Role of Ascophyllum nodosum and Fucus vesiculosus in Improving the Stress Resistance of Lactiplantibacillus plantarum
Abstract
1. Introduction
2. Results
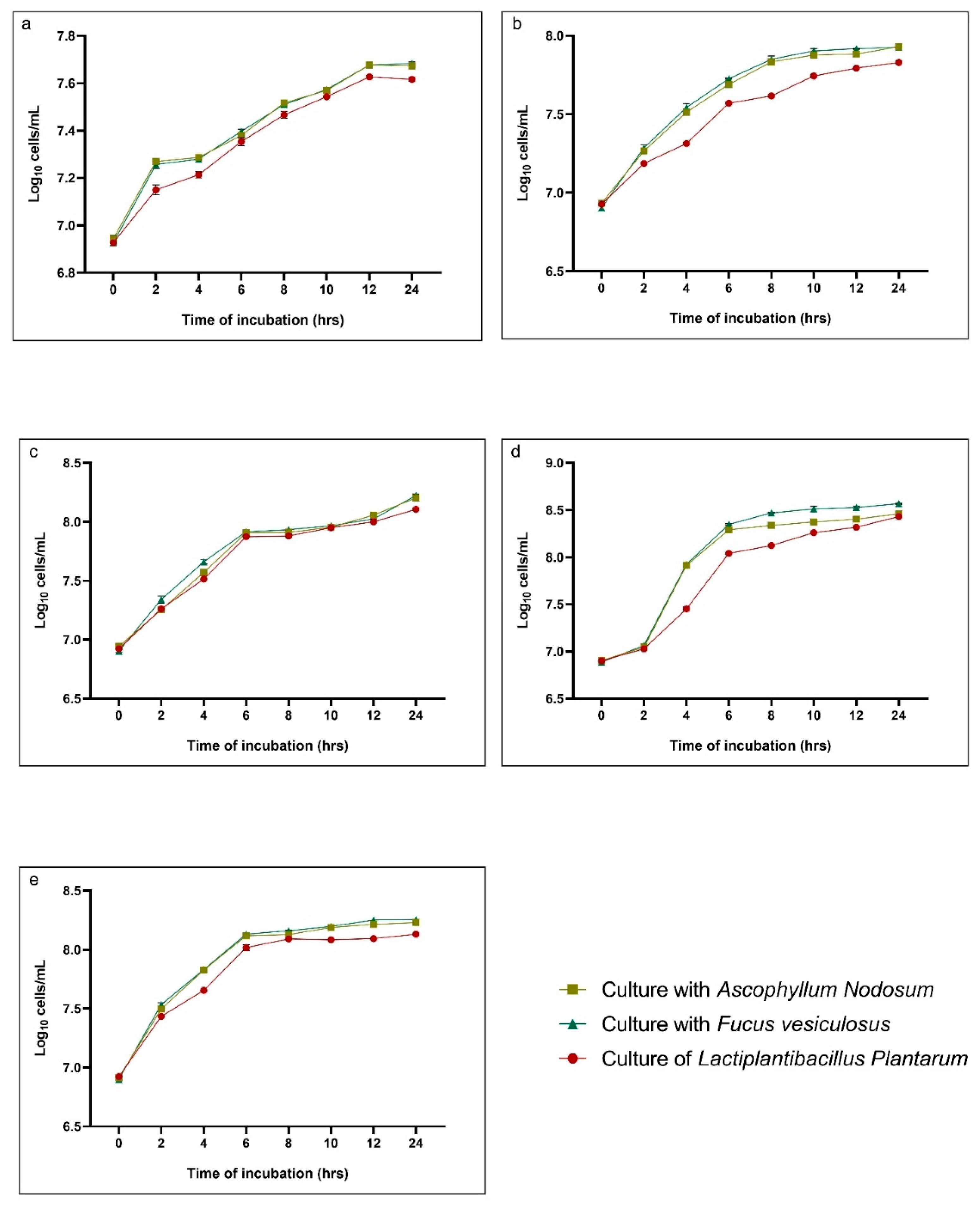
2.1. Temperature Tolerance
2.2. pH Tolerance
2.3. Enzymatic Tolerance Assay
3. Discussion
4. Materials and Methods
4.1. Seaweed Biomass
4.2. Bacterial Strain and Culture Condition
4.3. Survival of Lactiplantibacillus plantarum Cultured in the Presence of Seaweed Biomass Under Adverse Environmental Conditions
4.3.1. Temperature Tolerance Assay
4.3.2. pH Tolerance Assay
4.3.3. Enzyme Tolerance Assay
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; WHO. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland, 2006; ISBN 9251055130. [Google Scholar]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An Overview of Beneficial Effects. Antonie Van. Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Bustos, A.Y.; Taranto, M.P.; Gerez, C.L.; Agriopoulou, S.; Smaoui, S.; Varzakas, T.; El Enshasy, H.A. Recent Advances in the Understanding of Stress Resistance Mechanisms in Probiotics: Relevance for the Design of Functional Food Systems. Probiotics Antimicrob. Proteins 2025, 17, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Charalampopoulos, D.; Wang, R.; Pandiella, S.S.; Webb, C. Application of Cereals and Cereal Components in Functional Foods: A Review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic Functional Foods: Survival of Probiotics during Processing and Storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Sedgwick, H.; Gibson, G.; Adams, J.; Wijeyesekera, A. Seaweed-Derived Bioactives: Gut Microbiota Targeted Interventions for Immune Function. J. Funct. Foods 2025, 125, 106696. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Keleszade, E.; Patterson, M.; Trangmar, S.; Guinan, K.J.; Costabile, A. Clinical Efficacy of Brown Seaweeds Ascophyllum nodosum and Fucus vesiculosus in the Prevention or Delay Progression of the Metabolic Syndrome: A Review of Clinical Trials. Molecules 2021, 26, 714. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from Marine Macroalgae for Human and Animal Health Applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Frazzini, S.; Torresani, M.C.; Hejna, M.; Di Dio, M.; Rossi, L. Ascophyllum nodosum and Lithothamnium calcareum and Their Prebiotic Potential on Lactobacillus Strains. J. Funct. Foods 2024, 118, 106257. [Google Scholar] [CrossRef]
