Exploring Sources, Biological Functions, and Potential Applications of the Ubiquitous Marine Cyclic Dipeptide: A Concise Review of Cyclic Glycine-Proline
Abstract
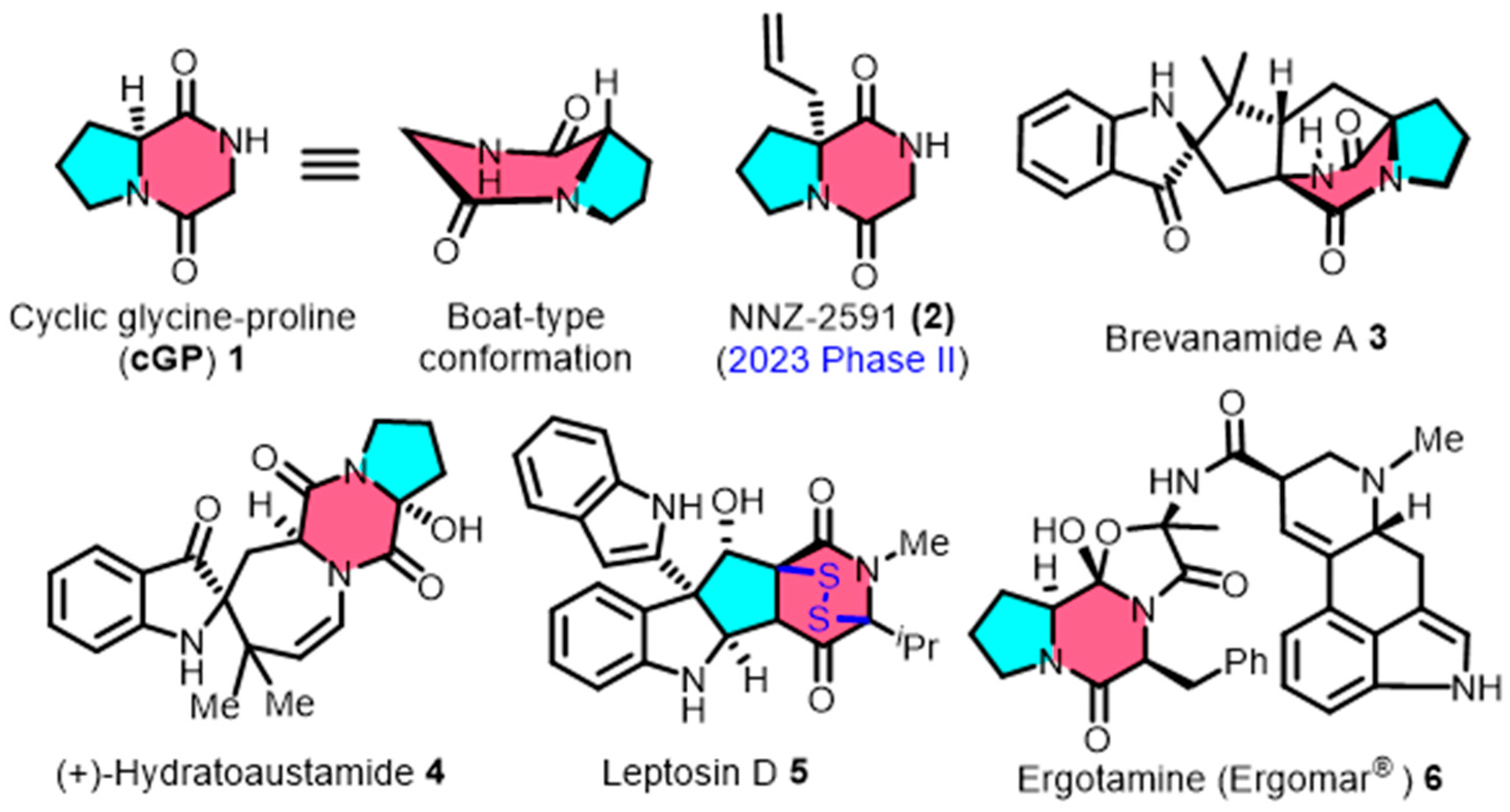
1. Introduction
2. Sources: Marine Origins, Endogenous Biological Transformation, and Chemical Synthesis
2.1. Marine Origins
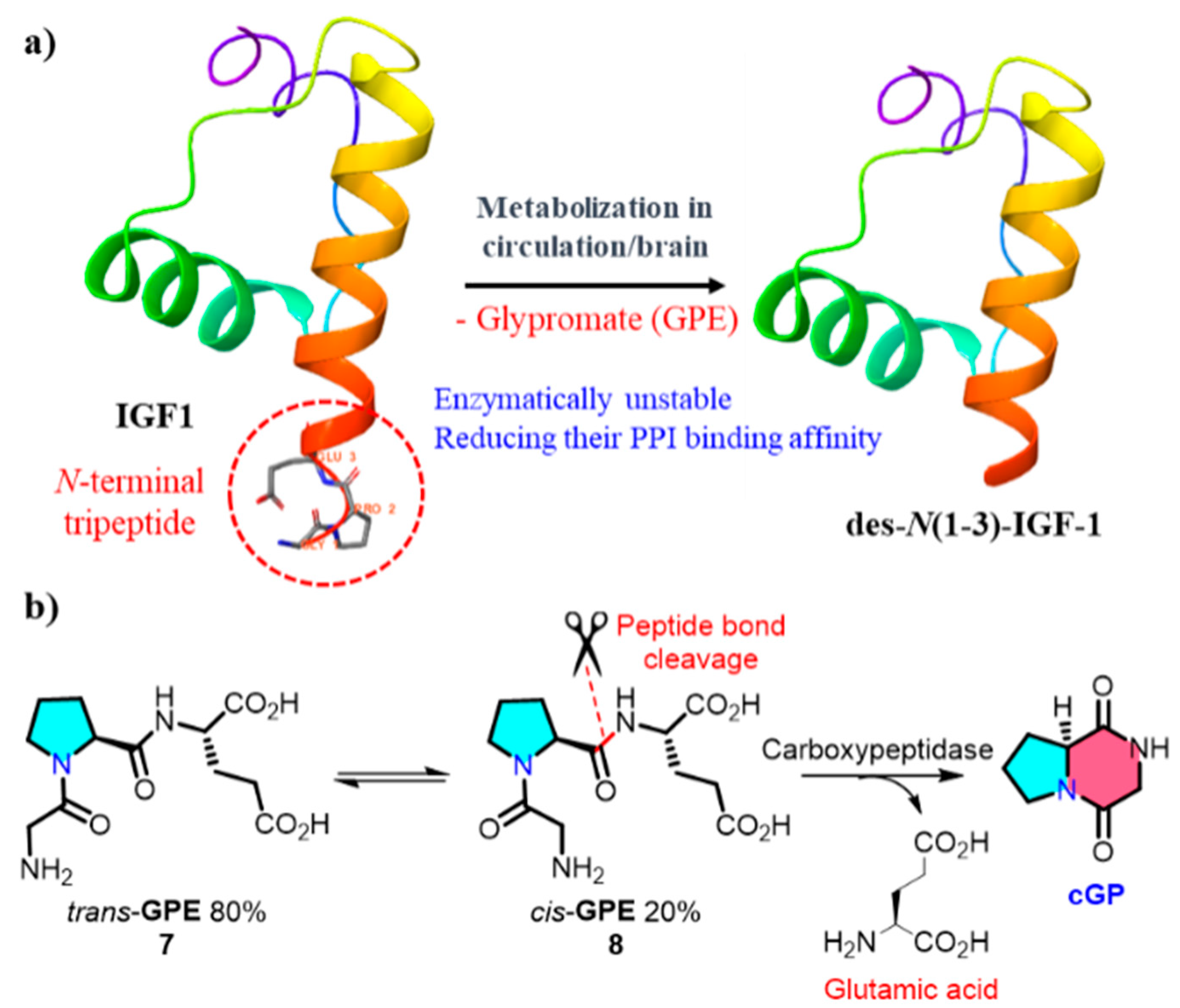
2.2. Endogenous Biological Transformation
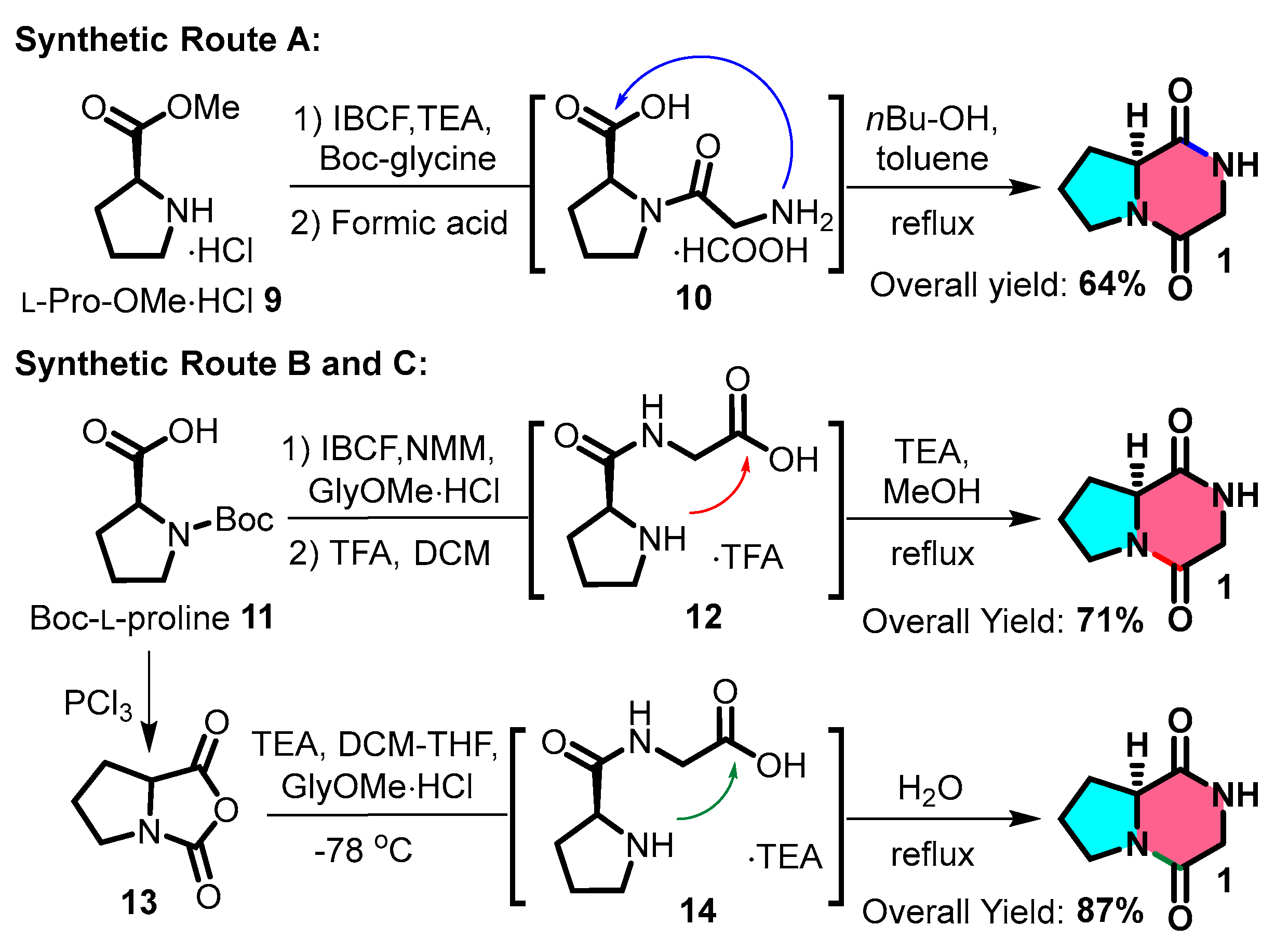
2.3. Chemical Synthesis
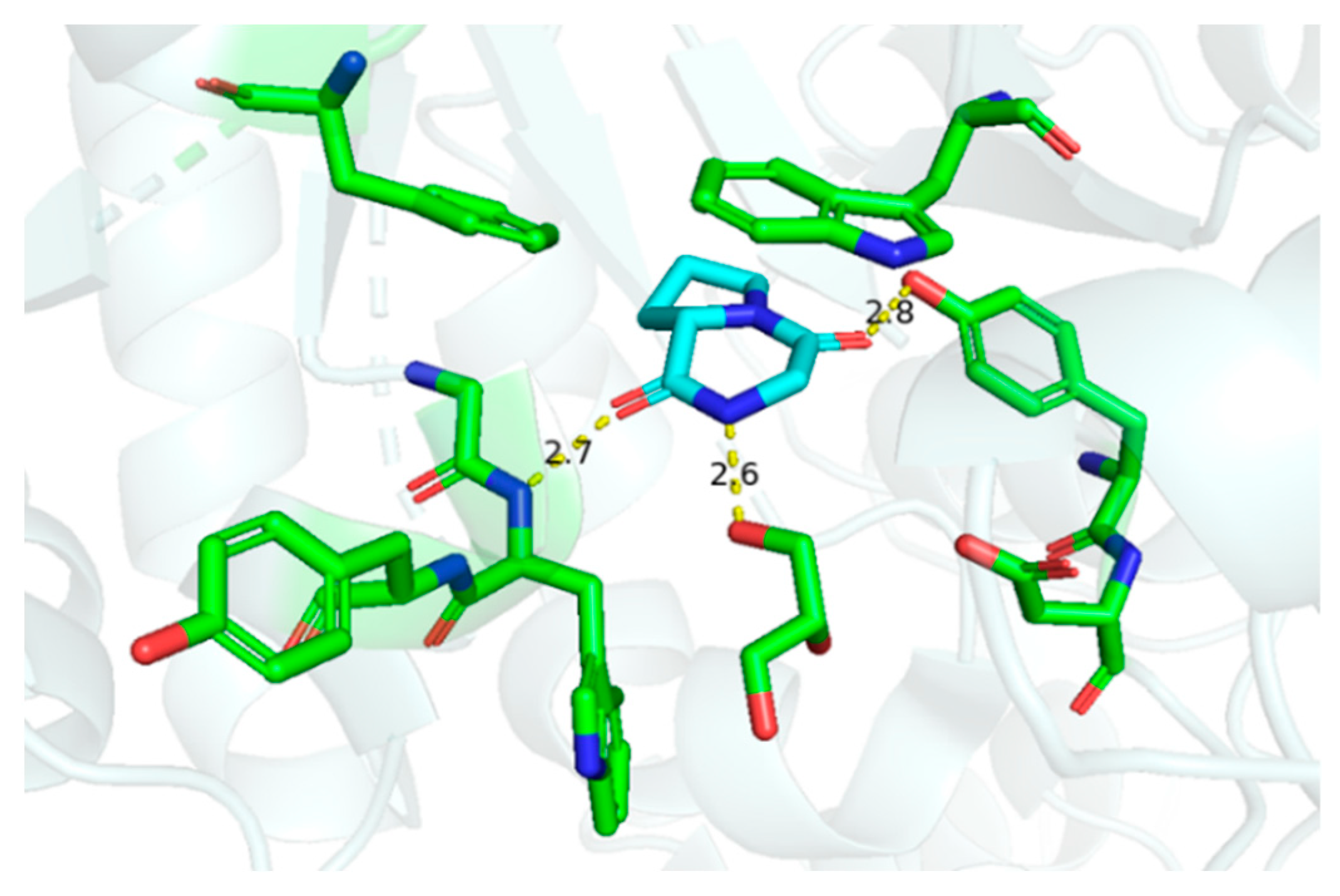
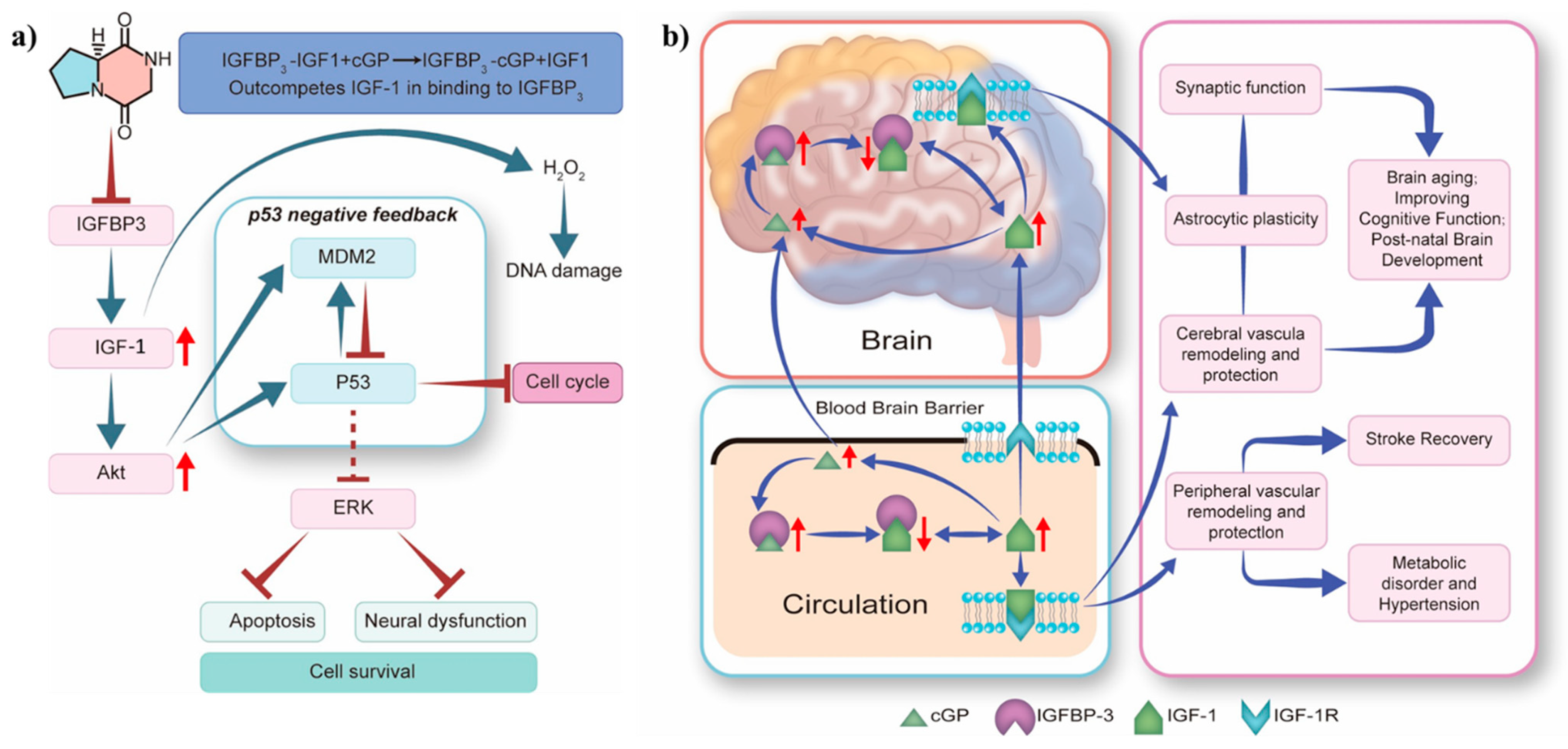
3. In Vitro and In Vivo Effects
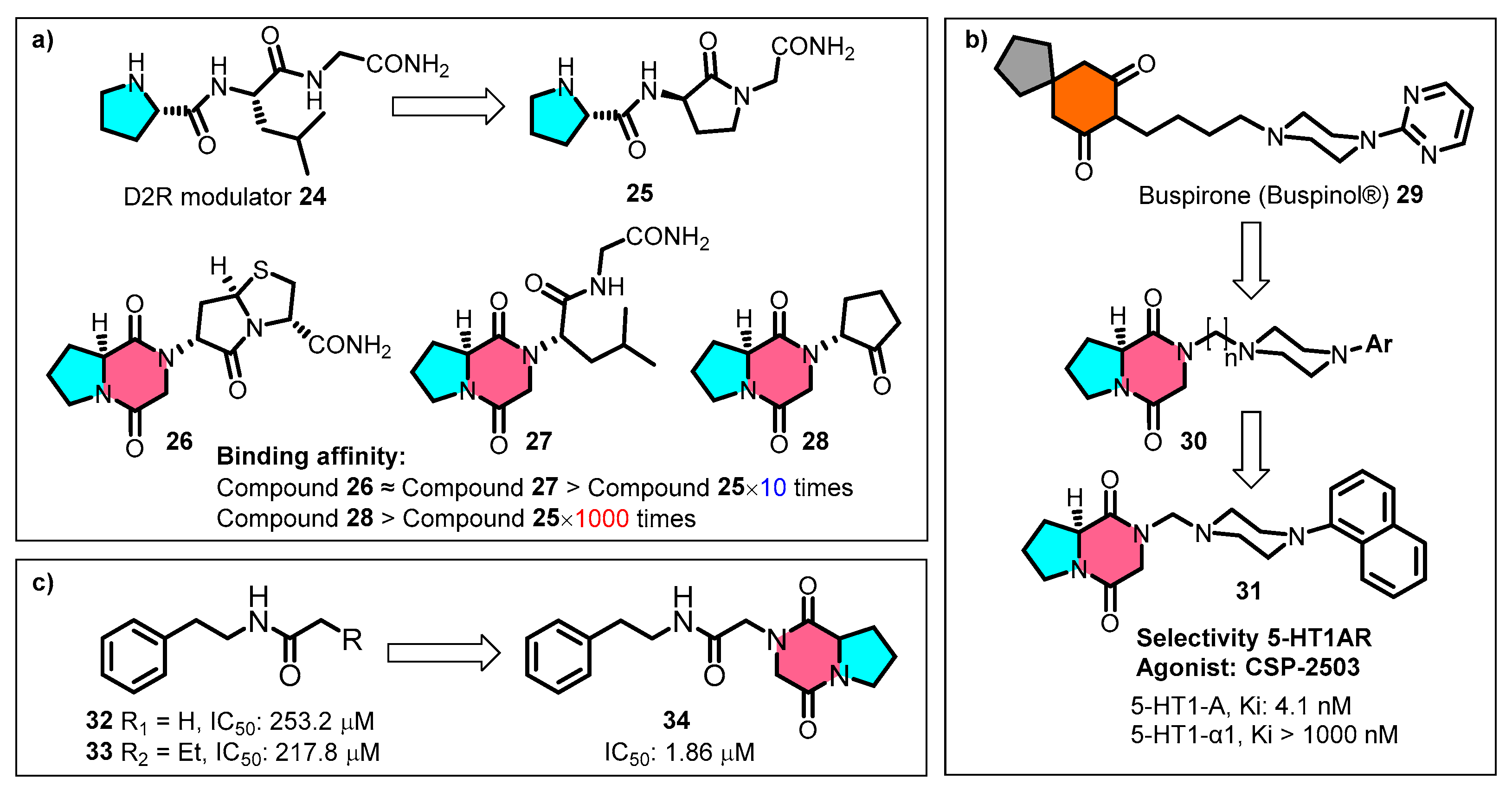
4. Exploring Applications for Building Blocks
5. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruggieri, G.D. Drugs from the sea: Marine organisms with novel chemical constituents are excellent sources of new drugs. Science 1976, 194, 491–497. [Google Scholar] [CrossRef]
- McGivern, J.G. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 2007, 3, 69–85. [Google Scholar] [CrossRef]
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.D.; Mateos, M.V.; Martín, A. Plitidepsin: Design, development, and potential place in therapy. Drug Des. Devel. Ther. 2017, 11, 253–264. [Google Scholar] [CrossRef]
- Costa, L.; Sousa, E.; Fernandes, C. Cyclic peptides in pipeline: What future for these great molecules? Pharmaceuticals 2023, 16, 996. [Google Scholar] [CrossRef]
- Sindhikara, D.; Borrelli, K. High throughput evaluation of macrocyclization strategies for conformer stabilization. Sci. Rep. 2018, 8, 6585. [Google Scholar] [CrossRef]
- Buckton, L.K.; Rahimi, M.N.; McAlpine, S.R. Cyclic peptides as drugs for intracellular targets: The next frontier in peptide therapeutic development. Chem. Eur. J. 2021, 27, 1487–1513. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S. Cyclic peptide drugs approved in the last two decades (2001–2021). RSC Chem. Biol. 2022, 3, 18–31. [Google Scholar] [CrossRef]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Song, Z.; Hou, Y.; Yang, Q.; Li, X.; Wu, S. Structures and biological activities of diketopiperazines from marine organisms: A review. Mar. Drugs 2021, 19, 403. [Google Scholar] [CrossRef] [PubMed]
- Gizzo, L.; Bliss, G.; Palaty, C.; Kolevzon, A. Caregiver perspectives on patient-focused drug development for Phelan-McDermid syndrome. Orphanet J. Rare Dis. 2024, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Bednarski, M.; Sapa, J.; Nowak, G. Ergotamine and nicergoline-facts and myths. Pharmacol. Rep. 2015, 67, 360–363. [Google Scholar] [CrossRef]
- Williams, R.M.; Cox, R.J. Paraherquamides, brevianamides, and asperparalines: Laboratory synthesis and biosynthesis. An interim report. Acc. Chem. Res. 2003, 36, 127–139. [Google Scholar] [CrossRef]
- Baran, P.S.; Corey, E.J. A short synthetic route to (+)-austamide,(+)-deoxyisoaustamide, and (+)-hydratoaustamide from a common precursor by a novel palladium-mediated indole → dihydroindoloazocine cyclization. J. Am. Chem. Soc. 2002, 124, 7904–7905. [Google Scholar] [CrossRef]
- Steyn, P.S. The structures of five diketopiperazines from Aspergillus ustus. Tetrahedron 1973, 29, 107–120. [Google Scholar] [CrossRef]
- Takahashi, C.; Minoura, K.; Yamada, T.; Numata, A.; Kushida, K.; Shingu, T.; Hagishita, S.; Nakai, H.; Sato, T.; Harada, H. Potent cytotoxic metabolites from a leptosphaeria species. Structure determination and conformational analysis. Tetrahedron 1995, 51, 3483–3498. [Google Scholar] [CrossRef]
