Novel Sulfated Oligosaccharide DP9 from Marine Algae, Gracilaria lemaneiformis: A Potent Galectin-3 Inhibitor for Pancreatic Cancer Therapy
Abstract
1. Introduction
2. Results
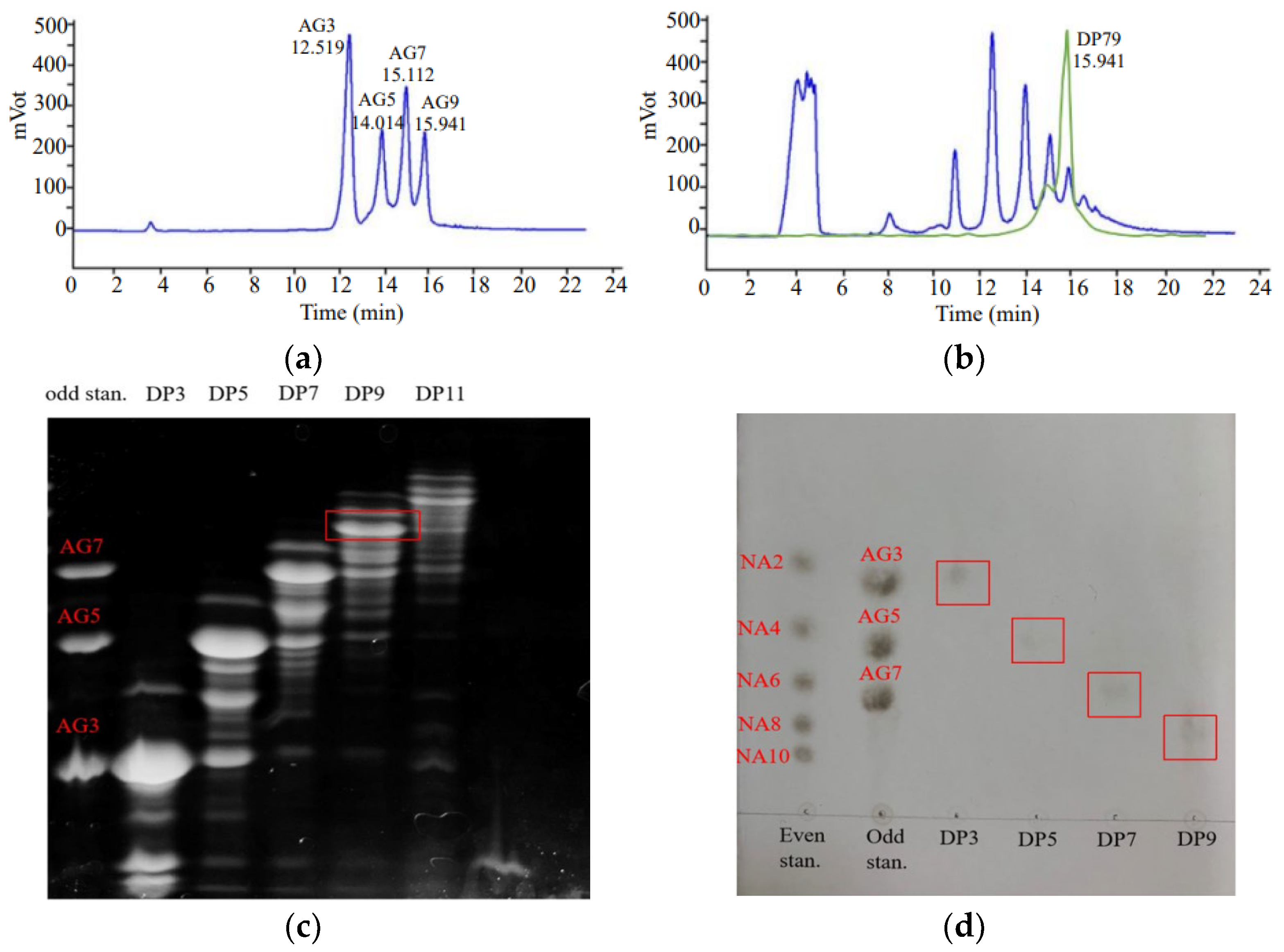
2.1. Separation and Identification of Acidolysis Oligosaccharides from G. lemaneiformis
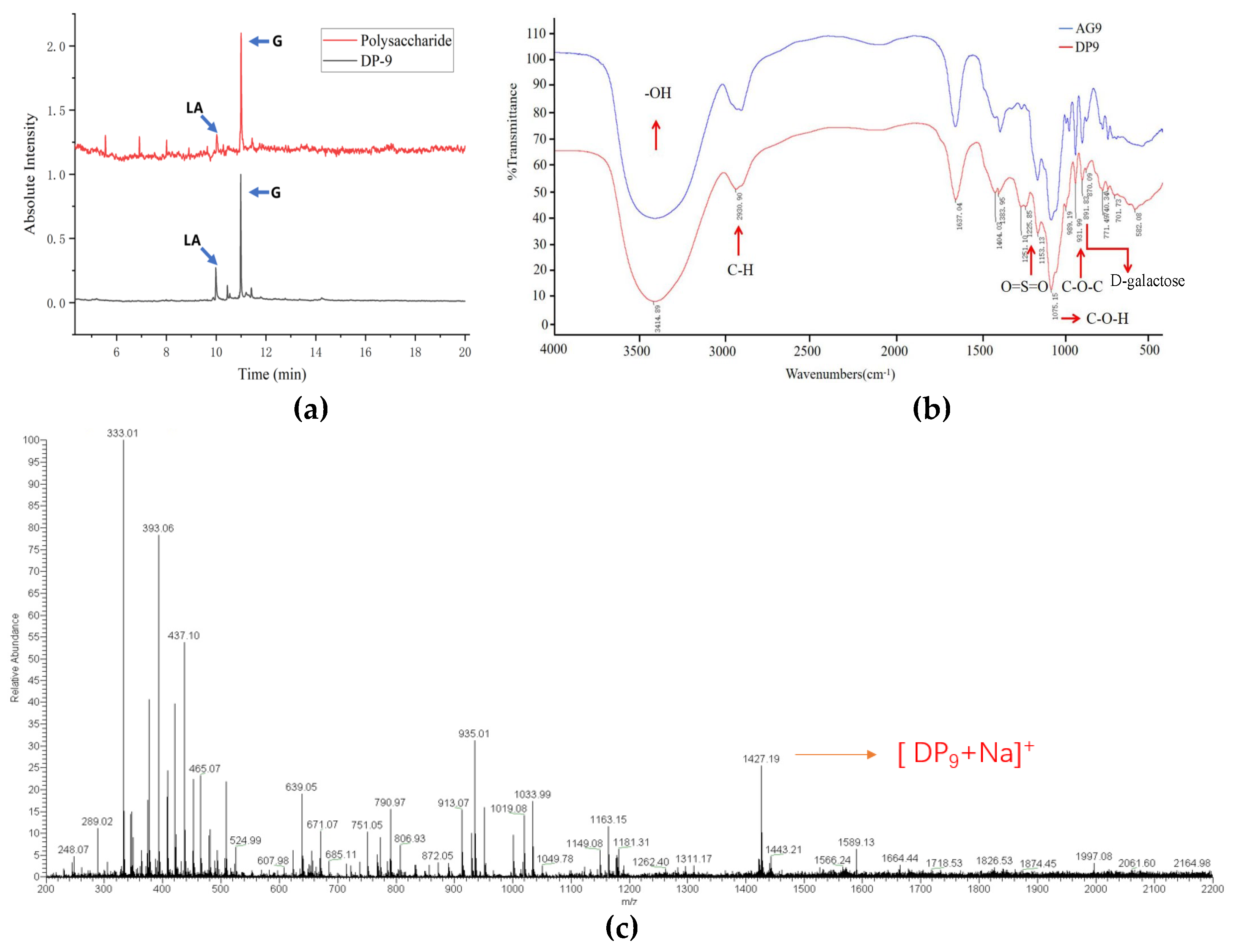
2.2. Analysis of DP9 Structural Characteristics
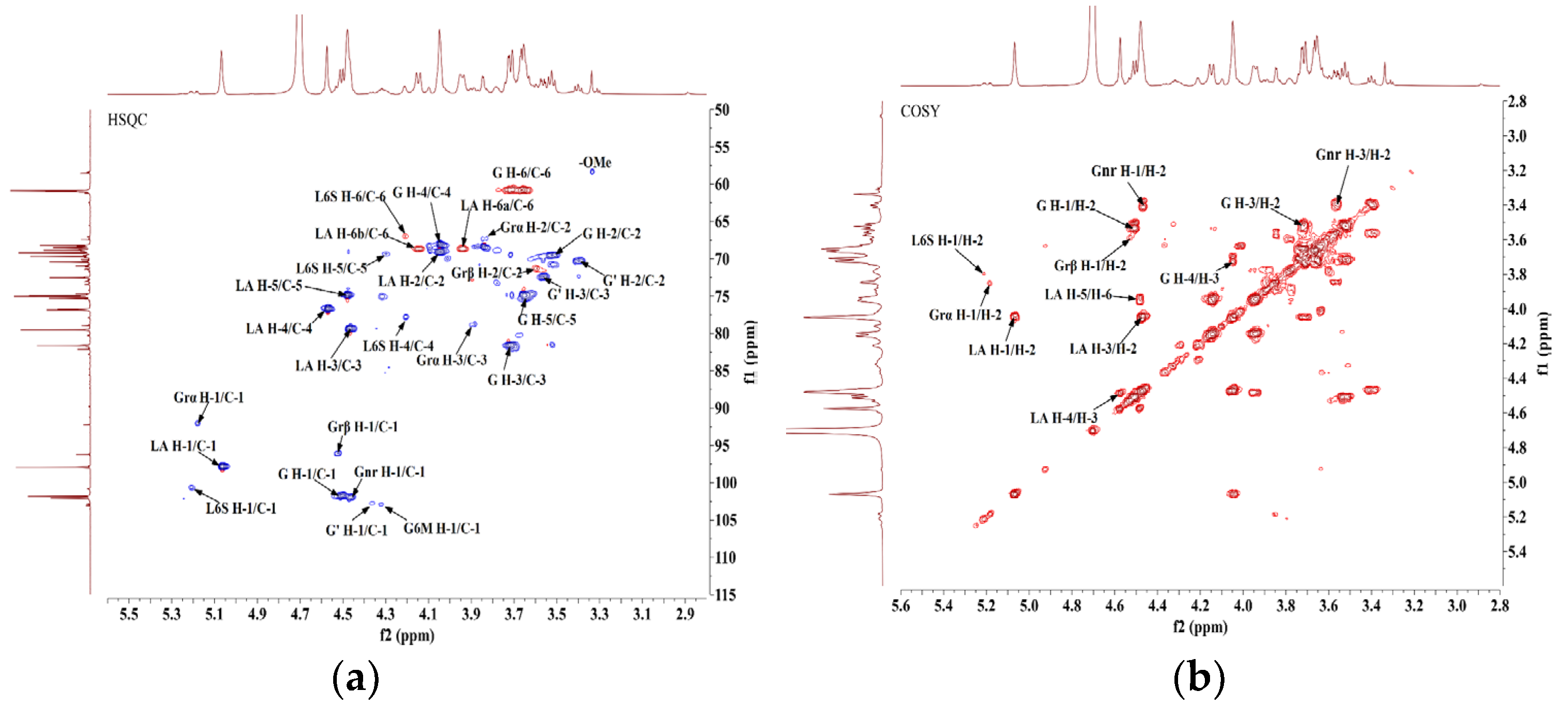
2.3. Structural Elucidation of DP9 by Multidimensional NMR Spectroscopy
- (1)
- Reductive Galactose Terminal Residues (Grα/Grβ) [31,34]: Cross-peaks at δ 5.18/92.37 ppm (H-1/C-1) and δ 4.52/96.15 ppm (H-1/C-1) in the HSQC (Figure 4a) anomeric region, consistent with α/β-configured reducing galactose terminals (Correc et al., 2011), were assigned to →3)-α-D-Galp (Grα) and →3)-β-D-Galp (Grβ), respectively. Chemical shifts were confirmed via COSY (Figure 4b)/HSQC.
- (2)
- →4)-α-L-AnGalp-(1→ Residue (LA) [29,34]: A strong HSQC cross-peak at δ 5.06/98.06 ppm (H-1/C-1) matched α-L-AnGalp [28,29,30]. Sequential assignment via COSY/HSQC revealed H-1–H-5 shifts (δ 5.06, 4.05, 4.46, 4.57, 4.48 ppm) and C-1–C-6 shifts (δ 98.06, 69.11, 79.63, 76.89, 74.91, 68.72 ppm). Downfield shifts at C-1, C-3, C-4, and C-6 indicated →4)-α-L-AnGalp-(1→ (LA).
