA) on lipid metabolism and health status of juvenile grass carp, Ctenopharyngodon idellus. Aquaculture 2014, 430, 57–65. [Google Scholar] [CrossRef]
- Wang, X.-X.; Li, Y.-J.; Hou, C.-L.; Gao, Y.; Wang, Y.-Z. Influence of different dietary lipid sources on the growth, tissue fatty acid composition, histological changes and peroxisome proliferator-activated receptor γ gene expression in large yellow croaker (Pseudosciaena crocea R.). Aquac. Res. 2012, 43, 281–291. [Google Scholar] [CrossRef]
- You, C.; Jiang, D.; Zhang, Q.; Xie, D.; Wang, S.; Dong, Y.; Li, Y. Cloning and expression characterization of peroxisome proliferator-activated receptors (PPARs) with their agonists, dietary lipids, and ambient salinity in rabbitfish Siganus canaliculatus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2017, 206, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Valenzuela, R.; Bustamante, A.; Álvarez, D.; Ortiz, M.; Espinosa, A.; Illesca, P.; Gonzalez-Mañan, D.; Videla, L.A. High-fat diet induces mouse liver steatosis with a concomitant decline in energy metabolism: Attenuation by eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation and the additive effects upon EPA and HT co-administration. Food Funct. 2019, 10, 6170–6183. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Yao, L.; Zhang, X.; Zhang, X.; Ye, C.; Jiang, H.; He, J.; Zhu, Y.; Ai, D. Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. Br. J. Pharmacol. 2017, 174, 2358–2372. [Google Scholar] [CrossRef]
- Chang, M.; Zhang, T.; Han, X.; Tang, Q.; Yanagita, T.; Xu, J.; Xue, C.; Wang, Y. Comparative analysis of EPA/DHA-PL forage and liposomes in orotic acid-induced nonalcoholic fatty liver rats and their related mechanisms. J. Agric. Food Chem. 2018, 66, 1408–1418. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Okar, D.A.; Wu, C.; Lange, A.J. Regulation of the regulatory enzyme, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Adv. Enzyme Regul. 2004, 44, 123–154. [Google Scholar] [CrossRef]
- Meng, J.; Feng, M.; Dong, W.; Zhu, Y.; Li, Y.; Zhang, P.; Wu, L.; Li, M.; Lu, Y.; Chen, H.; et al. Identification of HNF-4α as a key transcription factor to promote ChREBP expression in response to glucose. Sci. Rep. 2016, 6, 23944. [Google Scholar] [CrossRef]
- Zhuang, P.; Li, H.; Jia, W.; Shou, Q.; Zhu, Y.; Mao, L.; Wang, W.; Wu, F.; Chen, X.; Wan, X.; et al. Eicosapentaenoic and docosahexaenoic acids attenuate hyperglycemia through the microbiome-gut-organs axis in db/db mice. Microbiome 2021, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Salgado, M.C.; Metón, I.; Anemaet, I.G.; González, J.D.; Fernández, F.; Baanante, I.V. Hepatocyte nuclear factor 4alpha transactivates the mitochondrial alanine aminotransferase gene in the kidney of Sparus aurata. Mar. Biotechnol. 2012, 14, 46–62. [Google Scholar] [CrossRef]
