Identifying a Marine-Derived Small-Molecule Nucleoprotein Inhibitor Against Influenza A Virus
Abstract
1. Introduction
2. Results
2.1. Screening of Marine Derived Compounds Targeting Virus RNP Complex
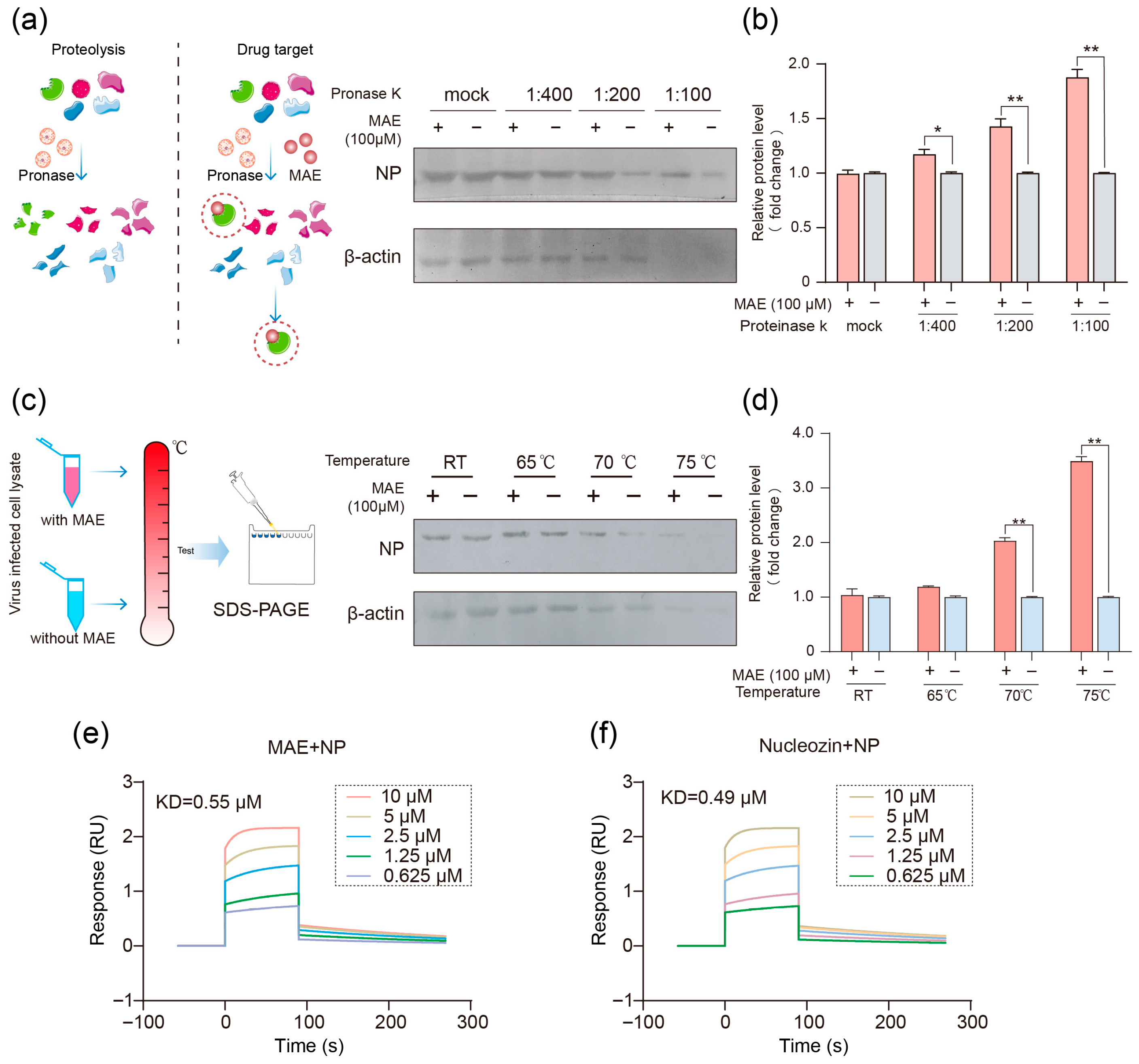
2.2. Compound MAE May Block IAV Infection Through Direct Interaction with NP Protein
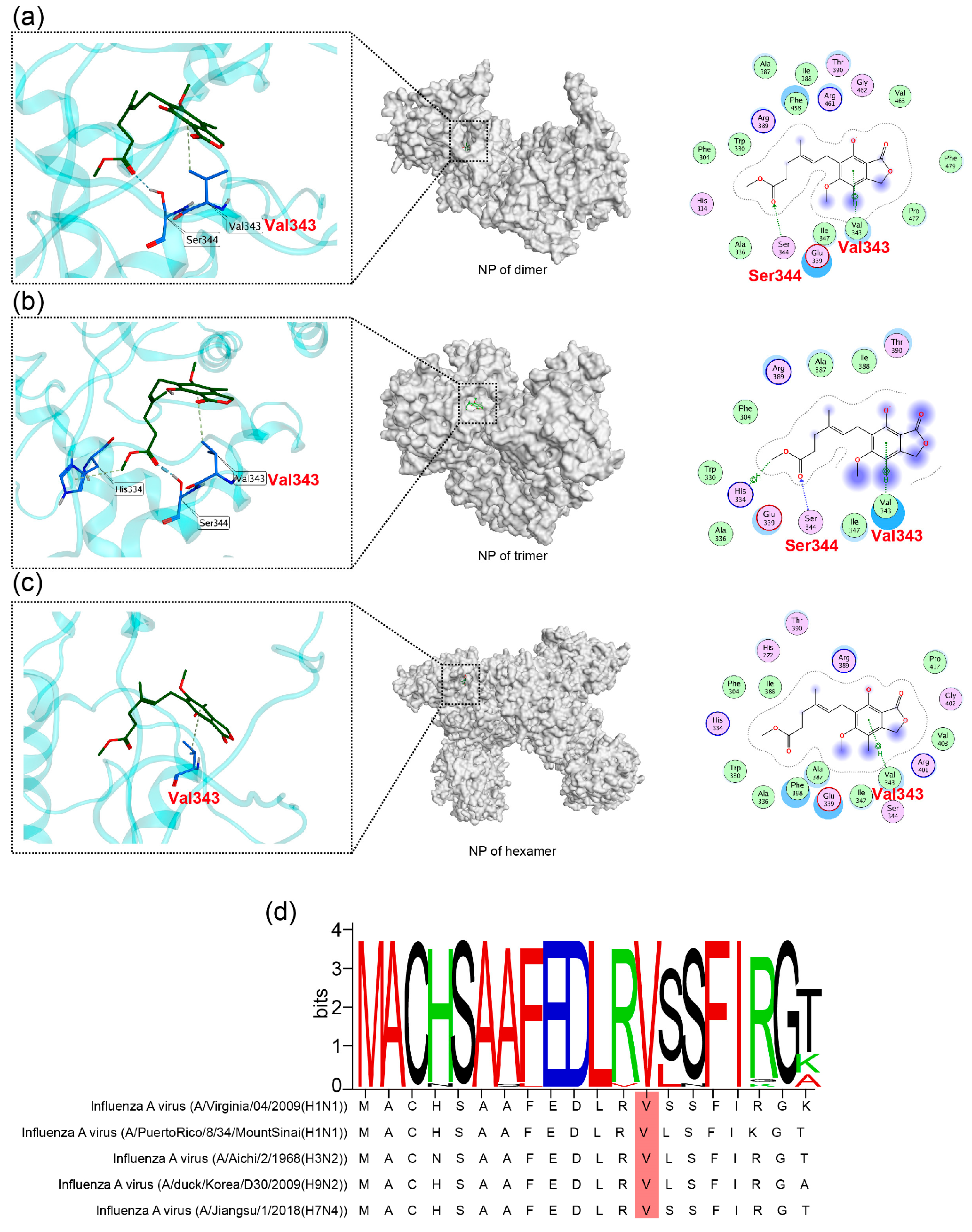
2.3. MAE May Exert Its Inhibition Effects by Binding to Valine 343 of NP
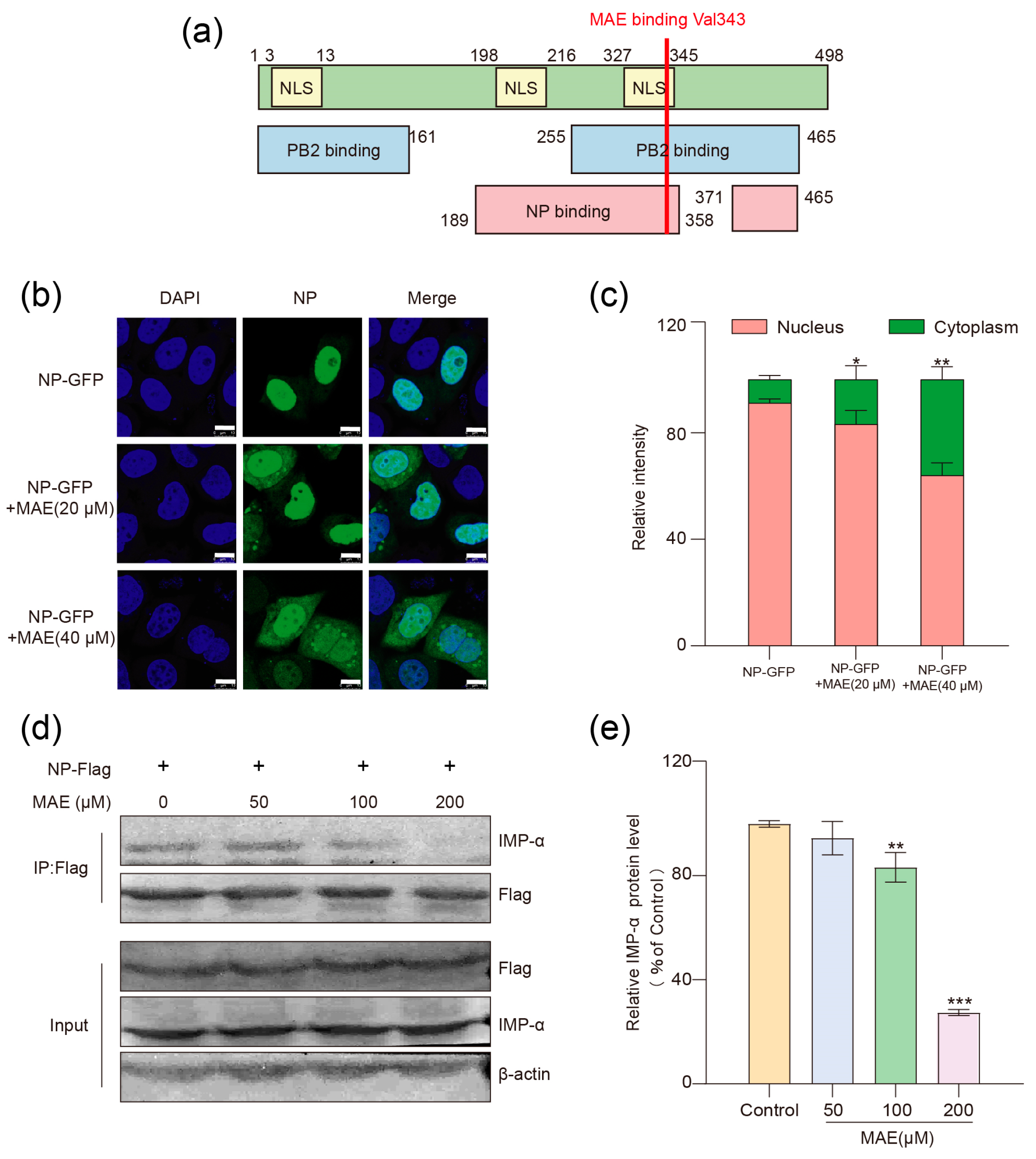
2.4. MAE Inhibits the Nuclear Entry of NP by Interfering with Its Binding to Importin α
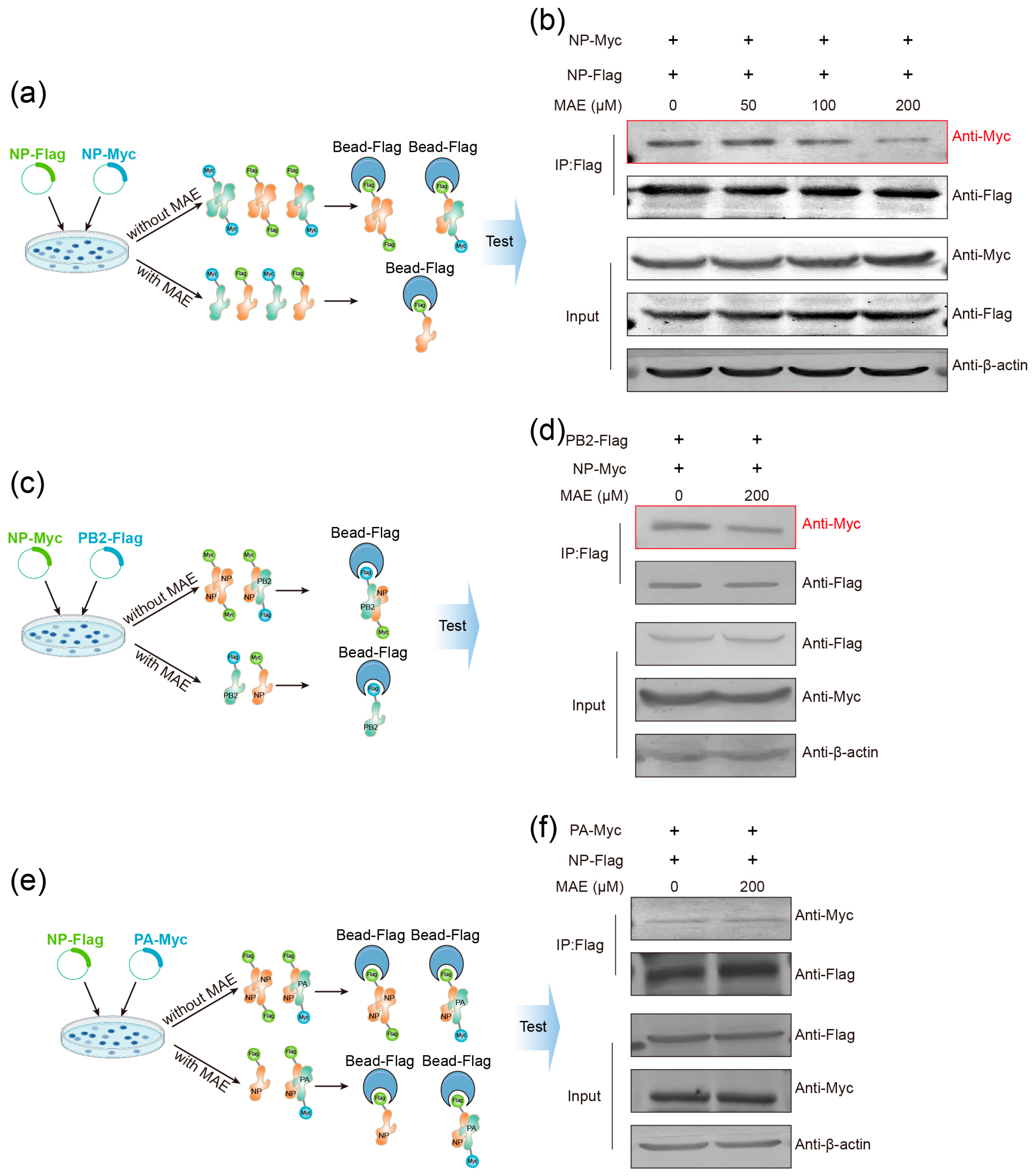
2.5. MAE Can Also Bind NP to Block the Assembly of vRNP
3. Discussion
4. Materials and Methods
4.1. Compounds and Reagents
4.2. Cell Culture and Viral Infection
4.3. Mini-Genome Assay
4.4. Surface Plasmon Resonance (SPR) Assay
4.5. Drug Affinity Responsive Target Stability (DARTS) Assay
4.6. Thermal Shift Assay (TSA)
4.7. Computer-Aided Virtual Docking Experiment
4.8. Pull Down Assay
4.9. Virucidal Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDC | 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Hydrochloride |
| FBS | Fetal Bovine Serum |
| IAV | Influenza A Virus |
| IMP-α | Importin-α |
| KD | Binding Affinity (Kd = Kd(Dissociation Rate Constant)/Ka (Association Rate Constant)) |
| MAE | Mycophenolic Acid Methyl Ester |
| MOE | Molecular Operating Environment |
| NHS | N-Hydroxysuccinimide |
| NLS | Nuclear Localization Signal Region |
| NP | Nucleoprotein |
| PA | Polymerase Acid Protein |
| PB1 | Polymerase Basic Protein 1 |
| PB2 | Polymerase Basic Protein 2 |
| PDB | The Brookhaven Protein Data Bank |
| SPR | Surface Plasmon Resonance |