- Venardou, B.; O’Doherty, J.V.; Garcia-Vaquero, M.; Kiely, C.; Rajauria, G.; McDonnell, M.J.; Ryan, M.T.; Sweeney, T. Evaluation of the Antibacterial and Prebiotic Potential of Ascophyllum nodosum and Its Extracts Using Selected Bacterial Members of the Pig Gastrointestinal Microbiota. Mar. Drugs 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, P.; Saxena, M.J. Feed Additives in Animal Health. In Nutraceuticals in Veterinary Medicine, 1st ed; Gupta, R., Srivastava, A., Lall, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 345–362. [Google Scholar]
- Agriopoulou, S.; Tarapoulouzi, M.; Varzakas, T.; Jafari, S.M. Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation. Microorganisms 2023, 11, 2896. [Google Scholar] [CrossRef]
- Sweeney, T.; Dillon, S.; Fanning, J.; Egan, J.; O’Shea, C.J.; Figat, S.; Gutierrez, J.J.M.; Mannion, C.; Leonard, F.; O’Doherty, J.V. Evaluation of Seaweed-Derived Polysaccharides on Indices of Gastrointestinal Fermentation and Selected Populations of Microbiota in Newly Weaned Pigs Challenged with Salmonella typhimurium. Anim. Feed. Sci. Technol. 2011, 165, 85–94. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The Role of Probiotics, Prebiotics and Synbiotics in Animal Nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Siezen, R.J.; van Hylckama Vlieg, J.E. Genomic Diversity and Versatility of Lactobacillus plantarum, a Natural Metabolic Engineer. Microb. Cell Fact. 2011, 10, S3. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- De Angelis, M.; Di Cagno, R.; Huet, C.; Crecchio, C.; Fox, P.F.; Gobbetti, M. Heat Shock Response in Lactobacillus plantarum. Appl. Environ. Microbiol. 2004, 70, 1336–1346. [Google Scholar] [CrossRef]
- Gökmen, G.G.; Sarıyıldız, S.; Cholakov, R.; Nalbantsoy, A.; Baler, B.; Aslan, E.; Düzel, A.; Sargın, S.; Göksungur, Y.; Kışla, D. A Novel Lactiplantibacillus plantarum Strain: Probiotic Properties and Optimization of the Growth Conditions by Response Surface Methodology. World J. Microbiol. Biotechnol. 2024, 40, 66. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed Components as Potential Modulators of the Gut Microbiota. Mar. Drugs 2021, 19, 358. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.; Bae, J.-H.; Seo, J.S.; Kim, S.-A.; Kim, T.-J.; Han, N.S. Comparative Analysis of Prebiotic Effects of Seaweed Polysaccharides Laminaran, Porphyran, and Ulvan Using in Vitro Human Fecal Fermentation. J. Funct. Foods 2019, 57, 408–416. [Google Scholar] [CrossRef]
- Ricós-Muñoz, N.; Maicas, S.; Pina-Pérez, M.C. Probiotic Lactobacillus reuteri Growth Improved under Fucoidan Exposure. In Proceedings of the 1st International Electronic Conference on Food Science and Functional Foods, Basel, Switzerland, 10 November 2020; Proceedings MDPI: Basel, Switzerland; p. 106. [Google Scholar]
