Therapeutic Potential of DPHC, A Brown Seaweed Polyphenol, Against TNF-α-Induced Inflammatory Muscle Loss
Abstract
1. Introduction
2. Results
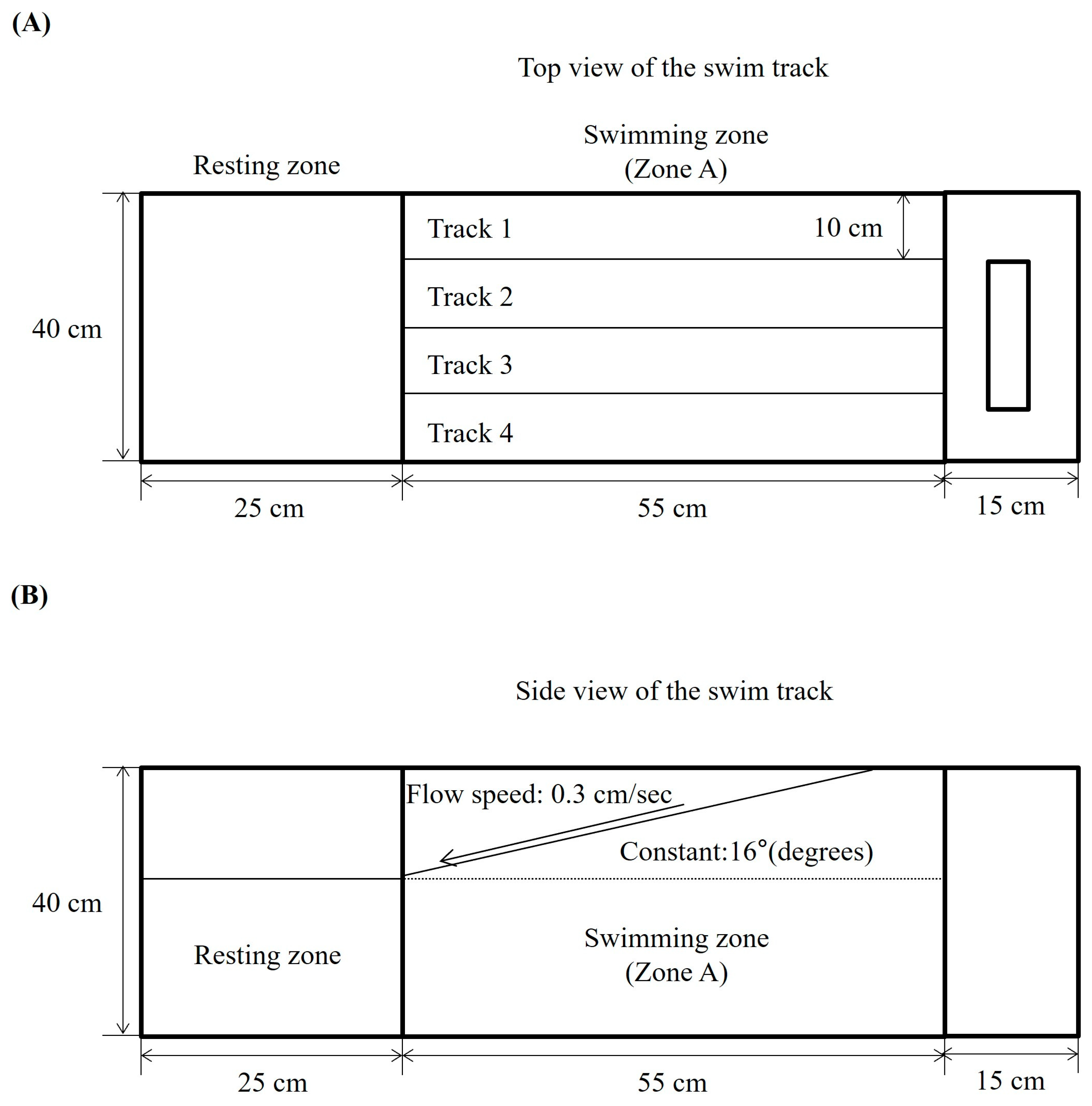
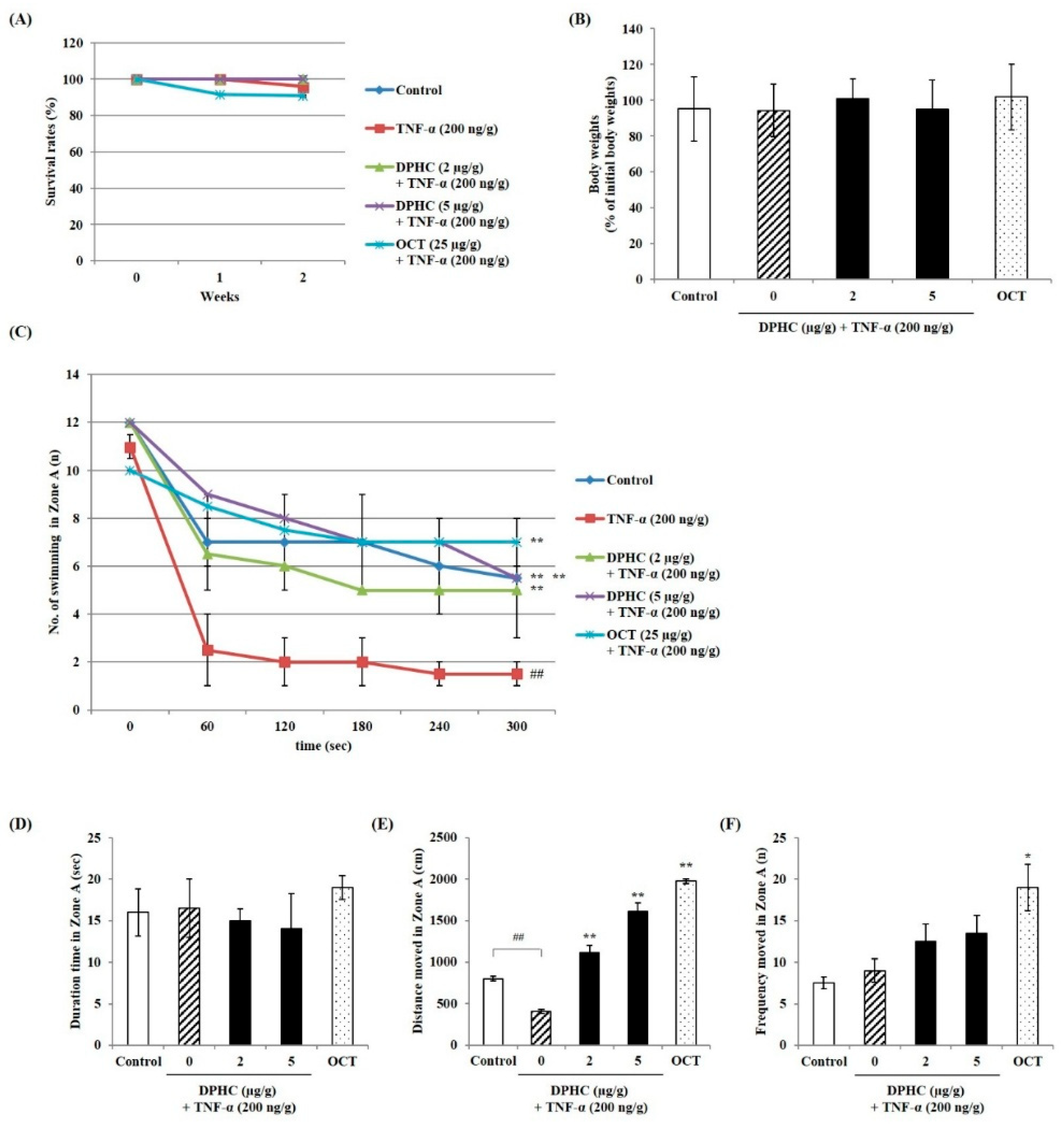
2.1. Effect of DPHC on Swimming Performance of TNF-α-Induced Zebrafish
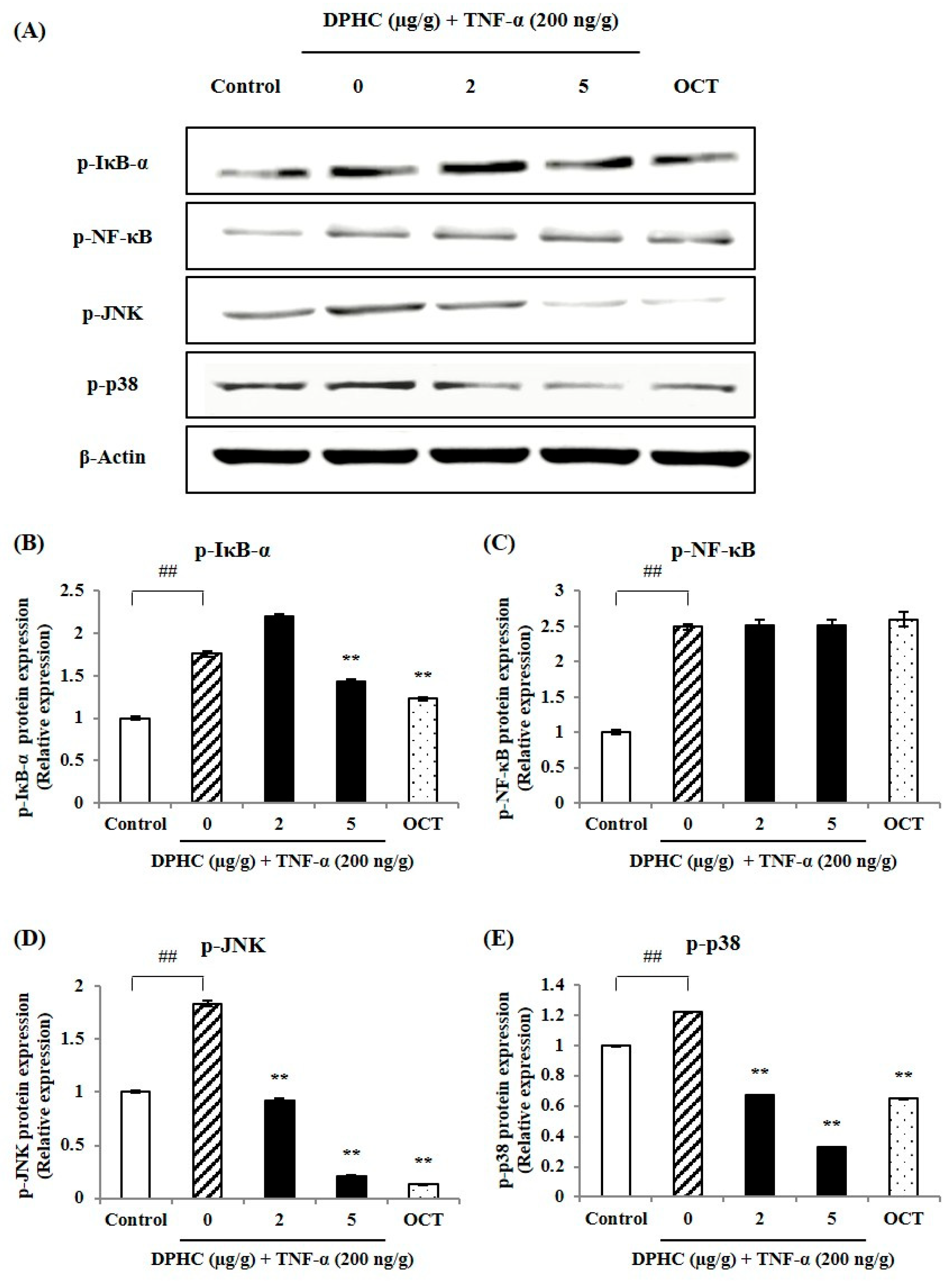
2.2. Effect of DPHC on Inflammatory Signaling in Skeletal Muscle Tissue of TNF-α-Induced Zebrafish
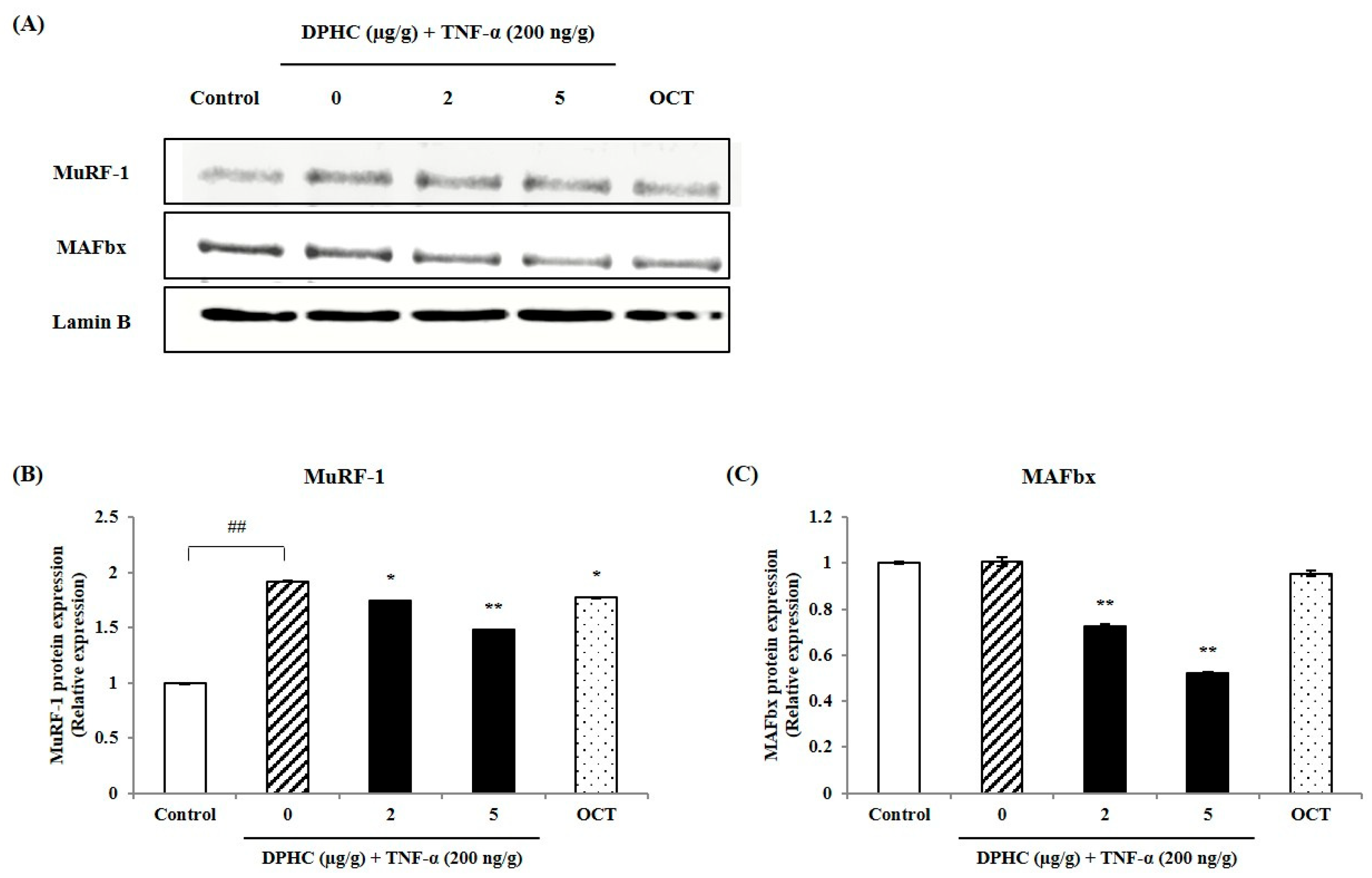
2.3. Protective Effect of DPHC Against Inflammatory Muscle Loss: Modulation of MuRF-1 and MAFbx in Skeletal Muscle Tissue of TNF-α-Induced Zebrafish
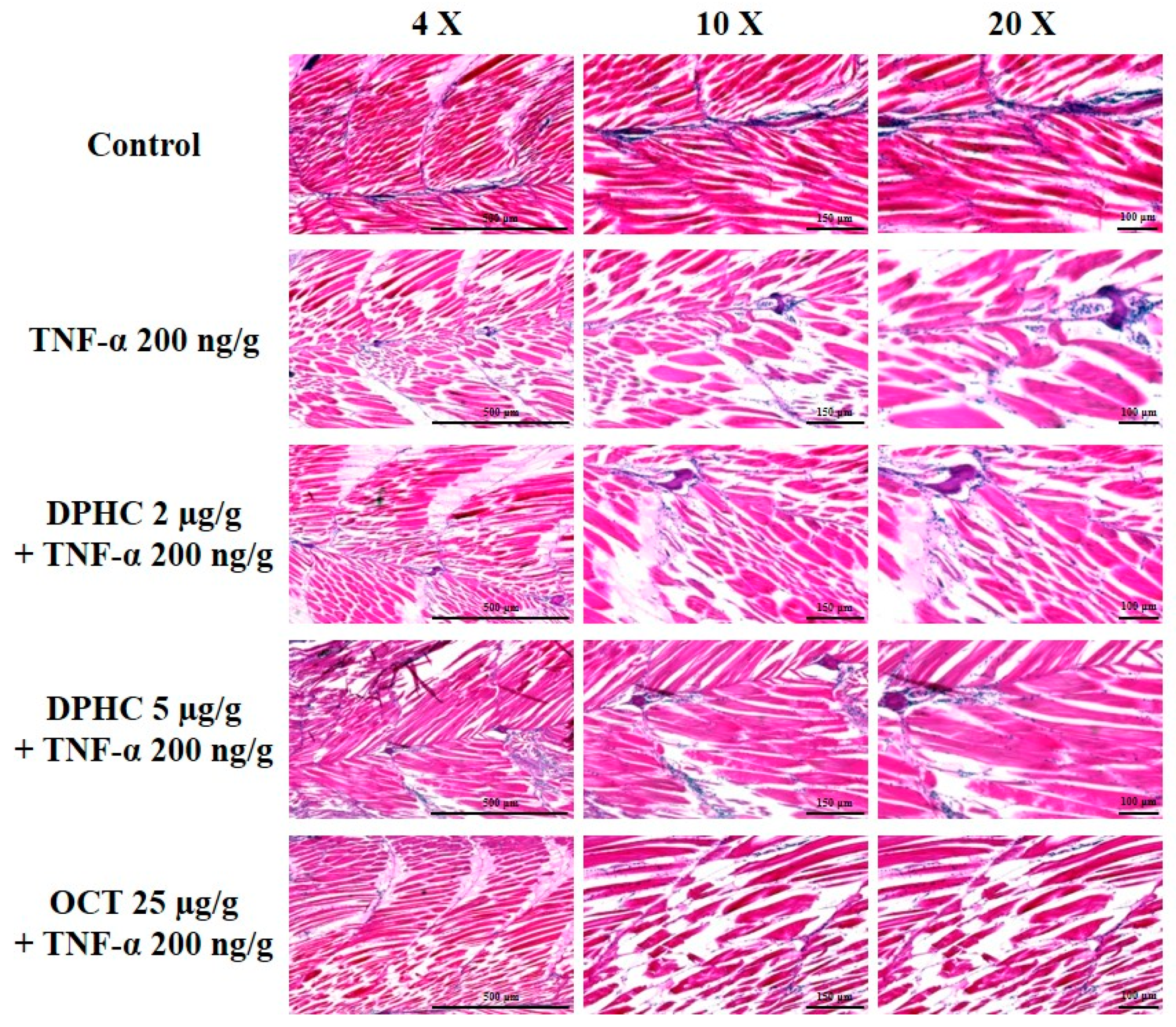
2.4. Protective Effect of DPHC on Skeletal Muscle Tissue Damage: Histological Analysis of TNF-α-Induced Inflammatory Muscle Loss
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Isolation of DPHC from I. okamurae
4.3. Western Blot Analysis
4.4. In Vivo Design
4.5. Survival Rate, Body Weight, and Swimming Performance Assessment in Zebrafish