- Ordóñez, M.; Torres-Hernández, F.; Viveros-Ceballos, J.L. Highly diastereoselective synthesis of cyclic α-aminophosphonic and α-aminophosphinic acids from glycyl-L-Proline 2,5-diketopiperazine. Eur. J. Org. Chem. 2019, 2019, 7378–7383. [Google Scholar] [CrossRef]
- Von Dreele, R.B. The crystal structure of cyclo-L-prolylglycyl: A refinement of high-angle diffraction data. Acta Crystallogr. B 1975, 31, 966–970. [Google Scholar] [CrossRef]
- Li, R.S.; Shi, K.L.; Long, K.H.; Mak, T.C.W. Studies on chemical constituents of Chinese marine folk remedies-isolation and X-ray structural characterization of cyclo-L-prolyl-glycyl from Diodon Novemacultus (Bleeker). J. Struct. Chem. 1985, 4, 210–213. [Google Scholar] [CrossRef]
- Trigos, A.; Reyna, S.; Gutierrez, M.L.; Sanchez, M. Diketopiperazines from cultures of the fungus Colletotrichum gloesporoides. Nat. Prod. Lett. 1997, 11, 13–16. [Google Scholar] [CrossRef]
- Trigos, A.; Reyna, S.; Cervantes, L. Three diketopiperazines from the cultivated fungus Fusarium oxysporum. Nat. Prod. Lett. 1995, 6, 241–246. [Google Scholar] [CrossRef]
- Furtado, N.A.; Pupo, M.T.; Carvalho, I.; Campo, V.L.; Duarte, M.C.T.; Bastos, J.K. Diketopiperazines produced by an Aspergillus fumigatus Brazilian strain. J. Braz. Chem. Soc. 2005, 16, 1448–1453. [Google Scholar] [CrossRef]
- Tan, Q.W.; Gao, F.; Wang, F.R.; Chen, Q.J. Anti-TMV activity of malformin A1, a cyclic penta-peptide produced by an endophytic fungus Aspergillus tubingensis FJBJ11. Int. J. Mol. Sci. 2015, 16, 5750–5761. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, H.; Yang, J. 2,5-Diketopiperazines from Aspergillus sp., the endophytic fungus of Astragalus membranaceus and their anticancer assay. Chem. Nat. Compd. 2020, 56, 583–585. [Google Scholar] [CrossRef]
- Lin, S.; Geng, M.; Liu, X.; Tan, J.; Yang, H. On the control of Microcystis aeruginosa and Synechococccus species using an algicidal bacterium, Stenotrophomonas F6, and its algicidal compounds cyclo-(Gly-Pro) and hydroquinone. J. Appl. Phycol. 2016, 28, 345–355. [Google Scholar] [CrossRef]
- Cao, X.T.; Wang, D.; Wang, N.; Cui, Z. Water-soluble constitutions from the skin of Bufo bufo gargarizans Cantor. Chin. J. Nat. Med. 2009, 7, 181–183. [Google Scholar] [CrossRef]
- Guigoz, Y.; Solms, J. Bitter peptides, occurrence and structure. Chem. Senses 1976, 2, 71–84. [Google Scholar] [CrossRef]
- Ginz, M.; Engelhardt, U.H. Identification of new diketopiperazines in roasted coffee. Eur. Food Res. Technol. 2001, 213, 8–11. [Google Scholar] [CrossRef]
- Chen, M.Z.; Dewis, M.L.; Kraut, K.; Merritt, D.; Reiber, L.; Trinnaman, L.; Da Costa, N.C. 2,5-Diketopiperazines (cyclic dipeptides) in beef: Identification, synthesis, and sensory evaluation. J. Food Sci. 2009, 74, C100–C105. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Alamri, Y.; Liu, K.; MacAskill, M.; Harris, P.; Brimble, M.; Dalrymple-Alford, J.; Prickett, T.; Menzies, O.; Laurenson, A.; et al. Supplementation of blackcurrant anthocyanins increased cyclic glycine-proline in the cerebrospinal fluid of Parkinson patients: Potential treatment to improve insulin-like growth factor-1 function. Nutrients 2018, 10, 714. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Q.; Ma, J.; Kang, W. Chemical constituents of Bacillus coagulans LL1103. Chem. Nat. Compd. 2018, 54, 419–420. [Google Scholar] [CrossRef]
