Tracing the Impact of Domestic Storage Conditions on Antioxidant Activity and Lipid Profiles in the Edible Microalgae Chlorella vulgaris and Tetraselmis chui
Abstract
1. Introduction
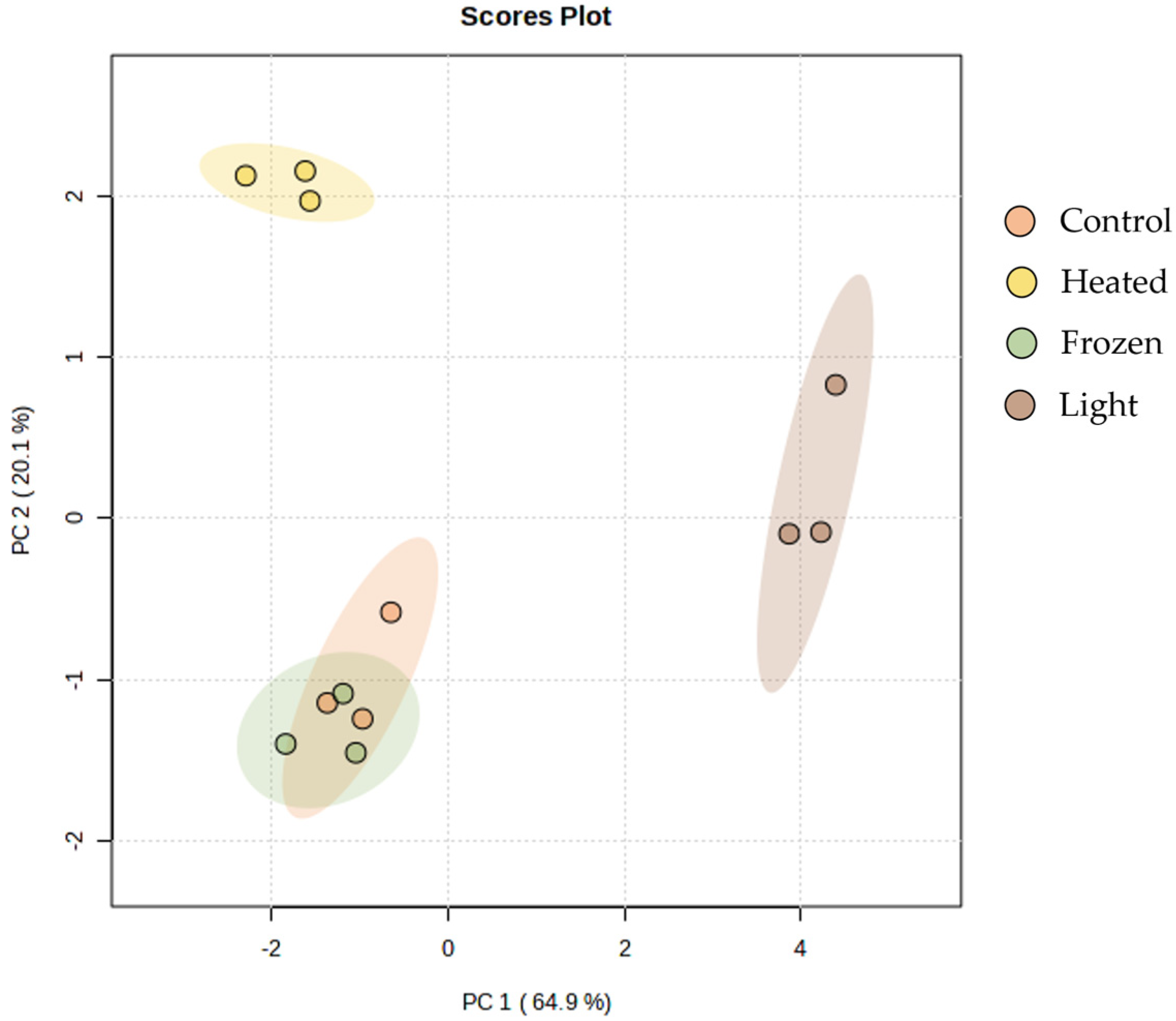
2. Results
2.1. Fatty Acids Profile and Lipid Quality Indices
2.2. Lipid Ratios
2.3. Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Microalgae Samples
4.2. Evaluated Conditions
4.3. Lipid Extraction
4.4. Analysis of Esterified Fatty Acid Analysis by Gas Chromatography–Mass Spectrometry
4.5. Analysis of Lipid by C18-MS
4.6. Evaluation of Antioxidant Activity of Lipid Extracts by DPPH and ABTS Radical Scavenging Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a Future Food Source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health Applications of Bioactive Compounds from Marine Microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Draaisma, R.B.; Wijffels, R.H.; Slegers, P.E.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food Commodities from Microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef]
- Paterson, S.; Gómez-Cortés, P.; de la Fuente, M.A.; Hernández-Ledesma, B. Bioactivity and Digestibility of Microalgae Tetraselmis sp. and Nannochloropsis sp. as Basis of Their Potential as Novel Functional Foods. Nutrients 2023, 15, 477. [Google Scholar] [CrossRef] [PubMed]
- Canelli, G.; Tarnutzer, C.; Carpine, R.; Neutsch, L.; Bolten, C.J.; Dionisi, F.; Mathys, A. Biochemical and Nutritional Evaluation of Chlorella and Auxenochlorella Biomasses Relevant for Food Application. Front. Nutr. 2020, 7, 565996. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, A.D.; Riaño-Pachón, D.M.; Mattiello, L.; Giraldi, L.A.; Winck, F.V.; Franco, T.T. Analysis of Autotrophic, Mixotrophic and Heterotrophic Phenotypes in the Microalgae Chlorella vulgaris Using Time-Resolved Proteomics and Transcriptomics Approaches. Algal Res. 2020, 51, 102060. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Rahman, N.A.; Khatoon, H.; Yusuf, N.; Banerjee, S.; Haris, N.A.; Lananan, F.; Tomoyo, K. Tetraselmis chuii Biomass as a Potential Feed Additive to Improve Survival and Oxidative Stress Status of Pacific White-Leg Shrimp Litopenaeus Vannamei Postlarvae. Int. Aquat. Res. 2017, 9, 235–247. [Google Scholar] [CrossRef]
- Roy, S.S.; Pal, R. Microalgae in Aquaculture: A Review with Special References to Nutritional Value and Fish Dietetics; Springer: Berlin/Heidelberg, Germany, 2015; Volume 68, pp. 1–8. [Google Scholar]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in Aquafeeds for a Sustainable Aquaculture Industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F. Update of the List of QPS-recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 15: Suitability of Taxonomic Units Notified to EFSA until September 2021. EFSA J. 2022, 20, e07045. [Google Scholar] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F. Scientific Opinion on the Update of the List of QPS-recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA (2017–2019). EFSA J. 2020, 18, e05966. [Google Scholar] [PubMed]
- European Comission Commission Implementing Regulation (EU) 2017/2470 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. OJ L 351. 30 December 2017, pp. 72–201. Available online: https://eur-lex.europa.eu/eli/reg_impl/2017/2470/oj (accessed on 1 November 2023).