- Venardou, B.; O’Doherty, J.V.; Garcia-Vaquero, M.; Kiely, C.; Rajauria, G.; McDonnell, M.J.; Ryan, M.T.; Sweeney, T. In Vitro Evaluation of Brown Seaweed Laminaria Spp. as a Source of Antibacterial and Prebiotic Extracts That Could Modulate the Gastrointestinal Microbiota of Weaned Pigs. Animals 2023, 13, 823. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ma, Q.; Wang, D.; Jiang, Q.; Wang, P.; Ge, Z.; Wang, J.; Qin, P.; Zhao, X. Different Microbiota Modulation and Metabolites Generation of Five Dietary Glycans during in Vitro Gut Fermentation Are Determined by Their Monosaccharide Profiles. Food Res. Int. 2024, 196, 115011. [Google Scholar] [CrossRef]
- McGuire, Z.; Revuru, S.; Zhang, S.; Blankenberger, A.; Rasheed, M.; Hosen, J.D.; Lin, G.; Verma, M.S. Modelling Complex Growth Profiles of Bacteroides fragilis and Escherichia coli on Various Carbohydrates in an Anaerobic Environment. bioRxiv 2023. [Google Scholar] [CrossRef]
- Manuguerra, S.; Arena, R.; Curcuraci, E.; Renda, G.; Rannou, M.; Hellio, C.; Messina, C.M.; Santulli, A. In Vitro Potential of Antioxidant Extracts from Gracilaria gracilis Cultivated in Integrated Multi-Trophic Aquaculture (IMTA) for Marine Biobased Sector. Water 2024, 16, 2667. [Google Scholar] [CrossRef]
- Carpena, M.; Pereira, C.S.G.P.; Silva, A.; Barciela, P.; Jorge, A.O.S.; Perez-Vazquez, A.; Pereira, A.G.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Prieto, M.A. Metabolite Profiling of Macroalgae: Biosynthesis and Beneficial Biological Properties of Active Compounds. Mar. Drugs 2024, 22, 478. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Francis, P.; Foysal, M.J.; Francis, D.S. Dietary Seaweed Extract Mitigates Oxidative Stress in Nile Tilapia by Modulating Inflammatory Response and Gut Microbiota. Front. Immunol. 2024, 15, 1471261. [Google Scholar] [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Development and Application of an in Vitro Methodology to Determine the Transit Tolerance of Potentially Probiotic Lactobacillus and Bifidobacterium Species in the Upper Human Gastrointestinal Tract. J. Appl. Microbiol. 1998, 84, 759–768. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, Y.; Xie, Q.; Chen, H.; Zhang, M.; Yu, L.; Yan, G.; Chen, Y.; Lin, X.; Zhang, Y.; et al. Protective Effects of Laminaria japonica Polysaccharide Composite Microcapsules on the Survival of Lactobacillus plantarum during Simulated Gastrointestinal Digestion and Heat Treatment. Mar. Drugs 2024, 22, 308. