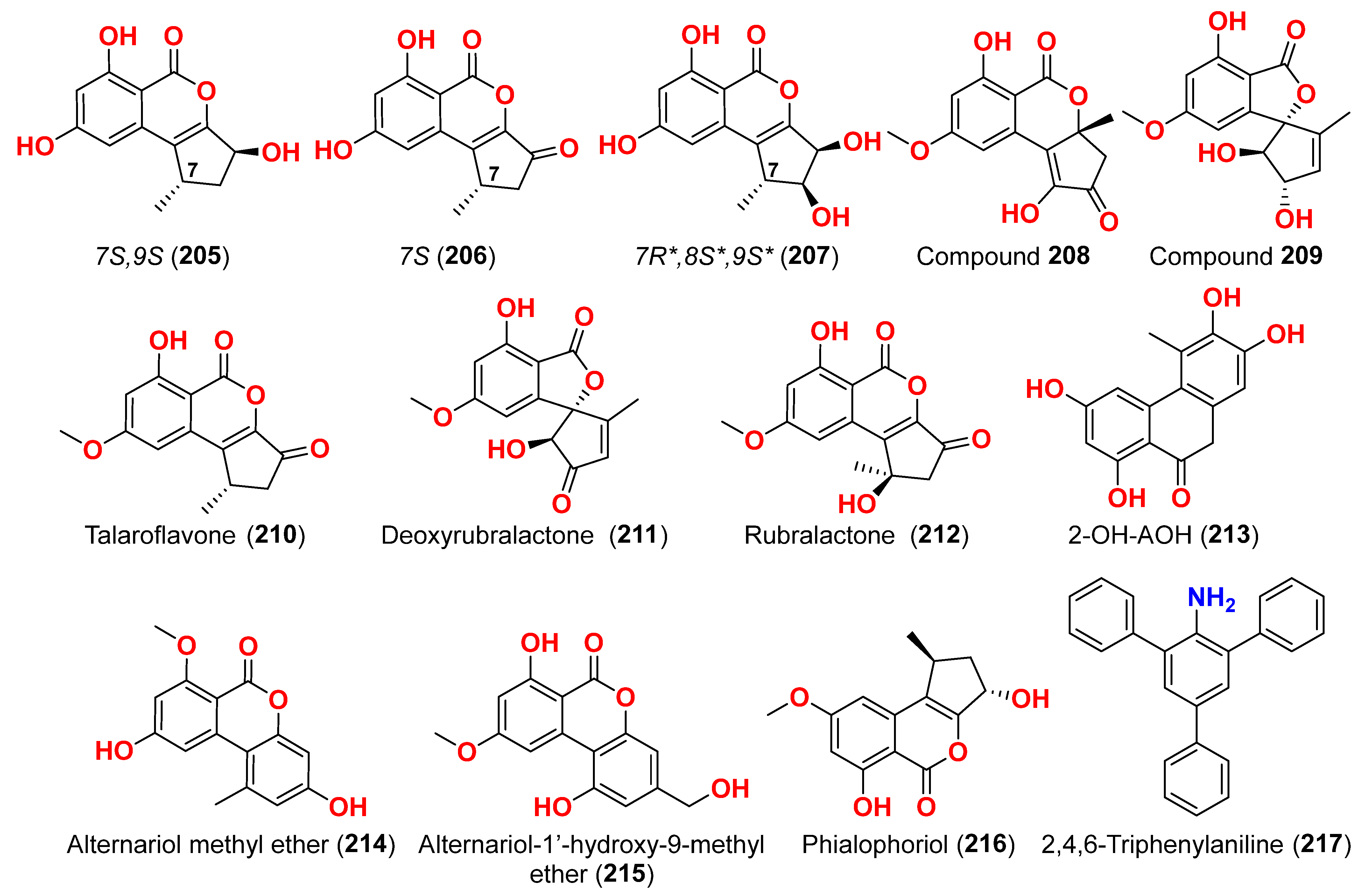
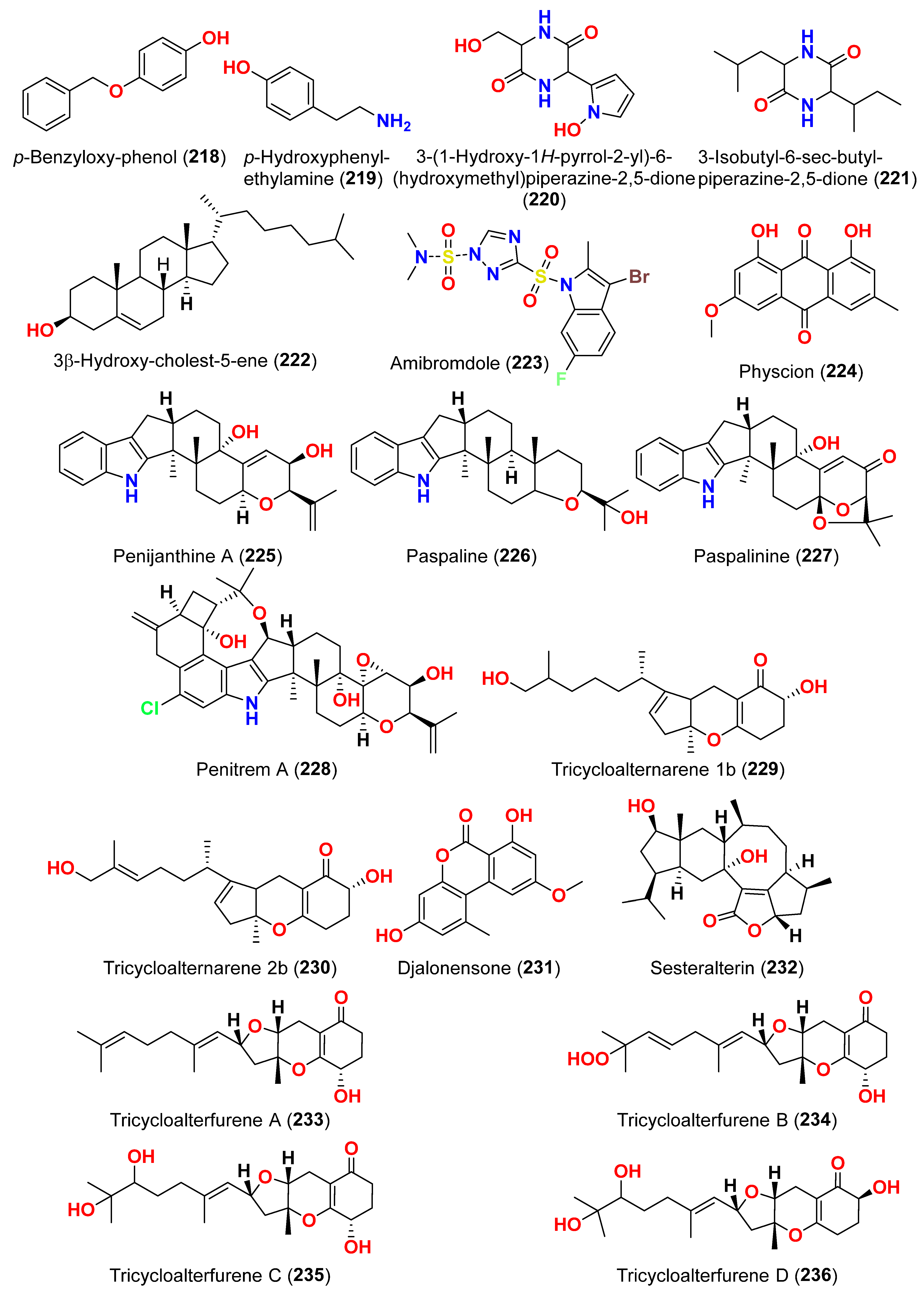
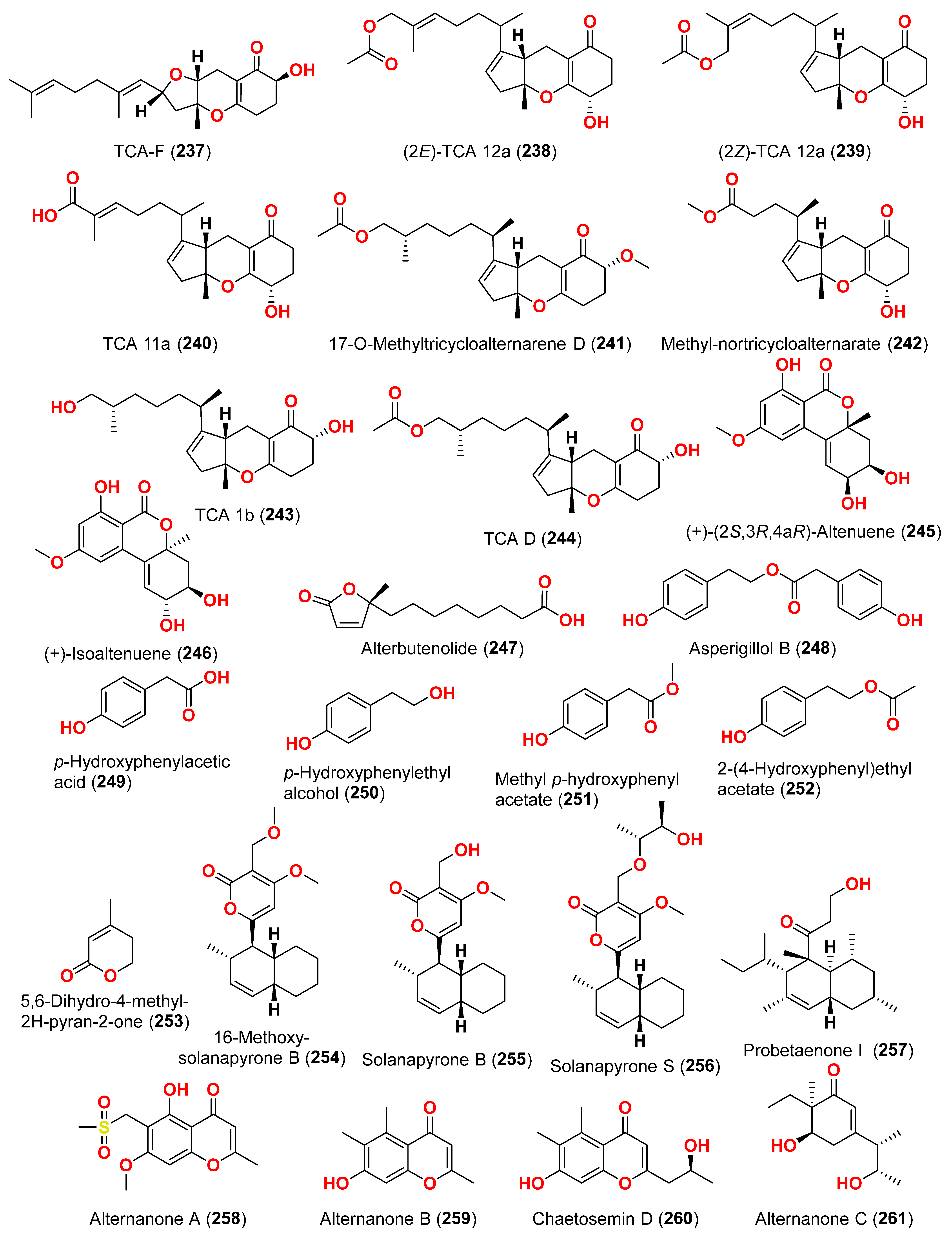
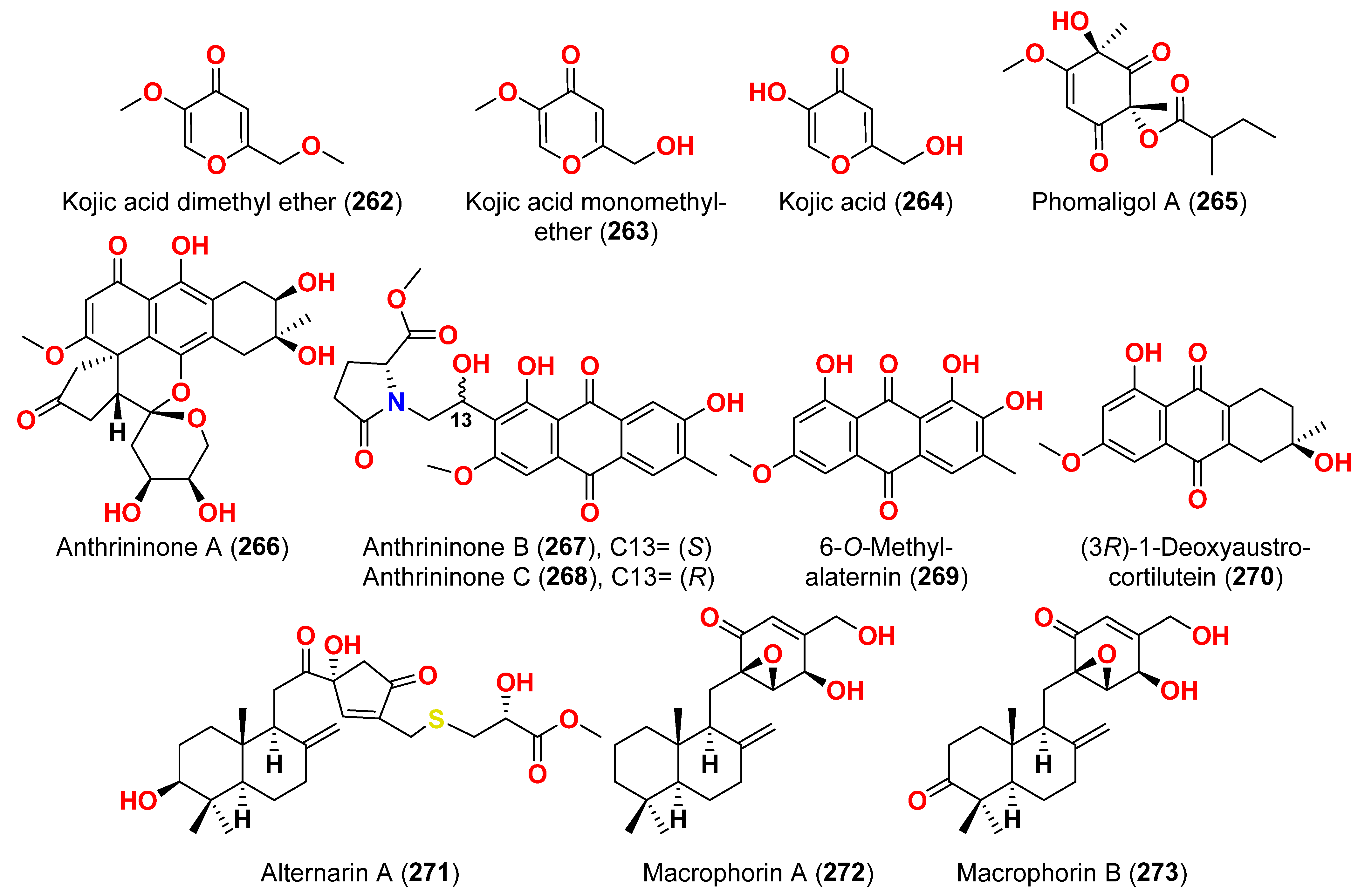
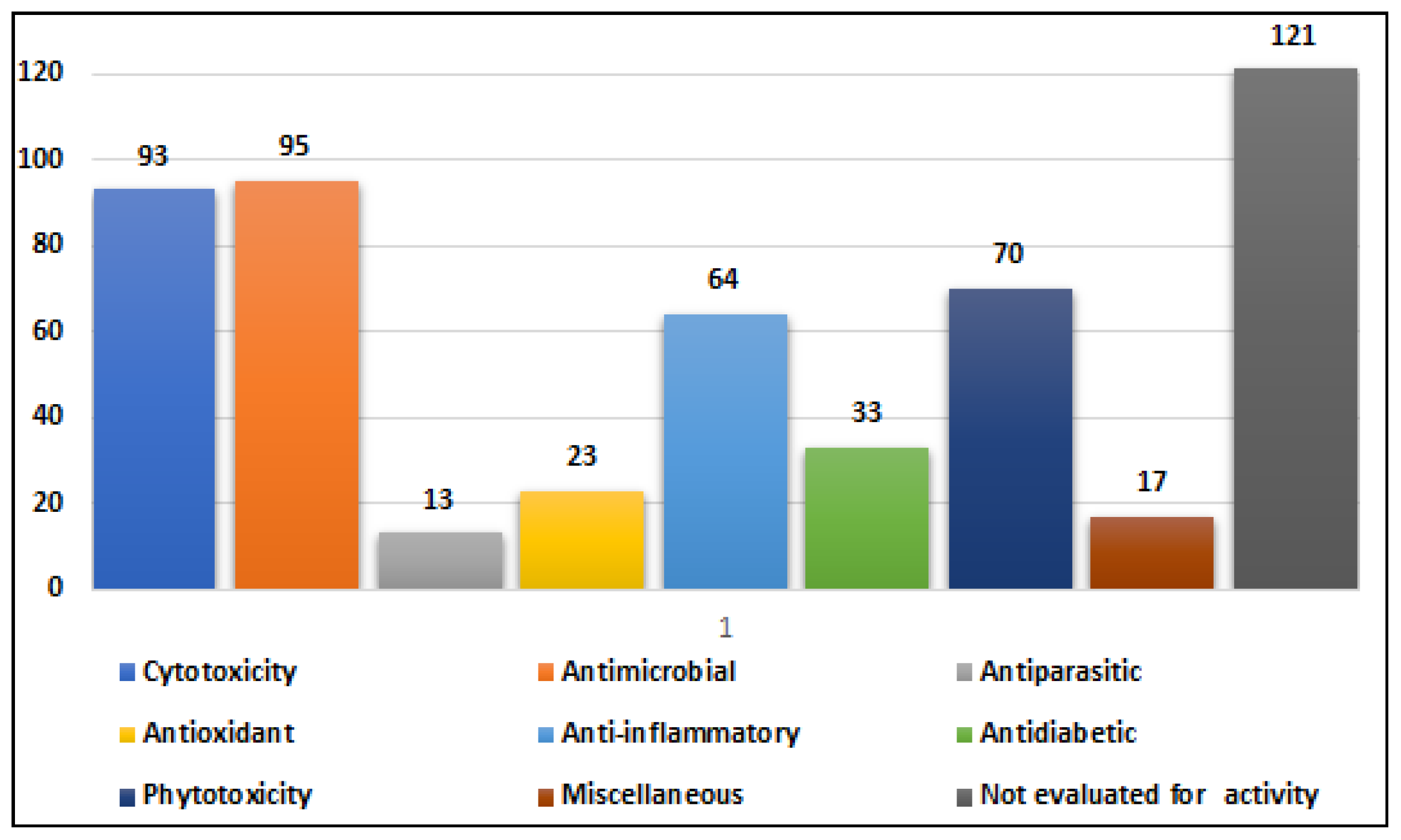
2.1. Compounds with Cytotoxic Activity (Table 1)
Different natural compounds reported from marine-derived
Alternaria species have exhibited varying degrees of cytotoxic activity. In total, 93 compounds (
1–
93) (
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6) have been identified and evaluated for their cytotoxic or growth-inhibitory effects against various cancer cell lines using diverse screening platforms, with or without reference control drugs [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
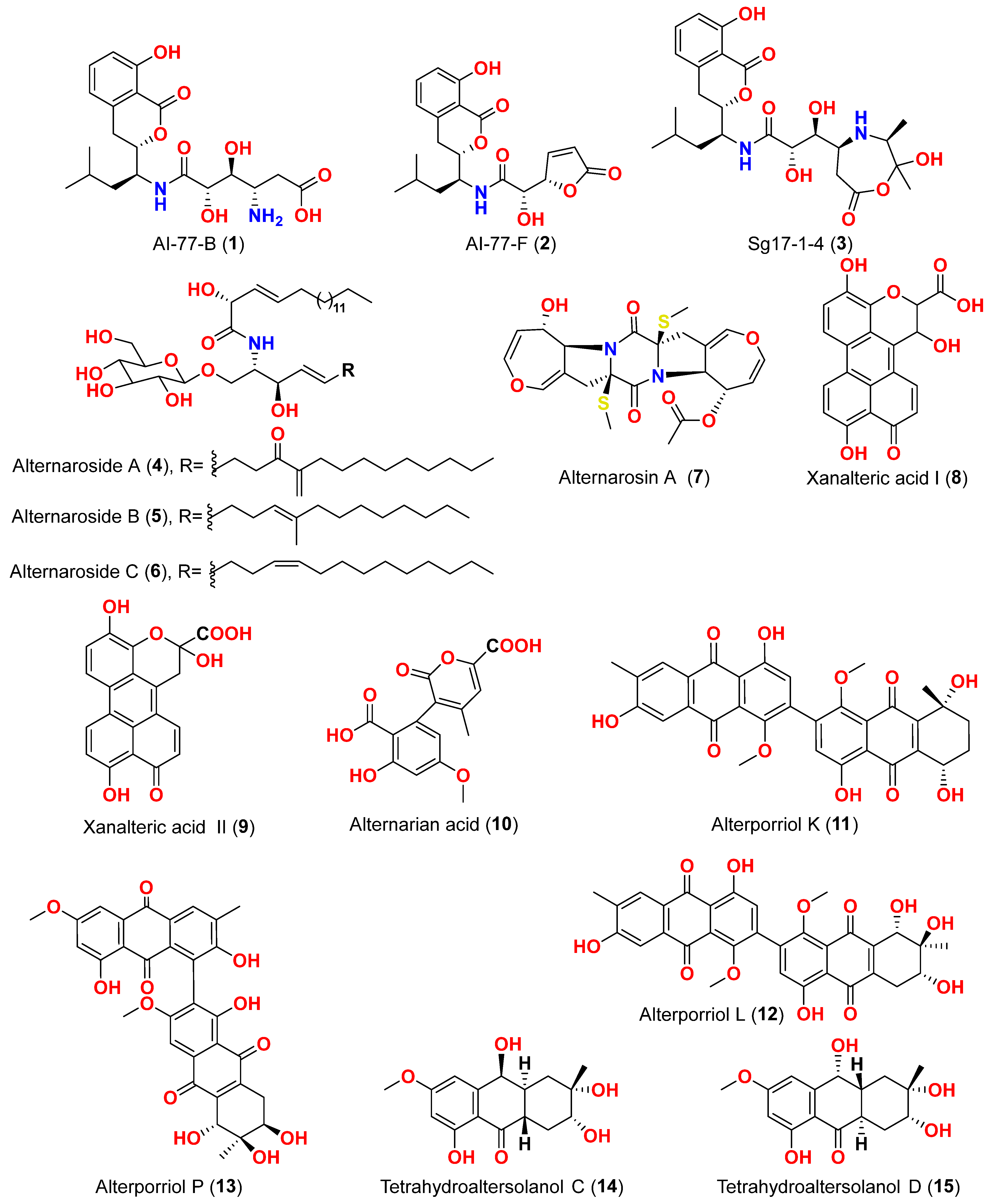
31]. Two isocoumarins, AI-77-B (
1) and AI-77-F (
2), with isocoumarin Sg17-1-4 (
3), were yielded from the fungus
Alternaria tenuis Sg17-1-4 obtained from a marine alga collected on Zhoushan Island, China [
7,
8]. In the MTT assay, cytotoxicity against human malignant A375-S2 and human cervical cancer Hela cells was assessed. AI-77-B (
1) showed IC
50 values of 100 and 20 µM against these cells, demonstrating potent activity. The IC
50 values for Sg17-1-4 (
3) were 300 and 50 µM, while AI-77-F (
2) showed weak activity on Hela cells, with an IC
50 value of 400 µM [
7,
8]. The marine-derived fungus
Alternaria raphani (THW-18) was isolated from sediment collected in the Qingdao Sea salt field, Qingdao, China [
9]. The cerebrosides alternarosides A–C (
4–
6) and the diketopiperazine alkaloid alternarosin A (
7) were isolated from this fungus. The SRB method was used to measure cytotoxicity against cancer cells P388 and HL-60, while the MTT method was used to measure cytotoxicity against a cancer cell A549 and a normal cell BEL-7402. No cytotoxic effect was observed in the four cancer cell lines (IC
50 > 100 μM) [
9]. Extracts of the fungus
Alternaria sp. JCM9.2, isolated from the mangrove
Sonneratia alba collected in the Dong Zhai Gang Mangrove Garden on the Chinese island of Hainan, generated three carboxylic acids: xanalteric acids I (
8) and II (
9) and alternarian acid (
10). Using the MTT test, the cytotoxicity of these compounds against L5178Y cells was determined. However, none of the compounds displayed significant efficacy [
10]. Alterporriols K and L (
11 and
12) are dimeric bianthraquinone derivatives with C-2-C-2′ connections, obtained from extract fungus
Alternaria sp. ZJ9-6B, obtained from mangroves of
Aegiceras corniculatum collected in the South China Sea [
11,
12]. According to preliminary bioassays, compounds
11 and
12 have modest cytotoxic effects on human breast cancer cell lines. The IC
50 values for compound
11 were 26.97 and 29.11 µM against MDA-MB-435 and MCF-7 cells, respectively. However, compound
12 reduced the development of MDA-MB-435 and MCF-7 with IC
50 values of 13.11 and 20.04 µM, respectively. Furthermore, alterporriol L (
12) showed significant inhibition of growth rate in both cell lines in a dose-dependent manner compared to control cells. More than 86% of cells were inhibited by compound
12 at a concentration of 50 µM [
11,
12]. Alterporriol P (
13) is an anthraquinone dimer derivative, identified in China by culture of the endophytic fungus
Alternaria sp. ZJ-2008003 from
Sarcophyton sp. soft coral [
13]. Alterporriol P (
13) inhibited the proliferation of the human prostate cancer cell line PC-3 and the colon cancer cell line HCT-116 [
13]. In contrast, the fungus
Alternaria sp. ZJ-2008003, isolated from
Sarcophyton sp., a soft coral in the South China Sea, afforded tetrahydroaltersolanols C-F (
14–
17), dihydroaltersolanol A (
18), tetrahydroaltersolanol B (
19), altersolanol B (
20), altersolanol L (
22), and ampelanol (
23) with an oxidized C-10 and a reduced C-9 fragment, which were inert (IC
50 > 100 μM) [
14]. The anthraquinone derivative with a paraquinone group, altersolanol C (
21), exhibited cytotoxicity against human colon carcinoma HCT-116, human breast cancer MCF-7/ADR, human prostatic cancer PC-3, and human hepatoma HepG2 and Hep3B cell lines with IC
50 values ranging from 2.2 to 8.9 μM [
14]. These results suggest that the presence of a paraquinone group is crucial for the observed cytotoxicity. Additionally, among the alterporriol-type dimers, alterporriols N, O, P (
24,
25,
13), alterporriol P (
13) showed cytotoxicity against PC-3 and HCT-116 with IC
50 values of 6.4 and 8.6 μM, respectively. However, alterporriol C (
26) was determined to be inactive (IC
50 > 20 μM) [
14].
