Origin of the 6/5/6/5 Tetracyclic Cyclopiazonic Acids
Abstract
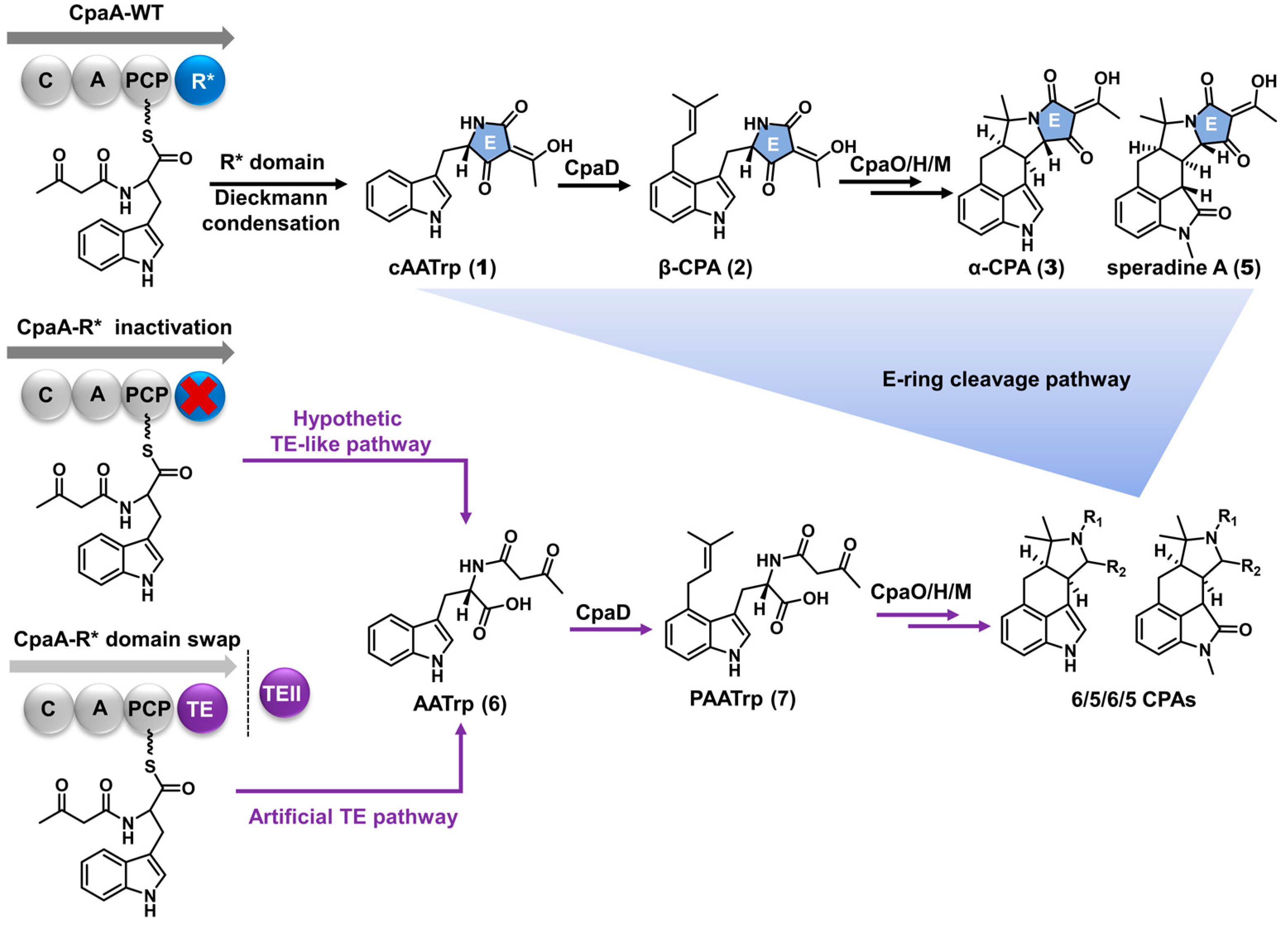
1. Introduction
2. Results and Discussion
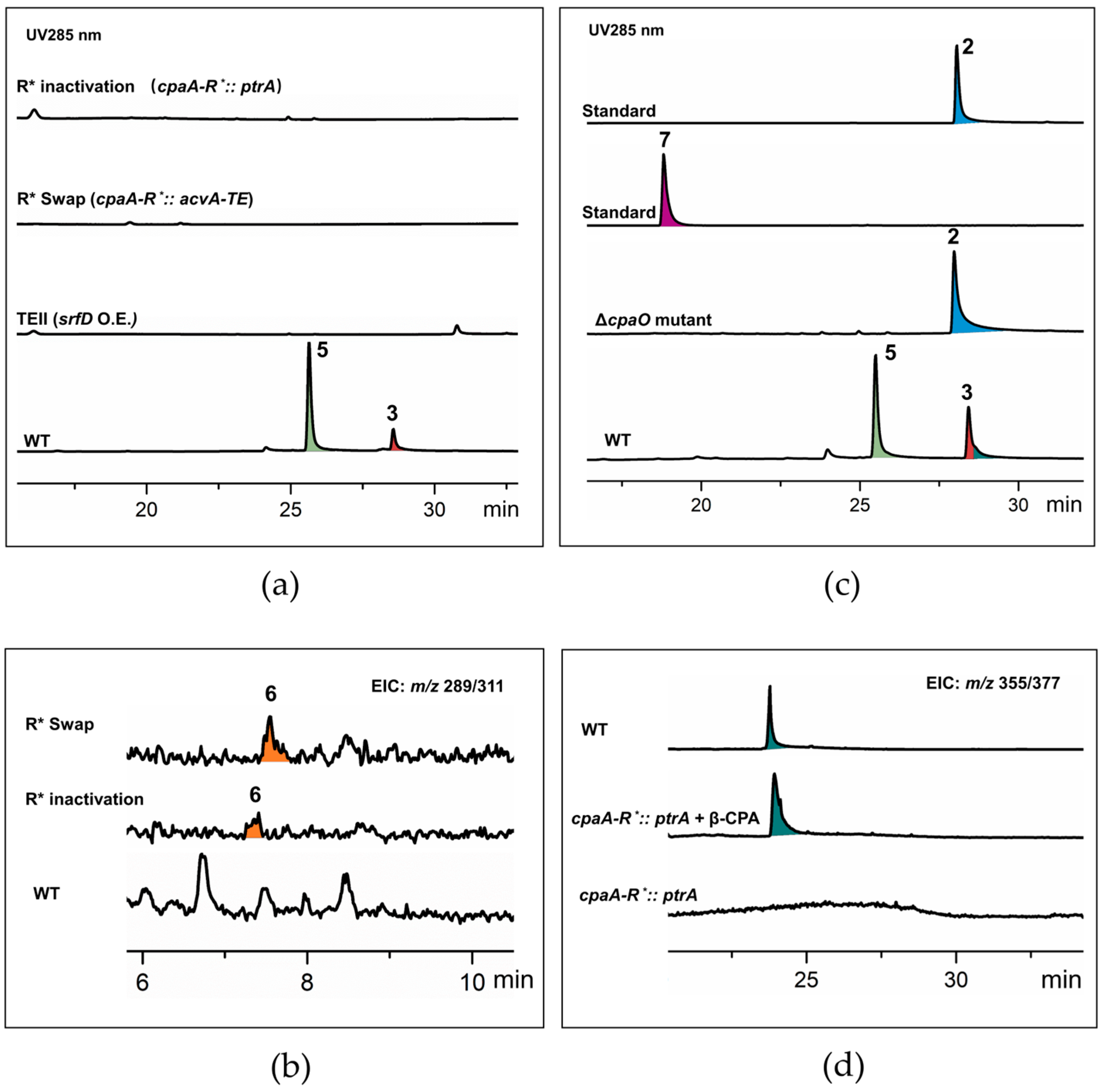
2.1. The 6/5/6/5 Skeleton Is Not Derived from the TE-like Pathway
2.2. AATrp Is a Shunt Metabolite
2.3. The E-Ring Cleavage Occurs after 6/5/6/5/5 Skeleton Formation
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Strains and Reagents
3.3. HPLC and LC-TOFMS Analysis
3.4. Engineering of the cpaA-R* Domain
3.5. Biotransformation Assays
3.6. Isolation and Purification
3.7. Synthesis of AATrp and PAATrp
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holzapfel, C.W. The isolation and structure of cyclopiazonic acid, a toxic metabolite of Penicillium cyclopium Westling. Tetrahedron 1968, 24, 2101–2119. [Google Scholar] [CrossRef]
- Goeger, D.E.; Riley, R.T.; Dorner, J.W.; Cole, R.J. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem. Pharmacol. 1988, 37, 978–981. [Google Scholar] [CrossRef]
- Aguayo-Ortiz, R.; Espinoza-Fonseca, L.M. Linking biochemical and structural states of SERCA: Achievements, challenges, and new opportunities. Int. J. Mol. Sci. 2020, 21, 4146. [Google Scholar] [CrossRef]
- Fusi, F.; Saponara, S.; Gagov, H.; Sgaragli, G. 2,5-Di-t-butyl-1,4-benzohydroquinone (BHQ) inhibits vascular L-type Ca2+ channel via superoxide anion generation. Br. J. Pharmacol. 2001, 133, 988–996. [Google Scholar] [CrossRef]
- Hossain, Z.; Busman, M.; Maragos, C.M. Immunoassay utilizing imaging surface plasmon resonance for the detection of cyclopiazonic acid (CPA) in maize and cheese. Anal. Bioanal. Chem. 2019, 411, 3543–3552. [Google Scholar] [CrossRef]
- Navale, V.; Vamkudoth, K.R.; Ajmera, S.; Dhuri, V. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicol. Rep. 2021, 8, 1008–1030. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.B.; Nováková, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenář, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Qian, X.; Yang, T.; Fang, D.; Qin, Z.; Ren, B.; Li, G. Cyclopiazonic acid and okaramine analogues, including chlorinated compounds, from Chrysosporium undulatum YT-1. J. Nat. Prod. 2022, 85, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, S.