Abstract
The increase in antimicrobial resistance (AMR) in microorganisms is a significant global health concern. Various factors contribute to AMR, including alterations in cell membrane permeability, increased efflux pump activity, enzymatic modification or inactivation of antibiotics, target site changes, alternative metabolic pathways, and biofilm formation. Marine environments, with their extensive biodiversity, provide a valuable source of natural products with a wide range of biological activities. Marine-derived antimicrobial compounds show significant potential against drug-resistant bacteria and fungi. This review discusses the current knowledge on marine natural products such as microorganisms, sponges, tunicates and mollusks with antibacterial and antifungal properties effective against drug-resistant microorganisms and their ecological roles. These natural products are classified based on their chemical structures, such as alkaloids, amino acids, peptides, polyketides, naphthoquinones, terpenoids, and polysaccharides. Although still in preclinical studies, these agents demonstrate promising in vivo efficacy, suggesting that marine sources could be pivotal in developing new drugs to combat AMR, thereby fulfilling an essential medical need. This review highlights the ongoing importance of marine biodiversity exploration for discovering potential antimicrobial agents.
1. Introduction
Antimicrobial resistance (AMR) is an escalating global health issue, significantly undermining the efficacy of current antibiotics and increasing the risk of untreatable infections [1,2,3]. This problem stems from the widespread overuse and misuse of antibiotics in both medical and agricultural settings, leading to the emergence of multidrug-resistant (MDR) pathogens [4,5]. The World Health Organization (WHO) projects that, without intervention, AMR could cause up to 10 million deaths annually by 2050 [6]. Bacteria can develop resistance through several mechanisms, including changes in cell membrane permeability, activation of efflux pumps, enzymatic degradation of antibiotics, modifications to target sites, alternative metabolic pathways, and biofilm formation [7,8,9]. The antibiotic resistance mechanism of currently available antibiotics against microbial pathogens is shown in Figure 1a. These strategies collectively reduce the effectiveness of traditional antimicrobial treatments.
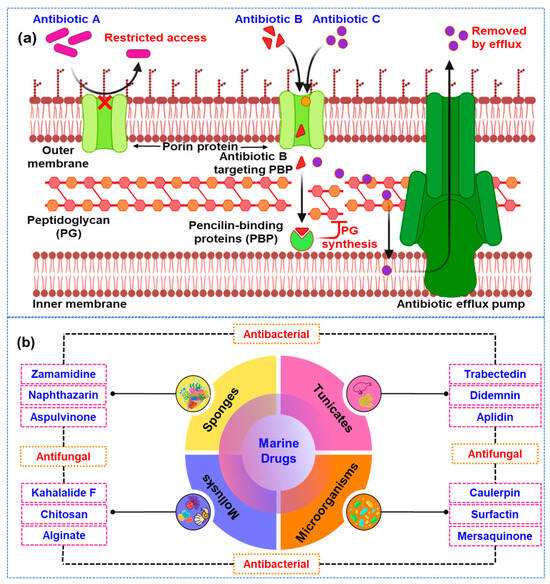
Figure 1.
(a) The antibiotic resistance mechanisms of currently available antibiotics against pathogenic microorganisms. The image, created with BioRender.com (accessed on 25 May 2024), illustrates various mechanisms by which pathogens develop resistance, including alteration of antibiotic targets, enzymatic degradation of antibiotics, and efflux pump activation. Each mechanism is depicted with representative examples of affected antibiotics and the corresponding microbial adaptations, (b) Different sources of marine drugs with their potential compounds for antibacterial and antifungal properties. These marine-derived compounds play a crucial role in the development of new therapeutic agents to combat microbial infections.
The vast and largely untapped biodiversity of marine environments offers a promising avenue for discovering new antimicrobial agents [10]. Marine organisms, especially microorganisms, have evolved unique biochemical pathways to survive the extreme conditions of their habitats. These environments include high pressure, low light, varying temperatures, and high salinity, which have driven marine life to develop novel adaptations [11,12]. As a result, these organisms produce a wide array of bioactive compounds not typically found in terrestrial environments. Marine microorganisms, in particular, have become a focal point of research due to their ability to synthesize secondary metabolites with remarkable biological activities [13,14]. These secondary metabolites are not directly involved in the normal growth, development, or reproduction of the organism. Instead, they often assist ecological functions, such as defense mechanisms against predators, competition with other microorganisms, or communication within their communities [15]. The unique properties of these compounds have garnered significant interest for their potential therapeutic applications. Among the various bioactive compounds produced by marine microorganisms, several classes stand out for their antimicrobial properties [16]. For example, peptides are short chains of amino acids that can disrupt microbial cell membranes, leading to cell death [17,18]. Polyketides are another class of compounds known for their structural diversity and potent biological activities [19]. Alkaloids, which contain nitrogen atoms, have been found to possess a range of pharmacological effects, including antimicrobial activity [20]. Terpenoids, derived from isoprene units, and polysaccharides are long carbohydrate molecules, which also contribute to the arsenal of bioactive compounds with potential antimicrobial applications (Figure 1b) [21,22].
Recent research has increasingly turned to marine microbial compounds for potential antibiotics. This shift is driven by the unique, high-stress environments of marine habitats, which promote the production of metabolites distinct from those in terrestrial organisms, and advancements in bioprospecting technologies that enable the collection of samples from previously inaccessible areas like deep-sea vents and polar regions [23,24,25]. For example, secondary metabolites from marine-derived Streptomyces sp. have shown significant activity against drug-resistant strains of Enterococcus faecium, Staphylococcus aureus, and Mycobacterium tuberculosis [26,27]. In the fight against AMR, exploring marine natural products is particularly critical due to the urgent need for new antimicrobial agents with unique mechanisms of action [28]. Compounds such as nocardiopsistins, stremycins, and chlororesistoflavins from marine sources have demonstrated effectiveness against methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococci (VRE), and multidrug-resistant M. tuberculosis (MDR-TB) [26,27,29]. These marine-derived compounds not only offer new mechanisms of action but also possess structural features that are absent in conventional antibiotics, making them less likely to encounter cross-resistance [30,31]. The exploration and characterization of such compounds are essential for expanding our target of antimicrobial agents and providing unique solutions to combat AMR.
Marine compounds contain unusual ring systems, halogenated compounds, highly branched molecules, and sulfated polysaccharides, which offer different modes of action compared to traditional antibiotics and also it can reduce the likelihood of cross-resistance [30]. Halogen atoms are able to establish non-covalent interactions with biomolecules, ensuring binding, specificity and enhanced drug efficacy against AMR pathogens [1]. Some marine-derived compounds can also enhance the efficacy of existing antibiotics by inhibiting bacterial efflux pumps, a common resistance mechanism. This synergistic effect not only restores the potency of conventional antibiotics but also broadens their spectrum of activity. For instance, an antibiotic that was previously ineffective against a particular strain of bacteria due to resistance mechanisms might regain its effectiveness when used in combination with a marine-derived efflux pump inhibitor [32,33].
While previous reviews address the isolation of marine-derived compounds for antibacterial and antifungal activities [12,13,14,15,16,21,24,25,26], this review stands out by focusing on recent research articles highlighting the extraction or isolation of marine-derived compounds with potential antimicrobial activity against multidrug-resistant (MDR) pathogens. Unlike previous reviews that focus on a single type of compound from specific marine sources, this review provides a detailed summary of compounds derived from various marine sources. It spans recent advancements and research articles from approximately 2010 to 2024, highlighting significant progress in marine-derived compounds as potent antibacterial and antifungal agents. This paper emphasizes the vast potential of marine biodiversity, showcasing marine-derived compounds with unique structures and mechanisms of action against resistant pathogens. It categorizes these compounds based on their chemical structures, including alkaloids, amino acids, peptides, polyketides, naphthoquinones, terpenoids, and polysaccharides, each with distinct mechanisms of action. Additionally, this review details how the unique chemical structures of marine-derived compounds result in different modes of action compared to traditional antibiotics. Furthermore, this review explores the connection between the antimicrobial properties of these compounds and their ecological roles, providing insights into their evolutionary significance and potential applications. Also, this review highlights significant progress and discoveries in marine-derived compounds as potent antibacterial and antifungal agents.
2. Marine-Derived Alkaloids
Marine organisms, such as sponges, tunicates, mollusks, algae, and microorganisms like bacteria and fungi, generate a diverse range of alkaloids [34,35,36,37,38]. These compounds have evolved as part of the organisms’ defense mechanisms, resulting in the unique structures and potent biological activities. Sponges are a prolific source of bioactive compounds, most notably alkaloids, which have garnered significant attention for their potent antibacterial and antifungal activities [39,40]. These sessile invertebrates inhabit diverse marine environments, from shallow reefs to the deep sea, where they have evolved complex chemical defense mechanisms to survive against a myriad of microbial threats. The unique structural diversity of sponge-derived alkaloids underpins their broad-spectrum bioactivity, making them promising candidates for developing new antimicrobial agents [41]. The chemical structure of various marine-derived alkaloids is shown in Figure 2.
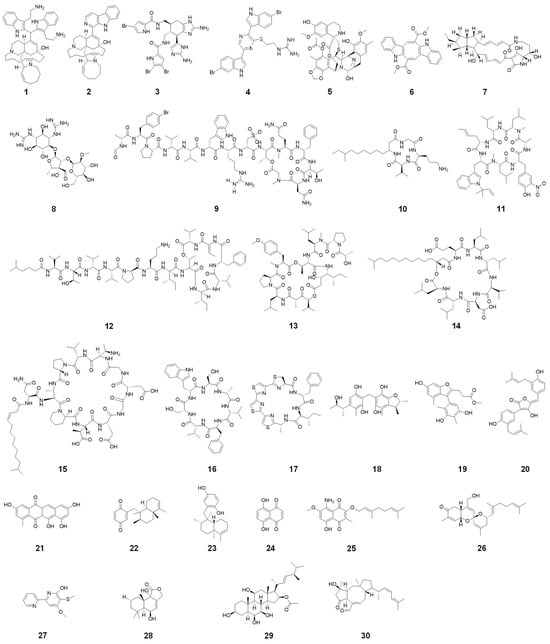
Figure 2.