The mycelium of the Chinese marine fungus
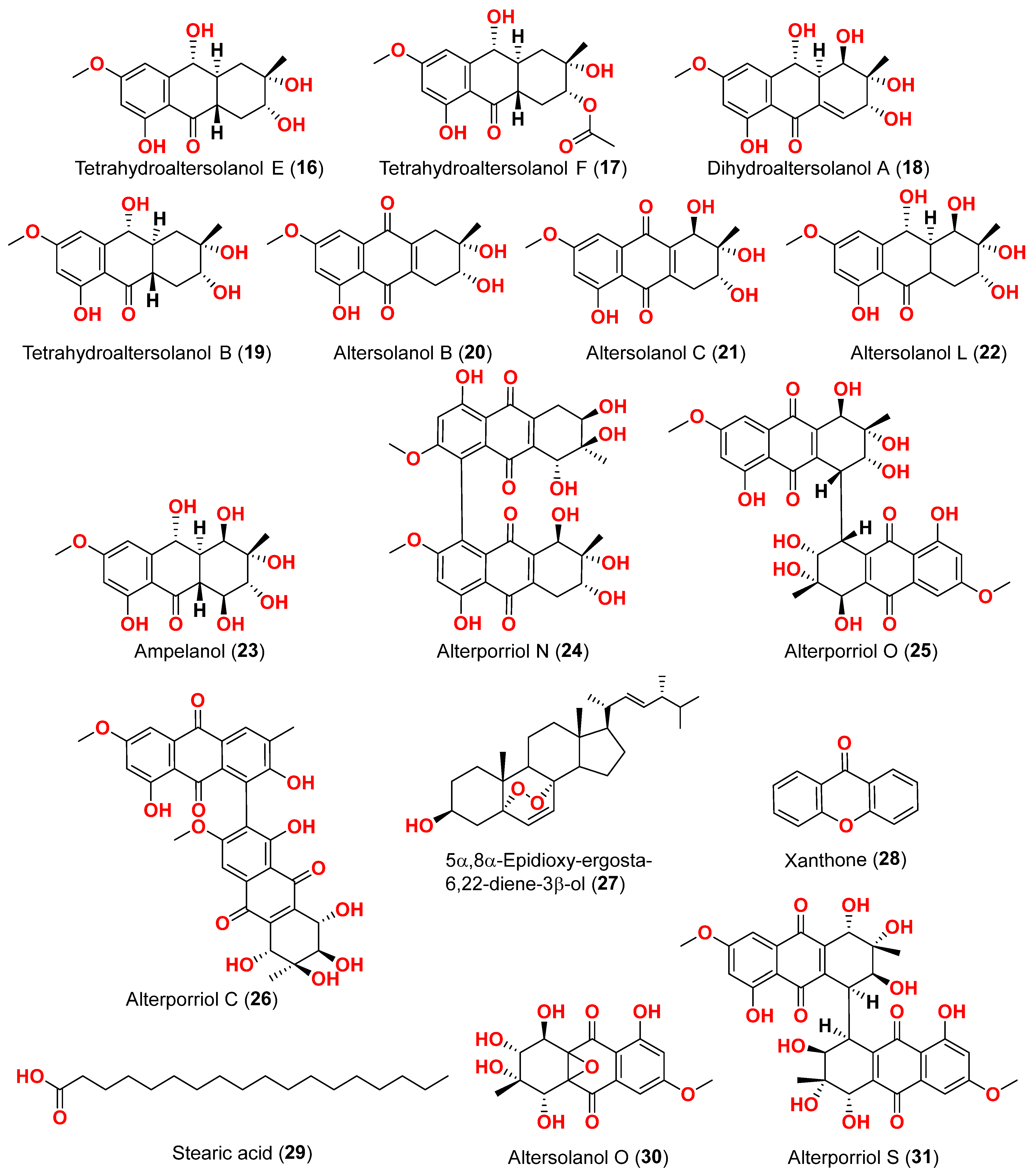
Alternaria sp. MNP801 was extracted to produce three compounds including 5α,8α-epidioxy-ergosta-6,22-diene-3-ol (
27), xanthone (
28), and stearic acid (
29). The IC
50 values of compound
27 against H460, 3T3, PC12, and U937A tumor cells were 119.6, 96.2, 27.1, and 34.1 μM, respectively. 5α,8α-Epodioxyergosta-6,22-diene-3β-ol (
27) was equally potent against H460 and 3T3 tumor cells and more effective than the VP16 in combating PC12 and U937A tumor cells [
15]. The fungus
Alternaria sp. XZSBG-1 was isolated from Salt Lake in Bange, Tibet, China. The anthraquinone derivatives, altersolanol C (
21), alterporriol N (
24), altersolanol O (
30), alterporriol S (
31), alterporriol T (
32), alterporriol U (
33), alterporriol E (
34), alterporriol D (
35), alterporriol A (
36), altersolanol A (
37), and macrosporin (
38), were isolated and identified [
16]. MTT assays were used to assess the cytotoxic activities of compounds
21,
24, and
30–
38 against MCF-7/ADR, HeLa, and HCT-116 cell lines. Altersolanol C (
21) exhibited potent inhibitory action against HCT-116 and HeLa cell lines, with IC
50 values of 3.0 and 8.0 μM, respectively. The remaining compounds did not exhibit any significant inhibitory effect against the cancer cell lines examined [
16]. A fraction of EtOAc extract recovered from the culture broth of the fungus
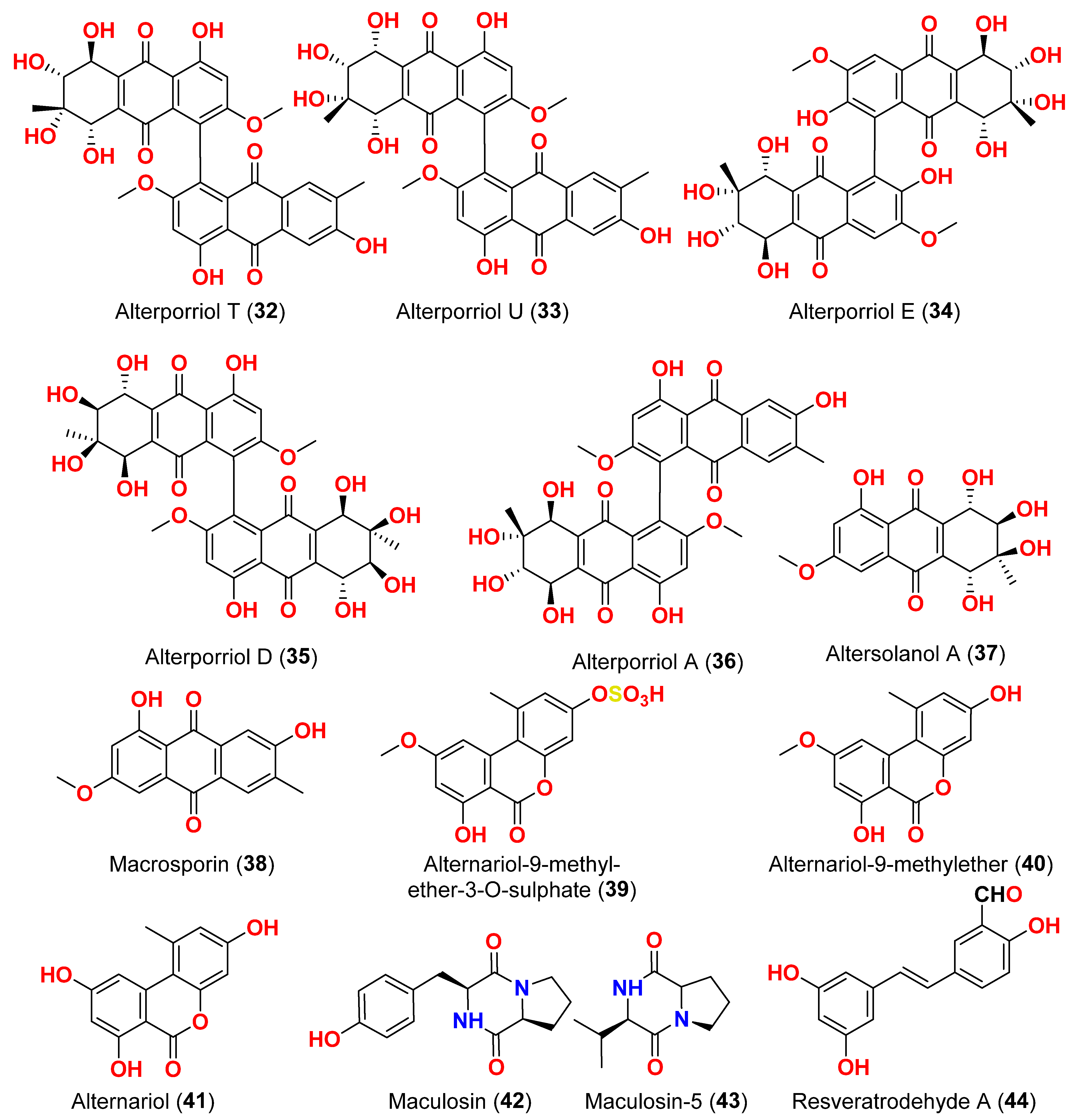
Alternaria alternata, isolated from the Egyptian Red Sea soft coral
Litophyton arboretum, afforded alternariol-9-methyl ether-3-
O-sulphate (
39), alternariol-9-methyl ether (
40), alternariol (
41), maculosin (
42), and maculosin-5 (
43) [
17]. Using the disk diffusion test, all compounds were evaluated for anticancer activity against two leukemia cells (murine L1210 and human CCRFCEM), four solid cancers (murine colon 38, human colon HCT116, human lung H125, and human liver HEPG2), and a normal human cell (CFUGM). Bioassay revealed that compound
39 was marginally selective against solid tumor HEPG2 compared to normal human cells (CFUGM) when 3 μg/disk of alternariol-9-methyl ether-3-
O-sulphate (
39) was used. In contrast, the fungal extracts (30 μg/disk), alternariol-9-methyl ether (
40) and alternariol (
41) inhibited normal cell growth [
17]. Three resveratrol derivatives, resveratrodehydes A–C (
44–
46), were obtained from the fungus
Alternaria sp. R6, isolated from the mangrove
Myoporum bontioides found in Guangdong Province, China [
18]. These compounds exhibited inhibitory effects against breast MDA-MB-435, liver HepG2, and colon HCT-116 human cells, as determined by the MTT assay. The antitumor effects of the compounds exhibit superiority in vitro compared to the positive control, resveratrol at concentrations below 50 μM. Compounds
44 and
45 displayed significant cytotoxicity against both the MDA-MB-435 and HCT-116 cell lines, with IC
50 values < 10 μM [
18]. A decalin derivative, altercrasin A (
47), with spiro skeletons isolated from a strain of
Alternaria sp. OUPS-117D-1, was isolated from the sea urchin
Anthocidaris crassispina, Japan [
19]. Altercrasin A (
47) inhibited human HL-60 leukemia and taurine L1210 leukemia cell lines with IC
50 values of 21.5 and 22.1 µM, respectively [
19]. Compound AS2-1 (
48), a polysaccharide with a molecular weight of 27.4 kDa was isolated from the fungus
Alternaria sp. SP-32, obtained from a sponge collected from the South China Sea [
20]. AS2-1 (
48) has a concentration-dependent cytotoxic effect on tested cell lines. The IC
50 values of compound
48 in Hela, HL-60, and K562 cell lines using the MTT and SRB methods were 6.4, 5.2, and 16.7 μM, respectively [
20]. Alterbrasone (
49) was separated from the fungus
Alternaria brassicae 93, isolated from crinoid
Comanthina schlegeli collected from the South China Sea [
21]. Cytotoxicity against two human cancer cell lines including human breast carcinoma cell line (MDA-MB-435) and human lung cancer cell line (A549) displayed no activity against these cells in the MTT assay [
21].
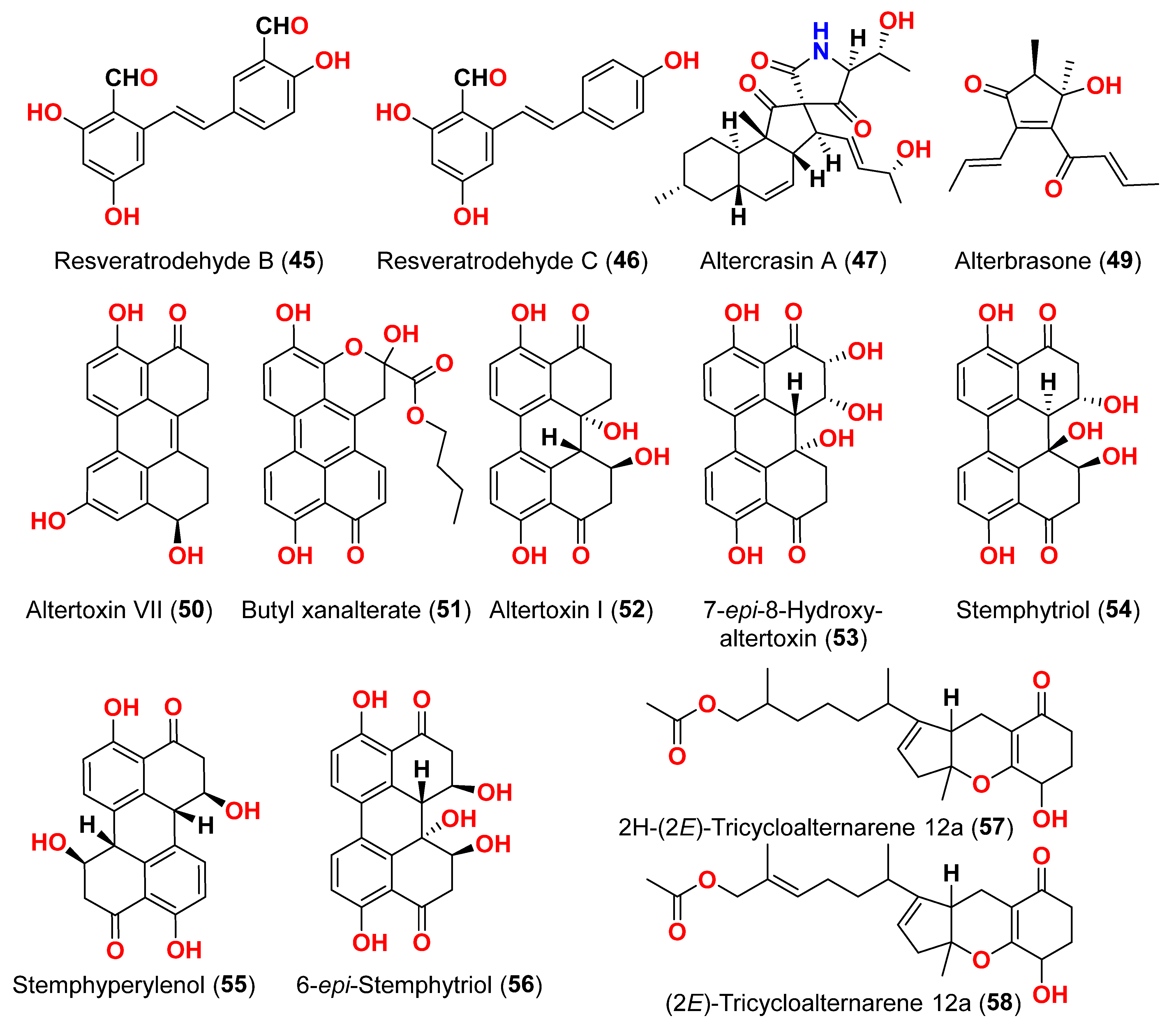
Two derivatives of perylenequinone, altertoxin VII (
50) and butyl xanalterate (
51), as well as five compounds, altertoxin I (
52), 7-
epi-8-hydroxyaltertoxin (
53), stemphytriol (
54), stemphyperylenol (
55), and 6-
epi-stemphytriol (
56), were isolated from the fungus
Alternaria sp. SCSIO41014 derived from the sponge
Callyspongia sp. collected from a coastal province in China [
22]. Using the CCK-8 assay, the cytotoxic effects of these compounds against human erythroleukemia (K562), human gastric carcinoma (SGC-7901), and hepatocellular cancer cells (BEL-7402) were investigated. Paclitaxel as the positive control showed IC
50 values of 0.21, 01.04, and 0.63 µM, respectively. Among the studied compounds, altertoxin VII (
50) was cytotoxic to the K562, SGC-7901, and BEL-7402 cell lines with corresponding IC
50 values of 82.6, 27.1, and 40.9 µM, respectively. Selective cytotoxic action against K562 with an IC
50 of 53.2 µM was exhibited by 6-
epi-stemphytriol (
56) [
22]. The fungus
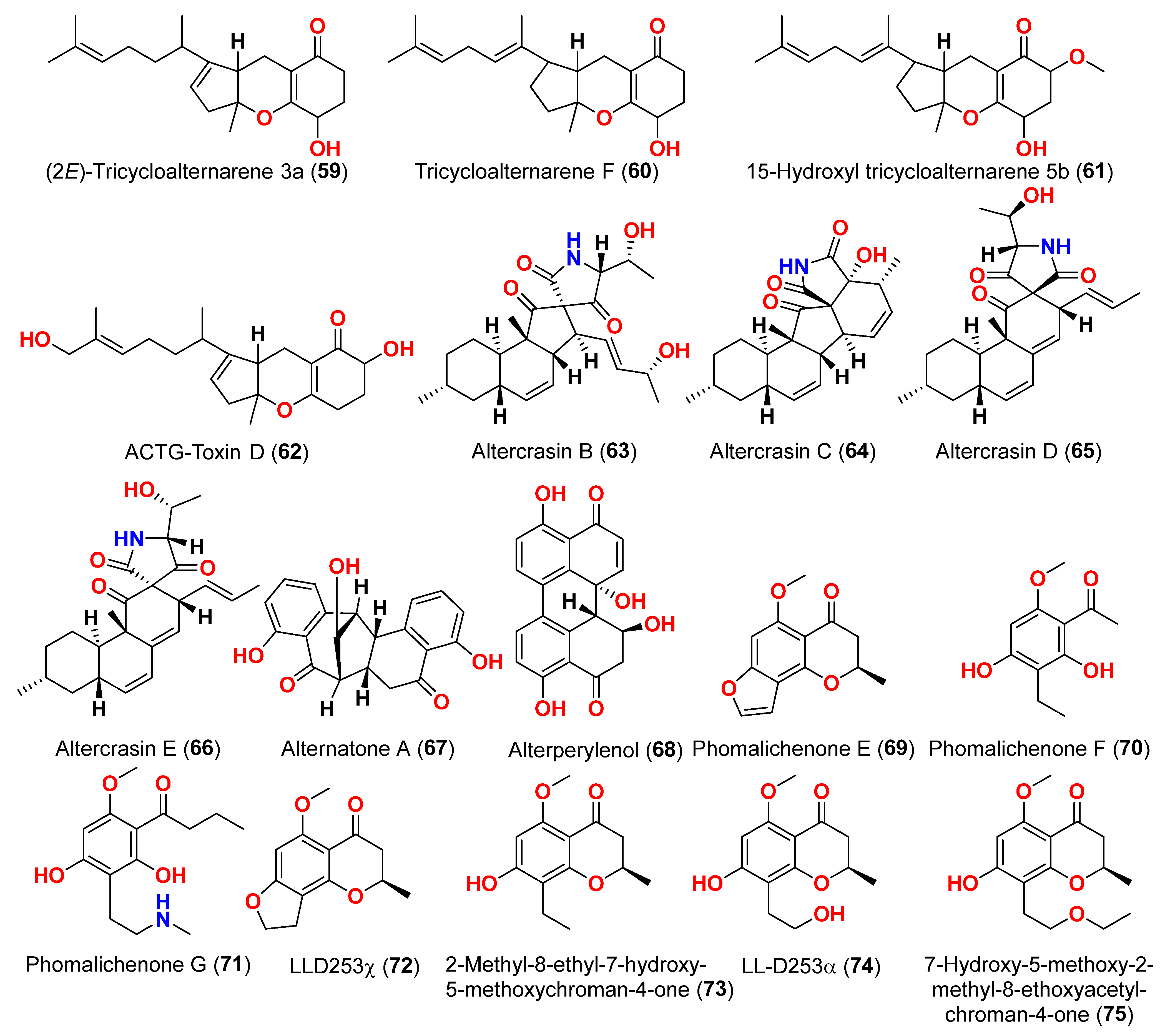
Alternaria sp. W-1 associated with the Chinese alga
Laminaria japonica produced 2H-(2
E)-tricycloalternarene 12a (
57), as well as five analogs, (2
E)-tricycloalternarene 12a (
58), tricycloalternarene 3a (
59), tricycloalternarene F (
60), 15-hydroxyl tricycloalternarene 5b (
61), and ACTG-Toxin D (
62) [
23]. The MTT assay evaluated cytotoxicity against the human hepatocellular carcinoma SMMC-7721 and the human gastric carcinoma SGC-7901 cell lines. Compounds 2H-(2
E)-tricycloalternarene 12a (
57), (2
E)-tricycloalternarene 3a (
59), and tricycloalternarene F (
60) decreased SMMC-7721 cell growth with corresponding IC
50 values of 127.4, 138.7, and 243.3 µM, while cisplatin had an IC
50 value of 21.5 µM. (2
E)-Tricycloalternarene 3a (
59) and ACTG-Toxin D (
62) exhibited a moderate antiproliferation action against SGC-7901 cells, with IC
50 values of 15.7 and 101.4 µM, respectively, compared to the IC
50 value of cisplatin of 14.9 µM. Further analysis revealed that the anticancer action of (2
E)-tricycloalternarene 3a (
59) against SMMC-7721 cells was related to G1 phase inhibition and cell apoptosis, using both the mitochondrial and death receptor pathways [
23]. The fungus
Alternaria sp. OUPS-117D-1, which was isolated from the sea urchin
Anthocidaris crassispina from Japan, developed four decalin derivatives, classified as altercrasins B–E (
63–
66) [
24]. The chemical pairings altercrasin B/altercrasin C (
63/
64) and altercrasin D/altercrasin E (
65/
66) were determined to be respective stereoisomers. The cytotoxic actions of altercrasins B-E (
63–
66) and 5-fluorouracil were investigated. Consequently, their cytotoxicity against murine L1210 leukemia, murine P388 leukemia, and human HL-60 leukemia cell lines revealed that compounds
65 and
66, containing a diene moiety (C-6 to C-8), exhibited strong cytotoxic activity against these cancer cells, especially the HL-60 cell line. In particular, the activity of compound
65 was comparable to that of 5-fluorouracil [
24]. Alternatone A (
67), having an unusual tricyclo[6.3.1.02,7]dodecane structure, was isolated from a soft coral-derived fungus
Alternaria alternata L3111′, along with three known perylenequinones, altertoxin I (
52), stemphyperylenol (
55), and alterperylenol (
68) [
25]. All compounds were exposed to a cytotoxic activity evaluation against human lung carcinoma (A-549), human colon cancer (HCT-116), and human cervical carcinoma (HeLa) cell lines. Alterperylenol (
68) exhibited cytotoxicity against A-549, HCT-116, and HeLa cell lines with corresponding IC
50 values of 2.6, 2.4, and 3.1 μM, respectively. However, the remaining compounds did not display cytotoxic actions. This demonstrates how double bonds in perylenequinones are crucial for their cytotoxicity [
25].
