Abstract
A marine-derived fungal strain, Aspergillus sp. ITBBc1, was isolated from coral collected from the South China Sea in Hainan province. Intensive chemical investigation of the fermentation extract of this strain afforded four new secondary metabolites (1–4), named megastigmanones A–C and prenylterphenyllin H, along with four known compounds (5–8). Their structures were elucidated by extensive spectroscopic analysis including one-and two-dimensional (1D and 2D) NMR spectroscopy and high-resolution electrospray ionization mass spectrometry (HR-ESI-MS). The modified Mosher’s method was undertaken to determine the absolute configurations of new compounds. The phytotoxic activity test showed that compounds 6–8 exhibited significant antagonistic activity against the germination of Triticum aestivum L. and Oryza sativa L. seeds with a dose-dependent relationship.
1. Introduction
Fungi are ubiquitous in nature, with a wide variety and large quantities. Many fungi are beneficial to the environment, humans and animals, while some are harmful due to the production of toxic metabolites (mycotoxins) [1]. More than 300 mycotoxins have been identified so far [2], and most of them are produced by well-known genera including Aspergillus, Fusarium and Penicillium [3,4,5,6,7]. Mycotoxins not only carry the potential to cause contaminations and result in serious annual yield and quality losses but also pose life-threatening risks to human and animal health [8]. Therefore, they have become a major issue in the sciences [9,10]. With synthetic agrochemicals becoming increasingly controversial due to their environmental and toxicological problems [11], mycotoxins have drawn great attention because of their potential as natural herbicides [12,13,14]. Modern agriculture heavily relies on natural herbicides for weed control due to their high efficiency and low cost [15]. Thus, herbicidal mycotoxins are considered environmentally friendly and sustainable alternative agents for controlling weeds. However, little is known about the occurrence and mechanisms of biosynthesis under various environmental conditions [16]. Therefore, excavating these mycotoxins has a vital impact on exploring the great potential of new natural herbicides.
With the expansion of research scope, more and more fungal mycotoxins with herbicidal potential have been discovered from marine fungi [17,18]. Many fungal mycotoxins can also be used as lead compounds, based on their structure, to obtain more active toxic compounds through chemical synthesis. The synthesis of Cinnacidin analogues is a typical case, and the synthesized analogues have better phytotoxic activity against weeds, demonstrating their potential application value as herbicides [19].
As part of our continuing exploration of structurally novel and biologically interesting secondary metabolites from marine-derived microorganisms [20,21,22], a coral-associated fungus, Aspergillus sp. ITBBc1, has drawn our attention. Furthermore, intensive chemical investigation of this fungus led to the identification of four new compounds (1–4) and four known compounds (5–8) (Figure 1). Herein, we report the isolation, structural elucidation, and biological activity evaluation of these compounds.
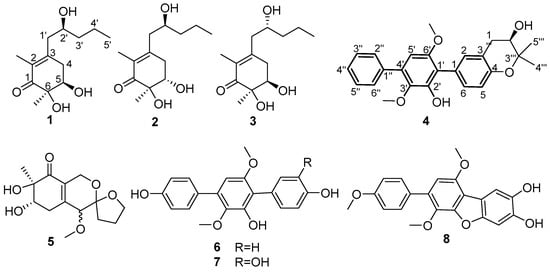
Figure 1.
Chemical structures of compounds 1–8.
2. Results
The EtOAc extract of the fungus Aspergillus sp. ITBBc1 was subjected to repeated column chromatography (CC) (silica gel, Sephadex LH-20) and semi-preparative HPLC to yield four new compounds (1–4) along with four known analogues (5–8). The known compounds were rapidly characterized as peniazaphilin A (5) [23], terphenyllin (6) [24], 3-hydroxyterphenyllin (7) [25], and candidusin C (8) [26] (Figure 1), respectively, via comparison of their experimental spectroscopic data with those reported in the literature.
