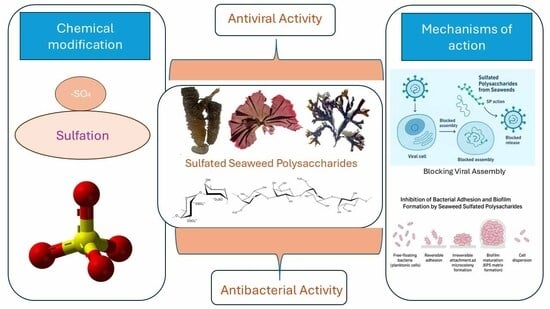
Beyond Nutrition: The Therapeutic Promise of Seaweed-Derived Polysaccharides Against Bacterial and Viral Threats
Abstract
1. Introduction
1.1. Overview of Seaweed-Derived Polysaccharides and Their Growing Significance
1.2. Importance of Investigating Their Antimicrobial and Antiviral Properties
1.3. Literature Search Strategy
2. Structural Characteristics of Seaweed Polysaccharides
2.1. Classification Based on Seaweed Type (Red, Brown, Green)
2.2. Chemical Composition and Sulfation Patterns
2.3. Structure-Activity Relationships Relevant to Bioactivity
3. Mechanisms of Antibacterial and Antiviral Action
3.1. Cellular Interactions and Inhibition of Pathogen Adhesion
3.2. Immunomodulatory Effects and Antiviral Pathways
3.3. Influence on Gut Microbiota and Prebiotic Benefits
4. Antibacterial Activity of Seaweed Polysaccharides
4.1. Summary of In Vitro and In Vivo Studies
4.2. Potential Applications in Medicine and Food Preservation
5. Antiviral Activity of Seaweed Polysaccharides
5.1. Overview of Studies Demonstrating Antiviral Properties
5.2. Mechanisms Targeting Viral Adsorption, Replication, and Immune Modulation
6. Extraction, Purification, and Commercial Applications
6.1. Current Methods for Isolation and Processing
6.2. Industrial-Scale Applications and Market Potential
7. Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BoHV-1 | Bovine alphaherpesvirus 1 |
| ECHO-1 | Enteric cytopathic human orphan virus 1 |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HIV | Human immunodeficiency viruses |
| HPV | Human papillomavirus |
| HSV | Herpes simplex virus |
| H5N1 | Influenza A virus subtype (Avian influenza) |
| IAV | Influenza A virus |
| IL-6 | Interleukin 6 |
| IL-12 | Interleukin 12 |
| IFN-α | Interferon alpha |
| IFN-γ | Interferon gamma |
| MAE | Microwave-assisted extraction |
| MHC | Major histocompatibility complex |
| NK | Natural killer cells |
| PRRs | Pattern recognition receptors |
| RNA | Ribonucleic acid |
| RSV | Respiratory syncytial virus |
| SARs | Structure–activity relationships |
| SCFAs | Short-chain fatty acids |
| SuHV | Suid herpes virus 1 |
| TLRs | Toll-like receptors |
| TNF-α | Tumor necrosis factor-alpha |
| UAE | ultrasound-assisted extraction |
References
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Ferrara, L. Seaweeds are a future resource in food as a source of raw materials and bio functional compounds. IJPRA 2023, 8, 512–527. [Google Scholar]
- Akter, A.; Sobuj, M.K.A.; Islam, M.S.; Chakroborty, K.; Tasnim, N.; Ayon, M.H.; Hossain, M.F.; Rafiquzzaman, S.M. Seaweed polysaccharides: Sources, structure and biomedical applications with special emphasis on antiviral potentials. Future Foods 2024, 10, 100440. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Nurkolis, F. Marine bioactives: Pioneering sustainable solutions for advanced cosmetics and therapeutics. Pharmacol. Res. 2025, 218, 107868. [Google Scholar] [CrossRef]
- Wang, P.; Liu, C.; Zheng, L. Unlocking the potential of microalgae-derived therapeutic carriers: Characteristics, types, and nanomedical applications. Mater. Today Bio 2025, 33, 102037. [Google Scholar] [CrossRef]
- Adarshan, S.; Sree, V.S.S.; Muthuramalingam, P.; Nambiar, K.S.; Sevanan, M.; Satish, L.; Venkidasamy, B.; Jeelani, P.G.; Shin, H. Understanding Macroalgae: A Comprehensive Exploration of Nutraceutical, Pharmaceutical, and Omics Dimensions. Plants 2024, 13, 113. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef]
- Kraithong, S.; Bunyameen, N.; Jaisan, C.; Liu, Y.; Sangsawad, P.; Huang, R.; Shi, X. Gelling, thickening, and biological properties of marine algal polysaccharides: Implications for food applications. Carbohydr. Polym. 2025, 369, 124309. [Google Scholar] [CrossRef]
- Zaitseva, O.O.; Sergushkina, M.I.; Khudyakov, A.N.; Polezhaeva, T.V.; Solomina, O.N. Seaweed sulfated polysaccharides and their medicinal properties. Algal Res. 2022, 68, 102885. [Google Scholar] [CrossRef]
- Mendes, M.; Cotas, J.; Pacheco, D.; Ihle, K.; Hillinger, A.; Cascais, M.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Red Seaweed (Rhodophyta) Phycocolloids: A Road from the Species to the Industry Application. Mar. Drugs 2024, 22, 432. [Google Scholar] [CrossRef]
- Li, S.-B.; Yao, Q.-H.; Ye, X.-Q.; Balasubramanian, B.; Liu, W.-C. Unraveling of Seaweed Bioactive Substances and Their Nutritional Regulation Functions for Poultry. Mar. Drugs 2025, 23, 324. [Google Scholar] [CrossRef]
- Gericke, M.; Amaral, A.J.R.; Budtova, T.; De Wever, P.; Groth, T.; Heinze, T.; Höfte, H.; Huber, A.; Ikkala, O.; Kapuśniak, J.; et al. The European Polysaccharide Network of Excellence (EPNOE) research roadmap 2040: Advanced strategies for exploiting the vast potential of polysaccharides as renewable bioresources. Carbohydr. Polym. 2024, 326, 121633. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Barriga, E.J.; Fields, R.D. Sulfated polysaccharides as multi target molecules to fight COVID 19 and comorbidities. Heliyon 2023, 9, e13797. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.J.; Xie, C.; Ng, K.; Suleria, H.A.R. Unraveling the bioactive interplay: Seaweed polysaccharide, polyphenol and their gut modulation effect. Crit. Rev. Food Sci. Nutr. 2025, 65, 382–405. [Google Scholar] [CrossRef] [PubMed]
- Bose, I.; Nousheen Roy, S.; Yaduvanshi, P.; Sharma, S.; Chandel, V.; Biswas, D. Unveiling the Potential of Marine Biopolymers: Sources, Classification, and Diverse Food Applications. Materials 2023, 16, 4840. [Google Scholar] [CrossRef]
- Carrasqueira, J.; Bernardino, S.; Bernardino, R.; Afonso, C. Marine-Derived Polysaccharides and Their Potential Health Benefits in Nutraceutical Applications. Mar. Drugs 2025, 23, 60. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Alaoui Mdarhri, H.; Benmessaoud, R.; Yacoubi, H.; Seffar, L.; Guennouni Assimi, H.; Hamam, M.; Boussettine, R.; Filali-Ansari, N.; Lahlou, F.A.; Diawara, I.; et al. Alternatives Therapeutic Approaches to Conventional Antibiotics: Advantages, Limitations and Potential Application in Medicine. Antibiotics 2022, 11, 1826. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour. Technol. Rep. 2021, 13, 100623. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Cotas, J. Seaweed: A sustainable solution for greening drug manufacturing in the pursuit of sustainable healthcare. Explor. Drug Ssci. 2024, 2, 50–84. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. Harnessing the power of seaweed: Unveiling the potential of marine algae in drug discovery. Explor. Drug Ssci. 2023, 1, 475–496. [Google Scholar] [CrossRef]
- Cooney, O.C.; Morrin, S.T.; Buck, R.H.; Owens, R.A.; Hickey, R.M. Seaweed-derived polysaccharides as antibacterial and antiviral ingredients. Int. J. Biol. Macromol. 2025, 321, 145823. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef]
- Cadar, E.; Popescu, A.; Dragan, A.-M.-L.; Pesterau, A.-M.; Pascale, C.; Anuta, V.; Prasacu, I.; Velescu, B.S.; Tomescu, C.L.; Bogdan-Andreescu, C.F.; et al. Bioactive Compounds of Marine Algae and Their Potential Health and Nutraceutical Applications: A Review. Mar. Drugs 2025, 23, 152. [Google Scholar] [CrossRef]
- Usov, A.I. Chapter 4-Polysaccharides of the red algae. In Advances in Carbohydrate Chemistry and Biochemistry; Horton, D., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 65, pp. 115–217. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Chen, X.; Fu, X.; Huang, L.; Xu, J.; Gao, X. Agar oligosaccharides: A review of preparation, structures, bioactivities and application. Carbohydr. Polym. 2021, 265, 118076. [Google Scholar] [CrossRef]
