Effects of Marine Natural Products on Liver Diseases
Abstract
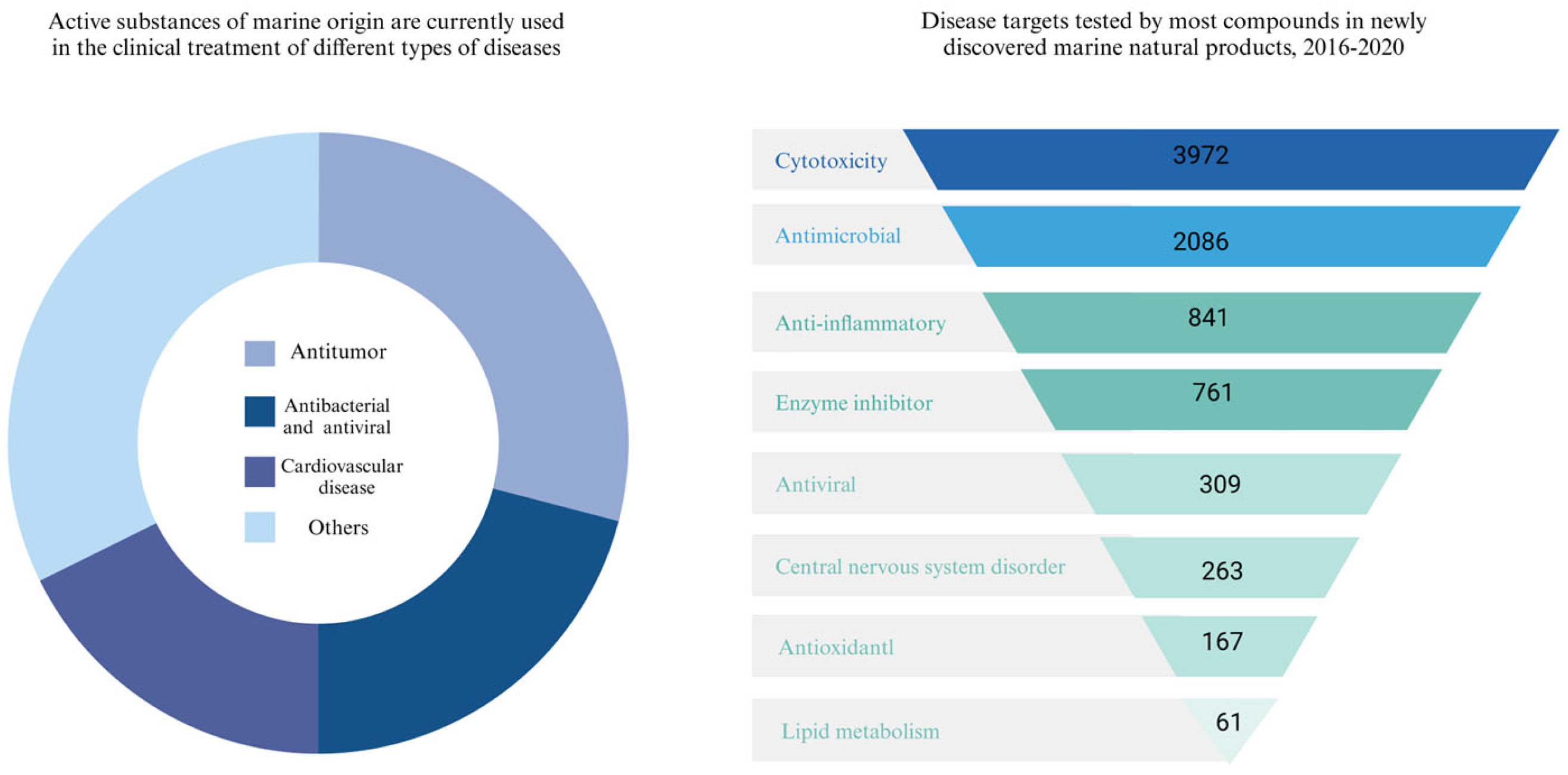
1. Introduction
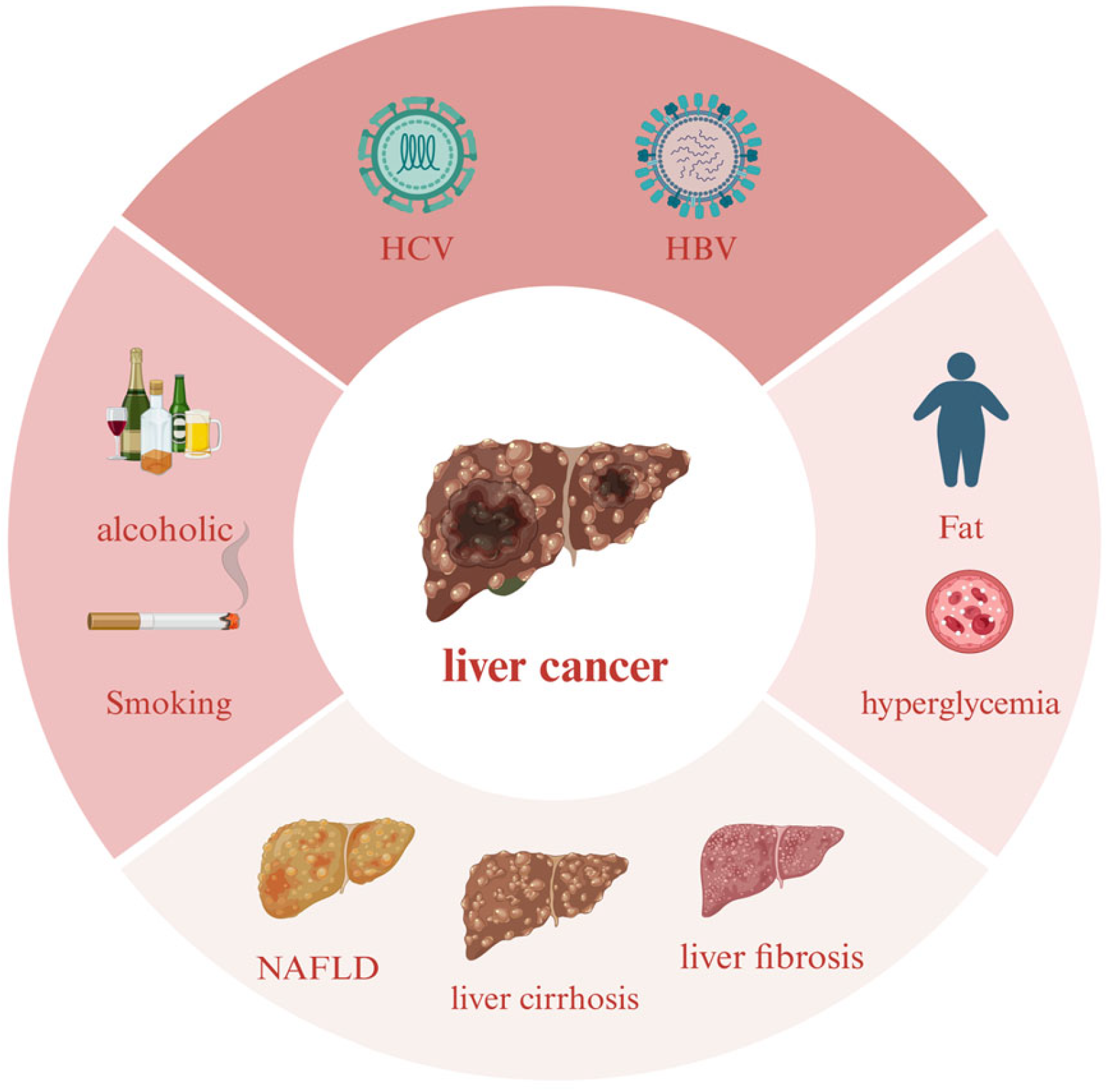
2. Liver Diseases
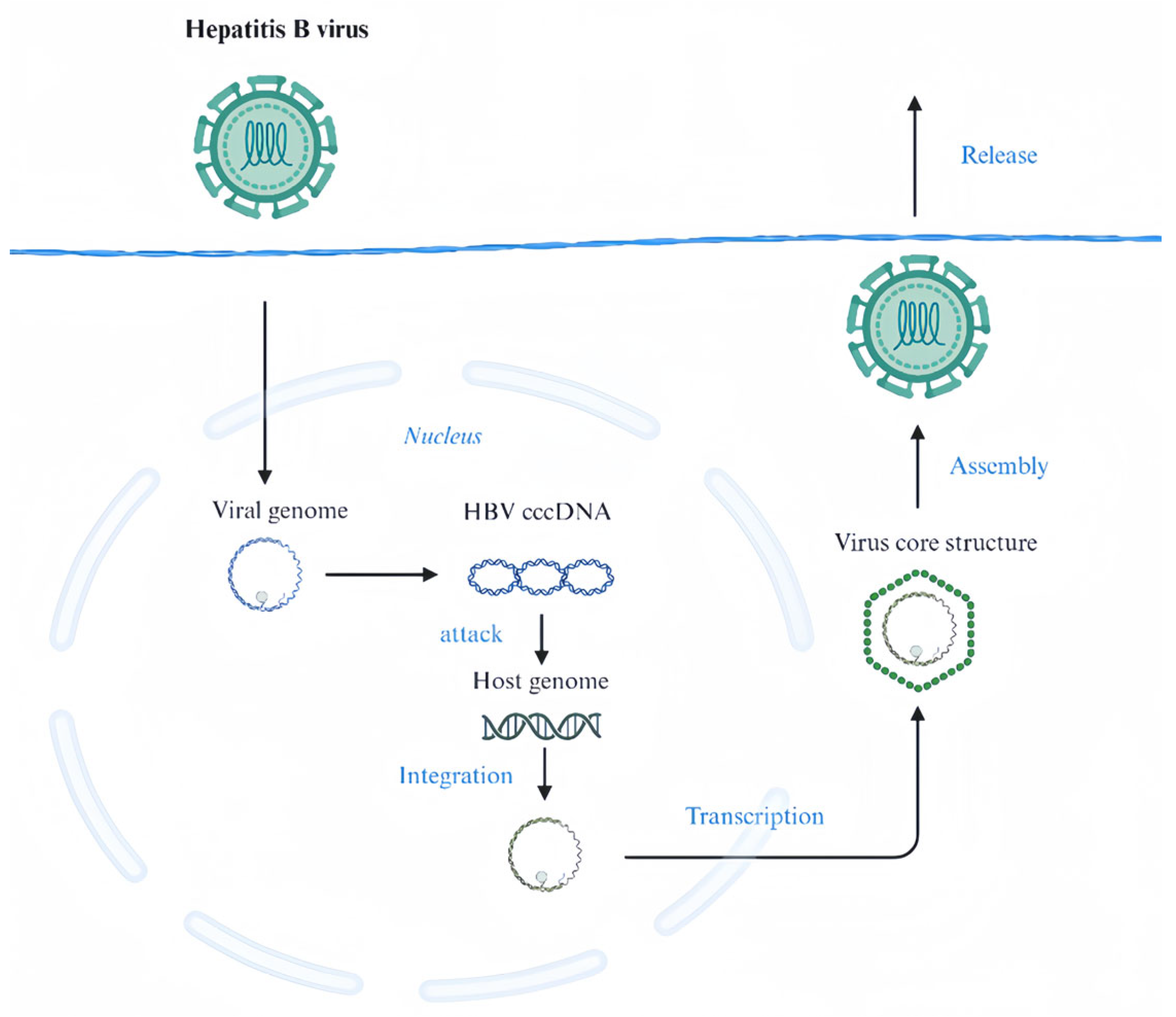
2.1. HCV and HBV
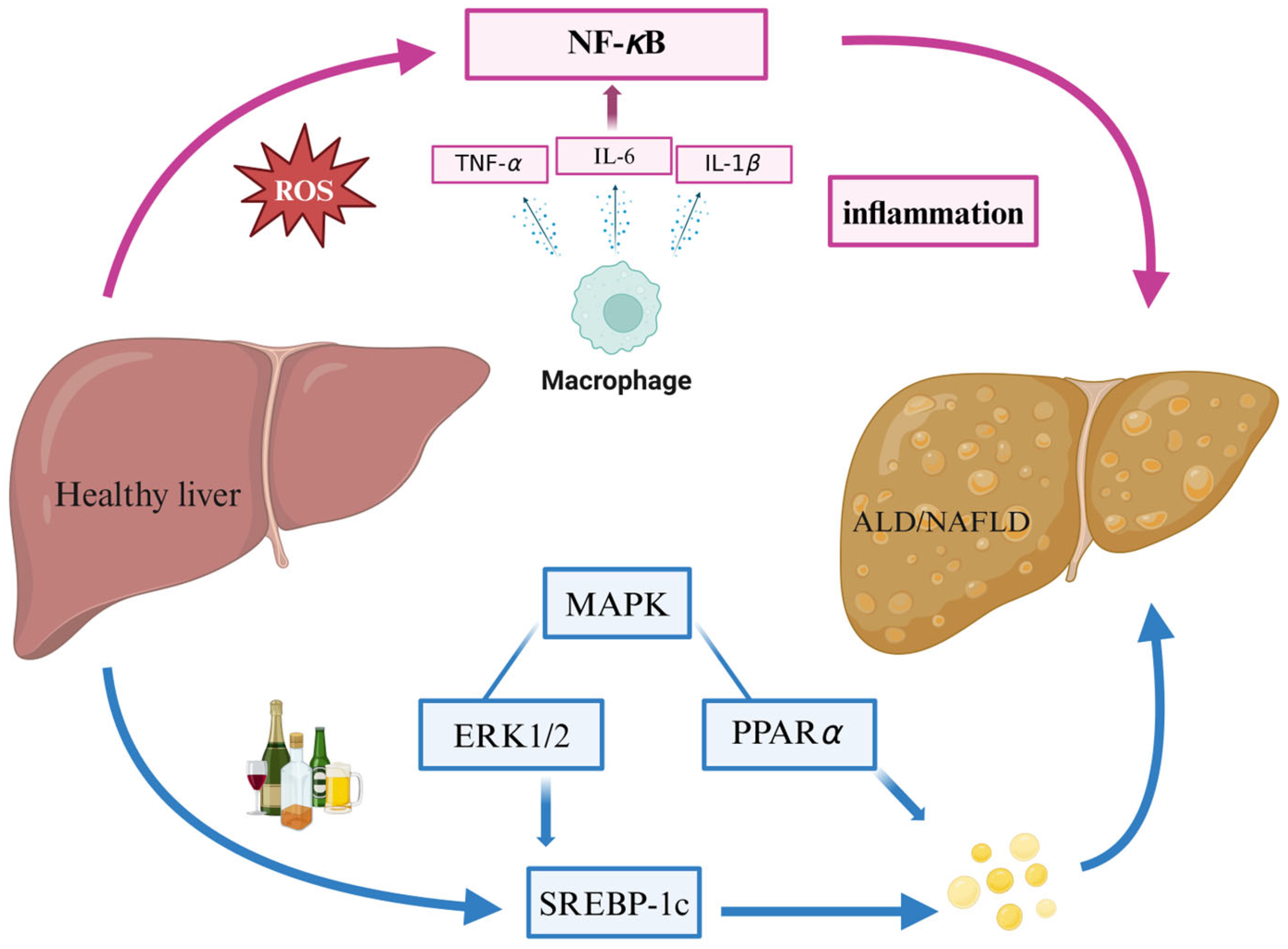
2.2. ALD
2.3. NAFLD
2.4. Liver Fibrosis
2.5. Liver Injury
2.6. HCC
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-S.; Zeng, X.-F.; Liu, Z.-N.; Zhao, Q.-H.; Tan, Y.-T.; Gao, J.; Li, H.-L.; Xiang, Y.-B. Diet and liver cancer risk: A narrative review of epidemiological evidence. Br. J. Nutr. 2020, 124, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Hepatitis Report 2024: Action for Access in Low-and Middle-Income Countries; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/publications/i/item/9789240091672 (accessed on 3 April 2024).