- Dao, P.T.; Huong, D.T.M.; Murphy, B.; Van Minh, C.; Van Cuong, P. Compounds from culture broth of marine bacterium Oceanisphaera sp. Vietnam J. Chem. 2015, 53, 120–123. [Google Scholar]
- Jiang, Z.; Boyd, K.G.; Mearns-Spragg, A.; Adams, D.R.; Wright, P.C.; Burgess, J.G. Two diketopiperazines and one halogenated phenol from cultures of the marine bacterium, Pseudoalteromonas luteoviolacea. Nat. Prod. Lett. 2000, 14, 435–440. [Google Scholar] [CrossRef]
- Rosandy, A.R.; Ying, Y.C.; Kqueen, C.Y.; Lim, S.J.; Latip, J.; Murad, A.; Bakar, M.A.; Khalid, R.M. (-)-Glaciantarcin, a new dipeptide and some secondary metabolites from the psychrophilic yeast Glaciozyma antarctica PI12. Sains Malays. 2018, 47, 2693–2698. [Google Scholar] [CrossRef]
- Mitova, M.; Popov, S.; De Rosa, S. Cyclic peptides from a Ruegeria strain of bacteria associated with the sponge Suberites domuncula. J. Nat. Prod. 2004, 67, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.N.; Chen, G.; Bai, J.; Hua, H.M.; Xu, W.F.; Pei, Y.H. Isolation and identification of secondary metabolites from Penicillium oxalicum HSY-P-1. J. Shenyang Pharm. Univ. 2014, 31, 360–362. (In Chinese) [Google Scholar]
- Xie, L.R.; Li, D.Y.; Wang, P.L.; Hua, H.M.; Wu, X.; Li, Z.L. A new 3,4-seco-lanostane triterpenoid from a marine-derived fungus Ascotricha sp. ZJ-M-5. Acta Pharm. Sin. B 2013, 48, 89–93. [Google Scholar]
- Liu, H.B.; Gao, H.; Wang, N.L.; Lin, H.P.; Hong, K.; Yao, X.S. Cyclic dipeptides from the mangrove fungus Penicillium oxalicum (092007). J. Shenyang Pharm. Univ. 2007, 24, 474–478. (In Chinese) [Google Scholar]
- Wang, Y.; Zhang, Y.Q.; Xu, J.F.; Bai, Q.Q.; Chen, W.H.; Chen, G.Y. Study on diketopiperazine secondary metabolites of endophytic fungus Aspergillus terreus HT-1. Chin. J. Mar. Drugs 2022, 41, 10–16. (In Chinese) [Google Scholar]
- Li, H.J.; Lin, Y.C.; Liu, X.H.; Zhou, S.N. Peptide components of mangrove endogenous fungus No. 2524 (I). Acta Sci. Nat. Univ. Sunyatseni 2002, 41, 110–112. (In Chinese) [Google Scholar]
- Danh, C.D.; Dao, P.T.; Huong, D.T.M.; Thach, T.D.; Anh, N.M.; Minh, L.T.H.; Anh, T.T.; Van Cuong, P. Cyclopeptides from marine actinomycete Streptomyces sp. G261. Vietnam J. Chem. 2018, 56, 570–573. [Google Scholar] [CrossRef]
- Hue, N.T.; Linh, N.T.; Minh, L.T.H.; Minh, L.T.H.; Huyen, V.T.T.; Huong, D.T.M.; Cuong, P.V. Chemical constituents of marine-derived cctinomycete Streptomyces fradiae G650. Vietnam J. Sci. Technol. 2022, 60, 974–981. [Google Scholar]
- Chen, C.; Ye, Y.; Wang, R.; Zhang, Y.; Wu, C.; Debnath, S.C.; Ma, Z.; Wang, J.; Wu, M. Streptomyces nigra sp. nov. is a novel actinobacterium isolated from mangrove soil and exerts a potent antitumor activity in vitro. Front Microbiol. 2018, 9, 1587. [Google Scholar] [CrossRef]
- Chen, J.H.; Lan, X.P.; Liu, Y.; Jia, A.Q. The effects of diketopiperazines from Callyspongia sp. on release of cytokines and chemokines in cultured J774A.1 macrophages. Bioorg. Med. Chem. Lett. 2012, 22, 3177–3180. [Google Scholar]
- Chen, Y.; Peng, Y.; Gao, C.; Huang, R. A new diketopiperazine from South China Sea marine sponge Callyspongia sp. Nat. Prod. Res. 2014, 28, 1010–1014. [Google Scholar] [CrossRef]
- Huang, R.; Yan, T.; Peng, Y.; Zhou, X.; Yang, X.; Liu, Y. Diketopiperazines from the marine sponge Axinella sp. Chem. Nat. Compd. 2014, 50, 191–193. [Google Scholar] [CrossRef]