Three phomalichenones E-G (
69–
71) and seven analogs, including LL-D253γ (
72), 2-methyl-8-ethyl-7-hydroxy-5-methoxychroman-4-one (
73), LL-D253α (
74), 7-hydroxy-5-methoxy-2-methyl-8-ethoxyacetylchroman-4-one (
75), phomalichenone A (
76), deoxyphomalone (
77), and phomalone (
78) originated from an
Alternaria sp. fungus MCCC 3A00467 isolated from deep sediment in the Pacific Ocean [
26]. The MTT test was used to assess the cytotoxicity of compounds
69–
78 against human myeloma cancer U266, human liver cancer (HepG2), and human lung cancer (A549) cells. Compounds
70,
74, and
76–
78 inhibited the growth of U266 and HepG2 human cells, while phomalone (
78) exhibited the highest cytotoxic action against three cancer cell lines, with IC
50 values ranging from 55.0 to 60.8 µM. Based on IC
50 values greater than 396.8–431.0 µM for compounds
69 and
72–
75 against U266 and HepG2 cells, the number of hydroxyl groups can influence cytotoxicity [
26]. Compounds
74 and
76–
78 inhibited U266 and HepG2 cells with IC
50 values between 55.0 and 256.7 µM, while phomalichenones E (
69) and LL-D253γ (
72) without hydroxyl groups are inactive (IC
50 > 427.0–431.0 µM). LL-D253α (
74) was more cytotoxic than compounds
73 and
75 against three cell lines, indicating that the hydroxyl group at C-2′ may play an essential role in antitumor action. Comparing the IC
50 values of compounds
74 and
78 revealed that the open pyrone ring or the presence of phenolic hydroxyl group at position C-2 had no impact on the activity against U266 and HepG2 cells but had a substantial effect on A549 cells. Compounds
70 and
78 demonstrated that the methylamino group at C-2′ decreased the inhibitory effect [
26]. Through investigation of the fungal extract of
Alternaria sp. 114-1G, compounds pachybasin (
79), cyclo(Gla-Tyr) (
80), cyclo(Ala-Ile) (
81), and thymidine (
82) were purified and identified [
27]. The most efficient inhibitory impact on HeLa cells was observed by pachybasin (
79) with a 57.8% inhibition rate at 420.1 µM [
27]. The fungus
Alternaria longipes, isolated from the mangrove of
Kandelia candel in Guangxi, China, afforded one chromanone derivative, alterchromone A (
83), and four curvularin-type macrolides curvularin (
84), 11-β-methoxycurvularin (
85), β,γ-dehydrocurvularin (
86), and α,β-dehydrocurvularin (
87) [
28]. Compounds
83–
87 were evaluated for their cytotoxicity against four human tumor cell lines, including HeLa (human cervical carcinoma cell line), HepG2 (human hepatocellular carcinoma cell line), MCF-7 (human breast cancer cell line), and ACHN (human renal carcinoma cell line). Interestingly, the 100 μM concentration of these chemicals had no detectable inhibitory impact on the tested cell lines [
28].
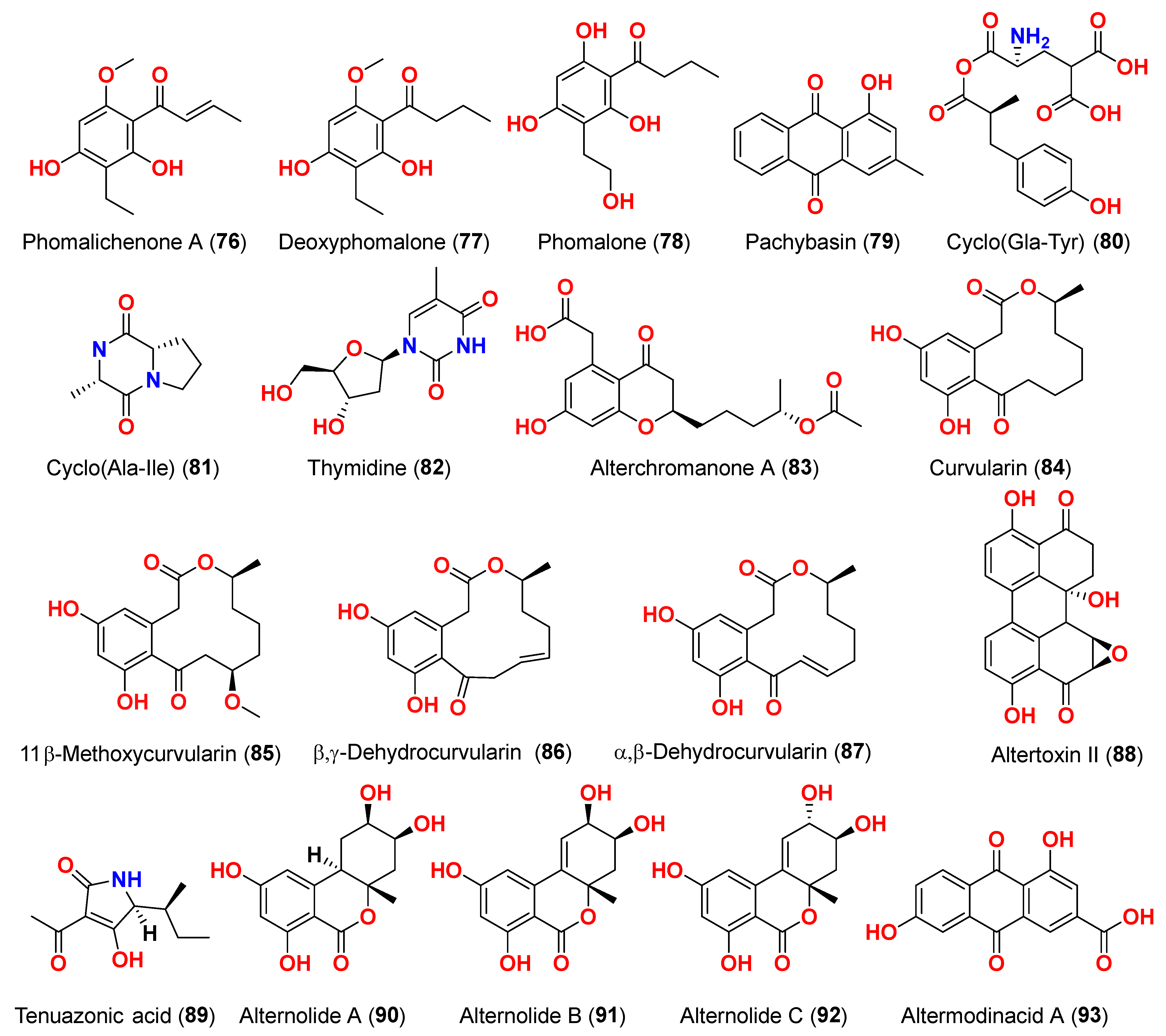
Isolation of five polyketides, alternariol (
41), alternariol-9-methyl ether (
40), altertoxin I (
52), altertoxin II (
88), and tenuazonic acid (
89), from the marine endophytic fungus
Alternaria sp. LV52 isolated from
Cystoseira tamariscifolia collected from the Red Sea, Egypt, was reported [
29]. However, all compounds exhibited cytotoxicity against HepG2 with corresponding EC
50 values ranging from 27.8 to 172.0 μM. The cytotoxicity of alternariol-9-methyl ether (
40), altertoxin II (
88), and tenuazonic acid (
89) was evaluated against A549 and PC3 cells [
29]. Thus, the EC
50 of alternariol-9-methyl ether (
40) and altertoxin II (
88) were 1.43 and 1.14 μM against A549 and 0.65 and 0.34 μM against PC3, respectively. Tenuazonic acid (
89) showed moderate activity against A549 and PC3 cells. Moreover, compound
89 was the only chemical identified as cytotoxic for the HeLa cell line with an EC
50 value of 109.1 μM [
29]. The fungus
Alternaria alternata LW37, a marine-derived fungus obtained from deep-sea sediment on the southwest Indian Ridge, produced three dibenzo-α-pyrone compounds, alternolides A–C (
90–
92). The cytotoxicity of compounds
90–
92 was assessed against MCF-7 (human breast cancer cells), B16 (mouse melanoma cells), and HepG2 (human hepatocellular carcinoma cells). The compounds exhibited no detectable inhibitory effect on the investigated cell lines at 50 µM [
30]. The cytotoxic activity of anthraquinone, altermodinacid A (
93), was assessed following its discovery in the fungus
Alternaria sp. X112, which was isolated from a marine fish
Gadus macrocephalus from Yangma Island, China. No cytotoxic effects (IC
50 > 40 µM) were observed against MCF-7, MKN-45, TE-1, and HCT116 cells [
31].
In conclusion, from the above discussion and from the data presented in
Table 1, perylenequinones, altertoxins, and altersolanols represent the most cytotoxic agents among the evaluated compounds in this section.
Among the 93 evaluated compounds, findings were as follows: Perylenequinones and altertoxins: Altertoxins I (
52) and II (
88) exhibited strong cytotoxicity against A549 and PC3 cells, with EC
50 values as low as 0.34–1.14 μM, while altertoxins also showed activity against HepG2 cells at slightly higher EC
50 values (42.8–131.7 μM) [
29]. Alternariol derivatives: Alternariol (
41) and alternariol-9-methyl ether (
40) were cytotoxic against HepG2, HeLa, A549, and PC3 cells, with EC
50 values as low as 0.65 μM (PC3) and 1.43 μM (A549) [
29]. Altersolanols: Altersolanol C (
21) showed broad cytotoxicity across HCT-116, MCF-7/AD, PC-3, HepG2, and Hep3B cells, with IC
50 values of 2.2–8.9 μM. Additional assays with
A. sp. XZSBG-1 confirmed strong cytotoxicity against MCF-7/ADR, HeLa, and HCT-116 cells (IC
50 = 3.0–8.0 μM) [
14,
16]. Resveratrodehydes: Resveratrodehydes A–C (
44–
46) exhibited potent cytotoxicity against MDA-MB-435, HepG2, and HCT-116 cells, with IC
50 values ranging from 6.9 to 18.6 μM [
18]. Alterporriols: Alterporriol P (
13) showed strong inhibitory effects against PC-3 and HCT-116 cells (IC
50 = 6.4 and 8.6 μM) [
13]. Alterporriols K (
11) and L (
12) demonstrated notable cytotoxicity against MDA-MB-435 and MCF-7 cells, with IC
50 values of 26.9–29.1 μM and 13.1–20.0 μM, respectively; alterporriol L killed 86% of cells at 50 μM [
11,
12].
Among the moderately active compounds are compound AI-77-B (
1), which displayed IC
50 values of 20 and 100 μM (Hela and A375-S2), while AI-77-F (
3) was less active (IC
50 = 50 and 300 μM) [
7,
8]. Xanalteric acids I (
8) and II (
9) exhibited IC
50 values of 45.0 and 87.5 μM against murine L5178Y cells [
10]. Altercrasin A (
47) and polysaccharide AS2-1 (
48) showed IC
50 values of 21.5–22.1 μM and 5.2–16.7 μM, respectively, against leukemia and HeLa cells [
19,
20]. Altertoxin VII (
50) exhibited IC
50 values of 27.1–82.8 μM against K562, SGC-7901, and BEL-7402 cells [
22].
Weakly active compounds included 6-epi-stemphytriol (
56) with an IC
50 of 53.2 μM (K562), while tricycloalternarenes
57–
60 and ACTG-toxin D (
62) displayed IC
50 values >100 μM [
23]. Altercrasins B–E (
63–
66) exhibited IC
50 values ranging from 15.3 to 165.8 μM, depending on the cell line [
24]. Phomalichenones (
70,
76), deoxyphomalone (
77), and phomalone (
78) showed IC
50 values between 55.0 and 381.9 μM [
26]. Pachybasin (
79) inhibited 57.8% of HeLa cells at 420.1 μM [
27]. Tenuazonic acid (
89) exhibited weaker activity, with EC
50 values above 100 μM in HepG2 and HeLa cells.
Overall, compounds based on perylenequinone, altertoxin, and altersolanol scaffolds represent the most potent cytotoxins, frequently exhibiting IC50/EC50 values below 10 μM. Other structural classes, including tetramic acid derivatives, diphenyl ethers, and ergosterol-type compounds, show moderate to weak cytotoxic effects. Most reported metabolites fall within the moderate range (50–100 μM), suggesting opportunities for structure optimization to improve potency and selectivity.
Cytotoxicity Mechanistic Insight. Many cytotoxic
Alternaria metabolites, especially perylenequinones (e.g., altertoxins, stemphyperylenol, alterperylenol), are known to act as photosensitizers that generate reactive oxygen species (ROS) under light, contributing to cell damage [
32]. The production of ROS leading to oxidative stress is believed to play a role in their anticancer effects, as demonstrated by altertoxins encouraging lipid peroxidation in cell membranes [
15]. Other compounds, like alternariol (
41) and alternariol monomethyl ether (
138), have been shown to intercalate DNA and inhibit eukaryotic topoisomerase enzymes, leading to DNA strand breaks in cancer cells [
16]. The relatively planar structures of these anthraquinones facilitate such interactions. It is also notable that slight structural modifications can alter potency: e.g., alternariol and its methyl ether differ in activity, suggesting that the hydroxylation pattern on the aromatic rings influences their ability to bind DNA or other targets. However, beyond a few cases studied, detailed pharmacological target data are lacking for most
Alternaria cytotoxins, representing an area for future research (See Conclusions and Future Trends).
Table 1 shows only the compounds with proven cytotoxic activity.
Table 1.
Reported compounds with proven cytotoxic activities.
Table 1.
Reported compounds with proven cytotoxic activities.
| Compound | Cell Line Used | Biological Activity | Fungus Name | Host Organism | Reference |
|---|
| AI-77-B (1) | A375-S2, HeLa | IC50 = 100, 20 μM | Alternaria tenuis, Sg17-1 | Unspecified alga | [7,8] |
| AI-77-F (2) | IC50 = 400 μM (Hela) |
| Sg17-1-4 (3) | IC50 = 300, 50 μM |
| Xanalteric acid I (8) | L5178Y | IC50 = 45.0 μM | Alternaria sp. JCM9.2 | Mangrove Sonneratia alba | [10] |
| Xanalteric acid II (9) | IC50 = 87.5 μM |
| Alternarian acid (10) | IC50 = 99.2 μM |
| Alterporriol K (11) | MDA-MB-435, MCF-7 | IC50 = 26.9, 29.1 μM | Alternaria sp. ZJ9-6B | Mangrove Aegiceras corniculatum | [11,12] |
| Alterporriol L (12) | IC50 = 13.1, 20.0 μM
86% cells killed at 50 μM |
| Alterporriol P (13) | PC-3, HCT-116 | IC50 = 6.4, 8.6 μM (PC-3, HCT-116) | Alternaria sp. ZJ-2008003 | Sarcophyton sp. soft coral | [13,14] |
| Altersolanol C (21) | HCT-116, MCF-7/AD, PC-3, HepG2, Hep3B | IC50 = 2.2–8.9 μM | Alternaria sp. ZJ-2008003 | Sarcophyton sp. soft coral | [14] |
| MCF-7/ADR, HeLa, HCT-116 | IC50 = 3.0, 8.0 μM
(HCT-116, HeLa) | Alternaria sp. XZSBG-1 | Sediment | [16] |
| 5α,8α-Epidioxy-ergosta-6,22-dien-3β-ol (27) | H460, 3T3, PC12, U937 | IC50 = 119.6, 96.2, 20.3, 34.1 μM | Alternaria sp. MNP801 | | [15] |
| Alternariol-9-methyl ether-3-O-sulphate (39) | Two leukemias (L1210, CCRFCEM); four solid tumors (murine colon 38, HCT116, H125, HEPG2); one normal cell (CFU-GM) | 400 zu against HEP-G2 compared to 100 zu against CFU-GM at 3 μg/disk | Alternaria alternata | Soft coral Litophyton arboreum | [17] |
| Alternariol-9-methyl ether (40) | Cytotoxic to CFU-GM |
| HepG2, Hela, A549, PC3 | EC50 = 108.5 μM (HepG2)
EC50 = 1.43 μM (A549)
EC50 = 0.65 μM (PC3) | Alternaria sp. LV52 | Cystoseira tamariscifolia | [29] |
| Alternariol (41) | CFU-GM | Cytotoxic to CFU-GM | Alternaria alternata | Soft coral Litophyton arboreum | [17] |
| EC50 = 37.9 μM (HepG2) | EC50 = 37.9 μM (HepG2) | Alternaria sp. LV52 | Cystoseira tamariscifolia | [29] |
| Resveratrodehyde A (44) | MDA-MB-435, HepG2, HCT-116 | IC50 = 8.5, 7.8 μM (MDA-MB-435, HCT-116) | Alternaria sp. R6 | Mangrove Myoporum bontioides | [18] |
| Resveratrodehyde B (45) | IC50 = 7.6, 6.9 μM (MDA-MB-435, HCT-116) |
| Resveratrodehyde C (46) | IC50 = 16.4, 18.6 μM (MDA-MB-435, HCT-116) |
| Altercrasin A (47) | Human HL-60 leukemia, taurine L1210 leukemia | IC50 = 21.5, 22.1 μM | Alternaria sp. OUPS-117D-1 | Sea urchin Anthocidariscrassispina | [19] |
| Polysaccharide AS2-1 (48) | Hela, HL-60, K562 | IC50 = 6.4, 5.2, 16.7 μM | Alternaria sp. SP-32 | Unspecified sponge | [20] |
| Altertoxin VII (50) | K562, SGC-7901, BEL-7402 | IC50 = 82.8, 27.1, 40.9 µM | Alternaria sp. SCSIO41014 | Callyspongia sp. sponge | [22] |
| 6-epi-Stemphytriol (56) | IC50 = 53.2 µM (K562) |
| 2H-(2E)-Tricycloalternarene 12a (57) | SMMC-7721, SGC-7901 | IC50 = 127.4 µM (SMMC-7721) | Alternaria sp. W-1 | Algae Laminaria japonica | [23] |
| (2Z)-Tricycloalternarene 3a (59) | IC50 = 138.7, 15.7 µM (SMMC-7721, SGC-7901) |
| Tricycloalternarene F (60) | IC50 = 243.3 µM (SMMC-7721) |
| ACTG-Toxin D (62) | IC50 = 101.4 µM (SGC-7901) |
| Altercrasin B (63) | P388, HL-60, L1210 | IC50 = 57.8, 29.1, 19.2 µM | Alternaria sp. OUPS-117D-1 | Urchin Anthocidaris crassispina | [24] |
| Altercrasin C (64) | IC50 = 165.8, 112.7, 73.4 µM |
| Altercrasin D (65) | IC50 = 24.4, 15.3, 21.1 µM |
| Altercrasin E (66) | IC50 = 39.0, 15.6, 25.9 µM |
| Phomalichenone F (70) | U266, HepG2, A549 | IC50 = 118.5, 157.6, 381.9 µM | Alternaria sp. MCCC 3A00467 | Deep ocean sediment | [26] |
| LL-D253α (74) | IC50 = 61.1, 132.9 µM (U266, HepG2) |
| Phomalichenone A (76) | IC50 = 62.2, 66.5, 86.4 µM |
| Deoxyphomalone (77) | IC50 = 98.3, 65.5, 256.7 µM |
| Phomalone (78) | IC50 = 55.0, 60.8, 101.2 µM |
| Pachybasin (79) | HeLa | 57.8% inhibition rate at 420.1 µM | Alternaria sp. 114-1G | Ocean | [27] |
| Altertoxin I (52) | HepG2, Hela, A549, PC3 | EC50 = 131.7 μM (HepG2) | Alternaria sp. LV52 | Cystoseira tamariscifolia | [29] |
| Altertoxin II (88) | EC50 = 42.8 μM (HepG2)
EC50 = 1.14 μM (A549)
EC50 = 0.34 μM (PC3) |
| Tenuazonic acid (89) | EC50 = 146.1 μM (HepG2)
EC50 = 109.1 μM (HeLa) |
2.2. Compounds with Antimicrobial Activity (Table 2)
The secondary metabolites produced by members of the genus
Alternaria are essential for survival in the marine environment. Consequently, they may have medicinal applications, including antibacterial, antifungal and antiviral activities. Compounds evaluated for their activity in this section are displayed in
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 10 and
Figure 11 and compounds with proven antimicrobial activity have been shown in
Table 2. In addition, alongside those compounds, several compounds from
Section 2.1. were evaluated for their antimicrobial potential. Alternarosides A–C (
4–
6) and alternarosin A (
7) were identified from the fungus
Alternaria raphani THW-18, which was obtained from sediments collected in the Hongdao sea salt field, Qingdao, China [
9]. Using the agar dilution technique, these compounds produced weak antibacterial activity against
E. coli,
B. subtilis, and
C. albicans, with MIC values ranging from 70 to 400 µM [
9]. The compounds xanalteric acids I (
8) and II (
9), alternarian acid (
10), alternarienonic acid (
94), altenusin (
95), altertoxin I (
52), altenuene (
96), 4′-
epi-altenuene (
97), alternariol (
41), alternariol-5-
O-methyl ether (
98), alterperylenol (
68), and stemphyperylenol (
55) were purified and characterized from the endophytic fungus
Alternaria sp. JCM9.2, which was isolated from
Sonneratia alba mangrove collected in China [
10]. Antibiotic activity against multi-resistant bacterial and fungal strains was evaluated for all compounds against
E. coli,
S. aureus,
K. pneumoniae,
E. cloacae,
E. faecium,
Pseudomonas aeruginosa,
S. pneumonia,
A. baumanii,
C. krusei,
C. albicans,
A. fumigatus, and
Aspergillus faecalis. In these analyses, the MIC values for xanalteric acids I (
8) and II (
9) against
S. aureus ranged from 343.4 to 686.8 µM. Altenusin (
95) showed extensive antibacterial activity against various resistant pathogens with MIC values of 107.7–431.0 µM, while all other compounds had no antibiotic action against the bacteria and fungi tested [
10].
The fungus
Alternaria sp. SF-5016, which was separated from Masan Bay shoreline sediment, provided a cyclic pentadepsipeptide, alternaramide (
99) [
33]. The antimicrobial action of compound
99 at 400 μg/disk was investigated against
S. aureus and
Bacillus subtilis, producing inhibition zones of 8 and 13 mm, respectively. In addition, compound
99 did not show comparable antimicrobial activity against
C. albicans,
Filobasidiella neoformans, or
Proteus vulgaris [
33]. The perylene derivatives, 7-
epi-8-hydroxyaltertoxin I (
53) stemphyperylenol (
55) and 6-
epi-stemphytriol (
56) were purified from the fungus
Alternaria alternata, derived from the algal genus
Laurencia sp. collected in the South China Sea on Weizhou Island [
34]. Compounds
53,
56, and
55 were evaluated for their antibacterial and antifungal activities against
E. coli,
S. aureus, and
A. niger. However, none of them exhibited discernible activity [
34]. A cyclic peptide (
100) obtained from the marine sediment-derived fungus
Alternaria sp. SF-5016 displayed antibacterial activity against
Staphylococcus aureus and
B. subtilis [
35]. Tetrahydroaltersolanols C-F (
14–
17), dihydroaltersolanol A (
18), and alterporriols N, O, P, Q, and R (
24,
25,
13,
101, and
102), in addition to seven analogs including tetrahydroaltersolanol B (
19), altersolanol B (
20), altersolanol C (
21), altersolanol L (
22), ampelanol (
23), alterporriol C (
26), and macrosporin (
38) were isolated and identified from the culture broth and the mycelia of the fungus
Alternaria sp. ZJ-2008003, a fungus from a
Sarcophyton sp. soft coral, which was collected from the South China Sea [
14]. The antibacterial activity of these compounds was evaluated against seven pathogenic bacteria (
E. coli,
S. aureus,
S. albus,
B. subtilis,
B. cereus,
Micrococcus tetragenus, and
Micrococcus luteus) and two marine pathogenic bacteria (
V. anguillarum and
V. parahemolyticus); only altersolanol C (
21), alterporriol C (
26), and macrosporin (
38) showed strong antibacterial activity against
E. coli and
V. parahemolyticus, with MIC values between 0.6 and 2.5 μM. Antiviral activity against porcine reproductive and respiratory syndrome virus (PRRSV) was investigated. Tetrahydroaltersolanol C (
14), alterporriol C (
26), and alterporriol Q (
101) showed IC
50 values of 65, 39, and 22 μM, respectively [
14].