- Hayes, C.N.; Imamura, M.; Chayama, K. Management of HCV patients in cases of direct-acting antiviral failure. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Morishita, A.; Himoto, T.; Masaki, T. Nutritional Support for Alcoholic Liver Disease. Nutrients 2023, 15, 1360. [Google Scholar] [CrossRef] [PubMed]
- Vancells Lujan, P.; Viñas Esmel, E.; Sacanella Meseguer, E. Overview of Non-Alcoholic Fatty Liver Disease (NAFLD) and the Role of Sugary Food Consumption and Other Dietary Components in Its Development. Nutrients 2021, 13, 1442. [Google Scholar] [CrossRef]
- Guo, P.C.; Zuo, J.; Huang, K.K.; Lai, G.Y.; Zhang, X.; An, J.; Li, J.X.; Li, L.; Wu, L.; Lin, Y.T.; et al. Cell atlas of CCl (4)-induced progressive liver fibrosis reveals stage-specific responses. Zool. Res. 2023, 44, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Foerster, F.; Gairing, S.J.; Ilyas, S.I.; Galle, P.R. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology 2022, 75, 1604–1626. [Google Scholar] [CrossRef]
- Jophlin, L.L.; Singal, A.K.; Bataller, R.; Wong, R.J.; Sauer, B.G.; Terrault, N.A.; Shah, V.H. ACG Clinical Guideline: Alcohol-Associated Liver Disease. Am. J. Gastroenterol. 2024, 119, 30–54. [Google Scholar] [CrossRef]
- Vannier, A.G.L.; Fomin, V.; Chung, R.T.; Patel, S.J.; Schaefer, E.; Goodman, R.P.; Luther, J. Substance use disorder is associated with alcohol-associated liver disease in patients with alcohol use disorder. Gastro Hep Adv. 2022, 1, 403–408. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, C.; Terrana, F.; Panzeca, G.; Parrino, B.; Cascioferro, S.; Diana, P.; Giovannetti, E.; Carbone, D. Nortopsentins as Leads from Marine Organisms for Anticancer and Anti-Inflammatory Agent Development. Molecules 2023, 28, 6450. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, S.; Javeed, A.; Jian, C.; Liu, Y.; Sun, J.; Wu, S.; Fu, P.; Han, B. Structures and Anti-Allergic Activities of Natural Products from Marine Organisms. Mar. Drugs 2023, 21, 152. [Google Scholar] [CrossRef]
- Voser, T.M.; Campbell, M.D.; Carroll, A.R. How different are marine microbial natural products compared to their terrestrial counterparts? Nat. Prod. Rep. 2022, 39, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Y.; Li, H.J.; Li, Q.Y.; Wu, Y.C. Application of marine natural products in drug research. Bioorg. Med. Chem. 2021, 35, 116058. [Google Scholar] [CrossRef]
- D’Souza, S.; Lau, K.C.K.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hu, S.; Park, Y.K.; Lee, J.Y. Health Benefits of Carotenoids: A Role of Carotenoids in the Prevention of Non-Alcoholic Fatty Liver Disease. Prev. Nutr. Food Sci. 2019, 24, 103–113. [Google Scholar] [CrossRef]
- Milosevic, I.; Todorovic, N.; Filipovic, A.; Simic, J.; Markovic, M.; Stevanovic, O.; Malinic, J.; Katanic, N.; Mitrovic, N.; Nikolic, N. HCV and HCC Tango-Deciphering the Intricate Dance of Disease: A Review Article. Int. J. Mol. Sci. 2023, 24, 16048. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, Z.; Kim, Y.-C.; Kumar, R.; Yuan, F.; Shi, P.-Y.; Kao, C.; Luo, G. Stimulation of Hepatitis C Virus (HCV) Nonstructural Protein 3 (NS3) Helicase Activity by the NS3 Protease Domain and by HCV RNA-Dependent RNA Polymerase. J. Virol. 2005, 79, 8687–8697. [Google Scholar] [CrossRef][Green Version]
- Abdelaleem, E.R.; Samy, M.N.; Ali, T.F.S.; Mustafa, M.; Ibrahim, M.A.A.; Bringmann, G.; Ahmed, S.A.; Abdelmohsen, U.R.; Desoukey, S.Y. NS3 helicase inhibitory potential of the marine sponge Spongia irregularis. RSC Adv. 2022, 12, 2992–3002. [Google Scholar] [CrossRef]
- Ahmed, E.F.; Rateb, M.E.; Abou El-Kassem, L.T.; Hawas, U.W. Anti-HCV Protease of Diketopiperazines Produced by the Red Sea Sponge-Associated Fungus Aspergillus versicolor. Appl. Biochem. Microbiol. 2017, 53, 101–106. [Google Scholar] [CrossRef]
- Hao, X.; Li, S.; Li, J.; Wang, G.; Li, J.; Peng, Z.; Gan, M. Acremosides A–G, Sugar Alcohol-Conjugated Acyclic Sesquiterpenes from a Sponge-Derived Acremonium Species. J. Nat. Prod. 2024, 87, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Y.; Hao, X.; Tan, J.; Li, F.; Qiao, X.; Chen, S.; Xiao, C.; Chen, M.; Peng, Z.; et al. Raistrickindole A, an Anti-HCV Oxazinoindole Alkaloid from Penicillium raistrickii IMB17-034. J. Nat. Prod. 2019, 82, 1391–1395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Hao, X.; Li, S.; Jia, J.; Guan, Y.; Peng, Z.; Bi, H.; Xiao, C.; Cen, S.; et al. Broad-Spectrum Antiviral Natural Products from the Marine-Derived Penicillium sp. IMB17-046. Molecules 2019, 24, 2821. [Google Scholar] [CrossRef] [PubMed]
- Tsukuda, S.; Watashi, K. Hepatitis B virus biology and life cycle. Antivir. Res. 2020, 182, 104925. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tian, Z. HBV-Induced Immune Imbalance in the Development of HCC. Front. Immunol. 2019, 10, 2048. [Google Scholar] [CrossRef] [PubMed]
- Höner Zu Siederdissen, C.; Maasoumy, B.; Cornberg, M. What is new on HBsAg and other diagnostic markers in HBV infection? Best Pract. Res. Clin. Gastroenterol. 2017, 31, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Tang, Y.; Lin, L.; Xie, Z.; Zhou, J.; Zhang, L.; Zhang, X.; Zhao, X.; Chen, Z.; et al. Fucoidan from Fucus vesiculosus suppresses hepatitis B virus replication by enhancing extracellular signal-regulated Kinase activation. Virol. J. 2017, 14, 178. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moreno, A.; Ploss, A. Mechanisms of Hepatitis B Virus cccDNA and Minichromosome Formation and HBV Gene Transcription. Viruses 2024, 16, 609. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Cheng, W.; Wang, J.; Zhang, H.; Lu, F.; Chen, X.; Lin, W. Junceellolide B, a novel inhibitor of Hepatitis B virus. Bioorganic Med. Chem. 2020, 28, 115603. [Google Scholar] [CrossRef]