| TNC | Tris-Nacl-Cacl2 |
| vRNP | Viral Ribonucleoprotein Complex |
References
- Bi, Y.H.; Suchard, M.A.; Lai, A.; Zhang, M.; Wang, L.; Zhu, Y.H.; Ma, L.; Li, H.P.; Haerheng, A.; Qi, Y.R.; et al. Zoonotic infections by avian influenza virus: Changing global epidemiology, investigation, and control. Lancet Infect. Dis. 2024, 24, e522–e531. [Google Scholar] [CrossRef]
- Javanian, M.; Barary, M.; Ghebrehewet, S.; Koppolu, V.; Vasigala, V.; Ebrahimpour, S. A brief review of influenza virus infection. J. Med. Virol. 2021, 93, 4638–4646. [Google Scholar] [CrossRef]
- Spackman, E. A Brief Introduction to Avian Influenza Virus. Methods Mol. Biol. 2020, 2123, 83–92. [Google Scholar] [CrossRef]
- Blacksell, S.D.; Dhawan, S.; Kusumoto, M.; Le, K.K.; Summermatter, K.; O’Keefe, J.; Kozlovac, J.P.; Almuhairi, S.S.; Sendow, I.; Scheel, C.M.; et al. Laboratory-acquired infections and pathogen escapes worldwide between 2000 and 2021: A scoping review. Lancet Microbe 2024, 5, e194–e202. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. The 1918 Influenza Pandemic and Its Legacy. Cold Spring Harb. Perspect. Med. 2020, 10, a038695. [Google Scholar] [CrossRef]
- Tan, T.K.; Rijal, P.; Huang, K.A.; Hyde, S.C.; Gill, D.R.; Townsend, A.R. Single-cycle, pseudotyped reporter influenza virus to facilitate evaluation of treatment strategies for avian influenza, Ebola and other highly infectious diseases in vivo. Front. Immunol. 2025, 16, 1608074. [Google Scholar] [CrossRef]
- Altman, M.O.; Angeletti, D.; Yewdell, J.W. Antibody Immunodominance: The Key to Understanding Influenza Virus Antigenic Drift. Viral Immunol. 2018, 31, 142–149. [Google Scholar] [CrossRef]