- Lu, H.; Lei, X.; Winkler, R.; John, S.; Kumar, D.; Li, W.; Alnouti, Y. Crosstalk of hepatocyte nuclear factor 4a and glucocorticoid receptor in the regulation of lipid metabolism in mice fed a high-fat-high-sugar diet. Lipids Health Dis. 2022, 21, 46. [Google Scholar] [CrossRef]
- Boyle, K.E.; Magill-Collins, M.J.; Newsom, S.A.; Janssen, R.C.; Friedman, J.E. Maternal fat-1 transgene protects offspring from excess weight gain, oxidative stress, and reduced fatty acid oxidation in response to high-fat diet. Nutrients 2020, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- López-Vicario, C.; Rius, B.; Alcaraz-Quiles, J.; García-Alonso, V.; Lopategi, A.; Titos, E.; Clària, J. Pro-resolving mediators produced from EPA and DHA: Overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur. J. Pharmacol. 2016, 785, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Guo, X.F.; Gao, J.L.; Li, J.M.; Li, D. Fat-1 mice prevent high-fat plus high-sugar diet-induced non-alcoholic fatty liver disease. Food Funct. 2017, 8, 4053–4061. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Y.; Wang, S.; Zhou, J.; Zhou, J.; Lu, X.; Bai, X.; Wang, X.-Y.; Chen, Z.; Zuo, D. Endogenous n-3 polyunsaturated fatty acids attenuate T cell-mediated hepatitis via autophagy activation. Front. Immunol. 2016, 7, 350. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, F.; Kovanen, P.T.; Mardani, R.; Gheibi-hayat, S.M.; Bo, S.; Sahebkar, A. Resolvins: Emerging players in autoimmune and inflammatory diseases. Clin. Rev. Allergy Immunol. 2020, 58, 82–91. [Google Scholar] [CrossRef]
- Anderson, E.K.; Hill, A.A.; Hasty, A.H. Stearic acid accumulation in macrophages induces toll-like receptor 4/2-independent inflammation leading to endoplasmic reticulum stress-mediated apoptosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1687–1695. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Hernández-Rodas, M.C.; Valenzuela, R.; Echeverría, F.; Rincón-Cervera, M.Á.; Espinosa, A.; Illesca, P.; Muñoz, P.; Corbari, A.; Romero, N.; Gonzalez-Mañan, D.; et al. Supplementation with docosahexaenoic acid and extra virgin olive oil prevents liver steatosis induced by a high-fat diet in mice through PPAR-α and Nrf2 upregulation with concomitant SREBP-1c and NF-kB downregulation. Mol. Nutr. Food Res. 2017, 61, 1700479. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Lii, C.K.; Wei, Y.L.; Li, C.C.; Lu, C.Y.; Liu, K.L.; Chen, H.W. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-κB pathways. J. Nutr. Biochem. 2013, 24, 204–212. [Google Scholar] [CrossRef]