-H.; Shin, Y.; Bae, M.; Kim, B.-Y.; Lee, S.; Oh, K.-B.; Shin, J.; Oh, D.-C. A new benzofuran glycoside and indole alkaloids from a sponge-associated rare actinomycete, Amycolatopsis sp. Mar. Drugs 2014, 12, 2326–2340. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Shaala, L.A.; Almohammadi, A.; Elhady, S.S.; Alzughaibi, T.A.; Alshali, K.Z. Characterization of bioactive compounds from the Red Sea tunicate-derived fungus Penicillium commune DY004. Lett. Org. Chem. 2022, 19, 144–149. [Google Scholar] [CrossRef]
- Yang, G.-Y.; Dai, J.-M.; Mi, Q.-L.; Li, Z.-J.; Li, X.-M.; Zhang, J.-D.; Wang, J.; Li, Y.-K.; Wang, W.-G.; Zhou, M.; et al. Cyclopiazonic acid type indole alkaloids from Nicotiana tabacum-derived fungus Aspergillus versicolor and their anti-tobacco mosaic virus activities. Phytochemistry 2022, 198, 113137. [Google Scholar] [CrossRef]
- Chen, W.-H.; Li, K.-L.; Lin, X.-P.; Liao, S.-R.; Yang, B.; Zhou, X.-F.; Wang, J.-J.; Liu, Y.-H.; Wang, J.-F. Antioxidant CPA-type indole alkaloids produced from the deep-sea derived fungus Aspergillus sp. SCSIO 41024. Nat. Prod. Res. 2021, 35, 5266–5270. [Google Scholar] [CrossRef]
- Xiang, Y.; Zeng, Q.; Mai, Z.-M.; Chen, Y.-C.; Shi, X.-F.; Chen, X.-Y.; Zhong, W.-M.; Wei, X.-Y.; Zhang, W.-M.; Zhang, S.; et al. Asperorydines N-P, three new cyclopiazonic acid alkaloids from the marine-derived fungus Aspergillus flavus SCSIO F025. Fitoterapia 2021, 150, 104839. [Google Scholar] [CrossRef]
- Tokuoka, M.; Seshime, Y.; Fujii, I.; Kitamoto, K.; Takahashi, T.; Koyama, Y. Identification of a novel polyketide synthase–nonribosomal peptide synthetase (PKS–NRPS) gene required for the biosynthesis of cyclopiazonic acid in Aspergillus oryzae. Fungal Genet. Biol. 2008, 45, 1608–1615. [Google Scholar] [CrossRef]