- Wong, K.H.; Lal, S.K. Alternative antiviral approaches to combat influenza A virus. Virus Genes 2023, 59, 25–35. [Google Scholar] [CrossRef]
- Stevenson-Leggett, P.; Adams, L.; Greenwood, D.; Lofts, A.; Libri, V.; Williams, B.; Gandhi, S.; Swanton, C.; Gamblin, S.; Carr, E.J.; et al. Crick Neutralization Consortium; Legacy Investigators; Crick Neutralization Consortium and Legacy Investigators. Investigation of Influenza A(H5N1) Virus Neutralization by Quadrivalent Seasonal Vaccines, United Kingdom, 2021–2024. Emerg Infect Dis 2025, 31, 1202–1206. [Google Scholar] [CrossRef]
- Bonomini, A.; Mercorelli, B.; Loregian, A. Antiviral strategies against influenza virus: An update on approved and innovative therapeutic approaches. Cell. Mol. Life Sci. 2025, 82, 75. [Google Scholar] [CrossRef]
- Yin, H.; Jiang, N.; Shi, W.; Chi, X.; Liu, S.; Chen, J.L.; Wang, S. Development and Effects of Influenza Antiviral Drugs. Molecules 2021, 26, 810. [Google Scholar] [CrossRef]
- Checkmahomed, L.; M’hamdi, Z.; Carbonneau, J.; Venable, M.C.; Baz, M.; Abed, Y.; Boivin, G. Impact of the Baloxavir-Resistant Polymerase Acid I38T Substitution on the Fitness of Contemporary Influenza A(H1N1)pdm09 and A(H3N2) Strains. J. Infect. Dis. 2020, 221, 63–70. [Google Scholar] [CrossRef]
- Liu, C.; Hu, L.; Dong, G.; Zhang, Y.; Ferreira da Silva-Júnior, E.; Liu, X.; Menéndez-Arias, L.; Zhan, P. Emerging drug design strategies in anti-influenza drug discovery. Acta Pharm. Sin. B 2023, 13, 4715–4732. [Google Scholar] [CrossRef]
- Müller, K.H.; Kakkola, L.; Nagaraj, A.S.; Cheltsov, A.V.; Anastasina, M.; Kainov, D.E. Emerging cellular targets for influenza antiviral agents. Trends Pharmacol. Sci. 2012, 33, 89–99. [Google Scholar] [CrossRef]