Chemical structures of zamamidine D (1), manzamine A (2), bromoageliferin (3), dragmacidin G (4), trabectedin (5), caulerpin (6), alteramide A (7), streptomycin (8), halicylindramide A (9), rhodopeptins C1 (10), ilamycin B1 (11), kahalalide F (12), didemnin B (13), surfactin (14), friulimicin B (15), asperversiamide A (16), marthiapeptide A (17), dicitrinone E (18), talarominine A (19), aspulvinones H (20), mersaquinone (21), avarone (22), avarol (23), 5,8-Dihydroxy-1,4-naphthoquinone (24), flaviogeranin B (25), phorbaketal A (26), collismycin C (27), strobilactone A (28), penicisteroid A (29), and ophiobolin K (30).
Takaaki Kubota et al. [42] demonstrated the isolation and analysis of zamamidine D (1). Compound 1 was obtained at a yield of 1.7 mg from 0.68 kg of wet sponge material, which corresponds to 0.00025% of the wet weight. The antimicrobial assays showed that zamamidine D is highly potent, with an MIC of 0.032 mg/mL for E. coli, and 0.008 mg/mL for S. aureus, B. subtilis, and M. luteus, and IC50 values of 0.016 mg/mL for C. albicans and A. niger, and 0.002 mg/mL for C. neoformans. These results underscore the compound’s significant antimicrobial potential. Other notable sponge-derived alkaloids include manzamine A (2) [43], which has shown promising antibacterial activity against M. tuberculosis. Research by Rateb et al. [44] underscores the potential of manzamine A as a lead compound for developing new anti-TB drugs. Alkaloids isolated from marine sponges exhibit a wide range of chemical structures, including pyrrole, quinoline, isoquinoline, and indole derivatives, each contributing to their potent biological activities [45,46]. These compounds are often synthesized as secondary metabolites, serving as chemical defenses to protect the sponges from pathogenic microorganisms and predators. This evolutionary pressure has resulted in the production of alkaloids with highly specialized and effective antimicrobial properties [47,48].
Recently, Pech-Puch et al. [49] isolated eight alkaloids from the sponge Agelas dilatata, exhibiting potent antibacterial activity. Bromoageliferin (3) notably exhibited strong antibacterial effects against P. aeruginosa. Quantitative analyses showed that the MICs for bromoageliferin against different strains of P. aeruginosa ranged from 0.008 to 0.032 mg/mL, highlighting its potency. These results indicate that bromoageliferin holds promise as a lead compound for developing new antibacterial treatments targeting multidrug-resistant pathogens.
The pharmaceutical potential of sponge-derived alkaloids extends beyond their antimicrobial properties. The unique chemical structures of these compounds provide a scaffold for the progress of novel drugs with better efficacy and reduced resistance. For instance, researchers have been exploring the synthetic modification of sponge alkaloids to enhance their stability, bioavailability, and selectivity for microbial targets, paving the way for new therapeutic applications. A study by Hong et al. [50] discusses the potential for synthetic chemistry to unlock new derivatives of sponge alkaloids with enhanced pharmacological properties.
Moreover, the ecological role of alkaloids in marine sponges underscores their importance in maintaining marine biodiversity and health. By producing bioactive compounds, sponges contribute to the regulation of microbial populations in their habitats, preventing the overgrowth of harmful pathogens and promoting a balanced ecosystem. This ecological function highlights the potential benefits of preserving marine biodiversity, as the loss of sponge species could mean the loss of valuable bioactive compounds with significant medical applications [51]. Leal et al. [52] emphasize the ecological significance of sponge-derived alkaloids and the need for conservation efforts to protect these valuable marine resources.
Recent advancements in marine biotechnology have facilitated the sustainable extraction and synthesis of sponge alkaloids, reducing the environmental impact of bioprospecting. Advances in aquaculture and microbial fermentation techniques are enabling the large-scale production of these compounds, making them more accessible for pharmaceutical research and development. A review by Li et al. [53] discusses the potential of microbial symbionts in sponges as an alternative source of bioactive alkaloids, highlighting innovative approaches to sustainable drug discovery. Compound 4 shown in Figure 2 is dragmacidin G. Dragmacidin G isolated from a deep-water sponge of the genus Spongosorties exhibited broad-spectrum antibacterial activity against MRSA and M. tuberculosis [54].
Marine tunicates, commonly known as sea squirts, are a notable source of bioactive alkaloids with significant antibacterial and antifungal properties [55,56]. Prominent tunicate-derived alkaloids include trabectedin (5), which has been noted for its antifungal activity, highlighting its potential for new antifungal therapeutics [57]. Additionally, Blunt et al. [40] discussed the ecological importance of these alkaloids and their role in the chemical defense of tunicates, reinforcing the value of marine biodiversity in discovering new bioactive compounds. Moreover, researchers explored the biosynthetic pathways of tunicate alkaloids, providing insights into their complex structures and potential applications in synthetic biology. Recently, several researchers demonstrated the use of microbial fermentation techniques to sustainably produce marine-derived alkaloids, which reduces the environmental impact typically associated with traditional bioprospecting. Their study highlights the potential of using engineered microorganisms to replicate the biosynthesis pathways of these marine compounds, thus enabling large-scale production without the need to harvest marine organisms directly [58,59].
Additionally, Xiong et al. [60] reviewed the potential of genome mining to identify new alkaloid-producing genes, highlighting innovative strategies for drug discovery. These advancements not only improve access to these compounds for pharmaceutical research but also contribute to marine ecosystem conservation [61]. Continued exploration of marine alkaloids holds great promise for developing novel antibacterial and antifungal agents, addressing the urgent need for new treatments in combating infectious diseases.
Marine microorganisms, including algae, bacteria, and fungi, are prolific producers of bioactive alkaloids with significant antibacterial and antifungal properties. Algae-derived alkaloids, particularly from green algae like Caulerpa sp., have demonstrated remarkable antibacterial activity against several pathogens, including multi-drug resistant strains. For instance, caulerpin (6), an indole alkaloid derived from Caulerpa, has demonstrated effectiveness against E. coli, S. aureus, Streptococcus sp., and Salmonella sp. The antimicrobial activity of caulerpin was assessed, revealing a MIC of 5.25 mg/mL for E. coli, S. aureus, and Salmonella sp. However, the MIC value against Streptococcus sp. was higher, at 15.50 mg/mL [62].
Alteramides are a general term for a class of compounds, and compound 7 shown in Figure 2 is alteramide A [63]. Additionally, marine bacteria, particularly those from the genera Streptomyces produce alkaloids like streptomycin (8), which exhibit extensive antibacterial and antifungal activities [64]. For example, four indole alkaloids, designated streptoindoles A–D, were obtained from Streptomyces sp. ZZ1118, which was cultured on a rice solid medium derived from a gut sample of marine shrimp (Penaeus sp.). Streptoindole C showed strong inhibition against E. coli and C. albicans, with a MIC of 0.007 mg/mL. Streptoindole D displayed weak activity solely against MRSA with an MIC of 0.025 mg/mL. Streptoindoles A and B were effective against all three pathogens, with MIC values ranging from 0.007 to 0.025 mg/mL [65]. These compounds often disrupt essential cellular processes in pathogens, such as protein synthesis, and DNA replication.
Marine-derived fungi also contribute significantly to the arsenal of bioactive alkaloids with antimicrobial properties. Species from marine environments, such as Penicillium and Aspergillus, produce various alkaloids which have shown strong antifungal activities against C. albicans and A. niger [66,67]. The ecological and biochemical diversity of marine organisms presents a valuable resource for developing novel antibacterial and antifungal agents, addressing the growing challenge of antimicrobial resistance. Their unique properties and bioactivities, derived from the challenging marine environments, make them promising candidates for addressing antibiotic resistance and other infectious diseases.
3. Marine-Derived Amino Acids
Marine-derived amino acids represent a rapidly growing area of research in the quest for new antimicrobial agents [68]. These amino acids are produced by a variety of marine organisms, including bacteria, fungi, and algae, and have shown significant antibacterial and antifungal activities [51]. Recent studies have demonstrated the effectiveness of these compounds in combating resistant microbial strains. Halicylindramides are a general term for a class of compounds, and compound 9 shown in Figure 2 is halicylindramide A [69]. Halicylindramides isolated from the Japanese marine sponge H. cylindruta exhibited in vitro antifungal activity against M. ramanniana at a concentration of 0.0075 mg/disk. Notably, the macrocyclic structure of compounds is crucial for their antifungal properties [70]. Rhodopeptins are a general term for a class of compounds, and compound 10 shown in Figure 2 is Rhodopeptins C1 [71]. Recently, Rhodopeptins C1, C2 C3, C4, and B5 were isolated from Rhodococcus sp. These antifungal cyclic lipotetrapeptides consist of a β-amino acid and three standard α-amino acids. The Rhodopeptin exhibited in vitro antifungal activity against C. albicans with MIC between 0.00125 and 0.005 mg/mL, and against C. neoformans with an MIC values ranging from 0.00063 to 0.00125 mg/mL [72].
In addition to their antifungal properties, marine-derived amino acids have demonstrated significant antibacterial activities. Four cyclic heptapeptides, identified as L-156,373 and its derivatives, were obtained from a marine Streptomyces sp. culture. These heptapeptides showed notable activity against pathogens including S. aureus, MRSA, B. Calmette-Guérin, and B. subtilis, with MIC values ranging from 0.00025 to 0.00125 mg/mL [73]. The continued exploration of marine-derived amino acids and their conjugates could lead to the identification of novel antimicrobial agents with specific activities against resistant bacterial and fungal pathogens. Furthermore, advancements in cultivation and chemical profiling techniques, as demonstrated by the integrated strategy used in these studies, are crucial for the effective discovery and optimization of these bioactive compounds.
4. Marine-Derived Peptides
Marine-derived peptides are emerging as potent candidates in combating bacterial and fungal infections, especially given the increasing challenge of AMR [74]. Ilamycins are a general term for a class of compounds, and the name of compound 11 is ilamycin B1 [75]. Cyclic oligopeptides such as ilamycins, sourced from marine bacteria like Streptomyces atratus, have shown considerable effectiveness against drug-resistant pathogens including M. tuberculosis [76]. Notable examples include kahalalide F (12), which is effective against C. albicans, and surfactin, which targets MRSA [77].