- Zhang, H.; Lai, W.; Guan, Z.B.; Liao, X.J.; Zhao, B.X.; Xu, S.H. A new thiodiketopiperzaine from the marine sponge Tedania sp. Nat. Prod. Res. 2020, 34, 1113–1117. [Google Scholar]
- Friess, D.A.; Rogers, K.; Lovelock, C.E.; Krauss, K.W.; Hamilton, S.E.; Lee, S.Y.; Lucas, R.; Primavera, J.; Rajkaran, A.; Shi, S. The state of the world’s mangrove forests: Past, present, and future. Annu. Rev. Environ. Resour. 2019, 44, 89–115. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Boyko, S.S.; Akparov, V.K.; Ostrovskaya, R.U.; Skoldinov, S.P.; Rozantsev, G.G.; Voronina, T.A.; Zherdev, V.P.; Seredenin, S.B. Identification of a novel endogenous memory facilitating cyclic dipeptide cyclo-prolylglycine in rat brain. FEBS Lett. 1996, 391, 149–152. [Google Scholar] [CrossRef]
- Keating, G.M. Mecasermin. BioDrugs 2008, 22, 177–188. [Google Scholar] [CrossRef]
- Fan, D.; Krishnamurthi, R.; Harris, P.; Barber, P.A.; Guan, J. Plasma cyclic glycine proline/IGF-1 ratio predicts clinical outcome and recovery in stroke patients. Ann. Clin. Transl. Neurol. 2019, 6, 669–677. [Google Scholar] [CrossRef]
- Guan, J.; Gluckman, P.D. IGF-1 derived small neuropeptides and analogues: A novel strategy for the development of pharmaceuticals for neurological conditions. Br. J. Pharmacol. 2009, 157, 881–891. [Google Scholar] [CrossRef]
- Guan, J.; Harris, P.; Brimble, M.; Lei, Y.; Lu, J.; Yang, Y.; Gunn, A.J. The role for IGF-1-derived small neuropeptides as a therapeutic target for neurological disorders. Expert Opin. Ther. Targets 2015, 19, 785–793. [Google Scholar] [CrossRef]
- Hayasaka, F.; Yamamoto, S.; Sakai, Y. Production method for cyclic dipeptide derived from native collagen. Food Sci. Technol. 2016, 22, 477–483. [Google Scholar] [CrossRef]
- Ishizu, T.; Tokunaga, M.; Fukuda, M.; Matsumoto, M.; Goromaru, T.; Takemoto, S. Molecular capture and conformational change of diketopiperazines containing proline residues by epigallocatechin-3-O-gallate in water. Chem. Pharm. Bull. 2021, 69, 585–589. [Google Scholar] [CrossRef]
- de Costa, B.R.; He, X.; Linders, J.T.M.; Dominguez, C.; Gu, Z.Q.; Williams, W.; Bowen, W. Synthesis and evaluation of conformationally restricted N-[2-(3, 4-dichlorophenyl) ethyl]-N-methyl-2-(1-pyrrolidinyl) ethylamines at. sigma. receptors. 2. Piperazines, bicyclic amines, bridged bicyclic amines, and miscellaneous compounds. J. Med. Chem. 1993, 36, 2311–2320. [Google Scholar] [CrossRef]
- Hendea, D.; Laschat, S.; Baro, A.; Frey, W. Diastereoselective alkylation of a proline-derived bicyclic lactim ether. Helv. Chim. Acta 2006, 89, 1894–1909. [Google Scholar] [CrossRef]
- Ahonen, K.; Lahtinen, M.; Kolehmainen, E. Cyclic dipeptides: Catalyst/promoter-free, rapid and environmentally benign cyclization of free amino acids. Green Chem. 2011, 13, 1203–1209. [Google Scholar]
- Poonia, B.K.; Sidhu, A.; Sharma, A.B. Cyclo (L-proline-L-serine) dipeptide suppresses seed borne fungal pathogens of rice: Altered cellular membrane integrity of fungal hyphae and seed quality benefits. J. Agric. Food Chem. 2022, 70, 2160–2168. [Google Scholar] [CrossRef]
- Ying, J.; Lin, R.; Xu, P.; Wu, Y.; Liu, Y.; Zhao, Y. Prebiotic formation of cyclic dipeptides under potentially early earth conditions. Sci. Rep. 2018, 8, 936. [Google Scholar] [CrossRef]
- Guo, Y.; Ying, J.; Sun, D.; Zhang, Y.; Zheng, M.; Ding, R.; Liu, Y.; Zhao, Y. Cyclic Dipeptides formation from linear dipeptides under potentially prebiotic earth conditions. Front. Chem. 2021, 9, 675821. [Google Scholar] [CrossRef]