- Echeverría, F.; Valenzuela, R.; Espinosa, A.; Bustamante, A.; Álvarez, D.; Gonzalez-Mañan, D.; Ortiz, M.; Soto-Alarcon, S.A.; Videla, L.A. Reduction of high-fat diet-induced liver proinflammatory state by eicosapentaenoic acid plus hydroxytyrosol supplementation: Involvement of resolvins RvE1/2 and RvD1/2. J. Nutr. Biochem. 2019, 63, 35–43. [Google Scholar] [CrossRef]
- Yang, W.; Liu, R.; Xia, C.; Chen, Y.; Dong, Z.; Huang, B.; Li, R.; Li, M.; Xu, C. Effects of different fatty acids on BRL3A rat liver cell damage. J. Cell. Physiol. 2020, 235, 6246–6256. [Google Scholar] [CrossRef]
- Jiang, X.; He, M.; Bai, J.; Chan, C.B.; Wong, A.O.L. Signal Transduction for TNFα-induced type II SOCS expression and its functional implication in growth hormone resistance in carp hepatocytes. Front. Endocrinol. 2020, 11, 20. [Google Scholar] [CrossRef]
- Ren, M.; Habte-Tsion, H.M.; Liu, B.; Miao, L.; Ge, X.; Xie, J.; Liang, H.; Zhou, Q.; Pan, L. Dietary leucine level affects growth performance, whole body composition, plasma parameters and relative expression of TOR and TNF-α in juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 2015, 448, 162–168. [Google Scholar] [CrossRef]
- Mu, H.; Wei, C.; Xu, W.; Gao, W.; Zhang, W.; Mai, K. Effects of replacement of dietary fish oil by rapeseed oil on growth performance, anti-oxidative capacity and inflammatory response in large yellow croaker Larimichthys crocea. Aquac. Rep. 2020, 16, 100251. [Google Scholar] [CrossRef]
- Li, X.; Huang, W.; Gu, J.; Du, X.; Lei, L.; Yuan, X.; Sun, G.; Wang, Z.; Li, X.; Liu, G. SREBP-1c overactivates ROS-mediated hepatic NF-κB inflammatory pathway in dairy cows with fatty liver. Cell. Signal. 2015, 27, 2099–2109. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Zhao, Z.; Lu, H.; Ke, B.; Ye, X.; Wu, B.; Ye, J. NF- B/HDAC1/SREBP1c pathway mediates the inflammation signal in progression of hepatic steatosis. Acta Pharm. Sin. B 2020, 10, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.-F.; Ding, J.; Liu, J.; Yin, C.; Xu, W.-P.; Cong, W.-M.; Zhang, Q.; Chen, F.; Han, T.; Deng, X.; et al. Hepatocyte nuclear factor 4α-nuclear factor-κB feedback circuit modulates liver cancer progression. Hepatology 2014, 60, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidou-Neokosmidou, V.; Zannis, V.I.; Kardassis, D. Inhibition of hepatocyte nuclear factor 4 transcriptional activity by the nuclear factor κB pathway. Biochem. J. 2006, 398, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Administration of Chitosan-Tripolyphosphate-DNA Nanoparticles Overexpressing Key Enzymes to Improve Omega-3 Long-Chain Polyunsaturated Fatty Acid Synthesis in Gilthead Sea Bream (Sparus aurata). Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2023. [Google Scholar]