2.1. Structural Elucidation
Megastigmanone A (1) was obtained as colorless oil. Its molecular formula, C13H22O4, was determined based on the HR-ESI-MS ion at m/z 265.1410 [M + Na]+ (calcd, C13H22NaO4, 265.1410), indicating three degrees of unsaturation. The 1H NMR spectrum (Figure S1) revealed the existence of three methyls at δH 1.79 (3H, s, CH3-2), 1.23 (3H, s, CH3-6), and 0.91 (3H, t, J = 7.1Hz, H-5′), two oxygenated protons at δH 3.90 (1H, m, H-2′) and 3.98 (1H, dd, J = 3.7, 2.9 Hz, H-5), eight aliphatic protons and three hydroxyl groups (Table 1). The 13C and DEPT135 NMR data showed that there were 13 carbon resonances, which were assigned to one carbonyl at δC 202.2 (C-1), three nonprotonated carbons at δC 152.7 (C-3), 128.8 (C-2), and 76.4 (C-6), two methines at δC 74.2 (C-5) and 70.0 (C-2′), four methylenes at δC 43.7 (C-1′), 41.0 (C-3′), 37.1 (C-4) and 19.6 (C-4′), and three methyls at δC 23.3 (CH3-6), 14.3 (C-5′) and 11.6 (CH3-2) (Table 1). The planar structure of compound 1 was proposed by HMBC (Figure S5) and 1H-1H COSY spectra (Figure S6). Analysis of the 1H-1H COSY spectrum revealed the existence of two separate spin systems: H2-4-H-5 and H2-1′-H3-5′ (Figure 2). HMBC correlations from H-4 to C-2, C-3 and C-6, from H-5 to C-1, C-3, C-6 and CH3-5′, from H-1′ to C-2, C-3, C-4 and C-3′, from H-2′ to C-3, from H-4′ to C-2′, and from H-5′ to C-3′ were observed. The HMBC correlation from CH3-2 to C-1, C-2 and C-3 suggested the methyl group at C-2, and that from CH3-6 to C-1, C-5 and C-6 suggested the methyl group at C-6. The ROESY correlations of H-5/CH3-6, H-5/H2-4,H-4/H-2′ and CH3-2/H2-1′ (Figure 2) could further support the relative configuration. The whole interpretation of 1D and 2D NMR data allowed the confirmation of the planar structure of compound 1, as shown in Figure 1. In order to further determine the absolute configuration of compound 1, the modified Mosher’s method was used to determine the absolute configurations of secondary alcohols at C-5 and C-2′. Compound 1 was reacted with (R)/(S)-MTPA-Cl to obtain (S) and (R)-Mosher esters. The difference in chemical shifts of the Mosher esters of compound 1 indicate that the stereocenters at C-5 and C-2′ were R and S (Figure 3; Figures S8–S11), respectively. Therefore, the complete structure of compound 1 was established as (5R, 6R)-5,6-dihydroxy-3-((S)-2′-hydroxypentyl)-2, 6-dimethylcyclohex-2- en-1-one.

Table 1.
1H (500 MHz) and 13C (125 MHz) NMR data for compounds 1–3 in acetone-d6.
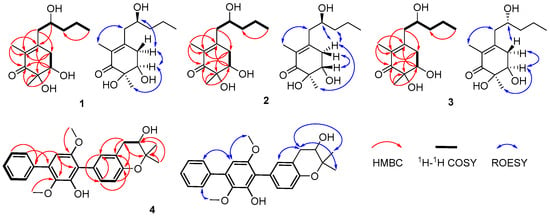
Figure 2.
Key HMBC, 1H-1H COSY and ROESY correlations of new compounds 1–4.
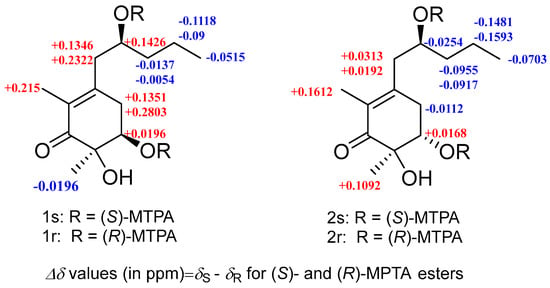
Figure 3.
ΔδRS values for the MTPA derivatives of compounds 1 and 2 in pyridine-d5.
Megastigmanone B (2) was isolated as colorless oil. Its molecular formula, C13H22O4, was determined based on the HR-ESI-MS ion at m/z 265.1447 [M + Na]+ (calcd, C13H22NaO4, 265.1410), indicating three degrees of unsaturation. Comparison of its 1D and 2D NMR data with those for compound 1 revealed that compound 2 shared the same skeleton with compound 1, except for the different configurations of H-5, revealed by the ROESY correlation between CH3-6 and H-5 in 2. The absolute configuration of C-5 and C-2′ in compound 2 was also determined by the modified Mosher’s method and has been determined to be (5S, 6R)-5, 6-dihydroxy-3-((S)-2′-hydroxypentyl)-2, 6-dimethylcyclohex-2-en-1-one according to the ΔδRS value analysis of the 1H NMR data of (S) and (R)-MTPA esters of compound 2 (Figure 3; Figures S20–S23).
