The Effect of Ultraviolet Light Irradiation on Pigment Performance in Microwave-Assisted Extraction of Arthrospira platensis
Abstract
1. Introduction
2. Results
2.1. Characterization of Arthrospira platensis
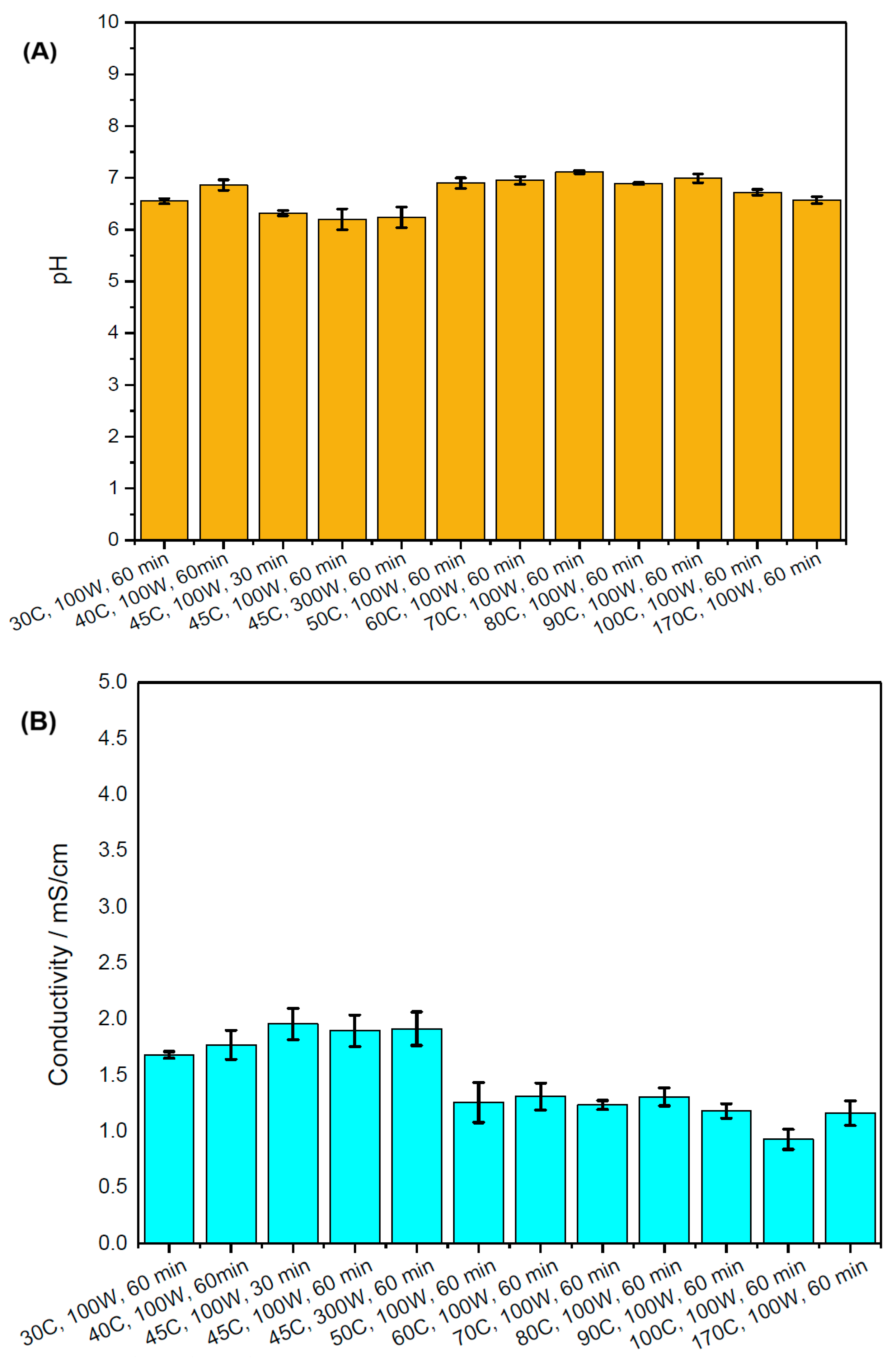
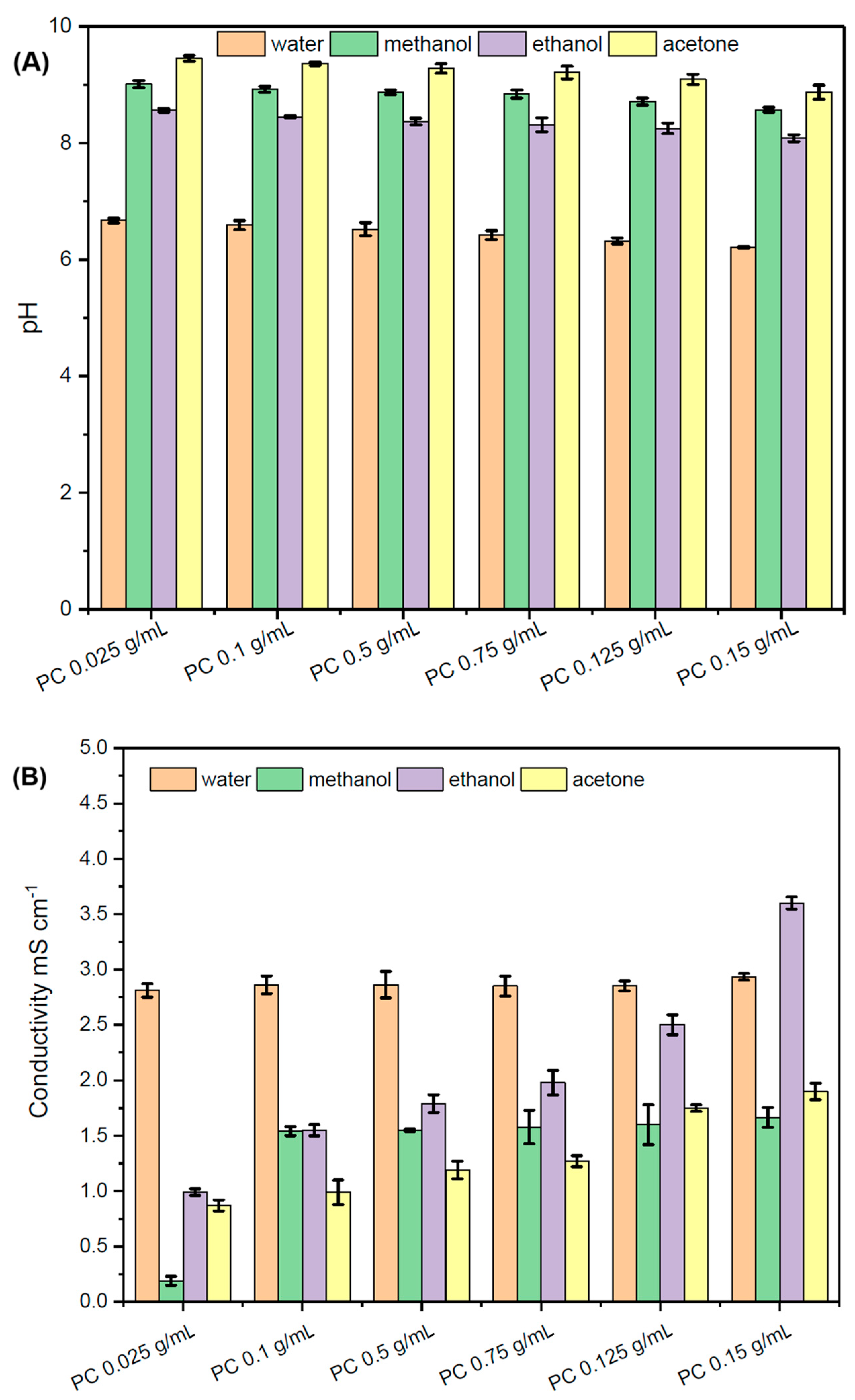
2.2. pH and Conductivity
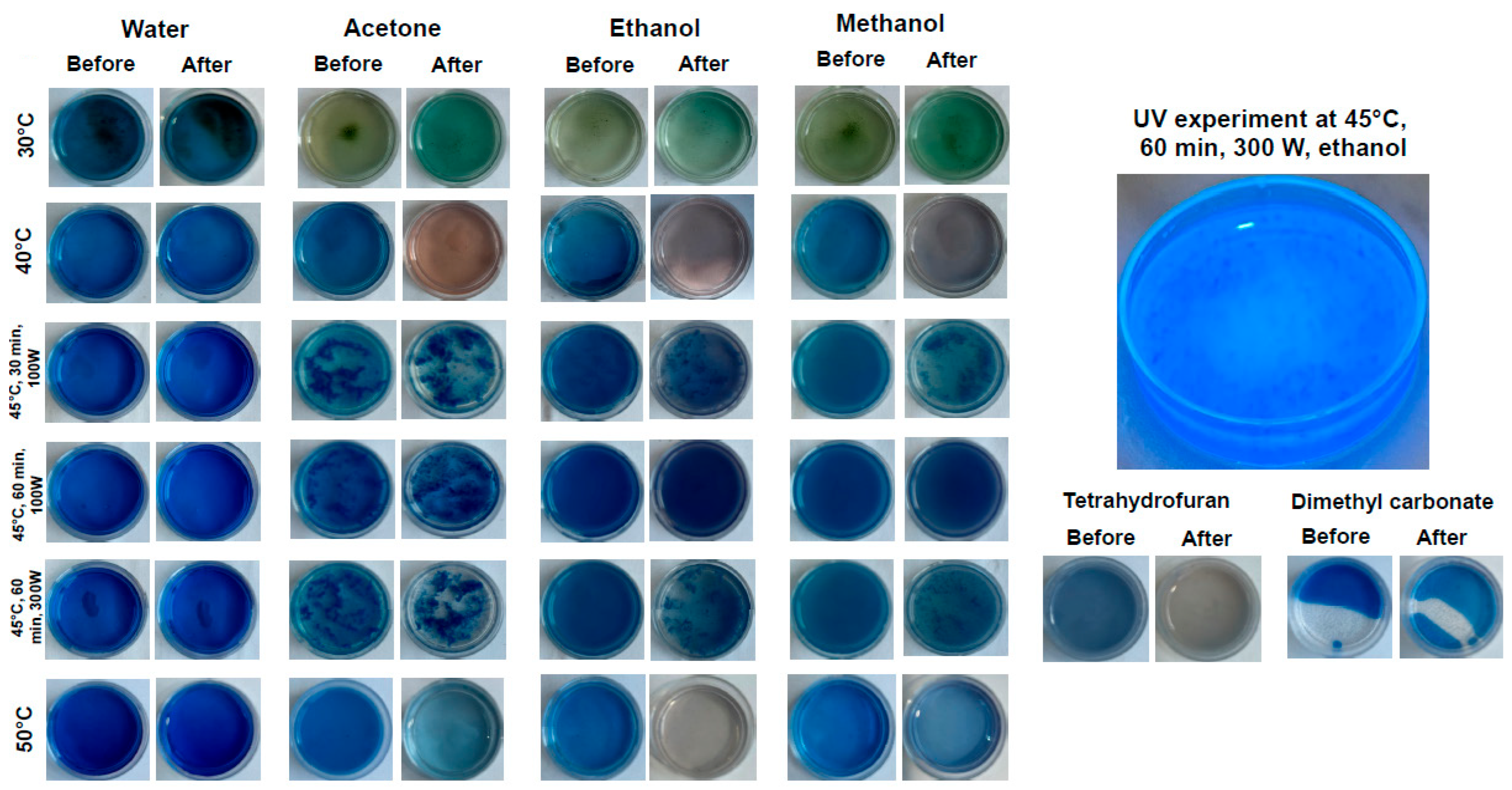
2.3. Solvent and Temperature Impact on Pigments
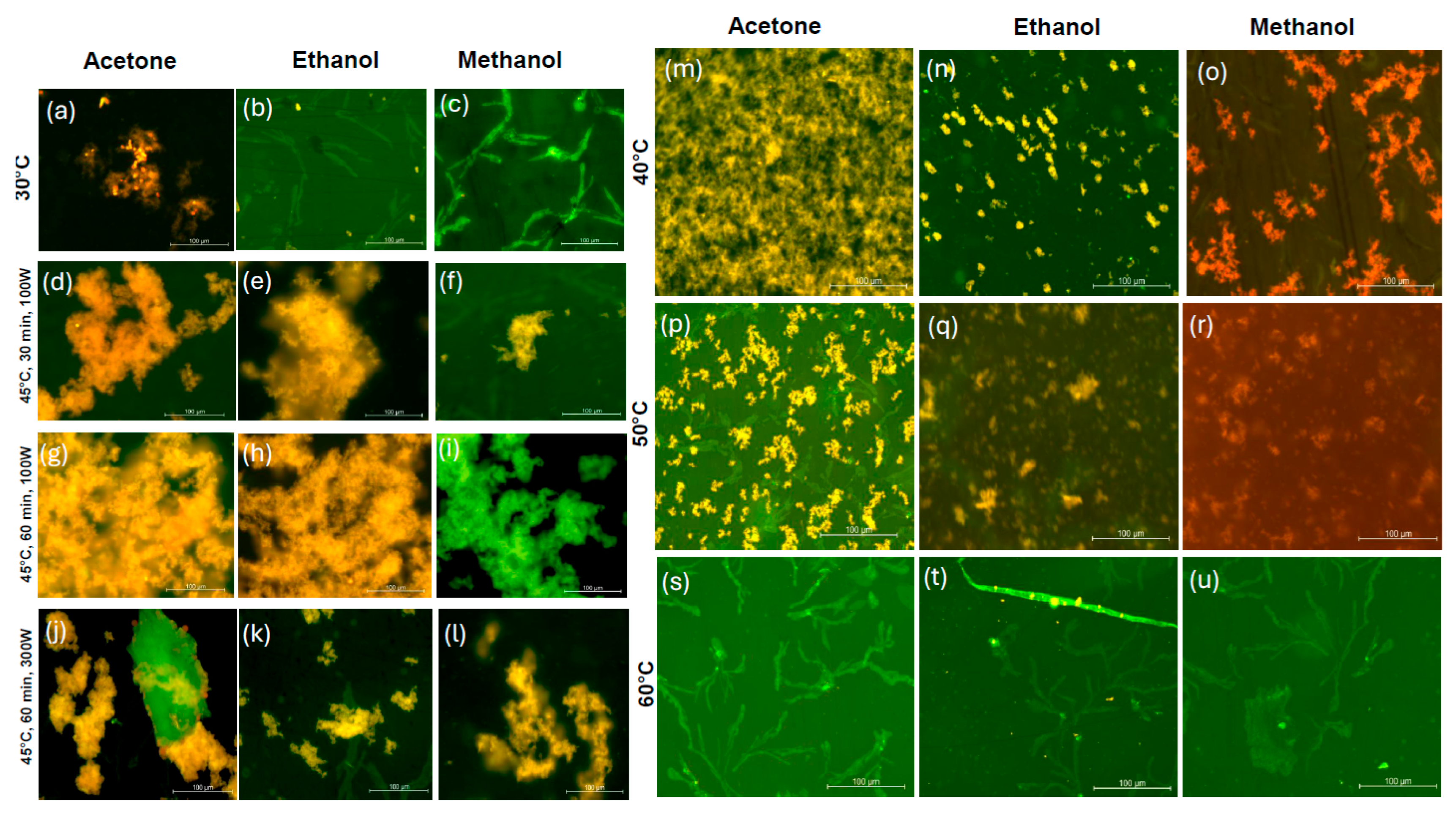
2.4. Fluorescence Microscopy
2.5. UV-Vis Spectroscopy
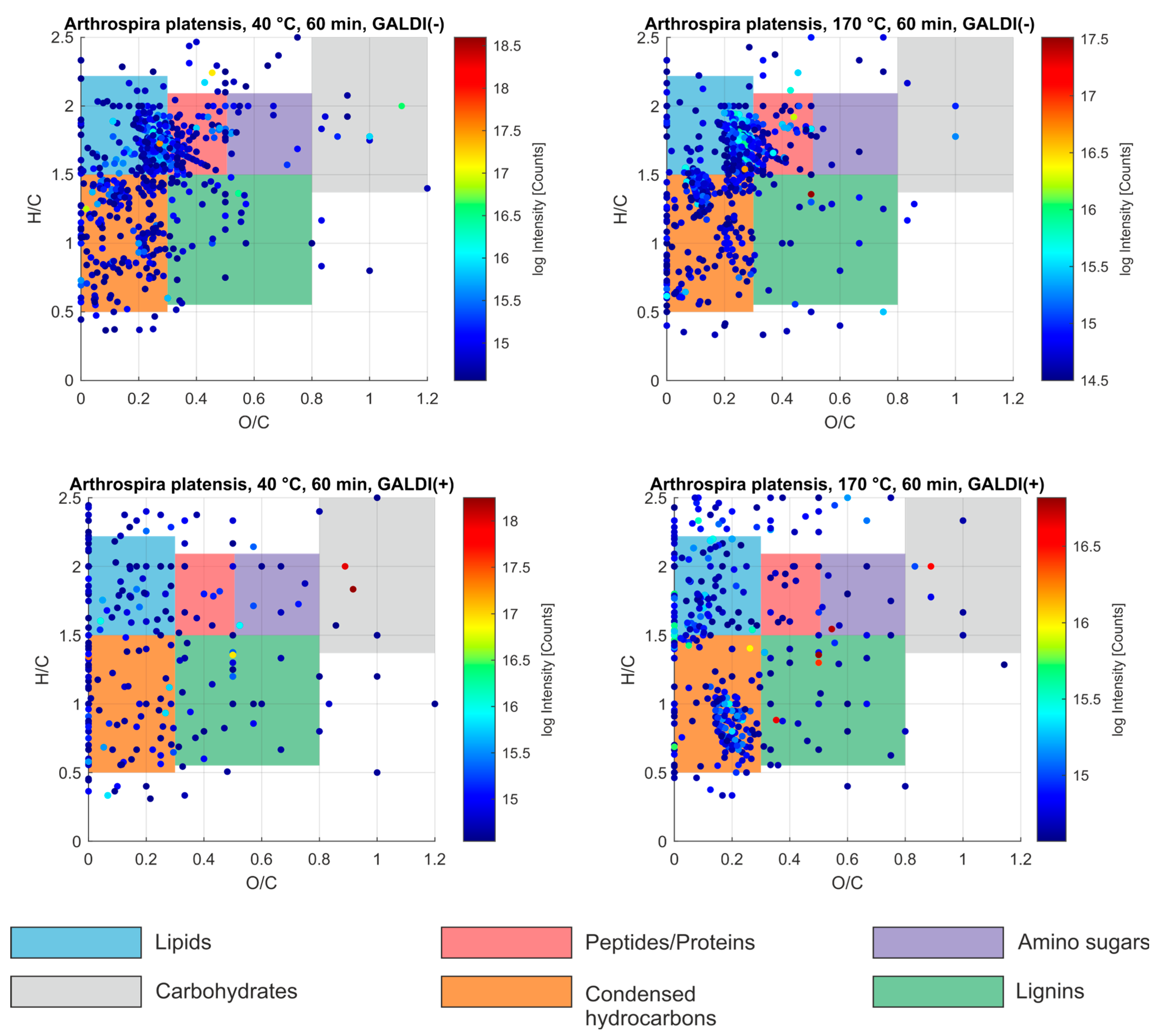
2.6. Chemical Composition of Aqueous Pigments Using FT-ICR-MS Analyses
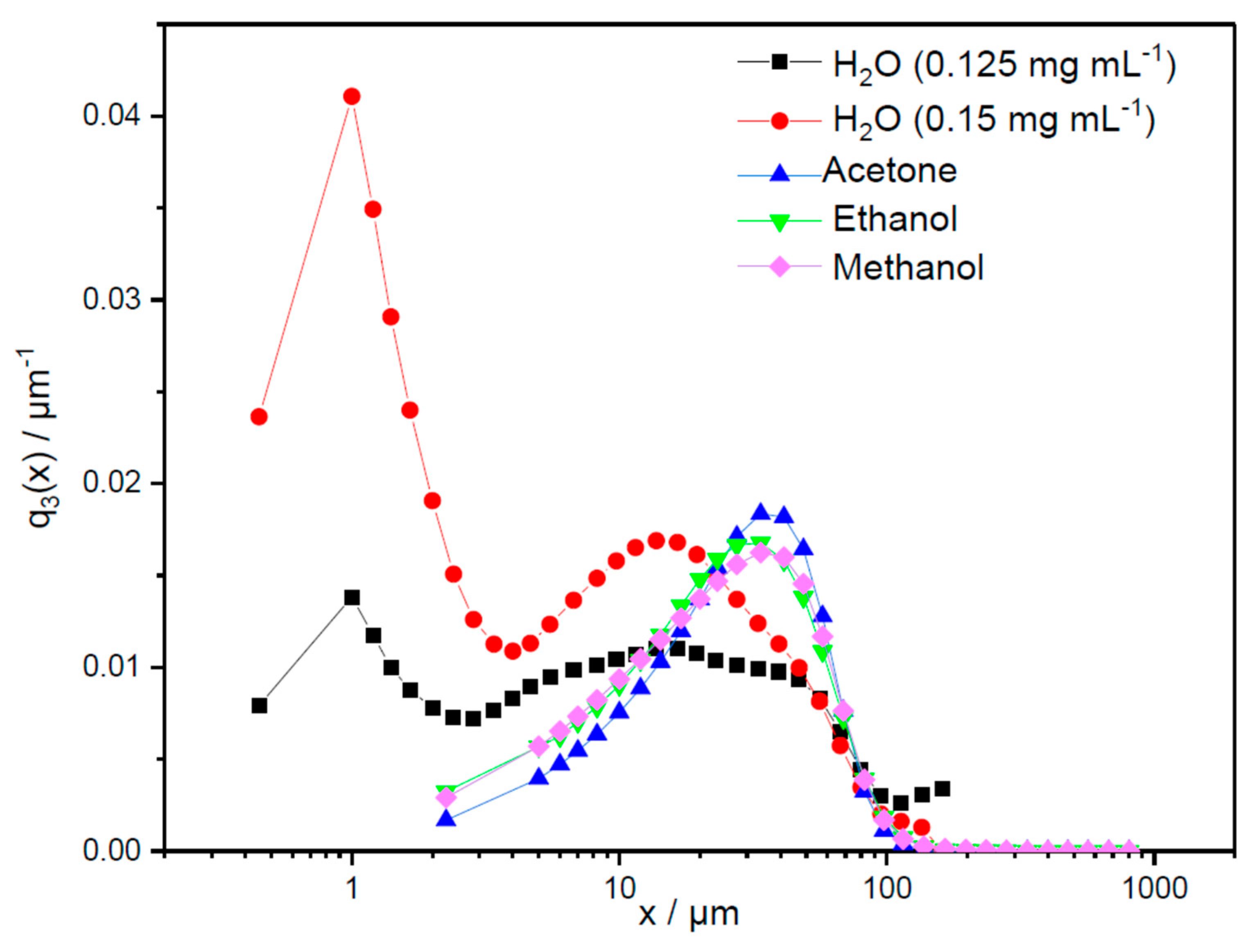
2.7. Particle Size Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Microwave-Assisted Extraction
4.3. Fluorescence Microscopy
4.4. UV-Vis Spectroscopy
4.5. FT-ICR-MS Analyses and Data Processing
4.6. Particle Size Analysis of Pigments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Clement, C.; Lonchamp, D.; Rebeller, M.; Van Landeghem, H. The development of Spirulina algae cultivation. Chem. Eng. Sci. 1980, 35, 119–126. [Google Scholar] [CrossRef]
- Jang, Z.A.; Kim, B. Protective Effect of Spirulina-Derived C-Phycocyanin against Ultraviolet B-Induced Damage in HaCaT Cells. Medicina 2021, 57, 273. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, L.; Wu, C.; Guo, K.; Li, J. Growth and antioxidant production of Spirulina in different NaCl concentrations. Biotechnol. Lett. 2016, 38, 1089–1096. [Google Scholar] [CrossRef]
- Forrest, H.; Van Baalen, C.; Myers, J. Isolation and identification of a new pteridine from a blue-green alga. Arch. Biochem. Biophys. 1958, 78, 95–99. [Google Scholar] [CrossRef]
- Matsuoka, T.; Kodera, Y.; Hiroto, M.; Matsushima, A.; Inada, Y.; Kageyama, H.; Ishii, A. Isolation of Biopterin-alpha-glucoside from Spirulina (Arthrospira) platensis and Its Physiologic Function. Mar. Biotechnol. 1994, 1, 185–188. [Google Scholar]
