5Z-7-Oxozeanol Isolated from the Fungus Curvularia sp. MDCW-1060 Inhibits the Proliferation of MDA-MB-231 Cells via the PI3K-Akt and MAPK Pathways
Abstract
1. Introduction
2. Results
2.1. 5Z-7-Oxozeaenol Structure
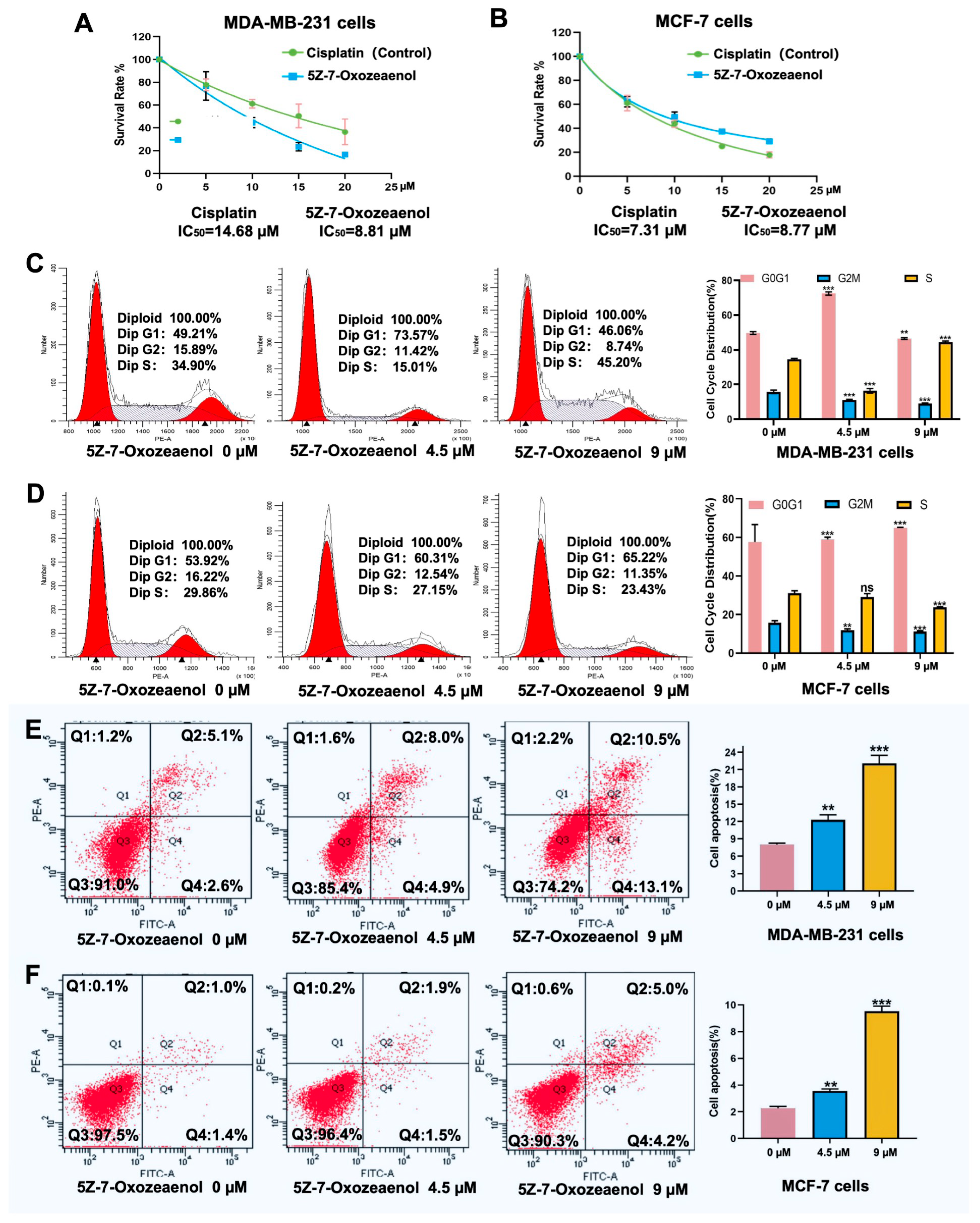
2.2. Antiproliferative Efficacy of 5Z-7-Oxozeaenol on Breast Cancer Cells
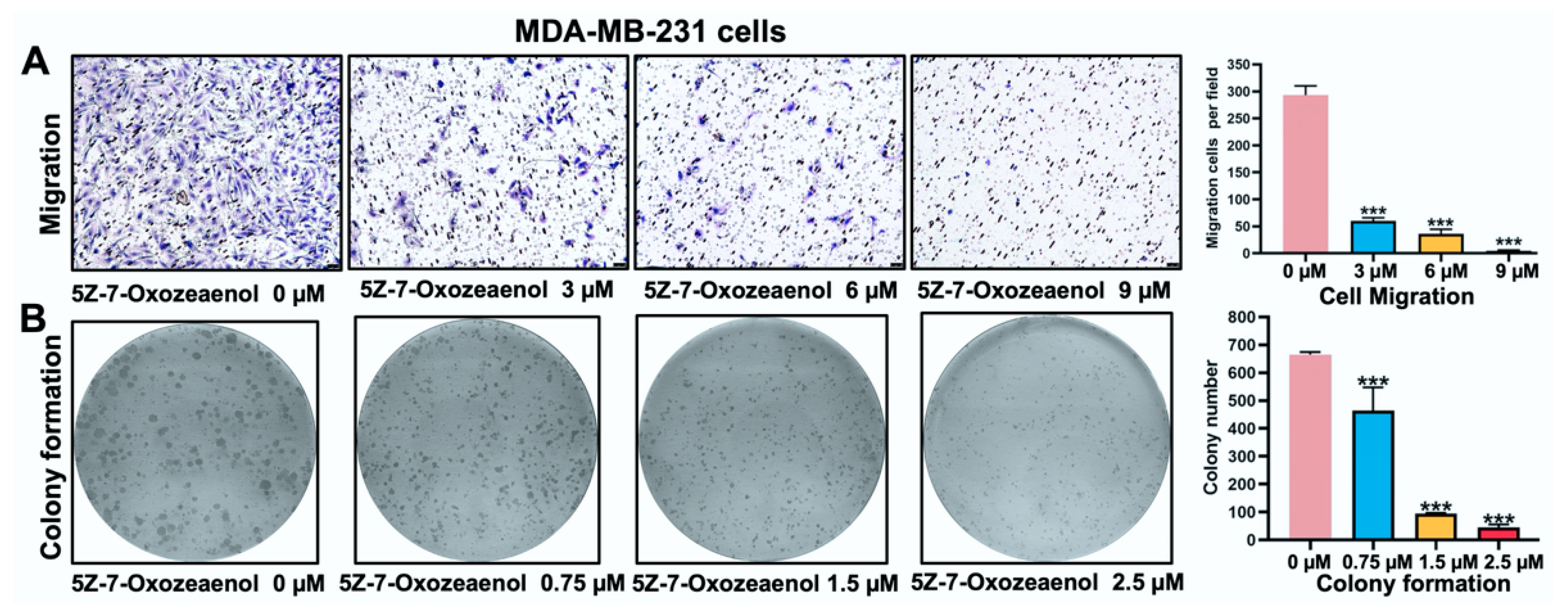
2.3. 5Z-7-Oxozeaenol Inhibited MDA-MB-231 Cells Migration and Growth
2.4. RNA-Seq Analysis of MDA-MB-231 Cells Treated with 5Z-7-Oxozeaenol
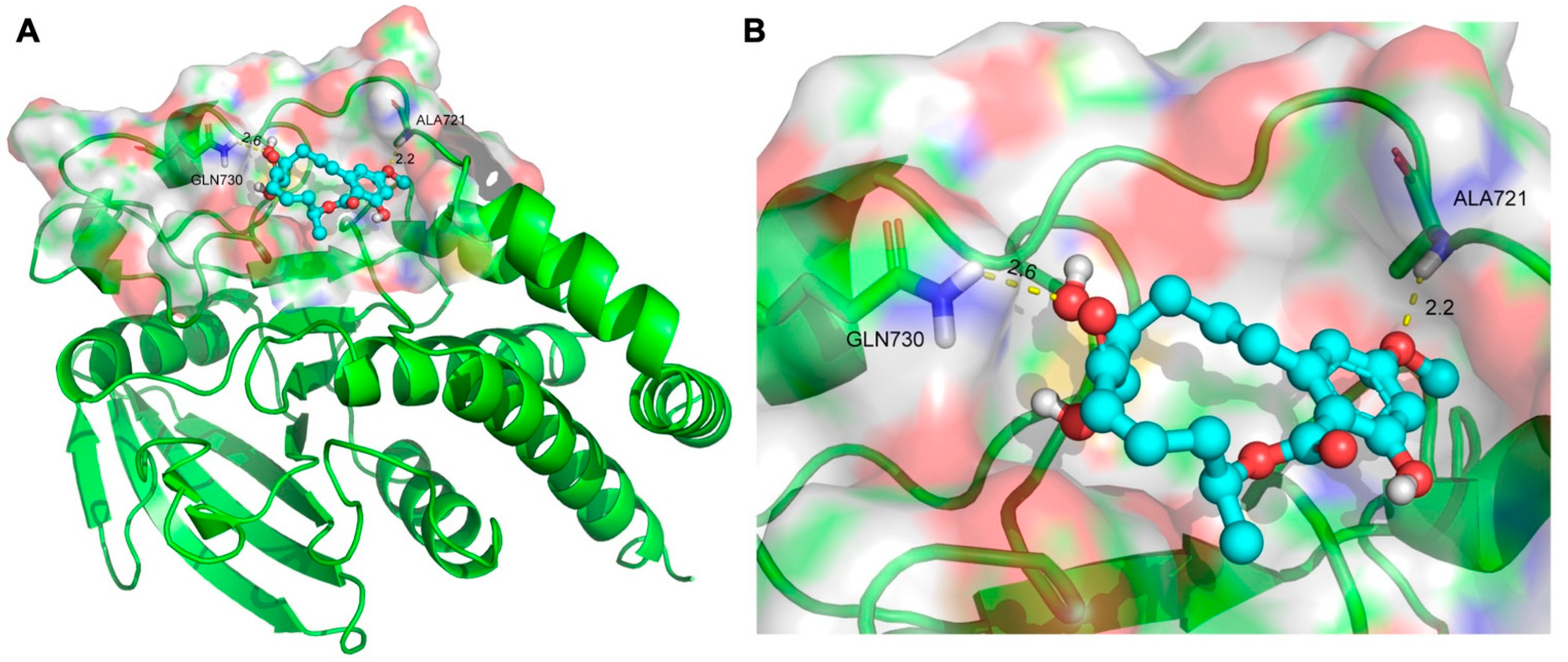
2.5. Molecular Docking for 5Z-7-Oxozeaenol with the Protein Tyrosine Phosphatase Receptor Type N (PTPRN)
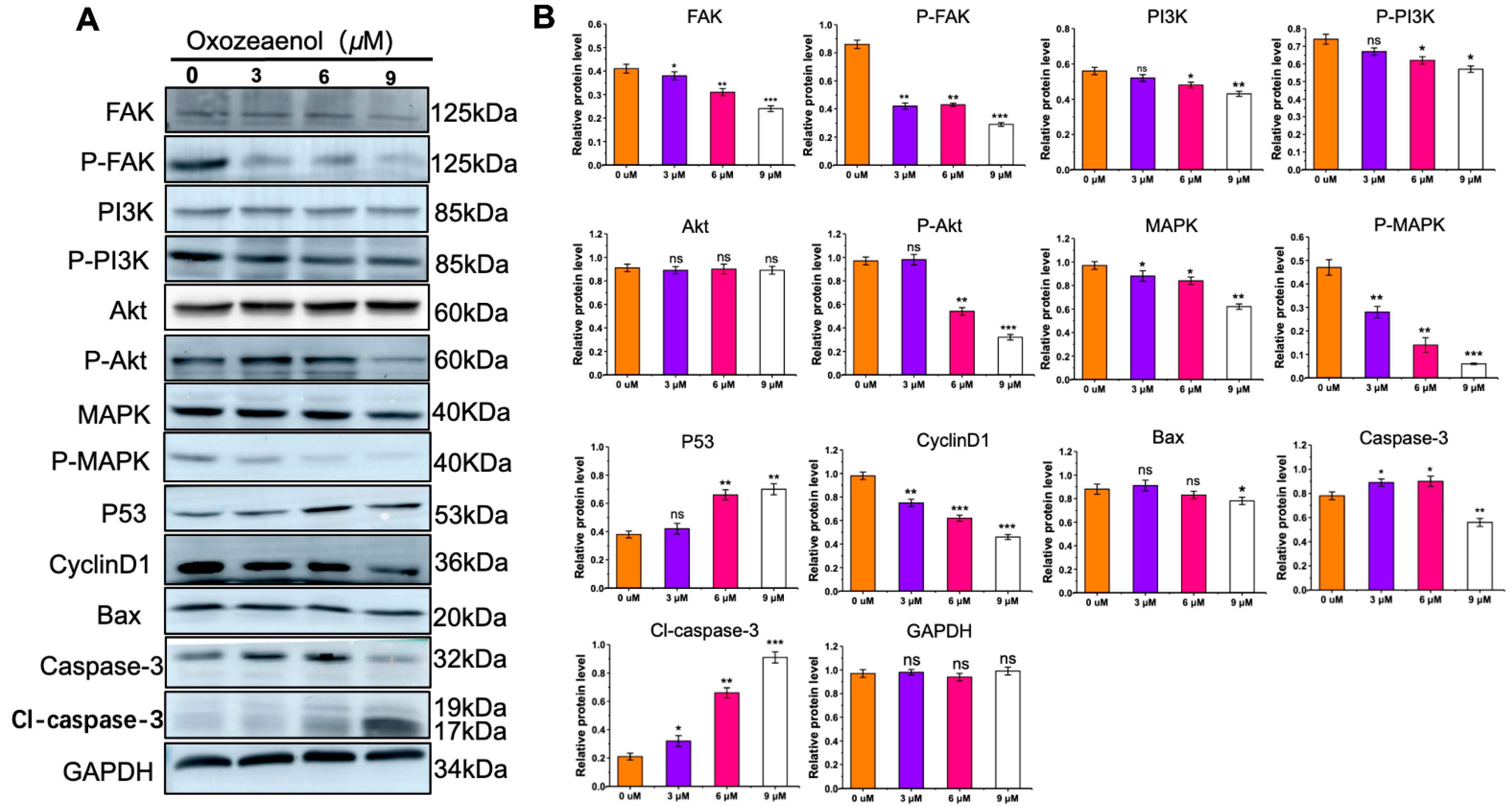
2.6. Western Blot Analysis of Relative FAK, PI3K-Akt, MAPK Protein Expression
3. Discussion
4. Materials and Methods
4.1. Fungal Materials
4.2. Fermentation and Extraction
4.3. Isolation
4.4. Cell Culture and Reagents
4.5. Cell Proliferation Assays
4.6. Cell Cycle Assay
4.7. Apoptosis Assays
4.8. Cell Migration Assays
4.9. Cell Colony Formation Assay
4.10. RNA-Seq
4.11. Construction of the Protein—Protein Interaction (PPI) Network
4.12. GO and KEGG Pathway Enrichment Analysis
4.13. Molecular Docking
4.14. Western Blot Analysis
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IARC | International agency for research on cancer |
| PI | Propidium iodide |
| PTPRN | Protein tyrosine phosphatase receptor type N |
| MTT | Methylthiazolyldiphenyl-tetrazolium bromide |
| DEGs | Differentially expressed genes |
| PPI | Protein–Protein interactions |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| GSEA | Gene set enrichment analysis |
| BP | Biological Processes |
| MF | Molecular functions |
| CC | Cellular components |
| MMP | Matrix metallopeptidase |
| RTKs | Receptor tyrosine kinases |
| FAK | Focal adhesion kinase |
| ECM | Extracellular Matrix |
| HT | Hyperthermia |
| ROS | Reactive oxygen species |