Megastigmanone C (3) was also obtained as colorless oil. Its molecular formula, C13H22O4, was determined based on the HR-ESI-MS ion at m/z 243.1595 [M + H]+ (calcd, C13H23O4, 243.1591), indicating three degrees of unsaturation. The 1D and 2D NMR data for compound 3 (Figures S26–S31) were very similar to those of compound 1, allowing the determination of the same planar structure as compounds 1 and 2, except for the different configuration of H-2′ through comparing the carbon values (C-5s in compounds 1 and 3 were δC 74.2, while the C-2′ in 3 was downshifted to δC 70.4) and ROESY data. Efforts were undertaken to establish the absolute configuration of C-2′ through the application of the modified Mosher’s method. However, these attempts were not successful. According to the ROESY experiment, the whole structure for 3 was tentatively determined to be (5R, 6R)-5,6-dihydroxy-3-((R)-2′-hydroxypentyl)-2, 6-dimethylcyclohex-2-en-1-one.
Prenylterphenyllin H (4) was isolated as a yellow amorphous solid. Its molecular formula, C25H26O5, was determined based on the HR-ESI-MS ion at m/z 429.1673 [M + Na]+ (calcd, C25H26NaO5, 429.1672), indicating 13 degrees of unsaturation. The 1H NMR spectrum (Figure S39) revealed the existence of two methyls at δH 1.38 (3H, s, C-4‴) and 1.28 (3H, s, C-5‴), two oxygenated methyls at δH 3.74 (3H, s, CH3-6′) and 3.38 (3H, s, CH3-3′), and nine olefinic protons at δH 7.67 (2H, d, J = 7.4 Hz, H-2″,6″), 7.46 (2H, t, J = 7.4 Hz, H-3″,5″), 7.38 (1H, t, J = 7.4 Hz, H-4″), 7.14 (1H, dd, J = 8.3, 2.0 Hz, H-6), 7.11 (1H, d, J = 2.0 Hz, H-2), 6.74 (1H, d, J = 8.3 Hz, H-5) and 6.53 (1H, s, H-5′) (Table 2). The 13C and DEPT135 NMR data showed that there were 25 carbon resonances, which were assigned to ten nonprotonated carbons at δC 154.6 (C-6′), 152.9 (C-4), 149.2 (C-2′), 140.3 (C-3′), 139.5 (C-1″), 133.6 (C-4′), 126.4 (C-1), 120.2 (C-3), 118.4 (C-1′) and 77.8 (C-3‴), ten methines at δC 133.0 (C-2), 131.0 (C-6), 129.7 (C-2″, 6″), 129.2 (C-3″,5″), 128.1 (C-4″), 116.7 (C-5), 104.3 (C-5′), and 70.1 (C-2‴), one methylene at δC 32.3 (C-1‴), two methyl carbons at δC 26.7 (C-5‴) and 20.7 (C-4‴), and two methoxyls at δC 60.9 (OCH3-3′) and 20.7 (OCH3-6′) (Table 2). These NMR data were very similar with those of the previously reported sanshamycin C [20], except for the presence of a segment of -CH2-CHOH- through detailed analysis of the HMBC (Figure S43) and 1H-1H COSY spectra (Figure S44) of compound 4. Thus, the planar structure of compound 4 was proposed to be sansamycin C’s derivative. Analysis of the 1H-1H COSY spectrum revealed the existence of three separate spin systems: H-2″-H-6″, H-5-H-6 and H2-1‴-H-2‴ (Figure 2). The HMBC correlations from OCH3-3′ to C-3′, from OCH3-6′ to C-6′, and from CH3-4‴ to C-3‴, C-5‴, C-2‴, CH3-5‴ to C-3‴, C-4‴, C-2‴ and ROESY correlations from OCH3-3′ to H-6″, OCH3-6′ to H-5′, CH3-4‴and CH3-5‴ to H-1‴ revealed the position of four methyl groups. Overall analysis of the 1D and 2D NMR data permitted the structural assignment for compound 4, as shown in Figure 1. Attempts were also made to determine the absolute configuration of C-2‴ using the modified Mosher’s method, but with no success. So, we tried to compare the optical rotation of compound 4 with those of compounds bearing similar chiral moieties in the literature, such as clausenanisine B [27] and (−) microphyline Q [28], indicating R for C-2‴.

