Marine Natural Products in Preclinical Cancer Studies: Ten Years of Advanced Total Synthesis
Abstract
1. Introduction
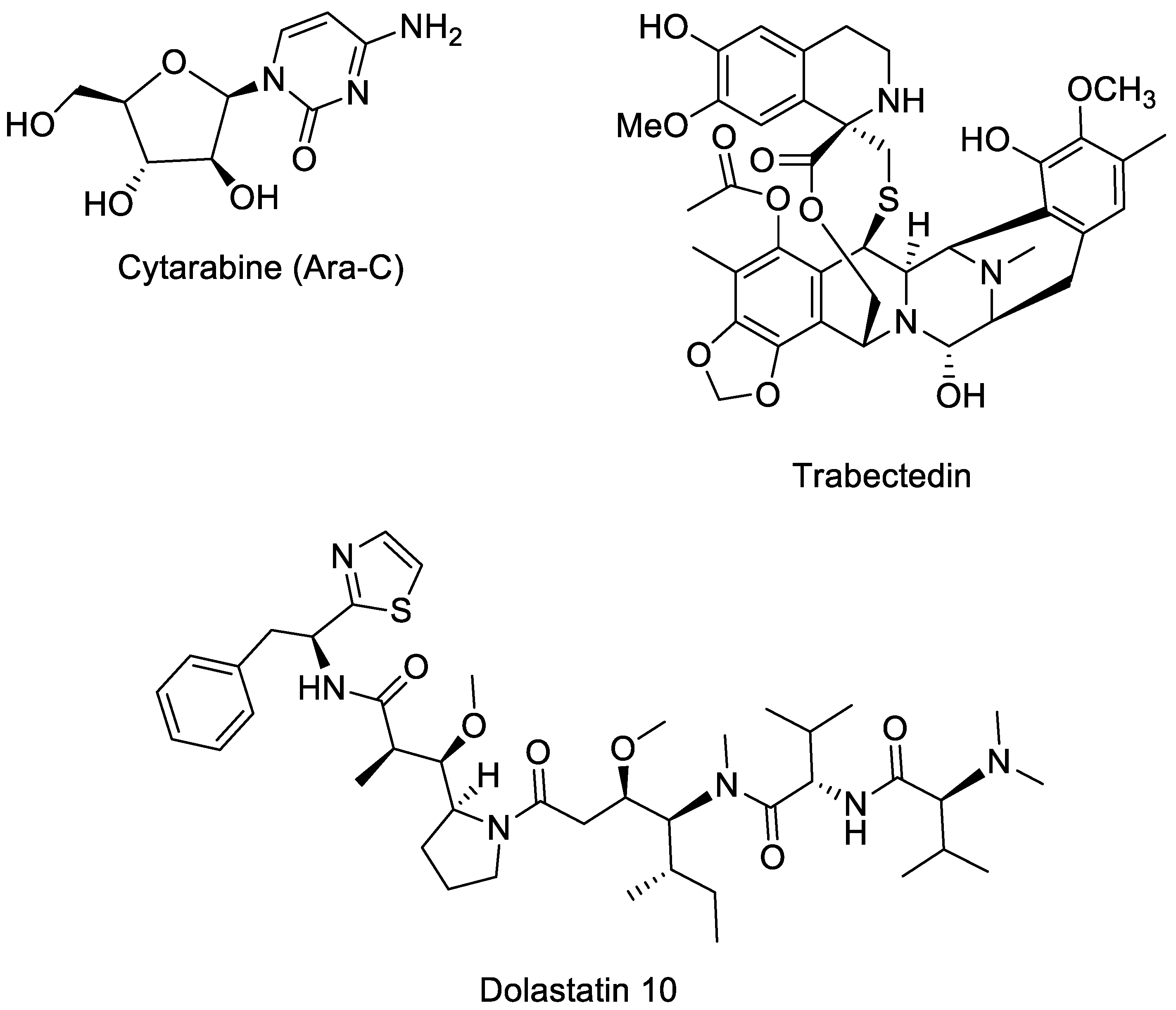
2. Marine Natural Products in Preclinical Cancer Research
2.1. Aromatic and Heterocyclic Alkaloids
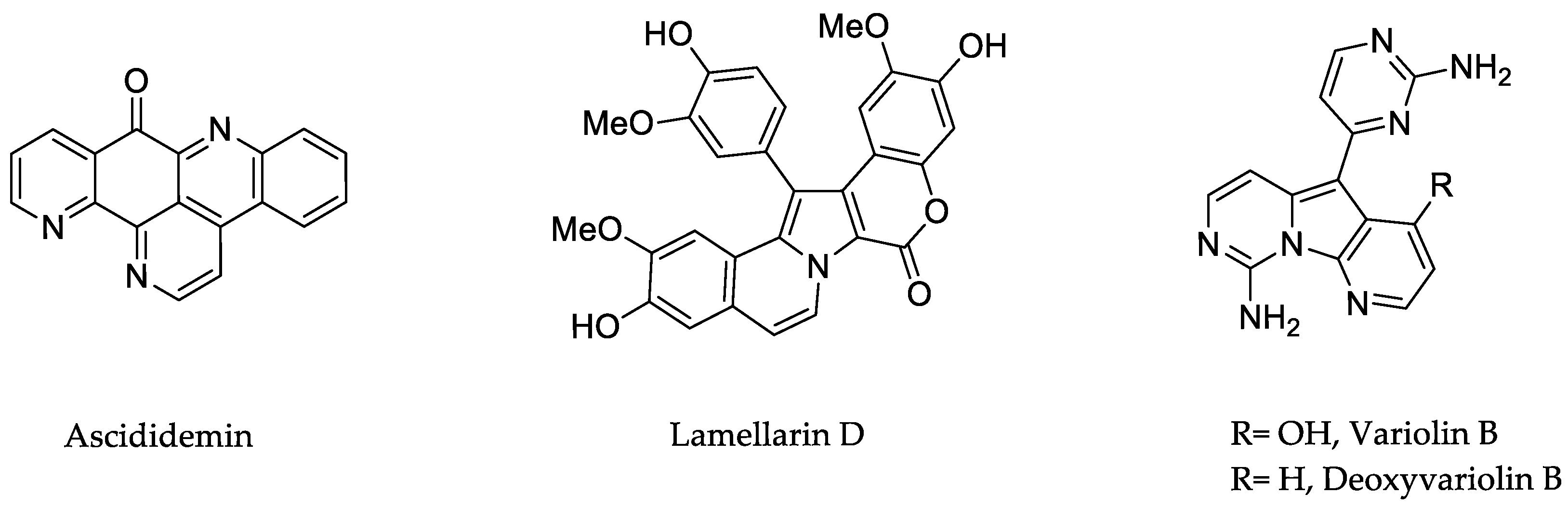
| Compound (Isolation) | Class | Source | Cancer Type |
|---|---|---|---|
| Ascididemin (1988) | Aromatic alkaloid | Didemnum sp. (sponge) | Leukaemia |
| Lamellarin D (1985) | Pyrrole alkaloid | Lamellaria sp. (mollusc/corals) | Leukaemia, colon cancer |
| Variolins (1994) | Heterocyclic alkaloid | Kirkpatrickia variolosa (sponge) | Leukaemia, brain tumours |
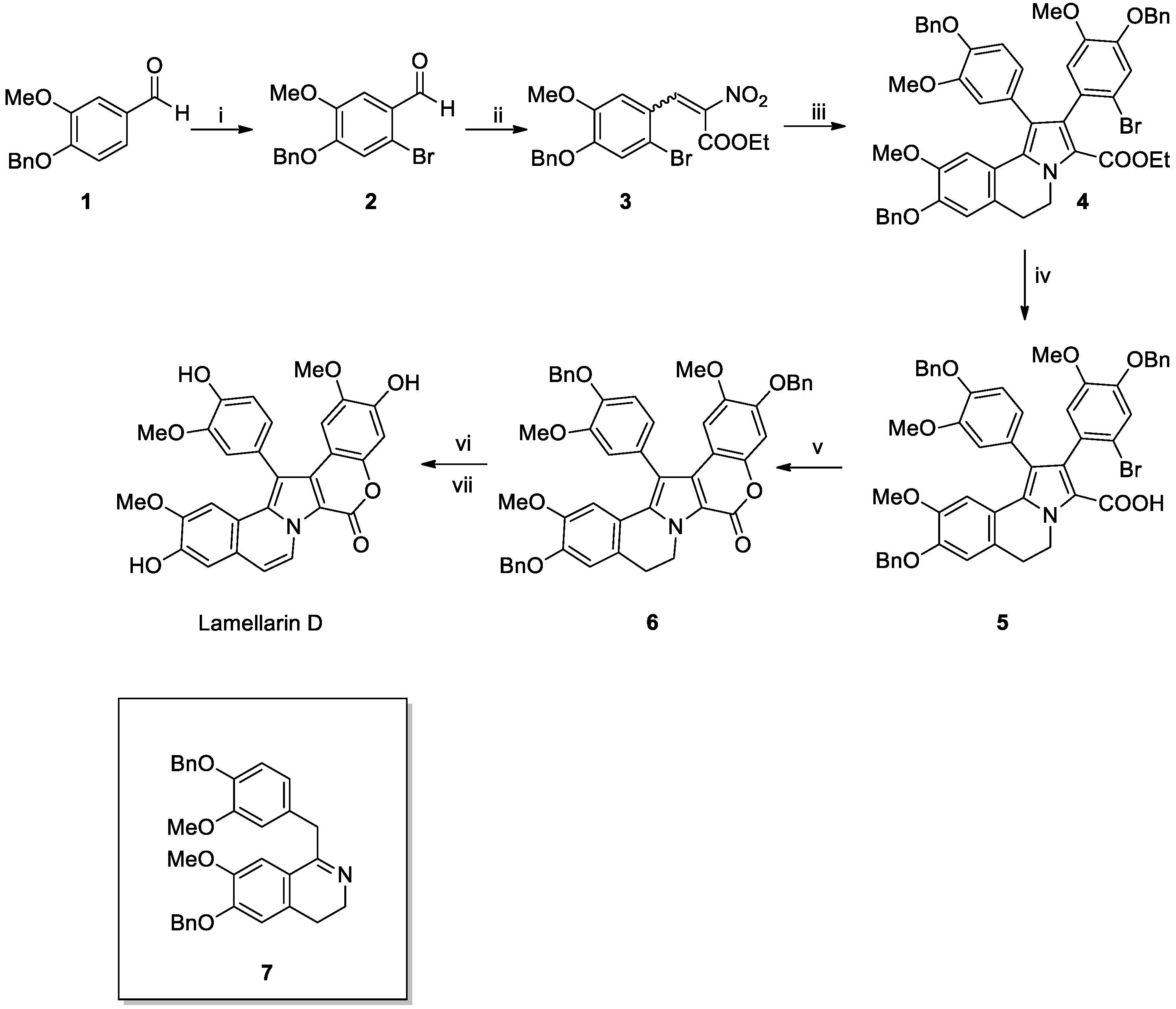
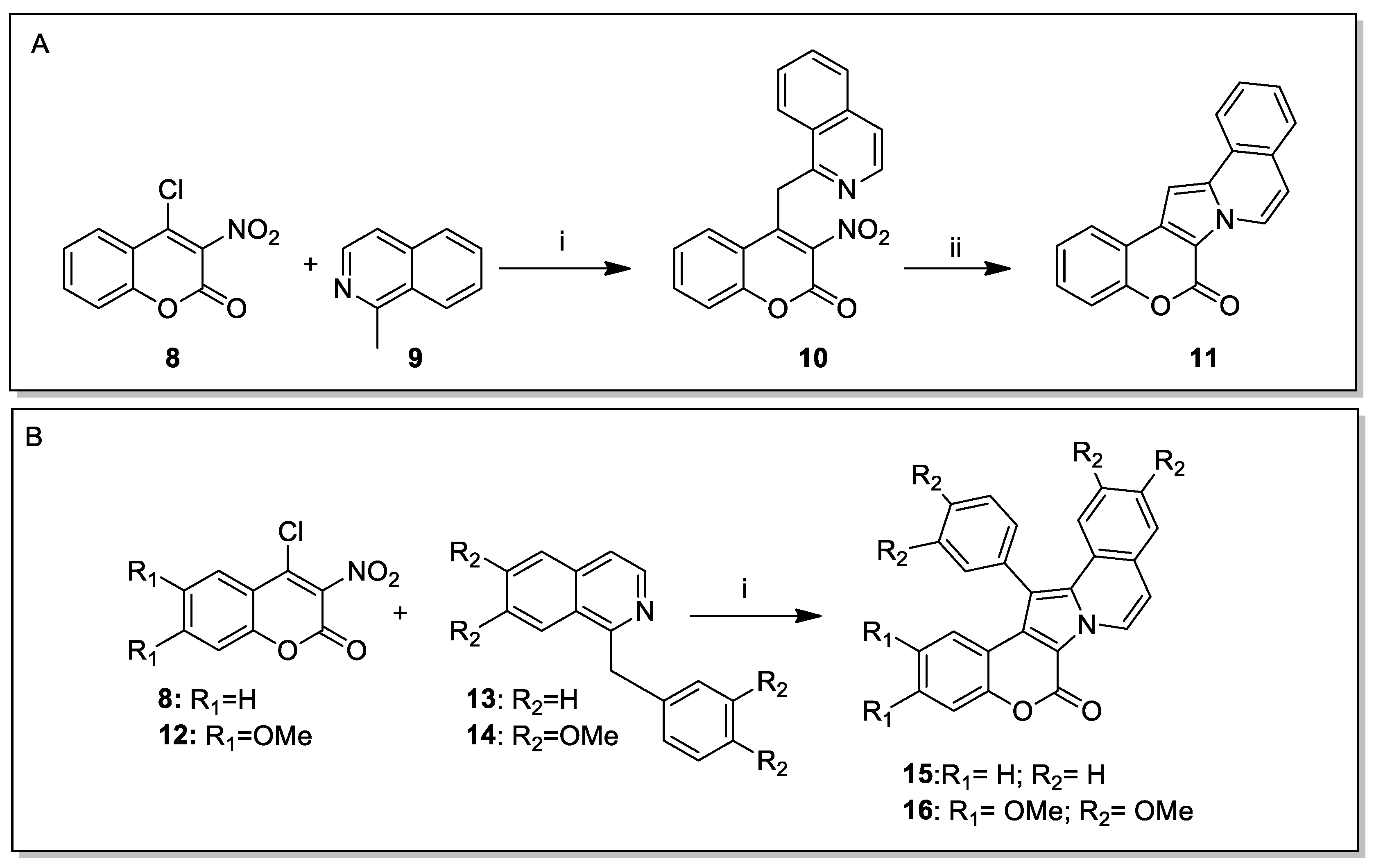
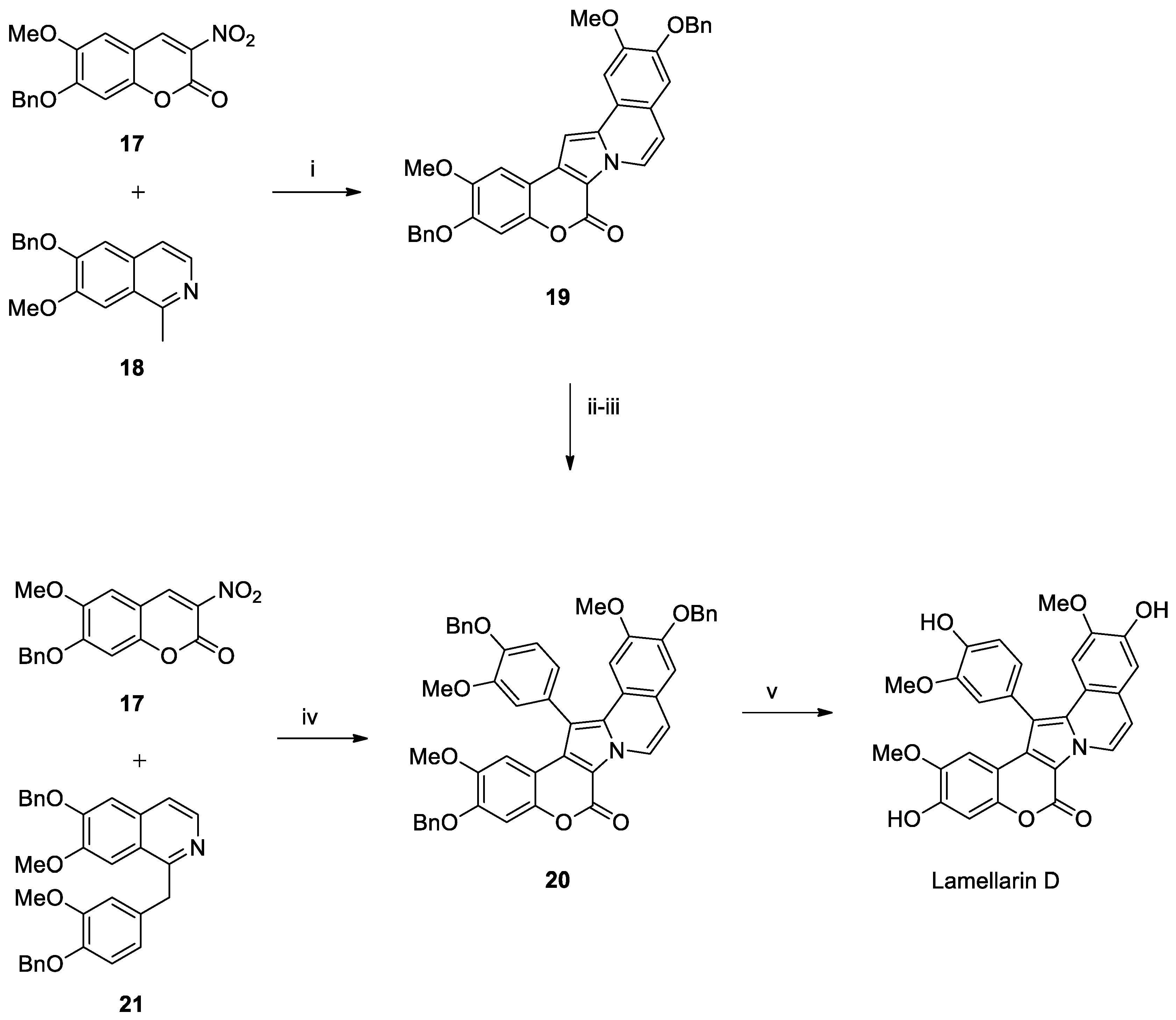
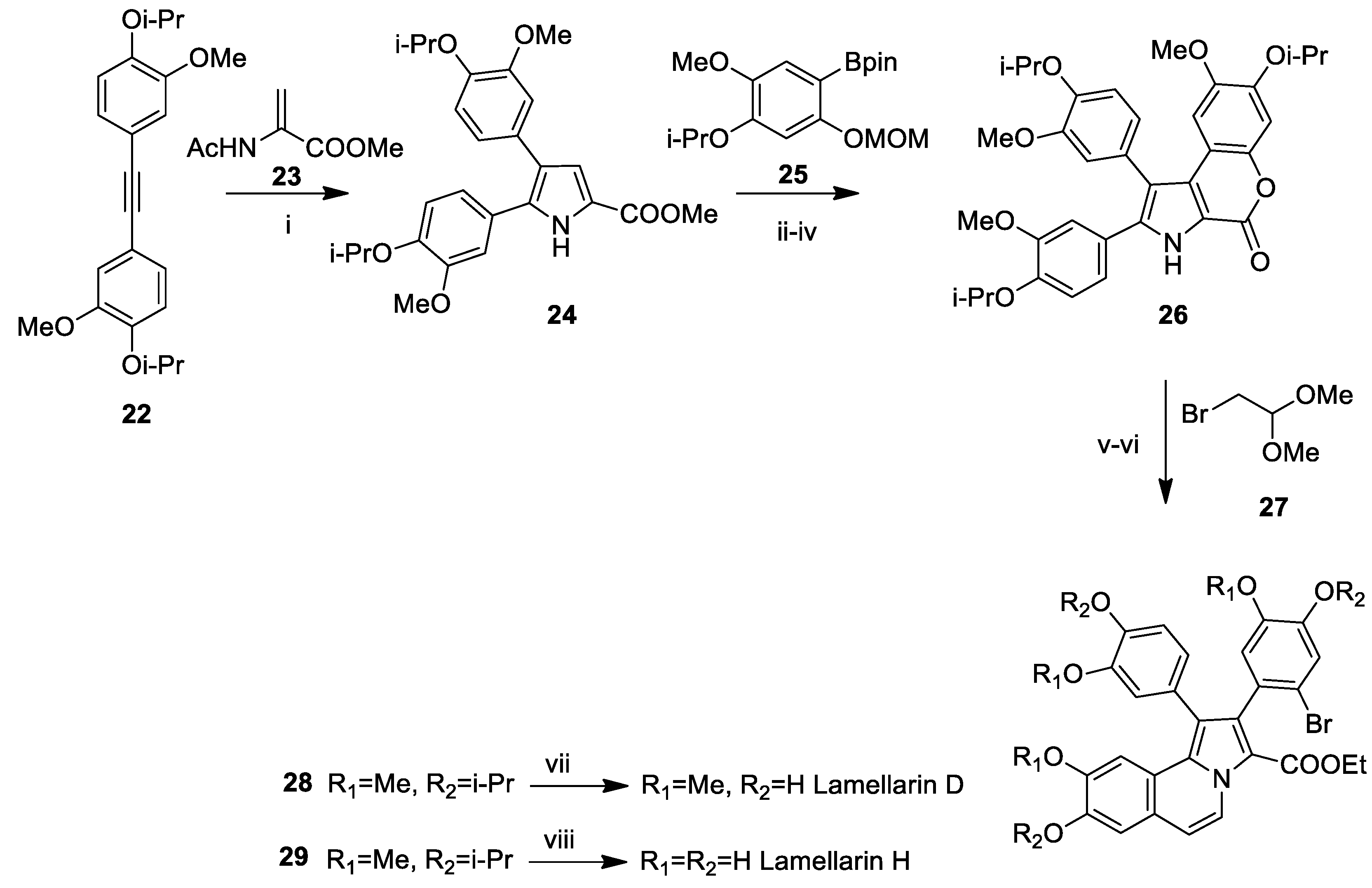
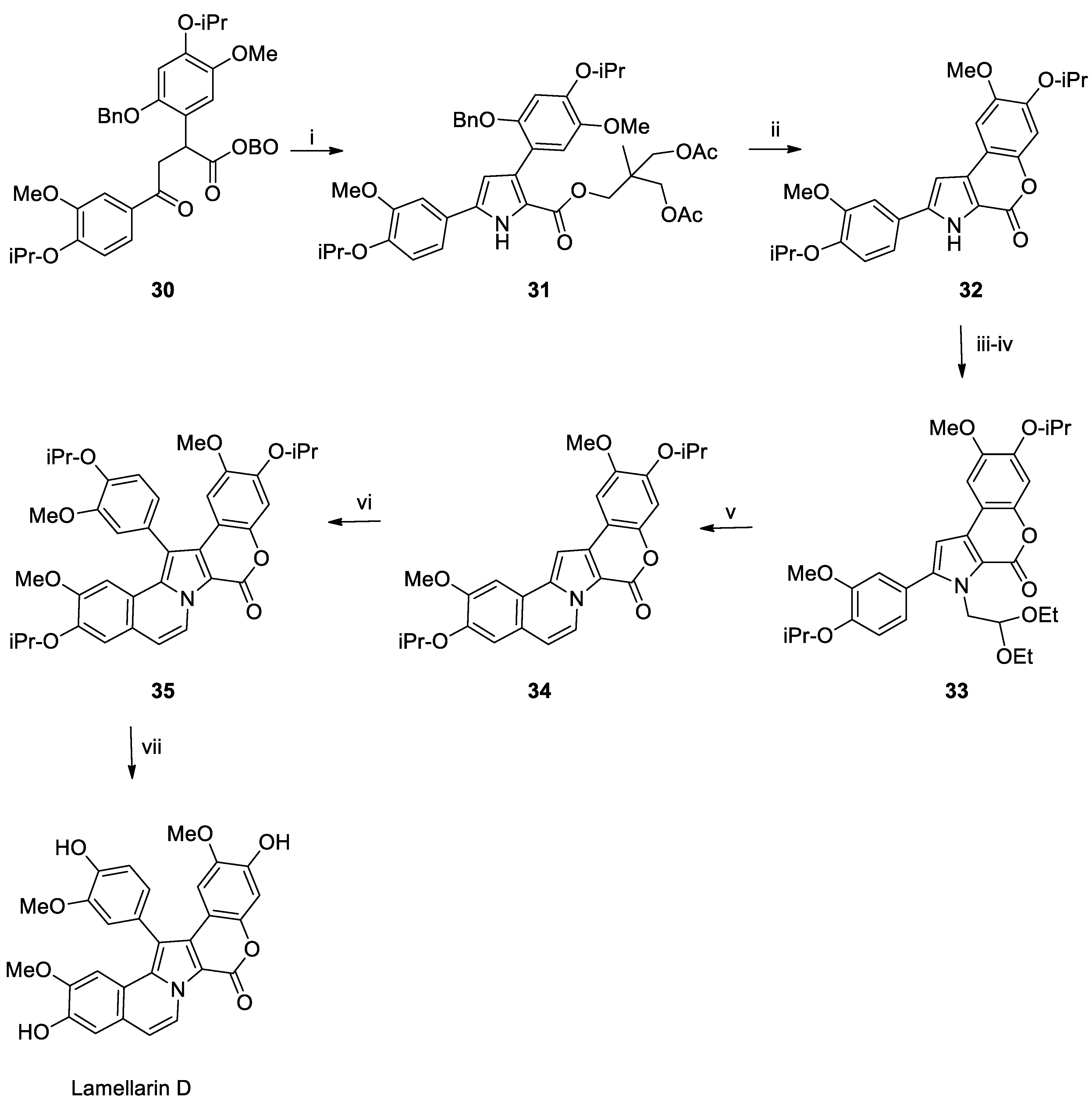
Lamellarin D Total Syntheses
2.2. Terpenes and Derivatives

| Compound (Isolation) | Class | Source | Cancer Type |
|---|---|---|---|
| Sarcodictyins (1987s) | Diterpene | Sarcodictyon roseum (sponge) | Ovarian, breast cancer, solid tumours |
| Eleutherobin 1997 | Diterpene glycoside | Eleutherobia sp./Erythropodium (sponge) | Leukaemia, breast cancer |
| Ircinolin A 2011 | Norsesterterpenoid | Sponge Ircinia sp. (sponge) | Leukaemia, colon cancer, hepatocellular carcinoma |
| 15-acetylirciformonin B 2011 | Furanosesterterpenoids | Sponge Ircinia sp. (sponge) | Leukaemia, colon cancer, hepatocellular carcinoma |
| 10-Acetylirciformonin B 2011 | Furanosesterterpenoids | Sponge Ircinia sp. (sponge) | Leukaemia, colon cancer, hepatocellular carcinoma |
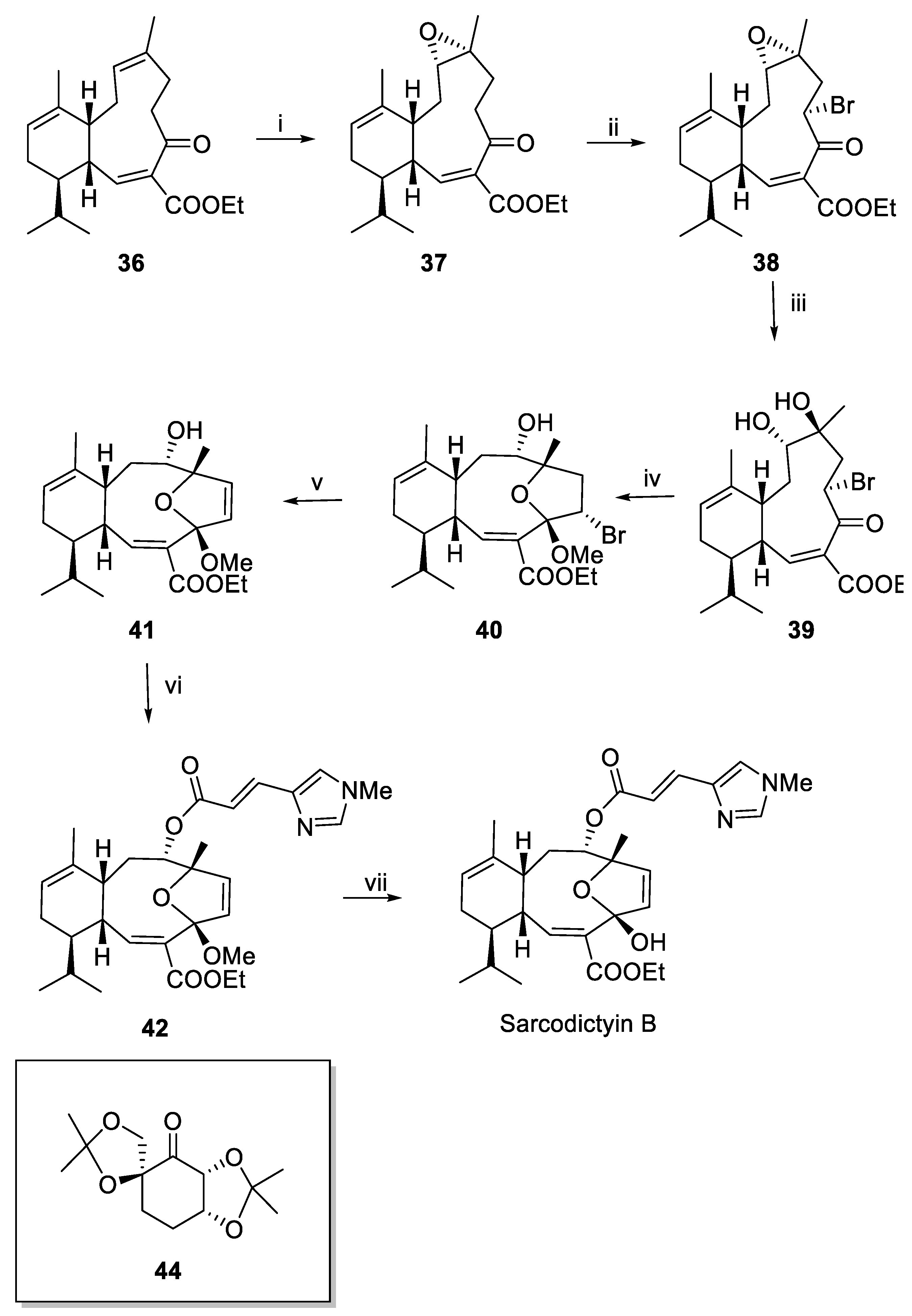
Recent Synthesis of Sarcodictyins and Eleutherobin
2.3. Macrolides
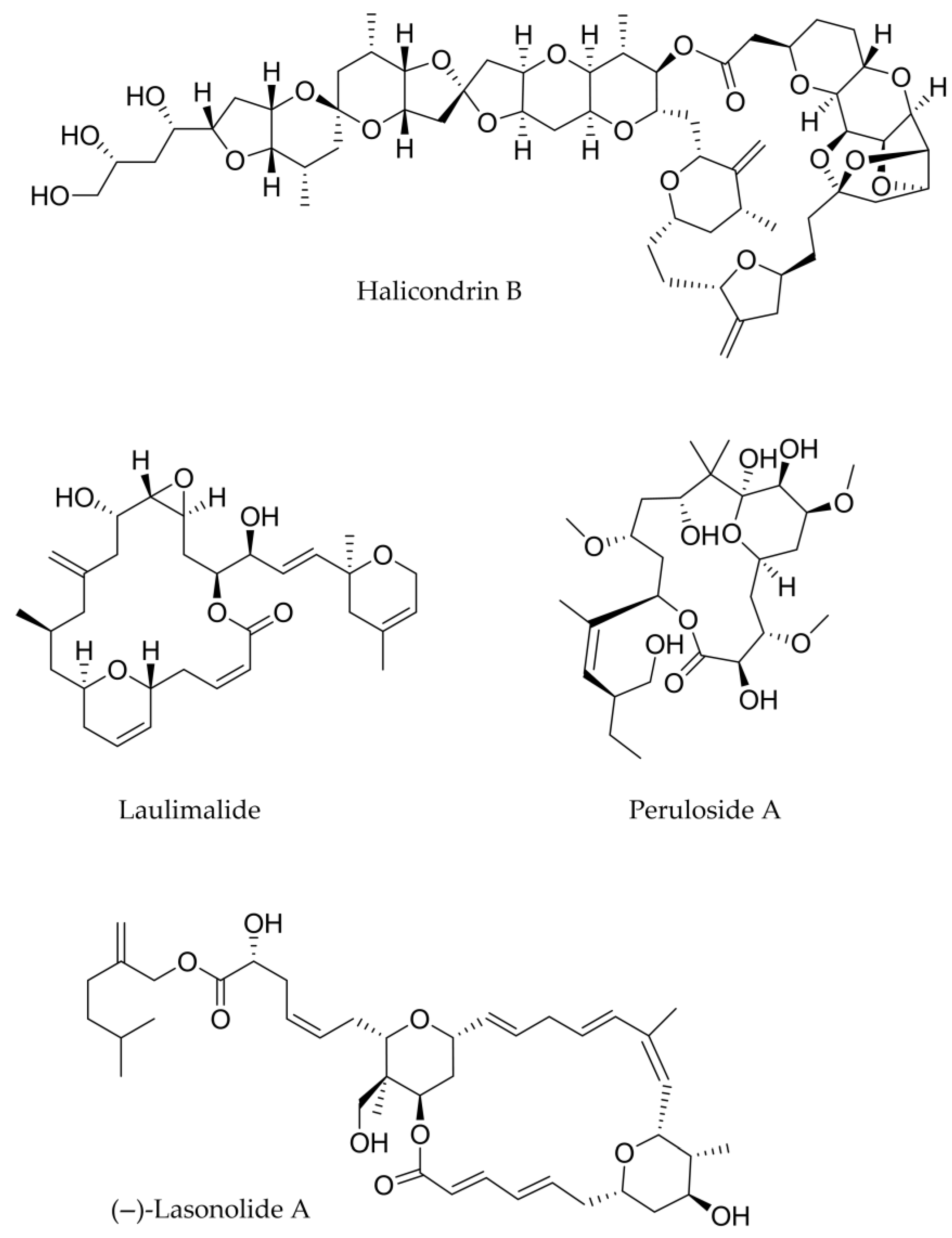
| Compound (Isolation) | Class | Source | Cancer Type |
|---|---|---|---|
| Halichondrin B (1986) | Macrolide | Halichondria okadai (sponge) | Breast, lung |
| Laulimalide 1988 | Macrolide | Cacospongia mycofijiensis (sponge) | Lung, ovarian |
| (−)-Lasonolide A 1994 | Macrolide | Forcepia sp. (sponge) | Lung |
| pancreas | |||
| Peloruside A 2003 | Macrolide | Mycale hentscheli (sponge) | Non-small cell, lung, ovarian |
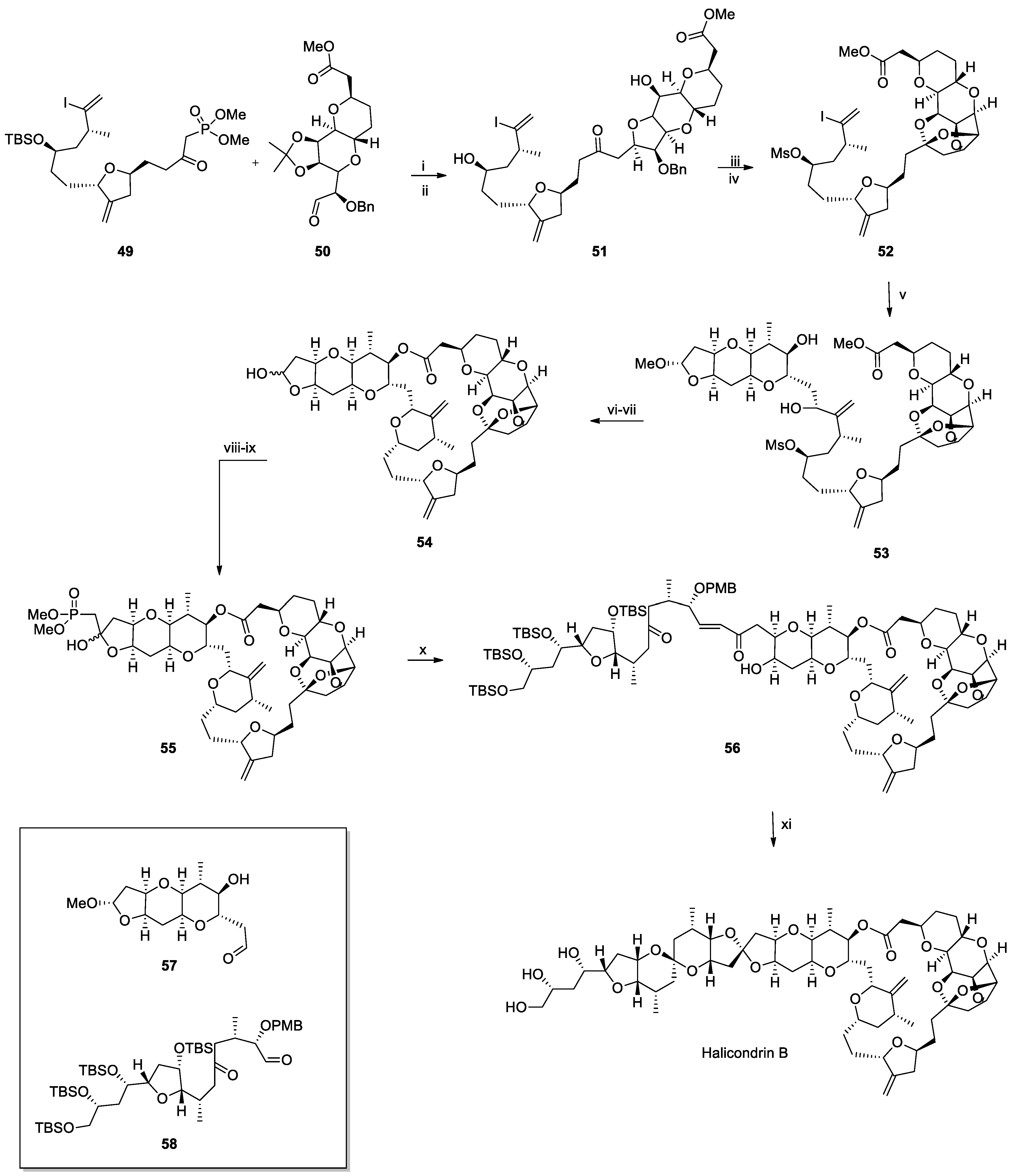
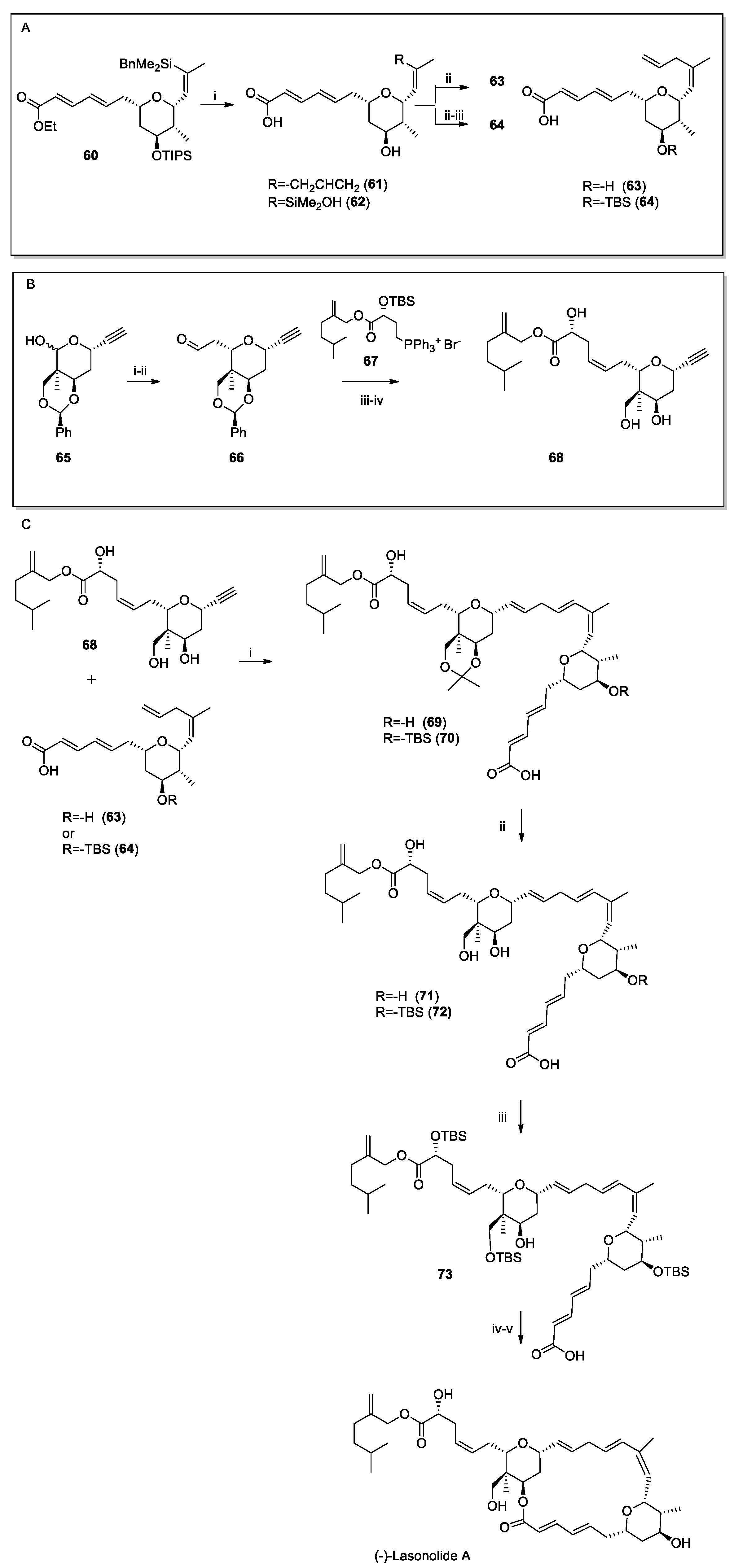
Recent Synthesis of Halichondrin B and (−)-Lasonolide A
2.4. Peptides: Linear, Cyclic, and Depsipeptides
| Compound (Isolation) | Class | Source | Cancer Type |
|---|---|---|---|
| Dolastatin 15 (1987) | Linear peptide | Dolabella auricularia (mollusc) | Lymphoma, breast cancer |
| Diazonamide (1994) | Cyclic peptide | Diazona angulata (tunicate) | Colon, pancreatic cancers |
| Thiocoraline (1999) | Depsipeptide | Micromonospora marina (bacterium) | Neuroblastoma, lung cancer |
| Vitilevuamide (2002) | Cyclic peptide | Didemnuin cuculiferum (tunicates) | Lung, breast cancers |
2.5. Other Classes

| Compound (Isolation) | Class | Source | Cancer Type |
|---|---|---|---|
| Curacin A (1994) | Thiazole lipid | Lyngbya majuscula (cyanobacterium) | Colon cancer |
| Spisulosine (ES-285) (2000) | Alkylamino alcohol | Mactromeris polynyma (mollusc) | Breast cancer |
| Dictyodendrins (1993–2012) | Pyrrolocarbazole derivatives | Dictyodendrilla verongiformis (sponge) | Melanoma, glioblastoma |
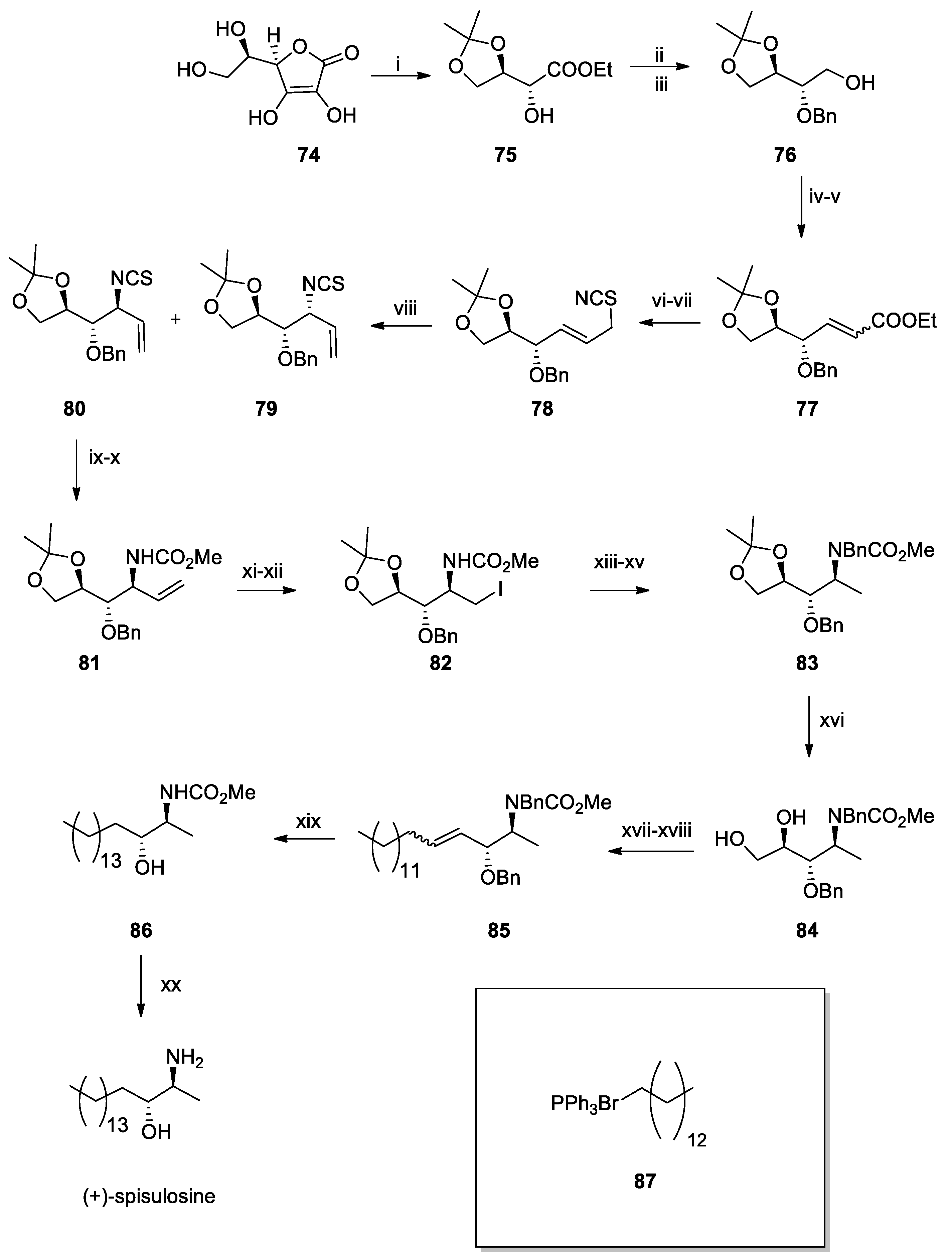
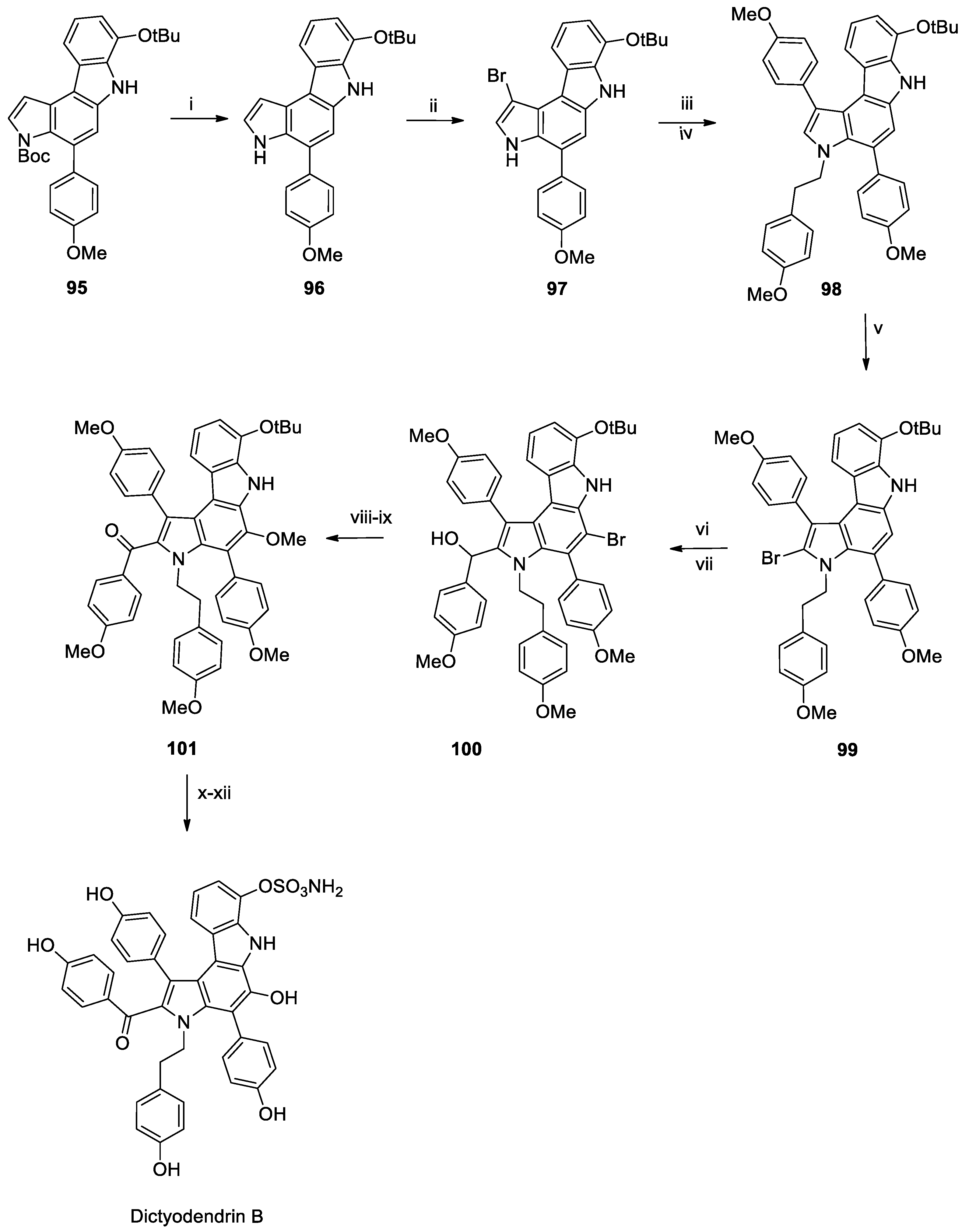
Recent Total Synthesis of Spisulosine and Dictyodendrin B
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altmann, K.-H. Drugs from the oceans: Marine natural products as leads for drug discovery. Chimia 2017, 71, 646. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.B.; Ettouati, L.; Litaudon, M.; Blunt, J.W.; Munro, M.H.; Parkin, S.; Hope, H. Alkaloids from the antarctic sponge Kirkpatrickia varialosa.: Part 1: Variolin b, a new antitumour and antiviral compound. Tetrahedron 1994, 50, 3987–3992. [Google Scholar] [CrossRef]