- Deniz, I.; Ozen, M.; Yesil-Celiktas, O. Supercritical fluid extraction of phycocyanin and investigation of cytotoxicity on human lung cancer cells. J. Supercrit. Fluids 2016, 108, 13–18. [Google Scholar] [CrossRef]
- Duangsee, R.; Phoopat, N.; Ningsanond, S. Phycocyanin extraction from Spirulina platensis and extract stability under various pH and temperature. Asian J. Food Agro-Ind. 2009, 2, 819–826. [Google Scholar]
- Martínez, J.; Luengo, E.; Saldaña, G.; Álvarez, I.; Raso, J. C-phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res. Inter. 2017, 99, 1042–1047. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Teixeira, I.R.; Damasceno Ferreira Marczak, L.; Mercali, G.D. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Res. Inter. 2021, 143, 110314. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.; Lorenzo, J.; Režek Jambrak, A.; Munekata, P.; Nutrizio, M.; Barba, F.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green. Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Le, H.Q.; Leppiniemi, J.; Koso, T.; Linder, M.; Pisano, I.; Välisalmi, T.; Dou, J.; Leahy, J.; Kontturi, E. Microwave hydrolysis, as a sustainable approach in the processing of seaweed for protein and nanocellulose management. Algal Res. 2024, 78, 103406. [Google Scholar] [CrossRef]
- Jain, A.; Upadhyay, C.S.; Mohite, P.M. Fiber breaking damage model for unidirectional fibrous composites using micromechanics. In Proceedings of the 16th National Seminar on Aerospace Structures (NASAS), IIT Bombay, Mumbai, India, 19–20 November 2009. [Google Scholar]
- Yoo, C.; Jun, S.; Ahn, C.; Oh, H.; Lee, J. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chen, K.; Chen, C. Study of the microwave lipid extraction from microalgae for biodiesel production. Chem. Eng. J. 2014, 250, 267–273. [Google Scholar] [CrossRef]
- Iqbal, J.; Theegala, C. Microwave assisted lipid extraction from microalgae using biodiesel as co-solvent. Algal Res. 2013, 2, 34–42. [Google Scholar] [CrossRef]