- Peng, R.; Xu, X.; Nepal, B.; Gong, Y.; Li, F.; Ferretti, M.B.; Zhou, M.; Lynch, K.W.; Burslem, G.M.; Kortagere, S.; et al. Molecular basis of influenza ribonucleoprotein complex assembly and processive RNA synthesis. Science 2025, 388, eadq7597. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Gordon, M.L. An overview of influenza A virus genes, protein functions, and replication cycle highlighting important updates. Virus Genes 2022, 58, 255–269. [Google Scholar] [CrossRef]
- De Vlugt, C.; Sikora, D.; Pelchat, M. Insight into Influenza: A Virus Cap-Snatching. Viruses 2018, 10, 641. [Google Scholar] [CrossRef]
- Monod, A.; Swale, C.; Tarus, B.; Tissot, A.; Delmas, B.; Ruigrok, R.W.; Crépin, T.; Slama-Schwok, A. Learning from structure-based drug design and new antivirals targeting the ribonucleoprotein complex for the treatment of influenza. Expert Opin. Drug Discov. 2015, 10, 345–371. [Google Scholar] [CrossRef]
- Makau, J.N.; Watanabe, K.; Otaki, H.; Mizuta, S.; Ishikawa, T.; Kamatari, Y.O.; Nishida, N. A Quinolinone Compound Inhibiting the Oligomerization of Nucleoprotein of Influenza A Virus Prevents the Selection of Escape Mutants. Viruses 2020, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.J.; Kao, R.Y.; Digard, P. Nucleozin targets cytoplasmic trafficking of viral ribonucleoprotein-Rab11 complexes in influenza A virus infection. J. Virol. 2013, 87, 4694–4703. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, J.; Zhang, J.; Tan, L.; Lu, G.; Luo, Y.; Pan, T.; Liang, J.; Li, Q.; Luo, B.; et al. Identification of a novel compound targeting the nuclear export of influenza A virus nucleoprotein. J. Cell. Mol. Med. 2018, 22, 1826–1839. [Google Scholar] [CrossRef]