- Jin, Y.; Qin, S.; Gao, H.; Zhu, G.; Wang, W.; Zhu, W.; Wang, Y. An anti-HBV anthraquinone from aciduric fungus Penicillium sp. OUCMDZ-4736 under low pH stress. Extrem. Life Under Extrem. Cond. 2018, 22, 39–45. [Google Scholar] [CrossRef]
- Narula, P.; Kiruthika, S.; Chowdhari, S.; Vivekanandan, P.; Chugh, A. Inhibition of Hepatitis B Virus (HBV) by Tachyplesin, a Marine Antimicrobial Cell-Penetrating Peptide. Pharmaceutics 2023, 15, 672. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Tamaki, M.; Kasai, H.; Tanaka, T.; Otoguro, T.; Ryo, A.; Maekawa, S.; Enomoto, N.; de Voogd, N.J.; Tanaka, J.; et al. Inhibitory effects of metachromin A on hepatitis B virus production via impairment of the viral promoter activity. Antivir. Res. 2017, 145, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Fujimoto, Y.; Tamaki, M.; Setiawan, A.; Tanaka, T.; Okuyama-Dobashi, K.; Kasai, H.; Watashi, K.; Wakita, T.; Toyama, M.; et al. Identification of Antiviral Agents Targeting Hepatitis B Virus Promoter from Extracts of Indonesian Marine Organisms by a Novel Cell-Based Screening Assay. Mar. Drugs 2015, 13, 6759–6773. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.S.; Fan, J.G.; Zhang, Z.; Gao, B.; Wang, H.Y. The global burden of liver disease: The major impact of China. Hepatology 2014, 60, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Alcohol and the liver: 1994 update. Gastroenterology 1994, 106, 1085–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Xing, R.; Liu, S.; Chen, X.; Li, P. Optimization of Oyster (Crassostrea talienwhanensis) Protein Hydrolysates Using Response Surface Methodology. Molecules 2020, 25, 2844. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xie, J.; Zhu, B.; Liu, X.; Wu, X. allograft inflammatory factor 1 functions as a pro-inflammatory cytokine in the oyster, Crassostrea ariakensis. PLoS ONE 2014, 9, e95859. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Hepatoprotective Effect of Oyster Peptide on Alcohol-Induced Liver Disease in Mice. Int. J. Mol. Sci. 2022, 23, 8081. [Google Scholar] [CrossRef]
- Xiao, C.; Zhou, L.; Gao, J.; Jia, R.; Zheng, Y.; Zhao, S.; Zhao, M.; Toldrá, F. Musculus senhousei as a promising source of bioactive peptides protecting against alcohol-induced liver injury. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2023, 174, 113652. [Google Scholar] [CrossRef]
- Jin, M.; Liu, H.; Hou, Y.; Chan, Z.; Di, W.; Li, L.; Zeng, R. Preparation, characterization and alcoholic liver injury protective effects of algal oligosaccharides from Gracilaria lemaneiformis. Food Res. Int. 2017, 100, 186–195. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, Y.; Lu, H.; Zhao, J.; Wen, C.; Song, S.; Ai, C.; Yang, J. The protective effect of Enteromorpha prolifera polysaccharide on alcoholic liver injury in C57BL/6 mice. Int. J. Biol. Macromol. 2024, 261, 129908. [Google Scholar] [CrossRef]
- Yang, S.; Chen, M.F.; Ryu, B.; Chen, J.; Xiao, Z.; Hong, P.; Sun, S.; Wang, D.; Qian, Z.J.; Zhou, C. The Protective Effect of the Polysaccharide Precursor, D-Isofloridoside, from Laurencia undulata on Alcohol-Induced Hepatotoxicity in HepG2 Cells. Molecules 2020, 25, 1024. [Google Scholar] [CrossRef]
- Song, H.; Song, C.; Yan, C.; Yang, J.; Song, S. Sea Cucumber Polysaccharide from Stichopus japonicu and Its Photocatalytic Degradation Product Alleviate Acute Alcoholic Liver Injury in Mice. Foods 2024, 13, 963. [Google Scholar] [CrossRef]
- Kim, D.K.; Rajan, P.; Cuong, D.M.; Choi, J.H.; Yoon, T.H.; Go, G.m.; Lee, J.W.; Noh, S.-W.; Choi, H.-K.; Cho, S.K. Melosira nummuloides Ethanol Extract Ameliorates Alcohol-Induced Liver Injury by Affecting Metabolic Pathways. J. Agric. Food Chem. 2024, 72, 8476–8490. [Google Scholar] [CrossRef]
- Ge, N.; Liang, H.; Zhao, Y.Y.; Liu, Y.; Gong, A.J.; Zhang, W.L. Aplysin Protects Against Alcohol-Induced Liver Injury Via Alleviating Oxidative Damage and Modulating Endogenous Apoptosis-Related Genes Expression in Rats. J. Food Sci. 2018, 83, 2612–2621. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, X.; Zhang, W.; Zheng, P.; Huang, F.; Ding, G.; Yang, Z. Protective Effects of Fucoxanthin against Alcoholic Liver Injury by Activation of Nrf2-Mediated Antioxidant Defense and Inhibition of TLR4-Mediated Inflammation. Mar. Drugs 2019, 17, 552. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Yan, L.; Wu, Y.; Zhang, L.; Li, B.; Tong, H.; Lin, X. Molecular Mechanisms of Fucoxanthin in Alleviating Lipid Deposition in Metabolic Associated Fatty Liver Disease. J. Agric. Food Chem. 2024, 72, 10391–10405. [Google Scholar] [CrossRef]
- Lin, L.; Yang, S.; Xiao, Z.; Hong, P.; Sun, S.; Zhou, C.; Qian, Z.J. The Inhibition Effect of the Seaweed Polyphenol, 7-Phloro-Eckol from Ecklonia Cava on Alcohol-Induced Oxidative Stress in HepG2/CYP2E1 Cells. Mar. Drugs 2021, 19, 158. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Afonso, M.B.; Rodrigues, P.M.; Mateus-Pinheiro, M.; Simão, A.L.; Gaspar, M.M.; Majdi, A.; Arretxe, E.; Alonso, C.; Santos-Laso, A.; Jimenez-Agüero, R.; et al. RIPK3 acts as a lipid metabolism regulator contributing to inflammation and carcinogenesis in non-alcoholic fatty liver disease. Gut 2021, 70, 2359–2372. [Google Scholar] [CrossRef]