- Pasquet, V.; Patrice, T.; Picot, L.; Cadoret, J.; Kaas, R.; Serive, B.; Piot, J.; Farhat, F.; Thiéry, V.; Chérouvrier, J.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Aftari, R.; Rezaei, K.; Mortazavi, A.; Bandani, A. The Optimized Concentration and Purity of Spirulina platensis C-Phycocyanin: A Comparative Study on Microwave-Assisted and Ultrasound-Assisted Extraction Methods. J. Food Process Preserv. 2015, 39, 3080–3091. [Google Scholar] [CrossRef]
- İlter, I.; Akyıl, S.; Demirel, Z.; Koc, M.; Conc-Dalay, M.; Kaymak-Ertekin, F. Optimization of phycocyanin extraction from Spirulina platensis using different techniques. J. Food Compos. Anal. 2018, 70, 78–88. [Google Scholar] [CrossRef]
- Akyıl, S.; İlter, I.; Koç, M.; Demirel, Z.; Erdoğan, A.; Conk-Dalay, M.; Ertekin, F.K. Effects of Extraction Methods and Conditions on Bioactive Compounds Extracted from Phaeodactylum tricornutum. Acta Chim. 2020, 67, 1250–1261. [Google Scholar] [CrossRef]
- Larrosa, A.; Camara, A.; Moura, J.; Pinto, L. Spirulina sp. biomass dried/disrupted by different methods and their application in biofilms production. Food Sci. Biotechnol. 2018, 27, 1659–1665. [Google Scholar] [CrossRef]
- Rahman, M.; Hosano, N.; Hosano, H. Recovering Microalgal Bioresources: A Review of Cell Disruption Methods and Extraction Technologies. Molecules 2022, 27, 2786. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Ellison, C.; Trubetskaya, A.; Smith, M.; Shekhawat, D. Effect of microwave and thermal co-pyrolysis of low-rank coal and pine wood on product distributions and char structure. Energy Fuels 2019, 33, 7069–7082. [Google Scholar] [CrossRef]
- Fernandes, R.; Campos, J.; Serra, M.; Fidalgo, J.; Almeida, H.; Casas, A.; Toubarro, D.; Barros, A. Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals 2023, 16, 592. [Google Scholar] [CrossRef]
- Romay, C.; González, R.; Ledón, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Eriksen, N. Production of phycocyanin—A pigment with applications in biology, biotechnology, foods, and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef]