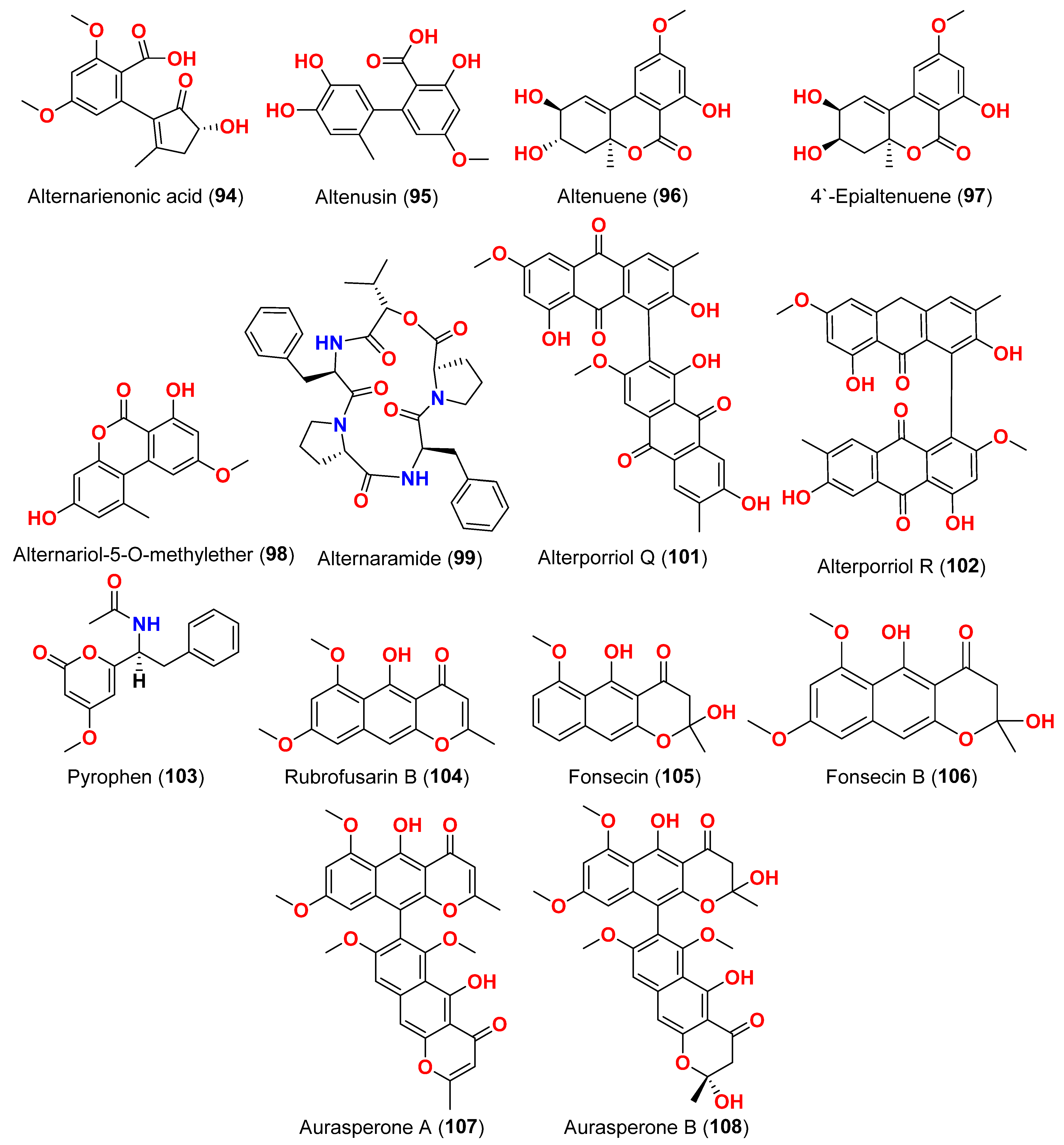
The compounds pyrophen (
103), rubrofusarin B (
104), fonsecin (
105), and fonsecin B (
106), together with dimers of naphtha-pyrones, aurasperone A (
107), aurasperone B (
108), aurasperone C (
109), and aurasperone F (
110), were obtained from the fungus
Alternaria alternata strain D2006 cultures [
36]. The fungus was isolated from a soft coral,
Denderonephthya hemprichi, collected from the Red Sea, Egypt [
36]. The antimicrobial activity of these compounds was assessed by the agar diffusion method against 11 microorganisms. The fungal strain’s crude extract was highly effective against bacteria and yeast. However, only three of the isolated metabolites revealed activity; pyrophen (
103) and rubrofusarin B (
104) exhibited significant antifungal activities with inhibition zones of 28 and 12 mm against
C. albicans, respectively. Additionally, aurosperone A (
107) was effective (inhibition zone = 13 mm) against the plant-pathogenic fungus
Rhizoctonia solani [
36]. Altenusin (
95) and a dibenzofuran derivative, porric acid D (
111), were recovered from the marine fungus
Alternaria sp. identified from Bohai Sea, Tianjin seawater [
37]. Using agar diffusion, the antimicrobial effect of the compounds against
Staphylococcus aureus was evaluated. Compounds
95 and
111 inhibited
S. aureus with MIC values of 86.2 and 347.2 μM, respectively [
37]. Alterporriol S (
31), an anthranoid dimer of the alterporriol class, was discovered in the mangrove plant
Excoecaria agallocha-associated fungus
Alternaria sp. SK11 in the South China Sea [
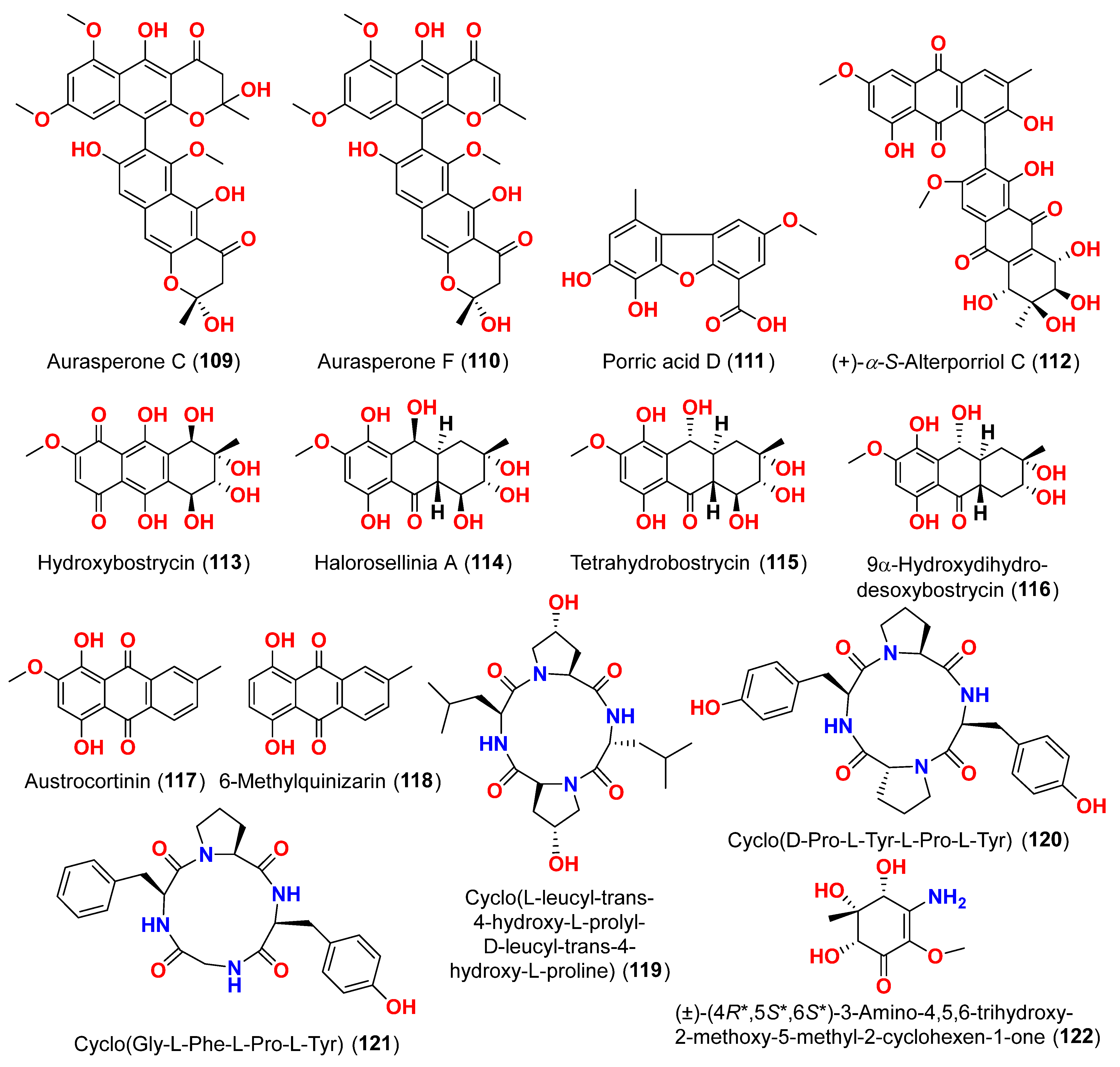
38]. In addition, seven anthraquinone derivatives, (+)-α-
S-alterporriol C (
112), hydroxybostrycin (
113), halorosellinia A (
114), tetrahydrobostrycin (
115), 9α-hydroxydihydrodesoxybostrycin (
116), austrocortinin (
117), and 6-methylquinizarin (
118) were also identified. All the compounds were evaluated for their ability to inhibit the
Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB), using sodium orthovanadate as a standard. The findings showed that compound
112 is a strong inhibitor of MptpB with an IC
50 value of 8.7 μM [
38]. A cyclic tetrapeptide cyclo(
l-leucyl-trans-4-hydroxy-
l-prolyl-
d-leucyl-trans-4-hydroxy-
l-proline) (
119) was identified from the co-culture broth of two mangrove fungi
Phomopsis sp. K38 and
Alternaria sp. E33 from Guangdong Province, China [
39]. The dilution approach revealed that compound
119 showed moderate to high inhibitory activity against four crop-threatening fungi, including
Rhizoctonia cerealis,
Gaeumannomyces graminis,
Fusarium graminarum, and
Helminthosporium sativum. The MIC values of compound
119 against
H. sativum were comparable to those of the positive control, triadimefon [
39]. Two tetracyclopeptides were extracted from the broth of two mangrove fungi,
Phomopsis sp. K38 and
Alternaria sp. E33: cyclo(
d-Pro-
l-Tyr-
l-Pro-
l-Tyr) (
120) and cyclo(Gly-
l-Phe-
l-Pro-
l-Tyr) (
121) [
40]. The dilution technique was utilized to examine antifungal activity and moderate to strong activity against
Candida albicans,
Gaeumannomyces graminis,
Helminthosporium sativum,
Rhzioctonia cerealis, and
Fusarium graminearum was observed, comparing to the positive control. Cyclo(Gly-l-Phe-
l-Pro-
l-Tyr) (
121) was more active (MIC = 53.8–538.7 µM) than cyclo(
d-Pro-
l-Tyr-
l-Pro-
l-Tyr) (
120) (MIC = 67.3–769.2 µM) [
40].
The fungus
Alternaria alternata, was isolated from
Litophyton arboreum soft coral collected from the coast of Egypt in the Red Sea. There are various antimicrobial properties of
Alternaria alternata broth extract and three isolated compounds: alternariol-9-methyl ether-3-
O-sulphate (
39), alternariol-9-methyl ether (
40), and alternariol (
41) were investigated [
17]. Using the agar diffusion technique, the antimicrobial effects were determined against Gram-positive bacteria
Bacillus megaterium,
Bacillus cereus,
Bacillus subtilis, and
Staphylococcus aureus; Gram-negative bacteria
Enterobacter cloacae,
Klebsiella pneumoniae, and
Escherichia coli; and yeasts
Candida albicans,
Saccharomyces cerevisiae, and
Aspergillus niger. The extract of
A. alternata had moderate activity against
B. megaterium and
E. coli and strong activity against
B. cereus with inhibition diameters of 20, 15, and 12 mm, respectively. Separated compounds correlated with antibacterial activities ranging from strong to moderate effects against the same pathogens at 50 μg/disk concentration. These compounds were also examined for their ability to block HCV protease NS3-NS4A, and hepatitis virus C NS3 protease inhibitor 2 was used as positive control. The IC
50 values of alternariol-9-methyl ether (
40) and alternariol (
41) against HCV NS3-NS4A were 118.3, and 46.5 μM, respectively. The IC
50 value for alternariol-9-methyl ether-3-
O-sulphate (
39) was 147.7 μM, making it less potent than alternariol (
41). These findings revealed that the inhibitory effect was reduced after C-9 methylation of alternariol (
41) [
17].
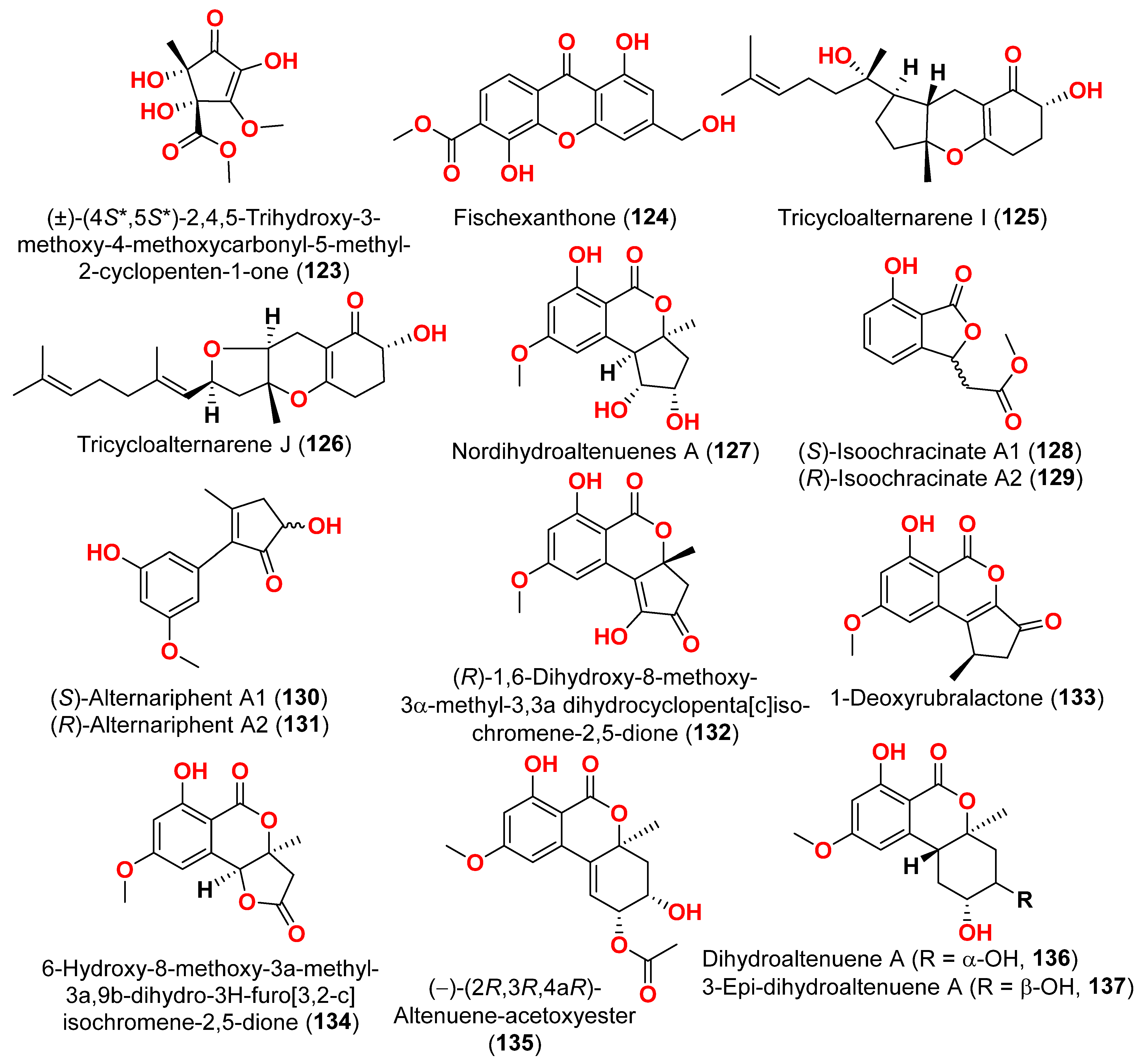
The racemic compounds of cyclohexenone and cyclopentenone derivatives, namely (±)-(4
R*,5
S*,6
S*)-3-amino-4,5,6-trihydroxy-2-methoxy-5-methyl-2-cyclohexen-1-one (
122) and (±)-(4
S*,5
S*)-2,4,5-trihydroxy-3-methoxy-4-methoxycarbonyl-5-methyl-2-cyclopenten1-one (
123), as well as fischexanthone (
124), were obtained from the fungus
Alternaria sp. R6, derived from the marine semi-mangrove plant
Myoporum bontioides A collected from Guangdong Province, China [
41]. The antimicrobial activity of these compounds was evaluated. Compounds
122–
124 displayed no activities either against Gram-positive bacterium
Staphyloccocus aureus nor against Gram-negative bacterium
Escherichia coli with MIC value ≥ 1265.82 μM [
41]. Investigation of the sediment-derived fungus
Alternaria sp. KJ749826 in the south Atlantic ridge revealed tricycloalternarenes I (
125) and J (
126) [
42]. The compounds were tested for their antibacterial activities against strains of
Streptococcus pyogenes,
Bacillus subtilis, and
Mycobacterium smegmatis. However, no activity was detected at a concentration of 172.4–173.4 μM [
42].
Two derivatives of perylenequinone—altertoxin VII (
50) and butylxanalterate (
51)—an altenusin derivative nordihydroaltenuene A (
127), and two phthalide racemates—(
S)-isoochracinate A1 (
128) and (
R)-isoochracinate A2 (
129)—together with (
S)-alternariphent A1 (
130), (
R)-alternariphent A2 (
131)
, altertoxin I (
52), 7-
epi-8-hydroxyaltertoxin (
53), stemphytriol (
54), 6-
epi-stemphytriol (
56), stemphyperylenol (
55), (
R)-1,6-dihydroxy-8-methoxy-3a-methyl-3,3a-dihydrocyclopenta[c]iso-chromene-2,5-dione (
132), 1-deoxyrubralactone (
133), 6-hydroxy-8-methoxy-3a-methyl-3a,9b-dihydro-3H-furo[3,2-c]isochromene-2,5-dione (
134), altenuene (
96), 4′-
epi-altenuene (
97), (−)-(2
R,3
R,4a
R)-altenuene-3-acetoxyester (
135), dihydroaltenuene A (
136), 3-
epi-dihydroaltenuene A (
137), alternariol (
41), alternariol monomethyl ether (
138), 3′-hydroxyalternariol-5-
O-methyl ether (
139), altenusin (
95), alterlactone (
140), altenuisol (
141), 5′-methoxy-6-methyl-biphenyl-3,4,3′-triol (
142), and 2,5-dimethyl-7-hydroxychromone (
143) were obtained from the fungus
Alternaria sp. SCSIO41014, which was isolated from
Callyspongia sp. sponge in Guangdong Province, China [
22]. Antibacterial activity against
Staphylococcus aureus was tested for all compounds using agar filter paper diffusion. Stemphytriol (
54) and alterlactone (
140), at 50 µg/disc, demonstrated inhibition zones with diameters of approximately 21 and 15 mm, respectively. Furthermore, the MIC value of compound
140 was 108.5 µM, and that of compound
54 was larger than 1265.8 µM, possibly due to its poor solubility. Ampicillin, with an MIC of 17.9 µM, was used as a positive control [
22].
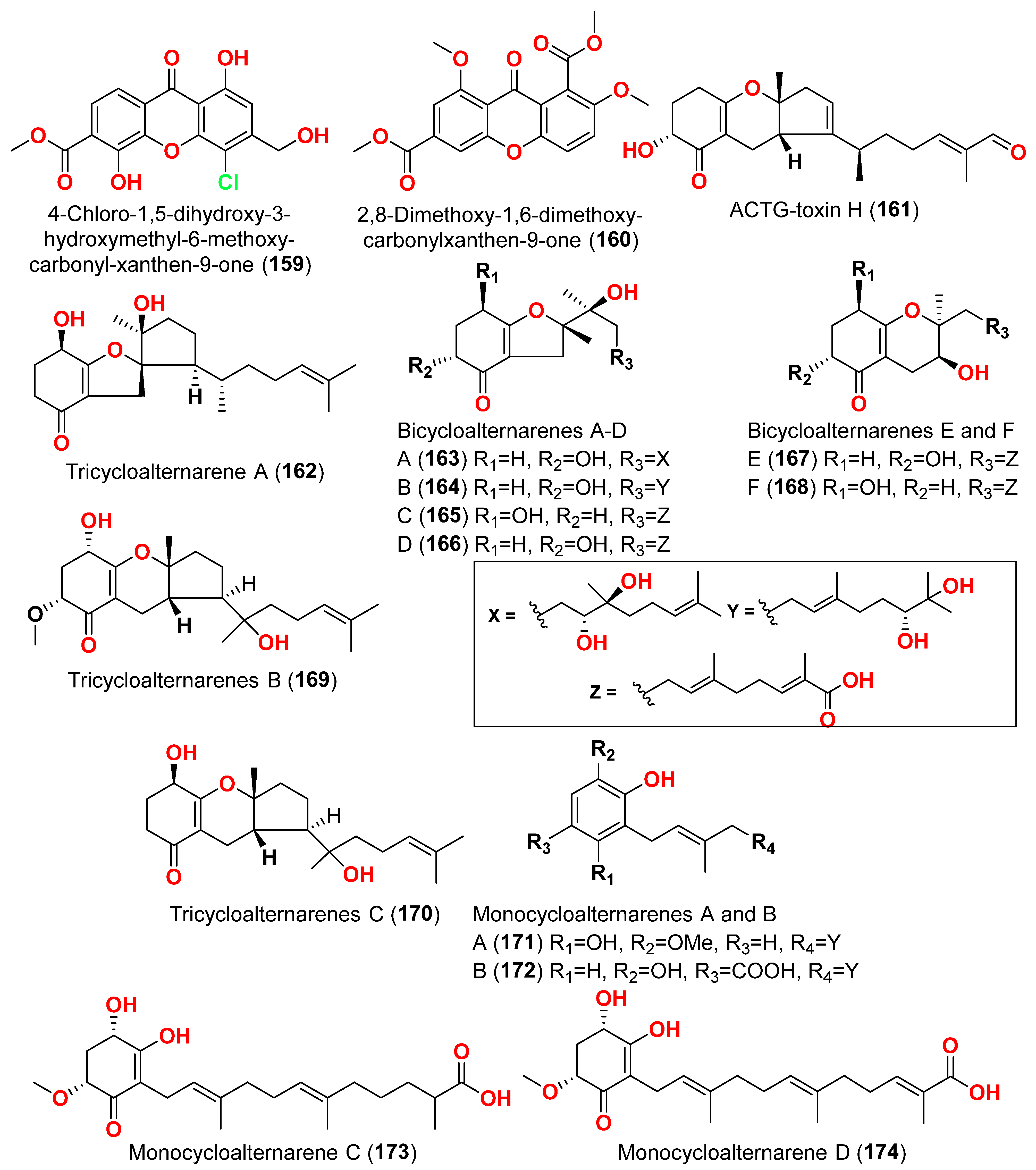
Tricycloalternarenes K (
144) and L (
145), two meroterpenoids, were obtained from the marine-derived fungus
Alternaria alternata ICD5-11, collected from the marine isopod
Ligia exotica, collected in Shandong Province, China [
43]. Compounds
144 and
145 were evaluated for antibacterial activity against
Staphylococcus aureus and
Bacillus subtilis using disk diffusion techniques. However, no activity was reported at 20 μg/disk [
43].