- Khan, R.; Basha, A.; Goverdhanam, R.; Rao, P.C.; Tanemura, Y.; Fujimoto, Y.; Begum, A.S. Attenuation of TNF-α secretion by L-proline-based cyclic dipeptides produced by culture broth of Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett. 2015, 25, 5756–5761. [Google Scholar] [CrossRef]
- Houston, D.R.; Synstad, B.; Eijsink, V.G.H.; Stark, M.J.R.; Eggleston, I.M.; Van Aalten, D.M.F. Structure-based exploration of cyclic dipeptide chitinase inhibitors. J. Med. Chem. 2004, 47, 5713–5720. [Google Scholar] [CrossRef]
- Varejão, E.V.V.; Demuner, A.J.; de Almeida Barbosa, L.C.; Barreto, R.W. Phytotoxic effects of metabolites from Alternaria euphorbiicola against its host plant Euphorbia heterophylla. Quim. Nova 2013, 36, 1004–1007. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, Y.H.; Yang, J.; Cui, W.Y.; He, P.J.; Munir, S.; He, P.F.; Wu, Y.X.; He, Y.Q. Acaricidal activity of cyclodipeptides from Bacillus amyloliquefaciens W1 against Tetranychus urticae. J. Agric. Food Chem. 2018, 66, 10163–10168. [Google Scholar] [CrossRef]
- Li, X.; Munir, S.; Xu, Y.; Wang, Y.; He, Y. Combined mass spectrometry-guided genome mining and virtual screening for acaricidal activity in secondary metabolites of Bacillus velezensis W1. RSC Adv. 2021, 11, 25441–25449. [Google Scholar] [CrossRef]
- Mendes, L.L.; Varejão, J.O.S.; de Souza, J.A.; de M. Carneiro, J.W.; Valdo, A.K.S.M.; Martins, F.T.; Ferreira, B.W.; Barreto, R.W.; da Silva, T.I.; Kohlhoff, M.; et al. 2,5-Diketopiperazines via intramolecular N-alkylation of Ugi adducts: A contribution to the synthesis, density functional theory study, X-ray characterization, and potential herbicide application. J. Agric. Food Chem. 2022, 70, 1799–1809. [Google Scholar]
- Murotomi, K.; Kagiwada, H.; Hirano, K.; Yamamoto, S.; Numata, N.; Matsumoto, Y.; Kaneko, H.; Namihira, M. Cyclo-glycylproline attenuates hydrogen peroxide-induced cellular damage mediated by the MDM2-p53 pathway in human neural stem cells. J. Cell. Physiol. 2023, 238, 434–446. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Ostrovskaya, R.U.; Maksimova, F.V.; Chuppin, A.V.; Trofimov, S.S.; Lezina, V.P.; Voronina, T.A.; Skoldinov, A.P. Proline-based topologic pyracetam analogs and their nootropic activity. Pharm. Chem. J. 1989, 23, 203–208. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Konstantinopol’skii, M.A.; Ostrovskaya, R.U.; Seredenin, S.B. Anxiolytic activity of endogenous nootropic dipeptide cycloprolylglycine in elevated plus-maze test. Bull. Exp. Biol. Med. 2001, 131, 464–466. [Google Scholar] [CrossRef]
- Ferro, J.N.; de Aquino, F.L.; de Brito, R.G.; dos Santos, P.L.; Quintans, J.S.S.; de Souza, L.C.; de Araújo, A.F.; Diaz, B.L.; Lucca--Júnior, W.; Quintans-Júnior, L.J.; et al. Cyclo-Gly-Pro, a cyclic dipeptide, attenuates nociceptive behaviour and inflammatory response in mice. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1287–1295. [Google Scholar] [CrossRef]