- Ferrandiz, M.; Moldovan, S.; Mira, E.; Pinchetti, J.; Rodriguez, T.; Abreu, H.; Rego, A.; Palomo, B.; Caro, P. Phycobiliproteins—New natural dyes from algae as a sustainable method. Fibres Text. 2016, 23, 56–61. [Google Scholar]
- Schirmer, T.; Bode, W.; Huber, R.; Sidler, W.; Zuber, H. X-ray crystallographic structure of the light-harvesting biliprotein C-phycocyanin from the thermophilic cyanobacterium Mastigocladus laminosus and its resemblance to globin structures. J. Mol. Biol. 1985, 184, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Furukawa, M.; Matsushima, A.; Kodera, Z.; Yamada, A.; Kanai, H.; Inada, Y. Alteration of properties of natural pigments by conjugation with fibroin or polyethylene glycol. Dye. Pigment. 1995, 27, 211–217. [Google Scholar] [CrossRef]
- Prates, J.; Mendes, A.; Spinola, M. Chemical Composition, Bioactivities, and Applications of Spirulina (Limnospira platensis) in Food, Feed, and Medicine. Foods 2024, 13, 3656. [Google Scholar] [CrossRef]
- Parshina, E.Y.; Liu, W.; Yusipovich, A.I.; Gvozdev, D.A.; He, Y.; Pirutin, S.K.; Klimanova, E.A.; Maksimov, E.G.; Maksimov, G.V. Spectral and conformational characteristics of phycocyanin associated with changes of medium pH. PhotoSynth. Res. 2024, 161, 93–103. [Google Scholar] [CrossRef]
- Ogbonda, K.H.; Aminigo, R.E.; Abu, G.O. Influence of temperature and pH on biomass production and protein biosynthesis in a putative Spirulina sp. Bioresour. Technol. 2007, 98, 2207–2211. [Google Scholar] [CrossRef]
- Ismaiel, M.; El-Ayouty, Y.; Piercey-Normore, M. Role of pH on antioxidants production by Spirulina (Arthrospira) platensis. Braz. J. Microbiol. 2016, 47, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, S.; Frey, W.; Krust, D.; Müller, G.; Gusbeth, C. Impact of incubation conditions on protein and C-Phycocyanin recovery from Arthrospira platensis post- pulsed electric field treatment. Bioresour. Technol. 2020, 306, 123099. [Google Scholar] [CrossRef]