Phragamide A (
146), phragamide B (
147), altechromone A (
148), tenuazonic acid (
89), altenusin (
95), alternariol (
41), alternariol monomethyl ether (
138), altertoxin I (
52), altertoxin II (
88), and alterperylenol (
68) were purified from the fungus
Alternaria alternata 13A, which was obtained from
Thalassia hemprichii and
Phragmites australis marine plants from a saline lake in the Wadi El Natrun, Egypt [
44]. Only phragamide A (
146) demonstrated potential antibacterial activity against Gram-positive strains. Phragamide B (
147) revealed considerable effectiveness against
Candida albicans, but low effect against bacterial pathogens. Tenuazonic acid (
89) showed modest action against Gram-positive bacteria. Altenusin (
95) and alternariol (
41) exhibited comparable antibacterial efficacy against
S. aureus,
B. subtilis,
P. aeruginosa, and
C. albicans. Additionally, both alternariol monomethyl ether (
138) and altertoxin I (
52) demonstrated mild antibacterial effects against
S. aureus and
C. albicans. Altertoxin I (
52), altertoxin II (
88), and alterperylenol (
68) displayed minimal antibacterial action towards Gram-positive bacteria. This result is due to the absence of synergism in the isolated compounds compared to the entire fungal extract [
44].
The isolation and characterization of five polyketides alternariol (
41)—alternariol-9-methyl ether (
40), altertoxin I (
52), altertoxin II (
88), and tenuazonic acid (
89)—from the marine endophytic fungus
Alternaria sp. LV52, which was obtained from the Red Sea algae
Cystoseira tamariscifolia in Egypt, was reported [
29]. The antibacterial activity of the extract and corresponding compounds was tested against a panel of tested organisms. Based on paper disk analyses, both the fungal extract and tenuazonic acid (
89) have low to moderate efficacy against various microbiological pathogens, including
Pseudomonas aeruginosa,
Staphylococcus aureus,
Bacillus subtilis,
Candida albicans, and
Saccharomyces cerevisiae compared to gentamycin. While other compounds were ineffective against the microorganisms studied up to 25 μL/disk [
29].
Alternarialone A (
149), curvularin derivative, alternariol 4-methyl ether (
150), and alternariol (
41) were obtained from the crude extract of the mangrove-derived fungus
Alternaria longipes, isolated from the branches of
Kandelia candel at Guangxi, China [
45]. Through broth microdilution assay, all compounds were assessed for their antibacterial activity against the
Helicobacter pylori standard strain G27 and a clinically isolated BHKS159 strain. Alternariol 4-methyl ether and alternariol (
150 and
41) exhibited antibacterial activity against
H. pylori G27 with MIC values of 14.3 and 62 μM, respectively, and alternariol (
41) also exhibited antibacterial activity against
H. pylori BHKS159 with an MIC value of 62.0 μM, while the positive control metronidazole exhibited an MIC value of 11.6 μM. However, alternarialone A (
149) demonstrated no inhibitory impact on the two
H. pylori strains [
45].
Altermodinacid A (
93), which is an anthraquinone, was obtained from the fungus
Alternaria sp. X112 that was isolated from a marine fish,
Gadus macrocephalus, residing in Yangma Island, China [
31]. No quorum sensing (QS)-inhibitory activity against
Chromobacterium violaceum (MIC > 40 µg/well) by altermodinacid A (
93) was observed. Furthermore, no antibacterial activity (MIC > 4 µg/well) was detected against the Gram-positive bacteria
Bacillus subtilis and
Staphylococcus aureus, as well as against the Gram-negative bacteria
Escherichia coli and
Pseudomonas aeruginosa [
31].
Based on the results summarized in
Table 2, marine-derived
Alternaria species produce a structurally diverse array of metabolites with antibacterial, antifungal, and antiviral activities. Many metabolites with antimicrobial effects also display cytotoxicity, suggesting overlapping mechanisms of action. For instance, alternariol (
41) and alternariol monomethyl ether (
138), previously discussed for their cytotoxic effects, also inhibit several bacterial strains.
Among the most potent antibacterial agents are altersolanol C (
21), with MIC values of 0.62 μM against
E. coli and 1.25 μM against
V. parahaemolyticus, and macrosporin (
38), active against the same organisms with MICs of 2.3 μM and 5.0 μM, respectively [
14]. Alterporriol C (
26) exhibited comparable potency (MIC = 2.5 μM) and additional antiviral activity against the porcine reproductive and respiratory syndrome virus (PRRSV) with IC
50 = 39 μM [
14]. Other metabolites, such as alterporriol Q (
101) and (+)-α-S-alterporriol C (112), inhibited PRRSV with IC
50 = 22 μM and 8.7 μM, respectively [
14,
37], while tetrahydroaltersolanol C (
14) also showed antiviral activity (IC
50 = 65 μM) and broad antibacterial effects [
14].
Within the antiviral spectrum, alternariol-9-methyl ether-3-
O-sulphate (
39), alternariol-9-methyl ether (
40), and alternariol (
41) inhibited
B. cereus,
B. megaterium, and
E. coli, with IC
50 values of 147.7 μM, 118.3 μM, and 46.5 μM, respectively, against the HCV protease NS3–NS4A [
17]. Moderately active metabolites include alterporriol S (
31) (IC
50 = 101.4 μM) [
38], altenusin (
95) (MIC = 86.2–431 μM) [
18,
37,
44], and porric acid D (
111) (MIC = 347.2 μM) [
37]. Weakly active compounds such as stemphytriol (
54) (MIC > 1265.8 μM) and the highly oxygenated derivatives (
122–
124) (MIC = 1724–1970 μM) showed limited activity [
22,
41]. Finally, alternariol 4-methyl ether (
150) exhibited notable antibacterial effects against
Helicobacter pylori G27 and BHKS159 with MIC = 14.3 μM [
45].
The overall range of antimicrobial activity shows that only a small subset of marine
Alternaria metabolites display strong potency, with low MIC values between 0.6 and 15 μM, whereas most fall within the moderate range of 80–450 μM. Although this level of activity is considered modest in early drug discovery, several metabolites may act through unique mechanisms. For instance, alternariol (
41) and alternariol monomethyl ether (
138) inhibit methicillin-resistant
Staphylococcus aureus (MRSA) by disrupting bacterial cell division through topoisomerase inhibition [
46], while maintaining low cytotoxicity toward mammalian cells [
46]. Such features make them promising scaffolds for next-generation antibiotics targeting multidrug-resistant (MDR) pathogens [
46].
Conclusively, marine-derived
Alternaria species yield structurally diverse metabolites spanning a wide range of antimicrobial potencies. Quinone- and perylene-based scaffolds appear central to their activity, indicating clear structure–activity relationships. Compounds such as altersolanol C, macrosporin, and alterporriol C represent particularly promising antibacterial leads. Further studies should focus on testing these metabolites against MDR clinical isolates—including MRSA, vancomycin-resistant
Enterococcus (VRE), and multidrug-resistant
P. aeruginosa—to assess their therapeutic potential. Continued investigation into their biosynthetic pathways and molecular targets may ultimately support the development of novel antimicrobial agents from marine
Alternaria species [
14,
17,
18,
22,
37,
38,
41,
44,
45]. Compounds with proven antimicrobial activities are listed in
Table 2.
Table 2.
Reported compounds with proven antimicrobial activities.
Table 2.
Reported compounds with proven antimicrobial activities.
| Compound | Organism Tested | Biological Activity | Fungus Name | Host Organism | Reference |
|---|
Alternarosides A–C (4–6)
Alernarosin A (7) | Escherichia coli, Bacillus subtilis, Candida albicans | MIC = 70–400 µM | Alternaria raphanin THW-18 | Sediment | [9] |
| Xanalteric acid I (8) | E. coli, Klebsiella pneumoniae, Enterococcus faecium, Enterococcus cloacae, Staphylococcus aureus, Streptococcus pneumonia, Pseudomonas aeruginosa, Acinetobacter baumanii, Candida albicans, Candida krusei, Aspergillus faecalis, Aspergillus fumigatus | MIC = 343.4 µM (S. aureus) | Alternaria sp. JCM9.2 | Mangrove Sonneratia alba | [10] |
| Xanalteric acid II (9) | MIC = 686.8 µM (S. aureus) |
| Altenusin (95) | MIC = 107.7–431.0 µM |
| Active (S. aureus, B. subtilis, P. aeruginosa, C. albicans); Antibiofilm (B. subtilis) | A. alternata 13A | Marine plant Phragmites australis and Thalassia hemprichii | [44] |
| MIC = 86.2 μM (S. aureus) | Alternaria sp. | Seawater | [37] |
| Alternaramide (99) | Bacillus subtilis, Staphylococcus aureus | 8 and 13 mm at 400 µg/disk | Alternaria sp. SF-5016 | Shoreline sediment | [33] |
| A cyclic peptide (100) | Bacillus subtilis, Staphylococcus aureus | Antibacterial activity | Alternaria sp. SF-5016 | Marine deposit | [35] |
| Tetrahydroaltersolanol C (14) | E. coli, S. aureus, S. albus, Bacillus subtilis, B. cereus, M. tetragenus, M. luteus, V. parahemolyticus, V. anguillarum
The porcine reproductive and respiratory syndrome virus (PRRSV) | IC50 = 65 μM (PRRSV) | Alternaria sp. ZJ-2008003 | Sarcophyton sp. soft coral | [14] |
| Altersolanol C (21) | MIC = 0.62 and 1.25 μM (E. coli, V. parahemolyticus) |
| Macrosporin (38) | MIC = 2.3 and 5.0 μM (E. coli, V. parahemolyticus) |
| Alterporriol Q (101) | IC50 = 22 μM (PRRSV) |
| Alterporriol C (26) | MIC = 2.5 and 2.5 μM (E. coli, V. parahemolyticus); IC50 = 39 μM (PRRSV) |
| Pyrophen (103) | B. subtilis, S. aureus, S. viridochromogenes, E. coli, C. albicans, M. miehi, C.vulgaris, C. sorokiniana, S. subspicatus, R. solani; P. ultimum | 28 mm at 40 μg/disk (C. albicans) | Alternaria alternata D2006 | Soft coral, Denderonep-hthya hemprichi | [36] |
| Rubrofusarin B (104) | 12 mm at 40 μg/disk (C. albicans) |
| Aurasperone A (107) | 13 mm at 40 μg/disk (R. solani) |
| Porric acid D (111) | Staphylococcus aureus | MIC = 347.2 μM | Alternaria sp. | Seawater | [37] |
| Alterporriol S (31) | Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB) | IC50 = 101.4 µM | Alternaria sp. (SK11) | Mangrove Excoecaria agallocha | [38] |
| (+)-α-S-Alterporriol C (112) | IC50 = 8.7 µM |
| cyclo(l-leucyl-trans-4-hydroxy-l-prolyl-d-leucyl-trans-4-hydroxy-l-proline) (119) | G. graminis, R. cerealis, H. sativum, F. graminearum | MIC = 287.6–553.0 µM | Phomopsis sp. K38, Alternaria sp. E33 | Mangrove | [39] |
| Cyclo(d-Pro-l-Tyr-l-Pro-l-Tyr) (120) | C. albicans, G. graminis, Rhzioctonia cerealis, H. sativum, F. graminearum | MIC = 67.3–769.2 µM | Phomopsis sp. K38, Alternaria sp. E33 | Mangrove | [40] |
| Cyclo(Gly-l-Phe-l-Pro-l-Tyr) (121) | MIC = 53.8–538.7 µM |
| Alternariol-9-methyl ether-3-O-sulphate (39) | B. megaterium, Bacillus cereus, B. subtilis, S. aureus, E. cloacae, K. pneumoniae, E. coli, C. albicans, S. cerevisiae, A. niger
HCV protease NS3-NS4A | 10–17 mm at 50 μg/disk (B. cereus, B. megaterium, E. coli); IC50 = 147.7 μM (HCV NS3-NS4A) | A. alternata | Soft coral Litophyton arboreum | [17] |
| Alternariol-9-methyl ether (40) | 10–15 mm at 50 μg/disk (B. cereus, B. megaterium, E. coli); IC50 = 118.3 μM (HCV NS3-NS4A) |
| Alternariol (41) | 10–14 mm at 50 μg/disk (B. cereus, B. megaterium, E. coli); IC50 = 46.5 μM (HCV NS3-NS4A) |
| MRSA (clinical) | Inhibition of MRSA DNA topoisomerase at 31.0–62.0 μM | A. alternata | Mangrove | [16,46] |
| S. aureus, B. subtilis, P. aeruginosa, C. albicans | Active (S. aureus, B. subtilis, P. aeruginosa, C. albicans) | A. alternata 13A | Phragmites australis, Thalassia hemprichii | [44] |
| H. pylori G27, BHKS159 | MIC = 62.0 µM (H. pylori G27 and BHKS159) | Alternaria longipes | Mangrove, Kandeliacandel | [45] |
| (±)-(4R*,5S*,6S*)-3-Amino-4,5,6-trihydroxy-2-methoxy-5-methyl-2-cyclohexen-1-one (122) | E. coli, S. aureus | MIC = 1970.4 μM | Alternaria sp. R6 | Mangrove Myoporum bontioides | [41] |
| (±)-(4S*,5S*)-2,4,5-Trihydroxy-3-methoxy-4-methoxycarbonyl-5-methyl-2-cyclopenten1-one (123) | MIC = 1724.1 μM |
| Fischexanthone (124) | MIC > 1265.8 μM |
| Stemphytriol (54) | S. aureus | 21 mm at 50 µg/disk
MIC > 1265.8 μM | Alternaria sp. SCSIO41014 | Callyspongia sp. sponge | [22] |
| Alterlactone (140) | 15 mm at 50 µg/disk
MIC = 108.5 µM |
| Tenuazonic acid (89) | P. aeruginosa, S. aureus, B. subtilis, C. albicans, S. cerevisiae | 8–11 mm at 25 μL/disk | Alternaria sp. LV52 | Algae, Cystoseirata mariscifolia | [29] |
| Antimicrobial activity Gram-positive bacteria (S. aureus, B. subtilis); Gram-negative bacteria (E. coli, P. aeruginosa, K. pneumonia, P. vulgaris); yeast (C. albicans) | Moderate activity (Gram-positive strains); Antibiofilm; Gram-positive (70–80%); Gram-negative strains (40–60%) | A. alternata 13A | Marine plant Phragmites australis and Thalassia hemprichii | [44] |
| Phragamide A (146) | Antimicrobial activity, Gram-positive bacteria (S. aureus, B. subtilis); Gram-negative bacteria (E. coli, P. aeruginosa, K. pneumonia, P. vulgaris); yeast (C. albicans) | Moderate activity (P. aeruginosa, C. albicans, and Gram-positive strains); Antibiofilm, Gram-positive (70–80%); Gram-negative strains (40–60%) |
| Phragamide B (147) | Moderate activity (C. albicans); Antibiofilm; Gram-positive (70–80%); Gram-negative strains (40–60%) |
| Altechromone A (148) | | Antibiofilm, Gram-positive (70–80%), Gram-negative strains (40–60%) | | | |
Alternariol monomethyl ether (138)
Altertoxin I (52) | Weakly active (S. aureus, C. albicans) |
Altertoxin II (88)
Alterperylenol (68) | Weakly active (Gram-positive strains) |
| Alternariol 4-methyl ether (150) | H. pylori G27, BHKS159 | MIC = 14.3 µM (H. pylori G27) | Alternaria longipes | Mangrove, Kandeliacandel | [45] |
| Altermodinacid A (93) | Quorum sensing (QS)-inhibitory activity against C. violaceum | MIC > 40 µg/well | Alternaria sp. X112 | Marine fish Gadus macroceph-alus | [31] |
| B. subtilis, S. aureus, E. coli, P. aeruginosa | MIC > 4 µg/well |
2.3. Compounds with Antiparasitic Activities (Table 3)
Compounds evaluated for their antiparasitic activity are shown in
Figure 3,
Figure 7,
Figure 10 and
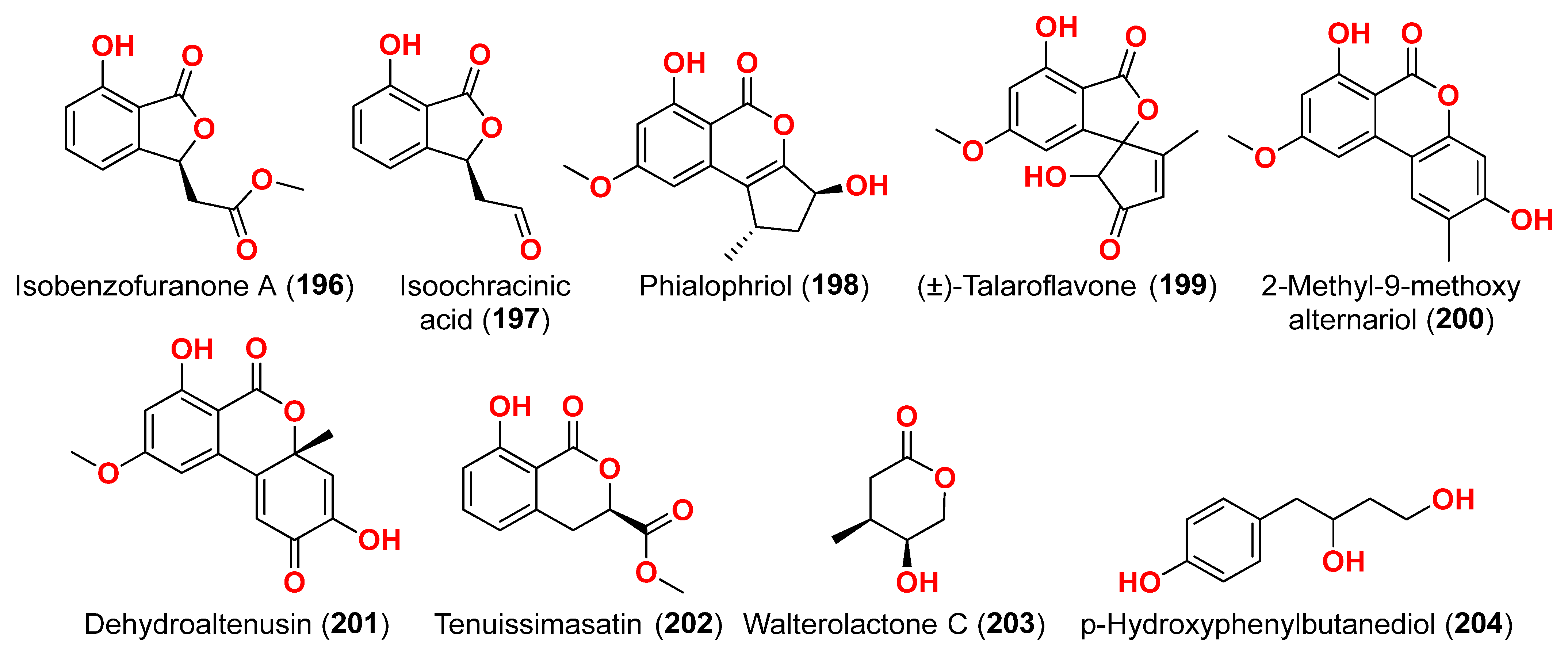
Figure 11. Two dimeric compounds of the alternariol class, (±)-alternarlactones A (
150) and B (
151), verrulactone B (
152), altenuisol (
141), alternariol (
41), 5-
O-methyl ether-3-hydroxyalternariol (
139), 4-methyl ether alternariol (
153), alterlactone (
140), altenuic acid II (
154), altenuic acid III (
155), 7-hydroxy-3-(2-hydroxypropyl)-5-methyl-isochromen-1-one (
156), 5′-methoxy-6-methyl-biphenyl-3,4,3′-triol (
142), and altenusin (
95) were separated from the fungus
Alternaria alternata P1210 obtained from halophyte
Salicornia sp. roots gathered from a salt marsh near Santa Pola, Spain [
47]. All isolated altenuisol derivatives were tested for their antiparasitic activities against
Trypanosoma cruzi,
Trypanosoma brucei rhodesiense,
Plasmodium falciparum, and
Leishmania donovani. All compounds, except
154–
156, showed inhibition towards
Trypanosoma or
Leishmania, indicating that antiparasitic actions require a large conjugated system with at least two aromatic rings. Monomers with a 2,3-dihydroxylphenyl group, such as alterlactone (
140), altenuisol (
141), 5′-methoxy-6-methyl-biphenyl-3,4,3′-triol (
142), and altenusin (
95) showed higher activity against
T. brucei rhodesiense (IC
50 < 10 µM) than compounds with a 3-hydroxylphenyl (
41 and
153) or 3,4-dihydroxylphenyl (
139) group. Structural dimerization, as seen in compounds
150–
152, limited the antiparasitic effect and led to the selective inhibition of
L. donovani and
P. falciparum. In contrast, altenuisol (
141) had a basic structure and broad-spectrum antiparasitic action [
47].
As shown above, several marine-derived compounds of the fungus
Alternaria displayed exceptional antiparasitic effects against
Trypanosoma brucei rhodesiense,
Trypanosoma cruzi,
Leishmania donovani, and
Plasmodium falciparum [
47]. The active metabolites belong mainly to the alternariol polyketide family, encompassing both monomeric and dimeric structures.
Altenuisol (
141) represents one of the most potent and broad-spectrum compounds with antiparasitic effects, with an IC
50 of 1.5 to 17.7 µM, demonstrating the strongest inhibition across multiple parasites. Likewise, verrulactone B (
152) demonstrated strong effects on
L. donovani and
P. falciparum, with IC
50 values of 2.4 and 13.5 µM, respectively. Further, (±)-alternarlactone A (
150) and (±)-alternarlactone B (
151) inhibited
L. donovani with IC
50 values of 4.7 and 8.9 µM, and
P. falciparum with IC
50 values of 5.9 and 9.7 µM. In addition, several of the monomeric phenolic compounds, altenusin (
95) and 5′-methoxy-6-methyl-biphenyl-3,4,3′-triol (
142) were highly active against
T. brucei rhodesiense, with IC
50 = 7.4 and 8.3 µM, respectively. Finally, IC
50 values of 7.2 and 11.7 µM were displayed by alterlactone (
140) against T
. brucei rhodesiense and
L. donovani, respectively [
47]. These results indicate that phenolic monomers containing extended conjugated systems and free hydroxyl groups represent key structural motifs for potent antiparasitic effects.
Moderately active compounds included alternariol (
41) (IC
50 of 15.4 µM against
L. donovani) and 5-
O-methyl ether-3-hydroxyalternariol (
139) (IC
50 of 7.5 µM against
L. donovani and 13.6 µM against
T. brucei rhodesiense). In contrast, 4-methyl ether alternariol (
153) showed a reduced effect, with an IC
50 of 31.1 µM against
P. falciparum, emphasizing that methylation tends to reduce the antiparasitic potency. As seen in (±)-alternarlactones A and B (
150–
151), structural dimerization resulted in reduced selectivity in comparison to their monomeric compounds. These findings imply that the antiparasitic effect depends greatly on molecular planarity and the existence of available phenolic hydroxyl functionalities, which accelerate redox and membrane interactions with parasitic targets [
47].
Some compounds, including altenuic acids II (154) and III (155) and 7-hydroxy-3-(2-hydroxypropyl)-5-methyl-isochromen-1-one (156), showed insignificant antiparasitic effect in the tested models, suggesting that the absence of conjugated aromatic moiety or phenolic groups is associated with reduced potency. This supports the conclusion that antiparasitic activity within Alternaria-derived compounds is closely coupled to their isocoumarin and phenolic core structures.
In conclusion,
Alternaria species afford a distinct array of phenolic polyketides with contrasting degrees of antiparasitic effect. The most potent compounds, such as altenuisol (
141), verrulactone B (
152), (±)-alternarlactones A–B (
150–
151), altenusin (
95), and alterlactone (
140), exhibit low µM-IC
50 values (<10 µM), rivaling known antiparasitic agents in in vitro potency. Moderately active secondary metabolites, including alternariol-type compounds, demonstrate activity in the 10–30 µM range and stay of pharmacological interest due to their broad spectrum and low cytotoxicity. The structure–activity relationships study indicate that extended aromatic conjugation and free hydroxyl functionalities are fundamental for activity, while methylation or dimerization reduces the potency. Overall, these results emphasize the potential of marine-derived
Alternaria species as a valuable source of lead compounds for antiparasitic drug discovery, warranting further studies on mechanisms of action, selectivity, and in vivo efficacy [
47].
Table 3 displays compounds with proven antiparasitic activities.
Table 3.
Compounds with proven antiparasitic activities.
Table 3.