Table 2.
1H (500 MHz) and 13C (125 MHz) NMR data for compound 4 in acetone-d6.
2.2. Antagonistic Evaluation
The phytotoxic activity of the isolated compounds was tested using Triticum aestivum L. (monocots), Oryza sativa L. (monocots) and Amaranthus retroflexus L. (dicotyledon) seeds. As a result, p-terphenyl derivatives of compounds 6–8 exhibited significant antagonistic activities against the germination of Triticum aestivum L. and Oryza sativa L. seeds as well as the root and shoot lengths of seedlings with a dose-dependent relationship (Figure 4 and Figure 5). Compound 7 exhibited almost identical phytotoxic activity, indicating that the hydroxyl group at position C-5 has no impact on their phytotoxicity. However, they have no significant effects against Amaranthus retroflexus L.
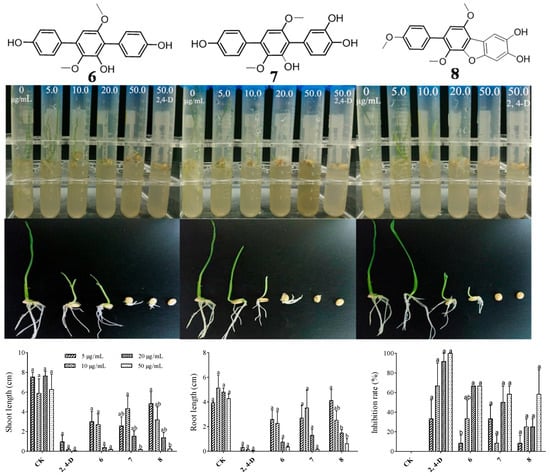
Figure 4.
Phytotoxic activity of compounds 6–8 against Triticum aestivum L. Different letters show statistical significance.
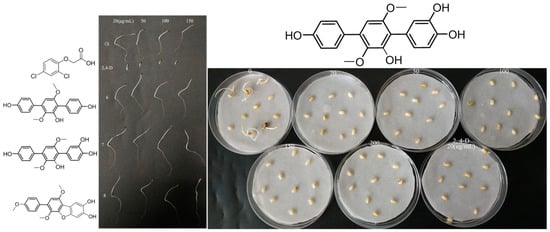
Figure 5.
Phytotoxic activity of compounds 6–8 against Amaranthus retroflexus L. (left); phytotoxic activity of compound 7 against Oryza sativa L. (right).
Combining the germination morphology and inhibition rate, as shown in Figure 4, it was found that there was a significant difference between Triticum aestivum L. seeds treated with 20 μg/L of compounds 6–8 and below 20 μg/L and the control. Shoot growth inhibition is more obvious than that of roots. The germination rate of Oryza sativa L. seeds treated with higher concentrations (50–200 μg/L) was significantly lower than the control or even no germination, and the development of roots and shoots was significantly inhibited compared to the control. The germination rate as well as the root and shoot lengths of seedlings of Amaranthus retroflexus L. seeds treated with higher concentrations (20–150 μg/L) were not affected. By comparing the inhibitory effects of compound 7 on Triticum aestivum L. and Oryza sativa L. seeds, it was found that Oryza sativa L. seeds are more sensitive to the treatment and can be effectively inhibited by compound 7 at a low concentration (20 μg/L). To sum up, the results indicated that the minimum inhibitory concentration for three germination inhibitors with significant inhibitory effects against Triticum aestivum L. is 20 μg/L. However, they have no significant inhibitory effects against Amaranthus retroflexus L., even at concentrations up to 150 μg/L. Therefore, it can be concluded that these compounds have significant selectivity for monocotyledonous and dicotyledonous plants seeds, and it is implied that they are more possibly only phytotoxic against monocots.