- Lu, W.-Y.; Li, H.-J.; Li, Q.-Y.; Wu, Y.-C. Application of marine natural products in drug research. Biorg. Med. Chem. 2021, 35, 116058. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. Contributions to the study of marine products. XXXII. The nucleosides of sponges. I. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Löwenberg, B.; Pabst, T.; Vellenga, E.; van Putten, W.; Schouten, H.C.; Graux, C.; Ferrant, A.; Sonneveld, P.; Biemond, B.J.; Gratwohl, A. Cytarabine dose for acute myeloid leukemia. N. Engl. J. Med. 2011, 364, 1027–1036. [Google Scholar] [CrossRef]
- Murphy, T.; Yee, K.W. Cytarabine and daunorubicin for the treatment of acute myeloid leukemia. Expert Opin. Pharmacother. 2017, 18, 1765–1780. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Parang, K. Development of cytarabine prodrugs and delivery systems for leukemia treatment. Expert Opin. Drug Deliv. 2010, 7, 1399–1414. [Google Scholar] [CrossRef]
- Moser, A.M.; Adamson, P.C.; Gillespie, A.J.; Poplack, D.G.; Balis, F.M. Intraventricular concentration times time (C × T) methotrexate and cytarabine for patients with recurrent meningeal leukemia and lymphoma. Cancer 1999, 85, 511–516. [Google Scholar] [CrossRef]
- Ganjoo, K.N.; Patel, S. Trabectedin: An anticancer drug from the sea. Expert Opin. Pharmacother. 2009, 10, 2735–2743. [Google Scholar] [CrossRef]
- Huryn, D.; Wipf, P. Natural product chemistry and cancer drug discovery. Cancer Drug Des. Discov. 2014, 2, 91–120. [Google Scholar]
- Pettit, G.R.; Kamano, Y.; Herald, C.L.; Tuinman, A.A.; Boettner, F.E.; Kizu, H.; Schmidt, J.M.; Baczynskyj, L.; Tomer, K.B.; Bontems, R.J. The isolation and structure of a remarkable marine animal antineoplastic constituent: Dolastatin 10. J. Am. Chem. Soc. 1987, 109, 6883–6885. [Google Scholar] [CrossRef]
- Turner, T.; Jackson, W.H.; Pettit, G.R.; Wells, A.; Kraft, A.S. Treatment of human prostate cancer cells with dolastatin 10, a peptide isolated from a marine shell-less mollusc. Prostate 1998, 34, 175–181. [Google Scholar] [CrossRef]
- Prabhu, R.H.; Patravale, V.B. Marine-derived pharmaceuticals for oncotherapy: Clinical trial and FDA-approved compounds. In Encyclopedia of Marine Biotechnology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; Volume 4, pp. 2607–2618. [Google Scholar]
- Kobayash, J.I.; Cheng, J.-F.; Nakamura, H.; Ohizumi, Y.; Hirata, Y.; Sasaki, T.; Ohta, T.; Nozoe, S. Ascididemin, a novel pentacyclic aromatic alkaloid with potent antileukemic activity from the okinawan tunicate didemnum sp. Tetrahedron Lett. 1988, 29, 1177–1180. [Google Scholar] [CrossRef]
- Dassonneville, L.; Wattez, N.; Baldeyrou, B.; Mahieu, C.; Lansiaux, A.; Banaigs, B.; Bonnard, I.; Bailly, C. Inhibition of topoisomerase II by the marine alkaloid ascididemin and induction of apoptosis in leukemia cells. Biochem. Pharmacol. 2000, 60, 527–537. [Google Scholar] [CrossRef]
- Bonnard, I.; Bontemps, N.; Lahmy, S.; Banaigs, B.; Combaut, G.; Francisco, C.; Colson, P.; Houssier, C.; Waring, M.; Bailly, C. Binding to DNA and cytotoxic evaluation of ascididemin, the major alkaloid from the Mediterranean ascidian Cystodytes dellechiajei. Anti-Cancer Drug Des. 1995, 10, 333–346. [Google Scholar]
- Dirsch, V.M.; Kirschke, S.O.; Estermeier, M.; Steffan, B.; Vollmar, A.M. Apoptosis signaling triggered by the marine alkaloid ascididemin is routed via caspase-2 and JNK to mitochondria. Oncogene 2004, 23, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Duc, D.X.; Quoc, N.V. Isolation, Bioactivities, and Synthesis of Lamellarin Alkaloids: A Review. Curr. Org. Chem. 2022, 26, 961–990. [Google Scholar] [CrossRef]
- Dias, N.; Vezin, H.; Lansiaux, A.; Bailly, C. Topoisomerase inhibitors of marine origin and their potential use as anticancer agents. In DNA Binders and Related Subjects; Springer: Berlin/Heidelberg, Germany, 2005; pp. 89–108. [Google Scholar]
- Chittchang, M.; Batsomboon, P.; Ruchirawat, S.; Ploypradith, P. Cytotoxicities and structure–activity relationships of natural and unnatural lamellarins toward cancer cell lines. ChemMedChem 2009, 4, 457–465. [Google Scholar] [CrossRef]