Compounds with proven antiparasitic activities.
| Compound | Organism Tested | Biological Activity | Fungus Name | Host Organism | Reference |
|---|
| (±)-Alternarlactone A (150) | Antiparasitic activity
T. brucei rhodesiense, T. cruzi, L. donovani, P. falciparum | IC50 = 4.7, 5.9 µM (L. donovani, P. falciparum) | Alternaria alternata P1210 | The halophyte Salicornia sp. | [47] |
| (±)-Alternarlactone B (151) | IC50 = 8.9, 9.7 µM (L. donovani, P. falciparum) |
| Verrulactone B (152) | IC50 = 2.4, 13.5 µM (L. donovani, P. falciparum) |
| Altenuisol (141) | IC50 = 1.5–17.7 µM |
| Alternariol (41) | IC50 = 15.4 µM (L. donovani) |
5-O-Methyl ether-3-hydroxy-
alternariol (139) | IC50 = 7.5 µM (L. donovani); IC50 = 13.6 µM (T. brucei rhodesiense) |
| 4-Methyl ether alternariol (153) | IC50 = 31.1 µM (P. falciparum) |
| Alterlactone (140) | IC50 = 11.7 µM (L. donovani); IC50 = 7.1 µM (T. brucei rhodesiense) |
| 5′-Methoxy-6-methyl-biphenyl-3,4,3′-triol (142) | IC50 = 8.3 µM (T. brucei rhodesiense) |
| Altenusin (95) | IC50 = 7.4 µM (T. brucei rhodesiense) |
2.4. Compounds with Antioxidant and Free Radical Scavenging Activity (Table 4)
Compounds evaluated for their antioxidant and free radical scavenging effects are displayed in
Figure 1,
Figure 3,
Figure 4,
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 10,
Figure 11 and
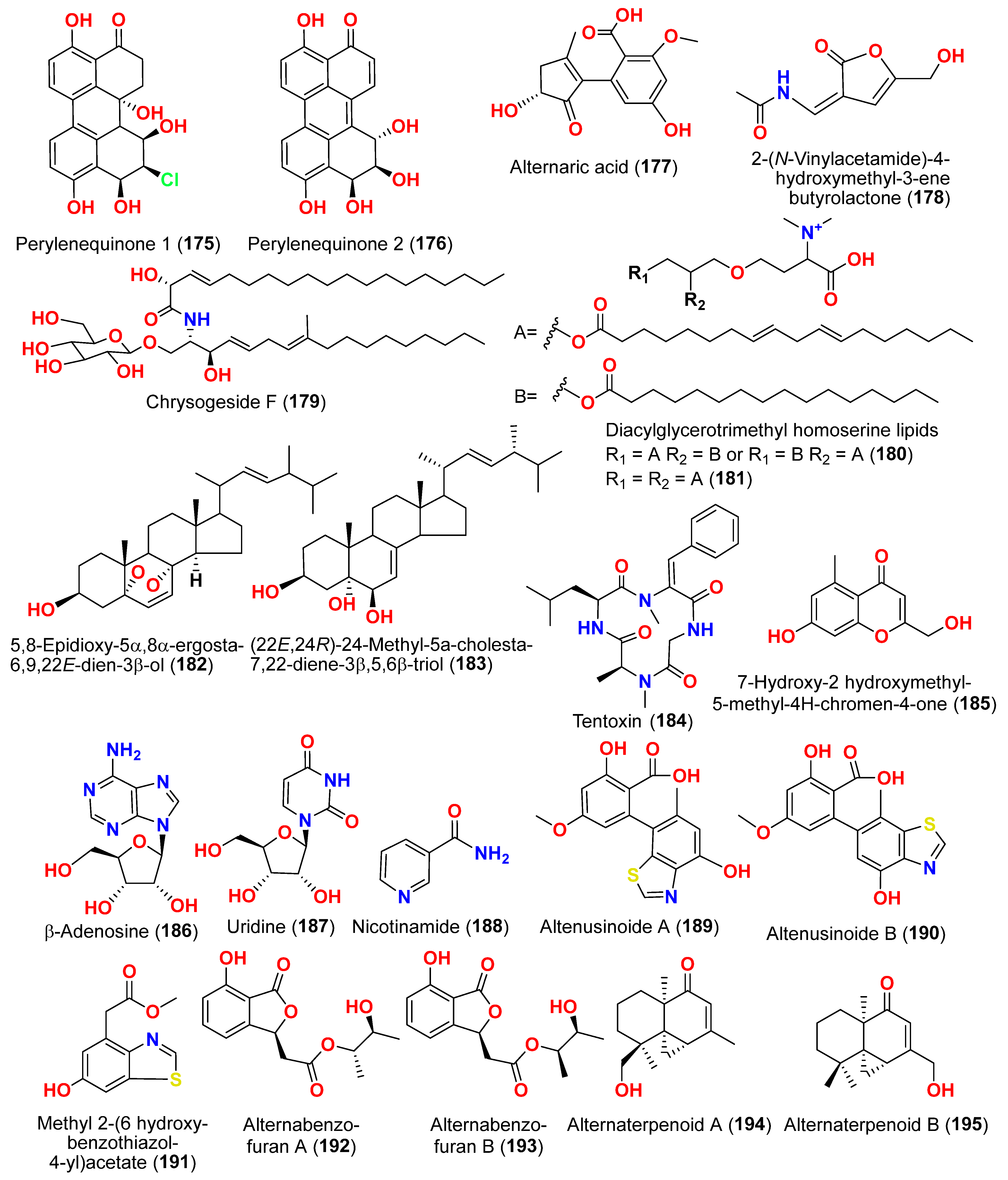
Figure 12. Marine fungi serve as a source of natural antioxidant active substances with significant growth potential. Investigation of the fungus
Alternaria raphanin, from sediment collected in Qingdao, China, afforded three cerebrosides, alternarosides A–C (
4–
6), and a diketopiperazine alkaloid, alternarosin A (
7) [
9]. The compounds were evaluated for their DPPH radical scavenging activity and did not show any activity (IC
50 > 500 μM) [
9].
The fungus
Alternaria sp., which was isolated from a marine sponge collected in China, afforded two polysaccharides, JJY-W (
157) and JJY-S (
158) [
48]. JJY-W (
157) consisted mainly of galactose and glucose, along with a trace quantity of mannose. JJY-S (
158) was mostly composed of mannose and glucose, with a trace of galactose. JJY-W (
157) had 46% total sugar and was free of uronic acid. JJY-S (
158) had 52% total sugar and 1.94% uronic acid. JJY-W (
157) had a greater variety of proteins than JJY-S (
158). The radical scavenging capabilities of compounds
157 and
158 against DPPH-free radicals and hydroxyl radicals were determined. Both compounds showed exceptional antioxidant activity. Moreover, compound
157 had a greater capacity for scavenging DPPH free radicals, while compound
158 had a greater capacity for scavenging hydroxyl radicals [
48].
Three resveratrol derivatives, resveratrodehydes A–C (
44–
46), were isolated from the fungus
Alternaria sp. R6, which was discovered in the mangrove plant
Myoporum bontioides located in Guangdong Province, China [
18]. Compounds
44 and
46 exhibited moderate antioxidant properties, as determined by the DPPH radical scavenging assay. The IC
50 values of resveratrodehydes A–C (
44–
46) for DPPH radical scavenging activity were determined to be 447.6, >900, and 572.6 μM, respectively. These values were relatively higher compared to the IC
50 value of the positive control resveratrol (70.2 μM) [
18].
Racemic mixtures of cyclohexenone and cyclopentenone derivatives, namely (±)-(4
R*,5
S*,6
S*)-3-amino-4,5,6-trihydroxy-2-methoxy-5-methyl-2-cyclohexen-1-one (
122) and (±)-(4
S*,5
S*)-2,4,5-trihydroxy-3-methoxy-4-methoxycarbonyl-5-methyl-2-cyclopenten1-one (
123), as well as two derivatives of xanthone, 4-chloro-1,5-dihydroxy-3-hydroxymethyl-6-methoxycarbonyl-xanthen-9-one (
159) and 2,8-dimethoxy-1,6-dimethoxycarbonyl-xanthen-9-one (
160), were obtained from mangrove-associated fungus
Alternaria sp. R6, derived from marine semi-mangrove plant
Myoporum bontioides A collected from Guangdong Province, China [
41]. The scavenging activities of
122 and
123 towards ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)] were found to be potent, with EC
50 values of 8.1 and 16.0 μM, respectively, surpassing the activity of ascorbic acid (EC
50 = 17.1 μM). On the other hand, compounds
159 and
160 did not exhibit any antioxidant activities (EC
50 > 500 μM) [
41].
The fungus
Alternaria sp. SP-32 was obtained initially from a sponge collected from the South China Sea [
20]. Study of the fungus revealed the extracellular polysaccharide, AS2-1 (
48). The scavenging ability of AS2-1 (
48) on DPPH and hydroxyl radicals was assessed and compared with those of ascorbic acid. The scavenging ability of
48 was concentration-dependent: at 36.4 µM, the effect on DPPH and hydroxyl radicals were 16.7%, and 19.2%, respectively. Similarly, the scavenging effect at a concentration of 328.4 µM was up to 90.5% on DPPH and like that of ascorbic acid on hydroxyl radicals. The EC
50 values of
48 on DPPH and hydroxyl radicals were approximately 124 and 153.2 µM, respectively. However, less scavenging activity was observed with AS2-1 (
48) than with ascorbic acid [
20].
The fungus
Alternaria sp. SCSIOS02F49, which was isolated from a sponge,
Callyspongia sp., in Guangdong, China, afforded altenusin (
95), 5′-methoxy-6-methyl-biphenyl-3,4,3′-triol (
142), and (
S)-alternariphent A1 (
130) [
49]. All substances were examined for their ability to scavenge DPPH free radicals. Compounds
95 and
142 exhibited significant DPPH free radical scavenging activity with IC
50 values of 10.7 µM and 100.6 µM, respectively [
49].
The fungus
Alternaria longipes, isolated from the branches of
Kandelia candel in Guangxi, China, provided one chromanone derivative—alterchromone A (
83)—and four curvularin-type macrolides—curvularin (
84), 11-β-methoxycurvularin (
85), β,γ-dehydrocurvularin (
86), and α,β-dehydrocurvularin (
87) [
28]. The 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging technique was used to evaluate the antioxidant capacities of compounds
83–
87. Only compound
83 displayed DPPH scavenging activity, with an IC
50 value of 160.8 μM, whereas the positive control ascorbic acid exhibited an IC
50 of 34.0 μM [
28].
Alternolides A–C (
90–
92), alternariol (
41), alternariol 5-
O-methyl ether (
98), 3′-hydroxyalternariol-5-
O-methyl ether (
139), alternariol-1′-hydroxy-9-methyl ether (
215), altenuisol (
141), 1-deoxyrubralactone (
133), and phialophoriol (
216) were purified and identified from the marine-derived fungus
Alternaria alternata LW37, which was isolated from a marine sediment [
30]. The compounds were screened for their DPPH scavenging activity. Compounds
139 and
215 exhibited excellent DPPH antioxidant scavenging abilities, with IC
50 values of 83.9 and 23.6 µM, respectively, while the positive control, ascorbic acid, had an IC
50 value of 23.7 µM [
30].
In summary, several compounds with antioxidant and radical scavenging effects have been reported from marine-derived
Alternaria, including macrolides, phenolic polyketides, cyclohexenones, and polysaccharides. The compounds were mainly assessed through DPPH, ABTS, and hydroxyl assays, and exhibited a broad range of potency, depending on structural motifs and the degree of aromatic conjugation. The cyclohexenone derivatives (±)-(4
R*,5
S*,6
S*)-3-amino-4,5,6-trihydroxy-2-methoxy-5-methyl-2-cyclohexen-1-one (
122) and (±)-(4
S*,5
S*)-2,4,5-trihydroxy-3-methoxy-4-methoxycarbonyl-5-methyl-2-cyclopenten-1-one (
123), which displayed strong ABTS radical scavenging with EC
50 of 8.1 and 16.0 μM, exceeding that of ascorbic acid (EC
50 = 17.1 μM), represent the most potent compounds [
41]. Equally, altenusin (
95) and alternariol-1′-hydroxy-9-methyl ether (
215) displayed high DPPH radical scavenging with IC
50 values of 10.7 and 23.6 μM, respectively, comparable to ascorbic acid (IC
50 = 23.7 μM) [
30,
49]. These findings indicate that para- and ortho-hydroxylated aromatic moieties foster single-electron transfer and hydrogen atom transfer, leading to stabilizing phenoxyl radicals through conjugation and enhancing redox reactivity.
With IC
50 values of 80 and 160 μM, alterchromone A (
83), 3′-hydroxyalternariol-5-
O-methyl ether (
139), and 5′-methoxy-6-methyl-biphenyl-3,4,3′-triol (
142) represent moderately effective compounds [
28,
30,
49]. The polysaccharide AS2-1 (
48) demonstrated moderate DPPH and hydroxyl radical scavenging with EC
50 = 124 and 153 μM, while JJY-W (
157) and JJY-S (
158) showed complementary effects, with JJY-S more active against hydroxyl radicals and JJY-W more active against DPPH [
20,
48]. Their antioxidant mechanisms are likely dependent on hydrogen-donating and metal ion chelation pathways, facilitated by uronic acid and protein residues that stabilize radicals.
In contrast, weak or inactive compounds included resveratrodehydes A–C (
44–
46), with IC
50 values of 447.6, >900, and 572.6 μM, respectively, and the xanthone derivatives (
159) and (
160), both showing EC
50 > 500 μM [
18,
41]. The diminished activity of these compounds is attributed to halogen substitution or methylation, which hinders electron delocalization and lowers hydrogen-donating ability.
Mechanistic insights suggest that phenolic compounds modulate oxidative signaling and quench radicals directly. For example, alternariol (
41) and altenusin (
95) reduce COX-2 and iNOS by suppressing the expression of ROS-induced activation of NF-κB and Nrf2 pathways, and thereby mitigating oxidative inflammation [
25,
49]. These dual antioxidant and anti-inflammatory mechanisms support their role as redox-regulating agents.
Finally, compounds from
Alternaria display a wide range of antioxidant effectiveness, from highly active, low-μM phenolics to moderately acting polysaccharides. Altenusin (
95), alternariol-1′-hydroxy-9-methyl ether (
215), and cyclohexenones (
122–
123) are the most potent candidates, competing with the activity of ascorbic acid, while hydroxyl-rich polysaccharides exhibit complementary radical scavenging through hydrogen donation and chelation. These results emphasize
Alternaria as a vital source of both small-molecule and macromolecular antioxidant effects, through the combined mechanisms of biological redox modulation and chemical quenching [
18,
20,
25,
28,
30,
41,
48,
49].
Table 4 illustrates compounds with proven antioxidant effects.
Table 4.
Reported compounds with antioxidants and free radical scavenging activities.
Table 4.
Reported compounds with antioxidants and free radical scavenging activities.
| Compound | Applied Assay | Biological Activity | Fungus Name | Host Organism | Reference |
|---|
| JJY-W (157) | DPPH free radical and hydroxyl radical scavenging activity | More scavenging capacity on DPPH free radical | Alternaria sp. | Sponge | [48] |
| JJY-S (158) | More hydroxyl radical scavenging capacity |
| Resveratrodehyde A (44) | DPPH scavenging
activity | IC50 = 447.6 μM | Alternaria sp. R6 | Mangrove Myoporum bontioides | [18] |
| Resveratrodehyde C (46) | IC50 = 572.6 μM |
| (±)-(4R*,5S*,6S*)-3-Amino-4,5,6-trihydroxy-2-methoxy-5-methyl-2-cyclohexen-1-one (122) | ABTS radical scavenging activity | EC50 = 8.1 μM | Alternaria sp. R6 | Mangrove Myoporum bontioides | [41] |
| (±)-(4S*,5S*)-2,4,5-Trihydroxy-3-methoxy-4-methoxycarbonyl-5-methyl-2-cyclopenten1-one (123) | EC50 = 16.0 μM |
| AS2-1 (48) | DPPH and hydroxyl
radical scavenging
activity | EC50 = 124 μM (DPPH)
EC50 = 153.2 μM (Hydroxyl radicals) | Alternaria sp. SP-32 | Unspecified sponge | [20] |
| Altenusin (95) | DPPH scavenging
activity | IC50 = 10.7 µM (DPPH) | Alternaria sp. SCSIOS02F49 | Sponge,
Callyspongia sp. | [49] |
| 5′-Methoxy-6-methyl-biphenyl-3,4,3′-triol (142) | IC50 = 100.6 µM (DPPH) |
| Alterchromone A (83) | DPPH scavenging
activity | IC50 = 160.8 μM | Alternaria longipes | Mangrove Kandelia candel | [28] |
| 3′-hydroxyalternariol-5-O-methyl ether (139) | DPPH scavenging
activity | IC50 = 83.9 µM | Alternaria alternata LW37 | Sediment | [30] |
| Alternariol-1′-hydroxy-9-methyl ether (215) | IC50 = 23.6 µM |
2.5. Compounds with Anti-Inflammatory Activity (Table 5)
Compounds evaluated for their anti-inflammatory effects are displayed in
Figure 7,
Figure 9,
Figure 10,
Figure 12,
Figure 13,
Figure 14 and
Figure 15. Alternaramide (
99) was isolated from the marine-derived fungus
Alternaria sp. SF-5016, which was obtained from a shoreline sediment sample in the Masan Bay region of Korea [
33]. Compound
99 weakly inhibited protein tyrosine phosphatase 1B (PTP1B) activity by 49% at 255.1 µM [
33].
Alternaramide (
99), which was purified from the fungus
Alternaria sp. SF-5016 extract, showed a significant decrease in LPS-stimulated RAW264.7 and BV2 cells, mRNA and protein levels of Toll-like receptor 4 (TLR4), and myeloid differentiation primary response gene 88 (MyD88) [
50]. Multiple TLR4-mediated inflammatory pathways were found to be affected by alternaramide (
99), indicating its potential to treat inflammatory and neuro-inflammatory diseases [
50].
ACTG-toxin H (AH) (
161) was obtained from a sponge-derived fungus
Alternaria alternata sp. tzp-11, which was gathered in China [
51]. The molecular mechanism underlying the anti-inflammatory properties of compound
161 was investigated. Interleukin-6, IL-1b, inducible nitric oxide synthase, cyclooxygenase-2 expression, and nitric oxide generation were reduced by compound
161 treatment in a dose-dependent manner when triggered by lipopolysaccharide (LPS). Additionally,
161 prevented the activation of P38 MAPK and Akt by LPS in RAW264.7 cells. According to electrophoretic mobility shift assays (EMSAs), compound
161 reduced the LPS-induced nuclear factor-jB (NFjB) DNA-binding activity. The transfection of toll-like receptor 4 (TLR4) increased LPS-induced NFjB transcription activity in 293T cells, determined by a transfection test and evaluation of an NFjB-sensitive promoter region. In TLR4-transfected cells, compound
161 dramatically inhibited LPS-induced NFjB activation. The anti-inflammatory properties of
161 resulted from inhibiting pro-inflammatory cytokines and enzyme production through the TLR4/NFjB signaling pathway. RAW264.7 macrophages exhibit reduced release of IL-1b, IL-6, iNOS, COX-2, and NO due to LPS-induced Akt and p38 MAPK activation [
51].
The fungus
Alternaria sp. JJY-32 was isolated from the sponge
Callyspongia sp. collected on the coast of Hainan Island, China [
52]. Investigation of the fungus revealed fifteen meroterpenoids, including tricycloalternarene A (
162), bicycloalternarenes A–F (
163–
168), tricycloalternarenes B and C (
169 and
170), ACTG-Toxins D and H (
62 and
161), and monocycloalternarenes A–D (
171–
174). The NF-κB inhibitory activities in the RAW264.7 cells of these compounds were evaluated. Compounds
162–
164 exhibited weak to moderate inhibition, with IC
50 values ranging from 52 to 85 μM, while compounds
62,
161, and
167–
172 showed IC
50 values from 39 to 76 μM compared to the positive control PDTC, which had an IC
50 value of 3 μM [
52].
The fungus
Alternaria sp. NH-F6 was identified from South China Sea deep sediment samples [
53]. Its ethyl acetate extract produced two perylenequinones—1 and 2 (
175 and
176)—alternaric acid (
177), 2-(
N-vinylacetamide)-4-hydroxymethyl-3-ene butyrolactone (
188), and a cerebroside, chrysogeside F (
179), together with alternarienonic acid (
94), talaroflavone (
210), alternariol (
41), alternariol 5-
O-methyl ether (
98), 4′-epialtenuene (
97), altenuene (
96), diacylglycerotrimethyl homoserine lipids (
180 and
181), 5,8-epidioxy-5α,8α-ergosta-6,22
E-dien-3β-ol (
27), 5,8-epidioxy-5α,8α-ergosta-6,9,22
E-dien-3β-ol (
182), (22
E,24
R)-24-methyl-5α-cholesta-7,22-diene-3β,5,6β-triol (
183), altenusin (
95), tentoxin (
184), tricycloalternarene A (
162), 2,5-dimethyl-7-hydroxychromone (
143), 7-hydroxy-2-hydroxymethyl-5-methyl-4H-chromen-4-one (
185), β-adenosine (
186), uridine (
187), and nicotinamide (
188). All compounds were assessed for their inhibitory effect on BRD4 protein. Compound
176 exhibited a potent inhibition rate of 88.1%. Compound
185 had a modest inhibition rate of 57.7%, while other compounds were below 35.0% at a concentration of 10 µM [
53].
The fungus
Alternaria sp. SCSIOS02F49—isolated from a sponge,
Callyspongia sp., in Guangdong, China—afforded two altenusin derivatives and thiazole hybrids—altenusinoide A (
189), altenusinoide B (
190), and methyl 2-(6-hydroxybenzothiazol-4-yl)acetate (
191)—altenusin (
95), 5′-methoxy-6-methyl-biphenyl-3,4,3′-triol (
142), and (
S)-alternariphent A1 (
130) [
49]. All substances were examined for their ability to inhibit COX-2. Compound
142 showed COX-2 inhibitory activity with an IC
50 value of 9.5 µM [
49].
The marine-derived fungus
Alternaria sp. 5102, was isolated from an Actiniae (sea anemone) collected on the Laishizhou island, Guangdong Province, China [
54]. Alternabenzofurans A and B (
192 and
193), alternaterpenoids A and B (
194 and
195), together with the polyketides, isobenzofuranone A (
196), isoochracinic acid (
197), (
R)-1,6-dihydroxy-8-methoxy-3a-methyl-3,3a-dihydrocyclopenta[c]isochromene-2,5-dione (
132), dihydroaltenuene A (
136), phialophriol (
198), (±)-talaroflavone (
199), alternariol-9-
O-methyl ether (
40), alternariol (
41), 2-methyl-9-methoxy alternariol (
200), 3′-hydroxyalternariol 5-
O-methyl ether (
139), alternariol-1′-hydroxy-9-methyl ether (
215), dehydroaltenusin (
201), alteryulactone (
140), tenuissimasatin (
202), 5′-methoxy-6-methyl-biphenyl-3,4,3′-triol (
142), altenusin (
95), 2,5-dimethyl-7-hydroxychromone (
143), and walterolactone C (
203) were purified and identified from the extract of this fungus [
52]. The compounds were tested for their ability to reduce the production of nitric oxide (NO) in RAW264.7 cells stimulated with lipopolysaccharide (LPS). Indomethacin was used as a positive control with an IC
50 of 35.8 µM in the Griess assay. Fourteen compounds showed higher anti-inflammatory efficacy than indomethacin. Among them, compounds
194,
132,
198, and
139 exhibited considerable inhibition of nitric oxide (NO) generation, with IC
50 values below 10 µM (ranging from 1.3 to 5.9 µM). Compounds
196 and
215 exhibited moderate anti-inflammatory activities with IC
50 values of 41.1 and 39.0 µM, respectively [
54]. The marine-derived fungus
Alternaria alternata 114-1G afforded
p-hydroxyphenylbutanediol (
204) with an anti-inflammatory effect [
55].
As shown from the discussion above, different marine-derived
Alternaria species afforded several compounds with prominent anti-inflammatory effects, mainly through the inhibition of pro-inflammatory mediators, including cytokines (TNF-α, IL-6), nitric oxide (NO), and enzymes including iNOS and COX-2, in LPS-stimulated microglial (BV2) cells or macrophages (RAW264.7). The NF-κB and TLR4 signaling pathways include other targets, suggesting diverse mechanisms of immunomodulation. Phialophriol (
198) presented as the most potent compound, with an IC
50 value of 1.3 μM, followed by alternaterpenoid A (
194), (
R)-1,6-dihydroxy-8-methoxy-3a-methyl-3,3a-dihydrocyclopenta[c]isochromene-2,5-dione (
132), and 3′-hydroxyalternariol 5-
O-methyl ether (
139), with IC
50 values of 2.4, 5.2, and 5.9 μM, respectively [
49,
54]. Further, as a potent COX-2 inhibitor, 5′-methoxy-6-methyl-biphenyl-3,4,3′-triol (
142) showed an IC
50 value of 9.5 μM [
49]. These values highlight the pharmacological promise of these
Alternaria-derived metabolites as anti-inflammatory leads.