- Garibova, T.L.; Gudasheva, T.A.; Seredenin, S.B. A new component in the mechanism of regulation of endogenous depressive-like states. Dokl. Biochem. Biophys. 2019, 488, 324–326. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Grigoriev, V.V.; Koliasnikova, K.N.; Zamoyski, V.L.; Seredenin, S.B. Neuropeptide cycloprolylglycine is an endogenous positive modulator of AMPA receptors. Dokl. Biochem. Biophys. 2016, 471, 387–389. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Povarnina, P.Y.; Koliasnikova, K.N.; Alyaeva, A.G.; Vorontsova, O.N.; Seredenin, S.B. The anxiolytic effect of the neuropeptide cycloprolylglycine is mediated by AMPA and TrkB receptors. Dokl. Biochem. Biophys. 2020, 493, 190–192. [Google Scholar] [CrossRef]
- Guan, J.; Li, F.; Kang, D.; Pitcher, T.; Dalrymple-Alford, J.; Shorten, P.; Singh-Mallah, G. Cyclic glycine-proline (cGP) normalises insulin-like growth factor-1 (IGF-1) function: Clinical significance in the ageing brain and in age-related neurological conditions. Molecules 2023, 28, 1021. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics Free Web Services. Slovensky Grob, Slovakia. Available online: https://www.molinspiration.com (accessed on 14 February 2024).
- Goldberg, W.; Kettle, J.G.; Kogej, T. Designing novel building blocks is an overlooked strategy to improve compound quality. Drug Discov. Today 2015, 20, 11–17. [Google Scholar] [CrossRef]
- Grygorenko, O.O.; Volochnyuk, D.M.; Ryabukhin, S.V.; Judd, D.B. The symbiotic relationship between drug discovery and organic chemistry. Chem. Eur. J. 2020, 26, 1196–1237. [Google Scholar] [CrossRef]
- Grygorenko, O.O.; Volochnyuk, D.M.; Vashchenko, B.V. Emerging building blocks for medicinal chemistry: Recent synthetic advances. Eur. J. Org. Chem. 2021, 2021, 6478–6510. [Google Scholar] [CrossRef]
- Wei, W.; Cherukupalli, S.; Jing, L.; Liu, X.; Zhan, P. Fsp3: A new parameter for drug-likeness. Drug Discov. Today 2020, 25, 1839–1845. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Whitty, A.; Zhong, M.; Viarengo, L.; Beglov, D.; Hall, D.R.; Vajda, S. Quantifying the chameleonic properties of macrocycles and other high-molecular-weight drugs. Drug Discov. Today 2016, 21, 712–717. [Google Scholar] [CrossRef]
- Clark, D.E.; Grootenhuis, P.D. Predicting passive transport in silico: History, hype, hope. Curr. Top. Med. Chem. 2003, 3, 1193–1203. [Google Scholar] [CrossRef]
- Lobell, M.; Molnar, L.; Keseru, G.M. Recent advances in the prediction of blood-brain partitioning from molecular structure. J. Pharm. Sci. 2003, 92, 360–370. [Google Scholar] [CrossRef]
- Baures, P.W.; Ojala, W.H.; Costain, W.J.; Ott, M.C.; Pradhan, A.; Gleason, W.B.; Mishra, R.K.; Johnson, R.L. Design, synthesis, and dopamine receptor modulating activity of diketopiperazine peptidomimetics of L-prolyl-L-leucylglycinamide. J. Med. Chem. 1997, 40, 3594–3600. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, M.L.; Morcillo, M.J.; Fernandez, E.; Benhamú, B.; Tejada, I.; Ayala, D.; Viso, A.; Campillo, M.; Pardo, L.; Delgado, M.; et al. Synthesis and structure-activity relationships of a new model of arylpiperazines. 8. computational simulation of ligand-receptor interaction of 5-HT1AR agonists with selectivity over α1-adrenoceptors. J. Med. Chem. 2005, 48, 2548–2558. [Google Scholar] [CrossRef]
- Sato, H.; Skelin, L.; Debonnel, G.; Diksic, M. Chronic buspirone treatment normalizes open field behavior in olfactory bulbectomized rats: Assessment with a quantitative autoradiographic evaluation of the 5-HT1A binding sites. Brain Res. Bull. 2008, 75, 545–555. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, M.L.; Morcillo, M.J.; Fernandez, E.; Benhamú, B.; Tejada, I.; Ayala, D.; Viso, A.; Olivella, M.; Pardo, L.; Delgado, M.; et al. Design and synthesis of S-(−)-2-[[4-(napht-1-yl) piperazin-1-yl] methyl]-1,4-dioxoperhydropyrrolo [1,2-a] pyrazine (CSP-2503) using computational simulation. A 5-HT1A receptor agonist. Bioorg. Med. Chem. Lett. 2003, 13, 1429–1432. [Google Scholar] [CrossRef]