- Liu, X.; Walsh, C.T. Cyclopiazonic Acid Biosynthesis in Aspergillus sp.: Characterization of a reductase-like R* domain in cyclopiazonate synthetase that forms and releases cyclo-acetoacetyl-L-tryptophan. Biochemistry 2009, 48, 8746–8757. [Google Scholar]
- Liu, X.; Walsh, C.T. Characterization of cyclo-acetoacetyl-L-tryptophan dimethylallyltransferase in cyclopiazonic acid biosynthesis: Substrate promiscuity and site directed mutagenesis studies. Biochemistry 2009, 48, 11032–11044. [Google Scholar] [CrossRef]
- Chang, P.-K.; Ehrlich, K.; Fujii, I. Cyclopiazonic acid biosynthesis of Aspergillus flavus and Aspergillus oryzae. Toxins 2009, 1, 74–99. [Google Scholar] [CrossRef] [PubMed]
- Seshime, Y.; Juvvadi, P.R.; Tokuoka, M.; Koyama, Y.; Kitamoto, K.; Ebizuka, Y.; Fujii, I. Functional expression of the Aspergillus flavus PKS–NRPS hybrid CpaA involved in the biosynthesis of cyclopiazonic acid. Bioorg. Med. Chem. Lett. 2009, 19, 3288–3292. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Tokuoka, M.; Shinohara, Y.; Kawatani, M.; Uramoto, M.; Seshime, Y.; Fujii, I.; Kitamoto, K.; Takahashi, T.; Takahashi, S.; et al. Genetic safeguard against mycotoxin cyclopiazonic acid production in Aspergillus Oryzae. ChemBioChem 2011, 12, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Tokuoka, M.; Kikuchi, T.; Shinohara, Y.; Koyama, A.; Iio, S.-I.; Kubota, T.; Kobayashi, J.I.; Koyama, Y.; Totsuka, A.; Shindo, H.; et al. Cyclopiazonic acid biosynthesis gene cluster gene cpaM is required for speradine A biosynthesis. Biosci. Biotechnol. Biochem. 2015, 79, 2081–2085. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, D.J.; Schabort, J.C.; Ferreira, P. β-Cyclopiazonate oxidocyclase from Penicillium cyclopium. III. Preliminary studies on the mechanism of action. Biochim. Biophys. Acta Enzymol. 1973, 309, 440–456. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, W.; Wang, N. Covalent flavoproteins: Types, occurrence, biogenesis and catalytic mechanisms. Chin. J. Nat. Med. 2022, 20, 749–760. [Google Scholar] [CrossRef]