- Ballot, C.; Kluza, J.; Lancel, S.; Martoriati, A.; Hassoun, S.M.; Mortier, L.; Vienne, J.-C.; Briand, G.; Formstecher, P.; Bailly, C. Inhibition of mitochondrial respiration mediates apoptosis induced by the anti-tumoral alkaloid lamellarin D. Apoptosis 2010, 15, 769–781. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Novel antitumor agents: Marine sponge alkaloids, their synthetic analogs and derivatives. Mini Rev. Med. Chem. 2005, 5, 319–336. [Google Scholar] [CrossRef]
- Anderson, R.J.; Hill, J.B.; Morris, J.C. Concise total syntheses of variolin B and deoxyvariolin B. J. Org. Chem. 2005, 70, 6204–6212. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.R.; Carter, E.J.; Huff, B.C.; Morris, J.C. Variolins and related alkaloids. Chem. Rev. 2009, 109, 3080–3098. [Google Scholar] [CrossRef] [PubMed]
- Simone, M.; Erba, E.; Damia, G.; Vikhanskaya, F.; Di Francesco, A.M.; Riccardi, R.; Bailly, C.; Cuevas, C.; Sousa-Faro, J.M.F.; D’Incalci, M. Variolin B and its derivate deoxy-variolin B: New marine natural compounds with cyclin-dependent kinase inhibitor activity. Eur. J. Cancer 2005, 41, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Bettayeb, K.; Tirado, O.M.; Marionneau-Lambot, S.; Ferandin, Y.; Lozach, O.; Morris, J.C.; Mateo-Lozano, S.; Drueckes, P.; Schächtele, C.; Kubbutat, M.H.; et al. Meriolins, a new class of cell death–inducing kinase inhibitors with enhanced selectivity for cyclin-dependent kinases. Cancer Res. 2007, 67, 8325–8334. [Google Scholar] [CrossRef]
- Imperatore, C.; Aiello, A.; D’Aniello, F.; Senese, M.; Menna, M. Alkaloids from marine invertebrates as important leads for anticancer drugs discovery and development. Molecules 2014, 19, 20391–20423. [Google Scholar] [CrossRef]
- Baeza, A.; Mendiola, J.; Burgos, C.; Alvarez-Builla, J.; Vaquero, J. Palladium-mediated C–N, C–C, and C–O functionalization of azolopyrimidines: A new total synthesis of Variolin B. Tetrahedron Lett. 2008, 49, 4073–4077. [Google Scholar] [CrossRef]
- Baeza, A.; Mendiola, J.; Burgos, C.; Alvarez-Builla, J.; Vaquero, J.J. Application of Selective Palladium-Mediated Functionalization of the Pyrido [3′,2′:4,5] pyrrolo [1,2-c] pyrimidine Heterocyclic System for the Total Synthesis of Variolin B and Deoxyvariolin B. EurJoc 2010, 1, 607–5618. [Google Scholar] [CrossRef]
- Ishibashi, F.; Miyazaki, Y.; Iwao, M. Total syntheses of lamellarin D and H. The first synthesis of lamellarin-class marine alkaloids. Tetrahedron 1997, 53, 5951–5962. [Google Scholar] [CrossRef]
- Komatsubara, M.; Umeki, T.; Fukuda, T.; Iwao, M. Modular synthesis of lamellarins via regioselective assembly of 3,4,5-differentially arylated pyrrole-2-carboxylates. J. Org. Chem. 2014, 79, 529–537. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, J.; Fan, A.; Cui, Y.; Jia, Y. Total synthesis of lamellarins D, H, and R and ningalin B. Org. Lett. 2011, 13, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Handy, S.T.; Zhang, Y. Approaches to the synthesis of the lamellarins and related natural products. A review. Org. Prep. Proced. Int. 2005, 37, 411–445. [Google Scholar] [CrossRef]
- Imbri, D.; Tauber, J.; Opatz, T. Synthetic approaches to the lamellarins—A comprehensive review. Mar. Drugs 2014, 12, 6142–6177. [Google Scholar] [CrossRef] [PubMed]
- Sivaganesan, P.; VL, S.; Sahoo, A.; Elanchezhian, C.; Nataraj, G.; Chaudhuri, S. A Comprehensive Review of Synthetic Approaches Toward Lamellarin D and its Analogous. ChemistrySelect 2024, 9, e202403112. [Google Scholar] [CrossRef]
- Theppawong, A.; Ploypradith, P.; Chuawong, P.; Ruchirawat, S.; Chittchang, M. Facile and divergent synthesis of lamellarins and lactam-containing derivatives with improved drug likeness and biological activities. Chem. Asian J. 2015, 10, 2631–2650. [Google Scholar] [CrossRef]
- Manjappa, K.B.; Syu, J.-R.; Yang, D.-Y. Visible-light-promoted and Yb (OTf) 3-catalyzed constructions of coumarin-pyrrole-(iso) quinoline-fused pentacycles: Synthesis of lamellarin core, lamellarin D trimethyl ether, and lamellarin H. Org. Lett. 2016, 18, 332–335. [Google Scholar] [CrossRef]