- Delgado, M.; Caicoya, A.G.; Greciano, V.; Benhamú, B.; López-Rodríguez, M.L.; Fernández-Alfonso, M.S.; Pozo, M.A.; Manzanares, J.; Fuentes, J.A. Anxiolytic-like effect of a serotonergic ligand with high affinity for 5-HT1A, 5-HT2A and 5-HT3 receptors. Eur. J. Pharmacol. 2005, 511, 9–19. [Google Scholar] [CrossRef]
- Hasan, A.; Yeom, H.S.; Ryu, J.; Bode, H.B.; Kim, Y. Phenylethylamides derived from bacterial secondary metabolites specifically inhibit an insect serotonin receptor. Sci. Rep. 2019, 9, 20358. [Google Scholar] [CrossRef]
- Kwon, M.J.; Steiniger, C.; Cairns, T.C.; Wisecaver, J.H.; Lind, A.L.; Pohl, C.; Regner, C.; Rokas, A.; Meyer, V. Beyond the biosynthetic gene cluster paradigm: Genome-wide coexpression networks connect clustered and unclustered transcription factors to secondary metabolic pathways. Microbiol. Spectr. 2021, 9, e00898-21. [Google Scholar] [CrossRef]
- Khaldi, N.; Seifuddin, F.T.; Turner, G.; Haft, D.; Nierman, W.C.; Wolfe, K.H.; Fedorova, N.D. SMURF: Genomic mapping of fungal secondary metabolite clusters. Fungal Genet. Biol. 2010, 47, 736–741. [Google Scholar] [CrossRef]
- Maiya, S.; Grundmann, A.; Li, S.M.; Turner, G. The fumitremorgin gene cluster of Aspergillus fumigatus: Identification of a gene encoding brevianamide F synthetase. ChemBioChem 2006, 7, 1062–1069. [Google Scholar] [CrossRef]
- Maiya, S.; Grundmann, A.; Li, S.M.; Turner, G. Improved tryprostatin B production by heterologous gene expression in Aspergillus nidulans. Fungal Genet. Biol. 2009, 46, 436–440. [Google Scholar] [CrossRef]
- Canu, N.; Moutiez, M.; Belin, P.; Gondry, M. Cyclodipeptide synthases: A promising biotechnological tool for the synthesis of diverse 2,5-diketopiperazines. Nat. Prod. Rep. 2020, 37, 312–321. [Google Scholar] [CrossRef]
- Gondry, M.; Sauguet, L.; Belin, P.; Thai, R.; Amouroux, R.; Tellier, C.; Tuphile, K.; Jacquet, M.; Braud, S.; Courçon, M.; et al. Cyclodipeptide synthases are a family of tRNA-dependent peptide bond-forming enzymes. Nat. Chem. Biol. 2009, 5, 414–420. [Google Scholar] [CrossRef]
- Dubois, P.; Correia, I.; Le Chevalier, F.; Dubois, S.; Jacques, I.; Canu, N.; Moutiez, M.; Thai, R.; Gondry, M.; Lequin, O.; et al. Reprogramming Escherichia coli for the production of prenylated indole diketopiperazine alkaloids. Sci. Rep. 2019, 9, 9208. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Lovering, F. Escape from flatland 2: Complexity and promiscuity. Med. Chem. Comm. 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Horrigan, J.P.; Tartaglia, N.; Hagerman, R.; Kolevzon, A.; Erickson, C.A.; Hatti, S.; Snape, M.; Yaroshinsky, A.; Stoms, G. A double-blind, randomized, placebo-controlled clinical study of Trofinetide in the treatment of Fragile X Syndrome. Pediatr. Neurol. 2020, 110, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Glaze, D.G.; Neul, J.L.; Percy, A.; Feyma, T.; Beisang, A.; Yaroshinsky, A.; Stoms, G.; Zuchero, D.; Horrigan, J.; Glass, L.; et al. A double-blind, randomized, placebo-controlled clinical study of Trofinetide in the treatment of rett syndrome. Pediatr. Neurol. 2017, 76, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Glaze, D.G.; Neul, J.L.; Kaufmann, W.E.; Berry-Kravis, E.; Condon, S.; Stoms, G.; Oosterholt, S.; Della Pasqua, O.; Glass, L.; Jones, N.E.; et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric rett syndrome. Neurology 2019, 92, e1912–e1925. [Google Scholar] [CrossRef] [PubMed]