- Cao, T.; Ling, J.; Liu, Y.; Chen, X.; Tian, X.; Meng, D.; Pan, H.; Hu, J.; Wang, N. Characterization and abolishment of the cyclopiazonic acids produced by Aspergillus oryzae HMP-F28. Biosci. Biotechnol. Biochem. 2018, 82, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Thaochan, N.; Hu, Q. Diversity of linear non-ribosomal peptide in biocontrol fungi. J. Fungi 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Long, J.; Wang, X.; She, J.; Liu, Y.; Li, Y.; Yang, B. Bioactive secondary metabolites isolated from the soft coral derived Penicillium sp. SCSIO 41038. Nat. Prod. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Uka, V.; Moore, G.G.; Arroyo-Manzanares, N.; Nebija, D.; De Saeger, S.; Diana Di Mavungu, J. Unravelling the diversity of the cyclopiazonic acid family of mycotoxins in Aspergillus flavus by UHPLC triple-TOF HRMS. Toxins 2017, 9, 35. [Google Scholar] [CrossRef]
- Liu, L.; Bao, L.; Wang, L.; Ma, K.; Han, J.; Yang, Y.; Liu, R.; Ren, J.; Yin, W.; Wang, W.; et al. Asperorydines A–M: Prenylated tryptophan-derived alkaloids with neurotrophic effects from Aspergillus oryzae. J. Org. Chem. 2018, 83, 812–822. [Google Scholar] [CrossRef]
- MacCabe, A.P.; van Liempt, H.; Palissa, H.; Unkles, S.E.; Riach, M.B.; Pfeifer, E.; von Döhren, H.; Kinghorn, J.R. Delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. Molecular characterization of the acvA gene encoding the first enzyme of the penicillin biosynthetic pathway. J. Biol. Chem. 1991, 266, 12646–12654. [Google Scholar]
- Steller, S.; Sokoll, A.; Wilde, C.; Bernhard, F.; Franke, P.; Vater, J. Initiation of surfactin biosynthesis and the role of the SrfD-thioesterase protein. Biochemistry 2004, 43, 11331–11343. [Google Scholar] [CrossRef]
- Li, D.; Tang, Y.; Lin, J.; Cai, W. Methods for genetic transformation of filamentous fungi. Microb. Cell Factories 2017, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Gama, F.H.S.; de Souza, R.O.M.A.; Garden, S.J. An efficient green protocol for the preparation of acetoacetamides and application of the methodology to a one-pot synthesis of Biginelli dihydropyrimidines. Expansion of dihydropyrimidine topological chemical space. RSC Adv. 2015, 5, 70915–70928. [Google Scholar]