Cyclic oligopeptides are a class of peptides consisting of 2–20 amino acids arranged in a cyclic structure. These peptides are primarily synthesized by non-ribosomal peptide synthetases, which contribute to their structural diversity and biological activity. One notable example is the ilamycin family, which includes ilamycin B1–F and ilamycins G–R. These peptides are produced by the marine-derived bacterium S. atratus and have shown potent activity against M. tuberculosis. The antimicrobial activity of ilamycins is ascribed to their ability to disrupt bacterial cell walls, making them effective against drug-resistant strains of M. tuberculosis [78]. Recent research by Wang et al. [24] has highlighted the structural and functional diversity of cyclic oligopeptides derived from marine microorganisms. The study discusses how modifications in the side chains of amino acids can significantly influence the antimicrobial potency of these peptides. For instance, the presence of specific side chain modifications in ilamycins have been shown to enhance their activity against M. tuberculosis, indicating the potential for structural optimization to improve therapeutic efficacy [79].
Cyclic depsipeptides are another important class of marine-derived peptides known for their potent antimicrobial properties [80,81]. These peptides contain ester bonds in addition to amide bonds, which contribute to their unique structural characteristics. Compound 13 shown in Figure 2 is didemnin B [82]. Compounds 12 and 13 has demonstrated significant antifungal activity against C. albicans [83,84]. The mechanism of action involves disrupting the fungal cell membrane, leading to cell lysis and death.
Cyclic lipopeptides are characterized by the presence of a lipid tail attached to a cyclic peptide core. These compounds exhibit strong surfactant properties, which enhance their ability to disrupt microbial cell membranes [24]. Marine-derived cyclic lipopeptides such as surfactin (14) produced by Bacillus sp., have shown remarkable antibacterial and antifungal activities. Surfactin has been effective against a range of Gram-positive bacteria, including MRSA. The antimicrobial action of surfactin involves the insertion of the lipid tail into the bacterial cell membrane, leading to increased membrane permeability and cell death [85]. Friulimicin B (15) is a natural cyclic lipopeptide composed of eleven amino acids, synthesized by the Actinoplanes friuliensis. It exhibits antibacterial activity against a broad range of Gram-positive bacteria, including antibiotic-resistant pathogens [86]. Asperversiamides are cyclic heptapeptides and the name of compound 16 is Asperversiamide A. Asperversiamide A, B and C extracted from a coral-derived fungal strain Aspergillus versicolor CHNSCLM-0063, exhibiting strong anti-M. marinum activity, with asperversiamide B also showing moderate anti-TB activity [87,88]. Asperheptatides A–B are structurally closest to asperversiamide B and also shows moderate anti-TB activity [89]. By using asperversiamide A as a core structure, various cinnamic acid groups were introduced onto the hydroxyl group of the serine side chain, resulting in cinnamic acid derivatives that exhibited an eight-fold increase in anti-TB action. This process also confirmed the anti-TB efficacy of the cinnamic acid structure [88].
The cyclic peptide marthiapeptide A (17) isolated from the deep-sea-derived Marinactinospora thermotolerans SCSIO 00652 showed antibacterial MICs of 0.004, 0.002, 0.002, and 0.008 mg/mL for B. subtilis, M. luteus, B. thuringiensis, and S. aureus, respectively, and showed no activity against Gram-negative bacteria like E. coli [90,91]. Marine-derived peptides often exhibit synergistic effects when combined with conventional antibiotics and antifungals. This synergy can enhance the overall antimicrobial efficacy and reduce the likelihood of resistance development. Some peptides inhibit bacterial efflux pumps, which are responsible for expelling antibiotics from bacterial cells. By blocking these pumps, peptides can restore the potency of existing antibiotics and expand their spectrum of activity. Additionally, marine peptides may target multiple bacterial pathways simultaneously, reducing the chances of resistance [92].
Understanding the structure-activity relationships (SARs) of marine-derived peptides is crucial for optimizing their antimicrobial properties. Structural modifications, such as altering amino acid residues or adding functional groups, can significantly impact the activity and stability of these peptides [93]. For instance, modifications to the side chains of amino acids in ilamycins have been shown to enhance their activity against M. tuberculosis. Similarly, altering the lipid tail length in cyclic lipopeptides can improve their ability to penetrate microbial membranes. A wide-ranging review by Wang et al. [24] discusses the SAR of various marine-derived peptides, emphasizing the importance of specific structural features in determining their antimicrobial efficacy. Their review highlights that fine-tuning the peptide structure can lead to the development of more potent antimicrobial properties.
5. Marine-Derived Polyketide
Marine-derived polyketides represent a promising frontier in the search for new antimicrobial agents [92]. These compounds, isolated from marine organisms such as bacteria, fungi, and algae, have shown significant potential due to their distinctive characteristics and various ways of combating pathogens. Unlike many terrestrial antibiotics, marine-derived polyketides often possess distinctive chemical features that reduce the likelihood of cross-resistance with existing drugs. Marine-derived polyketides including macrocyclic lactones, polyenes, and polyethers contribute to their potent biological activities and their ability to target pathogens in novel ways [92]. The antimicrobial mechanisms of marine-derived polyketides are varied and can include the inhibition of cell wall synthesis, disruption of membrane integrity, interference with protein and nucleic acid synthesis, and inhibition of critical metabolic pathways. This diversity in modes of action not only enhances their effectiveness but also reduces the risk of resistance development.
Dicitrinones are a general term for a class of compounds, and the compound 18 in Figure 2 is dicitrinone E [94]. Recently, dicitrinones were extracted from the starfish-associated Penicillium sp. GGF 16-1-2. These compounds demonstrated potent antifungal properties against Colletotrichum gloeosporioides, with LD50 values between 0.00958 mg/mL and 0.01614 mg/mL [95]. Five new polyketides (two chromones, two phenyl derivatives, and one tandyukusin derivative) were isolated alongside five known ones. Among these, few showed significant antifungal activities against Penicillium italicum. The study underscores the potential of mangrove-derived fungi in yielding bioactive compounds with significant antimicrobial properties [96]. A study by Song et al. [97] focused on aromatic polyketides from the endozoic fungus Talaromyces minioluteus, associated with deep-sea cold-seep mussels. Researchers identified five new aromatic polyketides, including a unique benzofuran derivative and four chromone analogs. Compound 19 shown in Figure 2 is talarominine A [97]. Talarominine A and its derivatives demonstrated notable antibacterial activity, particularly against MRSA and P. aeruginosa. The study emphasized the distinctive structures of these polyketides, which are essential for developing new antibacterial agents aimed at combating MRSA. Van Anh et al. [98] explored rifamycin-related polyketides from the marine-derived bacterium Salinispora arenicola, identifying eight polyketides, including three new derivatives. The rifamycin-related compounds exhibited moderate cytotoxic activity against various cancer cell lines, suggesting potential antibacterial applications due to their ability to inhibit bacterial RNA synthesis.
Aspulvinones are a general term for a class of compounds, and the compound 20 should be aspulvinones H [99]. Recently, four antimicrobial compounds named aspulvinones B’, H, R, and S were isolated from A. flavus KUFA1152, sourced from the marine sponge Mycale sp. These compounds demonstrate significant antibacterial properties against MREF ATCC 29212 and MRSA S. aureus ATCC 29213. In addition to their antibacterial effects, these compounds also prevent biofilm formation by these strains. The MIC values for aspulvinones B’, H, R, and S range between 0.004 and 0.064 mg/mL [100]. A study by Koch et al. [101] isolated 2-carboxymethyl-3-hexylmaleic acid anhydride from the endozoic fungus Aspergillus tubingensis OY907, found in the Mediterranean marine sponge Ircinia variabilis. This compound demonstrated inhibitory activity against Neurospora crassa. Tan et al. [102] emphasized the importance of marine-derived polyketides by identifying novel compounds from the marine-derived fungus Penicillium species, showing potent antifungal and antibacterial activities. These compounds were particularly effective against C. albicans and S. aureus, demonstrating broad-spectrum antimicrobial potential. The study suggested these polyketides could be developed into therapeutic agents for treating bacterial and fungal infections. In addition to their antibacterial properties, marine-derived polyketides show promise in disrupting biofilms, which protect bacterial communities from antibiotics, making infections difficult to treat. Wibowo et al. [26] highlighted the potential of marine-derived bacterial secondary metabolites including polyketides in antibiotic and antibiofilm applications. Their review underscores the importance of marine bacteria as a valuable resource for discovering new antimicrobial agents.
6. Marine-Derived Naphthoquinones
Marine-derived naphthoquinones are characterized by their naphthalene ring structure with two ketone groups. Isolated from various marine organisms, including sponges, algae, and bacteria, these compounds exhibit a wide range of biological activities [103]. Marine-derived naphthoquinones demonstrate a wide range of antimicrobial activities. They often disrupt the electron transport chain in microbial cells, hindering ATP production and causing cell death. Some naphthoquinones also intercalate into DNA, blocking replication and transcription, which prevents microorganisms from reproducing and functioning properly. Additionally, these compounds can induce the formation of ROS within microbial cells, leading to oxidative damage to proteins, lipids, and DNA, and resulting in cellular dysfunction and death [51,104,105].