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R. Viability of Microencapsuls Containing Lactic Acid Bacteria under Stimulated Gastrointestinal Conditions While Incorporated in Steamed Rice Cake. J. Nutri. Health Food Eng. 2015, 3, 309–314. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abdallah, N.A.; El-Shafei, K.; Tawfik, N.F.; El-Sayed, H.S. Survivability of Alginate-Microencapsulated Lactobacillus plantarum during Storage, Simulated Food Processing and Gastrointestinal Conditions. Heliyon 2020, 6, e03541. [Google Scholar] [CrossRef]
- Zou, T.; Yang, J.; Guo, X.; He, Q.; Wang, Z.; You, J. Dietary Seaweed-Derived Polysaccharides Improve Growth Performance of Weaned Pigs through Maintaining Intestinal Barrier Function and Modulating Gut Microbial Populations. J. Anim. Sci. Biotechnol. 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Ganchev, I. Impact of Spirulina Platensis Biomass on the Viability of Lactobacillus delbrueckii Subsp. Bulgaricus Strain during the Freeze-Drying Process. BioTechnologia 2024, 105, 109–119. [Google Scholar] [CrossRef]
- Wimmer, B.C.; Dwan, C.; De Medts, J.; Duysburgh, C.; Rotsaert, C.; Marzorati, M. Undaria pinnatifida Fucoidan Enhances Gut Microbiome, Butyrate Production, and Exerts Anti-Inflammatory Effects in an In Vitro Short-Term SHIME® Coupled to a Caco-2/THP-1 Co-Culture Model. Mar. Drugs 2025, 23, 242. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.; Li, S.; Hao, L.; Du, J.; Gao, D.; Kang, Q.; Lu, J. Extraction, Characterization and Biological Activity of Sulfated Polysaccharides from Seaweed Dictyopteris divaricata. Int. J. Biol. Macromol. 2018, 117, 256–263. [Google Scholar] [CrossRef]
- Castro-Pinheiro, C.; Junior, L.C.S.P.; Sanchez, E.F.; da Silva, A.C.R.; Dwan, C.A.; Karpiniec, S.S.; Critchley, A.T.; Fuly, A.L. Effect of Seaweed-Derived Fucoidans from Undaria pinnatifida and Fucus vesiculosus on Coagulant, Proteolytic, and Phospholipase A2 Activities of Snake Bothrops Jararaca, B. Jararacussu, and B. Neuwiedi Venom. Toxins 2024, 16, 188. [Google Scholar] [CrossRef]
- Kurochkina, L.; Pozdyshev, D.; Kusaykin, M.; Barinova, K.; Ermakova, S.; Semenyuk, P. Sulfated Polysaccharides Accelerate Gliadin Digestion and Reduce Its Toxicity. Biochem. Biophys. Res. Commun. 2024, 695, 149439. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Imbs, T.I.; Zvyagintseva, T.N.; Fedoreyev, S.A.; Ermakova, S.P. Brown Alga Metabolites—Inhibitors of Marine Organism Fucoidan Hydrolases. Chem. Nat. Compd. 