- Khan, B.M.; Qiu, H.-M.; Xu, S.-Y.; Liu, Y.; Cheong, K.-L. Physicochemical characterization and antioxidant activity of sulphated polysaccharides derived from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 145, 1155–1161. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D. Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Nagahawatta, D.P.; Fernando, I.P.S.; Kim, Y.-T.; Kim, J.-S.; Kim, W.-S.; Lee, J.S.; Jeon, Y.-J. A Review on Fucoidan Structure, Extraction Techniques, and Its Role as an Immunomodulatory Agent. Mar. Drugs 2022, 20, 755. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Sabir, A.; Wang, M.; Zhong, S.; Tan, K. Advancements in the Extraction, Characterization, and Bioactive Potential of Laminaran: A Review. Foods 2025, 14, 1683. [Google Scholar] [CrossRef] [PubMed]
- Bojorges, H.; López-Rubio, A.; Martínez-Abad, A.; Fabra, M.J. Overview of alginate extraction processes: Impact on alginate molecular structure and techno-functional properties. Trends Food Sci. Technol. 2023, 140, 104142. [Google Scholar] [CrossRef]
- Shahidi, F.; Rahman, M.J. Bioactives in seaweeds, algae, and fungi and their role in health promotion. J. Food Bioact. 2018, 2, 58–81. [Google Scholar] [CrossRef]
- Costa, S.P.; Cotas, J.; Pereira, L. Laminar Ulva Species: A Multi-Tool for Humankind? Appl. Sci. 2024, 14, 3448. [Google Scholar] [CrossRef]
- Corino, C.; Di Giancamillo, A.; Modina, S.C.; Rossi, R. Prebiotic Effects of Seaweed Polysaccharides in Pigs. Animals 2021, 11, 1573. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Cassani, L.; Carreira-Casais, A.; García Oliveira, P.; Barral Martínez, M.; Echave, J.; Otero, P.; García-Pérez, P.; Baamonde, S.; Saa, F.; et al. Optimization of Microwave-Assisted Extraction, from an Edible Marine Alga of the Galician Coastline, Using A Response Surface Methodology; Universidade de Vigo: Pontevedra, Spain, 2021; p. 288. [Google Scholar] [CrossRef]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 1901–1929. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Zhi, Z.; Wei, C.; Wang, W.; Ding, T.; Ye, X.; Hu, Y.; Linhardt, R.J.; Chen, S. Macromolecular properties and hypolipidemic effects of four sulfated polysaccharides from sea cucumbers. Carbohydr. Polym. 2017, 173, 330–337. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Bioactivity of sulfated polysaccharides from the edible red seaweed Mastocarpus stellatus. Bioact. Carbohydr. Diet. Fibre 2014, 3, 29–40. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Souto, S.; Flórez-Fernández, N.; Torres, M.D.; Bandín, I.; Domínguez, H. Antiviral Activity of Carrageenans and Processing Implications. Mar. Drugs 2021, 19, 437. [Google Scholar] [CrossRef]
- Mouga, T.; Fernandes, I.B. The Red Seaweed Giant Gelidium (Gelidium corneum) for New Bio-Based Materials in a Circular Economy Framework. Earth 2022, 3, 788–813. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gomez-Barrio, L.P.; Zhao, M.; Tiwari, B.; Knutsen, S.H.; Ballance, S.; Zobel, H.K.; Nilsson, A.E.; Krewer, C.; Östergren, K.; et al. Alternative protocols for the production of more sustainable agar-based extracts from Gelidium sesquipedale. Algal Res. 2021, 55, 102254. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Liu, X.; Elbahri, M. Antiviral polysaccharide and antiviral peptide delivering nanomaterials for prevention and treatment of SARS-CoV-2 caused COVID-19 and other viral diseases. J. Control. Release 2023, 358, 476–497. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.; Ferreira-Dias, S.; Tecelão, C.; Alves, V.D. Potential of Marine Biomolecules: Advances in Extraction and Applications of Proteins, Polysaccharides, and Antioxidant Compounds. Foods 2025, 14, 2555. [Google Scholar] [CrossRef]
- Fuentes, A.-L.; Millis, L.; Sigola, L.B. Laminarin, a soluble beta-glucan, inhibits macrophage phagocytosis of zymosan but has no effect on lipopolysaccharide mediated augmentation of phagocytosis. Int. Immunopharmacol. 2011, 11, 1939–1945. [Google Scholar] [CrossRef]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A Brief Review on the Development of Alginate Extraction Process and Its Sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Zhou, Q.-L.; Wang, Z.; Chen, W.-T.; Liu, X.-F.; Cheong, K.-L.; Zou, Y.-X.; Zhong, S.-Y.; Li, R. The structural characteristics, biological activities and mechanisms of bioactive brown seaweed polysaccharides: A review. J. Funct. Foods 2024, 119, 106303. [Google Scholar] [CrossRef]
- Krylova, N.V.; Kravchenko, A.O.; Iunikhina, O.V.; Pott, A.B.; Likhatskaya, G.N.; Volod’ko, A.V.; Zaporozhets, T.S.; Shchelkanov, M.Y.; Yermak, I.M. Influence of the Structural Features of Carrageenans from Red Algae of the Far Eastern Seas on Their Antiviral Properties. Mar. Drugs 2022, 20, 60. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Alfinaikh, R.S.; Alamry, K.A.; Hussein, M.A. Hussein. Sustainable and biocompatible hybrid materials-based sulfated polysaccharides for biomedical applications: A review. RSC Adv. 2025, 15, 4708–4767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Healy, L.; Wanigasekara, J.; Zhao, M.; Padamati, R.B.; Karuppusamy, S.; Curtin, J.F.; Sivagnanam, S.P.; Rai, D.K.; Sun, D.-W.; et al. Characterisation of laminarin extracted from brown seaweed Laminaria digitata, using optimized ultrasound- and ultrafiltration-assisted extraction method. Algal Res. 2023, 75, 103277. [Google Scholar] [CrossRef]
- Ibrahim, M.I.A.; Amer, M.S.; Ibrahim, H.A.H.; Zaghloul, E.H. Considerable Production of Ulvan from Ulva lactuca with Special Emphasis on Its Antimicrobial and Anti-fouling Properties. Appl. Biochem. Biotechnol. 2022, 194, 3097–3118. [Google Scholar] [CrossRef]
- Roy, A.; Roy, P.K.; Park, S.Y. The Role of Fucoidan in Controlling Listeria monocytogenes Biofilms on Seafood-Contact Surfaces. Appl. Sci. 2025, 15, 5799. [Google Scholar] [CrossRef]
- Palafox Félix, S.; Sandoval Larios, G.; Cabrera, R.; García-Galaz, A.; Huerta-Ocampo, J.Á.; Guzmán-Partida, A.M.; Armenta Corral, R.I.; Sarabia-Sainz, J.A.; Ramos Clamont Montfort, G. Effects of Fucoidan and Fucoidan Oligosaccharides in Growth and Quorum Sensing Mediated Virulence Factor of Campylobacter jejuni. Polysaccharides 2025, 6, 24. [Google Scholar] [CrossRef]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M. Cation-Alginate Complexes and Their Hydrogels: A Powerful Toolkit for the Development of Next-Generation Sustainable Functional Materials. Adv. Funct. Mater. 2025, 35, 2416390. [Google Scholar] [CrossRef]
- Kumar, A.; Soratur, A.; Kumar, S.; Venmathi Maran, B.A. A Review of Marine Algae as a Sustainable Source of Antiviral and Anticancer Compounds. Macromol 2025, 5, 11. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Lyu, Z.; Gu, H.; Li, D.; Chen, H. Glycosaminoglycans (GAGs) and GAG mimetics regulate the behavior of stem cell differentiation. Colloids Surf. B Biointerfaces 2017, 150, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed]
- Rupert, R.; Rodrigues, K.F.; Thien, V.Y.; Yong, W.T.L. Carrageenan from Kappaphycus alvarezii (Rhodophyta, Solieriaceae): Metabolism, Structure, Production, and Application. Front. Plant Sci. 2022, 13, 859635. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef]
- Frediansyah, A. The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review. Clin. Epidemiol. Glob. Health 2021, 12, 100826. [Google Scholar] [CrossRef]
- Pakan, P.D.; Siu, A.C.W.; Lee, H.; Singh, M.; De Rubis, G.; Yeung, S.; Kulkarni, M.P.; Goh, B.H.; Hsu, A.C.; Chellappan, D.K.; et al. Algal bioactives: Unlocking future frontiers in respiratory therapeutics. Food Biosci. 2025, 69, 106778. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Bhuyan, P.P.; Behera, P.K.; Mandal, A.K.; Behera, C.; Ki, J.-S.; Adhikary, S.P.; MubarakAli, D.; et al. A state-of-the-art review on fucoidan as an antiviral agent to combat viral infections. Carbohydr. Polym. 2022, 291, 119551. [Google Scholar] [CrossRef]
- Pari, R.F.; Uju, U.; Hardiningtyas, S.D.; Ramadhan, W.; Wakabayashi, R.; Goto, M.; Kamiya, N. Ulva Seaweed-Derived Ulvan: A Promising Marine Polysaccharide as a Sustainable Resource for Biomaterial Design. Mar. Drugs 2025, 23, 56. [Google Scholar] [CrossRef]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef]