- Aoude, C.; Grimi, N.; El Zakhem, H.; Vorobiev, E. Dewatering of Arthrospira platensis microalgae suspension by electrofiltration. Dry. Technol. 2023, 41, 1068–1080. [Google Scholar] [CrossRef]
- Aoude, C. Concentration of the Microalgae Arthrospira Platensis and Chlorella Vulgaris by Electrofiltration. Ph.D. Thesis, Universitéde Technologiede Compiègne, Compiègne, France, 2023. [Google Scholar]
- Zhuxin, L.; Biao, Y.; Yifan, C.; Badamkhand, D.; Xiao, X.; Honghong, S.; Mingqian, T.; Chongjiang, C.; Zhixiang, W. Carboxylated chitosan improved the stability of phycocyanin under acidified conditions. Int. J. Biol. Macromol. 2023, 233, 123474. [Google Scholar] [CrossRef]
- Yang, R.; Ma, T.; Shi, L.; Wang, Q.; Zhang, L.; Zhang, F.; Wang, Z.; Zhou, Z. The formation of phycocyanin-EGCG complex for improving the color protection stability exposing to light. Food Chem. 2022, 370, 130985. [Google Scholar] [CrossRef]
- Dai, J.; Liu, L.; Yang, Z.; Song, Y.; Liu, Z.; Lv, L. The study of phycocyanin-quercetin complex on color stability under light condition. LWT 2024, 211, 116931. [Google Scholar] [CrossRef]
- Buecker, S.; Grossmann, L.; Leeb, E.; Loeffler, M.; Weiss, J. Influence of storage temperature on the stability of heat treated phycocyanin-λ-carrageenan complexes in liquid formulations. Green. Chem. 2022, 24, 4174–4185. [Google Scholar] [CrossRef]
- Mou, S.; Xu, D.; Ye, N.; Zhang, X.; Liang, C.; Liang, Q.; Zheng, Z.; Zhuang, Z.; Miao, J. Rapid estimation of lipid content in an Antarctic ice alga (Chlamydomonas sp.) using the lipophilic fluorescent dye BODIPY505/515. J. Appl. Phycol. 2012, 24, 1169–1176. [Google Scholar] [CrossRef]