- (3)
- β-D-Galp Residues (G/Gnr) [30]: Cross-peaks at δ 4.51/101.84 ppm and δ 4.46/102.12 ppm (H-1/C-1) corresponded to β-Galp [25,31,32,33,34,35]. For δ 4.51/101.84 ppm, H-1–H-5 shifts (δ 4.51, 3.53, 3.72, 4.04, 3.65 ppm) and C-1–C-6 shifts (δ 101.84, 69.74, 81.70, 68.18, 75.03, 60.92 ppm) suggested →3)-β-D-Galp-(1→ (G). The δ 4.46/102.12 ppm signal was assigned to →4)-α-L-Galp6S-(1→ (L6S) with sulfation at C-6. The G→L6S linkage was weaker than G→LA, with the H-1 integral ratio (L6S:LA = 1:8.20), indicating G→LA as the dominant repeating unit.
- (4)
- Sulfated and Methylated Modifications [32,33,34]: The absence of cross-peaks in HSQC non-anomeric regions (δ 4.65–5.10 ppm/δ 60–90 ppm) ruled out sulfation at β-D-Galp C-2 or α-L-AnGalp C-2. A weak cross-peak at δ 5.21/100.80 ppm (H-1/C-1) was assigned to →4)-α-L-Galp6S-(1→ (L6S). A cross-peak at δ 3.33/58.57 ppm (-OMe) indicated β-D-Galp C-6 methylation [25,31,34,35] assigned to →4)-α-L-Galp6S-(1→ (G6M).
- (1)
- HMBC correlations: G-H1 (δ 4.51) ↔ LA-C4 (δ 76.89), LA-H4 (δ 4.57) ↔ G-C1 (δ 101.84); NOESY correlation: LA-H4 ↔ G-H1, indicating →3)-β-D-Galp-(1→4)-α-L-AnGalp-(1→ (G→LA).
- (2)
- HMBC correlations: LA-H1 (δ 5.06) ↔ G-C3 (δ 81.70), G-H3 (δ 3.72) ↔ LA-C1 (δ 98.06); NOESY correlation: LA-H1 ↔ G-H3, indicating →4)-α-L-AnGalp-(1→3)-β-D-Galp-(1→ (LA→G).
- (3)
- NOESY correlation: G’-H1 (δ 4.37) ↔ L6S-H4 (δ 4.20), indicating →3)-β-D-Galp-(1→4)-α-L-Galp6S-(1→ (G→L6S).
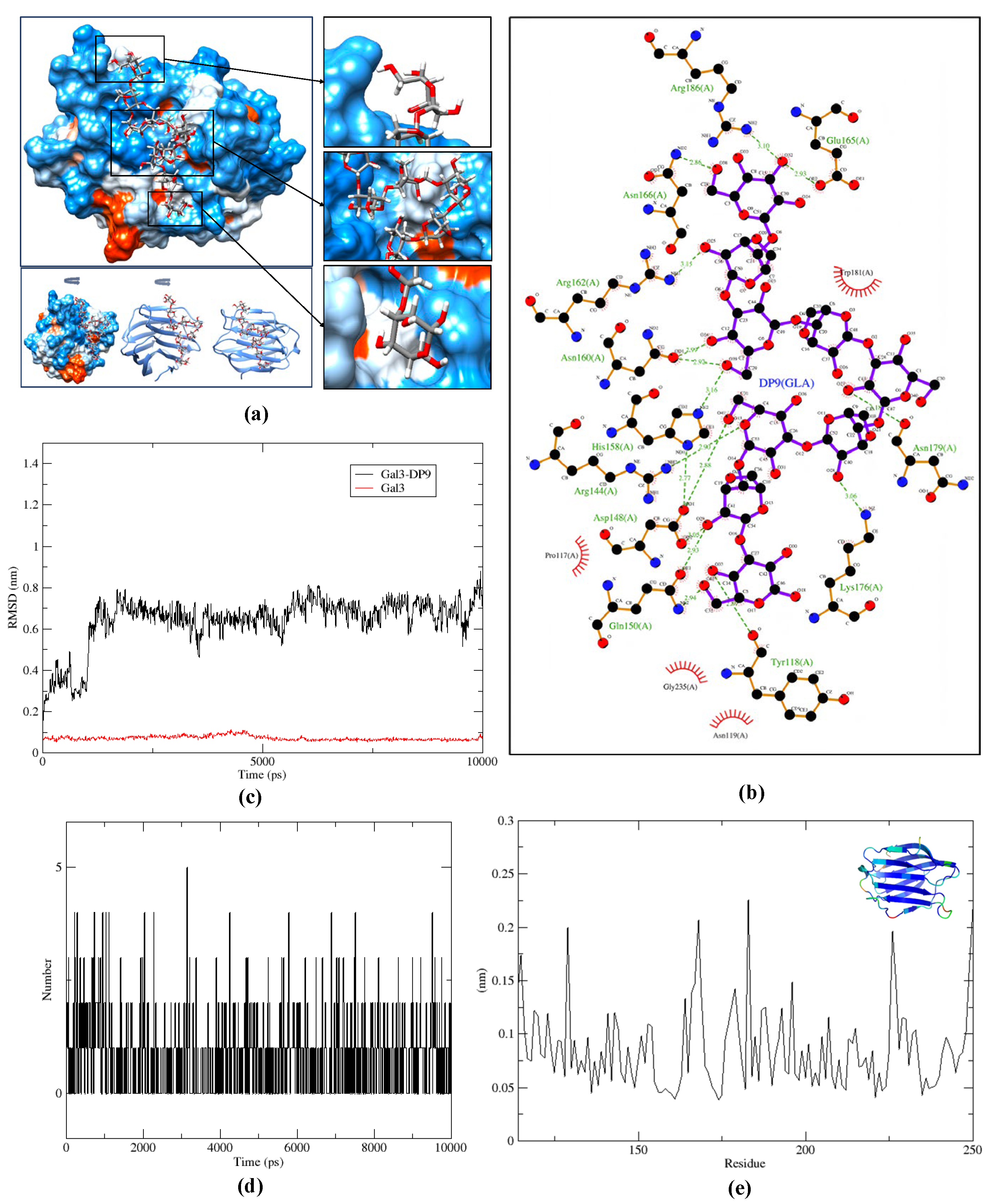
2.4. Molecular Docking and Dynamic Simulation
| Name | Chemical Structure |
|---|---|
| DP9(G-LA) |  |
| DP9(G-L6S)1 | 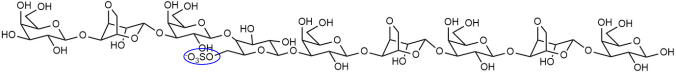 |
| DP9(G-L6S)2 | 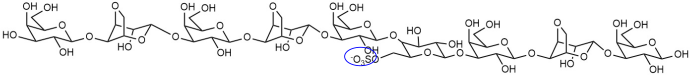 |
| DP9(G-L6S)3 |  |
| DP9(G-L6S)4 | 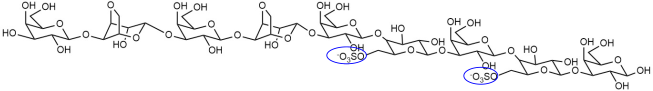 |
| DP9(G-L6S)5 | 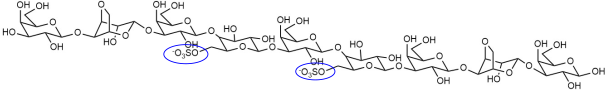 |
| DP9(G-L6S)6 |  |
| Ligand Name | LeDock Score (kcal/mol) | |||
|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | Average | |
| DP9(G-L6S)1 | −9.07 | −8.3 | −8.14 | −8.50 |
| DP9(G-L6S)2 | −7.55 | −8.2 | −7.38 | −7.71 |
| DP9(G-L6S)3 | −9.35 | −8.83 | −7.83 | −8.67 |
| DP9(G-L6S)4 | −8.84 | −9.22 | −9.44 | −9.17 |
| DP9(G-L6S)5 | −9.43 | −9.19 | −7.72 | −8.78 |
| DP9(G-L6S)6 | −9.77 | −10.45 | −10.05 | −10.09 |
| DP9(G-LA) | −8.82 | −8.15 | −8.4 | −8.46 |
2.5. Anti-Pancreatic Cancer Activity of DP9
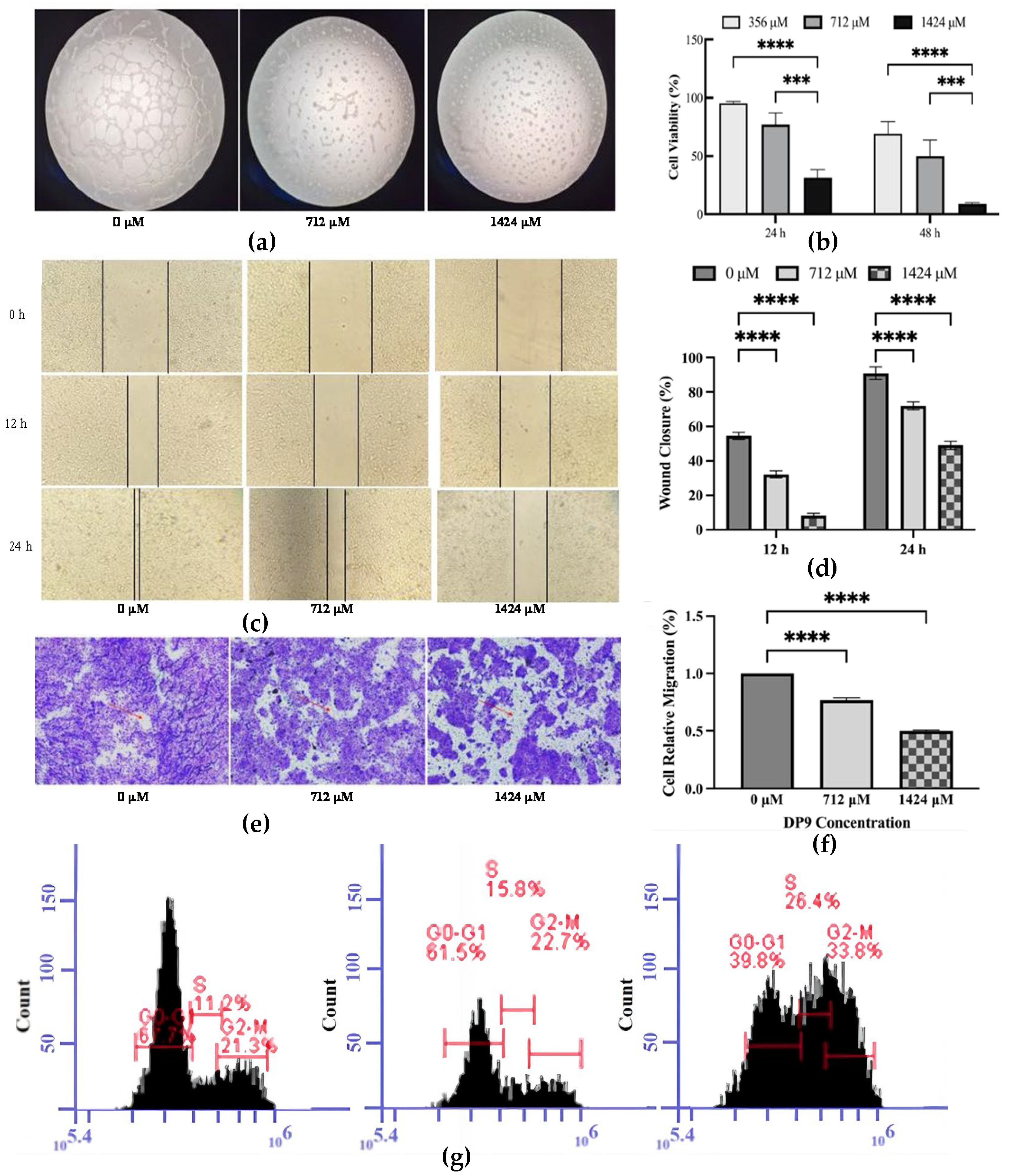
2.5.1. Inhibitory Effect of DP9 on Angiogenesis and the Proliferation of Pancreatic Cancer BxPC-3 Cells In Vitro
2.5.2. Inhibition Effect of DP9 on BxPC-3 Cell Migration
2.5.3. Effect of DP9 on S-Phase Arrest of BxPC-3 Cells
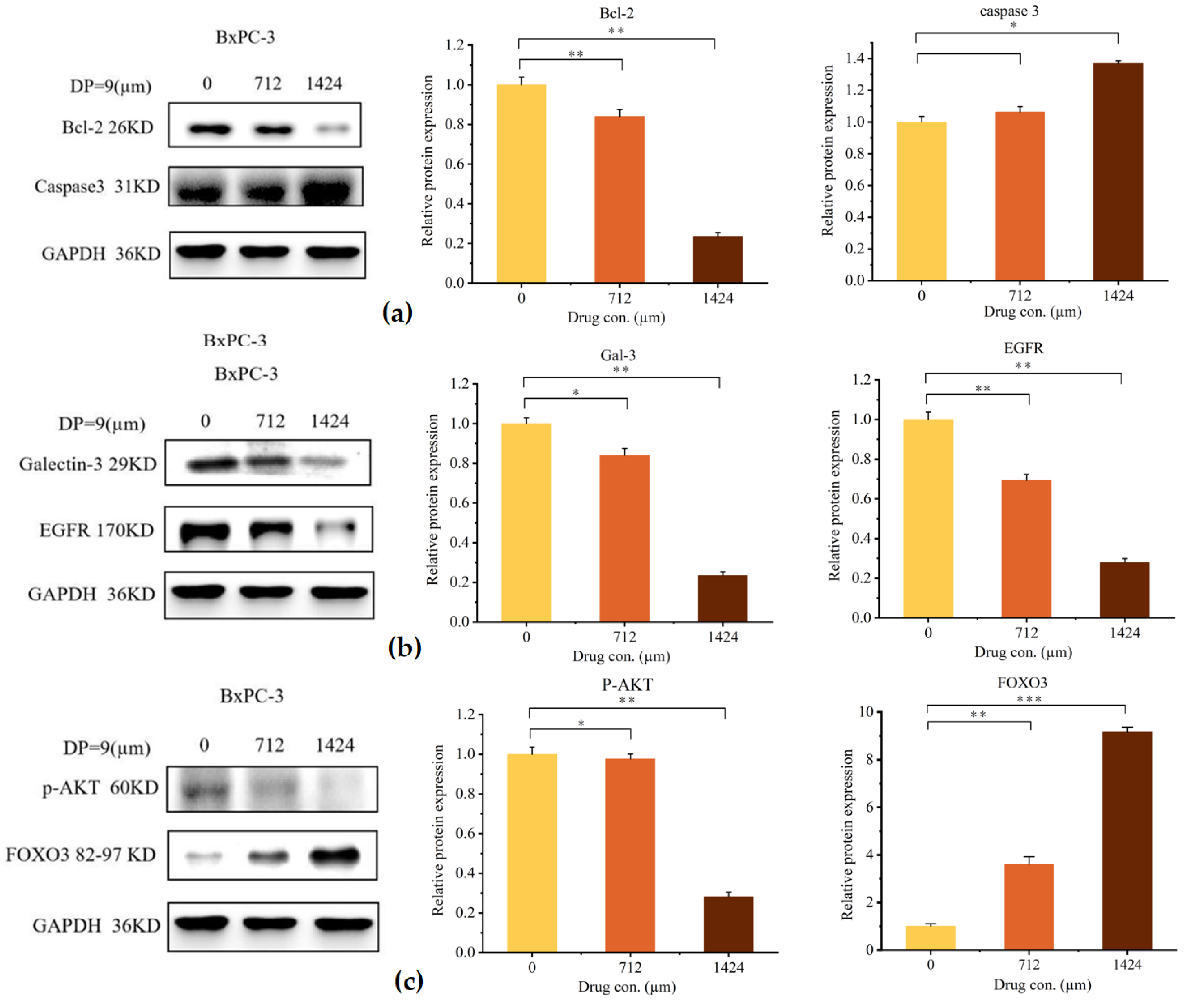
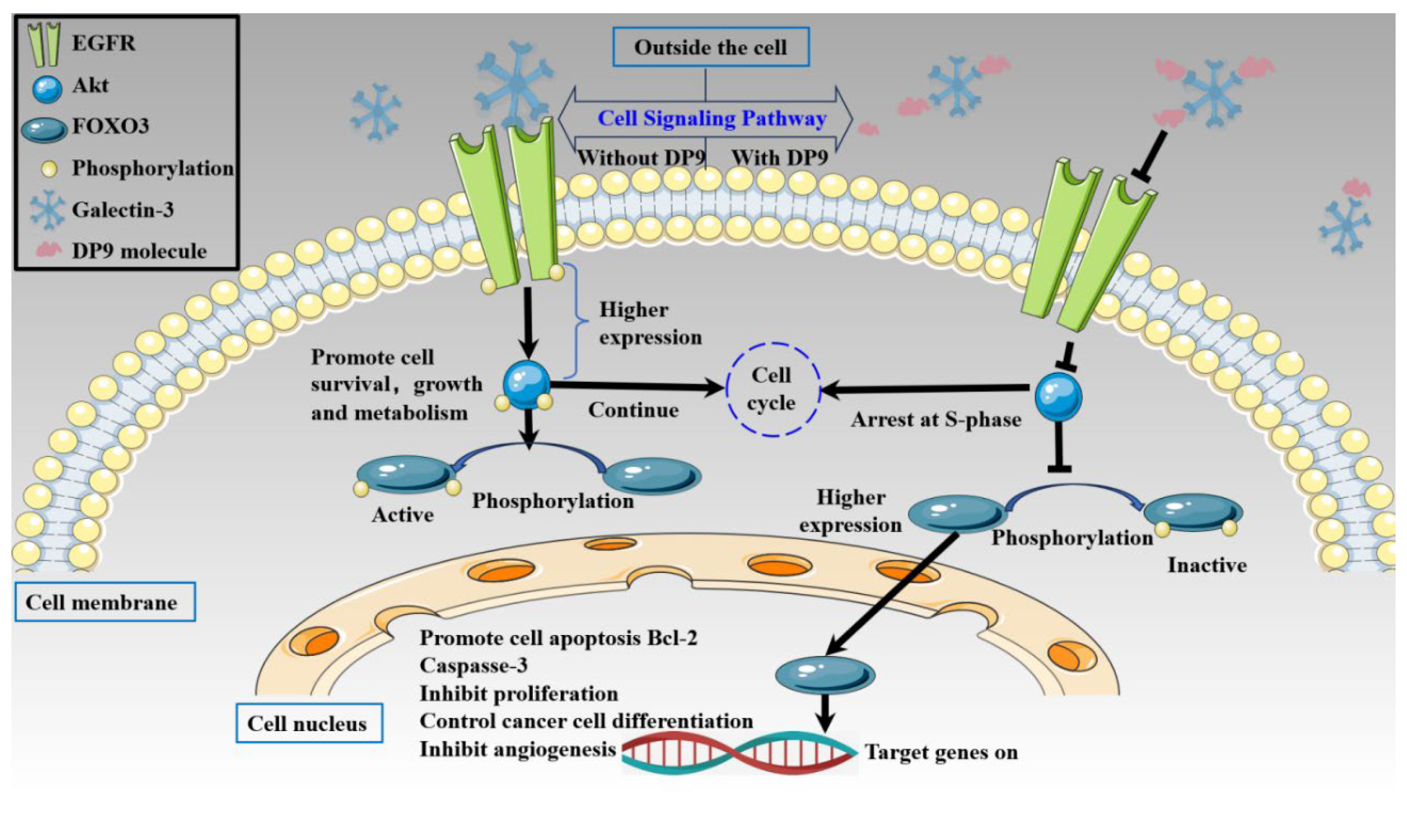
2.5.4. Anti-Pancreatic Cancer Activity of DP9 Through Targeting the Gal-3/EGFR/AKT/FOXO3 Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.1.1. General Reagents
4.1.2. Cell Lines and Cell Culture
4.2. Determination of DP9 Oligosaccharide Structural Characterization
4.2.1. Extraction of Crude Polysaccharides from G. lemaneiformis
4.2.2. Acidolysis of G. lemaneiformis Crude Polysaccharides