- Manjappa, K.B.; Lin, J.-M.; Yang, D.-Y. Construction of pentacyclic lamellarin skeleton via Grob reaction: Application to total synthesis of lamellarins H and D. J. Org. Chem. 2017, 82, 7648–7656. [Google Scholar] [CrossRef]
- Lade, D.M.; Pawar, A.B.; Mainkar, P.S.; Chandrasekhar, S. Total synthesis of lamellarin D trimethyl ether, lamellarin D, and lamellarin H. J. Org. Chem. 2017, 82, 4998–5004. [Google Scholar] [CrossRef]
- Mei, R.; Zhang, S.-K.; Ackermann, L. Concise Synthesis of Lamellarin Alkaloids by C–H/N–H Activation: Evaluation of Metal Catalysts in Oxidative Alkyne Annulation. Synlett 2017, 28, 1715–1718. [Google Scholar] [CrossRef][Green Version]
- Shirley, H.J.; Koyioni, M.; Muncan, F.; Donohoe, T.J. Synthesis of lamellarin alkaloids using orthoester-masked α-keto acids. Chem. Sci. 2019, 10, 4334–4338. [Google Scholar] [CrossRef]
- Liang, L.F.; Guo, Y.W. Terpenes from the soft corals of the genus Sarcophyton: Chemistry and biological activities. Chem. Biodivers. 2013, 10, 2161–2196. [Google Scholar] [CrossRef]
- Hamel, E.; Sackett, D.L.; Vourloumis, D.; Nicolaou, K. The coral-derived natural products eleutherobin and sarcodictyins A and B: Effects on the assembly of purified tubulin with and without microtubule-associated proteins and binding at the polymer taxoid site. Biochemistry 1999, 38, 5490–5498. [Google Scholar] [CrossRef] [PubMed]
- Pietra, F. Fighting cancer with microtubule-stabilizing agents: A computational investigation of the complex between β-tubulin and the microtubule-stabilizing, antitumor marine diterpenoid sarcodictyin A. Struct. Chem. 2020, 31, 927–935. [Google Scholar] [CrossRef]
- Risinger, A.L.; Giles, F.J.; Mooberry, S.L. Microtubule dynamics as a target in oncology. Cancer Treat. Rev. 2009, 35, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.; Pfefferkorn, J.; Xu, J.; Winssinger, N.; Ohshima, T.; Kim, S.; Hosokawa, S.; Vourloumis, D.; Van Delft, F.; Li, T. Total synthesis and chemical biology of the sarcodictyins. Chem. Pharm. Bull. 1999, 47, 1199–1213. [Google Scholar] [CrossRef]
- Lindel, T.; Jensen, P.R.; Fenical, W.; Long, B.H.; Casazza, A.M.; Carboni, J.; Fairchild, C.R. Eleutherobin, a new cytotoxin that mimics paclitaxel (Taxol) by stabilizing microtubules. J. Am. Chem. Soc. 1997, 119, 8744–8745. [Google Scholar] [CrossRef]
- Sosonyuk, S.E.; Peshich, A.; Tutushkina, A.V.; Khlevin, D.A.; Lozinskaya, N.A.; Gracheva, Y.A.; Glazunova, V.A.; Osolodkin, D.I.; Semenova, M.N.; Semenov, V.V. Synthesis and cytotoxicity of novel simplified eleutherobin analogues as potential antitumour agents. Org. Biomol. Chem. 2019, 17, 2792–2797. [Google Scholar] [CrossRef]
- Su, J.-H.; Tseng, S.-W.; Lu, M.-C.; Liu, L.-L.; Chou, Y.; Sung, P.-J. Cytotoxic C21 and C22 terpenoid-derived metabolites from the sponge Ircinia sp. J. Nat. Prod. 2011, 74, 2005–2009. [Google Scholar] [CrossRef]
- Nicolaou, K.; Winssinger, N.; Vourloumis, D.; Ohshima, T.; Kim, S.; Pfefferkorn, J.; Xu, J.-Y.; Li, T. Solid and solution phase synthesis and biological evaluation of combinatorial sarcodictyin libraries. J. Am. Chem. Soc. 1998, 120, 10814–10826. [Google Scholar] [CrossRef]
- Driedger, D.; Fers-Lidou, A.; Schroeder, M.; Elisia, I.; Krystal, G.; Britton, R. An Expedient Synthesis of the Antimitotic Natural Products Sarcodictyin and Eleutherobin, and Carbohydrate Analogues. J. Am. Chem. Soc. 2025, 147, 28117–28126. [Google Scholar] [CrossRef]
- Zhao, H.; McMillan, A.J.; Constantin, T.; Mykura, R.C.; Julia, F.; Leonori, D. Merging halogen-atom transfer (XAT) and cobalt catalysis to override E2-selectivity in the elimination of alkyl halides: A mild route toward contra-thermodynamic olefins. J. Am. Chem. Soc. 2021, 143, 14806–14813. [Google Scholar] [CrossRef]
- Nicolaou, K.; Van Delft, F.; Ohshima, T.; Vourloumis, D.; Xu, J.; Hosokawa, S.; Pfefferkorn, J.; Kim, S.; Li, T. Total synthesis of eleutherobin. Angew. Chem. Int. Ed. Engl. 1997, 36, 2520–2524. [Google Scholar] [CrossRef]
- Nicolaou, K.; Xu, J.-Y.; Kim, S.; Ohshima, T.; Hosokawa, S.; Pfefferkorn, J. Synthesis of the tricyclic core of eleutherobin and sarcodictyins and total synthesis of sarcodictyin A. J. Am. Chem. Soc. 1997, 119, 11353–11354. [Google Scholar] [CrossRef]