- Geesey, G.G.; Wigglesworth-Cooksey, B.; Cooksey, K.E. Influence of calcium and other cations on surface adhesion of bacteria and diatoms: A review. Biofouling 2000, 15, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies. Mar. Drugs 2022, 20, 772. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.-L.; Chen, W.; Wang, M.; Zhong, S.; Veeraperumal, S. Therapeutic Prospects of Undaria pinnatifida Polysaccharides: Extraction, Purification, and Functional Activity. Mar. Drugs 2025, 23, 163. [Google Scholar] [CrossRef]
- Seimon, T.A.; Obstfeld, A.; Moore, K.J.; Golenbock, D.T.; Tabas, I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc. Natl. Acad. Sci. USA 2006, 103, 19794–19799. [Google Scholar] [CrossRef]
- Cicinskas, E.; Kalitnik, A.A.; Karetin, Y.A.; Mohan Ram, M.S.G.; Achary, A.; Kravchenko, A.O. Immunomodulating Properties of Carrageenan from Tichocarpus crinitus. Inflammation 2020, 43, 1387–1396. [Google Scholar] [CrossRef]
- Ludwig, M.; Enzenhofer, E.; Schneider, S.; Rauch, M.; Bodenteich, A.; Neumann, K.; Prieschl-Grassauer, E.; Grassauer, A.; Lion, T.; Mueller, C.A. Efficacy of a Carrageenan nasal spray in patients with common cold: A randomized controlled trial. Respir. Res. 2013, 14, 124. [Google Scholar] [CrossRef]
- Lee, C. Carrageenans as Broad-Spectrum Microbicides: Current Status and Challenges. Mar. Drugs 2020, 18, 435. [Google Scholar] [CrossRef]
- Leibbrandt, A.; Meier, C.; König-Schuster, M.; Weinmüllner, R.; Kalthoff, D.; Pflugfelder, B.; Graf, P.; Frank-Gehrke, B.; Beer, M.; Fazekas, T.; et al. Iota-Carrageenan is a Potent Inhibitor of Influenza a Virus Infection. PLoS ONE 2010, 5, e14320. [Google Scholar] [CrossRef]
- Pereira, L.; Critchley, A.T. The COVID 19 novel coronavirus pandemic 2020: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020, 32, 1875–1877. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, P.; Baltrusch, K.L.; Díaz-Reinoso, B.; López-Álvarez, M.; Novoa-Carballal, R.; González, P.; González-Novoa, A.; Rodríguez-Montes, A.; Kennes, C.; Veiga, M.C.; et al. Hydrothermal extraction of ulvans from Ulva spp. in a biorefinery approach. Sci. Total Environ. 2024, 951, 175654. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Graves, B.; Child, R.; Rice, P.J.; Ma, Z.; Lowman, D.W.; Ensley, H.E.; Ryter, K.T.; Evans, J.T.; Williams, D.L. Immunoregulatory Activity of the Natural Product Laminarin Varies Widely as a Result of Its Physical Properties. J. Immunol. 2018, 200, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.D.; Allahgholi, L.; Dobruchowska, J.M.; Moenaert, A.; Guðmundsson, H.; Friðjónsson, Ó.; Karlsson, E.N.; Hreggviðsson, G.Ó.; Freysdottir, J. Laminarins and their derivatives affect dendritic cell activation and their crosstalk with T cells. Int. J. Biol. Macromol. 2025, 306, 141287. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Gonçalves, A.M.M. Antiviral Activity and Mechanisms of Seaweeds Bioactive Compounds on Enveloped Viruses—A Review. Mar. Drugs 2022, 20, 385. [Google Scholar] [CrossRef]
- Hwang, J.; Yadav, D.; Lee, P.C.; Jin, J.-O. Immunomodulatory effects of polysaccharides from marine algae for treating cancer, infectious disease, and inflammation. Phytother. Res. 2022, 36, 761–777. [Google Scholar] [CrossRef]
- Lin, Q.; Zhong, L.; Zeng, M.; Kraithong, S.; Xia, X.; Kuang, W.; Wang, Q.; Huang, R. Seaweed polysaccharides as potential Prebiotics: Rationale, factors, Prebiotic activity manifestations, gut health mechanisms and extraintestinal impacts. Trends Food Sci. Technol. 2025, 163, 105202. [Google Scholar] [CrossRef]
- Cherry, P.; Yadav, S.; Strain, C.R.; Allsopp, P.J.; McSorley, E.M.; Ross, R.P.; Stanton, C. Prebiotics from Seaweeds: An Ocean of Opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef]
- Ahmad, A.; Riaz, S.; Desta, D.T. Alginate’s ability to prevent metabolic illnesses, the degradation of the gut’s protective layer, and alginate-based encapsulation methods. Food Sci. Nutr. 2024, 12, 8692–8714. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jeon, Y.-J. Fucoidans as Scientifically and Commercially Important Algal Polysaccharides. Mar. Drugs 2021, 19, 284. [Google Scholar] [CrossRef]
- Vigors, S.; O’Doherty, J.V.; Rattigan, R.; McDonnell, M.J.; Rajauria, G.; Sweeney, T. Effect of a Laminarin Rich Macroalgal Extract on the Caecal and Colonic Microbiota in the Post-Weaned Pig. Mar. Drugs 2020, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, T.; Du, Y.; Jiang, L.; Yao, Z.; Ning, L.; Zhu, B. Ulvan and Ulva oligosaccharides: A systematic review of structure, preparation, biological activities and applications. Bioresour. Bioprocess. 2023, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Allur Subramaniyan, S.; Begum, N.; Kim, S.J.; Choi, Y.H.; Nam, T.-J. Biopeptides of Pyropia yezoensis and their potential health benefits: A review. Asian Pac. J. Trop. Biomed. 2021, 11, 375–384. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Ahmed, T.A.E.; Rjabovs, V.; Critchley, A.T.; Hincke, M.T.; Tuvikene, R. Green seaweed-derived polysaccharides: Insights into various bioactivities for biomedical applications. Int. J. Biol. Macromol. 2024, 282, 136858. [Google Scholar] [CrossRef]
- Yao, W.; Kong, Q.; You, L.; Zhong, S.; Hileuskaya, K. Polysaccharides from brown seaweed: Physicochemical properties, absorption in the intestine, and beneficial effects on intestinal barrier. Food Front. 2023, 4, 1547–1560. [Google Scholar] [CrossRef]
- Rajasekaran, J.; Viswanathan, P. Anti-bacterial and antibiofilm properties of seaweed polysaccharide-based nanoparticles. Aquac. Int. 2023, 31, 2799–2823. [Google Scholar] [CrossRef]
- Nshimiyumukiza, O.; Kang, S.-K.; Kim, H.-J.; Lee, E.-H.; Han, H.-N.; Kim, Y.; Kim, D.-H.; Kim, J.-H.; Eom, S.-H.; Kim, Y.-M. Synergistic Antibacterial Activity of Ecklonia cava (Phaeophyceae: Laminariales) against Listeria monocytogenes (Bacillales: Listeriaceae). Fish Aquat. Sci. 2015, 18, 1–6. [Google Scholar] [CrossRef]
- McGurrin, A.; Suchintita Das, R.; Soro, A.B.; Maguire, J.; Flórez Fernández, N.; Dominguez, H.; Torres, M.D.; Tiwari, B.K.; Garcia-Vaquero, M. Antimicrobial Activities of Polysaccharide-Rich Extracts from the Irish Seaweed Alaria esculenta, Generated Using Green and Conventional Extraction Technologies, Against Foodborne Pathogens. Mar. Drugs 2025, 23, 46. [Google Scholar] [CrossRef]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardena, T.U.; Yang, F.; Jayawardena, H.H.A.C.K.; Kurera, M.J.M.S.; Wang, F.; Fu, X.; Jeon, Y.-J. Functions and values of sulfated polysaccharides from seaweed. Algae 2023, 38, 217–240. [Google Scholar] [CrossRef]
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial Properties of Fucoidans from the Brown Algae Fucus vesiculosus L. of the Barents Sea. Biology 2021, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Anisha, G.S.; Padmakumari, S.; Patel, A.K.; Pandey, A.; Singhania, R.R. Fucoidan from Marine Macroalgae: Biological Actions and Applications in Regenerative Medicine, Drug Delivery Systems and Food Industry. Bioengineering 2022, 9, 472. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Rivas, S.; Torres, M.D.; Domínguez, H. Microwave-Assisted Extraction of Carrageenan from Sarcopeltis skottsbergii. Mar. Drugs 2023, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Garcia-Perez, P.; Garcia-Oliveira, P.; Chamorro, F.; Otero, P.; Lourenço-Lopes, C.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Biological properties and potential of compounds extracted from red seaweeds. Phytochem. Rev. 2023, 22, 1509–1540. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Dwan, C.; Wimmer, B.C.; Ahamed, S.K.; James, F.; Thinley, J.; Wilson, R.; Johnson, L.; Caruso, V. Anti-Inflammatory and Neuroprotective Effects of Undaria pinnatifida Fucoidan. Mar. Drugs 2025, 23, 350. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.; Cunha, S.A.; Ribeiro, T.; Borges, S.; Baptista-Silva, S.; Oliveira-Silva, P.; Pintado, M. Fucoidans: Exploring its neuroprotective mechanisms and therapeutic applications in brain disorders. Trends Food Sci. Technol. 2024, 143, 104300. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Kheraif, A.A.A.; Kang, K.-H.; Kim, S.-K. Seaweed polysaccharides and their potential biomedical applications. Starch-Stärke 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Anburaj, R.; Kathiresan, K.; Prasanna Kumar, C.; Arun. Exploring the depths: A comprehensive review of marine algae. In Recent Trends in Algae and Seaweeds; Scieng Publications: Tamil Nadu, India, 2024; pp. 176–187. ISBN 978-93-94766-96-9. [Google Scholar]