- Iizasa, S.; Nagao, K.; Tsuge, K.; Nagano, Y.; Yanagita, T. Identification of genes regulated by lipids from seaweed Susabinori (Pyropia yezoensis) involved in the improvement of hepatic steatosis: Insights from RNA-Seq analysis in obese db/db mice. PLoS ONE 2023, 18, e0295591. [Google Scholar] [CrossRef]
- Thakuri, L.S.; Park, C.M.; Kim, H.A.; Kim, H.J.; Park, J.W.; Park, J.C.; Rhyu, D.Y. Gracilaria chorda subcritical water ameliorates hepatic lipid accumulation and regulates glucose homeostasis in a hepatic steatosis cell model and obese C57BL/6J mice. J. Ethnopharmacol. 2024, 320, 117395. [Google Scholar] [CrossRef]
- Zaloga, G.P. Narrative Review of n-3 Polyunsaturated Fatty Acid Supplementation upon Immune Functions, Resolution Molecules and Lipid Peroxidation. Nutrients 2021, 13, 662. [Google Scholar] [CrossRef]
- Ulven, S.M.; Holven, K.B. Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc. Health Risk Manag. 2015, 11, 511–524. [Google Scholar] [CrossRef]
- Hwang, S.M.; Kim, Y.U.; Kim, J.K.; Chun, Y.S.; Kwon, Y.S.; Ku, S.K.; Song, C.H. Preventive and Therapeutic Effects of Krill Oil on Obesity and Obesity-Induced Metabolic Syndromes in High-Fat Diet-Fed Mice. Mar. Drugs 2022, 20, 483. [Google Scholar] [CrossRef]
- Chen, Y.F.; Fan, Z.K.; Gao, X.; Zhou, F.; Guo, X.F.; Sinclair, A.J.; Li, D. n-3 polyunsaturated fatty acids in phospholipid or triacylglycerol form attenuate nonalcoholic fatty liver disease via mediating cannabinoid receptor 1/adiponectin/ceramide pathway. J. Nutr. Biochem. 2024, 123, 109484. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Zhang, X.Q.; Liu, X.; Zhang, Z.X.; Zhu, Q.M.; Liu, J.Y.; Iqbal, M.O.; Ding, N.; Shao, C.L.; et al. Discovery of a natural small-molecule AMP-activated kinase activator that alleviates nonalcoholic steatohepatitis. Mar. Life Sci. Technol. 2023, 5, 196–210. [Google Scholar] [CrossRef]
- Vasarri, M.; Barletta, E.; Degl’Innocenti, D. Posidonia oceanica (L.) Delile Extract Reduces Lipid Accumulation through Autophagy Activation in HepG2 Cells. Pharmaceuticals 2021, 14, 969. [Google Scholar] [CrossRef]
- Tong, Y.; Zhu, W.; Wen, T.; Mukhamejanova, Z.; Xu, F.; Xiang, Q.; Pang, J. Xyloketal B Reverses Nutritional Hepatic Steatosis, Steatohepatitis, and Liver Fibrosis through Activation of the PPARα/PGC1α Signaling Pathway. J. Nat. Prod. 2022, 85, 1738–1750. [Google Scholar] [CrossRef]
- Li, T.; Hu, S.M.; Pang, X.Y.; Wang, J.F.; Yin, J.Y.; Li, F.H.; Wang, J.; Yang, X.Q.; Xia, B.; Liu, Y.H.; et al. The marine-derived furanone reduces intracellular lipid accumulation in vitro by targeting LXRα and PPARα. J. Cell. Mol. Med. 2020, 24, 3384–3398. [Google Scholar] [CrossRef]
- Fang, Y.; She, J.; Zhang, X.; Gu, T.; Xie, D.; Luo, X.; Yi, X.; Gao, C.; Liu, Y.; Zhang, C.; et al. Discovery of Anti-Hypercholesterolemia Agents Targeting LXRα from Marine Microorganism-Derived Natural Products. J. Nat. Prod. 2024, 87, 322–331. [Google Scholar] [CrossRef]
- Liu, J.; Wu, H.; Zhang, Y.; Hu, C.; Zhen, D.; Fu, P.; He, Y. Phycobiliprotein Peptide Extracts from Arthrospira platensis Ameliorate Nonalcoholic Fatty Liver Disease by Modulating Hepatic Lipid Profile and Strengthening Fat Mobilization. Nutrients 2023, 15, 4573. [Google Scholar] [CrossRef]
- Wan, M.C.; Qin, W.; Lei, C.; Li, Q.H.; Meng, M.; Fang, M.; Song, W.; Chen, J.H.; Tay, F.; Niu, L.N. Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ahn, E.Y.; Park, Y. Shape-dependent cytotoxicity and cellular uptake of gold nanoparticles synthesized using green tea extract. Nanoscale Res. Lett. 2019, 14, 129. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Cao, P.; Pan, H.; Ding, C.; Xiao, T.; Zhang, P.; Guo, J.; Su, Z. Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Mar. Drugs 2015, 13, 2732–2756. [Google Scholar] [CrossRef]
- Kim, M.B.; Lee, Y.; Bae, M.; Kang, H.; Hu, S.; Pham, T.X.; Lee, J.Y.; Park, Y.K. Sugar kelp (Saccharina latissima) inhibits hepatic inflammation and fibrosis in a mouse model of diet-induced nonalcoholic steatohepatitis. J. Nutr. Biochem. 2021, 97, 108799. [Google Scholar] [CrossRef]
- Guo, B.; Zhou, Y.; Liu, B.; He, Y.; Chen, F.; Cheng, K.W. Lipid-Lowering Bioactivity of Microalga Nitzschia laevis Extract Containing Fucoxanthin in Murine Model and Carcinomic Hepatocytes. Pharmaceuticals 2021, 14, 1004. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Mancuso, A.; Politi, F.; Maringhini, A. Portal Vein Thromboses in Cirrhosis: To Treat or Not to Treat? Gastroenterology 2018, 154, 758. [Google Scholar] [CrossRef]
- Li, J.; Chen, K.; Li, S.; Feng, J.; Liu, T.; Wang, F.; Zhang, R.; Xu, S.; Zhou, Y.; Zhou, S.; et al. Protective effect of fucoidan from Fucus vesiculosus on liver fibrosis via the TGF-β1/Smad pathway-mediated inhibition of extracellular matrix and autophagy. Drug Des. Dev. Ther. 2016, 10, 619–630. [Google Scholar] [CrossRef]
- Xu, S.; Mao, Y.; Wu, J.; Feng, J.; Li, J.; Wu, L.; Yu, Q.; Zhou, Y.; Zhang, J.; Chen, J.; et al. TGF-β/Smad and JAK/STAT pathways are involved in the anti-fibrotic effects of propylene glycol alginate sodium sulphate on hepatic fibrosis. J. Cell. Mol. Med. 2020, 24, 5224–5237. [Google Scholar] [CrossRef]
- Chale-Dzul, J.; Pérez-Cabeza de Vaca, R.; Quintal-Novelo, C.; Olivera-Castillo, L.; Moo-Puc, R. Hepatoprotective effect of a fucoidan extract from Sargassum fluitans Borgesen against CCl(4)-induced toxicity in rats. Int. J. Biol. Macromol. 2020, 145, 500–509. [Google Scholar] [CrossRef]