- Chen, X.-T.; Bhattacharya, S.K.; Zhou, B.; Gutteridge, C.E.; Pettus, T.R.; Danishefsky, S.J. The total synthesis of eleutherobin. J. Am. Chem. Soc. 1999, 121, 6563–6579. [Google Scholar] [CrossRef]
- Mowat, J.S. Studies Towards the Total Synthesis of Eleutherobin and Other Marine Natural Products. Ph.D. Thesis, Simon Fraser University, Vancouver, BC, Canada, 2012. [Google Scholar]
- Carter, R.; Hodgetts, K.; McKenna, J.; Magnus, P.; Wren, S. Studies on the stereoselective synthesis of the marine antitumor agent eleutherobin. Tetrahedron 2000, 56, 4367–4382. [Google Scholar] [CrossRef]
- Hickford, S.J.; Blunt, J.W.; Munro, M.H. Antitumour polyether macrolides: Four new halichondrins from the New Zealand deep-water marine sponge Lissodendoryx sp. Biorg. Med. Chem. 2009, 17, 2199–2203. [Google Scholar] [CrossRef]
- Dissanayake, D.S.; Nagahawatta, D.P.; Lee, J.-S.; Jeon, Y.-J. Immunomodulatory effects of halichondrin isolated from marine sponges and its synthetic analogs in oncological applications. Mar. Drugs 2024, 22, 426. [Google Scholar] [CrossRef]
- Newman, D.J. Drug discovery from natural sources. Curr. Pharmacol. Rep. 2023, 9, 67–89. [Google Scholar] [CrossRef]
- Corley, D.G.; Herb, R.; Moore, R.E.; Scheuer, P.J.; Paul, V.J. Laulimalides. New potent cytotoxic macrolides from a marine sponge and a nudibranch predator. J. Org. Chem. 1988, 53, 3644–3646. [Google Scholar] [CrossRef]
- Mooberry, S.L.; Tien, G.; Hernandez, A.H.; Plubrukarn, A.; Davidson, B.S. Laulimalide and isolaulimalide, new paclitaxel-like microtubule-stabilizing agents. Cancer Res. 1999, 59, 653–660. [Google Scholar]
- Mooberry, S.L.; Randall-Hlubek, D.A.; Leal, R.M.; Hegde, S.G.; Hubbard, R.D.; Zhang, L.; Wender, P.A. Microtubule-stabilizing agents based on designed laulimalide analogues. Proc. Natl. Acad. Sci. USA 2004, 101, 8803–8808. [Google Scholar] [CrossRef]
- Prota, A.E.; Bargsten, K.; Northcote, P.T.; Marsh, M.; Altmann, K.H.; Miller, J.H.; Díaz, J.F.; Steinmetz, M.O. Structural basis of microtubule stabilization by laulimalide and peloruside A. Angew. Chem. Int. Ed. 2014, 53, 1621–1625. [Google Scholar] [CrossRef]
- Horton, P.A.; Koehn, F.E.; Longley, R.E.; McConnell, O.J. Lasonolide A, a new cytotoxic macrolide from the marine sponge Forcepia sp. J. Am. Chem. Soc. 1994, 116, 6015–6016. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Guzman, E.A.; Pitts, T.P.; Wright, A.E. Early effects of lasonolide A on pancreatic cancer cells. J. Pharmacol. Exp. Ther. 2009, 331, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-W.; Ghosh, A.K.; Pommier, Y. Lasonolide A, a potent and reversible inducer of chromosome condensation. Cell Cycle 2012, 11, 4424–4435. [Google Scholar] [CrossRef] [PubMed]
- West, L.M.; Northcote, P.T.; Battershill, C.N. Peloruside A: A potent cytotoxic macrolide isolated from the New Zealand marine sponge Mycale sp. J. Org. Chem. 2000, 65, 445–449. [Google Scholar] [CrossRef]
- Liao, X.; Wu, Y.; De Brabander, J.K. Total synthesis and absolute configuration of the novel microtubule-stabilizing agent peloruside A. Angew. Chem. 2003, 42, 1648–1652. [Google Scholar] [CrossRef]
- McGowan, M.A.; Stevenson, C.P.; Schiffler, M.A.; Jacobsen, E.N. An enantioselective total synthesis of (+)-peloruside A. Angew. Chem. 2010, 49, 6147–6151. [Google Scholar] [CrossRef]
- Jin, M.; Taylor, R.E. Total synthesis of (+)-peloruside A. Org. Lett. 2005, 7, 1303–1305. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Xu, X.; Kim, J.-H.; Xu, C.-X. Enantioselective total synthesis of peloruside A: A potent microtubule stabilizer. Org. Lett. 2008, 10, 1001–1004. [Google Scholar] [CrossRef]
- Wilmes, A.; Bargh, K.; Kelly, C.; Northcote, P.T.; Miller, J.H. Peloruside A synergizes with other microtubule stabilizing agents in cultured cancer cell lines. Mol. Pharm. 2007, 4, 269–280. [Google Scholar] [CrossRef]
- Aicher, T.D.; Buszek, K.R.; Fang, F.G.; Forsyth, C.J.; Jung, S.H.; Kishi, Y.; Matelich, M.C.; Scola, P.M.; Spero, D.M.; Yoon, S.K. Total synthesis of halichondrin B and norhalichondrin B. J. Am. Chem. Soc. 1992, 114, 3162–3164. [Google Scholar] [CrossRef]