| PFs | Purkinje Fibers |
| PTP | Protein tyrosine phosphatase |
| LUAD | Lung adenocarcinoma |
| CRC | Colorectal cancer |
References
- Bashey, S.Z.; Kordon, A.; Ositelu, K.; Rao, A.; Akhter, N. The role of statins in breast cancer survivors. Breast Cancer Res. Tr. 2025, 210, 1–10. [Google Scholar] [CrossRef]
- Dee, E.C.; Laversanne, M.; Bhoo-Pathy, N.; Ho, F.D.; Feliciano, E.J.G.; Eala, M.A.B.; Ting, F.I.L.; Ginsburg, O.; Moraes, F.Y.; Gyawali, B.; et al. Cancer incidence and mortality estimates in 2022 in southeast Asia: A comparative analysis. Lancet Oncol. 2025, 26, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.Y.A.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.Y.; Zheng, R.S.; Zhang, S.W.; Chen, R.; Wang, S.M.; Sun, K.X.; Zeng, H.M.; Wei, W.Q.; He, J. Breast cancer incidence and mortality in women in China: Temporal trends and projections to 2030. Cancer Biol. Med. 2021, 18, 900–909. [Google Scholar] [CrossRef]
- Jing, Z.J.; Huang, W.Q.; Mei, J.; Bhushan, S.; Wu, X.H.; Yan, C.B.; Zheng, H.M.; Yang, Y.L. Advances in novel cell death mechanisms in breast cancer: Intersecting perspectives on ferroptosis, cuproptosis, disulfidptosis, and pyroptosis. Mol. Cancer 2025, 24, 224. [Google Scholar] [CrossRef]
- Zeng, C.; Lin, M.X.; Jin, Y.Z.; Zhang, J. Identification of Key Genes Associated with Brain Metastasis from Breast Cancer: A Bioinformatics Analysis. Med. Sci. Monit. 2022, 28, e935071-1–e935071-9. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lien, W.C.; Su, S.Y.; Jhuang, J.R.; Chiang, C.J.; Yang, Y.W.; Lee, W.C. Birth Cohort Effects in Breast Cancer Incidence: Global Patterns and Trends. Am. J. Epidemiol. 2022, 191, 1990–2001. [Google Scholar] [CrossRef]
- Puklin, L.S.; Ferrucci, L.M.; Harrigan, M. Improving Lifestyle Behaviors During Chemotherapy for Breast Cancer: The Lifestyle, Exercise, and Nutrition Early After Diagnosis (LEANer) Trial; American Cancer Society: Atlanta, GA, USA, 2024; Volume 130, pp. 2440–2452. [Google Scholar]