- Li, F.-R.; Lin, X.; Yang, Q.; Tan, N.-H.; Dong, L.-B. Efficient production of clerodane and ent-kaurane diterpenes through truncated artificial pathways in Escherichia Coli. Beilstein. J. Org. Chem. 2022, 18, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Unsöld, I.A.; Li, S.-M. Overproduction, purification and characterization of FgaPT2, a dimethylallyltryptophan synthase from Aspergillus Fumigatus. Microbiol. 2005, 151, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J.W.A.; Guo, C.-J.; Wang, C.C.C. Engineering fungal nonribosomal peptide synthetase-like enzymes by Heterologous expression and domain swapping. Org. Lett. 2016, 18, 6236–6239. [Google Scholar] [CrossRef] [PubMed]
- Stack, D.; Neville, C.; Doyle, S. Nonribosomal peptide synthesis in Aspergillus fumigatus and other fungi. Microbiology 2007, 153, 1297–1306. [Google Scholar] [CrossRef]
- Marahiel, M.A. A structural model for multimodular NRPS assembly lines. Nat. Prod. Rep. 2016, 33, 136–140. [Google Scholar] [CrossRef]
- Tripathi, A.; Choi, S.-S.; Sherman, D.H.; Kim, E.-S. Thioesterase domain swapping of a linear polyketide tautomycetin with a macrocyclic polyketide pikromycin in Streptomyces sp. CK4412. J. Ind. Microbiol. Biotechnol. 2016, 43, 1189–1193. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Jiang, X.; Wang, M.; Zhang, Z.; Wang, N. Origin of the 6/5/6/5 Tetracyclic Cyclopiazonic Acids. Mar. Drugs 2024, 22, 74. https://doi.org/10.3390/md22020074
Zhang W, Jiang X, Wang M, Zhang Z, Wang N. Origin of the 6/5/6/5 Tetracyclic Cyclopiazonic Acids. Marine Drugs. 2024; 22(2):74. https://doi.org/10.3390/md22020074
Chicago/Turabian StyleZhang, Wenyuan, Xuejian Jiang, Minjun Wang, Zhizhen Zhang, and Nan Wang. 2024. "Origin of the 6/5/6/5 Tetracyclic Cyclopiazonic Acids" Marine Drugs 22, no. 2: 74. https://doi.org/10.3390/md22020074
APA StyleZhang, W., Jiang, X., Wang, M., Zhang, Z., & Wang, N. (2024). Origin of the 6/5/6/5 Tetracyclic Cyclopiazonic Acids. Marine Drugs, 22(2), 74. https://doi.org/10.3390/md22020074