4.6. Hematoxylin and Eosin Staining
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, T.; Quan, H.; Kim, C.K.; Zeng, W. Role of nutrition in skeletal muscle atrophy and sarcopenia. Front. Nutr. 2024, 11, 1395491. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Rom, O.; Reznick, A.Z. The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass. Free Radic. Biol. Med. 2016, 98, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-G.; Kim, S.-Y.; Kim, K.-N.; Jeon, Y.-J.J.F.B. DPHC from Ishige okamurae mitigates oxidative stress-induced myopathy by regulating MuRF-1/MAFbx signaling in C2C12 cells. Food Biosci. 2024, 61, 104859. [Google Scholar] [CrossRef]
- Mascher, H.; Tannerstedt, J.; Brink-Elfegoun, T.; Ekblom, B.; Gustafsson, T.; Blomstrand, E. Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E43–E51. [Google Scholar] [CrossRef]
- Uruha, A.; Uruha, S. Update on pathology of inflammatory myopathy. Clin. Exp. Neuroimmunol. 2025, 16, 55–63. [Google Scholar] [CrossRef]
- Londhe, P.; Guttridge, D. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef]
- Tu, H.; Li, Y.-L. Inflammation balance in skeletal muscle damage and repair. Front. Immunol. 2023, 14, 1133355. [Google Scholar] [CrossRef]
- Sharma, B.; Dabur, R. Role of pro-inflammatory cytokines in regulation of skeletal muscle metabolism: A systematic review. Curr. Med. Chem. 2020, 27, 2161–2188. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K.; Choi, Y.H.; Nam, T.-J. Pyropia yezoensis protein protects against TNF-α-induced myotube atrophy in C2C12 myotubes via the NF-κB signaling pathway. Mol. Med. Rep. 2021, 24, 486. [Google Scholar] [CrossRef]
- Yasir, M.; Goyal, A.; Sonthalia, S. Corticosteroid Adverse Effects; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bagherniya, M.; Mahdavi, A.; Shokri-Mashhadi, N.; Banach, M.; Von Haehling, S.; Johnston, T.P.; Sahebkar, A. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2772–2790. [Google Scholar] [CrossRef]