- Yue, H.; Cai, W.; Li, Y.; Feng, X.; Dong, P.; Xue, C.; Wang, J. A Novel Sialoglycopeptide from Gadus morhua Eggs Prevents Liver Fibrosis Induced by CCl4 via Downregulating FXR/FGF15 and TLR4/TGF-β/Smad Pathways. J. Agric. Food Chem. 2021, 69, 13093–13101. [Google Scholar] [CrossRef]
- Tammam, M.A.; Pereira, F.; Aly, O.; Sebak, M.; Diab, Y.M.; Mahdy, A.; El-Demerdash, A. Investigating the hepatoprotective potentiality of marine-derived steroids as promising inhibitors of liver fibrosis. RSC Adv. 2023, 13, 27477–27490. [Google Scholar] [CrossRef]
- Cui, H.; Tang, Y.; Yang, C.; Deng, H.; Chen, L.; Fan, X.; Zhu, L.; Liu, Y.; Zhao, Z.; Su, T. Meroterpenoids from the marine-derived fungus Aspergillus terreus GZU-31-1 exerts anti-liver fibrosis effects by targeting the Nrf2 signaling in vitro. Phytochemistry 2024, 219, 113983. [Google Scholar] [CrossRef]
- Brancaccio, M.; D’Argenio, G.; Lembo, V.; Palumbo, A.; Castellano, I. Antifibrotic Effect of Marine Ovothiol in an In Vivo Model of Liver Fibrosis. Oxidative Med. Cell. Longev. 2018, 2018, 5045734. [Google Scholar] [CrossRef]
- Azam, M.; Hira, K.; Qureshi, S.A.; Khatoon, N.; Ara, J.; Ehteshamul-Haque, S. Ameliorative Effect of Marine Macroalgae on Carbon Tetrachloride-Induced Hepatic Fibrosis and Associated Complications in Rats. Turk. J. Pharm. Sci. 2022, 19, 116–124. [Google Scholar] [CrossRef]
- Henríquez, V.; Escobar, C.; Galarza, J.; Gimpel, J. Carotenoids in Microalgae. Sub-Cell. Biochem. 2016, 79, 219–237. [Google Scholar] [CrossRef]
- Yang, Y.; Bae, M.; Kim, B.; Park, Y.K.; Koo, S.I.; Lee, J.Y. Astaxanthin prevents and reverses the activation of mouse primary hepatic stellate cells. J. Nutr. Biochem. 2016, 29, 21–26. [Google Scholar] [CrossRef]
- Islam, M.A.; Al Mamun, M.A.; Faruk, M.; Ul Islam, M.T.; Rahman, M.M.; Alam, M.N.; Rahman, A.; Reza, H.M.; Alam, M.A. Astaxanthin Ameliorates Hepatic Damage and Oxidative Stress in Carbon Tetrachloride-administered Rats. Pharmacogn. Res. 2017, 9, S84–S91. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, B.; Park, Y.K.; Koo, S.I.; Lee, J.Y. Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression by inhibiting Smad3 activation in hepatic stellate cells. Biochim. Biophys. Acta 2015, 1850, 178–185. [Google Scholar] [CrossRef]
- Yang, Y.; Bae, M.; Park, Y.K.; Lee, Y.; Pham, T.X.; Rudraiah, S.; Manautou, J.; Koo, S.I.; Lee, J.Y. Histone deacetylase 9 plays a role in the antifibrogenic effect of astaxanthin in hepatic stellate cells. J. Nutr. Biochem. 2017, 40, 172–177. [Google Scholar] [CrossRef]
- Dietz, J.; Lutz, T.; Knecht, G.; Gute, P.; Berkowski, C.; Lange, C.M.; Khaykin, P.; Stephan, C.; Brodt, H.-R.; Herrmann, E.; et al. Evolution and function of the HCV NS3 protease in patients with acute hepatitis C and HIV coinfection. Virology 2015, 485, 213–222. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, X.; Tang, Y.; Han, T. Laminaria japonica fucoidan ameliorates cyclophosphamide-induced liver and kidney injury possibly by regulating Nrf2/HO-1 and TLR4/NF-κB signaling pathways. J. Sci. Food Agric. 2022, 102, 2604–2612. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Yang, W.C.; Lin, C.F.; Wang, C.M.; Liu, H.Y.; Lin, C.S.; Lin, J.W.; Lin, W.L.; Lin, T.C.; Fan, P.S.; et al. The Ameliorative Effects of Fucoidan in Thioacetaide-Induced Liver Injury in Mice. Molecules 2021, 26, 1937. [Google Scholar] [CrossRef]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Potential Beneficial Actions of Fucoidan in Brain and Liver Injury, Disease, and Intoxication-Potential Implication of Sirtuins. Mar. Drugs 2020, 18, 242. [Google Scholar] [CrossRef]
- Nabil-Adam, A.; Shreadah, M.A. Red algae natural products for prevention of lipopolysaccharides (LPS)-induced liver and kidney inflammation and injuries. Biosci. Rep. 2021, 41, BSR20202022. [Google Scholar] [CrossRef]
- Yang, F.; Cai, H.; Zhang, X.; Sun, J.; Feng, X.; Yuan, H.; Zhang, X.; Xiao, B.; Li, Q. An active marine halophenol derivative attenuates lipopolysaccharide-induced acute liver injury in mice by improving M2 macrophage-mediated therapy. Int. Immunopharmacol. 2021, 96, 107676. [Google Scholar] [CrossRef]
- Liang, Y.; Qiu, S.; Zou, Y.; Luo, L. Targeting ferroptosis with natural products in liver injury: New insights from molecular mechanisms to targeted therapies. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 122, 155134. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Cao, Y.; Chen, M.; Shi, Z.; Wu, M.; Feng, H.; Sun, L.; Ma, Z.; Tan, X.; et al. Bioactive Indole Alkaloid from Aspergillus amoenus TJ507 That Ameliorates Hepatic Ischemia/Reperfusion Injury. J. Nat. Prod. 2023, 86, 2059–2064. [Google Scholar] [CrossRef]
- Holczbauer, Á.; Wangensteen, K.J.; Shin, S. Cellular origins of regenerating liver and hepatocellular carcinoma. JHEP Rep. Innov. Hepatol. 2022, 4, 100416. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Zhi, X.; Ding, W.; Xiong, J.; Tao, T.; Yang, Y.; Zhang, H.; Zi, X.; Zhou, W.; et al. SOX9 enhances sorafenib resistance through upregulating ABCG2 expression in hepatocellular carcinoma. Biomed. Pharmacother. 2020, 129, 110315. [Google Scholar] [CrossRef]
- Xu, Z.P.; Liu, Y.; Wu, Z.R.; Gong, J.P.; Wang, Y.B. Prognostic and diagnostic value of SOX9 in cirrhotic and noncirrhotic hepatocellular carcinoma. Transl. Cancer Res. 2021, 10, 2738–2746. [Google Scholar] [CrossRef]
- Otto, J.; Verwaayen, A.; Penners, C.; Hundertmark, J.; Lin, C.; Kallen, C.; Paffen, D.; Otto, T.; Berger, H.; Tacke, F.; et al. Expression of Cyclin E1 in hepatic stellate cells is critical for the induction and progression of liver fibrosis and hepatocellular carcinoma in mice. Cell Death Dis. 2023, 14, 549. [Google Scholar] [CrossRef]