- Yang, F.; Pang, B.; Lai, K.K.; Cheung, N.N.; Dai, J.; Zhang, W.; Zhang, J.; Chan, K.H.; Chen, H.; Sze, K.H.; et al. Discovery of a Novel Specific Inhibitor Targeting Influenza A Virus Nucleoprotein with Pleiotropic Inhibitory Effects on Various Steps of the Viral Life Cycle. J. Virol. 2021, 95, e01432-20. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.F.; Yu, Y.; Zhang, Q.; Huang, L.; Hao, C.; Shao, C.L.; Wang, W. Inhibition of influenza A virus replication by a marine derived quinolone alkaloid targeting virus nucleoprotein. J. Med. Virol. 2023, 95, e28499. [Google Scholar] [CrossRef] [PubMed]
- Elsebai, M.F. Secondary metabolites from the marine-derived fungus Phaeosphaeria spartinae. Nat. Prod. Res. 2021, 35, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Walczak, J.; Iwaszkiewicz-Grześ, D.; Śliwka-Kaszyńska, M.; Kurdyn, A.; Augustin, E.; Viapiana, A.; Plenis, A.; Cholewiński, G. Direct synthesis of mycophenolic acid aryl esters with antioxidant and antiproliferative properties. Sci. Rep. 2025, 15, 26006. [Google Scholar] [CrossRef]
- Siebert, A.; Wysocka, M.; Krawczyk, B.; Cholewiński, G.; Rachoń, J. Synthesis and antimicrobial activity of amino acid and peptide derivatives of mycophenolic acid. Eur. J. Med. Chem. 2018, 143, 646–655. [Google Scholar] [CrossRef]
- Siebert, A.; Cholewiński, G.; Trzonkowski, P.; Rachon, J. Immunosuppressive properties of amino acid and peptide derivatives of mycophenolic acid. Eur. J. Med. Chem. 2020, 189, 112091. [Google Scholar] [CrossRef]