Several compounds displayed moderate anti-inflammatory activity in cell-based assays, with IC
50 values ranging from 14.9 to 26.3 μM, including walterolactone C (
203), alteryulactone (
140), alternariol (
41), 2,5-dimethyl-7-hydroxychromone (
143), alternabenzofuran B (
193), (±)-talaroflavone (
199), tenuissimasatin (
202), 2-methyl-9-methoxyalternariol (
200), altenusin (
95), and alternariol-1′-hydroxy-9-methyl ether (
215), with IC
50 values of 14.9, 16.2, 16.6, 17.3, 18.7, 23.9, 24.5, 25.4, and 26.3 μM, respectively [
49,
54,
55]. Also, ACTG-toxin D and H (
62,
161), tricycloalternarenes A, B, and C (
162,
169,
170), bicycloalternarenes A–D (
163–
166), and monocycloalternarenes A–D (
171–
174) inhibited NF-κB activation in LPS-stimulated RAW264.7 macrophages, with IC
50 values ranging from 39 to 85 μM [
52], indicating moderate but biologically relevant effects.
Alternaramide (
99), which inhibited PTP1B by 49% at 255.1 μM, represents example of weakly active candidate [
33].
Mechanistic studies suggest that many of these compounds achieve their anti-inflammatory effect by affecting the NF-κB signaling cascade, thereby lowering the downstream expression of COX-2 and iNOS. For example, COX-2 and iNOS expression in LPS-stimulated macrophages by blocking NF-κB nuclear translocation was significantly suppressed by alternariol (
41) [
25]. Similarly, the inhibition of TLR4/NF-κB activation in microglial and macrophage cells by ACTG-toxins D and H (
62 and
161) resulted in the reduced production of TNF-α and IL-1β [
51]. These results indicate that perylenequinone-type and chromone-based compounds could serve as scaffolds for the development of anti-inflammatory or neuroprotective leads.
In conclusion, the anti-inflammatory effects of marine-
Alternaria compounds reach a wide range of potencies. Compounds such as phialophriol (
198), alternaterpenoid A (
194), and hydroxylated isocoumarins display strong inhibition at sub-10 μM concentrations, exceeding the potency of standard drugs. Compounds with moderate effect, such as alternariol derivatives, present consistent μM inhibition of NO or cytokine production and are biologically relevant. Most of them act by suppressing the COX-2/iNOS or NF-κB pathways, underscoring their therapeutic potential. Further investigations into their selectivity, cytotoxicity, and in vivo anti-inflammatory efficacy are warranted to validate their potential as lead molecules for anti-inflammatory drug discovery [
32,
49,
51,
52,
53,
54,
55].
Table 5 displays reported compounds with proven anti-inflammatory activities.
Table 5.
Reported compounds with anti-inflammatory activities.
Table 5.
Reported compounds with anti-inflammatory activities.
| Compound | Applied Assay | Biological Activity | Fungus Name | Host Organism | Reference |
|---|
| Alternaramide (99) | Inhibition of protein tyrosine phosphatase 1B (PTP1B) | 49% inhibition at 255.1 µM | Alternaria sp. SF-5016 | Shoreline
sediment
sample | [32] |
| Anti-inflammatory effect in LPS-stimulated RAW264.7 and BV2 cells | Inhibits LPS-stimulated expression of TLR4 and MyD88 | [50] |
| ACTG-toxin H (AH) (161) | Pro-inflammatory cytokines and enzyme production by the TLR4/NFjB signaling pathway | Significantly inhibits LPS-induced NFjB activation in TLR4-transfected cells | Alternaria sp. tzp-11 | Sponge | [51] |
Tricycloalternarene A (162)
Bicycloalternarenes A–D (163–166) | NF-κB inhibitory activities in RAW264.7 cells | IC50 = 52–85 μM | Alternaria sp. JJY-32 | Sponge
Callyspongia sp. | [52] |
Tricycloalternarenes B, C (169, 170)
ACTG-toxin D, H (62, 161)
Monocycloalternarenes A–D (171–174) | IC50 = 39–76 μM |
| Perylenequinone 1 (175) | Inhibition of BRD4 protein | 57.7% inhibition rate | Alternaria sp. NH-F6 | Deep-sea
sediment | [53] |
| Perylenequinone 2 (176) | 88.1% inhibition rate |
| Alternaric acid (177); 2-(N-Vinylacetamide)-4-hydroxymethyl-3-ene -butyrolactone (188); Chrysogeside F (179); Alternarienonic acid (94); Talaroflavone (210); Alternariol (41); Alternariol 5-O-methyl ether (98); 4′-Epialtenuene (97); Altenuene (96); Diacylglycerotrimethyl Homoserine lipids (180, 181); 5,8-Epidioxy-5α,8α-ergosta-6,22E-dien-3β-ol (27); 5,8-Epidioxy-5α,8α-ergosta-6,9,22E-dien-3β-ol (182); (22E,24R)-24-Methyl-5α-cholesta-7,22-diene-3β,5,6β-triol (183); Altenusin (95); Tentoxin (184); Tricycloalternarene A (162); 2,5-Dimethyl-7-hydroxychromone (143); 7-Hydroxy-2-hydroxymethyl-5-methyl-4H-chromen-4-one (185); β-Adenosine (186); Uridine (187); Nicotinamide (188) | <35.0% inhibition rate |
| 5′-Methoxy-6-methyl-biphenyl-3,4,3′-triol (142) | COX-2 inhibition | IC50 = 9.5 µM (COX-2) | Alternaria sp. SCSIOS02F49 | Sponge,
Callyspongia sp. | [49] |
| Alternabenzofuran B (193) | Inhibition of NO production by lipopolysaccharide (LPS) activated in RAW264.7 cells | IC50 = 18.7 µM | Alternaria sp. 5102 | Actiniae | [54] |
| Alternaterpenoid A (194) | IC50 = 2.4 µM |
| Isobenzofuranone A (196) | IC50 = 41.1 µM |
| (R)-1,6-Dihydroxy-8-methoxy-3a-methyl-3,3a dihydrocyclopenta [c]isochromene-2,5-dione (132) | IC50 = 5.2 µM |
| Phialophriol (198) | IC50 = 1.3 µM |
| (±)-Talaroflavone (199) | IC50 = 23.9 µM |
| Alternariol-9-O-methyl ether (40) | IC50 = 39.0 µM |
| Alternariol (41) | IC50 = 16.6 µM |
| 2-Methyl-9-methoxy alternariol (200) | IC50 = 24.5 µM |
| 3′-Hydroxyalternariol 5-O-methyl ether (139) | IC50 = 5.9 µM |
| Alternariol-1′-hydroxy-9-methyl ether (215) | IC50 = 26.3 µM |
| Alteryulactone (140) | IC50 = 16.2 µM |
| Tenuissimasatin (202) | IC50 = 24.5 µM |
| Altenusin (95) | IC50 = 25.4 µM |
| 2,5-Dimethyl-7-hydroxychromone (143) | IC50 = 17.3 µM |
| Walterolactone C (203) | IC50 = 14.9 µM |
| p-Hydroxyphenylbutanediol (204) | Anti-inflammatory | Active | Alternaria alternata 114-1G | | [55] |
2.6. Compounds with Antidiabetic Activity (Table 6)
Compounds investigated for their antidiabetic potential are displayed in
Figure 2,
Figure 3,
Figure 6,
Figure 7,
Figure 13 and
Figure 15. The fungus
Alternaria sp. XZSBG-1 was isolated from the sediment of China’s Salt Lake in Bange, Tibet. Its chemical investigation afforded four anthraquinone derivatives, altersolanol O (
30), alterporriol S (
31), alterporriol T (
32), and alterporriol U (
33), as well as alterporriol E (
34), alterporriol D (
35), alterporriol N (
24), alterporriol A (
36), altersolanol C (
21), altersolanol A (
37), and macrosporin (
38) [
16]. The capacity of all compounds to inhibit α-glucosidase was evaluated. Alterporriol T (
32) effectively inhibits α-glucosidase, having an IC
50 value of 7.2 μM. The remaining compounds have no inhibitory effect on α-glucosidase [
16].
The fungus
Alternaria sp. SK6YW3L was identified from mangrove
Sonneratia caseolaris gathered in China’s Guangxi Province [
56]. The fungus yielded several compounds, which were evaluated for α-glucosidase inhibitory activity. Compounds 7
S (
206), 7
R,8
S,9
S (
177), and 2-hydroxyalternariol (2-OH-AOH) (
213) displayed the strongest inhibitory action, with respective IC
50 values of 78.2, 78.1, and 64.7 µM compared to other compounds and the positive control, acarbose (IC
50 = 553.7 µM). Compounds 7
S,9
S (
205), and rubralactone (
212) were two-fold more active than acarbose. Nonetheless, compound
208, talaroflavone (
210), and alternariol (
41) had moderate inhibitory action against α-glucosidase, with IC
50 values of 334.4, 348.4, and 474.3 µM, respectively. Other altenusin derivatives with a 6/5/5 ring skeleton, such as compound
209 and deoxyrubralactone (
211), exhibited poor activity (IC
50 > 500 µM). Compared to alternariol methyl ether (
214), which contains a methoxy group, the chelated hydroxyl group at C-3 (
213 and
41) increased the inhibitory effect [
56].
Three dibenzo-α-pyrone derivatives, alternolides A–C (
90–
92), and seven congeners—alternariol (
41), alternariol 5-
O-methyl ether (
98), 3′-hydroxyalternariol-5-
O-methyl ether (
139), alternariol-1′-hydroxy-9-methyl ether (
215), altenuisol (
141), 1-deoxyrubralactone (
133), and phialophoriol (
216)—were isolated from the marine-derived fungus
Alternaria alternata LW37, which was obtained from a marine sediment [
30]. All compounds were evaluated for their α-glucosidase inhibition effect. Compounds
91,
92,
215,
141, and
133 exhibited α-glucosidase inhibitory actions with inhibition rates of 36.6, 49.2, 93.7, 37.2, and 53.9%, respectively, at a concentration of 400 µM. Compounds
91 and
92 inhibited α-glucosidase with IC
50 values of 725.8 and 451.2 µM, respectively, while compound
215 demonstrated considerable inhibitory action with an IC
50 value of 6.27 µM (the positive control, acarbose, had an IC
50 value of 1.5 µM) [
30].
2,4,6-Triphenylaniline (
217) was purified from the fungus
Alternaria longipes strain VITN14G, which was obtained from the mangrove plant
Avicennia officinalis collected in the Chidambaram district, India [
57]. The antidiabetic activity of this compound was determined through in vitro analysis of its α-amylase and α-glucosidase inhibition. In terms of α-amylase inhibition rates, there was no significant difference observed between 2,4,6-triphenylaniline (
217) (51.5%) and acarbose (56.6%), which is the standard drug. Conversely, the α-glucosidase inhibition rate of compound
217 (78.8%) was slightly lower than that of acarbose (89.9%), but it displayed a significantly improved inhibitory activity towards α-glucosidase. 2,4,6-Triphenylaniline (
217) exhibited potent α-amylase inhibitory activity (IC
50 = 84.1 μM) compared to α-glucosidase inhibition (IC
50 = 121.4 μM) and compared to α-amylase, like the standard drug acarbose [
57].
As shown from the above discussion, marine-derived Alternaria species have delivered several chemically diverse compounds with noteworthy antidiabetic potential, largely through the inhibition of α-amylase and α-glucosidase, the carbohydrate-hydrolyzing enzymes. α-Amylase and α-glucosidase play fundamental roles in the regulation of postprandial glucose, and their inhibition signifies a proved therapeutic methodology for managing type 2 diabetes mellitus.
Alterporriol T (
32), with an IC
50 value of 7.2 μM against α-glucosidase, exceeds the effect of reference drug acarbose (IC
50 = 553.7 μM) [
16] and is therefore considered as a potent candidate. Similarly, the dibenzo-α-pyrone derivative alternariol-1′-hydroxy-9-methyl ether (
215) displayed significant α-glucosidase inhibition with an IC
50 value of 6.2 μM and with a 93.7% inhibition rate at 400 μM [
30]. Both compounds are considered as potential candidates for future in vivo studies and development.
Further, several phenolic and polyketide compounds, including 2-hydroxyalternariol (2-OH-AOH) (
213), 7
S (
206), and 7
R*,8
S*,9
S* (
207) displayed significant α-glucosidase inhibition, with IC
50 values of 64.7, 78.2, and 78.1 μM, respectively, are moderately active candidates and present a roughly seven-fold greater potency than acarbose [
56]. Other compounds with moderate effects are rubralactone (
212) (IC
50 = 194.4 μM) and 7
S,9
S (
205) (IC
50 = 235.2 μM), which are two- to three-fold more active than the control drug. Additional moderately active compounds are talaroflavone (
210) (IC
50 = 348.4 μM) and alternariol (
41) (IC
50 = 474.3 μM), while alternariol methyl ether (
214) and deoxyrubralactone (
211) are considered weakly active or inactive compounds (IC
50 > 500 μM) [
56]. These data suggest that the presence of a free hydroxyl group at C-3 improves enzyme interaction, whereas ring fusion or methylation tends to diminish inhibitory potency.
In addition, alternolides B (
91) and C (
92) exhibited modest α-glucosidase inhibition with IC
50 values of 725.8 and 451.2 μM, respectively, while altenuisol (
141) and 1-deoxyrubralactone (
133) showed inhibition rates of 37.2% and 53.9% at 400 μM [
30].
2,4,6-Triphenylaniline (
217) demonstrated dual enzyme inhibition, with IC
50 values of 84.1 and 121.4 μM for α-amylase and α-glucosidase. Its α-glucosidase inhibition rate (78.8%) was only slightly lower than that of acarbose (89.9%), while α-amylase inhibition (51.5%) was nearly equivalent to the standard drug (56.6%) [
57]. These results highlight the potential of non-phenolic aromatic scaffolds for multitargeted carbohydrate enzyme inhibition.
Collectively, the compounds display a wide range of α-amylase and α-glucosidase inhibition. The most active and potent candidates are alterporriol T (32) and alternariol-1′-hydroxy-9-methyl ether (215), with low micromolar IC50 values (6–7 μM), suggesting strong competitive inhibition equivalent to or surpassing standard inhibitors. Among the moderately active candidates are the hydroxylated anthraquinones and perylenequinones (IC50 = 60–250 μM), while methylated or dimeric analogs were less active. Structural analyses suggest that hydroxyl substitution and conjugated planar systems enhance enzyme binding through hydrogen bonding and π–π stacking, whereas methylation reduces affinity.
Mechanistically, several of these phenolic metabolites may exert dual antihyperglycemic effects by both antioxidant-mediated glucose regulation and enzyme inhibition, as previously observed for the polyketides [
25,
30,
56,
57]. Overall, these findings underline
Alternaria as a hopeful source of structurally diverse enzyme inhibitors with potential for antidiabetic drug development, particularly in targeting postprandial glucose control through α-amylase and α-glucosidase modulation [
16,
30,
56,
57]. Compounds that show antidiabetic activities are shown in
Table 6.
Table 6.
Reported compounds with antidiabetic activities.
Table 6.
Reported compounds with antidiabetic activities.
| Compound | Applied Assay | Biological Activity | Fungus Name | Host Organism | Reference |
|---|
| Alterporriol T (32) | Inhibition of
α-glucosidase | IC50 = 7.2 μM | Alternaria sp. XZSBG-1 | Sediment | [16] |
| 7S,9S (205) | IC50 = 235.2 μM | Alternaria sp. SK6YW3L | Mangrove Sonneratiacaseolaris | [56] |
| 7S (206) | IC50 = 78.2 μM |
| 7R*,8S*,9S* (207) | IC50 = 78.1 μM |
| Compound 208 | IC50 = 334.4 μM |
| Compound 209 | IC50 > 500 μM |
| Talaroflavone (210) | IC50 = 348.4 μM |
| Deoxyrubralactone (211) | IC50 > 500 μM |
| Rubralactone (212) | IC50 = 194.4 μM |
| 2-OH-AOH (213) | IC50 = 64.7 μM |
| Alternariol (41) | IC50 = 474.3 μM |
| Alternariol methyl ether (214) | IC50 > 500 μM |
| Alternolide B (91) | IC50 = 725.8 µM α-glucosidase), 36.62% inhibition rate | Alternaria alternata LW37 | Deep-sea
sediment | [30] |
| Alternolide C (92) | IC50 = 451.2 µM α-glucosidase), 49.24% inhibition rate |
| Alternariol-1′-hydroxy-9-methyl ether (215) | IC50 = 6.2 µM (α-glucosidase), 93.7% inhibition rate |
| Altenuisol (141) | 37.2% α-glucosidase inhibition rate |
| 1-Deoxyrubralactone (133) | 53.9% α-glucosidase inhibition rate |
| 2,4,6-Triphenylaniline (217) | Inhibition of
α-Amylase,
α-glucosidase | IC50 = 84.1 μM (α-amylase),
IC50 = 121.4 μM (α-glucosidase) | Alternaria longipes VITN14G | Mangrove plant Avicennia officinalis | [57] |
2.7. Compounds with Phytotoxic Activity (Table 7)
Chemical structures of the compounds evaluated for their phytotoxic activity are displayed in
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 10,
Figure 11,
Figure 16 and
Figure 17. Members of the genus
Alternaria are among the most common plant pathogens. Their secondary metabolites have phytotoxic properties, making them valuable for agricultural applications. The compounds
p-benzyloxy-phenol (
218),
p-hydroxyphenyl ethylamine (
219), 3-hydroxymethyl-8-hydroxy-pyrrolopiperazine-2,5-dione (
220), 3-isobutyl-6-sec-butyl-piperazine-2,5-dione (
221), 5α,8α-epidioxy-ergosta-6,22-diene-3β-ol (
27), and 3β-hydroxy-cholest-5-ene (
222) were purified from the marine fungus
Alternaria sp. [
58]. Compounds
218–
221 induced the morphological deformation of mycelia germinated from conidia of
Pyricularia oryzae [
58].
Four pyrone derivatives—pyrophen (
103), rubrofusarin B (
104), fonsecin (
105), and fonsecin B (
106)—and four dimers of naphtha-pyrones—aurasperones A–C (
107–
109) and F (
110)—were reported from the fungus
Alternaria alternata strain D2006, which was purified from the soft coral
Denderonephthya hemprichi [
36]. All compounds exhibited weak cytotoxicity (4–11% inhibition) in the brine shrimp assay at concentrations between 16.5 and 36.4 µM [
36].
Four polyketides amibromdole (
223)—altersolanol L (
22), altersolanol C (
21), and physcion (
224)—were derived from the fungus
Alternaria sp. which was isolated from a coral found in the South China Sea [
59]. All polyketides were evaluated for the ability to suppress the larval settling of
B. amphitrite larvae at concentrations between 35.9 and 176.0 μM. Moderate activity was shown by amibromdole (
223), with an EC
50 value of 35.9 μM [
59].
The fungus
Alternaria tenuissima EN-192 was isolated from the mangrove
Rhizophora stylosa on Hainan Island in the South China Sea [
60]. Investigation of the fungus afforded the following: four indole-diterpenoids—penijanthine A (
225), paspaline (
226), paspalinine (
227), and penitrem A (
228); three tricycloalternarene derivatives—tricycloalternarene 3a (
59), tricycloalternarene 1b (
229), and tricycloalternarene 2b (
230); and two alternariol derivatives—djalonensone (
231) and alternariol (
41) [
60]. The inhibitory activities of each isolated compound against the pathogenic bacterium
Vibrio anguillarum were evaluated. Using the disk diffusion method, compounds
59 and
231 showed moderate activity against
V. anguillarum, with inhibition zone diameters of 8 and 9 mm, respectively, at 100 μg/disk. In comparison, the positive control, chloramphenicol, demonstrated a significantly larger inhibition zone of 22 mm when used at a concentration of 20 µg/disk [
60].
The marine-derived fungus
Alternaria sp. WZL003, obtained from a gorgonian
Echinogorgia rebekka collected from the South China Sea, was investigated [
61]. Investigation of the fungal extract afforded macrosporin (
38). Investigation of the antimicrobial mechanism against the marine pathogenic
Vibrio anguillarum revealed that compound
38 effectively eradicates bacteria by causing destruction to both the cell wall and cytoplasmic membrane. This process causes an increase in cell permeability, causing the release of cellular content [
61].
Alterbrasone (
49) was isolated from the crinoid-associated fungus
Alternaria brassicae 93, named as
Comanthina schlegeli, collected from the South China Sea [
21]. Compound
49 was evaluated against twelve aquatic bacteria:
E. coli,
Shigella castellani,
Salmonella,
S. aureus,
Vibrio parahemolyticus,
Vibrio vulnificus,
Vibrio alginolyticus,
Vibrio cholera,
Citrobacter freundii,
Exiguobacterium aurantiacum,
Morganella morganii, and
Bacillus cereus. No antibacterial activity was observed for compound
49 against all aquatic bacteria, with IC
50 values below 60 μM [
21].
Racemic mixtures of cyclohexenone and cyclopentenone derivatives, namely (±)-(4
R*,5
S*,6
S*)-3-amino-4,5,6-trihydroxy-2-methoxy-5-methyl-2-cyclohexen-1-one (
122), (±)-(4
S*,5
S*)-2,4,5-trihydroxy-3-methoxy-4-methoxycarbonyl-5-methyl-2-cyclopenten1-one (
123), and fischexanthone (
124), along with 4-chloro-1,5-dihydroxy-3-hydroxymethyl-6-methoxycarbonyl-xanthen-9-one (
159) were obtained from the fungus
Alternaria sp. R6, derived from a marine semi-mangrove plant
Myoporum bontioides A collected from Guangdong Province, China [
41]. The antimicrobial activity of these compounds was evaluated. The activities of compounds
123,
124, and
159 were higher than the positive control triadimefon (MIC = 510.6 μM) against
Fusarium graminearum, with MIC values of 215.5, 474.6, and 107.1 μM, respectively. Compound
159 also exhibited more potent antifungal activity against
Calletotrichum musae (MIC = 214.2 μM) than triadimefon (MIC = 340.4 μM), while compounds
123 and
124 showed moderate activities, with MIC values of 862.0 and 474.6 μM, respectively. Compound
122 was inactive towards
Calletotrichum musae (MIC > 1970.4 μM). Compound
159 bears a chlorine group at C-4 and exhibits a greater antifungal activity against
Fusarium graminearum and
Calletotrichum musae than compound
124, which lacks the chlorine substitution at C-4, suggesting that the chlorine group at C-4 is the key reason for better antifungal activity [
41].
Sesteralterin (
232), tricycloalterfurenes A–D (
233–
236), and TCA-F (
237) were obtained from the fungal strain
Alternaria alternata k21-1, which was isolated from the marine red alga
Lomentaria hakodatensis collected from Kongdong Island [
62]. Compounds
232–
237 were examined for growth suppression against three marine phytoplanktons—
Chattonella marina,
Heterosigma akashiwo, and
Prorocentrum donghaiense—one marine zooplankton—
Artemia salina—and one marine-derived bacterium—
Pseudoalteromonas citrea—that can cause porphyrayezoensis green-spot disease. Among the marine plankton tested,
C. marina appeared more sensitive to compounds
232–
237, with weak to moderate inhibition at a concentration of 264.5 μM. Compound
232 showed 41–69% inhibition of the three phytoplankton, but was inactive with the zooplankton
A. salina. Compound
233 (64, 37, 46% inhibition) and compound
237 (70, 41, 52% inhibition) showed almost similar effects against the three phytoplanktons, respectively, indicating that the hydroxyl group’s position on ring C had minimal impact on their activities. Hydroxylation at C-2 and C-3 slightly reduced the inhibition of the three phytoplanktons by compounds
235 and
236, which showed inhibition ranges from 17 to 56%. In addition, all compounds did not exhibit activities against the
bacterium P. citrea at 20 μg/disk [
62].
Three isomeric tricycloalternaren—(2
E)-TCA 12a (
238), (2
Z)-TCA 12a (
239), and TCA 11a (
240)—were obtained from the fungus
Alternaria alternata k23-3 isolated from the marine red alga
Gelidiella acerosa collected from Kongtong Island, China [
63]. Compounds
238–
240 showed weak to moderate activity by inhibiting or killing the marine plankton tested,
Chattonella marina,
Heterosigma akashiwo, and
Prorocentrum donghaiense. However, compound
239 had a greater ability to suppress the three phytoplankton species and was less toxic to the zooplankton
Artemia salina than compound
238 [
63].