- Li, Q.L.; Zhang, P.P.; Wang, P.Q.; Yu, H.B.; Sun, F.; Hu, W.Z.; Wu, W.H.; Zhang, X.; Chen, F.; Chu, Z.Y.; et al. The cytotoxic and mechanistic effects of aaptamine on hepatocellular carcinoma. Anti-Cancer Agents Med. Chem. 2015, 15, 291–297. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Kumar, S.N.; Ramachandran, G.; Manoharan, N. Marine sponge alkaloid aaptamine enhances the anti-bacterial and anti-cancer activity against ESBL producing Gram negative bacteria and HepG 2 human liver carcinoma cells. Biocatal. Agric. Biotechnol. 2019, 17, 628–637. [Google Scholar] [CrossRef]
- Sun, M.; Zhou, D.; Wu, J.; Zhou, J.; Xu, J. Sdy-1 Executes Antitumor Activity in HepG2 and HeLa Cancer Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway. Mar. Drugs 2022, 20, 125. [Google Scholar] [CrossRef]
- Kudo, Y.; Sugimoto, M.; Arias, E.; Kasashima, H.; Cordes, T.; Linares, J.F.; Duran, A.; Nakanishi, Y.; Nakanishi, N.; L’Hermitte, A.; et al. PKCλ/ι Loss Induces Autophagy, Oxidative Phosphorylation, and NRF2 to Promote Liver Cancer Progression. Cancer Cell 2020, 38, 247–262.e211. [Google Scholar] [CrossRef]
- Chang, W.T.; Bow, Y.D.; Fu, P.J.; Li, C.Y.; Wu, C.Y.; Chang, Y.H.; Teng, Y.N.; Li, R.N.; Lu, M.C.; Liu, Y.C.; et al. A Marine Terpenoid, Heteronemin, Induces Both the Apoptosis and Ferroptosis of Hepatocellular Carcinoma Cells and Involves the ROS and MAPK Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 7689045. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Wang, X.; Zhang, Y.; Shen, Y.; Qian, Y. Macrophage-Derived MMP-9 and MMP-2 are Closely Related to the Rupture of the Fibrous Capsule of Hepatocellular Carcinoma Leading to Tumor Invasion. Biol. Proced. Online 2023, 25, 8. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.C.; Wu, W.T.; Lin, J.J.; Su, J.H.; Wu, Y.J. Stellettin B Isolated from Stelletta Sp. Reduces Migration and Invasion of Hepatocellular Carcinoma Cells through Reducing Activation of the MAPKs and FAK/PI3K/AKT/mTOR Signaling Pathways. Int. J. Cell Biol. 2022, 2022, 4416611. [Google Scholar] [CrossRef] [PubMed]
- Vasarri, M.; Barletta, E.; Stio, M.; Bergonzi, M.C.; Galli, A.; Degl’Innocenti, D. Ameliorative Effect of Posidonia oceanica on High Glucose-Related Stress in Human Hepatoma HepG2 Cells. Int. J. Mol. Sci. 2023, 24, 5203. [Google Scholar] [CrossRef] [PubMed]
- Makol, A.; Kaur, H.; Sharma, S.; Kanthaje, S.; Kaur, R.; Chakraborti, A. Vimentin as a potential therapeutic target in sorafenib resistant HepG2, a HCC model cell line. Clin. Mol. Hepatol. 2020, 26, 45–53. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Han, K.; Wang, L.; Liu, X. Induction of Apoptosis by Matrine Derivative ZS17 in Human Hepatocellular Carcinoma BEL-7402 and HepG2 Cells through ROS-JNK-P53 Signalling Pathway Activation. Int. J. Mol. Sci. 2022, 23, 15991. [Google Scholar] [CrossRef]
- Lu, I.T.; Lin, S.C.; Chu, Y.C.; Wen, Y.; Lin, Y.C.; Cheng, W.C.; Sheu, J.H.; Lin, C.C. (-)-Agelasidine A Induces Endoplasmic Reticulum Stress-Dependent Apoptosis in Human Hepatocellular Carcinoma. Mar. Drugs 2022, 20, 109. [Google Scholar] [CrossRef]
- Yu, H.-B.; Hu, B.; Wu, G.-F.; Ning, Z.; He, Y.; Jiao, B.-H.; Liu, X.-Y.; Lin, H.-W. Phyllospongianes A–E, Dinorscalarane Sesterterpenes from the Marine Sponge Phyllospongia foliascens. J. Nat. Prod. 2023, 86, 1754–1760. [Google Scholar] [CrossRef]
- Gao, Q.; Shi, Y.; Wen, H.; Zhang, D.; Yan, X.; He, S.; Ding, L. Asepterpenedol A, a novel indole sesquiterpene with a rare 7/6/5/5/6/6-hexacyclic scaffold from a marine-derived fungus Chloridium sp. NBU3282. J. Mol. Struct. 2024, 1296, 136723. [Google Scholar] [CrossRef]
- Carretero-Molina, D.; Ortiz-López, F.J.; Martín, J.; Oves-Costales, D.; Díaz, C.; de la Cruz, M.; Cautain, B.; Vicente, F.; Genilloud, O.; Reyes, F. New Napyradiomycin Analogues from Streptomyces sp. Strain CA-271078. Mar. Drugs 2020, 18, 22. [Google Scholar] [CrossRef]
- Sarmiento-Vizcaíno, A.; Braña, A.F.; Pérez-Victoria, I.; Martín, J.; de Pedro, N.; Cruz, M.; Díaz, C.; Vicente, F.; Acuña, J.L.; Reyes, F.; et al. Paulomycin G, a New Natural Product with Cytotoxic Activity against Tumor Cell Lines Produced by Deep-Sea Sediment Derived Micromonospora matsumotoense M-412 from the Avilés Canyon in the Cantabrian Sea. Mar. Drugs 2017, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wang, J.; Wang, J.; Shi, L.; Li, K.; Lin, X.; Min, Y.; Yang, B.; Tang, L.; Liu, Y.; et al. Cytotoxic and Antibacterial Eremophilane Sesquiterpenes from the Marine-Derived Fungus Cochliobolus lunatus SCSIO41401. J. Nat. Prod. 2018, 81, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-Z.; Zhang, B.-Q.; Wang, C.-F.; Yin, J.-N.; Haider, W.; Said, G.; Wei, M.-Y.; Lu, L. A Terphenyllin Derivative CHNQD-00824 from the Marine Compound Library Induced DNA Damage as a Potential Anticancer Agent. Mar. Drugs 2023, 21, 512. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, S.; Peng, H.; Zhao, W.; Chang, W.; Wang, H.; Chen, H.; Dai, H. p-Terphenyl and Diphenyl Ether Derivatives from the Marine-Derived Fungus Aspergillus candidus HM5-4. Mar. Drugs 2024, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Virués-Segovia, J.R.; Millán, C.; Pinedo, C.; González-Rodríguez, V.E.; Papaspyrou, S.; Zorrilla, D.; Mackenzie, T.A.; Ramos, M.C.; de la Cruz, M.; Aleu, J.; et al. New Eremophilane-Type Sesquiterpenes from the Marine Sediment-Derived Fungus Emericellopsis maritima BC17 and Their Cytotoxic and Antimicrobial Activities. Mar. Drugs 2023, 21, 634. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.-T.; Yang, L.; Kong, F.-D.; Ma, Q.-Y.; Xie, Q.-Y.; Dai, H.-F.; Yu, Z.-F.; Zhao, Y.-X. Cytotoxic Indole-Diterpenoids from the Marine-Derived Fungus Penicillium sp. KFD28. Mar. Drugs 2021, 19, 613. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.-Q.; Lan, W.-J.; Qiu, Y.; Guo, Q.; Feng, G.-K.; Deng, R.; Zhu, X.-F.; Li, H.-J.; Dong, J. Monarubins A–C from the Marine Shellfish-Associated Fungus Monascus ruber BB5. Mar. Drugs 2020, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.-P.; Li, X.-M.; Li, L.; Li, X.; Wang, B.-G. Cytotoxic Thiodiketopiperazine Derivatives from the Deep Sea-Derived Fungus Epicoccum nigrum SD-388. Mar. Drugs 2020, 18, 160. [Google Scholar] [CrossRef] [PubMed]