- Liu, X.; Xu, N.; Wang, J.; Chen, K.; Ke, H.; Xu, Y.; Feng, D.; Xiao, L.; Meng, X.; Chen, S.; et al. Marine natural product Methyl mycophenolate inhibits gastric cancer growth through regulating p53 and the downstream pathways. Cancer Cell Int. 2025, 25, 206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, L.; Zhao, H.; Sow, M.D.; Zhang, Y.; Wang, W. Inhibition Effects and Mechanisms of Marine Compound Mycophenolic Acid Methyl Ester against Influenza A Virus. Mar. Drugs 2024, 22, 190. [Google Scholar] [CrossRef]
- Martínez-Sobrido, L.; Peersen, O.; Nogales, A. Temperature Sensitive Mutations in Influenza A Viral Ribonucleoprotein Complex Responsible for the Attenuation of the Live Attenuated Influenza Vaccine. Viruses 2018, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Melen, K.; Fagerlund, R.; Franke, J.; Kohler, M.; Kinnunen, L.; Julkunen, I. Importin alpha nuclear localization signal binding sites for STAT1, STAT2, and influenza A virus nucleoprotein. J. Biol. Chem. 2003, 278, 28193–28200. [Google Scholar] [CrossRef]
- Cros, J.F.; García-Sastre, A.; Palese, P. An unconventional NLS is critical for the nuclear import of the influenza A virus nucleoprotein and ribonucleoprotein. Traffic 2005, 6, 205–213. [Google Scholar] [CrossRef]
- Ortega, J.; Martín-Benito, J.; Zürcher, T.; Valpuesta, J.M.; Carrascosa, J.L.; Ortín, J. Ultrastructural and functional analyses of recombinant influenza virus ribonucleoproteins suggest dimerization of nucleoprotein during virus amplification. J. Virol. 2000, 74, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Szeto, W.C.; Hsia, H.P.; Tang, Y.S.; Shaw, P.C. Interaction between influenza A virus nucleoprotein and PB2 cap-binding domain is mediated by RNA. PLoS ONE 2020, 15, e0239899. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.; Thwaites, R.S.; Openshaw, P.J.M. Seasonal and pandemic influenza: 100 years of progress, still much to learn. Mucosal Immunol. 2020, 13, 566–573. [Google Scholar] [CrossRef]