- Bharathi, D.; Lee, J. Recent Advances in Marine-Derived Compounds as Potent Antibacterial and Antifungal Agents: A Comprehensive Review. Mar. Drugs 2024, 22, 348. [Google Scholar] [CrossRef]
- Ye, S.; Xie, C.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Alginates from Brown Seaweeds as a Promising Natural Source: A Review of Its Properties and Health Benefits. Food Rev. Int. 2024, 40, 2682–2710. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef]
- Schütz, D.; Conzelmann, C.; Fois, G.; Groß, R.; Weil, T.; Wettstein, L.; Stenger, S.; Zelikin, A.; Hoffmann, T.K.; Frick, M.; et al. Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L750–L756. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, N.A.; Sequeira, R.A.; Moradiya, K.; Prasad, K. Seaweed Polysaccharide Based Products and Materials: An Assessment on Their Production from a Sustainability Point of View. Molecules 2021, 26, 2608. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; Rodríguez-Coello, A.; Latire, T.; Bourgougnon, N.; Torres, M.D.; Buján, M.; Muíños, A.; Muiños, A.; Meijide-Faílde, R.; Blanco, F.J.; et al. Anti-inflammatory potential of ulvan. Int. J. Biol. Macromol. 2023, 253, 126936. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Kumar, G.; Singh, R.; Selvaraj, M.; Malik, T.; Al Tawaha, A.R.M. Natural biopolymers in edible coatings: Applications in food preservation. Food Chem. X 2025, 25, 102171. [Google Scholar] [CrossRef]
- Ferreira, M.; Salgado, J.M.; Peres, H.; Belo, I. Valorization of Gelidium corneum by-product through solid-state fermentation. Food Bioprod. Process. 2024, 146, 205–212. [Google Scholar] [CrossRef]
- Jabeen, F.; Ahmad, R.; Mir, S.; Awwad, N.S.; Ibrahium, H.A. Carrageenan: Structure, properties and applications with special emphasis on food science. RSC Adv. 2025, 15, 22035–22062. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Song, H.; Zhang, H.; Niu, X. Regioselective syntheses of sulfated porphyrans from Porphyra haitanensis and their antioxidant and anticoagulant activities in vitro. Carbohydr. Polym. 2010, 79, 1124–1129. [Google Scholar] [CrossRef]
- Dong, X.; Qiu, Y.; Jia, N.; Wu, Y.; Nie, Q.; Wen, J.; Zhao, C.; Zhai, Y. Recent advances of edible marine algae-derived sulfated polysaccharides in antiviral treatments: Challenges vs. opportunities. Front. Nutr. 2025, 12, 1561119. [Google Scholar] [CrossRef]
- Hayashi, K.; Lee, J.-B.; Nakano, T.; Hayashi, T. Anti-influenza A virus characteristics of a fucoidan from sporophyll of Undaria pinnatifida in mice with normal and compromised immunity. Microbes Infect. 2013, 15, 302–309. [Google Scholar] [CrossRef]
- Richards, C.; Williams, N.A.; Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S.; Park, A.Y. Oral fucoidan attenuates lung pathology and clinical signs in a severe influenza a mouse model. Mar. Drugs 2020, 18, 246. [Google Scholar] [CrossRef]
- Synytsya, A.; Bleha, R.; Synytsya, A.; Pohl, R.; Hayashi, K.; Yoshinaga, K.; Hayashi, T. Mekabu fucoidan: Structural complexity and defensive effects against avian influenza A viruses. Carbohyd. Polym. 2014, 111, 633–644. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Zhang, X.; Hao, C.; Zhao, X.; Jiao, G.; Yu, G. Inhibition of influenza A virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci. Rep. 2017, 7, 40760. [Google Scholar] [CrossRef]
- Makarenkova, I.D.; Deriabin, P.G.; L’vov, D.K.; Zviagintseva, T.N.; Besednova, N.N. Antiviral activity of sulfated polysaccharide from the brown algae Laminaria japonica against avian influenza A (H5N1) virus infection in the cultured cells. Vopr. Virusol. 2010, 55, 41–45. [Google Scholar] [PubMed]
- Prokofjeva, M.M.; Imbs, T.I.; Shevchenko, N.M.; Spirin, P.V.; Horn, S.; Fehse, B.; Prassolov, V.S. Fucoidans as potential inhibitors of HIV-1. Mar. Drugs 2013, 11, 3000–3014. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.T.; Van Quang, N.; Tu, H.C.; Zheng, Y.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohyd. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef]
- Dinesh, S.; Menon, T.; Hanna, L.E.; Suresh, V.; Sathuvan, M.; Manikannan, M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016, 82, 83–88. [Google Scholar] [CrossRef]
- Mori, N.; Nakasone, K.; Tomimori, K.; Ishikawa, C. Beneficial effects of fucoidan in patients with chronic hepatitis C virus infection. World J. Gastroenterol. 2012, 18, 2225. [Google Scholar] [CrossRef]
- Ponce, N.M.; Flores, M.L.; Pujol, C.A.; Becerra, M.B.; Navarro, D.A.; Córdoba, O.; Stortz, C.A. Fucoidans from the phaeophyta Scytosiphon lomentaria: Chemical analysis and antiviral activity of the galactofucan component. Carbohyd. Res. 2019, 478, 18–24. [Google Scholar] [CrossRef]
- Sun, Q.-L.; Li, Y.; Ni, L.-Q.; Li, Y.-X.; Cui, Y.-S.; Jiang, S.-L.; Dong, C.-X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487. [Google Scholar] [CrossRef]
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Silchenko, A.S. The comparative analysis of antiviral activity of native and modified fucoidans from brown algae Fucus evanescens in vitro and in vivo. Mar. Drugs 2020, 18, 224. [Google Scholar] [CrossRef]
- Koenighofer, M.; Lion, T.; Bodenteich, A.; Prieschl-Grassauer, E.; Grassauer, A.; Unger, H.; Mueller, C.A.; Fazekas, T. Carrageenan nasal spray in virus confirmed common cold: Individual patient data analysis of two randomized controlled trials. Multidiscip. Respir. Med. 2014, 9, 57. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, P.; Yu, G.-L.; Li, C.-X.; Hao, C.; Qi, X.; Zhang, L.-J.; Guan, H.-S. Preparation and anti-influenza A virus activity of κ-carrageenan oligosaccharide and its sulphated derivatives. Food Chem. 2012, 133, 880–888. [Google Scholar] [CrossRef]
- Tang, F.; Chen, F.; Li, F. Preparation and potential in vivo anti-influenza virus activity of low molecular-weight κ-carrageenans and their derivatives. J. Appl. Polym. Sci. 2013, 127, 2110–2115. [Google Scholar] [CrossRef]
- Rodríguez, A.; Kleinbeck, K.; Mizenina, O.; Kizima, L.; Levendosky, K.; Jean-Pierre, N.; Teleshova, N. In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition. Antivir. Res. 2014, 108, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Morokutti-Kurz, M.; König-Schuster, M.; Koller, C.; Graf, C.; Graf, P.; Kirchoff, N.; Reutterer, B.; Seifert, J.-M.; Unger, H.; Grassauer, A.; et al. The Intranasal Application of Zanamivir and Carrageenan Is Synergistically Active against Influenza A Virus in the Murine Model. PLoS ONE 2015, 10, e0128794. [Google Scholar] [CrossRef]
- Diogo, J.V.; Novo, S.G.; González, M.J.; Ciancia, M.; Bratanich, A.C. Antiviral activity of lambda-carrageenan prepared from red seaweed (Gigartina skottsbergii) against BoHV-1 and SuHV-1. Res. Vet. Sci. 2015, 98, 142–144. [Google Scholar] [CrossRef]
- Cáceres, P.J.; Carlucci, M.A.J.; Damonte, E.B.; Matsuhiro, B.; Zúñiga, E.A. Carrageenans from chilean samples of Stenogramme interrupta (Phyllophoraceae): Structural analysis and biological activity. Phytochemistry 2000, 53, 81–86. [Google Scholar] [CrossRef]
- Carlucci, M.; Scolaro, L.; Noseda, M.; Cerezo, A.; Damonte, E. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antivir. Res. 2004, 64, 137–141. [Google Scholar] [CrossRef]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 2009, 83, 282–289. [Google Scholar] [CrossRef]