Two tricycloalternarene-type meroterpenes, 17-
O-methyltricycloalternarene D (
241) and methyl-nortricycloalternarate (
242), and two congeners, TCA 1b (
243) and TCA D (
244), were purified from the extract of the fungus
Alternaria alternata k21-1, isolated from the marine red alga
Lomentariah akodatensis collected from Kongdong Island [
64]. Evaluation of these compounds for the growth inhibition of three marine planktons—
Chattonella marina,
Heterosigma akashiwo, and
Prorocentrum donghaiense—and toxicity to one marine zooplankton—
Artemia salina—revealed weak or moderate inhibition. Among the phytoplankton species tested,
C. marina appeared to be more sensitive to compounds
241–
244 than
H. akashiwo and
P. donghaiense were, and than to compound
244 with an inhibitory rate of 78.5% at 256.4 μM. In addition, compound
244 was less toxic to the zooplankton
A. salina than the others, possibly due to its acetyl and hydroxy groups located at C-1 and C-17, respectively [
64].
The fungus
Alternaria sp. P8 was isolated from several unidentified marine plants collected from the Yellow Sea in Qingdao, China [
65]. The fungus afforded two perylenequinones, stemphyperylenol (
55) and alterperylenol (
68) [
63]. In the antifungal assay, six plant pathogenic fungal strains,
A. alternata (Fries) Keissler,
A. brassicicola,
P. parasitica var. nicotianae Tucker,
D. medusaea Nitschke,
A. niger van. Tiegh, and
P. theae, were used. Stemphyperylenol (
55) showed inhibitory activity against
P. theae and
A. brassicicola, with MIC values of 22.1 and 355.1 µM, respectively, which were like the positive control carbendazim. Alterperylenol (
68) showed significant antibacterial activity against
C. michiganensis, with an MIC of 5.57 µM, which was two times higher than the positive control of streptomycin (MIC = 6.7 µM) [
65].
The marine-derived fungus
Alternaria sp. P8 was isolated from seawater, sediments, animals, and algae collected from the Yellow Sea, South China Sea, and Chinese coastal marine environments [
66]. Chemical analysis of the fungal extract resulted in the discovery of one benzopyranone, (+)-(2
S,3
R,4a
R)-altenuene (
245), and seven compounds, (+)-isoaltenuene (
246), alternariol (
41), altenuisol (
141), alternariol-9-methyl ether (
40), altertoxin I (
52), stemphyperylenol (
55), and alterperylenol (
68). Compounds
245,
246,
52,
55, and
68 showed obvious phytotoxicity against the seedling growth of amaranth and lettuce at 200 ppm. The perylenequinones (
52,
55, and
68) were more active than the benzopyranones (
245 and
246), since they were able to block seed germination at 200 ppm and still exhibited high phytotoxicity at 50 ppm. It showed that separated chemicals hindered lettuce seedling development less than amaranth seedling growth. Their effects on root elongation were much more pronounced than on hypocotyl elongation. Due to the lack of observed phytotoxicity in compounds
40,
41, and
141, it is revealed that substituting benzene with cyclohexene could favorably affect phytotoxicity. In addition, anti-phytopathogenic properties for compound
245 displayed strong antifungal activity against
A. brassicicola, with an MIC of 428.0 μM, which was equivalent to that of the positive control carbendazim. Compound
52 exhibited moderate antifungal activity toward
D. medusaea, with an MIC of 177.5 μM, compared to 163.8 μM for carbendazim. None of the compounds showed antibacterial activity [
66].
A derivative of butenolide, known as alterbutenolide (
247), containing a long-chain aliphatic acid, was discovered together with seven phenolic compounds, namely alternariol (
41), asperigillol B (
248),
p-hydroxyphenylacetic acid (
249),
p-hydroxyphenylethyl alcohol (
250), methyl
p-hydroxyphenyl acetate (
251), 2-(4-hydroxyphenyl) ethyl acetate (
252), and 5,6-dihydro-4-methyl-2H-pyran-2-one (
253), from the sponge-derived fungus
Alternaria alternata I-YLW6-1 gathered from the Shandong Province of China [
67]. All compounds underwent growth inhibition tests against three marine phytoplanktons—
Chattonellamarina,
Heterosigma akashiwo, and
Prorocentrum donghaiense—as well as one marine zooplankton—
Artemia salina. Only compound
247 demonstrated moderate inhibition against
C. marina, with an IC
50 value of 144.1 μM. On the other hand, compounds
41 and
248 exhibited significant to moderate inhibitory activities against
C. marina,
H. akashiwo, and
P. donghaiense, with IC
50 values ranging from 11.6 to 140.3 μM. Additionally, compounds
248 and
249 showed weak toxicity against brine shrimp larvae
A. salina, with LC
50 values exceeding 367.6 and 657.8 μM, respectively [
67].
The fungus
Alternaria iridiaustralis, derived from the
Suaeda glauca marine plant found in the Yellow River Delta in Dongying, China, produced 16-methoxy solanapyrone B (
254), as well as solanapyrones B and S (
255 and
256), probetaenone I (
257), alternanones A and B (
258 and
259), chaetosemin D (
260), alternanone C (
261), and tenuazonic acid (
89) [
68]. These compounds have shown potential as herbicides, specifically in inhibiting the growth of
Echinochloa crusgalli seedlings. Of particular significance is compound
89, which exhibited inhibition rates exceeding 90% at concentrations of 101.5 and 203.0 µM, surpassing the effectiveness of the commonly used chemical herbicide acetochlor. Compound
259 also showed moderate inhibition rates of 60.3% and 72.6%, at of 98.0 and 196.0 µM, respectively. Additionally, compounds
258,
259, and
261 demonstrated antifungal properties against two carbendazim-resistant strains of
B. cinerea, with MIC values ranging from 141.5 to 313.7 µM, which were significantly superior to those of carbendazim (MIC = 1339 µM). Furthermore, compounds
259–
261 also displayed moderate antifungal activities against two
F. oxysporum strains [
68].
Altermodinacid A (
93), an anthraquinone, was initially isolated from the fungal extract of
Alternaria sp. X112, which was obtained from a marine fish
Gadus macrocephalus found in the vicinity of Yangma Island, China [
31]. However, the compound did not exhibit any significant antifungal activity against various agricultural pathogenic fungi, including
Alternaria solani,
Lasiodiplodia pseudotheobromae,
Fusarium oxysporum f. sp.
cubense,
Fusarium oxysporum f. sp
. phaseoli,
Fusarium foetens,
Fusarium graminearum,
Nectria sp.,
Fusarium mangiferae,
Colletotrichum asianum,
Colletotrichum musae, and
Colletotrichum coccodesand, as the MIC was found to be greater than 40 µg/well [
31].
As shown above, marine-derived
Alternaria species have emerged as prolific sources of chemically diverse secondary metabolites with broad-spectrum phytotoxic, and algicidal activities (
Table 7).
Highly potent compounds are represented by alterperylenol (
68) and stemphyperylenol (
55), with complete inhibition of the seed germination of amaranth and lettuce at 50–200 ppm and antimicrobial activities surpassing streptomycin with MIC values of 5.57 µM against
Clavibacter michiganensis and 22.1 µM against
P. theae [
65,
66]. Similarly, altertoxin I (
52) showed high phytotoxicity comparable to carbendazim at 200 ppm [
66]. Exceeding the effect of the commercial herbicide acetochlor, tenuazonic acid (
89) inhibited the growth of
Echinochloa crusgalli at 101.5–203 µM by >90% [
68]. Mechanistic studies showed that
89 acts through the inhibition of electron transport in photosystem II, leading to the accumulation of reactive oxygen species (ROS), chlorophyll degradation, lipid peroxidation, and necrosis. Similarly, macrosporin (
38) eradicates
Vibrio anguillarum by disrupting cytoplasmic membranes and cell walls [
61]. Also, the chlorinated xanthone derivative
159 displayed superior antifungal effect against
Fusarium graminearum and
Calletotrichum musae (MIC 107.1–214.2 µM) relative to the reference fungicide triadimefon [
41].
Amibromdole (
223) displayed moderate antifouling activity with an EC
50 of 35.9 µM, and altersolanol L (
22), altersolanol C (
21), and physcion (
224) are example of compounds with moderate activity (EC
50 78.1–176 µM) [
59]. Alterbutenolide (
247) and asperigillol B (
248) also displayed moderate algicidal effects, with IC
50 values between 11.6 and 144.1 µM and low toxicity to zooplankton [
67]. The benzopyranone derivatives altenuene (
245) and isoaltenuene (
246) showed moderate phytotoxicity against amaranth and lettuce at 200 ppm, while alternanones (
258–
261) showed moderate antifungal and herbicidal effects, including 60–72% inhibition of
E. crusgalli and MICs of 141.5–313.7 µM against
B. cinerea and
Fusarium oxysporum [
66,
68].
Conclusively, perylenequinones and tenuazonic acid appear as the most potent candidates for bioherbicidal and antifungal applications due to their potent activity and well-understood ROS-mediated mechanisms. Moderately active scaffolds, including benzopyranones, tricycloalternarenes, and alternanones, represent potential candidates for further chemical derivatization and optimization. Cooperatively, these compounds underscore the ecological, agricultural, and biotechnological relevance of members of
Alternaria as a source of bioactive compounds for sustainable crop protection.
Table 7 displays compounds with reported phytotoxic effects.
Table 7.
Reported compounds with proven phytotoxic activities.
Table 7.
Reported compounds with proven phytotoxic activities.
| Compound | Organism Used | Biological Activity | Fungus
Name | Host
Organism | Reference |
|---|
p-Benzyloxy-phenol (218)
p-Hydroxyphenyl ethylamine (219)
3-Hydroxymethyl-8-hydroxy-pyrrolopiperazine-2,5-dione (220)
3-Isobutyl-6-sec-butyl-piperazine-2,5-dione (221) | Pyricularia oryzae | Induce morphological deformation of mycelia germinated from conidia of Pyricularia oryzae | Alternaria sp. | | [58] |
| Pyrophen (103) | % Inhibition of Brine shrimp | 4–11% at 34.8 μM | Alternaria alternata D2006 | Soft coral
Denderonephthya hemprichi | [36] |
| Rubrofusarin B (104) | 4–11% at 34.9 μM |
| Fonsecin (105) | 4–11% at 36.4 μM |
| Fonsecin B (106) | 4–11% at 32.8 μM |
| Aurasperone A (107) | 4–11% at 17.5 μM |
| Aurasperone B (108) | 4–11% at 16.5 μM |
| Aurasperone C (109) | 4–11% at 16.8 μM |
| Aurasperone F (110) | 4–11% at 17.4 μM |
| Amibromdole (223) | Antilarval Settlement Activity | EC50 = 35.9 μM | Alternaria sp. | Coral | [59] |
| Altersolanol L (22) | EC50 = 154.3 μM |
| Altersolanol C (21) | EC50 = 78.1 μM |
| Physcion (224) | EC50 = 176.0 μM |
| Tricycloalternarene 3a (59) | Marine pathogenic Vibrio anguillarum | 8 mm at 100 μg/disk | Alternaria tenuissima EN-192 | Mangrove Rhizophora stylosa | [60] |
| Djalonensone (231) | 9 mm at 100 μg/disk |
| Macrosporin (38) | Marine pathogenic Vibrio anguillarum | Antimicrobial activity | Alternaria sp. WZL003 | Gorgonian Echinogorgia rebekka | [61] |
(±)-(4S*,5S*)-2,4,5-Trihydroxy-3-methoxy-4-methoxycarbonyl-
5-methyl-2-cyclopenten1-one (123) | Fusarium graminearum, Calletotrichum musae | MIC = 215.5, 862.0 μM | Alternaria sp. R6 | Mangrove
Myoporum
bontioides | [41] |
| Fischexanthone (124) | MIC = 474.6, 474.6 μM |
| 4-Chloro-1,5-dihydroxy-3-hydroxymethyl-6-methoxycarbonyl-xanthen-9-one (159) | MIC = 107.1, 214.2 μM |
| Sesteralterin (232) | Three marine phytoplankton (Chattonella marina, Heterosigma akashiwo, Prorocentrum donghaiense),
one marine zooplankton (Artemia salina); marine-derived bacterium (Pseudo-alteromonascitrea) | 41–69% inhibition of three marine phytoplankton | Alternaria alternata k21-1 | Red alga
Lomentaria
hakodatens | [62] |
| Tricycloalterfurene A (233) | 64, 37, 46% inhibition of three marine phytoplankton |
| Tricycloalterfurene B (234) | Weak to moderate at 264.5 μM, more sensitive to C. marina |
Tricycloalterfurene C (235)
Tricycloalterfurene D (236) | 17–56% inhibition of three marine phytoplankton |
| TCA-F (237) | 70, 41, 52% inhibition of three marine phytoplankton |
| (2E)-TCA 12a (238) | Marine phytoplanktons (Chattonella marina, Heterosigma akashiwo, Prorocentrum donghaiense); one marine zooplankton (Artemia salina) | 3.6–48.6% | Alternaria alternata k23-3 | Red alga
Gelidiella acerosa | [63] |
| (2Z)-TCA 12a (239) | 23.8–79.6% |
| TCA 11a (240) | 23.5–51.6% |
17-O-methyltricycloalternarene D (241)
Methyl-nortricycloalternarate (242)
TCA 1b (243) | Four marine plankton species (Chattonella marina, Heterosigma akashiwo, Prorocentrum donghaiense); marine zooplankton (Artemia salina) | Weak or moderate activity | Alternaria alternata k21-1 | Red alga
Lomentariaha
kodatens | [64] |
| TCA D (244) | 78.5% of C. marina at 256.4 μM
Less toxic to A. salina 12.6% |
| Stemphyperylenol (55) | A. alternata (Fries) Keissler, A. brassicicola, P. parasitica var. nicotianae Tucker, D. medusaea Nitschke, A. niger van. Tiegh, P. theae | MIC = 22.1, 355.1 µM (P. theae, A. brassicicola) | Alternaria sp. P8 | Marine plants | [65] |
| Alterperylenol (68) | MIC = 5.57 µM (C. michiganensis) |
| (+)-(2S,3R,4aR)-Altenuene (245) | Phytotoxicity against seed germination and seedling growth of amaranth and lettuce
A. alternata (Fries) Keissler, A. brassicicola, D. medusaea Nitschke, P. theae
Three plant pathogenic bacteria, A. avenae, P. syringae pv. lachrymans, and R. solanacearum | Phytotoxicity against the seedling growth of amaranth and lettuce at 200 ppm
A. brassicicola, MIC = 428.0 μM | Alternaria sp. P8 | Seawater, sediments, animals, and algae | [66] |
| (+)-Isoaltenuene (246) | Phytotoxicity against the seedling growth of amaranth and lettuce at 200 ppm |
| Altertoxin I (52) | Phytotoxicity against the seedling growth of amaranth and lettuce at 200 ppm
MIC = 177.5 μM (D. medusaea) |
| Stemphyperylenol (55) | Phytotoxicity against the seedling growth of amaranth and lettuce at 200 ppm |
| Alterperylenol (68) | Phytotoxicity against the seedling growth of amaranth and lettuce at 200 ppm |
| Alterbutenolide (247) | Chattonella marina, Heterosigma akashiwo, Prorocentrum donghaiense, Artemia salina | IC50 = 144.1 μM (C. marina) | Alternaria alternata I-YLW6-1 | Sponge | [67] |
| Alternariol (41) | IC50 = 11.6–140.3 μM |
| Asperigillol B (248) | IC50 = 31.2–88.9 μM
LC50 > 367.6 μM (A. salina) |
| p-Hydroxyphenylacetic acid (249) | LC50 > 657.8 μM (A. salina) |
| 16-Methoxy solanapyrone B (254) | Herbicidal against weed E. crusgalli
Antifungal against soil-borne B. cinerea from grape (BCG) and strawberry (BCS) F. oxysporum f. sp. cucumerinum (FOC) and F. oxysporum f. sp. Lycopersici (FOL) | 43.1–61.3% at 125.7 µM (E. crusgalli) | Alternaria iridiaustralis | Suaeda glauca plant | [68] |
| Solanapyrone B (255) | 43.1–61.3% at 131.5 µM (E. crusgalli) |
| Solanapyrone S (256) | 43.1–61.3% at 106.3 µM (E. crusgalli) |
| Probetaenone I (257) | 43.1–61.3% at 125.0 µM (E. crusgalli) |
| Alternanone A (258) | MIC = 214.7 µM (B. cinerea both strains) |
| Alternanone B (259) | 60.3% and 72.6% inhibition rate at 98.0 and 196.0 µM (E. crusgalli); MIC = 313.7 µM (B. cinerea both strains); MIC = 627.4 and >1000 µM (F. oxysporum (FOC) and (FOL)) |
| Chaetosemin D (260) | MIC > 1000 µM (F. oxysporum (FOC) and (FOL)) |
| Alternanone C (261) | MIC = 141.5 µM (B. cinerea both strains);
MIC = 566.3 and >1000 µM (F. oxysporum (FOC) and (FOL)) |
| Tenuazonic acid (89) | 90% inhibition rate at 101.5 and 203.0 µM against E. crusgalli |
2.8. Compounds with Miscellaneous Activities (Table 8)
Compounds evaluated for their miscellaneous effects are displayed in
Figure 1,
Figure 2 and
Figure 18. Some isolated marine natural products have served as potential lead compounds for clinically useful drugs and have been used as chemical probes for fundamental studies in life science. Kojic acid dimethyl ether (
262), kojic acid monomethyl ether (
263), kojic acid (
264), and phomaligol A (
265) were isolated from marine-derived fungus
Alternaria sp. MFA 898, which was collected from the green algae
Ulva pertusa on Jeju Island, Korea [
69]. Spectrophotometric analysis was used to determine the antityrosinase activity of the compounds. Among them, kojic acid (
264) was found to have significant antityrosinase activity with an IC
50 value of 12.0 µM. However, the remaining compounds were found to be inactive [
69]. Sg17-1-4 (
3) was yielded from the fungus
Alternaria tenuis Sg17-1-4, which was isolated from a marine alga collected on Zhoushan Island, China [
8]. Sg17-1-4 (
3) is an isocoumarin with a seven-numbered ring on its side chain, and displayed anti-ulcer activity [
8].
The hydroanthraquinone—anthrininone A (
266)—two anthraquinones—anthrininones B and C (
267 and
268)—in addition to 6-
O-methylalaternin (
269), were obtained from the fungus
Alternaria tenuissima DFFSCS013, which was isolated from a deep-sea sediment, collected from the South China Sea [
70]. Compounds
266–
269 had significant inhibition against indoleamine 2,3-dioxygenase 1 (IDO1), especially anthrininone C (
268), with an IC
50 value of 0.5 μM. Furthermore, compounds
267–
269 exhibited selective inhibitory activities against five different protein tyrosine phosphatases (PTPs), including TCPTP, SHP1, MEG2, SHP2, and PTP1B. The IC
50 value of compound
269 for PTP1B was 2.1 μM, which was 17.1- and 14.3-fold less than that for TCPTP (IC
50 = 35.3 μM) and PTP-MEG2 (IC
50 = 29.6 μM), respectively. The anthraquinone moiety is necessary for enzymatic inhibition, and the substituents on the structure can influence the observed activity. Moreover, compounds
266–
269, together with (3
R)-1-deoxyaustrocortilutein (
270), dihydroaltersolanol A (
18), altersolanol L (
22), ampelanol (
23), and altersolanol B (
20) were evaluated for their effects on intracellular calcium flux in HEK293 cells using a calcium imaging assay. Compound
266, at 10 μM concentration, stimulated intracellular calcium levels, and its fluorescence count was around 25% of calcium ionophore I (CA 1001). However, compound
266 at <10 μM concentration and other compounds at 10 μM concentration did not affect intracellular calcium levels [
70].
Alternarin A (
271) was isolated together with two analogs, macrophorins A (
272) and B (
273), from the fungus
Alternaria sp. ZH-15, which was isolated from a
Lobophytum crassum soft coral collected in the South China Sea [
71]. The isolated meroterpenoids were evaluated for their neuronal modulatory activity by testing the effect on spontaneous Ca
2+ oscillations (SCOs) in primary cultured neocortical neurons. Alternarin A (
271) concentration-dependently inhibited SCOs by decreasing both the spontaneous SCO frequency (IC
50 = 3.2 μM) and amplitude (IC
50 = 1.8 μM). Furthermore, compound
271 also effectively suppressed hyperactive SCOs induced by the seizurogenic agent 4-aminopyridine (4-AP) in cortical neurons, with IC
50 values of 10.0 μM for frequency and 4.6 μM for amplitude, respectively, indicating its inhibitory activity on neuronal excitability. Compounds
272 and
273 were inactive in modulating SCOs in neocortical neurons. The results showed that the reorganized meroterpenoid drimane (
271), with an unusual cyclopentenone moiety, is a novel neuroactive compound with potential anti-epileptic properties [
71].
The results shown above support the miscellaneous effect of marine
Alternaria-derived fungal metabolites. Kojic acid (
264) showed significant antityrosinase activity with IC
50 value of 12.0 µM. Conversely, the analogs, kojic acid dimethyl ether (
262) and kojic acid monomethyl ether (
263), were inactive [
69], suggesting the importance of the free hydroxyl group for enzymatic inhibition. Within the anthraquinone class, anthrininone C (
268) demonstrated strong inhibition of IDO1 (IC
50 = 0.5 µM), while 6-
O-methylalaternin (
269) selectively inhibited PTP1B (IC
50 = 2.1 µM), with reduced activity against other PTPs [
70]. These data suggest that the anthraquinone core is crucial for activity, and specific substituents modulate both potency and target selectivity. In the meroterpenoid drimane series, alternarin A (
271) displayed potent neuronal modulatory activity, suppressing spontaneous Ca
2+ oscillation frequency (IC
50 = 3.2 µM) and amplitude (IC
50 = 1.8 µM), as well as 4-AP-induced hyperactive oscillations, while its analogs, macrophorins A and B (
272 and
273), were inactive [
71], highlighting the functional importance of the cyclopentenone moiety.
Moderately active compounds in this group are represented by anthrininone A (
266), which induces intracellular calcium flux at 10 µM, and Sg17-1-4 (
3) which exhibits anti-ulcer activity [
8,
70]. These compounds, while less potent, still provide insight into functional group contributions to bioactivity.
Overall, structure–activity relationship analyses indicate that minor modifications, such as methylation, hydroxylation, or ring rearrangements, dramatically influence biological outcomes. For antityrosinase activity, free hydroxyl groups are required for copper chelation; for anthraquinones, the planar core is essential for enzyme binding, with substituents governing selectivity; and for meroterpenoid drimanes, the cyclopentenone ring is critical for neuroactivity. These observations underscore the potential of marine fungi as a source of structurally diverse bioactive compounds and provide a framework for the rational design of more potent and selective analogs. Compounds of reported miscellaneous activities are shown in
Table 8.
Table 8.
Reported compounds showing miscellaneous activities.
Table 8.
Reported compounds showing miscellaneous activities.
| Compound | Applied Assay | Biological Activity | Fungus Name | Host Organism | Reference |
|---|
| Kojic acid (263) | Tyrosinase inhibitory activity | IC50 = 12.0 μM | Alternaria sp. MFA 898 | The green alga Ulva pertusa | [69] |
| Sg17-1-4 (3) | Anti-ulcer activity | Active | Alternaria tenuis, Sg17-1 | Unspecified
marine alga | [8] |
| Anthrininone A (266) | Inhibition activity against five (PTPs), Indoleamine 2,3-dioxygenase 1 (IDO1)
Effects on intracellular calcium flux | Significant inhibition activity against IDO1
Stimulate intracellular calcium levels at 10 μM | Alternaria tenuissima DFFSCS013 | Deep sea
sediment | [70] |
| Anthrininone B (267) | Significant inhibition activity against IDO1
Selective inhibition activity against five PTPs |
| Anthrininone C (268) | Selective inhibition activity against five PTPs
Significant inhibition activity against IDO1, IC50 = 0.5 μM |
| 6-O-Methylalaternin (269) | Significant inhibition activity against IDO1
Selective inhibition activity against five PTPs, IC50 for PTP1B = 2.1 μM |
| Alternarin A (271) | Neuronal modulatory activity by testing the effect on spontaneous Ca2+ oscillations (SCOs) in primary cultured neocortical neurons | IC50 = 3.2 μM against SCO frequency IC50 = 1.8 μM against amplitude | Alternaria sp. ZH-15 | Soft coral
Lobophytum
crassum | [71] |