- Begolli, R.; Chatziangelou, M.; Samiotaki, M.; Goutas, A.; Barda, S.; Goutzourelas, N.; Kevrekidis, D.P.; Malea, P.; Trachana, V.; Liu, M.; et al. Transcriptome and proteome analysis reveals the anti-cancer properties of Hypnea musciformis marine macroalga extract in liver and intestinal cancer cells. Hum. Genom. 2023, 17, 71. [Google Scholar] [CrossRef]
- Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Gaspar, H.; Malcata, F.X.; Barreira, L.; Varela, J. Anti-Hepatocellular Carcinoma (HepG2) Activities of Monoterpene Hydroxy Lactones Isolated from the Marine Microalga Tisochrysis Lutea. Mar. Drugs 2020, 18, 567. [Google Scholar] [CrossRef]
- Kim, E.A.; Lee, J.H.; Heo, S.J.; Jeon, Y.J. Saringosterol acetate isolated from Hizikia fusiforme, an edible brown alga, suppressed hepatocellular carcinoma growth and metastasis in a zebrafish xenograft model. Chem. Biol. Interact. 2021, 335, 109362. [Google Scholar] [CrossRef] [PubMed]
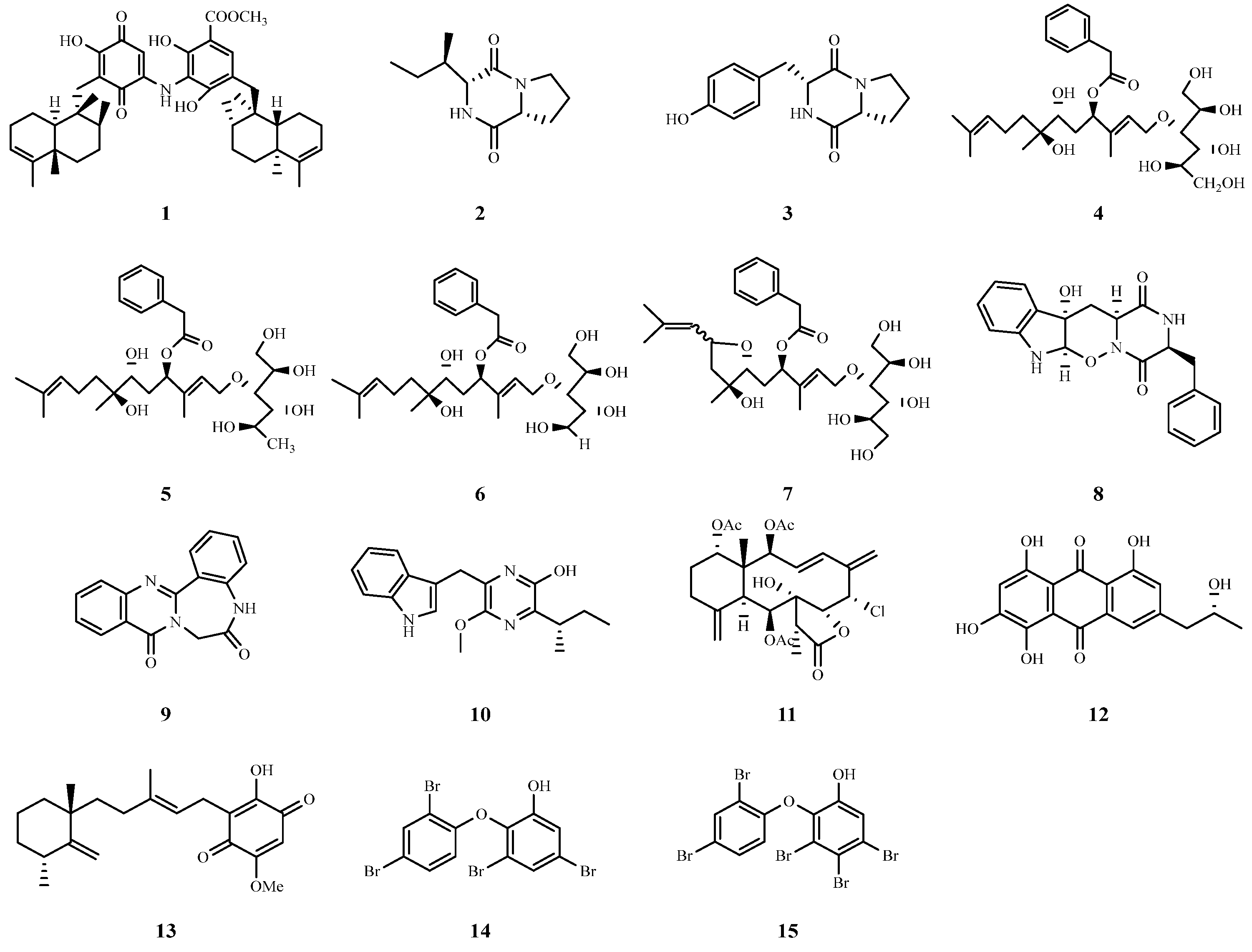

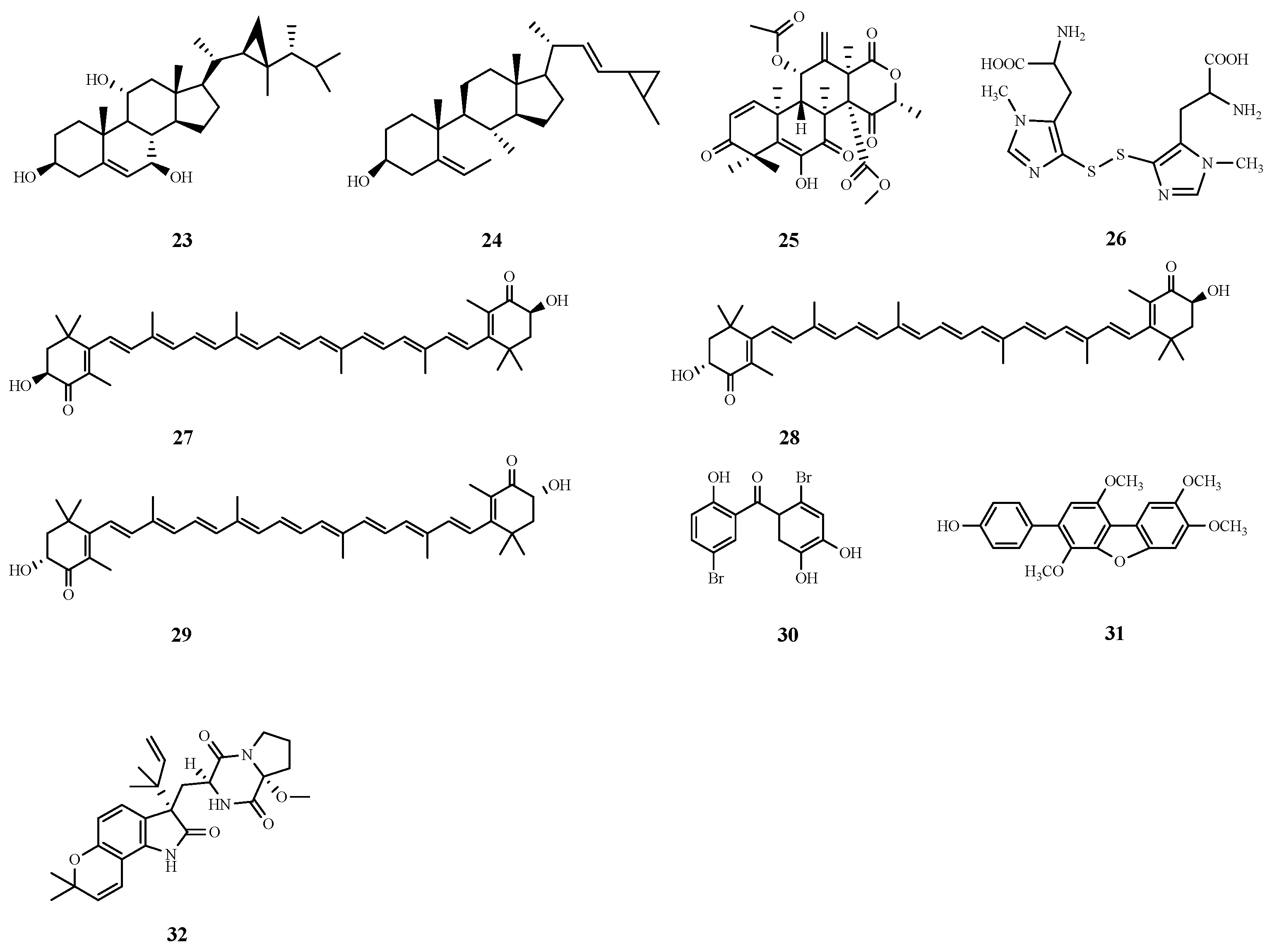
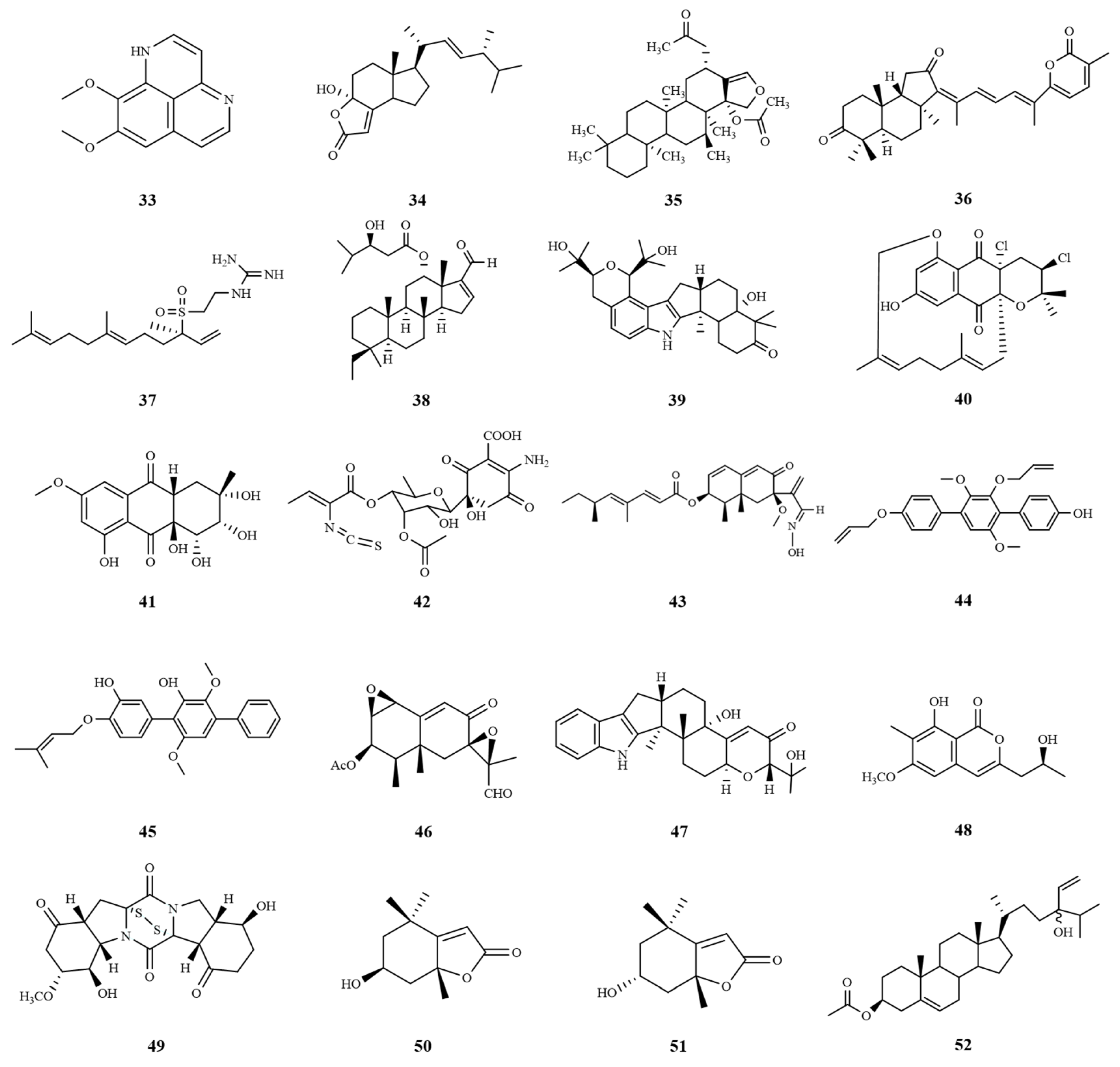
| Number | Name | Source | Disease | IC50 (μM) | EC50 (μM) |
|---|---|---|---|---|---|
| 1 | Nakijiquinone F | Spongia irregularis | HCV | 12.6 | — |
| 2 | Cyclic (L-tyrosine-L-proline) | Aspergillus versicolor | HCV | 8.2 | — |
| 3 | Cyclic (L-proline-L-enine) | Aspergillus versicolor | HCV | 13.4 | — |
| 4–7 | Acremosides A and C–E | Acremonium sp. IMB18-086 | HCV | — | 4.8~8.8 |
| 8 | Raistrickindole A | Penicillium raistrickii IMB17-034 | HCV | — | 5.7 |
| 9 | Raistrickin | Penicillium raistrickii IMB17-034 | HCV | — | 7.0 |
| 10 | Trypilepyrazinol | Penicillium sp. IMB 17-046 | HCV | 7.7 | — |
| 12 | (−)-2′R-1-hydroxyisorhodoptilometrin | Penicillium sp. OUCMDZ-4736 | HBV | 4.63 | — |
| 13 | Metachromin A | Dactylospongia metachromia | HBV | — | 0.8 |
| 14 | 3,5-dibromo-2-(2,4-dibromophenoxy)-phenol | Dysidea sp. | HBV | — | 0.23 |
| 15 | 3,4,5-tribromo-2-(2,4-dibromophenoxy)-phenol | Dysidea sp. | HBV | — | 0.80 |
| Number | Name | Source | Disease | Effects |
|---|---|---|---|---|
| 16 | Aplysin | Laurencia tristicha | ALD | Anti-oxidation Bcl-2, Bax |
| 17 | Fucoxanthin | Laminaria japonica Aresch | ALD | AST, ALT, KEAP1/Nrf2/ARE PGC1α/NRF1 |
| 18 | 7-Phloroeckol | Ecklonia cava | ALD | GSH, SOD, Bcl-2 |
| 19 | Xyloketal B | Xylaria sp. | NAFLD | PPARα/PGC1α |
| 20 | Furazone | Setosphaeria sp. SCSIO41009 | NAFLD | LXRα, PPARα |
| 21 | 12R,13S-dihydroxyfumitremorgin C | Aspergillus sp. SCSIO 41420 | NAFLD | LXRα |
| 22 | Tryprostatin A | Aspergillus sp. SCSIO 41420 | NAFLD | LXRα |
| 23 | Dissesterol | Sarcophyton glaucum | Liver fibrosis | GST, SOD |
| 24 | Glaucasterol | Sarcophyton glaucum | Liver fibrosis | GST, SOD |
| 25 | Aspermeroterpene B | Aspergillus terreus GZU-31-1 | Liver fibrosis | Nrf2 |
| 27 | 3S-3′S Astaxanthin | Haematococcus pluvialis | Liver fibrosis | Anti-oxidation NrF2 TGF-β1/Smad3 |
| 28 | 3R-3′S Astaxanthin | |||
| 29 | 3R-3′R Astaxanthin | |||
| 30 | LM49 | Galaxaura oblongata | Liver injury | NF-κB, MPO, LPO |
| 31 | HN-001 | Aspergillus sp. C1 | Liver injury | PLA2/IRE-1α/XBP-1s JNK |
| 32 | Notoamide Q | Aspergillus amoenus TJ507 | Liver injury | ALT, AST, LDH |
| Number | Name | Source | IC50 (μM) |
|---|---|---|---|
| 38 | Phyllospongiane C | Phyllospongia foliascens | 9.6 |
| 40 | Near-tearomycin D1 | Streptomyces sp. strain CA-271078 | 14.9 |
| 41 | Auxarthrols D | Sporendonema casei | 16.6 |
| 42 | Paulomycin G | Micromonospora matsumotoense M-412 | 4.3 |
| 43 | Dendryphiellin J | Cochliobolus lunatus SCSIO41401 | 5.9 |
| 44 | CHNQD-00824 | Aspergillus candidus (CHNSCLM-0393) | 7.64 |
| 45 | 4″-Deoxyterprenin | Aspergillus candidus HM5-4 | 6.69 |
| 46 | PR toxin | Emericellopsis maritima BC17 | 8.28 |
| 47 | Epipaxilline | Penicillium sp. KFD 28 | 5.3 |
| 48 | Monarubin B | Monascus ruber BB5 | 1.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Dong, Y.; Cui, X.; Guo, X.; Zhang, J.; Yu, C.; Zhang, M.; Wang, H. Effects of Marine Natural Products on Liver Diseases. Mar. Drugs 2024, 22, 288. https://doi.org/10.3390/md22070288
Sun Y, Dong Y, Cui X, Guo X, Zhang J, Yu C, Zhang M, Wang H. Effects of Marine Natural Products on Liver Diseases. Marine Drugs. 2024; 22(7):288. https://doi.org/10.3390/md22070288
Chicago/Turabian StyleSun, Yandi, Yansong Dong, Xiaohang Cui, Xiaohe Guo, Juan Zhang, Chong Yu, Man Zhang, and Haifeng Wang. 2024. "Effects of Marine Natural Products on Liver Diseases" Marine Drugs 22, no. 7: 288. https://doi.org/10.3390/md22070288
APA StyleSun, Y., Dong, Y., Cui, X., Guo, X., Zhang, J., Yu, C., Zhang, M., & Wang, H. (2024). Effects of Marine Natural Products on Liver Diseases. Marine Drugs, 22(7), 288. https://doi.org/10.3390/md22070288