Mersaquinone (21), a newly discovered derivative of tetracene, was isolated from Streptomyces sp. EG1, obtained from sediment off Egypt’s North Mediterranean coast. The chemical structure of mersaquinone was meticulously identified using HRESIMS, IR spectroscopy, and both one-dimensional and two-dimensional NMR spectroscopy. This substance demonstrated the ability to suppress the growth of the MRSA strain TCH1516, showing a MIC of 0.00336 mg/mL [106]. Marine sponges are rich sources of naphthoquinones. Compounds such as avarone (22) and avarol (23), isolated from Dysidea avara, have demonstrated potent antibacterial and antifungal activities. These compounds have shown effectiveness against marine bacteria (C. marina, M. stanieri, V. fischeri, and P. haloplanktis) and marine fungi (H. mediosetigera, A. cruciatus, L. uniseptate, and M. pelagica) [107]. Naphthazarins are a general term for a class of compounds, and compound 24 shown in Figure 2 is 5,8-Dihydroxy-1,4-naphthoquinone [108]. These compounds are mostly effective against a range of bacterial and fungal pathogens. Recent studies have demonstrated the significant antibacterial potential of marine-derived naphthoquinones, such as those isolated from Streptomyces and Talaromyces sp. For example, Park et al. [109] identified two new naphterpin derivatives from Streptomyces sp. CNQ-509, which exhibited potent antibacterial activity against several pathogens. The study highlighted the role of the naphthoquinone moiety in the antibacterial efficacy of these compounds. Flaviogeranins are a general term for a class of compounds, and compound 25 shown in Figure 2 is flaviogeranin B. Shen et al. [110] reported the isolation of a group of naphthoquinone-containing compounds, specifically seven flaviogeranin (25) congeners, including three novel compounds, from Streptomyces sp. B9173. Their structures were determined by a combination of spectroscopic techniques, including 1D and 2D NMR, and high-resolution mass spectrometry. The compounds flaviogeranin B and flaviogeranin C2 exhibited potent inhibitory activity against S. aureus and M. smegmatis, with MIC values ranging from 0.005 to 0.009 mg/mL, comparable to the MIC of the positive control, erythromycin.
Phorbaketals are a general term for a class of compounds, and compound 26 shown in Figure 2 is phorbaketals A. Kim et al. [111] explored the antibiofilm properties of phorbaketals (26) extracted from the Korean marine sponge Phorbas sp. against S. aureus, including MRSA. The study isolated six different phorbaketals (phorbaketal A, A acetate, B, B acetate, C, and C acetate) and tested for their ability to prevent biofilm formation. Specifically, compounds B and C demonstrated notable antibiofilm activity without bactericidal effects, thereby minimizing the potential for resistance. These compounds also suppressed the production of staphyloxanthin, a virulence factor aiding S. aureus in evading the immune system. The antibiofilm action was attributed to the downregulation of crucial genes associated with biofilm formation and virulence, including α-hemolysin (hla) and nuclease (nuc1). Consequently, phorbaketals B and C show promise as potential antibiofilm agents for treating infections caused by drug-resistant bacteria. Another recent study by Lee et al. [112] demonstrated the antibiofilm activity of collismycin C (27), a compound isolated from the marine bacterium Streptomyces sp. MC025, against MRSA. The study involved screening 79 Micronesian marine microorganisms for their ability to inhibit S. aureus biofilm formation. Among the isolated compounds, collismycin C was the most effective, inhibiting MRSA biofilm development by over 90% at 0.05 mg/mL. The biofilm suppression activity of collismycin C was attributed to its iron-chelating properties, which interfere with the biofilm formation process. In addition to their antibacterial properties, marine-derived naphthoquinones also exhibit strong antifungal activities, addressing the critical need for new antifungal agents amidst rising drug resistance. Liu et al. [113] studied naphthoquinone derivatives from the mangrove-derived endophytic fungus Talaromyces sp., identifying twelve 1,4-naphthoquinone derivatives, including two new compounds. These derivatives showed significant antifungal effects against C. albicans and A. niger, with some compounds demonstrating higher potency than standard antifungal agents. The study emphasized the dual functionality of these naphthoquinones in addressing both bacterial and fungal infections.
7. Marine-Derived Terpenoids
Marine-derived terpenoids are characterized by their diverse and complex structures, often consisting of multiple isoprene units. Marine-derived terpenoids frequently display unique modifications such as halogenation, glycosylation, and unusual ring systems. These modifications arise from the distinctive biosynthetic pathways found in marine environments, contributing to the enhanced biological activities of these compounds [114]. Marine-derived terpenoid derivatives are increasingly recognized for their potent antibacterial and antifungal activities, making them a promising frontier in the development of new antimicrobial agents. Their unique bioactivities and mechanisms of action make them valuable candidates for novel therapies to combat resistant microbial strains and biofilm-associated infections. Marine-derived terpenoids can disrupt microbial cell membranes, prevent cell wall synthesis, restrict protein and nucleic acid synthesis, and inhibit critical metabolic pathways. Additionally, some terpenoids can modulate the immune response, enhancing the host’s ability to fight off infections. For instance, compounds like squalene and its derivatives have been shown to disrupt microbial membranes, leading to cell lysis and death.
Terpenoid derivatives isolated from marine sponges have demonstrated strong antimicrobial properties. Compound 28 shown in Figure 2 is strobilactone A [115]. Four drimane-type sesquiterpenoid lactones were extracted from the fungus A. insuetus (OY-207), which was sourced from the Mediterranean sponge Psammocinia sp. These compounds were identified as derivatives of strobilactone A, including a new compound identified as (E)-6-(4′-hydroxy-2′-butenoyl)-strobilactone A. In addition, the known derivatives strobilactone A and (E,E)-6-(6′,7′-dihydroxy-2′,4′-octadienoyl)-strobilactone A, along with 2α,9α,11-trihydroxy-6-oxodrim-7-ene, were also isolated. The antifungal properties of these compounds were tested, revealing that strobilactone A and (E,E)-6-(6′,7′-dihydroxy-2′,4′-octadienoyl)-strobilactone A exhibited mild antifungal activity against the fungus Neurospora crassa [116].
Terpenoids from marine fungi and algae have revealed considerable effectiveness against fungal pathogens like C. albicans and A. niger. Research on the endophytic fungus P. chrysogenum QEN-24s, isolated from the marine red alga Laurenica sp., resulted in the discovery of two new polyoxygenated sterols, penicisteroid A and B, along with the previously known steroid, anicequol. These compounds were evaluated for their antifungal properties. The findings revealed that penicisteroid A (29) exhibited antifungal activity against both A. niger and Alternaria brassicae, while anicequol was active only against A. brassicae. In contrast, penicisteroid B showed no antifungal activity against either of the two fungi [117]. Generally, terpenoid derivatives often disrupt fungal cell membranes or inhibit ergosterol synthesis, a critical component of fungal cell membranes, leading to fungal cell death. This dual functionality underscores the versatility and broad-spectrum potential of marine-derived terpenoids in antimicrobial therapy.
Marine-derived terpenoid derivatives have shown promise in addressing biofilm-associated infections. Terpenoid derivatives have demonstrated the ability to disrupt biofilm formation and maintenance. By targeting the biofilm matrix and preventing the development of new biofilms, these compounds offer a viable solution for treating biofilm-related infections. This characteristic is particularly valuable in medical settings, where biofilm formation on medical devices and implants poses significant challenges. Three novel sesterterpenes belonging to the ophiobolin class were isolated from the marine-derived fungus Emericella variecolor strain GF-10, which was sourced from sediment collected at a depth of 70 meters in the Gokasyo Gulf, Japan. These compounds were identified as ophiobolin K, 6-epi-ophiobolin K, and 6-epi-ophiobolin G. They were found to inhibit the biofilm formation of M. smegmatis, with ophiobolin K (30) demonstrating the highest activity, showing a MIC of 1.84 mg/mL [118].
8. Marine-Derived Polysaccharides
Marine-derived polysaccharides are complex carbohydrates that consist of long chains of monosaccharide units. These polysaccharides often display unique structural features such as sulfation, branching, and acetylation, which contribute to their diverse biological activities. Marine-derived polysaccharides have garnered significant interest for their antibacterial and antifungal properties, leading to their exploration in various industrial applications, from pharmaceuticals to food packaging. Marine-derived polysaccharides can inhibit microbial adhesion and biofilm formation, disrupt cell membranes, and modulate immune responses. Additionally, some marine polysaccharides have been shown to enhance the efficacy of existing antibiotics, potentially reducing the required dosage and minimizing side effects. Recent studies have highlighted the effectiveness of key marine polysaccharides such as chitin, chitosan, cellulose, fucoidan, and alginate. Figure 3 schematically illustrates an overview of the key marine polysaccharides and their sources from marine organisms.
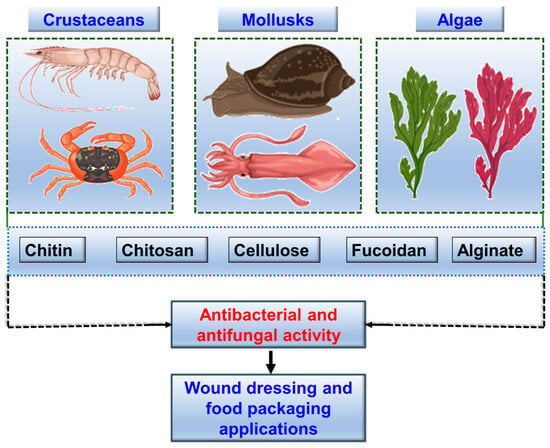
Figure 3.
The sources of selected key biopolymers and their applications. Crustaceans and mollusks provide chitin, chitosan and cellulose. Algae (such as green and red algae) provide cellulose, fucoidan and alginate. These biopolymers exhibit antibacterial and antifungal activities, making them suitable for use in wound dressing and food packaging applications.
Chitin is a polymer consisting of (1→4)-β-linked N-acetyl-D-glucosamine units. It is the most abundant amino-polysaccharide polymer found in nature and ranks as the second most abundant polysaccharide after cellulose. Marine ecosystems, particularly crustacean shells from crabs, shrimp, lobsters, and krill, are considered the primary sources of chitin [119]. A study by Abdel-Rahman isolated highly pure chitin from Brazilian Atlantic Coast shrimp shells by the chemical treatment method. The isolated chitin exhibited antibacterial activity against E. coli by the chemo-luminescence technique. Chitosan, derived from the deacetylation of chitin found in the exoskeletons of crustaceans, has been extensively studied for its antimicrobial properties [120,121]. Its polycationic nature allows chitosan to interact with negatively charged bacterial membranes, causing cell leakage and death. Recent research has demonstrated the effectiveness of chitosan in food packaging and biomedical applications. A recent study by Mohammadi et al. [122] extracted chitosan from shrimp waste using different methods: conventional extraction and microwave-assisted extraction. The antibacterial action of the extracted chitosan was evaluated using an agar disc diffusion assay. The chitosan obtained through conventional extraction displayed the highest antibacterial zone of inhibition (mm) against Listeria monocytogenes (9.48), E. coli (8.79), and S. Typhimurium (8.57). On the other hand, chitosan produced via microwave-assisted extraction showed superior activity against S. aureus (8.05) and E. coli (8.37) with a similar antibacterial property against L. monocytogenes (6.52) and S. Typhimurium (7.34). A recent study by Verma et al. [123] demonstrated a process of extracting chitosan from the shells of horse mussels, a common waste product from fisheries. The extracted chitosan displayed a degree of acetylation of 57.43%, making it a suitable biopolymer for biomedical applications. The research highlights the antimicrobial efficacy of the chitosan, particularly against E. coli and B. subtilis, demonstrating its potential as a valuable material in antimicrobial applications.