- Huang, G.; Feng, C.; Dong, W.; Wu, Z.X.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Wood, A.; Lipsen, M.; Coble, P. Fluorescence-based characterization of phycoerythrin-containing cyanobacterial communities in the Arabian Sea during the Northeast and early Southwest Monsoon (1994–1995). Deep. Sea Res. Part II Top. Stud. Oceanogr. 1999, 46, 1769–1790. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Chen, H.; Deng, J.; Li, L.; Liu, Z.; Xiong, P.; Sun, S. Recent Progress of Natural and Recombinant Phycobiliproteins as Fluorescent Probes. Mar. Drugs 2023, 21, 572. [Google Scholar] [CrossRef]
- Ashenafi, E.; Nyman, M.; Mattson, N.; Shelley, J. Spectral properties and stability of selected carotenoid and chlorophyll compounds in different solvent systems. Food Chem. Adv. 2023, 2, 100178. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Liu, Y.; Bianchi, T.; Tolić, N.; Jansson, C.; Paša-Tolić, L. Moving beyond the van Krevelen Diagram: A New Stoichiometric Approach for Compound Classification in Organisms. Anal. Chem. 2018, 90, 6152–6160. [Google Scholar] [CrossRef] [PubMed]
- Laszakovits, J.; MacKay, A. Data-Based Chemical Class Regions for Van Krevelen Diagrams. J. Am. Soc. Mass. Spectrom. 2022, 33, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Munawaroh, H.; Hazmatulhaq, F.; Gumilar, G.; Pratiwi, R.; Kurniawan, I.; Ningrum, A.; Hidayati, N.; Koyande, A.; Kumar, P.; Show, P. Microalgae as a potential sustainable solution to environment health. Chemosphere 2022, 295, 133740. [Google Scholar] [CrossRef]
- Cole, W.; Chapman, D.; Siegelman, H. Structure of phycocyanobilin. J. Am. Chem. Soc. 1967, 89, 3643–3645. [Google Scholar] [CrossRef]
- Munawaroh, H.; Gumilar, G.; Nurjanah, F.; Yuliani, G.; Aisyah, S.; Kurnia, D.; Wulandari, A.; Kurniawan, I.; Ningrum, A.; Koyande, A.; et al. In-vitro molecular docking analysis of microalgae extracted phycocyanin as an anti-diabetic candidate. Biochem. Eng. J. 2020, 161, 107666. [Google Scholar]
- Liu, F.; Fan, M.; Wei, X.; Zong, Z. Application of mass spectrometry in the characterization of chemicals in coal-derived liquids. Mass Spectrom. Rev. 2017, 36, 543–579. [Google Scholar] [CrossRef]