- Shen, S.; Yu, H.; Gan, L.; Ye, Y.; Lin, L. Natural constituents from food sources: Potential therapeutic agents against muscle wasting. Food Funct. 2019, 10, 6967–6986. [Google Scholar] [CrossRef]
- Heo, S.-J.; Hwang, J.-Y.; Choi, J.-I.; Han, J.-S.; Kim, H.-J.; Jeon, Y.-J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef]
- Kang, M.-C.; Ding, Y.; Kim, H.-S.; Jeon, Y.-J.; Lee, S.-H. Inhibition of adipogenesis by diphlorethohydroxycarmalol (DPHC) through AMPK activation in adipocytes. Mar. Drugs 2019, 17, 44. [Google Scholar] [CrossRef]
- Lu, Y.A.; Jiang, Y.; Yang, H.-W.; Hwang, J.; Jeon, Y.-J.; Ryu, B. Diphlorethohydroxycarmalol Isolated from Ishige okamurae exerts vasodilatory effects via calcium signaling and PI3K/Akt/eNOS pathway. Int. J. Mol. Sci. 2021, 22, 1610. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.-J.; Ryu, B. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Ahn, G.; Kim, H.-S.; Je, J.-G.; Kim, K.-N.; Jeon, Y.-J. Diphlorethohydroxycarmalol (DPHC) isolated from the brown alga Ishige okamurae acts on inflammatory myopathy as an inhibitory agent of TNF-α. Mar. Drugs 2020, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, M.; Chang, M.; Liu, R.; Qiu, J.; Wang, K.; Deng, C.; Shen, Y.; Zhu, J.; Wang, W. Inflammation: Roles in skeletal muscle atrophy. Antioxidants 2022, 11, 1686. [Google Scholar] [CrossRef]
- Fernando, I.; Kim, H.-S.; Sanjeewa, K.; Oh, J.-Y.; Jeon, Y.-J.; Lee, W.W.; Fernando, I.S.; Kim, H.-S.; Sanjeewa, K.A.; Oh, J.-Y. Inhibition of inflammatory responses elicited by urban fine dust particles in keratinocytes and macrophages by diphlorethohydroxycarmalol isolated from a brown alga Ishige okamurae. Algae 2017, 32, 261–273. [Google Scholar] [CrossRef]
- Habjan, E.; Schouten, G.K.; Speer, A.; van Ulsen, P.; Bitter, W. Diving into drug-screening: Zebrafish embryos as an in vivo platform for antimicrobial drug discovery and assessment. Fems Microbiol. Rev. 2024, 48, fuae011. [Google Scholar] [CrossRef] [PubMed]