- Jackson, K.L.; Henderson, J.A.; Motoyoshi, H.; Phillips, A.J. A total synthesis of norhalichondrin B. Angew. Chem. Int. Ed. 2009, 48, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Ueda, A.; Brémond, P.; Tiseni, P.S.; Kishi, Y. Total synthesis of halichondrin C. J. Am. Chem. Soc. 2012, 134, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Yahata, K.; Ye, N.; Ai, Y.; Iso, K.; Kishi, Y. Unified, Efficient, and Scalable Synthesis of Halichondrins: Zirconium/Nickel-Mediated One-Pot Ketone Synthesis as the Final Coupling Reaction. Angew. Chem. 2017, 129, 10936–10940. [Google Scholar] [CrossRef]
- Ueda, A.; Yamamoto, A.; Kato, D.; Kishi, Y. Total synthesis of halichondrin A, the missing member in the halichondrin class of natural products. J. Am. Chem. Soc. 2014, 136, 5171–5176. [Google Scholar] [CrossRef]
- Wang, Y.; Habgood, G.J.; Christ, W.J.; Kishi, Y.; Littlefield, B.A.; Yu, M.J. Structure–activity relationships of halichondrin B analogues: Modifications at C. 30–C. 38. Bioorg. Med. Chem. Lett. 2000, 10, 1029–1032. [Google Scholar] [CrossRef]
- Nicolaou, K.; Pan, S.; Shelke, Y.; Das, D.; Ye, Q.; Lu, Y.; Sau, S.; Bao, R.; Rigol, S. A reverse approach to the total synthesis of halichondrin B. J. Am. Chem. Soc. 2021, 143, 9267–9276. [Google Scholar] [CrossRef]
- Yoshimura, T.; Yakushiji, F.; Kondo, S.; Wu, X.; Shindo, M.; Shishido, K. Total synthesis of (+)-lasonolide A. Org. Lett. 2006, 8, 475–478. [Google Scholar] [CrossRef]
- Trost, B.M.; Stivala, C.E.; Hull, K.L.; Huang, A.; Fandrick, D.R. A concise synthesis of (−)-lasonolide A. J. Am. Chem. Soc. 2014, 136, 88–91. [Google Scholar] [CrossRef]
- Kang, S.H.; Kang, S.Y.; Choi, H.-W.; Kim, C.M.; Jun, H.-S.; Youn, J.-H. Stereoselective total synthesis of the natural (+)-lasonolide A. Synthesis 2004, 2004, 1102–1114. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Gong, G. Total Synthesis of Potent Antitumor Agent (−)-Lasonolide A: A Cycloaddition-Based Strategy. Chem. Asian J. 2008, 3, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Joo, J.M.; Kang, J.W.; Kim, D.-S.; Jung, C.-K.; Kwak, H.S.; Park, J.H.; Lee, E.; Hong, C.Y.; Jeong, S.; et al. Lasonolide A: Structural revision and total synthesis. J. Org. Chem. 2003, 68, 8080–8087. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Gong, G. Enantioselective total synthesis of macrolide antitumor agent (−)-lasonolide A. Org. Lett. 2007, 9, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kang, S.Y.; Kim, C.M.; Choi, H.-W.; Jun, H.-S.; Lee, B.M.; Park, C.M.; Jeong, J.W. Total synthesis of natural (+)-lasonolide A. Angew. Chem. 2003, 115, 4927–4930. [Google Scholar] [CrossRef]
- Trost, B.M.; Stivala, C.E.; Fandrick, D.R.; Hull, K.L.; Huang, A.; Poock, C.; Kalkofen, R. Total synthesis of (−)-lasonolide A. J. Am. Chem. Soc. 2016, 138, 11690–11701. [Google Scholar] [CrossRef]
- Trost, B.M.; Cregg, J.J. Ruthenium-catalyzed alkene–alkyne coupling of disubstituted olefins: Application to the stereoselective synthesis of trisubstituted enecarbamates. J. Am. Chem. Soc. 2015, 137, 620–623. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Fujii, Y.; Herald, C.L.; Inoue, M.; Brown, P.; Gust, D.; Kitahara, K.; Schmidt, J.M.; Doubek, D.L. Marine animal biosynthetic constituents for cancer chemotherapy. J. Nat. Prod. 1981, 44, 482–485. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Dufresne, C.; Cerny, R.L.; Herald, C.L.; Schmidt, J.M. Isolation and structure of the cytostatic linear depsipeptide dolastatin 15. J. Org. Chem. 1989, 54, 6005–6006. [Google Scholar] [CrossRef]
- Pettit, G.R.; Smith, T.H.; Arce, P.M.; Flahive, E.J.; Anderson, C.R.; Chapuis, J.-C.; Xu, J.-P.; Groy, T.L.; Belcher, P.E.; Macdonald, C.B. Antineoplastic agents. 599. Total synthesis of dolastatin 16. J. Nat. Prod. 2015, 78, 476–485. [Google Scholar] [CrossRef]
- Pika, J.; Faulkner, D.J. A reinvestigation of the didemnaketals from the Palauan ascidian Didemnum sp. Nat. Prod. Lett. 1995, 7, 291–296. [Google Scholar] [CrossRef]