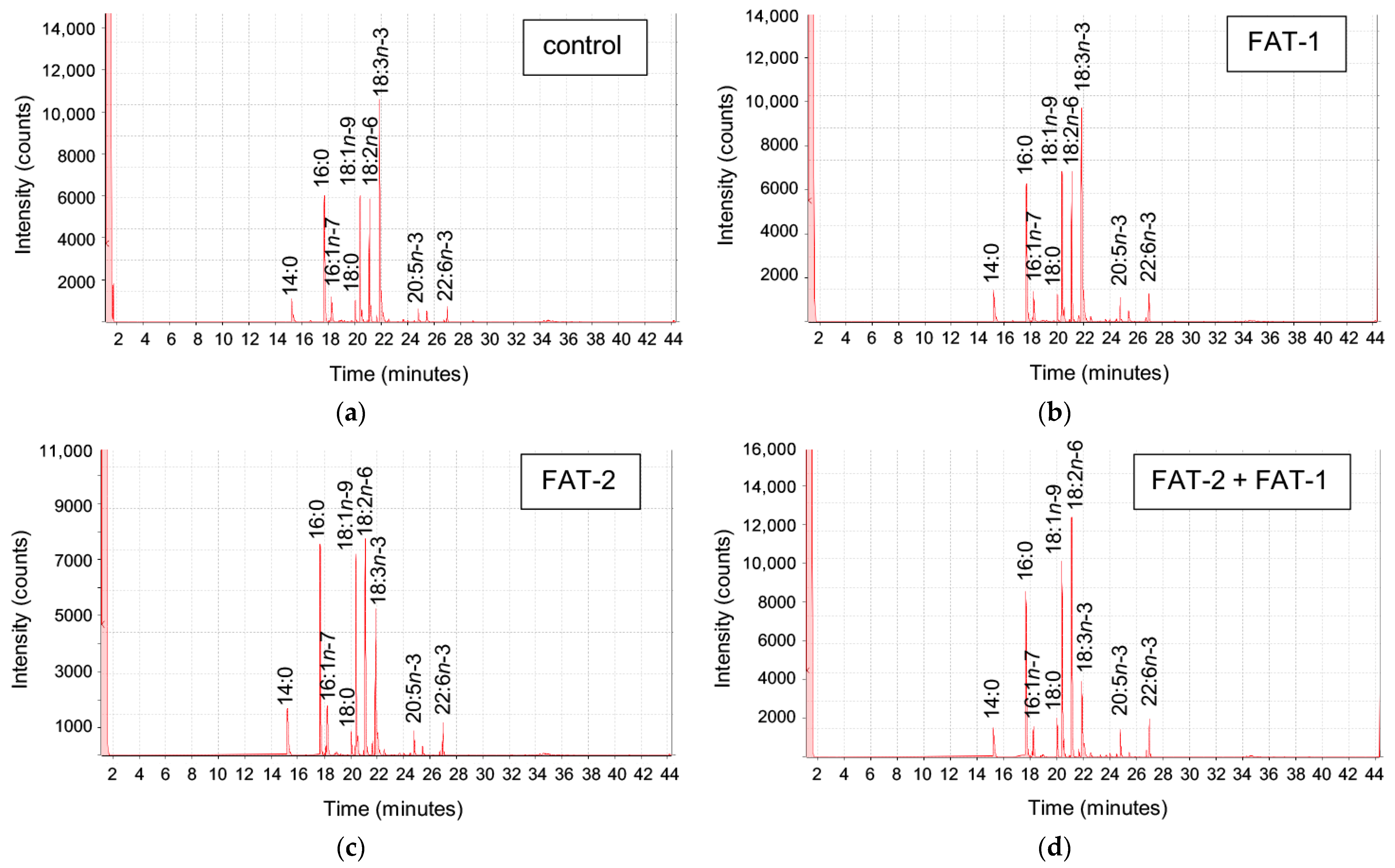


| Fatty Acid | pSG5 | FAT-1 | FAT-2 | FAT-1 + FAT-2 |
|---|---|---|---|---|
| 14:0 | 5.7 a ± 0.9 | 7.2 ab ± 0.1 | 8.7 b ± 0.7 | 7.4 ab ± 0.7 |
| 16:0 | 19.0 ± 2.9 | 20.3 ± 0.9 | 22.3 ± 0.5 | 24.4 ± 1.4 |
| 18:0 | 3.0 ± 0.7 | 3.2 ± 0.6 | 2.6 ± 0.3 | 4.0 ± 0.4 |
| 16:1n-7 | 2.6 a ± 0.7 | 4.2 b ± 0.3 | 5.4 b ± 0.2 | 4.9 b ± 0.3 |
| 18:1n-9 | 14.4 ± 2.7 | 16.1 ± 1.8 | 17.6 ± 1.4 | 18.8 ± 0.7 |
| 18:2n-6 | 16.0 a ± 3.3 | 18.2 ab ± 1.2 | 20.2 ab ± 1.4 | 24.0 b ± 1.1 |
| 18:3n-3 | 0.8 ± 0.3 | 1.3 ± 0.4 | 1.1 ± 0.2 | 1.0 ± 0.2 |
| 20:5n-3 | 1.6 a ± 0.4 | 2.4 b ± 0.1 | 2.3 ab ± 0.1 | 2.5 b ± 0.2 |
| 22:6n-3 | 1.7 a ± 0.2 | 2.6 b ± 0.2 | 2.6 bc ± 0.2 | 3.2 c ± 0.2 |
| SFA | 28.0 ± 4.6 | 30.9 ± 1.4 | 33.7 ± 0.7 | 36.1 ± 1.8 |
| MUFA | 17.7 ± 2.7 | 21.1 ± 2.2 | 23.8 ± 1.3 | 23.8 ± 0.7 |
| PUFA | 20.6 a ± 3.7 | 25.2 ab ± 1.5 | 27.0 ab ± 1.9 | 31.4 b ± 1.3 |
| n-3 | 4.1 a ± 0.6 | 6.2 b ± 0.4 | 6.0 b ± 0.5 | 6.9 b ± 0.3 |
| n-6 | 16.5 a ± 3.2 | 18.8 ab ± 1.2 | 20.7 ab ± 1.4 | 24.3 b ± 1.1 |
| n-9 | 14.8 ± 2.7 | 16.7 ± 1.9 | 18.3 ± 1.3 | 19.0 ± 0.6 |
| n-6/n-3 | 4.0 ± 0.5 | 3.0 ± 0.2 | 3.5 ± 0.2 | 3.5 ± 0.1 |
| Gene | Forward Sequences (5′ to 3′) | Reverse Sequences (5′ to 3′) | GenBank Accession |
|---|---|---|---|
| acaca | CCCAACTTCTTCTACCACAG | GAACTGGAACTCTACTACAC | JX073712 |
| acacb | TGACATGAGTCCTGTGCTGG | GCCTCAGTTCGTATGATGGT | JX073714 |
| actb | CTGGCATCACACCTTCTACAACGAG | GCGGGGGTGTTGAAGGTCTC | X89920 |
| chrebp | CTTCGACACGGTGAACAGGA | AGGGACATGCAGTCGAACAG | NC044199 |
| eef1a | CCCGCCTCTGTTGCCTTCG | CAGCAGTGTGGTTCCGTTAGC | AF184170 |
| elovl4a | AAGAACAGAGAGCCCTTCCAG | TGCCACCCTGACTTCATTG | MK610320 |
| elovl4b | TCTACACAGGCTGCCCATTC | CGAAGAGGATGATGAAGGTGAC | MK610321 |
| elovl5 | GGGATGGCTACTGCTCGACA | CAGGAGAGTGAGGCCCAGAT | AY660879 |
| fads2 | CACTATGCTGGAGAGGATGCC | TATTTCGGTCCTGGCTGGGC | AY055749 |
| fasn | GTAGAGGACACGCCCATCGAT | TGCGTATGACCTCTTGGTGTGCT | JQ277708 |
| fat-1 | TTCAACCCCATTCCTTTCAGCG | TAGGCGCACACGCAGCAGCA | ON374024 |
| fat-2 | AAGAGGACTACAACAACAGAACCGCCA | CGAACAGTCTGCTCCAAGGCCAA | ON374025 |
| fbp1 | CAGATGGTGAGCCGTGTGAGAAGGATG | GCCGTACAGAGCGTAACCAGCTGCC | AF427867 |
| g6pd | TGATGATCCAACAGTTCCTA | GCTCGTTCCTGACACACTGA | JX073711 |
| hmgcr | ACTGATGGCTGCTCTGGCTG | GGGACTGAGGGATGACGCAC | MN047456 |