Marine-derived cellulose and nanocellulose from algae such as Ulva lactuca shows significant antibacterial properties [124]. This cellulose is a promising antibacterial agent against K. pneumonia (ST627), S. aureus (ATCC6538), E. coli (ATCC25922) and coagulase-negative staphylococci. It disrupts bacterial membranes and adsorbs onto cells, effectively inhibiting growth. Its biocompatibility and antibacterial nature also make it suitable for wound dressings and food packaging, promoting sterility and extending shelf life [125,126,127]. Ongoing research aims to enhance these properties, making marine-derived cellulose a sustainable antibacterial material. Fucoidan is a type of sulfated polysaccharide found mainly in various species of brown seaweed and some marine invertebrates [128,129]. Fucoidan is primarily composed of fucose, a type of sugar, along with sulfate groups. It may also contain other monosaccharides like glucose, galactose, and mannose. Recent studies have shown its effectiveness against various bacterial pathogens, including S. aureus, E. coli, L. monocytogenes, and Salmonella enterica serovar Typhimurium. The antibacterial activity of fucoidan is attributed to its ability to disrupt bacterial cell walls and membranes, inhibit bacterial adhesion and biofilm formation, and interfere with bacterial metabolism. Its sulfate groups interfere with the replication of viruses and the growth of bacteria. Additionally, fucoidan has shown potential against fungal pathogens such as C. albicans by impairing cell wall integrity and preventing adhesion and biofilm formation [127,129]. Carrageenan, extracted from algae, is known for its antibacterial and antifungal properties [130]. It forms protective barriers on surfaces, inhibiting the adherence and penetration of pathogens. This polysaccharide is widely used in the food industry as a natural preservative to enhance product shelf life by preventing microbial growth [130].
Alginate is a naturally occurring biopolymer derived primarily from the cell walls of brown seaweed and contain linear copolymers of α-L-guluronic acid and β-D-mannuronic acid. Alginate forms gels in the presence of calcium ions and exhibits notable antimicrobial properties [131]. Alginate-based wound dressings are highly absorbent and maintain a moist atmosphere favorable to healing while protecting against microbial infections. In the food industry, alginate is used as a thickener and stabilizer, with its antimicrobial properties enhancing food safety and quality [132]. Recent developments in composite films combining marine-derived polysaccharides have shown enhanced antimicrobial efficacy. For instance, O-carboxymethyl and pectin films, when combined with neem extracts, demonstrated significant antimicrobial activity and improved tensile properties [133]. Such composite films are biodegradable and offer a sustainable solution for food packaging and other applications requiring antimicrobial properties.
Overall, a variety of marine-derived substances have demonstrated potential as antibacterial and antifungal agents. Table 1 provides a comprehensive overview of various marine-derived compounds, including their type, sources, properties, and modes of action.

Table 1.
List of marine-based drugs, outlining their characteristics and methods of action.
9. Conclusions
In conclusion, the rising threat of antimicrobial resistance (AMR) necessitates the discovery of novel antimicrobial agents. Our review highlights the untapped potential of marine environments, which harbor a diverse array of bioactive compounds. Marine-derived alkaloids, peptides, polyketides, naphthoquinones, terpenoids, and polysaccharides exhibit significant antibacterial and antifungal properties against resistant pathogens. Notable compounds such as zamamidine D, manzamine A, ilamycins, and kahalalide F show particular promise. The unique mechanisms of action and potential for synergistic effects with existing antibiotics make natural marine products a valuable resource in the fight against AMR. Future research should focus on the exploration and sustainable utilization of these marine-derived compounds to develop new, effective antimicrobial therapies.
10. Future Prospectives
The future of marine-derived antimicrobial research lies in several key areas:
- (a)
- Advanced Bioprospecting and Sustainable Harvesting
Advancements in bioprospecting technologies, including accessing previously unexplored marine habitats such as deep-sea vents and polar regions, can lead to the discovery of novel bioactive compounds. Emphasizing sustainable harvesting methods, including microbial fermentation and aquaculture, will minimize environmental impact and ensure a continuous supply of these valuable compounds.
- (b)
- Synthetic Biology and Genome Mining
Synthetic biology offers the potential to enhance the production and structural diversity of marine-derived compounds. Genome mining techniques can identify new biosynthetic pathways and gene clusters responsible for producing bioactive metabolites, leading to the discovery of new compounds and the optimization of existing ones for improved antimicrobial efficacy.
- (c)
- Structural Optimization and Drug Development
Understanding the SAR of marine-derived compounds is crucial for optimizing their antimicrobial properties. Structural modifications, such as altering amino acid residues or functional groups, can enhance the potency, stability, and bioavailability of these compounds. Collaborative efforts between chemists, biologists, and pharmacologists are essential to translate these compounds into clinically viable drugs.
- (d)
- Combination Therapies
Exploring the synergistic effects of marine-derived compounds with conventional antibiotics and antifungals can enhance overall antimicrobial efficacy and reduce resistance development due to different targets of drugs. Combination therapies could be particularly effective against multidrug-resistant pathogens and biofilm-associated infections.
- (e)
- Ecological and Conservation Considerations
Preserving marine biodiversity is paramount for maintaining the source of these bioactive compounds. Conservation efforts should focus on protecting marine ecosystems and mitigating the impacts of climate change, pollution, and overfishing. Additionally, understanding the ecological roles of these compounds can provide insights into their evolutionary significance and potential applications.
- (f)
- Clinical Trials and Regulatory Approval
Rigorous preclinical and clinical trials are necessary to evaluate the safety, efficacy, and pharmacokinetics of marine-derived compounds. Establishing regulatory frameworks will facilitate the approval and commercialization of new marine-based antimicrobial agents.
- (g)
- Toxicity Issues
Marine-derived compounds hold therapeutic potential but pose toxicity risks such as cytotoxicity, neurotoxicity, hepatotoxicity, and immunotoxicity. Future strategies to mitigate these issues include advanced bioprospecting to identify safer compounds, synthetic biology for producing less toxic analogs, and structural optimization to improve selectivity. Rigorous preclinical and clinical testing, combination therapies to reduce doses, and comprehensive regulatory frameworks are essential.
Author Contributions
Conceptualization, D.B. and J.L.; writing-original draft preparation, D.B.; writing-review and editing, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (2021R1A2C1008368).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Faleye, O.S.; Boya, B.R.; Lee, J.H.; Choi, I.; Lee, J. Halogenated antimicrobial agents to combat drug-resistant pathogens. Pharmacol. Rev. 2024, 76, 90–141. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Lee, J.H.; Ma, J.Y.; Tan, Y.; Lee, J. Antivirulence activities of retinoic acids against Staphylococcus aureus. Front. Microbiol. 2023, 14, 1224085. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Imani, S.; Zhou, A.; Zhao, Y.; Du, L.; Deng, S.; Li, J.; Wang, Q. Combatting resistance: Understanding multi-drug resistant pathogens in intensive care units. Biomed. Pharmacother. 2023, 167, 115564. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pradhan, D.; Halder, J.; Biswasroy, P.; Rai, V.K.; Dubey, D.; Kar, B.; Ghosh, G.; Rath, G. Metal nanoparticles against multi-drug-resistance bacteria. J. Inorg. Biochem. 2022, 237, 111938. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, P.; Jiang, C.; Cui, P.; Zhang, S. Antibacterial activity and modes of action of phosvitin-derived peptide pt5e against clinical multi-drug resistance bacteria. Fish Shellfish Immunol. 2016, 58, 370–379. [Google Scholar] [CrossRef] [PubMed]
- UN. International Agencies and Experts New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. WHO. 2019. Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis. (accessed on 10 May 2024).