- Karuppasamy, M.; English, K.G.; Henry, C.A.; Manzini, M.C.; Parant, J.M.; Wright, M.A.; Ruparelia, A.A.; Currie, P.D.; Gupta, V.A.; Dowling, J. Standardization of zebrafish drug testing parameters for muscle diseases. Dis. Model. Mech. 2024, 17, dmm050339. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Wang, L.; Jayawardena, T.U.; Kim, E.-A.; Heo, S.-J.; Fernando, I.S.; Kim, S.-Y.; Ahn, G.; Jeon, Y.-J.; Lee, N.-H. High-performance centrifugal partition chromatography (HPCPC) for efficient isolation of diphlorethohydroxycarmalol (DPHC) and screening of its antioxidant activity in a zebrafish model. Phytomedicine 2020, 88, 189–196. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.S.; Cho, M.; Jeon, Y.J. Enzymatic hydrolysates of Hippocampus abdominalis regulates the skeletal muscle growth in C2C12 cells and zebrafish model. J. Aquat. Food Prod. Technol. 2019, 28, 264–274. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Lee, W.-W.; Kim, K.-N.; Kim, Y.-M.; Jeon, Y.-J.; Yang, F.; Kim, S.-Y.; Lee, H.-G. Therapeutic Potential of DPHC, A Brown Seaweed Polyphenol, Against TNF-α-Induced Inflammatory Muscle Loss. Mar. Drugs 2025, 23, 376. https://doi.org/10.3390/md23100376
Kim M, Lee W-W, Kim K-N, Kim Y-M, Jeon Y-J, Yang F, Kim S-Y, Lee H-G. Therapeutic Potential of DPHC, A Brown Seaweed Polyphenol, Against TNF-α-Induced Inflammatory Muscle Loss. Marine Drugs. 2025; 23(10):376. https://doi.org/10.3390/md23100376
Chicago/Turabian StyleKim, Minji, Won-Woo Lee, Kil-Nam Kim, Young-Mog Kim, You-Jin Jeon, Fengqi Yang, Seo-Young Kim, and Hyo-Geun Lee. 2025. "Therapeutic Potential of DPHC, A Brown Seaweed Polyphenol, Against TNF-α-Induced Inflammatory Muscle Loss" Marine Drugs 23, no. 10: 376. https://doi.org/10.3390/md23100376
APA StyleKim, M., Lee, W.-W., Kim, K.-N., Kim, Y.-M., Jeon, Y.-J., Yang, F., Kim, S.-Y., & Lee, H.-G. (2025). Therapeutic Potential of DPHC, A Brown Seaweed Polyphenol, Against TNF-α-Induced Inflammatory Muscle Loss. Marine Drugs, 23(10), 376. https://doi.org/10.3390/md23100376