- Seo, Y.C.; Choi, W.S.; Park, J.H.; Park, J.O.; Jung, K.-H.; Lee, H.Y. Stable Isolation of Phycocyanin from Spirulina platensis Associated with High-Pressure Extraction Process. Int. J. Mol. Sci. 2013, 14, 1778–1787. [Google Scholar] [CrossRef]
- Mao, M.; Zhao, Y.; Zhao, Y.; Xu, X.; Han, G. A review of phycocyanin: Production, extraction, stability and food applications. Int. J. Biol. Macromol. 2024, 280, 135860. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Lian, X.; Li, Y.; Li, X.; Liang, Z.; Chang, X. Progress of Microencapsulated Phycocyanin in Food and Pharma Industries: A Review. Molecules 2022, 27, 5854. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, D.; Wu, C.; Duan, W.; Jing, H.; Long, J.; Huang, Y. Improving the stability and transdermal permeability of phycocyanin loaded cubosomes. Front. Nanotech 2024, 6, 1359219. [Google Scholar] [CrossRef]
- Kurniasih, R.; Purnamayati, L.; Amalia, U.; Dewi, E. Formulation and Characterization of Phycocyanin Microcapsules within Maltodextrin-Alginate. IndoChem Agritech 2018, 38, 23–29. [Google Scholar] [CrossRef]
- Wu, H.; He, H.; Li, T.; Wang, G.; Xiang, W. Stability and Antioxidant Activity of Food-Grade Phycocyanin Isolated from Spirulina platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef]
- Gore, A.H.; Prajapat, A.L. Biopolymer Nanocomposites for Sustainable UV Protective Packaging. Front. Mater. 2022, 9, 855727. [Google Scholar] [CrossRef]
- Adjali, A.; Clarot, I.; Chen, Z.; Marchioni, E.; Boudier, A. Physicochemical degradation of phycocyanin and means to improve its stability: A short review. J. Pharm. Anal. 2022, 12, 406–414. [Google Scholar] [CrossRef]
- BVL_L_17.00-15:2013-08, Untersuchung von Lebensmitteln—Bestimmung des Rohproteingehaltes in Brot einschließlich Kleingebäck aus Brotteigen—Kjeldahl-Verfahren, Saarbrücken: Deutsche Akkreditierungsstelle. 2013, p. 4. Available online: https://www.dinmedia.de/de/technische-regel/bvl-l-17-00-15/193412088 (accessed on 15 May 2025).