- Harrington, W.N.; Kackos, C.M.; Webby, R.J. The evolution and future of influenza pandemic preparedness. Exp. Mol. Med. 2021, 53, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Balasubramanian, S.; Oelschlaeger, T.A.; Grkovic, T.; Pham, N.B.; Quinn, R.J.; Hentschel, U. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect. Dis. 2017, 17, e30–e41. [Google Scholar] [CrossRef]
- Gribble, G.W. Biological Activity of Recently Discovered Halogenated Marine Natural Products. Mar. Drugs 2015, 13, 4044–4136. [Google Scholar] [CrossRef]
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, Y.; Xu, S.; Sun, L.; Zhao, H.; Wang, W. Identifying a Marine-Derived Small-Molecule Nucleoprotein Inhibitor Against Influenza A Virus. Mar. Drugs 2025, 23, 413. https://doi.org/10.3390/md23110413
Wang Z, Zhang Y, Xu S, Sun L, Zhao H, Wang W. Identifying a Marine-Derived Small-Molecule Nucleoprotein Inhibitor Against Influenza A Virus. Marine Drugs. 2025; 23(11):413. https://doi.org/10.3390/md23110413
Chicago/Turabian StyleWang, Zihan, Yang Zhang, Shangjie Xu, Lishan Sun, Hongwei Zhao, and Wei Wang. 2025. "Identifying a Marine-Derived Small-Molecule Nucleoprotein Inhibitor Against Influenza A Virus" Marine Drugs 23, no. 11: 413. https://doi.org/10.3390/md23110413
APA StyleWang, Z., Zhang, Y., Xu, S., Sun, L., Zhao, H., & Wang, W. (2025). Identifying a Marine-Derived Small-Molecule Nucleoprotein Inhibitor Against Influenza A Virus. Marine Drugs, 23(11), 413. https://doi.org/10.3390/md23110413







