Identification of Penexanthone A as a Novel Chemosensitizer to Induce Ferroptosis by Targeting Nrf2 in Human Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Structural Identification of PXA
2.2. Effect of PXA on CDDP-Induced Cytotoxicity and Apoptosis in CRC Cells
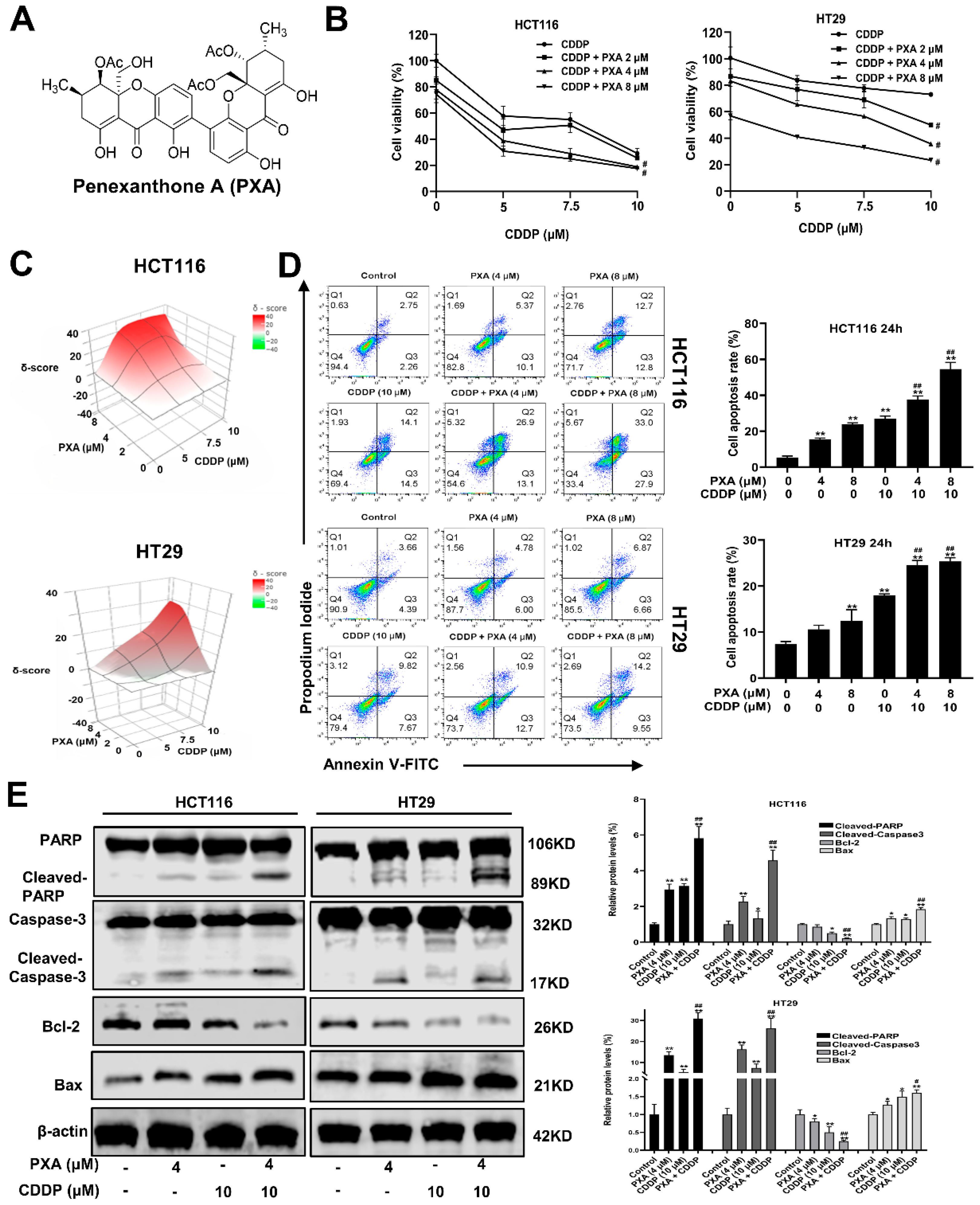
2.3. Effect of PXA on CDDP-Induced ROS in CRC Cells
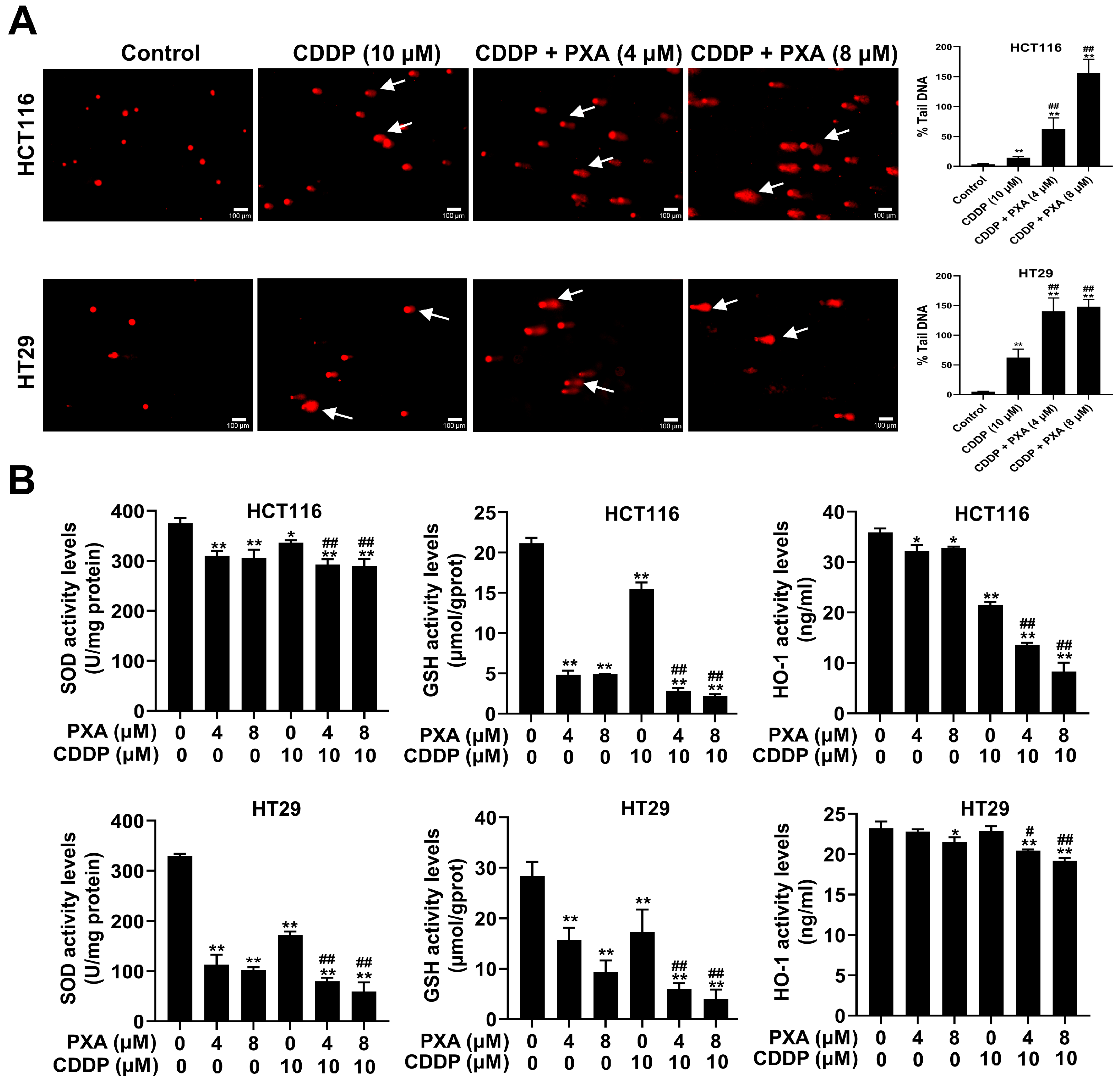
2.4. Effect of PXA on CDDP-Induced DNA Damage and Oxidative Stress in CRC Cells
2.5. Effect of PXA on Nrf2 Protein in CRC Cells
2.6. Overexpression of Nrf2 Reverses the Sensitization Effect of PXA on CDDP
2.7. Effect of Nrf2 on PXA Enhanced CDDP-Induced Ferroptosis
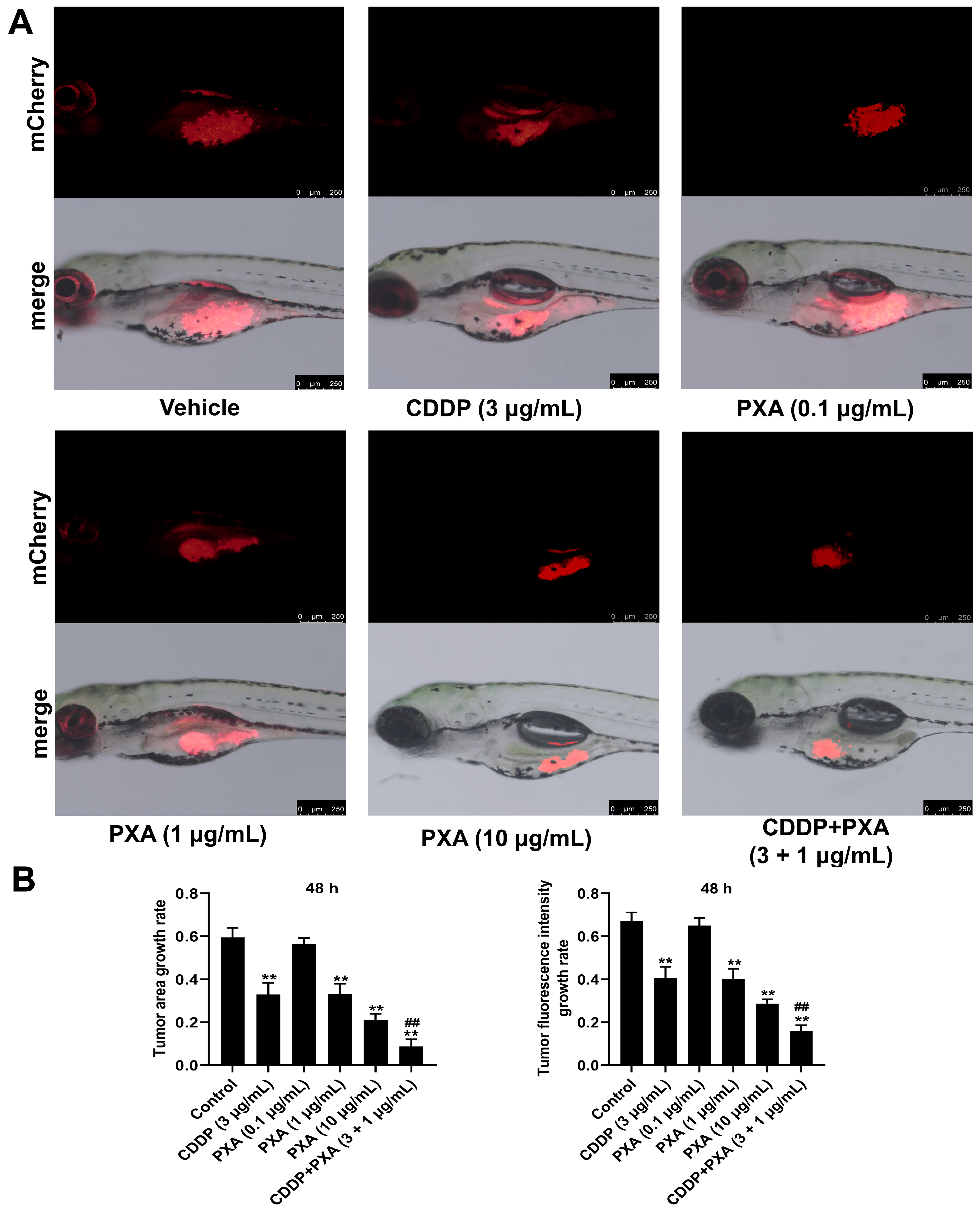
2.8. Effect of PXA and CDDP on CRC Xenograft Zebrafish

3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Plant Materials and Isolation of PXA
4.3. Cell Culture and Transfection
4.4. Cell Viability Assay
4.5. Flow Cytometry Assay
4.6. ELISA
4.7. Comet Assay
4.8. Cellular Thermal Shift Assay
4.9. Liperfluo Staining
4.10. Western Blot Analysis
4.11. CRC Xenograft Zebrafish Model
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Koberle, B.; Schoch, S. Platinum complexes in colorectal cancer and other solid tumors. Cancers 2021, 13, 2073. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Pan, J.; Ye, G. Mir-135b aggravates fusobacterium nucleatum-induced cisplatin resistance in colorectal cancer by targeting klf13. J. Microbiol. 2024, 62, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Pope, L.E.; Dixon, S.J. Regulation of ferroptosis by lipid metabolism. Trends Cell Biol. 2023, 33, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Zhou, J.; Zhu, Z.; Xu, Q.; Yin, Z.; Wang, Y.; Zheng, Z.; Zhao, H. Shikonin and cisplatin synergistically overcome cisplatin resistance of ovarian cancer by inducing ferroptosis via upregulation of hmox1 to promote Fe2+ accumulation. Phytomedicine 2023, 112, 154701. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Z.; Yao, F.; Liao, S.; Sun, K.; Sun, S.; Li, Z.; Wang, Z. Role of escin in breast cancer therapy: Potential mechanism for inducing ferroptosis and synergistic antitumor activity with cisplatin. Apoptosis 2023, 28, 1154–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sui, S.; Wang, L.; Li, H.; Zhang, L.; Xu, S.; Zheng, X. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J. Cell. Physiol. 2020, 235, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Bao, L.; Li, M.; Chang, K.; Yi, X. Erastin synergizes with cisplatin via ferroptosis to inhibit ovarian cancer growth in vitro and in vivo. J. Obstet. Gynaecol. Res. 2021, 47, 2481–2491. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.L. Targeting nrf2 for ferroptosis-based therapy: Implications for overcoming ferroptosis evasion and therapy resistance in cancer. Biochim. Biophys. Acta-Mol. Basis Dis. 2023, 1869, 166788. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. Nrf2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Yu, Y.; Liu, X.; Shang, X.; Du, Z.; Xu, M.L.; Zhang, T. Recent advances in the inhibition of membrane lipid peroxidation by food-borne plant polyphenols via the nrf2/gpx4 pathway. J. Agric. Food Chem. 2024, 72, 12340–12355. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.X.; Zhu, J.Q.; Dou, Y.X.; Xian, Y.F.; Lin, Z.X. Natural nrf2 inhibitors: A review of their potential for cancer treatment. Int. J. Biol. Sci. 2023, 19, 3029–3041. [Google Scholar] [CrossRef]
- Ballout, F.; Lu, H.; Chen, Z.; Hu, T.; Chen, L.; Washington, M.K.; El-Rifai, W.; Peng, D. Targeting nrf2 sensitizes esophageal adenocarcinoma cells to cisplatin through induction of ferroptosis and apoptosis. Antioxidants 2022, 11, 1859. [Google Scholar] [CrossRef]