- Boulho, R.; Marty, C.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Ana, P.; Nathalie, B.; Gilles, B.; Daniel, R.; Tomás, M.-S.; Yolanda, F.-P. Anti-Herpes simplex virus (HSV-1) activity and antioxidant capacity of carrageenan-rich enzymatic extracts from Solieria filiformis (Gigartinales, Rhodophyta). Int. J. Biol. Macromol. 2021, 168, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.A.; Scolaro, L.A.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiviral Activity of a Carrageenan from Gigartina skottsbergii against Intraperitoneal Murine Herpes simplex Virus Infection. Planta Med. 2006, 72, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Sanniyasi, E.; Venkatasubramanian, G.; Anbalagan, M.M.; Raj, P.P.; Gopal, R.K. In vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana J.V. Lamouroux and Turbinaria decurrens Bory). Sci. Rep. 2019, 9, 12185. [Google Scholar] [CrossRef]
- Binsuwaidan, R.; El-Masry, T.A.; El-Sheekh, M.; Seadawy, M.G.; Makhlof, M.E.M.; Aboukhatwa, S.M.; El-Shitany, N.A.; Elmorshedy, K.E.; El-Nagar, M.M.F.; El-Bouseary, M.M. Prospective Antiviral Effect of Ulva lactuca Aqueous Extract against COVID-19 Infection. Mar. Drugs 2024, 22, 30. [Google Scholar] [CrossRef]
- Ohta, Y.; Lee, J.-B.; Hayashi, K.; Hayashi, T. Isolation of Sulfated Galactan from Codium fragile and Its Antiviral Effect. Biol. Pharm. Bull. 2009, 32, 892–898. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Y.; Gao, W.; He, Y.; Wang, Y.; Sun, Y.; Kuang, H. Polysaccharides from Porphyra haitanensis: A Review of Their Extraction, Modification, Structures, and Bioactivities. Molecules 2024, 29, 3105. [Google Scholar] [CrossRef]
- Chen, M.-Z.; Xie, H.-G.; Yang, L.-W.; Liao, Z.-H.; Yu, J. In vitro anti-influenza virus activities of sulfated polysaccharide fractions from Gracilaria lemaneiformis. Virol. Sin. 2010, 25, 341–351. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Domingues, V.F.; Barroso, M.F.; Simal-Gandara, J.; et al. Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens. Antibiotics 2020, 9, 712. [Google Scholar] [CrossRef]
- Amri, S.; Bouslama, L.; Mzoughi, Z.; Nouira, F.; Majdoub, H.; Bouraoui, A. Chemical characterization and evaluation of antiviral activity of two fucoidans extracted from Mediterranean brown seaweeds, Padina pavonica and Dictyopteris membranacea. Lett. Appl. Microbiol. 2025, 78, ovaf002. [Google Scholar] [CrossRef]
- Gomaa, H.H.A.; Elshoubaky, G.A. Antiviral activity of sulfated polysaccharides carrageenan from some marine seaweeds. Bioresour. Technol. Rep. 2016, 7, 34–42. [Google Scholar]
- Wei, Q.; Fu, G.; Wang, K.; Yang, Q.; Zhao, J.; Wang, Y.; Ji, K.; Song, S. Advances in Research on Antiviral Activities of Sulfated Polysaccharides from Seaweeds. Pharmaceuticals 2022, 15, 581. [Google Scholar] [CrossRef] [PubMed]
- Nosik, M.N.; Krylova, N.V.; Usoltseva, R.V.; Surits, V.V.; Kireev, D.E.; Shchelkanov, M.Y.; Svitich, O.A.; Ermakova, S.P. In Vitro Anti-HIV-1 Activity of Fucoidans from Brown Algae. Mar. Drugs 2024, 22, 355. [Google Scholar] [CrossRef] [PubMed]
- Panggabean, J.A.; Adiguna, S.P.; Rahmawati, S.I.; Ahmadi, P.; Zainuddin, E.N.; Bayu, A.; Putra, M.Y. Antiviral Activities of Algal-Based Sulfated Polysaccharides. Molecules 2022, 27, 1178. [Google Scholar] [CrossRef]
- Bonfim-Mendonça, P.D.S.; Capoci, I.R.G.; Tobaldini-Valerio, F.K.; Negri, M.; Svidzinski, T.I.E. Overview of β-Glucans from Laminaria spp.: Immunomodulation Properties and Applications on Biologic Models. Int. J. Mol. Sci. 2017, 18, 1629. [Google Scholar] [CrossRef]
- Konjević, G.M.; Vuletić, A.M.; Mirjačić Martinović, K.M.; Larsen, A.K.; Jurišić, V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef]
- Guevara-Torrejón, V.; Chandía Parra, P.; Campos-Estrada, C.; Vera Quezada, W.E. Evaluation of the Immunostimulatory Effect of Ulvan Polysaccharide on Human Macrophages: Use as a Potential Vaccine Adjuvant. Mar. Drugs 2025, 23, 248. [Google Scholar] [CrossRef]
- Srivastava, H.; Bisht, B.; James, J.; Malhotra, R.K.; Kurbatova, A.; Dabral, A.; Upadhyay, S.; Kumar, V. Advanced extraction technologies and functional applications of algal polysaccharides in modern food systems. Discov. Food. 2025, 5, 272. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Effect of extraction conditions on the yield and purity of ulvan extracted from Ulva lactuca. Food Hydrocoll. 2013, 31, 375–382. [Google Scholar] [CrossRef]
- Lin, J.; Jiao, G.; Kermanshahi-pour, A. Algal Polysaccharides-Based Hydrogels: Extraction, Synthesis, Characterization, and Applications. Mar. Drugs 2022, 20, 306. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-assisted extractions of polyphenols—A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, X.; Zhang, J.; Zhang, Y.; Chen, J.; Chen, F.; Xiao, A. Pretreatment Techniques and Green Extraction Technologies for Agar from Gracilaria lemaneiformis. Mar. Drugs 2021, 19, 617. [Google Scholar] [CrossRef] [PubMed]
- Roobab, U.; Aadil, R.M.; Kurup, S.S.; Maqsood, S. Comparative evaluation of ultrasound-assisted extraction with other green extraction methods for sustainable recycling and processing of date palm bioresources and by-products: A review of recent research. Ultrason. Sonochem. 2025, 114, 107252. [Google Scholar] [CrossRef] [PubMed]
- Antonisamy, A.J.; Rajendran, K. Comparative study on the extraction methods, characterization, and bioactivity of crude fucoidan, a polysaccharide derived from Sargassum ilicifolium. Biochem. Eng. J. 2024, 209, 109398. [Google Scholar] [CrossRef]
- Catarino, M.D.; Circuncisão, A.R.; Silva, S.; Pinto, D.C.G.A.; Pereira, O.R.; Cardoso, S.M. Himanthalia elongata: An overview of its chemical composition and health-related benefits. J. Food Compos. Anal. 2025, 143, 107587. [Google Scholar] [CrossRef]
- Mensah, E.O.; Kanwugu, O.N.; Panda, P.K.; Adadi, P. Marine fucoidans: Structural, extraction, biological activities and their applications in the food industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Chadwick, M.; Carvalho, L.G.; Vanegas, C.; Dimartino, S. A Comparative Review of Alternative Fucoidan Extraction Techniques from Seaweed. Mar. Drugs 2025, 23, 27. [Google Scholar] [CrossRef]
- Şahin, O.I. Seaweed Polysaccharides: Structure, Extraction and Applications. In Polysaccharides: Properties and Applications; John Wiley & Sons, Inc.: New York, NY, USA, 2021; pp. 61–74. [Google Scholar] [CrossRef]
- Sharma, A.; Dubey, S.; Singh, K.; Mittal, R.; Quille, P.; Rajauria, G. Innovative Processing and Industrial Applications of Seaweed. Phycology 2025, 5, 10. [Google Scholar] [CrossRef]
- Galinskaitė, A.; Gruškienė, R.; Kavleiskaja, T.; Stanevičienė, R.; Servienė, E.; Sereikaitė, J. Bioactive Fucoidan-Based Three-Component Colloidal Particles for Food Safety. Food Bioprocess Technol. 2025, 18, 5621–5633. [Google Scholar] [CrossRef]
- Ianchis, R.; Marin, M.M.; Alexa, R.L.; Gifu, I.C.; Alexandrescu, E.; Pircalabioru, G.G.; Vlasceanu, G.M.; Teodorescu, G.M.; Serafim, A.; Preda, S.; et al. Nanoclay-reinforced alginate/salecan composite inks for 3D printing applications. Int. J. Bioprinting 2023, 10. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M.; Afonso, C. Seaweed bioactives potential as nutraceuticals and functional ingredients: A review. J. Food Compos. Anal. 2024, 133, 106453. [Google Scholar] [CrossRef]
- Malairaj, S.; Veeraperumal, S.; Yao, W.; Subramanian, M.; Tan, K.; Zhong, S.; Cheong, K.-L. Porphyran from Porphyra haitanensis Enhances Intestinal Barrier Function and Regulates Gut Microbiota Composition. Mar. Drugs 2023, 21, 265. [Google Scholar] [CrossRef]
- Nandi, P. Algae Products Market Research Report: By Application (Food and Beverages, Cosmetics and Personal Care, Nutraceuticals, Animal Feed, Biofuels), by Product Type (Spirulina, Chlorella, Carrageenan, Agar, Algal Oil), by Form (Powder, Liquid, Capsules, Granules), by Source (Microalgae, Macroalgae, Cyanobacteria) and by Regional (North America, Europe, South America, Asia Pacific, Middle East and Africa)-Forecast to 203. Available online: https://www.marketresearchfuture.com/reports/algae-products-market-4730 (accessed on 11 September 2025).