3. Materials and Methods
3.1. General Experimental Procedures
NMR spectra were recorded using a Bruker AVANCEIII-500 NMR spectrometer at 500 MHz for 1H and 125 MHz for 13C nuclei (Bruker Corporation, Karlsruhe, Germany). HRESIMS data were determined using an Agilent 6530 TOF LC-MS mass spectrometer (Agilent, Santa Clara, CA, USA) and Bruker compact mass spectrometer (Bruker, Bremen, Germany). Optical rotation values were recorded using an Anton Paar MCP5100 (Anton Paar, Germany). IR data were measured on a Nicolet 380 Infrared Spectrometer (Thermo Electron Corporation, Madison, WI, USA). UV and ECD data were collected on an MOS-500 Bio-Logic circular dichroic spectrometer (Bio-Logic, Seyssinet-Pariset, France). The semi-preparative HPLC was conducted on a Waters 1525 HPLC equipped with a XBridge C18 column (5 μm, 250.0 mm × 10.0 mm; Waters Corporation, Milford, MA, USA). Thin-layer chromatography (TLC) was performed on pre-coated glass plates (silica gel GF254, Qingdao Marine Chemical Inc., Qingdao, China). Column chromatography (CC) was performed on silica gel (45–75 μm; Qingdao Marine Chemical Inc., Qingdao, China), ODS (40–60 μm; Osaka Soda Co., Ltd., Hyogo, Japan) and Sephadex LH-20 (Cytiva, Uppsala, Sweden).
3.2. Fungal Material and Fermentation
The symbiotic epiphytic fungal strain of Aspergillus sp. ITBBc1 was previously isolated from coral collected from the South China Sea in Hainan and identified as Aspergillus sp. by internal transcribed spacer (ITS) sequencing (GenBank accession number OP614945) [20]. The strain was cultivated on ME medium (malt extract 10.0 g/L, sucrose 10.0 g/L, tryptone 1.0 g/L) cultured on a rotary shaker (180 rpm) at 28 °C for 3 days to afford a seed culture, which was then inoculated into rice solid media (rice 90.0 g and 0.1 L water) in 1L-Erlenmeyer flasks and fermented at 28 °C for 45 d under static conditions.
3.3. Extraction and Isolation
The fungal culture was exhaustively extracted using ethyl acetate (EA) to obtain a crude extract, which was fractionated via silica gel CC with the eluting gradient of petroleum ether (PE)/EA(100:0, 50:1, 25:1, 10:1, 5:1, 2:1, 1:1, 0:1) to yield eight fractions (Fractions (Frs.) A–H). Fraction F (Fr. F) was purified by Sephadex LH-20 (MeOH) and then further purified by using semipreparative HPLC to obtain compound 4 (1.8 mg, tR 15.8 min) (80% MeOH–H2O). Fraction G (Fr. G) was purified via CC over RP-C18 eluting with a MeOH–H2O gradient (from 4:6 to 1:0) to obtain six subfractions (Fr. G-1 to G-6). G-4 was purified by Sephadex LH-20 (MeOH) and then further purified by using semipreparative HPLC (40% MeOH–H2O) to obtain compounds 1 (7.8 mg, tR 15.8 min), 2 (5.7 mg, tR 18.1 min), and 3 (3.3 mg, tR 23.8 min).
3.3.1. Megastigmanone A (1)
Colorless oil; [α] −9.0 (c 0.10, MeOH); UV (MeOH) λmax (log ε) 257 (2.32), 260 (2.29) nm (Figure S13); CD (c 0.05, MeOH) λmax (∆ε): 233 (−3.61), 266 (+0.27), 315 (−0.51) nm (Figure S49). IR (KBr) νmax 3396, 2931, 1669, 1089 cm−1 (Figure S14); 1H and 13C NMR data, see Table 1; HR-ESI-MS m/z: 265.1441 [M + Na]+ (calcd for C13H22NaO4, 265.1410) (Figure S12).
3.3.2. Megastigmanone B (2)
Colorless oil; [α] +42.0(c 0.10, MeOH); UV (MeOH) λmax (log ε) 251 (2.36), 266 (2.01) nm (Figure S27); CD (c 0.05, MeOH) λmax (∆ε): 214(−7.04), 251 (+6.36), 319 (−0.32) nm (Figure S49). IR (KBr) νmax 2958, 2930, 1664, 1095 cm−1 (Figure S28); 1H and 13C NMR data, see Table 1; HR-ESI-MS m/z: 265.1447 [M + Na]+ (calcd, C13H22NaO4, 265.1410) (Figure S26).
3.3.3. Megastigmanone C (3)
Colorless oil; [α] −24.0 (c 0.10, MeOH); UV (MeOH) λmax (log ε) 251 (2.24), 265 (1.97) nm (Figure S37); CD (c 0.05, MeOH) λmax (∆ε): 242 (−4.90), 285 (+0.17) nm (Figure S49). IR (KBr) νmax 3484, 2922, 1725, 1660, 1385 cm−1 (Figure S38); 1H and 13C NMR data, see Table 1; HR-ESI-MS m/z: 243.1595 [M + H]+ (calcd for C13H23O4, 243.1591) (Figure S36).