- Li, T.; Wang, Z.; Guo, J.; de la Fuente-Nunez, C.; Wang, J.; Han, B.; Tao, H.; Liu, J.; Wang, X. Bacterial resistance to antibacterial agents: Mechanisms, control strategies, and implications for global health. Sci. Total Environ. 2023, 860, 160461. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Pierce, M.L.; Howe, K.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2018: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Pharmacol. Res. 2022, 183, 106391. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.R.; Buckley, B.A.; Eppley, S.M.; Reysenbach, A.L.; Stedman, K.M.; Wagner, J.T. Life on the edge—The biology of organisms inhabiting extreme environments: An introduction to the symposium. Integr. Comp. Biol. 2016, 56, 493–499. [Google Scholar] [CrossRef][Green Version]
- Giordano, D. Bioactive molecules from extreme environments. Mar. Drugs 2021, 19, 642. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Sheikh, M.A.; Ubaid, M.; Chauhan, P.; Kumar, K.; Choudhary, S. Comprehensive exploration of marine algae diversity, bioactive compounds, health benefits, regulatory issues, and food and drug applications. Meas. Food 2024, 14, 100163. [Google Scholar] [CrossRef]
- Chinnathambi, A.; Salmen, S.H.; Al-Garadi, M.A.; Wainwright, M.; Ali Alharbi, S. Marine actinomycetes: An endless source of potentially therapeutic novel secondary metabolites and other bioactive compounds. J. King Saud Univ. Sci. 2023, 35, 102931. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Pharmaceutically active secondary metabolites of marine Actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Song, Z.; Hou, Y.; Liu, S.; Li, X.; Yang, Q.; Wu, S. Secondary metabolites of the genus Nigrospora from terrestrial and marine habitats: Chemical diversity and biological activity. Fitoterapia 2022, 161, 105254. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, X.; Teng, D.; Mao, R.; Hao, Y.; Yang, N.; Chen, H.; Wang, X.; Wang, J. Improved antibacterial activity of a marine peptide-n2 against intracellular salmonella typhimurium by conjugating with cell-penetrating peptides-blfcin6/tat11. Eur. J. Med. Chem. 2018, 145, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, S.; Chugh, A. Engraulisin: A novel marine derived cell penetrating peptide with activity against drug resistant bacteria. Biochim. Biophys. Acta Biomembr. 2024, 1866, 184255. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M. Polyketide-derived macrobrevins from marine macroalga-associated bacillus amyloliquefaciens as promising antibacterial agents against pathogens causing nosocomial infections. Phytochemistry 2022, 193, 112983. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Gou, X.; Jia, J.; Wei, J.; Zheng, M.; Ding, W.; Bi, H.; Wu, B.; Tang, J. New diketopiperazine alkaloid and polyketides from marine-derived fungus Penicillium sp. TW58-16 with antibacterial activity against Helicobacter pylori. Fitoterapia 2022, 156, 105095. [Google Scholar] [CrossRef]
- Gozari, M.; Alborz, M.; El-Seedi, H.R.; Jassbi, A.R. Chemistry, Biosynthesis and biological activity of terpenoids and meroterpenoids in bacteria and fungi isolated from different marine habitats. Eur. J. Med. Chem. 2021, 210, 112957. [Google Scholar] [CrossRef]
- Gopu, M.; Selvam, K. Polysaccharides from marine red algae Amphiroa Rigida and their biomedical potential: An in-vitro study. Biocatal. Agric. Biotechnol. 2020, 29, 101769. [Google Scholar] [CrossRef]
- Mohamed, S.S.; Abdelhamid, S.A.; Ali, R.H. Isolation and Identification of marine microbial products. J. Genet. Eng. Biotechnol. 2021, 19, 162. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gu, L.; Wang, J.; Hu, X.; Wei, B.; Zhang, H.; Wang, H.; Chen, J. Recent advances in polypeptide antibiotics derived from marine microorganisms. Mar. Drugs 2023, 21, 547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. Marine microbial-derived antibiotics and biosurfactants as potential new agents against catheter-associated urinary tract infections. Mar. Drugs 2021, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, J.T.; Bayu, A.; Aryati, W.D.; Fernandes, C.; Yanuar, A.; Kijjoa, A.; Putra, M.Y. Secondary metabolites from marine-derived bacteria with antibiotic and antibiofilm activities against drug-resistant pathogens. Mar. Drugs 2023, 21, 50. [Google Scholar] [CrossRef]
- Sharma, M.; Manhas, R.K. Purification and characterization of actinomycins from Streptomyces strain m7 active against methicillin resistant Staphylococcus aureus and vancomycin resistant Enterococcus. BMC Microbiol. 2019, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.J.; Khan, F.; Tabassum, N.; Cho, K.J.; Kim, Y.M. Marine-derived bioactive materials as antibiofilm and antivirulence agents. Trends Biotechnol. 2024, 2024, S0167–S7799. [Google Scholar] [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-Da-silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as antibiotic delivery systems: A promising nanotechnological strategy against antimicrobial resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Yin, Q.; Liang, J.; Zhang, W.; Zhang, L.; Hu, Z.L.; Zhang, Y.; Xu, Y. Butenolide, a marine-derived broad-spectrum antibiofilm agent against both gram-positive and gram-negative pathogenic bacteria. Mar. Biotechnol. 2019, 21, 88–98. [Google Scholar] [CrossRef]
- Labes, A. Marine resources offer new compounds and strategies for the treatment of skin and soft tissue infections. Mar. Drugs 2023, 21, 387. [Google Scholar] [CrossRef]
- Durães, F.; Szemerédi, N.; Kumla, D.; Pinto, M.; Kijjoa, A.; Spengler, G.; Sousa, E. Metabolites from marine-derived fungi as potential antimicrobial adjuvants. Mar. Drugs 2021, 19, 475. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Maimaitiming, M.; Zhou, Y.; Li, H.; Wang, P.; Liu, Y.; Schäberle, T.F.; Liu, Z.; Wang, C.Y. Discovery of marine natural products as promising antibiotics against Pseudomonas aeruginosa. Mar. Drugs 2022, 20, 192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, Y.; Li, Y.; Song, X.; Wang, R.; Zhang, D. The potential of marine-derived piperazine alkaloids: Sources, structures and bioactivities. Eur. J. Med. Chem. 2024, 265, 116081. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Sun, Y.; Wang, W.; Song, X.; Zhang, D. Chemical diversity and biological activities of marine-derived sulphur containing alkaloids: A comprehensive update. Arab. J. Chem. 2023, 16, 105011. [Google Scholar] [CrossRef]
- Singh, K.S.; Majik, M.S. Bioactive alkaloids from marine sponges. In Marine Sponges: Chemicobiological And biomedical Applications, 1st ed.; Springer: New Delhi, India, 2016; Volume 1, pp. 257–286. [Google Scholar]
- Elissawy, A.M.; Dehkordi, E.S.; Mehdinezhad, N.; Ashour, M.L.; Pour, P.M. Cytotoxic alkaloids derived from marine sponges: A comprehensive review. Biomolecules 2021, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.S.; Zhang, D.; de Souza, F.Z.R.; Liu, L. Recent advances in the synthesis of marine-derived alkaloids via enzymatic reactions. Mar. Drugs 2022, 20, 368. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Li, Y.; Liao, S.; Liu, Y. Exploring Diverse Bioactive secondary metabolites from marine microorganisms using co-culture strategy. Molecules 2023, 28, 6371. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed]
- Rajivgandhi, G.; Kumar, S.N.; Ramachandran, G.; Manoharan, N. Marine sponge alkaloid aaptamine enhances the anti-bacterial and anti-cancer activity against esbl producing gram negative bacteria and hepg 2 human liver carcinoma cells. Biocatal. Agric. Biotechnol. 2019, 17, 628–637. [Google Scholar] [CrossRef]
- Kubota, T.; Nakamura, K.; Kurimoto, S.I.; Sakai, K.; Fromont, J.; Gonoi, T.; Kobayashi, J. Zamamidine D, a manzamine alkaloid from an okinawan Amphimedon sp. marine sponge. J. Nat. Prod. 2017, 80, 1196–1199. [Google Scholar] [CrossRef]
- Pierce, F.; Jefford, C.W.; Bernardinelli, G. Antitumor alkaloid. 1986, 6405, 6404–6405.
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Edrada, R.A.; Ebel, R. Drugs from the Sea—Current status and microbiological implications. Appl. Microbiol. Biotechnol. 2002, 59, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Lagos, R.; Tello, M.; Mercado, G.; Garcia, V.; Monasterio, O. Antibacterial and antitumorigenic properties of microcin e492, a pore- forming bacteriocin. Curr. Pharm. Biotechnol. 2009, 10, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Rohde, S.; Nietzer, S.; Schupp, P.J. Prevalence and mechanisms of dynamic chemical defenses in tropical sponges. PLoS ONE 2015, 10, e0132236. [Google Scholar] [CrossRef] [PubMed]
- Helber, S.B.; Hoeijmakers, D.J.J.; Muhando, C.A.; Rohde, S.; Schupp, P.J. Sponge Chemical defenses are a possible mechanism for increasing sponge abundance on reefs in zanzibar. PLoS ONE 2018, 13, e0197617. [Google Scholar] [CrossRef] [PubMed]
- Pech-Puch, D.; Pérez-Povedano, M.; Martinez-Guitian, M.; Lasarte-Monterrubio, C.; Vázquez-Ucha, J.C.; Bou, G.; Rodríguez, J.; Beceiro, A.; Jimenez, C. In vitro and in vivo assessment of the efficacy of bromoageliferin, an alkaloid isolated from the sponge agelas dilatata, against Pseudomonas aeruginosa. Mar. Drugs 2020, 18, 326. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.-L.; Ding, Y.-F.; Zhang, W.; Lin, H.-W. Chemical and biological diversity of new natural products from marine sponges: A review (2009–2018). Mar. Life Sci. Technol. 2022, 4, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; She, J.; Fu, J.; Wang, J.; Ye, Y.; Yang, B.; Liu, Y.; Zhou, X.; Tao, H. Advances in natural products from the marine-sponge-associated microorganisms with antimicrobial activity in the last decade. Mar. Drugs 2023, 21, 236. [Google Scholar] [CrossRef]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.M.; Calado, R. Trends in the discovery of new marine natural products from invertebrates over the last two decades—Where and what are we bioprospecting? PLoS ONE 2012, 7, e30580. [Google Scholar] [CrossRef]