2017, 53, 345–350. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, M.; Ouyang, D.; Tong, H.; Wu, M.; Su, L. Research Progress on the Protective Effect of Brown Algae-Derived Polysaccharides on Metabolic Diseases and Intestinal Barrier Injury. Int. J. Mol. Sci. 2022, 23, 10784. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Gaspar, D.; Ferreira, S.S.; Correia, A.; Vilanova, M.; Perrineau, M.-M.; Kerrison, P.D.; Gachon, C.M.M.; Domingues, M.R.; Coimbra, M.A.; et al. Water-Soluble Saccharina latissima Polysaccharides and Relation of Their Structural Characteristics with In Vitro Immunostimulatory and Hypocholesterolemic Activities. Mar. Drugs 2023, 21, 183. [Google Scholar] [CrossRef]
- Matin, M.; Koszarska, M.; Atanasov, A.G.; Król-Szmajda, K.; Jóźwik, A.; Stelmasiak, A.; Hejna, M. Bioactive Potential of Algae and Algae-Derived Compounds: Focus on Anti-Inflammatory, Antimicrobial, and Antioxidant Effects. Molecules 2024, 29, 4695. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Dwan, C.; Wimmer, B.; Wilson, R.; Johnson, L.; Caruso, V. Fucoidan from Undaria pinnatifida Enhances Exercise Performance and Increases the Abundance of Beneficial Gut Bacteria in Mice. Mar. Drugs 2024, 22, 485. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anno, M.; Giromini, C.; Reggi, S.; Cavalleri, M.; Moscatelli, A.; Onelli, E.; Rebucci, R.; Sundaram, T.S.; Coranelli, S.; Spalletta, A.; et al. Evaluation of Adhesive Characteristics of L. plantarum and L. reuteri Isolated from Weaned Piglets. Microorganisms 2021, 9, 1587. [Google Scholar] [CrossRef] [PubMed]
- Oguntoyinbo, F.A.; Narbad, A. Multifunctional Properties of Lactobacillus plantarum Strains Isolated from Fermented Cereal Foods. J. Funct. Foods 2015, 17, 621–631. [Google Scholar] [CrossRef]
- Singhal, N.; Singh, N.S.; Mohanty, S.; Kumar, M.; Virdi, J.S. Rhizospheric Lactobacillus plantarum (Lactiplantibacillus plantarum) Strains Exhibit Bile Salt Hydrolysis, Hypocholestrolemic and Probiotic Capabilities in Vitro. Sci. Rep. 2021, 11, 15288. [Google Scholar] [CrossRef]
- Kouadio, N.J.; Zady, A.L.O.; Kra, K.A.S.; Diguță, F.C.; Niamke, S.; Matei, F. In Vitro Probiotic Characterization of Lactiplantibacillus plantarum Strains Isolated from Traditional Fermented Dockounou Paste. Fermentation 2024, 10, 264. [Google Scholar] [CrossRef]
| Survival Rate (%) | ||||
|---|---|---|---|---|
| Time | pH | L. plantarum | L. plantarum with A. nodosum | L. plantarum with F. vesiculosus |
| 1 h | 2.5 | 97.20 ± 0.133 a, B | 99.07 ± 0.133 b, B | 99.29 ± 0.077 b, B |