- Radwan, R.M.; Schuster, A.L.R.; Hertz, D.L.; Lustberg, M.B.; Vachhani, H.R.; Zacholski, E.H.; Sheppard, V.B.; Bridges, J.F.P.; Salgado, T.M. Tolerance for chemotherapy-induced peripheral neuropathy among women with metastatic breast cancer: A discrete-choice experiment. Breast Cancer Res. Treat. 2025, 212, 149–159. [Google Scholar] [CrossRef]
- Liu, X.X.; Zhuang, C.Y.; Liu, L.; Xiong, L.; Xie, X.; He, P.; Li, J.J.; Wei, B.; Yan, X.; Tian, T.L.; et al. Exploratory phase II trial of an anti-PD-1 antibody camrelizumab combined with a VEGFR-2 inhibitor apatinib and chemotherapy as a neoadjuvant therapy for triple-negative breast cancer (NeoPanDa03): Efficacy, safety and biomarker analysis. Signal Transduct. Target. Ther. 2025, 10, 237. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Ma, C.; Xu, A.S.; Zuo, L.P.; Li, Q.; Fan, F.J.; Hu, Y.; Sun, C.Y. Methionine Dependency and Restriction in Cancer: Exploring the Pathogenic Function and Therapeutic Potential. Pharmaceuticals 2025, 18, 640. [Google Scholar] [CrossRef]
- Acuña, U.M.; Wittwer, J.; Ayers, S.; Pearce, C.J.; Oberlies, N.H.; De Blanco, E.J.C. Effects of (5Z)-7-Oxozeaenol on the Oxidative Pathway of Cancer Cells. Anticancer Res. 2012, 32, 2665–2671. [Google Scholar]
- Ayers, S.; Graf, T.N.; Adcock, A.F.; Kroll, D.J.; Matthew, S.; de Blanco, E.J.C.; Shen, Q.; Swanson, S.M.; Wani, M.C.; Pearce, C.J.; et al. Resorcylic Acid Lactones with Cytotoxic and NF-κB Inhibitory Activities and Their Structure-Activity Relationships. J. Nat. Prod. 2011, 74, 1126–1131. [Google Scholar] [CrossRef]
- Yuan, W.H.; Yu, R.C.; Sun, W. Unique climate features of the rainfall center along the northern coast of the Beibu Gulf. Clim. Dyn. 2025, 63, 169. [Google Scholar] [CrossRef]