4.2.3. High-Performance Liquid Chromatography-Evaporative Light-Scattering Detector (HPLC-ELSD) Analysis of Acid Hydrolysis Products
4.2.4. Separation of Oligosaccharides from G. lemaneiformis
4.2.5. Thin-Layer Chromatography (TLC) Analysis of Oligosaccharides
4.2.6. Fluorophore-Assisted Carbohydrate Electrophoresis (Face) Analysis of Oligosaccharides
4.2.7. Analysis of Monosaccharides and Their Ratios (Anhydro-Galactose/Galactose, Angal/Gal) in Dp9
4.2.8. Fourier-Transform Infrared (FTIR) Analysis of DP9
4.2.9. Electrospray Ionization Mass Spectrometry (ESI-MS) Analysis of DP9
4.2.10. Nuclear Magnetic Resonance (NMR) Analysis of DP9
4.3. Computational Analysis of Molecular Interactions Between DP9 and Human Gal-3
4.3.1. Molecular Docking
4.3.2. Molecular Dynamic Simulation
4.4. Gal-3 Inhibitory Effect and In Vitro Anti-Pancreatic Cancer Activity of DP9
4.4.1. Gal-3 Inhibitory Effect of DP9 Through Hemagglutination Assay
4.4.2. Effect of DP9 on BxPC-3 Cell Proliferation
4.4.3. Effect of DP9 on BxPC-3 Cell Migration
4.4.4. Effect of DP9 on BxPC-3 Cell Cycle
4.4.5. Effect of DP9 on Scratch Wound Assay
4.4.6. Effect of DP9 on Angiogenesis
4.4.7. Effect of DP9 on Western Blot Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Takenaka, Y.; Fukumori, T.; Raz, A. Galectin-3 and metastasis. Glycoconj. J. 2002, 19, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Sakamoto, M.; Ino, Y.; Shimada, K.; Kosuge, T.; Sato, Y.; Tanaka, K.; Sekihara, H.; Hirohashi, S. Clinicopathological significance of galectin-3 expression in ductal adenocarcinoma of the pancreas. Clin. Cancer Res. 2002, 8, 2570–2575. [Google Scholar] [PubMed]
- Mariño, K.V.; Cagnoni, A.J.; Croci, D.O.; Rabinovich, G.A. Targeting galectin-driven regulatory circuits in cancer and fibrosis. Nat. Rev. Drug Discov. 2023, 22, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Sturgill, E.R.; Rolig, A.S.; Linch, S.N.; Mick, C.; Kasiewicz, M.J.; Sun, Z.; Traber, P.G.; Shlevin, H.; Redmond, W.L. Galectin-3 inhibition with belapectin combined with anti-OX40 therapy reprograms the tumor microenvironment to favor anti-tumor immunity. Oncoimmunology 2021, 10, 1892265. [Google Scholar] [CrossRef]
- Curti, B.D.; Koguchi, Y.; Leidner, R.S.; Rolig, A.S.; Sturgill, E.R.; Sun, Z.; Wu, Y.; Rajamanickam, V.; Bernard, B.; Hilgart-Martiszus, I.; et al. Enhancing clinical and immunological effects of anti-PD-1 with belapectin, a galectin-3 inhibitor. J. Immunother. Cancer 2021, 9, e002371. [Google Scholar] [CrossRef]
- Zetterberg, F.R.; MacKinnon, A.; Brimert, T.; Gravelle, L.; Johnsson, R.E.; Kahl-Knutson, B.; Leffler, H.; Nilsson, U.J.; Pedersen, A.; Peterson, K.; et al. Discovery and optimization of the first highly effective and orally available galectin-3 inhibitors for treatment of fibrotic disease. J. Med. Chem. 2022, 65, 12626–12638. [Google Scholar] [CrossRef]