- Potts, B.C.; Faulkner, D.J.; Chan, J.A.; Simolike, G.C.; Offen, P.; Hemling, M.E.; Francis, T.A. Didemnaketals A and B, HIV-1 protease inhibitors from the ascidian Didemnum sp. J. Am. Chem. Soc. 1991, 113, 6321–6322. [Google Scholar] [CrossRef]
- Nicolaou, K.; Bella, M.; Chen, D.Y.K.; Huang, X.; Ling, T.; Snyder, S.A. Total synthesis of diazonamide A. Angew. Chem. 2002, 114, 3645–3649. [Google Scholar] [CrossRef]
- Nicolaou, K.; Bheema Rao, P.; Hao, J.; Reddy, M.V.; Rassias, G.; Huang, X.; Chen, D.Y.K.; Snyder, S.A. The second total synthesis of diazonamide A. Angew. Chem. Int. Ed. 2003, 42, 1753–1758. [Google Scholar] [CrossRef]
- Knowles, R.R.; Carpenter, J.; Blakey, S.B.; Kayano, A.; Mangion, I.K.; Sinz, C.J.; MacMillan, D.W. Total synthesis of diazonamide A. Chem. Sci. 2010, 2011, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S. Applied Microbiology; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Erba, E.; Bergamaschi, D.; Ronzoni, S.; Faretta, M.; Taverna, S.; Bonfanti, M.; Catapano, C.; Faircloth, G.; Jimeno, J.; D’incalci, M. Mode of action of thiocoraline, a natural marine compound with anti-tumour activity. Br. J. Cancer 1999, 80, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R.; Dahiya, S.; Fuloria, N.K.; Jankie, S.; Agarwal, A.; Davis, V.; Sahadeo, V.; Radhay, V.; Ramsubhag, Y.; Mullings, W. Natural Thiazoline-based cyclodepsipeptides from marine cyanobacteria: Chemistry, bioefficiency and clinical aspects. Curr. Med. Chem. 2021, 28, 7887–7909. [Google Scholar] [CrossRef] [PubMed]
- Boger, D.L.; Ichikawa, S. Total syntheses of thiocoraline and BE-22179: Establishment of relative and absolute stereochemistry. J. Am. Chem. Soc. 2000, 122, 2956–2957. [Google Scholar] [CrossRef]
- Dahiya, R.; Dahiya, S.; Kumar, P.; Kumar, R.V.; Dahiya, S.; Kumar, S.; Saharan, R.; Basu, P.; Mitra, A.; Sharma, A. Structural and biological aspects of natural bridged macrobicyclic peptides from marine resources. Arch. Pharm. 2021, 354, 2100034. [Google Scholar] [CrossRef]
- Edler, M.C.; Fernandez, A.M.; Lassota, P.; Ireland, C.M.; Barrows, L.R. Inhibition of tubulin polymerization by vitilevuamide, a bicyclic marine peptide, at a site distinct from colchicine, the vinca alkaloids, and dolastatin 10. Biochem. Pharmacol. 2002, 63, 707–715. [Google Scholar] [CrossRef]
- Wipf, P.; Reeves, J.T.; Day, B.W. Chemistry and biology of curacin A. Curr. Pharm. Des. 2004, 10, 1417–1437. [Google Scholar] [CrossRef]
- Rinehart, K.; Fregeau, N.; Warwick, R.; Garcia Gravalos, D.; Avila, J.; Faircloth, G. Spisulosine Compounds Having Antitumor Activity. PCT WO0052521 A, 31 August 1999. Volume 1, p. 19991021. [Google Scholar]
- Ganesher, A.; Chaturvedi, P.; Sahai, R.; Meena, S.; Mitra, K.; Datta, D.; Panda, G. New Spisulosine Derivative promotes robust autophagic response to cancer cells. Eur. J. Med. Chem. 2020, 188, 112011. [Google Scholar] [CrossRef]
- Gonda, J.; Fazekašová, S.; Martinková, M.; Mitríková, T.; Roman, D.; Pilátová, M.B. Synthesis and biological activity of sphingosines with integrated azobenzene switches. Org. Biomol. Chem. 2019, 17, 3361–3373. [Google Scholar] [CrossRef]
- Warabi, K.; Matsunaga, S.; van Soest, R.W.M.; Fusetani, N. Dictyodendrins A−E, the First Telomerase-Inhibitory Marine Natural Products from the Sponge Dictyodendrilla v erongiformis. J. Org. Chem. 2003, 68, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Gowan, S.M.; Harrison, J.R.; Patterson, L.; Valenti, M.; Read, M.A.; Neidle, S.; Kelland, L.R. A G-quadruplex-interactive potent small-molecule inhibitor of telomerase exhibiting in vitro and in vivo antitumor activity. Mol. Pharmacol. 2002, 61, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Fürstner, A.; Domostoj, M.M.; Scheiper, B. Total synthesis of dictyodendrin B. J. Am. Chem. Soc. 2005, 127, 11620–11621. [Google Scholar] [CrossRef] [PubMed]
- Fürstner, A.; Domostoj, M.M.; Scheiper, B. Total syntheses of the telomerase inhibitors dictyodendrin B, C, and E. J. Am. Chem. Soc. 2006, 128, 8087–8094. [Google Scholar] [CrossRef]