- Chen, B.Y.; Lu, F.T.; Zhang, X.L.; Wu, C.W.; Lü, L.G.; Jefferson, T.A. The largest known population of Eden’s whale aggregates in the Beibu Gulf, southern China. Mar. Mammal Sci. 2025, 41, e13226. [Google Scholar] [CrossRef]
- Che, W.X.; Zhao, H.; Man, Y.; Tan, X. Spatial characteristics of microbial communities and their functions in sediments of subtropical Beibu Gulf, China. Mar. Environ. Res. 2025, 207, 107077. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Qin, Y.N.; Lin, M.P.; Song, Y.Y.; Lu, H.M.; Xu, X.Y.; Liu, Y.H.; Zhou, X.F.; Gao, C.H.; Luo, X.W. Marine Natural Products from the Beibu Gulf: Sources, Chemistry, and Bioactivities. Mar. Drugs 2023, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2025, 42, 257–297. [Google Scholar] [CrossRef]
- Brito, M.F.; Fagundes, T.S.F.; Jimenez, P.C.; Valverde, A.L. Marine Natural Product as a Source of New Drugs: 15 Years and One Pandemic Later. Quim. Nova 2025, 48, e20250167. [Google Scholar] [CrossRef]
- Alves, C.; Diederich, M. Marine Natural Products as Anticancer Agents 3.0. Mar. Drugs 2025, 23, 243. [Google Scholar] [CrossRef]
- Acuña, U.M.; Wittwer, J.; Ayers, S.; Pearce, C.J.; Oberlies, N.H.; de Blanco, E.J.C. Effects of (5Z)-7-Oxozeaenol on MDA-MB-231 Breast Cancer Cells. Anticancer Res. 2012, 32, 2415–2421. [Google Scholar] [PubMed]
- Li, P.; Zhao, Q.L.; Jawaid, P.; Rehman, M.U.; Sakurai, H.; Kondo, T. Enhancement of hyperthermia-induced apoptosis by 5Z-7-oxozeaenol, a TAK1 inhibitor, in A549 cells. Cell Stress Chaperon. 2016, 21, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.Y.; Chien, C.T.; Wang, S.S. Progressive thermopreconditioning attenuates rat cardiac ischemia/reperfusion injury by mitochondria-mediated antioxidant and antiapoptotic mechanisms. J. Thorac. Cardiov. Sur. 2014, 148, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.H.; Cheng, J.; Vasudevan, S.A.; Patel, R.H.; Liang, L.; Xu, X.; Zhao, Y.L.; Jia, W.; Lu, F.M.; Zhang, H.; et al. TAK1 inhibitor 5Z-7-oxozeaenol sensitizes neuroblastoma to chemotherapy. Apoptosis 2013, 18, 1224–1234. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Liu, H.T.; Song, Y.S.; Gao, N.; Gao, P.; Hui, Y.R.; Li, Y.H.; Fan, T.L. Luteolin enhances drug chemosensitivity by downregulating the FAK/PI3K/AKT pathway in paclitaxel-resistant esophageal squamous cell carcinoma. Int. J. Mol. Med. 2024, 54, 77. [Google Scholar] [CrossRef]
- Liu, J.Y.; Liu, T.; Qian, X.; Ma, D.Y.; Chen, L.L.; Li, A.L.; Liang, X.R.; Hu, Z.; Qi, C.X.; Qi, W. Tuina Inhibits Chondrocyte Apoptosis by Regulating the FAK/PI3K/Akt Pathway in Rabbits with IVDD. Comb. Chem. High Throughput Screen. 2025, 28, 1746–1753. [Google Scholar] [CrossRef]
- Sun, L.H.; Chen, W.; Yuan, W.X.; Huang, Q.W.; Yang, H.; Zhang, W.; Tang, J.J.; Hu, P. Ginkgetin inhibits the proliferation and migration of lung cancer cells via FAK/STAT3/AKT pathway. Mol. Biol. Rep. 2025, 52, 458. [Google Scholar] [CrossRef]
- Tan, J.C.; Lin, G.Q.; Zhang, R.; Wen, Y.T.; Luo, C.Y.; Wang, R.; Wang, F.Y.; Peng, S.J.; Zhang, J.G. Bufotalin Induces Oxidative Stress-Mediated Apoptosis by Blocking the ITGB4/FAK/ERK Pathway in Glioblastoma. Antioxidants 2024, 13, 1179. [Google Scholar] [CrossRef]
- Guo, J.; Santiago-O’Farrill, J.M.; Orellana, V.; Ozyurt, R.; Yang, H.L.; Pina, M.; Bildik, G.; Mao, W.Q.; Bast, R.C.; Lu, Z. DIRAS3 Inhibits Ovarian Cancer Cell Growth by Blocking the Fibronectin-Mediated Integrin β1/FAK/AKT Signaling Pathway. Cells 2025, 14, 1250. [Google Scholar] [CrossRef]
- Zhou, Q.W.; You, Y.; Zhao, Y.Y.; Xiao, S.X.; Song, Z.Q.; Huang, C.X.; Qian, J.L.; Lu, W.Q.; Tong, H.X.; Zhang, Y.; et al. TRPV4 drives the progression of leiomyosarcoma by promoting ECM1 generation and co-activating the FAK/PI3K/AKT/GSK3β pathway. Cell. Oncol. 2025, 48, 455–470. [Google Scholar] [CrossRef]