- Abedi, E.; Sahari, M.A. Long-chain Polyunsaturated Fatty Acid Sources and Evaluation of Their Nutritional and Functional Properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Melo, T.; Conde, T.A.; Costa, M.; Silva, J.; Domingues, M.R.M.; Domingues, P. Chemoplasticity of the Polar Lipid Profile of the Microalgae Chlorella vulgaris Grown under Heterotrophic and Autotrophic Conditions. Algal Res. 2021, 53, 102128. [Google Scholar] [CrossRef]
- Maurício, T.; Couto, D.; Lopes, D.; Conde, T.; Pais, R.; Batista, J.; Melo, T.; Pinho, M.; Moreira, A.S.; Trovão, M. Differences and Similarities in Lipid Composition, Nutritional Value, and Bioactive Potential of Four Edible Chlorella vulgaris Strains. Foods 2023, 12, 1625. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Esquivel, P.; Rodriguez-Amaya, D.B. Comprehensive Review on Carotenoid Composition: Transformations during Processing and Storage of Foods. Food Res. Int. 2023, 169, 112773. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kaur, A.; Bansal, A.; Gill, B.S. Positional Effects on Soybean Seed Composition during Storage. J. Food Sci. Technol. 2013, 50, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Siriwardane, D.A.; Wang, C.; Jiang, W.; Mudalige, T. Quantification of Phospholipid Degradation Products in Liposomal Pharmaceutical Formulations by Ultra Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS). Int. J. Pharm. 2020, 578, 119077. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cerrato, A.; Aita, S.E.; Montone, C.M.; Piovesana, S.; Laganà, A.; Cavaliere, C. Degradation of the Polar Lipid and Fatty Acid Molecular Species in Extra Virgin Olive Oil during Storage Based on Shotgun Lipidomics. J. Chromatogr. A 2021, 1639, 461881. [Google Scholar] [CrossRef]
- Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Effect of Light Exposure on the Quality of Extra Virgin Olive Oils According to Their Chemical Composition. Food Chem. 2017, 229, 726–733. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Implications of Domestic Food Practices for the Presence of Bioactive Components in Meats with Special Reference to Meat-Based Functional Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Liu, K.; Zhang, H. Lipid Oxidation in Foods and Its Implications on Proteins. Front. Nutr. 2023, 10, 1192199. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, R.; Zhang, J.; Zhou, P. Heat-Induced Denaturation and Bioactivity Changes of Whey Proteins. Int. Dairy J. 2021, 123, 105175. [Google Scholar] [CrossRef]
- Wazir, H.; Chay, S.Y.; Zarei, M.; Hussin, F.S.; Mustapha, N.A.; Wan Ibadullah, W.Z.; Saari, N. Effects of Storage Time and Temperature on Lipid Oxidation and Protein Co-Oxidation of Low-Moisture Shredded Meat Products. Antioxidants 2019, 8, 486. [Google Scholar] [CrossRef]
- Couto, D.; Melo, T.; Conde, T.A.; Moreira, A.S.; Ferreira, P.; Costa, M.; Silva, J.; Domingues, R.; Domingues, P. Food Grade Extraction of Chlorella vulgaris Polar Lipids: A Comparative Lipidomic Study. Food Chem. 2022, 375, 131685. [Google Scholar] [CrossRef]
- Conlon, T.; Parkes, R.; Fierli, D.; Touzet, N. Comparative Pigment and Fatty Acid Profiling of Marine Species within the Chlorophyte Genus Tetraselmis. Food Biosci. 2024, 58, 103660. [Google Scholar] [CrossRef]