- Buchgraber, P.; Domostoj, M.M.; Scheiper, B.; Wirtz, C.; Mynott, R.; Rust, J.; Fürstner, A. Synthesis-driven mapping of the dictyodendrin alkaloids. Tetrahedron 2009, 65, 6519–6534. [Google Scholar] [CrossRef]
- Okano, K.; Fujiwara, H.; Noji, T.; Fukuyama, T.; Tokuyama, H. Total synthesis of dictyodendrin A and B. Angew. Chem. Int. Ed. 2010, 49, 5925–5929. [Google Scholar] [CrossRef]
- Tokuyama, H. Construction of N-heterocycles fused with a highly substituted benzene ring by a benzyne-mediated cyclization/functionalization cascade reaction and its application to the total synthesis of marine natural products. Chem. Pharm. Bull. 2021, 69, 707–716. [Google Scholar] [CrossRef]
- Hirao, S.; Yoshinaga, Y.; Iwao, M.; Ishibashi, F. A formal total synthesis of the telomerase inhibitor dictyodendrin B. Tetrahedron Lett. 2010, 51, 533–536. [Google Scholar] [CrossRef]
- Ayats, C.; Soley, R.; Albericio, F.; Alvarez, M. Synthesis of the pyrrolo [2,3-c] carbazole core of the dictyodendrins. Org. Biomol. Chem. 2009, 7, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Hirao, S.; Sugiyama, Y.; Iwao, M.; Ishibashi, F. Synthetic approach to telomerase inhibitor dictyodendrin B: Synthesis of the pyrrolo [2,3-c] carbazole core. Biosci. Biotechnol. Biochem. 2009, 73, 1764–1772. [Google Scholar] [CrossRef]
- Liang, J.; Hu, W.; Tao, P.; Jia, Y. Total synthesis of dictyodendrins B and E. J. Org. Chem. 2013, 78, 5810–5815. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, P.; Shaw, A.K. An efficient total synthesis of the anticancer agent (+)-spisulosine (ES-285) from Garner’s aldehyde. Tetrahedron Lett. 2010, 51, 4140–4142. [Google Scholar] [CrossRef]
- Amarante, G.W.; Cavallaro, M.; Coelho, F. Highly diastereoselective total synthesis of the anti-tumoral agent (±)-Spisulosine (ES285) from a Morita–Baylis–Hillman adduct. Tetrahedron Lett. 2010, 51, 2597–2599. [Google Scholar] [CrossRef]
- Dinda, S.K.; Das, S.K.; Panda, G. Asymmetric total syntheses of spisulosine, its diastereo-and regio-isomers. Tetrahedron 2010, 66, 9304–9309. [Google Scholar] [CrossRef]
- Fabišíková, M.; Martinková, M.; Hirková, S.; Gonda, J.; Pilátová, M.B.; Gönciová, G. Total synthesis and the anticancer activity of (+)-spisulosine. Carbohydr. Res. 2016, 435, 26–36. [Google Scholar] [CrossRef]
- Abushanab, E.; Vemishetti, P.; Leiby, R.W.; Singh, H.K.; Mikkilineni, A.B.; Wu, D.C.; Saibaba, R.; Panzica, R.P. The chemistry of L-ascorbic and D-isoascorbic acids. 1. The preparation of chiral butanetriols and-tetrols. J. Org. Chem. 1988, 53, 2598–2602. [Google Scholar] [CrossRef]
- Pitts, A.K.; O’Hara, F.; Snell, R.H.; Gaunt, M.J. A Concise and Scalable Strategy for the Total Synthesis of Dictyodendrin B Based on Sequential C- H Functionalization. Angew. Chem. Int. Ed. 2015, 54, 5451–5455. [Google Scholar] [CrossRef]
- Matsuoka, J.; Inuki, S.; Matsuda, Y.; Miyamoto, Y.; Otani, M.; Oka, M.; Oishi, S.; Ohno, H. Total Synthesis of Dictyodendrins A–F by the Gold-Catalyzed Cascade Cyclization of Conjugated Diyne with Pyrrole. Chem. Eur. J. 2020, 26, 11150–11157. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colarusso, E.; Giordano, A.; Chini, M.G.; Bifulco, G.; Lauro, G. Marine Natural Products in Preclinical Cancer Studies: Ten Years of Advanced Total Synthesis. Mar. Drugs 2025, 23, 430. https://doi.org/10.3390/md23110430
Colarusso E, Giordano A, Chini MG, Bifulco G, Lauro G. Marine Natural Products in Preclinical Cancer Studies: Ten Years of Advanced Total Synthesis. Marine Drugs. 2025; 23(11):430. https://doi.org/10.3390/md23110430
Chicago/Turabian StyleColarusso, Ester, Assunta Giordano, Maria Giovanna Chini, Giuseppe Bifulco, and Gianluigi Lauro. 2025. "Marine Natural Products in Preclinical Cancer Studies: Ten Years of Advanced Total Synthesis" Marine Drugs 23, no. 11: 430. https://doi.org/10.3390/md23110430
APA StyleColarusso, E., Giordano, A., Chini, M. G., Bifulco, G., & Lauro, G. (2025). Marine Natural Products in Preclinical Cancer Studies: Ten Years of Advanced Total Synthesis. Marine Drugs, 23(11), 430. https://doi.org/10.3390/md23110430