- Wu, T.J.; Lin, C.Y.; Tsai, C.H.; Huang, Y.L.; Tang, C.H. Glucose suppresses IL-1β-induced MMP-1 expression through the FAK, MEK, ERK, and AP-1 signaling pathways. Environ. Toxicol. 2018, 33, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Nafees, S.; Rahman, A.U.; Akhtar, J.; Khan, A.A.; Sharma, A. Itrifal-e-Aftimoon potentiates imatinib-induced anti-leukemic effect by influencing FAK/STAT/Akt/ERK signalling pathways against chronic myeloid leukaemia in vitro. J. Pharm. Pharmacol. 2022, 74, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.L.; Yang, K.C.; Peng, J.Y.; Hong, C.L.; Li, P.C.; Chye, S.M.; Lu, F.J.; Shih, C.W.; Chen, C.H. Parecoxib Enhances Resveratrol against Human Colorectal Cancer Cells through Akt and TXNDC5 Inhibition and MAPK Regulation. Nutrients 2024, 16, 3020. [Google Scholar] [CrossRef] [PubMed]
- Skopek, R.; Özcan, S.G.; Chmiel, P.; Morgner, S.; Schütt, J.; Zadegan, F.G.; Stanko, C.; Palusinska, M.; Maslinska-Gromadka, K.; Sbirkov, Y.; et al. Synergistic potential of CDK4/6 inhibitors and ATRA in non-APL AML. Br. J. Haematol. 2025, 207, 1279–1288. [Google Scholar] [CrossRef]
- Cardona-Benavides, I.J.; Cristobal-Vargas, S.; De Ramón, C.; Rojas, E.A.; Misiewicz-Krzeminska, I.; Isidro, I.; Pérez, J.J.; Paiva, B.; Puig, N.; Alcoceba, M.; et al. Exploring the Role of Cyclin D1 in the Pathogenesis of Multiple Myeloma Beyond Cell Cycle Regulation; Molecular Oncology: Heidelberg, Germany, 2025. [Google Scholar]
- Suzuki, Y.; Usuki, S.; Nishizawa, M.; Tanaka, N.; Suhara, Y.; Yajima, I. Acyclic Retinoid Overcomes Vemurafenib Resistance in Melanoma Cells via Dual Inhibition of MAPK and PI3K/AKT/mTOR Pathways. Anticancer Res. 2025, 45, 2265–2278. [Google Scholar] [CrossRef]
- Kambaru, A.; Chaudhary, N. Role of Protein Tyrosine Phosphatase in Regulation of Cell Signaling Cascades Affecting Tumor Cell Growth: A Future Perspective as Anti-Cancer Drug Target. Curr. Pharm. Biotechnol. 2022, 23, 920–931. [Google Scholar] [CrossRef]