| 3.5 | 98.40 ± 0.133 a, C | 99.29 ± 0.077 b, B | 99.47 ± 0.133 b, B | |
| 4.5 | 100.18 ± 0.154 a, A | 100.84 ± 0.154 b, C | 103.11 ± 0.077 c, C | |
| 6.5 | 100.04 ± 0.539 a, A | 101.78 ± 0.770 b, A | 104.76 ± 0.154 c, A | |
| 8.5 | 99.60 ± 0.231 a, A | 101.47 ± 0.231 b, AC | 103.20 ± 0.533 c, C | |
| 2 h | 2.5 | 89.90 ± 0.221 a, B | 93.22 ± 0.128 b, B | 93.48 ± 0.128 b, B |
| 3.5 | 93.22 ± 0.128 a, C | 94.63 ± 0.128 b, C | 94.76 ± 0.126 b, C | |
| 4.5 | 97.66 ± 0.450 a, D | 98.59 ± 0.128 b, D | 101.41 ± 0.130 c, D | |
| 6.5 | 100.00 ± 0.256 a, A | 102.98 ± 0.195 b, A | 103.41 ± 0.074 b, A | |
| 8.5 | 98.72 ± 0.256 a, E | 102.47 ± 0.391 b, A | 102.13 ± 0.195 b, E | |
| 3 h | 2.5 | 83.37 ± 0.120 a, B | 85.41 ± 0.120 b, B | 86.12 ± 0.120 c, B |
| 3.5 | 85.29 ± 0.207 a, C | 86.60 ± 0.120 b, C | 87.32 ± 0.120 c, C | |
| 4.5 | 92.46 ± 0.120 a, D | 94.30 ± 0.276 b, D | 96.65 ± 0.207 c, D | |
| 6.5 | 100.08 ± 0.276 a, A | 101.91 ± 0.120 b, A | 101.91 ± 0.120 b, A | |
| 8.5 | 96.37 ± 0.276 a, E | 98.29 ± 0.069 b, E | 97.93 ± 0.069 c, E | |
| Viability (log10 CFU/mL) | ||||
|---|---|---|---|---|
| Concentration | Time | L. plantarum | L. plantarum with A. nodosum | L. plantarum with F. vesiculosus |
| 0.3 mg/mL | 0 | 7.46 ± 0.015 a, A | 7.47 ± 0.017 a, A | 7.46 ± 0.029 a, A |
| 1 | 7.37 ± 0.012 a, B | 7.46 ± 0.029 b, A | 7.42 ± 0.040 ab, AB | |
| 2 | 7.35 ± 0.031 a, B | 7.47 ± 0.021 b, A | 7.40 ± 0.036 a, AB | |
| 3 | 7.32 ± 0.020 a, B | 7.47 ± 0.047 b, A | 7.35 ± 0.031 ab, B | |
| 0.5 mg/mL | 0 | 7.43 ± 0.023 a, A | 7.43 ± 0.021 a, A | 7.43 ± 0.026 a, A |
| 1 | 7.37 ± 0.017 a, B | 7.50 ± 0.030 b, B | 7.51 ± 0.006 b, B | |
| 2 | 7.40 ± 0.023 a, AB | 7.52 ± 0.026 b, AB | 7.53 ± 0.015 b, B | |
| 3 | 7.39 ± 0.020 a, AB | 7.47 ± 0.035 b, AB | 7.48 ± 0.12 b, B | |
| 1 mg/mL | 0 | 7.59 ± 0.002 a, A | 7.59 ± 0.002 a, A | 7.59 ± 0.001 a, A |
| 1 | 7.48 ± 0.046 a, B | 7.58 ± 0.026 b, AB | 7.53 ± 0.023 b, B | |
| 2 | 7.45 ± 0.035 a, B | 7.56 ± 0.029 b, AB | 7.53 ± 0.015 b, B | |
| 3 | 7.45 ± 0.015 a, B | 7.50 ± 0.017 b, B | 7.50 ± 0.021 b, B | |
| Viability (log10 CFU/mL) | ||||
|---|---|---|---|---|
| Concentration | Time | L. plantarum | L. plantarum with A. nodosum | L. plantarum with F. vesiculosus |
| 0.1 mg/mL | 0 | 7.54 ± 0.010 a, A | 7.52 ± 0.015 a, A | 7.51 ± 0.015 a, A |
| 1 | 7.50 ± 0.012 a, AB | 7.61 ± 0.006 b, B | 7.60 ± 0.076 b, B | |
| 2 | 7.48 ± 0.012 a, AB | 7.60 ± 0.044 b, AB | 7.52 ± 0.046 ab, AB | |
| 3 | 7.43 ± 0.053 a, B | 7.54 ± 0.045 b, AB | 7.52 ± 0.055 b, AB | |