- Lou, J.S.; Zhao, L.P.; Huang, Z.H.; Chen, X.Y.; Xu, J.T.; Tai, W.C.; Tsim, K.; Chen, Y.T.; Xie, T. Ginkgetin derived from ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the nrf2/ho-1 axis in egfr wild-type non-small-cell lung cancer. Phytomedicine 2021, 80, 153370. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, J.; Meesakul, P.; Zhou, J.; Liu, J.; Liu, S.; Wang, C.; Cao, S. Cytotoxic compounds from marine fungi: Sources, structures, and bioactivity. Mar. Drugs 2024, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Z.; Xie, Y.; Liu, G.; Shang, X.; Zhan, N. Transcriptome and metabolome profiling provide insights into flavonoid synthesis in acanthus ilicifolius linn. Genes 2023, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Yuan, J.; Huang, X.; Wen, W.; Zhu, X.; Liu, Y.; Li, H.; Lu, Y.; He, L.; Tan, H.; et al. New dimeric members of the phomoxanthone family: Phomolactonexanthones a, b and deacetylphomoxanthone c isolated from the fungus phomopsis sp. Mar. Drugs 2013, 11, 4961–4972. [Google Scholar] [CrossRef]
- Cao, S.; Mcmillin, D.W.; Tamayo, G.; Delmore, J.; Mitsiades, C.S.; Clardy, J. Inhibition of tumor cells interacting with stromal cells by xanthones isolated from a costa rican penicillium sp. J. Nat. Prod. 2012, 75, 793–797. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.; Vellayappan, B.A.; Jeyasekharan, A.D. Ros and the dna damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ge, H.; Shi, Y.; Yuan, F.; Yue, H. Cafs secrete cxcl12 to accelerate the progression and cisplatin resistance of colorectal cancer through promoting m2 polarization of macrophages. Med. Oncol. 2023, 40, 90. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Leng, S.; Mao, P. Cisplatin in the era of parp inhibitors and immunotherapy. Pharmacol. Ther. 2024, 258, 108642. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, B.; Zhou, L.; Zhang, Z.; Shen, Y.; Lv, H.; Alqudsy, L.; Shang, P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020, 483, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ensoy, M.; Bumin, Z.S.; Jama, H.A.; Cansaran-Duman, D. The regulation role of ferroptosis mechanism of anti-cancer drugs and noncoding rnas. Curr. Med. Chem. 2023, 30, 1638–1656. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Feng, Y.; Zandkarimi, F.; Wang, H.; Zhang, Z.; Kim, J.; Cai, Y.; Gu, W.; Stockwell, B.R.; Jiang, X. Ferroptosis surveillance independent of gpx4 and differentially regulated by sex hormones. Cell 2023, 186, 2748–2764. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The coq oxidoreductase fsp1 acts parallel to gpx4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Zeng, C.; Lin, J.; Zhang, K.; Ou, H.; Shen, K.; Liu, Q.; Wei, Z.; Dong, X.; Zeng, X.; Zeng, L.; et al. Sharpin promotes cell proliferation of cholangiocarcinoma and inhibits ferroptosis via p53/slc7a11/gpx4 signaling. Cancer Sci. 2022, 113, 3766–3775. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, W.; Guan, J.; Liu, H.; Dan, Y.; Zhu, L.; Song, Y.; Zhou, Y.; Zhao, X.; Zhang, Y.; et al. Socs2-enhanced ubiquitination of slc7a11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma. Cell Death Differ. 2023, 30, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Qiao, Y.; Yang, H.; Nan, Q.; Qu, W.; Feng, F.; Liu, W.; Chen, Y.; Sun, H. Small molecular nrf2 inhibitors as chemosensitizers for cancer therapy. Future Med. Chem. 2020, 12, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, W.; Yang, L.; Wang, T.; Li, C.; Yu, J.; Zhang, P.; Yin, Y.; Li, R.; Tao, K. Nrf2 inhibition increases sensitivity to chemotherapy of colorectal cancer by promoting ferroptosis and pyroptosis. Sci. Rep. 2023, 13, 14359. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Lin, B.; Jin, W.; Tang, L.; Hu, S.; Cai, R. Nrf2, a superstar of ferroptosis. Antioxidants 2023, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Yao, G.; Zhao, H.; Wu, S. Nrf2 is essential for iron-overload stimulated osteoclast differentiation through regulation of redox and iron homeostasis. Cell Biol. Toxicol. 2023, 39, 3305–3321. [Google Scholar] [CrossRef] [PubMed]