- Zhao, W.; Ajani, J.A.; Sushovan, G.; Ochi, N.; Hwang, R.; Hafley, M.; Johnson, R.L.; Bresalier, R.S.; Logsdon, C.D.; Zhang, Z.; et al. Galectin-3 mediates tumor cell-stroma interactions by activating pancreatic stellate cells to produce cytokines via integrin signaling. Gastroenterology 2018, 154, 1524–1537.e6. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Qin, Y.; Cong, Q.; Shao, C.; Du, Z.; Ni, X.; Li, P.; Ding, K. RN1, a novel galectin-3 inhibitor, inhibits pancreatic cancer cell growth in vitro and in vivo via blocking galectin-3 associated signaling pathways. Oncogene 2017, 36, 1297–1308. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, L.; Liao, W.; Chen, H.; Du, Z.; Shao, C.; Wang, P.; Ding, K. HH1-1, a novel Galectin-3 inhibitor, exerts anti-pancreatic cancer activity by blocking Gal-3/EGFR/AKT/FOXO3 signaling pathway. Carbohydr. Polym. 2019, 204, 111–123. [Google Scholar] [CrossRef]
- Zhang, X.; Aweya, J.J.; Huang, Z.X.; Kang, Z.Y.; Bai, Z.H.; Li, K.H.; He, X.T.; Liu, Y.; Chen, X.Q.; Cheong, K.L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 2020, 234, 115849. [Google Scholar] [CrossRef]
- Shi, F.; Yan, X.; Cheong, K.L.; Liu, Y. Extraction, purification, and characterization of polysaccharides from marine algae Gracilaria lemaneiformis with anti-tumor activity. Process. Biochem. 2018, 73, 197–203. [Google Scholar] [CrossRef]
- Lu, S.Y.; Liu, Y.; Tang, S.; Zhang, W.; Yu, Q.; Shi, C.; Cheong, K.L. Gracilaria lemaneiformis polysaccharides alleviate colitis by modulating the gut microbiota and intestinal barrier in mice. Food Chem. X 2022, 13, 100197. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.T.; Zhang, X.; Liu, Y.; Chen, X.Q.; Cheong, K.L. Quantifcation of 3,6-anhydro-galactose in red seaweed polysaccharides and their potential skin-whitening activity. 3 Biotech 2020, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Veeraperumal, S.; Qiu, H.M.; Zeng, S.-S.; Yao, W.Z.; Wang, B.-P.; Liu, Y.; Cheong, K.L. Polysaccharides from Gracilaria lemaneiformis promote the HaCaT keratinocytes wound healing by polarised and directional cell migration. Carbohydr. Polym. 2020, 241, 116310. [Google Scholar] [CrossRef]
- Jiang, C.; Cheng, D.; Liu, Z.; Sun, J.; Mao, X. Advances in agaro-oligosaccharides preparation and bioactivities for revealing the structure-function relationship. Food Res. Int. 2021, 145, 110408. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.L. Preparation methods, biological activities, and potential applications of marine algae oligosaccharides: A review. FSHW 2023, 12, 359–370. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef]
- Zou, M.Y.; Nie, S.P.; Yin, J.Y.; Xie, M.Y. Ascorbic acid induced degradation of polysaccharide from natural products: A review. Int. J. Biol. Macromol. 2020, 151, 483–491. [Google Scholar] [CrossRef]
- Gao, B.; Li, L.; Wu, H.; Zhu, D.; Jin, M.; Qu, W.; Zeng, R. A novel strategy for efficient agaro-oligosaccharide production based on the enzymatic degradation of crude agarose in flammeovirga pacifica WPAGA1. Front. Microbiol. 2019, 10, 1231. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.R.; Hong, S.K. Implications of agar and agarase in industrial applications of sustainable marine biomass. Appl. Microbiol. Biotechnol. 2020, 104, 2815–2832. [Google Scholar] [CrossRef]