- Song, X.Y.; Jiao, X.; Yan, H.; Yu, L.F.; Jiang, L.Y.; Zhang, M.; Chen, L.Z.; Ju, M.Y.; Wang, L.; Wei, Q.; et al. Overexpression of PTPRN Promotes Metastasis of Lung Adenocarcinoma and Suppresses NK Cell Cytotoxicity. Front. Cell Dev. Biol. 2021, 9, 622018. [Google Scholar] [CrossRef]
- Garranzo-Asensio, M.; Solís-Fernández, G.; Montero-Calle, A.; García-Martínez, J.M.; Fiuza, M.C.; Pallares, P.; Palacios-Garcia, N.; García-Jiménez, C.; Guzman-Aranguez, A.; Barderas, R. Seroreactivity Against Tyrosine Phosphatase PTPRN Links Type 2 Diabetes and Colorectal Cancer and Identifies a Potential Diagnostic and Therapeutic Target. Diabetes 2022, 71, 497–510. [Google Scholar] [CrossRef]
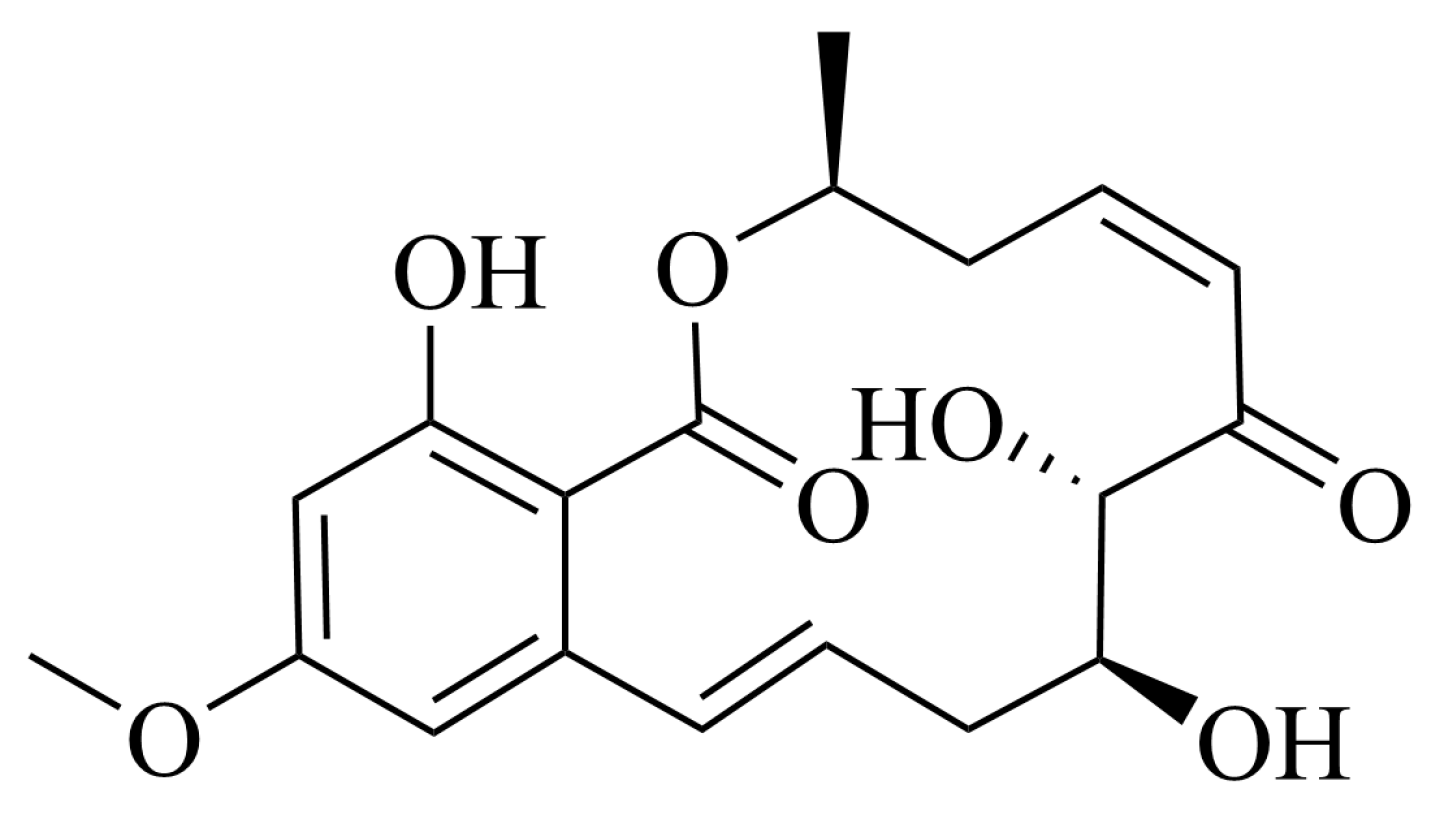

| No. | δC, Type | δH, Mult. (J in Hz) |
|---|---|---|
| 1 | 170.4 (C) | |
| 3 | 73.8 (CH) | 5.20, qdd (12.0, 5.9, 1.9) |
| 4 | 36.3 (CH2) | 2.58, m |
| 3.20, m | ||
| 5 | 142.4 (CH) | 6.19, ddd (13.6, 10.0, 3.5) |
| 6 | 126.7 (CH) | 6.43, dd (11.3, 2.6) |
| 7 | 200.9 (C) | |
| 8 | 81.7 (CH) | 4.38, m |
| 9 | 72.7 (CH) | 3.85, m |
| 10 | 36.0 (CH2) | 2.02, m |
| 11 | 132.4 (CH) | 6.06, ddd (14.9, 9.2, 5.2) |
| 12 | 131.0 (CH) | 6.74, dd (15.3) |
| 13 | 143.9 (C) | |
| 14 | 106.1 (CH) | 6.42, d (2.5) |
| 15 | 163.4 (C) | |
| 16 | 100.2 (CH) | 6.38, d (2.5) |
| 17 | 163.7 (C) | |
| 18 | 104.5 (C) | |
| 19 | 20.1 (CH3) | 1.37, d (6.1) |
| 20 | 55.5 (CH3) | 3.78, s |
| 8-OH | 4.98, d (4.9) | |
| 9-OH | 5.06, d (6.1) | |
| 17-OH | 11.77, s |
| No. | Primary Antibody | Dilution | Isotype | Source |
|---|---|---|---|---|
| 1 | FAK | 1:1000 | Rabbit IgG (#71433) | Cell Signaling Technology |
| 3 | P-FAK | 1:1000 | Rabbit IgG (#8556) | Cell Signaling Technology |
| 4 | PI3K | 1:1000 | Rabbit IgG (#4257) | Cell Signaling Technology |
| 5 | P-PI3K | 1:1000 | Tyr458/199 (#4228) | Cell Signaling Technology |
| 6 | Akt | 1:1000 | Rabbit IgG (#4691) | Cell Signaling Technology |
| 7 | P-Akt | 1:2000 | Rabbit IgG (#4060) | Cell Signaling Technology |