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Kontogiorgos, V. Seaweed Polysaccharides (Agar, Alginate Carrageenan). In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 240–250. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Maya-Ramírez, C.E.; Cotas, J.; Pereira, L. Cosmeceutical Significance of Seaweed: A Focus on Carbohydrates and Peptides in Skin Applications. Phycology 2024, 4, 276–313. [Google Scholar] [CrossRef]
- Mamede, M.; Cotas, J.; Bahcevandziev, K.; Pereira, L. Seaweed Polysaccharides in Agriculture: A Next Step towards Sustainability. Appl. Sci. 2023, 13, 6594. [Google Scholar] [CrossRef]
- Shin, H.J.; Cho, H.U.; Park, J.M. Alginate as a Soil Conditioner: Properties, Mechanisms, and Agricultural Applications. Biotechnol. Bioprocess Eng. 2023, 28, 734–749. [Google Scholar] [CrossRef]
- Velho, A.C.; Dall’Asta, P.; de Borba, M.C.; Magnin-Robert, M.; Reignault, P.; Siah, A.; Stadnik, M.J.; Randoux, B. Defense responses induced by ulvan in wheat against powdery mildew caused by Blumeria graminis f. sp. tritici. J. Plant Physiol. Biochem. 2022, 184, 14–25. [Google Scholar] [CrossRef]
- Mishra, A.; Sahni, S.; Kumar, S.; Prasad, B.D. Seaweed-An Eco-friendly Alternative of Agrochemicals in Sustainable Agriculture. Curr. J. Appl. Sci. Technol. 2020, 39, 71–78. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Bahramian, B.; Tavassoli, M.; Majlesi, M.; Ghaderi, S.; Assadpour, E.; Zhang, F.; Sadeghi, E.; Bangar, S.P.; Jafari, S.M. Alginate-based edible films/coatings/nanofibers in food packaging: A comprehensive review of recent advances. Carbohydr. Polym. Tech. Appl. 2025, 11, 100955. [Google Scholar] [CrossRef]
- Khorami, F.; Babaei, S.; Valizadeh, S.; Naseri, M.; Golmakani, M.-T. Bilayer coatings for extension of the shelf life of fish fillets: Incorporating seaweed sulfated polysaccharides in chitosan-alginate LbL structures. Food Sci. Nutr. 2024, 12, 2511–2522. [Google Scholar] [CrossRef]
- Balti, R.; Ben Mansour, M.; Zayoud, N.; Le Balc’h, R.; Brodu, N.; Arhaliass, A.; Massé, A. Active exopolysaccharides based edible coatings enriched with red seaweed (Gracilaria gracilis) extract to improve shrimp preservation during refrigerated storage. Food Biosci. 2020, 34, 100522. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Marine Seaweed Polysaccharides-Based Engineered Cues for the Modern Biomedical Sector. Mar. Drugs 2020, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Lee, Z.J.; Ye, S.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. A Review on Seaweeds and Seaweed-Derived Polysaccharides: Nutrition, Chemistry, Bioactivities, and Applications. Food Rev. Int. 2024, 40, 1312–1347. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed Components as Potential Modulators of the Gut Microbiota. Mar. Drugs 2021, 19, 358. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Santhiravel, S.; Dave, D.; Shahidi, F. Bioactives from marine resources as natural health products: A review. Pharmacol. Rev. 2025, 77, 100006. [Google Scholar] [CrossRef]
- Cotas, J.; Lomartire, S.; Gonçalves, A.M.M.; Pereira, L. From Ocean to Medicine: Harnessing Seaweed’s Potential for Drug Development. Int. J. Mol. Sci. 2024, 25, 797. [Google Scholar] [CrossRef]
- Gengatharan, A.; Mohamad, N.V.; Zahari, C.N.M.C.; Vijayakumar, R. Seaweeds as emerging functional foods and therapeutics for colorectal cancer management. Disc. Food 2025, 5, 128. [Google Scholar] [CrossRef]
- Bhuyan, P.P.; Nayak, R.; Patra, S.; Abdulabbas, H.S.; Jena, M.; Pradhan, B. Seaweed-Derived Sulfated Polysaccharides; The New Age Chemopreventives: A Comprehensive Review. Cancers 2023, 15, 715. [Google Scholar] [CrossRef]
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C.R.K. Seaweed biorefinery: A sustainable process for valorising the biomass of brown seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Ayub, A.; Rahayu, F.; Khamidah, A.; Antarlina, S.S.; Iswari, K.; Supriyadi, K.; Mufidah, E.; Singh, A.; Chopra, C.; Wani, A.K. Harnessing microalgae as a bioresource for nutraceuticals: Advancing bioactive compound exploration and shaping the future of health and functional food innovation. Discov. Appl. Sci. 2025, 7, 389. [Google Scholar] [CrossRef]