- Sun, M.; Sun, C.; Li, T.; Liu, K.; Yan, S.; Yin, H. Characterization of a novel bifunctional mannuronan C-5 epimerase and alginate lyase from Pseudomonas mendocina. sp. DICP-70. Int. J. Biol. Macromol. 2020, 150, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Zheng, L.; Yan, X. The preparation and bioactivity research of agaro-oligosaccharides. Food Technol. Biotechnol. 2005, 43, 29–36. [Google Scholar]
- Yang, B.; Yu, G.; Zhao, X.; Jiao, G.; Ren, S.; Chai, W. Mechanism of mild acid hydrolysis of galactan polysaccharides with highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides. FEBS J. 2009, 276, 2125–2137. [Google Scholar] [CrossRef]
- Agrawal, P.K. NMR-spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.L.; Qiu, H.M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef]
- Ciancia, M.; Matulewicz, M.C.; Tuvikene, R. Structural diversity in galactans from red seaweeds and its influence on rheological properties. Front. Plant Sci. 2020, 11, 559986. [Google Scholar] [CrossRef]
- Chen, X.; Fu, X.; Huang, L.; Xu, J.; Gao, X. Agar oligosaccharides: A review of preparation, structures, bioactivities and application. Carbohydr. Polym. 2021, 265, 118076. [Google Scholar] [CrossRef]
- Jiang, F.; Xu, X.; Xiao, Q.; Li, Z.; Weng, H.; Chen, F.; Xiao, A. Fabrication, structure, characterization and emulsion application of citrate agar. Int. J. Biol. Macromol. 2024, 268 Pt 1, 131451. [Google Scholar] [CrossRef]
- Alencar, P.O.C.; Lima, G.C.; Barros, F.C.N.; Costa, L.E.C.; Ribeiro, C.V.P.E.; Sousa, W.M.; Sombra, V.G.; Abreu, C.M.W.S.; Abreu, E.S.; Pontes, E.O.B.; et al. A novel antioxidant sulfated polysaccharide from the algae Gracilaria caudata: In vitro and in vivo activities. Food Hydrocoll. 2019, 90, 28–34. [Google Scholar] [CrossRef]
- Chagas, F.; Lima, G.C.; Dos Santos, V.I.N.; Costa, L.E.C.; de Sousa, W.M.; Sombra, V.G.; de Araújo, D.F.; Barros, F.C.N.; Marinho-Soriano, E.; de Andrade Feitosa, J.P.; et al. Sulfated polysaccharide from the red algae Gelidiella acerosa: Anticoagulant, antiplatelet and antithrombotic effects. Int. J. Biol. Macromol. 2020, 159, 415–421. [Google Scholar] [CrossRef]
- Atya, M.E.; Yang, X.; Tashiro, Y.; Tuvikene, R.; Matsukawa, S. Structural and physicochemical characterization of funoran extracted from Gloiopeltis furcata by different methods. Algal Res. 2023, 76, 103334. [Google Scholar] [CrossRef]
- Rodríguez Sánchez, R.A.; Canelón, D.J.; Cosenza, V.A.; Fissore, E.N.; Gerschenson, L.N.; Matulewicz, M.C.; Ciancia, M. Gracilariopsis hommersandii, a red seaweed, source of agar and sulfated polysaccharides with unusual structures. Carbohydr. Polym. 2019, 213, 138–146. [Google Scholar] [CrossRef]
- Darko, C.N.S.; Humayun, S.; Premarathna, A.D.; Howlader, M.M.; Rjabovs, V.; Tuvikene, R. Rheology and characterization of sulfated agarans from the edible epiphytic red alga, Vertebrata lanosa (truffle seaweed). Food Hydrocoll. 2024, 151, 109770. [Google Scholar] [CrossRef]