- Li, Z. Advances in marine microbial symbionts in the china sea and related pharmaceutical metabolites. Mar. Drugs 2009, 7, 113–129. [Google Scholar] [CrossRef]
- Wright, A.E.; Killday, K.B.; Chakrabarti, D.; Guzmán, E.A.; Harmody, D.; McCarthy, P.J.; Pitts, T.; Pomponi, S.A.; Reed, J.K.; Roberts, B.F.; et al. Dragmacidin G, a bioactive bis-indole alkaloid from a deep-water sponge of the genus spongosorites. Mar. Drugs 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, C.; Tulasi, B.R.; Raju, M.; Thakur, N.; Dufossé, L. Marine natural products from tunicates and their associated microbes. Mar. Drugs 2021, 19, 308. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Khong, H.Y.; Mao, W.; Chen, X.; Bao, L.; Wen, X.; Xu, Y. Tunicates as sources of high-quality nutrients and bioactive compounds for food/feed and pharmaceutical applications: A review. Foods 2023, 12, 3684. [Google Scholar] [CrossRef] [PubMed]
- Mannarino, L.; Ravasio, N.; D’Incalci, M.; Marchini, S.; Masseroli, M. In-Silico identification of novel pharmacological synergisms: The trabectedin case. Int. J. Mol. Sci. 2024, 25, 2059. [Google Scholar] [CrossRef]
- Lv, D.; Xia, J.; Guan, X.; Lai, Q.; Zhang, B.; Lin, J.; Shao, Z.; Luo, S.; Zhangsun, D.; Qin, J.J.; et al. Indole diketopiperazine alkaloids isolated from the marine-derived fungus Aspergillus chevalieri MCCC M23426. Front. Microbiol. 2022, 13, 950857. [Google Scholar] [CrossRef]
- Yang, J.; Gong, L.; Guo, M.; Jiang, Y.; Ding, Y.; Wang, Z.; Xin, X.; An, F. Bioactive indole diketopiperazine alkaloids from the marine endophytic fungus Aspergillus sp. YJ191021. Mar. Drugs 2021, 19, 157. [Google Scholar] [CrossRef]
- Xiong, Z.Q.; Wang, J.F.; Hao, Y.Y.; Wang, Y. Recent advances in the discovery and development of marine microbial natural products. Mar. Drugs 2013, 11, 700–717. [Google Scholar] [CrossRef] [PubMed]
- Bourgade, B.; Stensjö, K. Synthetic biology in marine cyanobacteria: Advances and challenges. Front. Microbiol. 2022, 13, 994365. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, T.; Vairappan, C.S. Nutritional and bioactive properties of three edible species of green algae, Genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027. [Google Scholar] [CrossRef]
- Shigemori, H.; Bae, M.A.; Yazawa, K.; Sasaki, T.; Kobayashi, J. Alteramide A, A new tetracyclic alkaloid from a bacterium Alteromonas sp. Associated with TheMarine Sponge Halichondria Okadai. J. Org. Chem. 1992, 57, 4317–4320. [Google Scholar] [CrossRef]
- Dharmaraj, S. Marine streptomyces as a novel source of bioactive substances. World J. Microbiol. Biotechnol. 2010, 26, 2123–2139. [Google Scholar] [CrossRef]
- Newaz, A.W.; Yong, K.; Lian, X.Y.; Zhang, Z. streptoindoles a–d, novel antimicrobial indole alkaloids from the marine-associated actinomycete Streptomyces sp. ZZ1118. Tetrahedron 2022, 104, 132598. [Google Scholar] [CrossRef]
- Youssef, F.S.; Alshammari, E.; Ashour, M.L. Bioactive alkaloids from genus Aspergillus: Mechanistic interpretation of their antimicrobial and potential SARS-CoV-2 inhibitory activity using molecular modelling. Int. J. Mol. Sci. 2021, 22, 1866. [Google Scholar] [CrossRef] [PubMed]
- Hang, S.; Lu, H.; Jiang, Y. Marine-derived metabolites act as promising antifungal agents. Mar. Drugs 2024, 22, 180. [Google Scholar] [CrossRef] [PubMed]
- Stonik, V.A.S.; Stonik, I.V. Marine excitatory amino acids: Structure, properties, biosynthesis and recent approaches to their syntheses. Molecules 2020, 25, 3049. [Google Scholar] [CrossRef]
- Seo, H.; Lim, D. Total Synthesis of halicylindramide A. J. Org. Chem. 2009, 74, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Halichondria, M.S. Halicylindramides A-C, antifungal and cytotoxic depsipeptides from the marine sponge Halichondria cylindrata. J. Med. Chem. 1995, 38, 338–343. [Google Scholar]
- Ribeiro, R.; Pinto, E.; Fernandes, C.; Sousa, E. Marine cyclic peptides: Antimicrobial activity and synthetic strategies. Mar. Drugs 2022, 20, 397. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Agematu, H.; Kaneto, R.; Terasawa, T.; Sakai, K.; Dobashi, K.; Yoshioka, T. Rhodopeptins (Mer-N1033), Novel cyclic tetrapeptides activity from Rhodococcus sp. with antifungal. J. Antibiot. 1999, 52, 695–699. [Google Scholar] [CrossRef][Green Version]
- Morgan, K.D.; Andersen, R.J.; Ryan, K.S. Piperazic acid-containing natural products: Structures and biosynthesis. Nat. Prod. Rep. 2019, 36, 1628–1653. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N.; Konosu, S. Amino Acid composition of discodermin a, an antimicrobial peptide, from the marine sponge Discodermia kiiensis. J. Nat. Prod. 1985, 48, 236–241. [Google Scholar] [CrossRef]
- Parry, R.; Nishino, S.; Spain, J. Naturally-occurring nitro compounds. Nat. Prod. Rep. 2011, 28, 152–167. [Google Scholar] [CrossRef]
- Sun, C.; Liu, Z.; Zhu, X.; Fan, Z.; Huang, X.; Wu, Q.; Zheng, X.; Qin, X.; Zhang, T.; Zhang, H.; et al. Antitubercular ilamycins from marine-derived Streptomyces atratus SCSIO ZH16 ilar. J. Nat. Prod. 2020, 83, 1646–1657. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; Zheng, G.F.; Chen, L.C.; Yang, N.; Xin, X.J.; Ma, J.Y.; Ju, J.H.; Wu, H.; Zhao, M.; Wang, R.; et al. Efficient ilamycins production utilizing Enteromorpha Prolifera by metabolically engineered Streptomyces atratus. Biotechnol. Biofuels Bioprod. 2023, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huang, H.; Xie, Y.; Liu, Z.; Zhao, J.; Zhang, C.; Jia, Y.; Zhang, Y.; Zhang, H.; Zhang, T.; et al. Biosynthesis of ilamycins featuring unusual building blocks and engineered production of enhanced anti-tuberculosis agents. Nat. Commun. 2017, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Schlecker, A.; Ma, D. Total synthesis of antimicrobial and antitumor cyclic depsipeptides. Chem. Commun. 2010, 46, 5403–5420. [Google Scholar] [CrossRef]
- Bionda, N.; Pitteloud, J.P.; Cudic, P. Cyclic Lipodepsipeptides: A new class of antibacterial agents in the battle against resistant bacteria. Future Med. Chem. 2013, 5, 1311–1330. [Google Scholar] [CrossRef]
- Li, W.-R.; Ewing, W.R.; Harris, B.D.; Joullie, M.M. Total Synthesis and structural investigations of didemnins A, B, and C. J. Am. Chem. Soc. 1990, 112, 7659–7672. [Google Scholar] [CrossRef]
- Gao, J.; Hamann, M.T. Chemistry and biology of kahalalides. Chem. Rev. 2011, 111, 3208–3235. [Google Scholar] [CrossRef]
- Shilabin, A.S.; Hamann, M.T. In vitro and in vivo evaluation of select kahalalide f analogs with antitumor and antifungal activities. Bioorg. Med. Chem. 2011, 15, 6628–6632. [Google Scholar] [CrossRef]
- Meena, K.R.; Kanwar, S.S. Lipopeptides as the antifungal and antibacterial agents: Applications in food safety and therapeutics. BioMed Res. Int. 2015, 2015, 473050. [Google Scholar] [CrossRef] [PubMed]
- Vertesy, L.; Ehlers, E.; Kogler, H.; Kurz, M.; Meiwes, J.; Seibert, G.; Vogel, M.; Hammann, P. Friulimicins: Novel lipopeptide antibiotics with peptidoglycan synthesis inhibiting activity from actinoplanes Friuliensis sp. Nov. II. isolation and structural characterization. J. Antibiot. 2000, 53, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Q.; Zhang, Q.; Xu, W.F.; Hai, Y.; Chao, R.; Wang, C.F.; Hou, X.M.; Wei, M.Y.; Gu, Y.C.; Wang, C.Y.; et al. Targeted isolation of antitubercular cycloheptapeptides and an unusual pyrroloindoline-containing new analog, asperpyrroindotide A, using lc–ms/ms-based molecular networking. Mar. Life Sci. Technol. 2023, 5, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Chao, R.; Hou, X.M.; Xu, W.F.; Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Targeted isolation of asperheptatides from a coral-derived fungus using lc-ms/ms-based molecular networking and antitubercular activities of modified cinnamate derivatives. J. Nat. Prod. 2021, 84, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, C.; Liu, Z.; Liu, Q.; Zhang, T.; Ju, J.; Ma, J. Production of antitubercular depsipeptides via biosynthetic engineering of cinnamoyl units. J. Nat. Prod. 2020, 83, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, H.; Chen, Y.; Tan, J.; Song, Y.; Zou, J.; Tian, X.; Hua, Y.; Ju, J. Marthiapeptide A, an anti-infective and cytotoxic polythiazole cyclopeptide from a 60 l scale fermentation of the deep sea-derived Marinactinospora thermotolerans SCSIO 00652. J. Nat. Prod. 2012, 75, 2251–2255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Islam, M.A.; Mcalpine, S.R. Synthesis of the natural product marthiapeptide A. Org. Lett. 2015, 17, 5149–5151. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, J.; Meesakul, P.; Zhou, J.; Liu, J.; Liu, S.; Wang, C.; Cao, S. Cytotoxic compounds from marine fungi: Sources, structures, and bioactivity. Mar. Drugs 2024, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.G.; Gelain, F. Structure–activity relationships of antibacterial peptides. Microb. Biotechnol. 2023, 16, 757–777. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Yu, G.; Sun, Z.; Zhang, G.; Gu, Q.; Zhu, T.; Che, Q.; Guan, H.; Li, D. Dicitrinones E and F, citrinin dimers from the marine derived fungus Penicillium citrinum HDN-152-088. Tetrahedron Lett. 2019, 60, 151182. [Google Scholar] [CrossRef]