- Augusto, A.; Lemos, M.F.L.; Silva, S.F.J. Exploring Marine-Based Food Production: The Challenges for a Sustainable and Fast Biotechnology-Based Development. Appl. Sci. 2024, 14, 8255. [Google Scholar] [CrossRef]
- Cheng, J.; Li, X.; Ke, T.; Gong, J.; Jiang, T.; Chen, L. Advances in polysaccharide-based probiotic delivery systems: A review. Carbohydr. Polym. Technol. Appl. 2025, 10, 100804. [Google Scholar] [CrossRef]



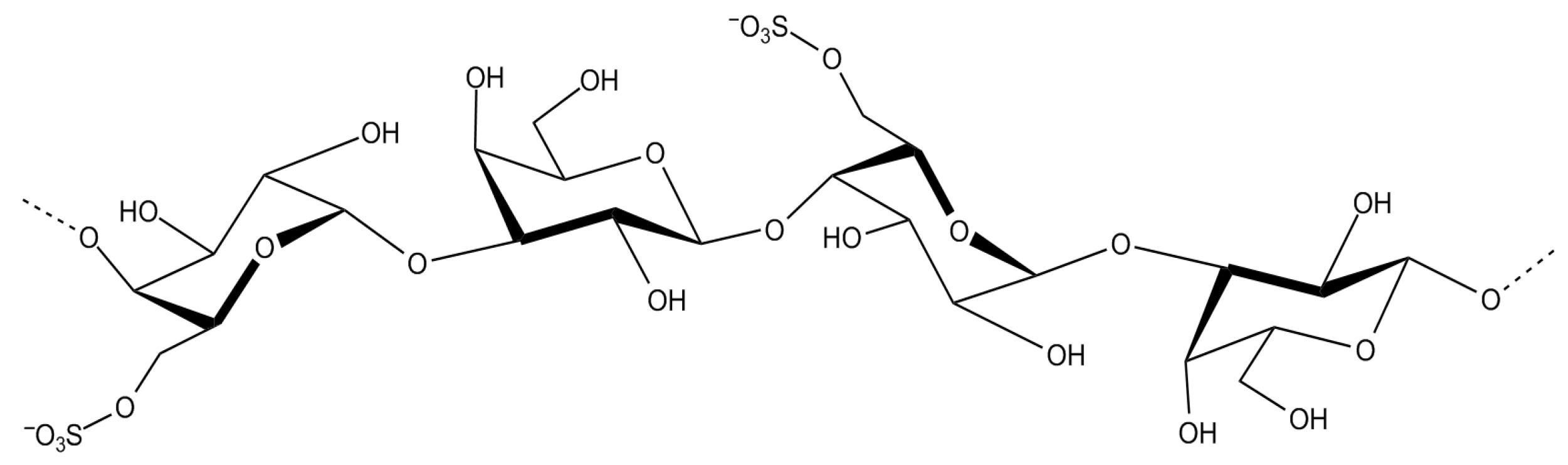
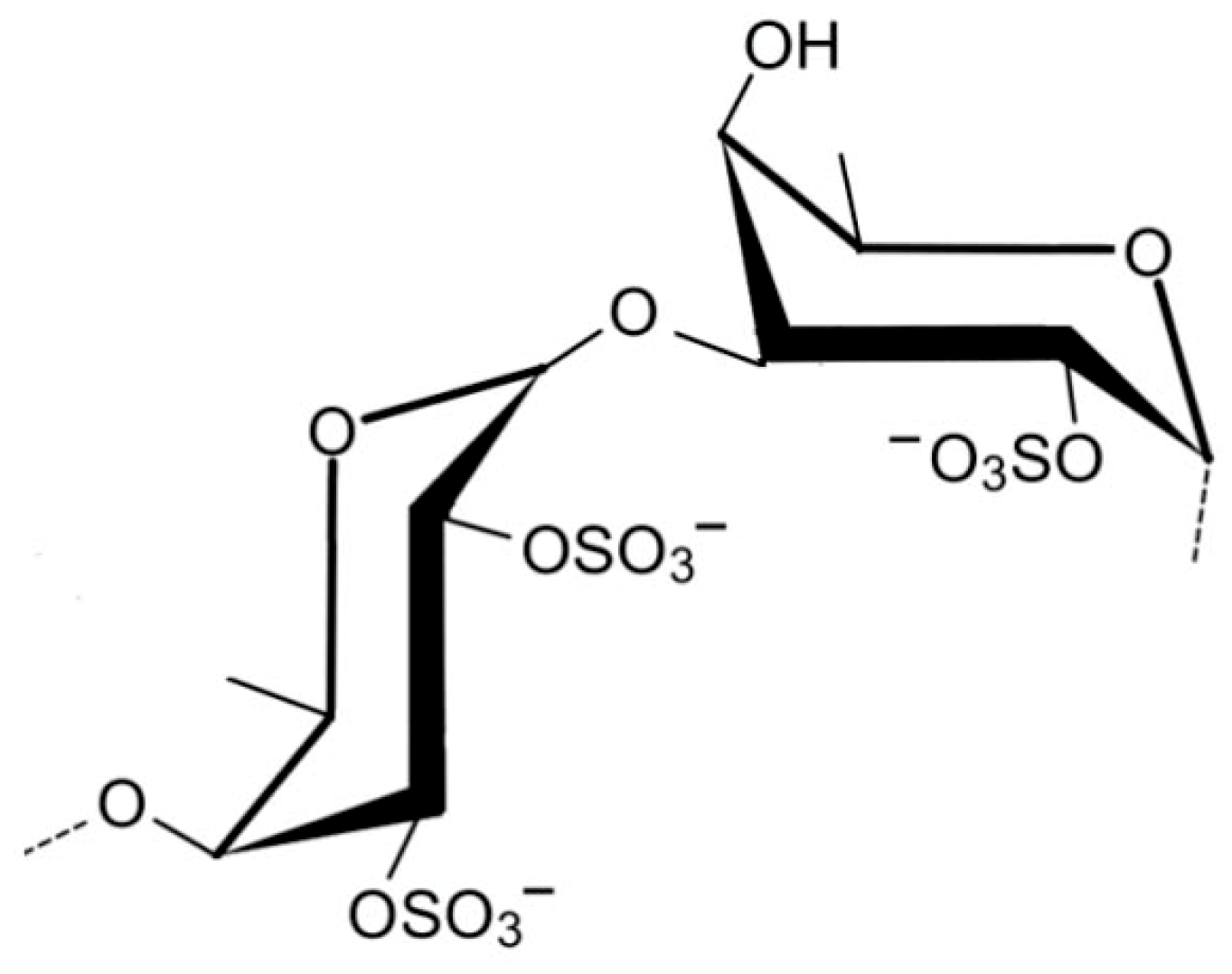
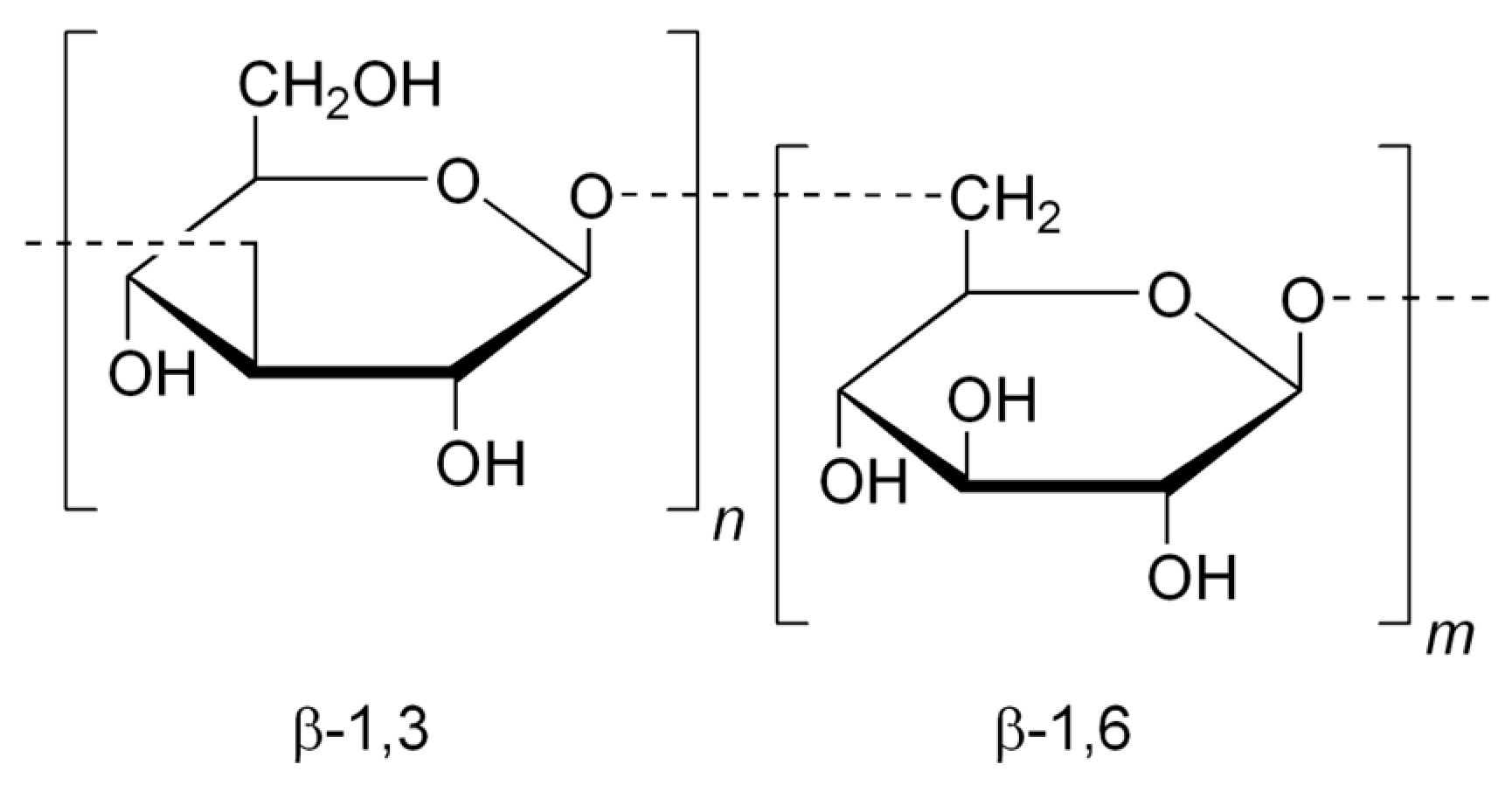

















| Sulfated Polysaccharide | Source | Dose/IC50 (µg mL−1) | Bacterial Infection and Effect of Treatment | Ref. |
|---|---|---|---|---|
| Fucoidan | Fucus vesiculosus (brown algae) | IC50: 250 µg/mL | Inhibits Staphylococcus aureus and Escherichia coli; disrupts biofilm formation | [99] |
| Methanolic extract | Ecklonia cava (brown algae) | IC50: 256 μg/mL | Effective against Listeria monocytogenes; reduces bacterial adhesion | [100] |
| Carrageenan (κ-type) | Kappaphycus alvarezii (red algae) | IC50: 150 µg/mL | Suppresses Pseudomonas aeruginosa growth and biofilm formation | [101] |
| Ulvan | Ulva lactuca (green algae) | IC50: 300 µg/mL | Inhibits Salmonella typhimurium; interferes with quorum sensing | [101] |
| Sulfated galactan | Gracilariopsis longissima (red algae) | IC50: 180 µg/mL | Active against Bacillus subtilis and E. coli; damages cell membrane integrity | [99] |
| Alginic acid | Macrocystis pyrifera (brown algae) | IC50: 220 µg/mL | Inhibits Vibrio cholerae and E. coli; interferes with cell wall synthesis | [100] |
| Sulfated rhamnan | Monostroma nitidum (green algae) | IC50: 160 µg/mL | Effective against Streptococcus pyogenes; inhibits bacterial proliferation | [101] |
| Porphyran | Neopyropia yezoensis (red algae) | IC50: 190 µg/mL | Inhibits Helicobacter pylori; disrupts membrane potential | [99] |
| Sulfated xylogalactan | Eucheuma denticulatum (red algae) | IC50: 210 µg/mL | Active against Klebsiella pneumoniae; reduces biofilm formation | [100] |
| Sulfated arabinogalactan | Codium fragile (green algae) | IC50: 170 µg/mL | Targets Enterococcus faecalis; inhibits cell division | [101] |
| Fucoidan | Fucus vesiculosus (brown algae) | IC50: 250 µg/mL | Inhibits Staphylococcus aureus and Escherichia coli; disrupts biofilm formation | [99] |
| Laminarin sulfate | Laminaria digitata (brown algae) | IC50: 200 µg/mL | Effective against Listeria monocytogenes; reduces bacterial adhesion | [100] |
| Carrageenan (κ-type) | Kappaphycus alvarezii (red algae) | IC50: 150 µg/mL | Suppresses Pseudomonas aeruginosa growth and biofilm formation | [101] |
| Ulvan | Ulva lactuca (green algae) | IC50: 300 µg/mL | Inhibits Salmonella typhimurium; interferes with quorum sensing | [101] |
| Sulfated galactan | Gracilaria verrucosa (red algae) | IC50: 180 µg/mL | Active against Bacillus subtilis and E. coli; damages cell membrane integrity | [99] |
| Alginic acid | Macrocystis pyrifera (brown algae) | IC50: 220 µg/mL | Inhibits Vibrio cholerae and E. coli; interferes with cell wall synthesis | [100] |
| Sulfated rhamnan | Monostroma nitidum (green algae) | IC50: 160 µg/mL | Effective against Streptococcus pyogenes; inhibits bacterial proliferation | [101] |
| Porphyran | Porphyra yezoensis (red algae) | IC50: 190 µg/mL | Inhibits Helicobacter pylori; disrupts membrane potential | [99] |
| Sulfated xylogalactan | Eucheuma denticulatum (red algae) | IC50: 210 µg/mL | Active against Klebsiella pneumoniae; reduces biofilm formation | [100] |
| Sulfated arabinogalactan | Codium fragile (green algae) | IC50: 170 µg/mL | Targets Enterococcus faecalis; inhibits cell division | [101] |
| Fucoidan | Fucus vesiculosus (brown algae) | IC50: 250 µg/mL | Inhibits Staphylococcus aureus and Escherichia coli; disrupts biofilm formation | [99] |
| Sulfated Polysaccharide | Source | Dose/IC50 (µg mL−1) | Virus Infection and Effect of Treatment | Refs. |
|---|---|---|---|---|
| Fucoidan | Undaria pinnatifida (P) | In vivo study with 5 mg day−1 twice a day for 14 days | Anti-IAV activity; Positive effect on production of antigen-specific antibody; Inhibition of virus attachment and blocking virus penetration | [121,122,123] |