- Trubetskaya, A.; Shchukarev, A.; Umeki, K.; Larsen, F.H.; Ståhl, K. Potassium and soot interaction in fast biomass pyrolysis at high temperatures. Fuel 2018, 225, 89–94. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Attard, T.; Budarin, V.; Arshadi, M.; Grams, J.; Hunt, A. Supercritical Extraction of Biomass—A Green and Sustainable Method to Control the Pyrolysis Product Distribution. ACS Sust. Chem. Eng. 2021, 9, 5278–5287. [Google Scholar] [CrossRef]
- Kiss, G.C.; Forgács, E.; Cserháti, T.; Mota, T.; Morais, H.; Ramos, A. Optimisation of the microwave-assisted extraction of pigments from paprika (Capsicum annuum L.) powders. J. Chromatogr. A 2000, 889, 41–49. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Zuber, J.; Cascabulho, P.L.; Piperni, S.G.; Amaral, R.C.D.; Vogt, C.; Carre, V.; Hertzog, J.; Kontturi, E.; Trubetskaya, A. Fast, Easy, and Reproducible Fingerprint Methods for Endotoxin Characterization in Nanocellulose and Alginate-Based Hydrogel Scaffolds. Biomacromolecules 2024, 25, 6762–6772. [Google Scholar] [CrossRef] [PubMed]
- Herzsprung, P.; Hertkorn, N.; von Tümpling, W.; Harir, M.; Friese, K.; Schmitt-Kopplin, P. Understanding molecular formula assignment of Fourier transform ion cyclotron resonance mass spectrometry data of natural organic matter from a chemical point of view. Anal. Bioanal. Chem. 2014, 406, 7977–7987. [Google Scholar] [CrossRef] [PubMed]
- Herzsprung, P.; Tümpling, W.; Hertkorn, N.; Harir, M.; Friese, K.; Schmitt-Kopplin, P. High-Field FTICR-MS Data Evaluation of Natural Organic Matter: Are CHON5S2 Molecular Class Formulas Assigned to (13)C Isotopic m/z and in Reality CHO Components? Anal. Chem. 2015, 87, 9563–9566. [Google Scholar] [CrossRef] [PubMed]

| Moisture, wt.% (as received) | 7.1 |
| Ash, wt.% (as received) | 9.3 |
| Protein g/100 g | 64.7 |
| Carbohydrate g/100 g | 8.6 |
| Ultimate analysis % (on a dry basis) | |
| Carbon | 45.9 |
| Hydrogen | 6.2 |
| Oxygen | 25.4 |
| Nitrogen | 6.1 |
| Ash composition in mg kg−1 | |
| Cl | 17,200 |
| I | 900 |
| S | 27,000 |
| Al | 20,600 |
| Ca | 11,600 |
| Cr | 30 |
| Cu | 70 |
| Fe | 10,000 |
| K | 6000 |
| Mg | 4000 |
| Mn | 200 |
| Na | 7600 |
| Ni | 5 |
| Zn | 300 |
| As | 8 |
| Hg | 10 |
| Mo | 11 |
| P | 14,000 |
| P | 14,000 |
| Se | 0 |
| Solvent | Phycocyanin Concentrations (mg mL−1) with Standard Deviation | |||||||
|---|---|---|---|---|---|---|---|---|
| 30 °C | 40 °C | 45 °C | 45 °C | 45 °C | 50 °C | 60 °C | 70 °C | |
| 60 min | 60 min | 60 min | 30 min | 60 min | 60 min | 60 min | 60 min | |
| 100 W | 100 W | 100 W | 100 W | 300 W | 100 W | 100 W | 100 W | |
| Water | 3.1 ± 0.007 | 1.9 ± 0.016 | 2.1 ± 0.01 | 1.8 ± 0.005 | 1.7 ± 0.007 | 0.7 ± 0.017 | 0.4 ± 0.005 | 0.3 ± 0.010 |
| Acetone | 2.2 ± 0.017 | 2.1 ± 0.016 | 1.7 ± 0.009 | 1.8 ± 0.012 | 1.7 ± 0.004 | 2.1 ± 0.126 | 0.6 ± 0.009 | 0.4 ± 0.009 |
| Ethanol | 2.1 ± 0.015 | 2.0 ± 0.009 | 1.8 ± 0.008 | 1.9 ± 0.008 | 1.8 ± 0.012 | 1.7 ± 0.007 | 0.6 ± 0.019 | 0.5 ± 0.004 |
| Methanol | 2.0 ± 0.006 | 1.8 ± 0.006 | 1.9± 0.011 | 1.9 ± 0.016 | 1.7 ± 0.005 | 1.9 ± 0.009 | 0.5 ± 0.008 | 0.4 ± 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trubetskaya, A.; Haseneder, R.; Lippold, M.; van Haren, R.J.F.; Herdegen, V.; Ditscherlein, L.; Leahy, J.J.; Pisano, I.; Joseph, Y.; Vogt, C.; et al. The Effect of Ultraviolet Light Irradiation on Pigment Performance in Microwave-Assisted Extraction of Arthrospira platensis. Mar. Drugs 2025, 23, 391. https://doi.org/10.3390/md23100391
Trubetskaya A, Haseneder R, Lippold M, van Haren RJF, Herdegen V, Ditscherlein L, Leahy JJ, Pisano I, Joseph Y, Vogt C, et al. The Effect of Ultraviolet Light Irradiation on Pigment Performance in Microwave-Assisted Extraction of Arthrospira platensis. Marine Drugs. 2025; 23(10):391. https://doi.org/10.3390/md23100391
Chicago/Turabian StyleTrubetskaya, Anna, Roland Haseneder, Maximilian Lippold, Rob J. F. van Haren, Volker Herdegen, Lisa Ditscherlein, James J. Leahy, Italo Pisano, Yvonne Joseph, Carla Vogt, and et al. 2025. "The Effect of Ultraviolet Light Irradiation on Pigment Performance in Microwave-Assisted Extraction of Arthrospira platensis" Marine Drugs 23, no. 10: 391. https://doi.org/10.3390/md23100391
APA StyleTrubetskaya, A., Haseneder, R., Lippold, M., van Haren, R. J. F., Herdegen, V., Ditscherlein, L., Leahy, J. J., Pisano, I., Joseph, Y., Vogt, C., & Zuber, J. (2025). The Effect of Ultraviolet Light Irradiation on Pigment Performance in Microwave-Assisted Extraction of Arthrospira platensis. Marine Drugs, 23(10), 391. https://doi.org/10.3390/md23100391