3.3.4. Prenylterphenyllin H (4)
Yellow amorphous solid; [α] −14.0 (c 0.10, MeOH); UV (MeOH) λmax (log ε) 222 (2.56), 249 (2.32), 273 (2.39) (Figure S47); CD (c 0.05, MeOH) λmax (∆ε): 220 (−1.01), 229 (−2.20), 249 (−1.62) nm; IR (KBr) νmax 3420, 2924, 1617, 1066 cm−1 (Figure S48); 1H and 13C NMR data, see Table 2; HR-ESI-MS m/z: 429.1673 [M + Na]+ (calcd, C25H26NaO5, 429.1672) (Figure S46).
3.4. Modified Mosher’s Reaction
The (S)-MTPA and (R)-MTPA esters for compounds 1–4 were prepared using modified Mosher’s method as previously reported [29]. Samples of compound 1 (1.0 mg, 0.0041 mmol) in pyridine-d5 (0.5 mL) and (R)-MTPACl (7.8 μL, 0.0410 mmol), compound 2 (1.0 mg, 0.0041 mmol) in pyridine-d5 (0.5 mL) and (R)-MTPACl (7.8 μL, 0.0410 mmol), compound 3 (1.0 mg, 0.0044 mmol) in pyridine-d5 (0.5 mL) and (R)-MTPACl (8.2 μL, 0.0440 mmol), and compound 4 (1.0 mg, 0.0041 mmol) in pyridine-d5 (0.5 mL) and (R)-MTPACl (4.6 μL, 0.0410 mmol) were reacted in NMR tubes at room temperature for 24 h, respectively. (R)-MTPA esters were also prepared in a similar way using (S)-MTPACl.
3.5. Antagonistic Bioassay
The Triticum aestivum L. (monocots), Oryza sativa L. (monocots) and Amaranthus retroflexus L. (dicotyledon) seeds were disinfected with 8% sodium hypochlorite solution for 15 min and then washed three times with sterilized water. Then, Triticum aestivum L. seeds were placed in a 15 mL tube containing 5 mL of 1/2 MS medium with four concentrations (0, 5, 10, 20, and 50 μg/mL). Oryza sativa L. and A. retroflexus seeds were evenly placed on sterilized filter paper in separate 120 mm Petri dishes containing 2 mL of compound 7 with four concentrations (0, 20, 50, 100, 150, and 200 μg/mL). Seeds cultivated at a 25 ℃ growth chamber for upright growth for 5 d (16 h/8 h light/dark cycle). Then, the shoot length and root length of each treatment were measured, and the germination inhibition rates were calculated. The commonly used synthetic herbicide 2, 4-dichlorophenoxyacetic acid (2, 4-D) was used as a positive control. The inhibition percentage was calculated using the formula as follows:
inhibition percentage (%) = (Lcontrol − Ltreatment)/Lcontrol × 100
4. Conclusions
In summary, this study focused on the isolation and structural elucidation of four new compounds and four known compounds from the marine fungus Aspergillus sp. ITBBc1. The identification of these compounds was accomplished through the application of diverse spectroscopic techniques and comparison with published data. Compounds 1–3 have a similar structure to compounds reported by González Coloma et al., obtained from Stemphylium solani [30], which have biocidal activity simultaneously against more than one category of harmful organisms affecting plants. Compound 4 belongs to p-terphenyl, possessing the same skeleton with prenylterphenyllin G [31] and modified by hydroxy group at C-2‴. Compound 5 is an anazaphilone derivative that was first reported by Zhang et al. [23]. Compounds 6 and 7 are p-terphenyl derivatives and have the ability to inhibit wheat coleoptile growth significantly according to the previous reports [32]. Compound 7 has also been reported as an important anti-proliferative and pro-apoptotic agent for ovarian cancer [33]. Compound 8 is a terphenyllin derivative. Our results showed that compounds 6–8 exhibited remarkable antagonistic activities against the germination of Triticum aestivum L. and Oryza sativa L. seeds, but did not show effects against and Amaranthus retroflexus L. Therefore, it can be roughly concluded that compounds 6–8 are only phytotoxic against monocots. These bioactive compounds can be considered for weed control as herbicides in the field of dicotyledonous crops as they are only toxic to monocots, so they can be mixed with fertilizer to inhibit the germination of monocotyledonous weeds in the soil. Taken together, the unique structures of new compounds and the biologically active compound could provide an interesting foundation for further investigations.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/md22060270/s1: Figures S1–S49: HR-ESI-MS, 1H NMR, 13C NMR, DEPT 135, HSQC, HMBC, 1H-1H COSY, ROESY, UV, IR spectra of compounds 1–4 and ECD spectra of compounds 1–3.