- Dwivedi, R.; Maurya, A.K.; Ahmed, H.; Farrag, M.; Pomin, V.H. Nuclear magnetic resonance-based structural elucidation of novel marine glycans and derived oligosaccharides. Magn. Reason. Chem. 2024, 62, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Zhao, J.; Wang, C.; Wei, M.; Dang, T.; Deng, Y.; Sun, J.; Song, S.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of the degradation products from Porphyra haitanensis polysaccharides. Process Biochem. 2018, 74, 185–193. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kawai, S.; Murata, K. Strategies for the production of high concentrations of bioethanol from seaweeds: Production of high concentrations of bioethanol from seaweeds. Bioengineered 2013, 4, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Koneru, J.K.; Sinha, S.; Mondal, J. Molecular dynamics simulations elucidate oligosaccharide recognition pathways by galectin-3 at atomic resolution. J. Biol. Chem. 2021, 297, 101271. [Google Scholar] [CrossRef]
- Miller, M.C.; Cai, C.; Wichapong, K.; Bhaduri, S.; Pohl, N.L.B.; Linhardt, R.J.; Gabius, H.-J.; Mayo, K.H. Structural insight into the binding of human galectins to corneal keratan sulfate, its desulfated form and related saccharides. Sci. Rep. 2020, 10, 15708. [Google Scholar] [CrossRef]
- Mahanti, M.; Pal, K.B.; Kumar, R.; Schulze, M.; Leffler, H.D.; Logan, T.; Nilsson, U.J. Ligand Sulfur Oxidation State Pro-gressively Alters Galectin-3-Ligand Complex Conformations to Induce Affinity-Influencing Hydrogen Bonds. J. Med. Chem. 2023, 66, 14716–14723. [Google Scholar] [CrossRef]
- Du, W.W.; Yang, W.; Liu, E.; Yang, Z.; Dhaliwal, P.; Yang, B.B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016, 44, 2846–2858. [Google Scholar] [CrossRef]
- Bodur, C.; Karakas, B.; Timucin, A.C.; Tezil, T.; Basaga, H. AMP-activated protein kinase couples 3-bromopyruvate-induced energy depletion to apoptosis via activation of FoxO3a and upregulation of proapoptotic Bcl-2 proteins. Mol. Carcinog. 2016, 55, 1584–1597. [Google Scholar] [CrossRef]
- Potente, M.; Urbich, C.; Sasaki, K.-i.; Hofmann, W.K.; Heeschen, C.; Aicher, A.; Kollipara, R.; DePinho, R.A.; Zeiher, A.M.; Dimmeler, S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J. Clin. Investig. 2005, 115, 2382–2392. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, H.; Yu, X.; Collins, P.M.; Bum-Erdene, K. Galectin-3 inhibitors: A patent review (2008–present). Expert. Opin. Ther. Pat. 2014, 24, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Lu, G.; Zheng, Y.; Xie, W.; Li, S.; Hu, Z. Aquimarina agarilytica sp. nov., an agarolytic species isolated from a red alga. Int. J. Syst. Evol. Micr. 2012, 62 Pt 4, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.-L.; Wu, D.-T.; Deng, Y.; Leong, F.; Zhao, J.; Zhang, W.-J.; Li, S.-P. Qualitation and quantification of specific polysaccharides from Panax species using GC-MS, saccharide mapping and HPSEC-RID-MALLS. Carbohydr. Polym. 2016, 153, 47–54. [Google Scholar] [CrossRef]