| 8 | MAPK | 1:1000 | Rabbit IgG (#8690) | Cell Signaling Technology |
| 9 | P-MAPK | 1:1000 | Rabbit IgG (#4377) | Cell Signaling Technology |
| 10 | P53 | 1:1000 | Rabbit IgG (#2527) | Cell Signaling Technology |
| 11 | CyclinD1 | 1:1000 | Rabbit IgG (#55506) | Cell Signaling Technology |
| 12 | Bax | 1:1000 | Rabbit IgG (#5023) | Cell Signaling Technology |
| 13 | Caspase-3 | 1:1000 | Rabbit IgG (#14220) | Cell Signaling Technology |
| 14 | Cleaved-caspase-3 | 1:1000 | Rabbit IgG (#9664) | Cell Signaling Technology |
| 15 | GAPDH | 1:1000 | Mouse IgG (#T0004) | Cell Signaling Technology |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, J.; Xu, C.; Liu, K.; Xie, J.; He, Z.; Liu, Y.; Wang, C.; Qu, X. 5Z-7-Oxozeanol Isolated from the Fungus Curvularia sp. MDCW-1060 Inhibits the Proliferation of MDA-MB-231 Cells via the PI3K-Akt and MAPK Pathways. Mar. Drugs 2025, 23, 414. https://doi.org/10.3390/md23110414
Zhang H, Wang J, Xu C, Liu K, Xie J, He Z, Liu Y, Wang C, Qu X. 5Z-7-Oxozeanol Isolated from the Fungus Curvularia sp. MDCW-1060 Inhibits the Proliferation of MDA-MB-231 Cells via the PI3K-Akt and MAPK Pathways. Marine Drugs. 2025; 23(11):414. https://doi.org/10.3390/md23110414
Chicago/Turabian StyleZhang, Hong, Jianjian Wang, Chang Xu, Kai Liu, Jufang Xie, Zhoucheng He, Yonghong Liu, Cong Wang, and Xinjian Qu. 2025. "5Z-7-Oxozeanol Isolated from the Fungus Curvularia sp. MDCW-1060 Inhibits the Proliferation of MDA-MB-231 Cells via the PI3K-Akt and MAPK Pathways" Marine Drugs 23, no. 11: 414. https://doi.org/10.3390/md23110414
APA StyleZhang, H., Wang, J., Xu, C., Liu, K., Xie, J., He, Z., Liu, Y., Wang, C., & Qu, X. (2025). 5Z-7-Oxozeanol Isolated from the Fungus Curvularia sp. MDCW-1060 Inhibits the Proliferation of MDA-MB-231 Cells via the PI3K-Akt and MAPK Pathways. Marine Drugs, 23(11), 414. https://doi.org/10.3390/md23110414