Author Contributions
Conceptualization, H.G. and Z.G.; methodology, A.A. and R.W.; software, A.A.; validation, A.A., Z.X. and S.Z.; formal analysis, A.A. and Z.G.; investigation, A.A., R.W. and Z.X.; resources, R.W., Y.L., H.G. and Z.G.; data curation, A.A. and Z.G.; writing—original draft preparation, A.A.; writing—review and editing, H.G. and Z.G.; visualization, A.A. and Z.X.; supervision, Z.X. and S.Z.; project administration, Z.G.; funding acquisition, R.W., Y.L., H.G. and Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hainan Province Science and Technology Special Fund (ZDYF2024SHFZ091, ZDYF2023SHFZ107), the Major Science and Technology Program of Hainan Province (ZDKJ2021008), Social Public-interest Scientific Institution Reform Special Fund (Study on the bioactive metabolites of marine microorganism from South China Sea), Central Public-interest Scientific Institution Basal Research Fund (1630052024022, 1630052022016), and Chinese Academy of Tropical Agricultural Sciences for Science and Technology Innovation Team of National Tropical Agricultural Science Center (CATASCXTD202312).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials; further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Public Technology Research and Sharing Center of the Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences for equipment sharing and technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Juraschek, L.M.; Kappenberg, A.; Amelung, W. Mycotoxins in soil and environment. Sci. Total Environ. 2022, 814, 152425. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Vianello, A.; Macri, F. Inhibition of plant cell membrane transport phenomena induced by zearalenone (F-2). Planta 1978, 143, 51–57. [Google Scholar] [CrossRef]
- Pekkarinen, A.; Mannonen, L.; Jones, B.L.; Niku-Paavola, M.L. Production of proteases by Fusarium species grown on barley grains and in media containing cereal proteins. J. Cereal Sci. 2000, 31, 253–261. [Google Scholar] [CrossRef]
- Abbas, H.K.; Duke, S.O.; Tanaka, T. Phytotoxicity of fumonisins and related compounds. J. Toxicol. Toxin Rev. 1993, 12, 225–251. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr.; Sullards, M.C.; Wang, E.; Voss, K.A.; Riley, R.T. Sphingolipid metabolism: Roles in signal transduction and disruption by fumonisins. Environ. Health. Perspect 2001, 109, 283–289. [Google Scholar]
- Brodal, G.; Hofgaard, I.S.; Eriksen, G.S.; Bernhoft, A.; Sundheim, L. Mycotoxins in organically versus conventionally produced cereal grains and some other crops in temperate regions. World Mycotoxin J. 2016, 9, 755–770. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. The fate of mycotoxins during the processing of wheat for human consumption. Compr. Rev. Food Sci. Food Saf. 2018, 17, 556–593. [Google Scholar] [CrossRef]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health 2016, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cheng, J.; Lu, Y.; Wang, H.; Gao, Y.; Shi, J.; Yin, C.; Wang, X.; Chen, S.; Strasser, R.J.; et al. Novel action targets of natural product gliotoxin in photosynthetic apparatus. Front. Plant Sci. 2020, 10, 1688. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef]
- Evidente, A. Specialized metabolites produced by phytotopatogen fungi to control weeds and parasite plants. Microorganisms 2023, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.H.; Gou, J.Y.; Zhao, D.L.; Wang, D.; Liu, J.; Ma, G.Y.; Li, Y.Q.; Zhang, C.S. Phytotoxicity and anti-phytopathogenic activities of marine-derived fungi and their secondary metabolites. RSC Adv. 2018, 8, 37573–37580. [Google Scholar] [CrossRef] [PubMed]
- Du, F.Y.; Li, X.M.; Sun, Z.C.; Meng, L.H.; Wang, B.G. Secondary metabolites with agricultural antagonistic potentials from Beauveria felina, a marine-derived entomopathogenic fungus. J. Agric. Food Chem. 2020, 68, 14824–14831. [Google Scholar] [CrossRef] [PubMed]