| Sources | Species | Habitats | Bioactivities | Refs |
|---|---|---|---|---|
| Bacteria | B.coagulans | Yellow croaker, Zhejiang, China | -- a | [30] |
| Oceanisphaera sp. | Halong Bay sediment, Vietnam | -- a | [31] | |
| Pseudoalteromonas luteoviolacea | Hawaiian seaweed a | Stimulate bacteria for quorum-sensing signals | [32] | |
| Psychrophilic yeast Glaciozyma antarctica PI12 | Casey Research Station, Antarctica | Weak cytotoxicity or anti-oxidation | [33] | |
| Ruegeria Strain | Sponge Suberites domuncula a | No antimicrobial or antifungal | [34] | |
| Fungus | Penicillium oxalicum | Coastal intertidal zone, Tsingtao, China | -- a | [35] |
| Ascotricha sp. | Mudflat soil, Zhejiang, China | Weak cytotoxicity | [36] | |
| Penicillium oxalicum | Mangrove plant root, Hainan, China | No cytotoxicity | [37] | |
| Aspergillus terreus HT-1 | Mangrove plant root, Hainan, China | -- a | [38] | |
| Mangrove endophytic fungus | Mangrove plant seed, Hong Kong, China | -- a | [39] | |
| Actinomycetes | Streptomyces sp. G261 | Sediment, Cham Islands, Vietnam | -- a | [40] |
| Streptomyces fradiae | Prickly pen shell, Van Phong, Vietnam | -- a | [41] | |
| Streptomyces sp. | Mangrove rhizosphere soil, Fujian, China | HepG2 cells (IC50: 101.8 μM) | [42] | |
| Sponge | Callyspongia sp. | South China Sea | Mild macrophage cytokines stimulator | [43] |
| Callyspongia sp. | South China Sea | No cytotoxicity | [44] | |
| Axinella sp. | South China Sea | -- a | [45] | |
| Tedania sp. | Zhanjiang, China | -- a | [46] |
| ADME Parameters a | cGP | “Rule of Two” [79] | Lipinski’s Rule [81] | Veber’s Rule [82] | Clark&Lobell’s Rule [85,86] |
|---|---|---|---|---|---|
| MW | 154.2 | <200 | <500 | <500 | <450 |
| cLogP | −1.37/−0.29 c | <2 | ≤5 | -- | 1–3 |
| H Don | 2 | ≤2 | ≤5 | Sum ≤ 10 | -- |
| Hacc | 1 | ≤4 | ≤10 | <6 | |
| Rot B | 0 | ≤4 | -- | ≤10 | -- |
| Fsp3 | 0.73 | 0.2~1 | -- | -- | -- |
| TPSA b | 49.4 Å2 | -- | -- | <140 Å2 | <60–70 Å2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Lin, J.; Qin, F.; Xu, L.; Luo, L. Exploring Sources, Biological Functions, and Potential Applications of the Ubiquitous Marine Cyclic Dipeptide: A Concise Review of Cyclic Glycine-Proline. Mar. Drugs 2024, 22, 271. https://doi.org/10.3390/md22060271
Hu L, Lin J, Qin F, Xu L, Luo L. Exploring Sources, Biological Functions, and Potential Applications of the Ubiquitous Marine Cyclic Dipeptide: A Concise Review of Cyclic Glycine-Proline. Marine Drugs. 2024; 22(6):271. https://doi.org/10.3390/md22060271
Chicago/Turabian StyleHu, Lei, Jing Lin, Fei Qin, Li Xu, and Lianzhong Luo. 2024. "Exploring Sources, Biological Functions, and Potential Applications of the Ubiquitous Marine Cyclic Dipeptide: A Concise Review of Cyclic Glycine-Proline" Marine Drugs 22, no. 6: 271. https://doi.org/10.3390/md22060271
APA StyleHu, L., Lin, J., Qin, F., Xu, L., & Luo, L. (2024). Exploring Sources, Biological Functions, and Potential Applications of the Ubiquitous Marine Cyclic Dipeptide: A Concise Review of Cyclic Glycine-Proline. Marine Drugs, 22(6), 271. https://doi.org/10.3390/md22060271