- Moser, G.A.O.; Barrera-Alba, J.J.; Ortega, M.J.; Alves-de-Souza, C.; Bartual, A. Comparative Characterization of Three Tetraselmis chui (Chlorophyta) Strains as Sources of Nutraceuticals. J. Appl. Phycol. 2022, 34, 1–15. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.-S. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Chaula, D.; Jacobsen, C.; Laswai, H.S.; Chove, B.E.; Dalsgaard, A.; Mdegela, R.; Hyldig, G. Changes in Fatty Acids during Storage of Artisanal-processed Freshwater Sardines (Rastrineobola argentea). Food Sci. Nutr. 2023, 11, 3040–3047. [Google Scholar] [CrossRef]
- Kumar, R.R.; Bhargava, D.; Pandit, K.; Goswami, S.; Shankar, S.M.; Singh, S.P.; Rai, G.K.; Satyavathi, C.T.; Praveen, S. Lipase–The Fascinating Dynamics of Enzyme in Seed Storage and Germination—A Real Challenge to Pearl Millet. Food Chem. 2021, 361, 130031. [Google Scholar] [CrossRef]
- Reyes-Reyes, A.L.; Valero Barranco, F.; Sandoval, G. Recent Advances in Lipases and Their Applications in the Food and Nutraceutical Industry. Catalysts 2022, 12, 960. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; González-Barriga, V.; Romero, J.; Rojas, R.; López-Arana, S. Quantification and Distribution of Omega-3 Fatty Acids in South Pacific Fish and Shellfish Species. Foods 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Wijendran, V.; Hayes, K. Dietary N-6 and n-3 Fatty Acid Balance and Cardiovascular Health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, Y.; Zhang, B.; Li, D.; Yan, J.; Yang, J.; Sun, J.; Cao, H.; Wang, Y.; Zhang, F. Effects of Different n-6/n-3 Polyunsaturated Fatty Acids Ratios on Lipid Metabolism in Patients with Hyperlipidemia: A Randomized Controlled Clinical Trial. Front. Nutr. 2023, 10, 1166702. [Google Scholar] [CrossRef] [PubMed]
- Manuela, A.G.; Robert, V.-A.; Yoannis, N.; Elvira, L.; Rossana, R.; Roberta, M.; Giacomo, D.; Daniele, G. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices, of Raw Roe of Blue Fin Tuna (Thunnus thynnus L.) and Their Salted Product “Bottarga”. Food Nutr. Sci. 2011, 2011, 7235. [Google Scholar]
- Khalili Tilami, S.; Kouřimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef] [PubMed]
- Turan, H.; Sönmez, G.; Kaya, Y. Fatty Acid Profile and Proximate Composition of the Thornback Ray (Raja clavata, L. 1758) from the Sinop Coast in the Black Sea. J. Fish. Sci. 2007, 1, 97–103. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as Sustainable Bio-Factories of Healthy Lipids: Evaluating Fatty Acid Content and Antioxidant Activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef]
- Testi, S.; Bonaldo, A.; Gatta, P.P.; Badiani, A. Nutritional Traits of Dorsal and Ventral Fillets from Three Farmed Fish Species. Food Chem. 2006, 98, 104–111. [Google Scholar] [CrossRef]
- Fernandes, C.E.; da Silva Vasconcelos, M.A.; de Almeida Ribeiro, M.; Sarubbo, L.A.; Andrade, S.A.C.; de Melo Filho, A.B. Nutritional and Lipid Profiles in Marine Fish Species from Brazil. Food Chem. 2014, 160, 67–71. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, X.; He, X.; Sun, X.; Yu, X.; Cheng, Y.; Yu, R.-Q.; Wu, Y. Fatty Acid Composition Analyses of Commercially Important Fish Species from the Pearl River Estuary, China. PLoS ONE 2020, 15, e0228276. [Google Scholar] [CrossRef] [PubMed]
- Conde, T.; Aveiro, S.; Melo, T.; Santos, T.; Neves, B.; Domingues, P.; Varela, J.; Pereira, H.; Domingues, M.R. Cross-Stress Lipid Response of Tetraselmis striata CTP4 to Temperature and Salinity Variation. Algal Res. 2023, 74, 103218. [Google Scholar] [CrossRef]