- Irvine, N.M.; Yerkes, C.N.; Graupner, P.R.; Roberts, R.E.; Hahn, D.R.; Pearce, C.; Gerwick, B.C. Synthesis and characterization of synthetic analogs of cinnacidin, a novel phytotoxin from Nectria sp. Pest Manag. Sci. 2008, 64, 891–899. [Google Scholar] [CrossRef]
- Guo, Z.K.; Abulaizi, A.; Huang, L.; Xiong, Z.J.; Zhang, S.Q.; Liu, T.; Wang, R. Discovery of p-terphenyl metabolites as potential phosphodiesterase PDE4D inhibitors from the coral-associated fungus Aspergillus sp. ITBBc1. Mar. Drugs 2022, 20, 679. [Google Scholar] [CrossRef]
- Xiong, Z.J.; Wang, R.; Xia, T.F.; Zhang, S.Q.; Ma, S.; Guo, Z.K. Natural products and biological activity from actinomycetes associated with marine algae. Molecules 2023, 28, 5138. [Google Scholar] [CrossRef]
- Guo, Z.K.; Ma, S.; Khan, S.; Zhu, H.J.; Zhang, B.; Zhang, S.; Jiao, R.H. Zhaoshumycins A and B, two unprecedented antimycin-type depsipeptides produced by the marine-derived Streptomyces sp. ITBB-ZKa6. Mar. Drugs 2021, 19, 624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, J.; Wang, X.; Zhao, L.; Liu, H.; Wei, Y.; You, X.; Cen, S.; Yu, L. Peniazaphilin A, a new azaphilone derivative produced by Penicillium sp. CPCC 400786. J. Antibiot. 2018, 71, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Marchelli, R.; Vining, L.C. Terphenyllin, a novel p-terphenyl metabolite from Aspergillus candidus. J. Antibiot. 1975, 28, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Kurobane, I.; Vining, L.C.; Mcinnes, A.G.; Smith, D.G. 3-Hydroxyterphenyllin, a new metabolite of Aspergillus candidus. Structure elucidation by H and C nuclear magnetic resonance spectroscopy. J. Antibiot. 1979, 32, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Rahbaek, L.; Frisvad, J.C.; Christophersen, C. An amendment of Aspergillus section Candidi based on chemotaxonomical evidence. Phytochemistry 2000, 53, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Li, Y.J.; Zhao, Y.Y.; Guo, J.M.; Liu, Y.Y.; Wang, X.P.; Shen, Z.Y.; Qiang, L.; Fu, Y.H. Carbazole alkaloids from the fruits of Clausena anisum-olens with potential PTP1B and α-glucosidase inhibitory activities. Bioorg. Chem. 2021, 110, 104775. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Zhu, S.S.; Liu, Y.; Chen, H.W.; Shi, Y.T.; Zeng, K.W.; Tu, P.F.; Jiang, Y. Carbazole alkaloids with potential cytotoxic activities targeted on PCK2 protein from Murraya microphylla. Bioorg. Chem. 2021, 114, 105113. [Google Scholar] [CrossRef]
- Guo, Z.K.; Wang, T.; Guo, Y.; Song, Y.C.; Tan, R.X.; Ge, H.M. Cytotoxic angucyclines from Amycolatopsis sp. HCa1, a rare actinobacteria derived from Oxya chinensis. Planta Med. 2011, 77, 2057–2060. [Google Scholar] [CrossRef]
- González Coloma, A.; Yeves, M.F.; Diaz Hernandez, C.E.; Reina Artiles, M.; Lacret Pimienta, R.; Cabrera Perez, R.; Gimenez Mariño, C.; Kaushik, N. Natural Borad-Spectrum Biocides. WO2017068223A1, 27 April 2017. [Google Scholar]
- Zhou, G.; Chen, X.; Zhang, X.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Prenylated p-Terphenyls from a mangrove endophytic fungus, Aspergillus candidus LDJ-5. J. Nat. Prod. 2020, 83, 8–13. [Google Scholar] [CrossRef]
- Cutler, H.G.; Lefiles, J.H.; Crumley, F.G.; Cox, R.H. Hydroxyterphenyllin: A novel fungal metabolite with plant growth inhibiting properties. J. Agric. Food Chem. 1978, 26, 632–635. [Google Scholar] [CrossRef]
- Wang, Y.; Compton, C.; Rankin, G.O.; Cutler, S.J.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. 3-Hydroxyterphenyllin, a natural fungal metabolite, induces apoptosis and S phase arrest in human ovarian carcinoma cells. Int. J. Oncol. 2017, 50, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).