- Fan, H.; Shi, Z.M.; Lei, Y.H.; Si-Tu, M.X.; Zhou, F.G.; Feng, C.; Wei, X.; Shao, X.H.; Chen, Y.; Zhang, C.X. Rare carbon-bridged citrinin dimers from the starfish-derived symbiotic fungus Penicillium sp. GGF16-1-2. Mar. Drugs 2022, 20, 443. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tan, Q.; Wu, J.; Chen, T.; Yang, W.; She, Z.; Wang, B. The polyketides with antimicrobial activities from a mangrove endophytic fungus Trichoderma lentiforme ML-P8-2. Mar. Drugs 2023, 21, 566. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Yang, S.Q.; Li, X.M.; Hu, X.Y.; Li, X.; Wang, B.G. Aromatic polyketides from the deep-sea cold-seep mussel associated endozoic fungus Talaromyces Minioluteus CS-138. Mar. Drugs 2022, 20, 529. [Google Scholar] [CrossRef] [PubMed]
- Van Anh, C.; Kang, J.S.; Yang, J.W.; Kwon, J.H.; Heo, C.S.; Lee, H.S.; Shin, H.J. Rifamycin-related polyketides from a marine-derived bacterium Salinispora arenicola and their cytotoxic activity. Mar. Drugs 2023, 21, 494. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Seto, S.; Ojima, N.; Ogura, K. Purification and characterization of dimethylallyl pyrophosphate: Aspulvinone dimethylallyltransferase from Aspergillus terreus. Biochemistry 1978, 17, 2696–2702. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.P.; Kumla, D.; Pereira, J.A.; Sousa, E.; Dethoup, T.; Freitas-Silva, J.; Costa, P.M.; Mistry, S.; Silva, A.M.S.; Kijjoa, A. Prenylated phenylbutyrolactones from cultures of a marine sponge-associated fungus Aspergillus Flavipes KUFA1152. Phytochemistry 2021, 185, 112709. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Lodin, A.; Herold, I.; Ilan, M.; Carmeli, S.; Yarden, O. Sensitivity of Neurospora Crassa to a marine-derived Aspergillus tubingensis anhydride exhibiting antifungal activity that is mediated by the mas1 protein. Mar. Drugs 2014, 12, 4713–4731. [Google Scholar] [CrossRef]
- Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Streptomyces bacteria as potential probiotics in aquaculture. Front. Microbiol. 2016, 7, 79. [Google Scholar] [CrossRef]
- Zhang, Z.; Harunari, E.; Igarashi, Y. Iseoic acids and bisiseoate: Three new naphthohydroquinone/naphthoquinone-class metabolites from a coral-derived Streptomyces. J. Antibiot. 2023, 76, 618–622. [Google Scholar] [CrossRef]
- Nweze, J.A.; Mbaoji, F.N.; Huang, G.; Li, Y.; Yang, L.; Zhang, Y.; Yang, D. Antibiotics development and the potentials of marine-derived compounds to stem the tide of and protozoa. Mar. Drugs 2020, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Eshboev, F.; Mamadalieva, N.; Nazarov, P.A.; Hussain, H.; Katanaev, V.; Egamberdieva, D.; Azimova, S. Antimicrobial action mechanisms of natural compounds isolated from endophytic microorganisms. Antibiotics 2024, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Mrsa, S.; Kim, M.C.; Cullum, R.; Hebishy, A.M.S.; Mohamed, H.A.; Faraag, A.H.I.; Salah, N.M.; Abdelfattah, M.S.; Fenical, W. Mersaquinone, a new tetracene derivative from the activity against methicillin-resistant. Antibiotics 2020, 9, 252. [Google Scholar] [CrossRef]
- Tsoukatou, M.; Maréchal, J.P.; Hellio, C.; Novaković, I.; Tufegdzic, S.; Sladić, D.; Gašić, M.J.; Clare, A.S.; Vagias, C.; Roussis, V. Evaluation of the activity of the sponge metabolites avarol and avarone and their synthetic derivatives against fouling micro- and macroorganisms. Molecules 2007, 12, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Karcz, W.; Burdach, Z.; Rudnicka, M. The Effects of 1,4-naphthoquinone (nq) and naphthazarin (5,8-dihydroxy-1,4-naphthoquinone, dhnq) individually and in combination on growth and oxidative stress in maize (Zea mays L.) seedlings. Plants 2023, 12, 900. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kwon, H.C. New Naphthoquinone terpenoids from marine actinobacterium, Streptomyces sp. CNQ-509. Mar. Drugs 2018, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, X.; Huang, T.; Deng, Z.; Lin, S. Naphthoquinone-Based Meroterpenoids from Marine-Derived Streptomyces sp. B9173. Biomolecules 2020, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Lee, J.H.; Lee, S.; Lee, Y.K.; Hwang, B.S.; Lee, J. Antibiofilm activity of phorbaketals from the marine sponge Phorbas sp. against Staphylococcus aureus. Mar. Drugs 2021, 19, 301. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, E.; Choi, H.; Lee, J. Collismycin C from the micronesian marine bacterium Streptomyces sp. MC025 inhibits Staphylococcus aureus biofilm formation. Mar. Drugs 2017, 15, 387. [Google Scholar] [CrossRef]
- Liu, H.; Yan, C.; Li, C.; You, T.; She, Z. Naphthoquinone derivatives with anti-inflammatory activity from mangrove-derived endophytic fungus Talaromyces sp. SK-S009. Molecules 2020, 25, 576. [Google Scholar] [CrossRef]
- Elissawy, A.M.; El-Shazly, M.; Ebada, S.S.; Singab, A.N.B.; Proksch, P. Bioactive terpenes from marine-derived fungi. Mar. Drugs 2015, 13, 1966–1992. [Google Scholar] [CrossRef] [PubMed]
- Shiono, Y.; Hiramatsu, F.; Murayama, T.; Koseki, T.; Funakoshi, T.; Ueda, K.; Yasuda, H. Two drimane-type sesquiterpenes, strobilactones a and b, from the liquid culture of the edible mushroom Strobilurus ohshimae. Zeitschrift fur Naturforsch. Sect. B J. Chem. Sci. 2007, 62, 1585–1589. [Google Scholar] [CrossRef]
- Cohen, E.; Koch, L.; Thu, K.M.; Rahamim, Y.; Aluma, Y.; Ilan, M.; Yarden, O.; Carmeli, S. Novel terpenoids of the fungus aspergillus insuetus isolated from the mediterranean sponge psammocinia sp. collected along the coast of israel. Bioorg. Med. Chem. 2011, 19, 6587–6593. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Li, X.M.; Li, C.S.; Proksch, P.; Wang, B.G. Penicisteroids A and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus Penicillium chrysogenum qen-24s. Bioorg. Med. Chem. Lett. 2011, 21, 2894–2897. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Niikawa, H.; Kobayashi, M. Marine-derived fungal sesterterpenes, ophiobolins, inhibit biofilm formation of Mycobacterium species. J. Nat. Med. 2013, 67, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood Waste: A Source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Yu, D.; Basumatary, I.B.; Kumar, S.; Ye, F.; Dutta, J. Chitosan modified with bio-extract as an antibacterial coating with uv filtering feature. Int. J. Biol. Macromol. 2023, 230, 123145. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.M.; Hrdina, R.; Abdel-Mohsen, A.M.; Fouda, M.M.G.; Soliman, A.Y.; Mohamed, F.K.; Mohsin, K.; Pinto, T.D. Chitin and chitosan from brazilian atlantic coast: Isolation, characterization and antibacterial activity. Int. J. Biol. Macromol. 2015, 80, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Taghavi, E.; Foong, S.Y.; Rajaei, A.; Amiri, H.; de Tender, C.; Peng, W.; Lam, S.S.; Aghbashlo, M.; Rastegari, H.; et al. Comparison of shrimp waste-derived chitosan produced through conventional and microwave-assisted extraction processes: Physicochemical properties and antibacterial activity assessment. Int. J. Biol. Macromol. 2023, 242, 124841. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Vasudevan, S. Extraction, Characterization, and antimicrobial activity of chitosan from horse mussel Modiolus modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Yousuf, W.E.; Kenawy, E.R.; Mohamed, T.M. Biosynthesis of cellulose from Ulva lactuca, manufacture of nanocellulose and its application as antimicrobial polymer. Sci. Rep. 2023, 13, 10188. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Recent advances and future prospects of cellulose, starch, chitosan, polylactic acid and polyhydroxyalkanoates for sustainable food packaging applications. Int. J. Biol. Macromol. 2022, 221, 163–182. [Google Scholar] [CrossRef]
- Zubair, M.; Hussain, A.; Shahzad, S.; Arshad, M.; Ullah, A. Emerging trends and challenges in polysaccharide derived materials for wound care applications: A review. Int. J. Biol. Macromol. 2024, 270, 132048. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Garg, Y.; Mahmood, S.; Chopra, S.; Bhatia, A. Marine-derived polysaccharides and their therapeutic potential in wound healing application—A review. Int. J. Biol. Macromol. 2023, 253, 127331. [Google Scholar] [CrossRef]
- Behzadnia, A.; Moosavi-Nasab, M.; Oliyaei, N. Anti-Biofilm activity of marine algae-derived bioactive compounds. Front. Microbiol. 2024, 15, 1270174. [Google Scholar] [CrossRef]
- Arokiarajan, M.S.; Thirunavukkarasu, R.; Joseph, J.; Ekaterina, O.; Aruni, W. Advance research in biomedical applications on marine sulfated polysaccharide. Int. J. Biol. Macromol. 2022, 194, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Ki, J.S. Biological activity of algal derived carrageenan: A comprehensive review in light of human health and disease. Int. J. Biol. Macromol. 2023, 238, 124085. [Google Scholar] [CrossRef]
- Ye, S.; Xie, C.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Alginates from brown seaweeds as a promising natural source: A review of its properties and health benefits. Food Rev. Int. 2023, 00, 1–29. [Google Scholar] [CrossRef]
- Froelich, A.; Jakubowska, E.; Wojtyłko, M.; Jadach, B.; Gackowski, M.; Gadziński, P.; Napierała, O.; Ravliv, Y.; Osmałek, T. Alginate-based materials loaded with nanoparticles in wound healing. Pharmaceutics 2023, 15, 1142. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Lal, S.; Kumar, S.; Kumar, P.; Nagar, J.K.; Kennedy, J.F. Utilization of marine and agro-waste materials as an economical and active food packaging: Antimicrobial, mechanical and biodegradation studies of o-carboxymethyl chitosan/pectin/neem composite films. Int. J. Biol. Macromol. 2024, 254, 128038. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Riswanto, R.; Won, T.H.; Kim, H.; Elya, B.; Sim, C.J.; Oh, D.C.; Oh, K.B.; Shin, J. Manzamine alkaloids from an Acanthostrongylophora sp. Sponge. J. Nat. Prod. 2017, 80, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).