- Zhou, L.; Ma, P.; Shuai, M.; Huang, J.; Sun, C.; Yao, X.; Chen, Z.; Min, X.; Zhang, T. Analysis of the water-soluble polysaccharides from Camellia japonica pollen and their inhibitory effects on galectin-3 function. Int. J. Biol. Macromol. 2020, 159, 455–460. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New docking methods, expanded force field, and Python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Wicaksono, D.; Taslim, N.A.; Lau, V.; Syahputra, R.A.; Alatas, A.I.; Putra, P.P.; Tallei, T.E.; Tjandrawinata, R.R.; Tsopmo, A.; Kim, B.; et al. Elucidation of anti-human melanoma and anti-aging mechanisms of compounds from green seaweed Caulerpa racemosa. Sci. Rep. 2024, 14, 27534. [Google Scholar] [CrossRef]
- Si, Y.; Li, Y.; Yang, T.; Li, X.; Ayala, G.J.; Mayo, K.H.; Tai, G.; Su, J.; Zhou, Y. Structure-function studies of galectin-14, an important effector molecule in embryology. FEBS J. 2021, 288, 1041–1055. [Google Scholar] [CrossRef]



| Residue | Chemical Shifts δ (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | 1 | 2 | 3 | 4 | 5 | 6 | -OMe | ||
| G | →3)-β-D-Galp-(1→ | H | 4.51 | 3.53 | 3.72 | 4.04 | 3.65 | 3.66/3.72 | |
| C | 101.84 | 69.74 | 81.70 | 68.18 | 75.03 | 60.92 | |||
| LA | →4)-α-L-AnGalp-(1→ | H | 5.06 | 4.05 | 4.46 | 4.57 | 4.48 | 3.93/4.15 | |
| C | 98.06 | 69.11 | 79.63 | 76.89 | 74.91 | 68.72 | |||
| G′ | →3)-β-D-Galp-(1→ | H | 4.37 | 3.63 | 3.68 | 4.04 | 3.65 | 3.66/3.72 | |
| G→L6S | C | 102.86 | 70.77 | 80.28 | 68.18 | 75.03 | 60.92 | ||
| L6S | →4)-α-L-Galp6S-(1→ | H | 5.21 | 3.79 | 3.87 | 4.20 | 4.30 | 4.21 | |
| C | 100.80 | 68.96 | 68.57 | 77.92 | 69.49 | 67.01 | |||
| G6M | →3)-β-D-Galp-6-OMe-(1→ | H | 4.32 | 3.52 | 3.72 | 4.04 | 3.78 | 3.60 | 3.33 |
| C | 103.15 | 70.92 | 81.70 | 68.18 | 73.26 | 71.45 | 58.57 | ||
| Gnr | β-D-Galp-(1→ | H | 4.46 | 3.40 | 3.56 | 3.84 | 3.65 | 3.66/3.72 | |
| C | 102.12 | 70.38 | 72.48 | 68.57 | 75.03 | 60.92 | |||
| Grα | →3)-α-D-Galp | H | 5.18 | 3.88 | 3.89 | 4.10 | 3.71 | 3.66/3.72 | |
| C | 92.37 | 68.57 | 78.85 | 68.72 | 69.49 | 60.92 | |||
| Grβ | →3)-β-D-Galp | H | 4.52 | 3.59 | 3.69 | 4.01 | 3.70 | 3.66/3.72 | |
| C | 96.15 | 71.31 | 82.09 | 69.88 | 74.94 | 60.92 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Li, F.; Liu, Z.; Liu, Y. Novel Sulfated Oligosaccharide DP9 from Marine Algae, Gracilaria lemaneiformis: A Potent Galectin-3 Inhibitor for Pancreatic Cancer Therapy. Mar. Drugs 2025, 23, 423. https://doi.org/10.3390/md23110423
Liu P, Li F, Liu Z, Liu Y. Novel Sulfated Oligosaccharide DP9 from Marine Algae, Gracilaria lemaneiformis: A Potent Galectin-3 Inhibitor for Pancreatic Cancer Therapy. Marine Drugs. 2025; 23(11):423. https://doi.org/10.3390/md23110423
Chicago/Turabian StyleLiu, Pingting, Fengyuan Li, Zhicong Liu, and Yang Liu. 2025. "Novel Sulfated Oligosaccharide DP9 from Marine Algae, Gracilaria lemaneiformis: A Potent Galectin-3 Inhibitor for Pancreatic Cancer Therapy" Marine Drugs 23, no. 11: 423. https://doi.org/10.3390/md23110423
APA StyleLiu, P., Li, F., Liu, Z., & Liu, Y. (2025). Novel Sulfated Oligosaccharide DP9 from Marine Algae, Gracilaria lemaneiformis: A Potent Galectin-3 Inhibitor for Pancreatic Cancer Therapy. Marine Drugs, 23(11), 423. https://doi.org/10.3390/md23110423