| hnf4a | GTGGACAAAGACAAGCGAAATC | GCATTGATGGATGGTAAACTGC | FJ360721 |
| il1b | CGTCATCGCCATGGAGAGGT | TGAGCTGGTTTTGCAGTGCG | AJ277166 |
| il6 | CGGAGCAGCATCGTCACTTT | TTCTGCATGGCACACACAAT | EU244588 |
| myd88 | GCGACGCCTGTGACTTTCAG | GGGTGTAGTCGCAGAGGGTG | XM_030399037 |
| nfkb1 | GCTGCTGCTCGGGATCAAAC | GTCCACACTGAGCCACTGGA | XM_030396749 |
| nfkb2 | GTGTGTTGCTGCCGTGTGAC | GTCTGTCCGTCTGCTCCCTC | XM_030441891 |
| nr1h3 | GCATCTGGACGAGGCTGAATAC | ACTTAGTGTGCGAAGGCTCACC | FJ502320 |
| pck1 | CAGCGATGGAGGAGTGTGGTGGGA | GCCCATCCCAATTCCCGCTTCTGTGCTCCGGCTGGTCAGTGT | AF427868 |
| pfkfb1 | TGCTGATGGTGGGACTGCCG | CTCGGCGTTGTCGGCTCTGAAG | U84724 |
| pfk1 | TGCTGGGGACAAAACGAACTCTTCC | AAACCCTCCGACTACAAGCAGAGCT | KF857580 |
| pklr | CAAAGTGGAAAGCCGGCAAGGG | GTCGCCCCTGGCAACCATAAC | KF857579 |
| pparg | TGCGAGGGCTGTAAGGGTTTC | GTTTCTCCTTCTCCGCCTGGG | AY590304 |
| srebf1 | CAGCAGCCCGAACACCTACA | TTGTGGTCAGCCCTTGGAGTTG | JQ277709 |
| scd1a | TCCCTTCCGCATCTCCTTTG | TTGTGGTGAACCCTGTGGTCTC | JQ277703 |
| tnfa | GTCTTCCGCCCCTCAGATCC | GAAAGCCGAAGCATGAGCCC | AJ413189 |
| 18s | TTACGCCCATGTTGTCCTGAG | AGGATTCTGCATGATGGTCACC | AM490061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Rashidpour, A.; Duan, W.; Fàbregas, A.; Almajano, M.P.; Metón, I. Chitosan-Mediated Expression of Caenorhabditis elegans fat-1 and fat-2 in Sparus aurata: Short-Term Effects on the Hepatic Fatty Acid Profile, Intermediary Metabolism, and Proinflammatory Factors. Mar. Drugs 2025, 23, 434. https://doi.org/10.3390/md23110434
Wu Y, Rashidpour A, Duan W, Fàbregas A, Almajano MP, Metón I. Chitosan-Mediated Expression of Caenorhabditis elegans fat-1 and fat-2 in Sparus aurata: Short-Term Effects on the Hepatic Fatty Acid Profile, Intermediary Metabolism, and Proinflammatory Factors. Marine Drugs. 2025; 23(11):434. https://doi.org/10.3390/md23110434
Chicago/Turabian StyleWu, Yuanbing, Ania Rashidpour, Wenwen Duan, Anna Fàbregas, María Pilar Almajano, and Isidoro Metón. 2025. "Chitosan-Mediated Expression of Caenorhabditis elegans fat-1 and fat-2 in Sparus aurata: Short-Term Effects on the Hepatic Fatty Acid Profile, Intermediary Metabolism, and Proinflammatory Factors" Marine Drugs 23, no. 11: 434. https://doi.org/10.3390/md23110434
APA StyleWu, Y., Rashidpour, A., Duan, W., Fàbregas, A., Almajano, M. P., & Metón, I. (2025). Chitosan-Mediated Expression of Caenorhabditis elegans fat-1 and fat-2 in Sparus aurata: Short-Term Effects on the Hepatic Fatty Acid Profile, Intermediary Metabolism, and Proinflammatory Factors. Marine Drugs, 23(11), 434. https://doi.org/10.3390/md23110434