| 0.5 mg/mL | 0 | 7.65 ± 0.010 a, A | 7.65 ± 0.007 a, A | 7.64 ± 0.010 a, A |
| 1 | 7.55 ± 0.031 a, B | 7.63 ± 0.012 b, AB | 7.59 ± 0.006 a, B | |
| 2 | 7.53 ± 0.026 a, B | 7.60 ± 0.021 b, B | 7.58 ± 0.025 b, B | |
| 3 | 7.52 ± 0.020 a, B | 7.59 ± 0.015 b, C | 7.58 ± 0.006 b, B | |
| 1 mg/mL | 0 | 7.59 ± 0.002 a, A | 7.59 ± 0.002 a, A | 7.59 ± 0.001 a, A |
| 1 | 7.56 ± 0.006 a, B | 7.58 ± 0.006 b, AB | 7.57 ± 0.010 ab, B | |
| 2 | 7.53 ± 0.006 a, B | 7.57 ± 0.012 b, B | 7.57 ± 0.010 b, B | |
| 3 | 7.50 ± 0.010 a, C | 7.57 ± 0.012 b, B | 7.55 ± 0.012 b, B | |
| Viability (log10 CFU/mL) | ||||
|---|---|---|---|---|
| Concentration | Time | L. plantarum | L. plantarum with A. nodosum | L. plantarum with F. vesiculosus |
| 0.3% (w/v) | 0 | 7.73 ± 0.010 a, A | 7.73 ± 0.006 a, A | 7.72 ± 0.006 a, A |
| 1 | 7.67 ± 0.015 a, B | 7.72 ± 0.010 b, A | 7.70 ± 0.025 ab, A | |
| 2 | 7.60 ± 0.026 a, C | 7.69 ± 0.010 b, A | 7.64 ± 0.006 ab, B | |
| 3 | 7.55 ± 0.036 a, C | 7.67 ± 0.017 b, A | 7.63 ± 0.012 b, B | |
| 0.5% (w/v) | 0 | 7.81 ± 0.010 a, A | 7.83 ± 0.006 a, A | 7.82 ± 0.006 a, A |
| 1 | 7.80 ± 0.015 a, AB | 7.82 ± 0.021 a, A | 7.82 ± 0.029 a, AB | |
| 2 | 7.77 ± 0.012 a, B | 7.80 ± 0.020 b, A | 7.78 ± 0.015 ab, B | |
| 3 | 7.69 ± 0.010 a, C | 7.75 ± 0.006 b, B | 7.74 ± 0.010 b, B | |
| 1% (w/v) | 0 | 7.97 ± 0.010 a, A | 7.97 ± 0.006 a, A | 7.96 ± 0.006 a, A |
| 1 | 7.92 ± 0.007 a, B | 7.96 ± 0.010 b, AB | 7.94 ± 0.010 c, B | |
| 2 | 7.92 ± 0.006 a, B | 7.94 ± 0.002 b, B | 7.93 ± 0.006 ab, B | |
| 3 | 7.92 ± 0.012 a, B | 7.93 ± 0.015 a, B | 7.92 ± 0.010 a, B | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazzini, S.; Dell’Anno, M.; Rossi, L. Role of Ascophyllum nodosum and Fucus vesiculosus in Improving the Stress Resistance of Lactiplantibacillus plantarum. Mar. Drugs 2025, 23, 373. https://doi.org/10.3390/md23100373
Frazzini S, Dell’Anno M, Rossi L. Role of Ascophyllum nodosum and Fucus vesiculosus in Improving the Stress Resistance of Lactiplantibacillus plantarum. Marine Drugs. 2025; 23(10):373. https://doi.org/10.3390/md23100373
Chicago/Turabian StyleFrazzini, Sara, Matteo Dell’Anno, and Luciana Rossi. 2025. "Role of Ascophyllum nodosum and Fucus vesiculosus in Improving the Stress Resistance of Lactiplantibacillus plantarum" Marine Drugs 23, no. 10: 373. https://doi.org/10.3390/md23100373
APA StyleFrazzini, S., Dell’Anno, M., & Rossi, L. (2025). Role of Ascophyllum nodosum and Fucus vesiculosus in Improving the Stress Resistance of Lactiplantibacillus plantarum. Marine Drugs, 23(10), 373. https://doi.org/10.3390/md23100373