- Rojo, D.L.V.M.; Chapman, E.; Zhang, D.D. Nrf2 and the hallmarks of cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, D.; Yan, J. Targeting ferroptosis using chinese herbal compounds to treat respiratory diseases. Phytomedicine 2024, 130, 155738. [Google Scholar] [CrossRef] [PubMed]
- Gjorgieva, A.D.; Maksimova, V.; Smilkov, K.; Buttari, B.; Arese, M.; Saso, L. Alkaloids as natural nrf2 inhibitors: Chemoprevention and cytotoxic action in cancer. Pharmaceuticals 2023, 16, 850. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Seidel, V.; Izabela, M.; Monserrat-Mequida, M.; Sureda, A.; Ormazabal, V.; Zuniga, F.A.; Mangalpady, S.S.; Pezzani, R.; Ydyrys, A.; et al. Phenolic compounds as nrf2 inhibitors: Potential applications in cancer therapy. Cell Commun. Signal. 2023, 21, 89. [Google Scholar] [CrossRef]
- Yang, C.; Lu, T.; Liu, M.; Yuan, X.; Li, D.; Zhang, J.; Zhou, L.; Xu, M. Tiliroside targets tbk1 to induce ferroptosis and sensitize hepatocellular carcinoma to sorafenib. Phytomedicine 2023, 111, 154668. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Liu, S.; Yin, H.; Duan, J.; Fan, L.; Zhao, X.; Jiang, B. Implications of withaferin a for the metastatic potential and drug resistance in hepatocellular carcinoma cells via nrf2-mediated emt and ferroptosis. Toxicol. Mech. Methods 2023, 33, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, G.; Meng, T.; Lv, Z.; Li, X.; Li, J.; Li, K. Eicosapentaenoic acid enhances the sensitivity of osteosarcoma to cisplatin by inducing ferroptosis through the dna-pkcs/akt/nrf2 pathway and reducing pd-l1 expression to attenuate immune evasion. Int. Immunopharmacol. 2023, 125, 111181. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Nong, F.; Wang, Y.; Huang, D.; Qin, J.; Chen, Y.F.; He, D.H.; Wu, P.E.; Huang, H.; Zhan, R.; et al. Brusatol has therapeutic efficacy in non-small cell lung cancer by targeting skp1 to inhibit cancer growth and metastasis. Pharmacol. Res. 2022, 176, 106059. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Pang, Y.C.; Wang, H.C.; Wu, P.E.; Chen, Z.J.; Huang, D.; Peng, D.L.; Yan, Y.M.; Liu, C.; Wu, L.C.; et al. Identification of arnicolide c as a novel chemosensitizer to suppress mtor/e2f1/fancd2 axis in non-small cell lung cancer. Br. J. Pharmacol. 2024, 181, 1221–1237. [Google Scholar] [CrossRef]
- Yang, C.; Xing, S.; Wei, X.; Lu, J.; Zhao, G.; Ma, X.; Dai, Z.; Liang, X.; Huang, W.; Liu, Y.; et al. 12-o-deacetyl-phomoxanthone a inhibits ovarian tumor growth and metastasis by downregulating pdk4. Biomed. Pharmacother. 2024, 175, 116736. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Liu, Y.; Wei, X.; Yang, C.; Lu, J.; Yan, S.; Ma, X.; Cheng, X.; You, Z.; Ding, Y.; et al. Identification of Penexanthone A as a Novel Chemosensitizer to Induce Ferroptosis by Targeting Nrf2 in Human Colorectal Cancer Cells. Mar. Drugs 2024, 22, 357. https://doi.org/10.3390/md22080357
Zhao G, Liu Y, Wei X, Yang C, Lu J, Yan S, Ma X, Cheng X, You Z, Ding Y, et al. Identification of Penexanthone A as a Novel Chemosensitizer to Induce Ferroptosis by Targeting Nrf2 in Human Colorectal Cancer Cells. Marine Drugs. 2024; 22(8):357. https://doi.org/10.3390/md22080357
Chicago/Turabian StyleZhao, Genshi, Yanying Liu, Xia Wei, Chunxia Yang, Junfei Lu, Shihuan Yan, Xiaolin Ma, Xue Cheng, Zhengliang You, Yue Ding, and et al. 2024. "Identification of Penexanthone A as a Novel Chemosensitizer to Induce Ferroptosis by Targeting Nrf2 in Human Colorectal Cancer Cells" Marine Drugs 22, no. 8: 357. https://doi.org/10.3390/md22080357
APA StyleZhao, G., Liu, Y., Wei, X., Yang, C., Lu, J., Yan, S., Ma, X., Cheng, X., You, Z., Ding, Y., Guo, H., Su, Z., Xing, S., & Zhu, D. (2024). Identification of Penexanthone A as a Novel Chemosensitizer to Induce Ferroptosis by Targeting Nrf2 in Human Colorectal Cancer Cells. Marine Drugs, 22(8), 357. https://doi.org/10.3390/md22080357