| Kjellmaniella crassifolia (P) | 250 µg mL−1 of fucoidan with purity of 92.8% | Inhibition of influenza A virus infection; targeting viral neuraminidase | [124] | |
| Saccharina japonica (P) | 50–500 µg mL−1 of fucoidan with 1.9% of uronic acids and 10.4% of sulfur in sulfate semi-esters | Antiviral activity against avian influenza A (H5N1) virus infection in the cultured cells | [125] | |
| Saccharina cichorioides, S. japonica (P) | 0.001–100 µg mL−1 | Anti-HIV activity. Prevention of attachment and cell-to-cell virus spread | [126] | |
| Sargassum mcclurei, Sargassum polycystum, Turbinaria ornata (P) | IC50 value 0.33–0.7 µg mL−1 | Fucoidans blocking the early steps of HIV entry into target cells | [127] | |
| Sargassum swartzii (P) | 1.56 and 6.25 μg mL−1 | Fucoidan fractions exhibit significant anti-HIV-1 activity | [128] | |
| Cladosiphon okamuranus (P) | 0.83 g day−1 | Anti-HCV activity. Inhibits virus replication | [129] | |
| Scytosiphon lomentaria (P) | IC50 value 0.76- 1.34 µg mL−1 | Anti-HSV activity; The galactofucan fractions of fucoidan showed antiviral activity because of the low uronic acid and high sulfate esters content; Inhibition of virus attachment | [130] | |
| Sargassum henslowianum (P) | IC50 value 0.89 and 0.82 µg mL−1 | [131] | ||
| Fucus distichus subsp. evanescens (P) | In vitro study with 0.25–250 µg mL−1 In vivo study with 10 mg kg−1 day−1 | Antivirus activity against HSV, ECHO-1, and HIV-1; Inhibiting virus replication | [132] | |
| Carrageenan | Commercial carrageenan | Iota-carrageenan | Anti-IAV; Inhibition virus replication | [133] |
| κ-carrageenan | Kappa carrageenan and sulfated derivatives; in vivo study with 40 mg kg−1 d−1 | Inhibition virus replication | [134] | |
| κ-carrageenan | Kappa, acetylated and sulfated derivatives; in vivo study with 30 mg kg−1 day−1 | Carrageenan displayed higher activity than Rabivirin at the dose of 30 mg/kg·d | [135] | |
| Lambda-carrageenan | Lambda-carrageenan IC50 1–20 ng mL−1 | Anti-HPV potential; Inhibition of virus attachment and blocking virus penetration | [136] | |
| Iota-carrageenan | Carrageenan and Zanamivir act in vitro synergistically against several influenza A virus strains (H1N1(09)pdm, H3N2, H5N1, H7N7). | Nasal spray containing only iota-carrageenan, or together with “zanamivir” provide treatment of upper respiratory tract infections in patients under suspicion of infection by influenza A (H1N1) | [82,137] | |
| Sarcopeltis Skottsbergii (R) | Lambda-carrageenan IC50 0.52 and 10.42 for BoHv-1 and SuHV-1, respectively | BoHV-1 and SuHV-1; Inhibition of virus attachment and blocking virus penetration | [138] | |
| Stenogramma Interruptum (R) | Kappa/iota and lambda-carrageenans 0.65–2.88 µg mL−1 | Anti-HSV activity; Inhibition of virus attachment and blocking virus penetration | [139] | |
| Sarcopeltis Skottsbergii (R) | Lambda-carrageenan 10 mg mL−1 | interfere with protein binding to the heparan sulfate coreceptor in host tissues | [140] | |
| Sarcothalia Atropurpurea (R) | Kappa and lambda-carrageenan 0.2–0.8 µg mL−1 | Interfere with protein binding to the heparan sulfate co-receptor in host tissues | [141] | |
| Solieria chordalis (R) | Anti-Herpes simplex virus (HSV-1) activity | Anti-Herpes simplex virus (HSV-1) activity | [142] | |
| Solieria filiformis (R) | Iota-carrageenan 4.5–11.7 µg mL−1 | Anti-Herpes simplex virus (HSV-1) activity | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, L.; Valado, A. Beyond Nutrition: The Therapeutic Promise of Seaweed-Derived Polysaccharides Against Bacterial and Viral Threats. Mar. Drugs 2025, 23, 407. https://doi.org/10.3390/md23100407
Pereira L, Valado A. Beyond Nutrition: The Therapeutic Promise of Seaweed-Derived Polysaccharides Against Bacterial and Viral Threats. Marine Drugs. 2025; 23(10):407. https://doi.org/10.3390/md23100407
Chicago/Turabian StylePereira, Leonel, and Ana Valado. 2025. "Beyond Nutrition: The Therapeutic Promise of Seaweed-Derived Polysaccharides Against Bacterial and Viral Threats" Marine Drugs 23, no. 10: 407. https://doi.org/10.3390/md23100407
APA StylePereira, L., & Valado, A. (2025). Beyond Nutrition: The Therapeutic Promise of Seaweed-Derived Polysaccharides Against Bacterial and Viral Threats. Marine Drugs, 23(10), 407. https://doi.org/10.3390/md23100407