- Couto, D.; Conde, T.A.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, R.; Domingues, P. The Chemodiversity of Polar Lipidomes of Microalgae from Different Taxa. Algal Res. 2023, 70, 103006. [Google Scholar] [CrossRef]
- Facchini, L.; Losito, I.; Cianci, C.; Cataldi, T.R.; Palmisano, F. Structural Characterization and Profiling of Lyso-phospholipids in Fresh and in Thermally Stressed Mussels by Hydrophilic Interaction Liquid Chromatography—Electrospray Ionization—Fourier Transform Mass Spectrometry. Electrophoresis 2016, 37, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Losito, I.; Facchini, L.; Catucci, R.; Calvano, C.D.; Cataldi, T.R.; Palmisano, F. Tracing the Thermal History of Seafood Products through Lysophospholipid Analysis by Hydrophilic Interaction Liquid Chromatography–Electrospray Ionization Fourier Transform Mass Spectrometry. Molecules 2018, 23, 2212. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, K.K.; Varakumar, P.; Pamuru, R.R.; Basha, S.J.; Mehta, S.; Rao, A.D. Plant Lipoxygenases and Their Role in Plant Physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Automatic Method for Determination of Total Antioxidant Capacity Using 2, 2-Diphenyl-1-Picrylhydrazyl Assay. Anal. Chim. Acta 2006, 558, 310–318. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Barreiros, L.; Maia, M.A.; Reis, S.; Segundo, M.A. Rapid Assessment of Endpoint Antioxidant Capacity of Red Wines through Microchemical Methods Using a Kinetic Matching Approach. Talanta 2012, 97, 473–483. [Google Scholar] [CrossRef]

| Fatty Acids | Control | Light | Frozen | Heated |
|---|---|---|---|---|
| 16:0 | 52.3 ± 4.8 | 35.1 ± 6.2 | 37.5 ± 2.9 | 46.2 ± 3.2 |
| 16:1 | 9.3 ± 0.7 | 6.2 ± 1.1 | 7.3 ± 0.9 | 9.2 ± 0.7 |
| 16:2n-6 | 21.4 ± 1.5 | 14.2 ± 2.4 | 16.9 ± 2.3 | 20.8 ± 1.2 |
| 16:3n-3 | 38.7 ± 2.7 a | 24.1 ± 4.0 b | 30.8 ± 4.7 a,b | 35.2 ± 2.0 a,b |
| 18:0 | 13.4 ± 5.3 | 8.4 ± 1.6 | 6.3 ±0.8 | 10.6 ± 0.4 |
| 18:1 | 26.9 ± 1.7 | 19.3 ± 3.0 | 21.2 ± 1.8 | 28.0 ± 2.3 |
| 18:2n-6 | 61.9 ± 3.5 | 46.3 ± 7.3 | 49.9 ± 5.8 | 62.7 ± 4.7 |
| 18:3n-3 | 72.3 ± 4.2 a | 45.9 ± 7.3 b | 56.7 ± 7.4 a,b | 65.5 ± 4.1 a,b |
| n-3 | 111.0 ± 6.9 | 70.0 ± 11.2 | 87.5 ± 12.1 | 100.7 ± 6.1 |
| n-6 | 83.3 ± 4.9 | 60.6 ± 9.7 | 66.8 ± 8.1 | 83.5 ± 5.8 |
| n-6/n-3 | 0.8 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 |
| SFA | 65.7 ± 9.9 | 43.7 ± 6.9 | 43.8 ± 3.5 | 56.8 ± 2.8 |
| MUFA | 36.2 ± 2.4 | 25.5 ± 4.1 | 28.5 ± 2.7 | 37.2 ± 2.9 |
| PUFA | 230.5 ± 14.3 | 156.0 ± 25.0 | 182.8 ± 22.8 | 221.4 ± 14.8 |
| AI | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| TI | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| h/H | 4.3 ± 0.4 | 4.3 ± 0.1 | 4.7 ± 0.2 | 4.6 ± 0.0 |
| Fatty Acids | Control | Light | Frozen | Heated |
|---|---|---|---|---|
| 16:0 | 67.6 ± 9.6 | 54.4 ± 5.6 | 51.9 ± 5.4 | 53.0 ± 3.6 |
| 16:1 | 6.6 ± 0.8 | 6.4 ± 0.8 | 7.1 ± 0.8 | 7.1 ± 0.5 |
| 16:3n-3 | 3.8 ± 0.4 | 3.7 ± 0.5 | 4.3 ± 0.6 | 4.4 ± 0.3 |
| 16:4n-3 | 37.0 ± 4.8 | 35.3 ± 4.6 | 41.5 ± 4.5 | 40.9 ± 4.1 |
| 18:0 | 21.5 ± 5.7 | 5.0 ± 2.2 | 3.8 ± 0.3 | 4.8 ± 1.2 |
| 18:1 | 35.6 ± 3.7 | 34.1 ± 4.2 | 35.6 ±3.5 | 37.8 ± 2.2 |
| 18:2n-6 | 18.9 ± 2.1 | 16.8 ± 2.1 | 19.3 ± 2.0 | 20.0 ± 1.3 |
| 18:3n-6 | 9.0 ± 1.1 | 8.3 ± 1.1 | 10 ± 1.1 | 9.8 ± 0.9 |
| 18:3n-3 | 47.5 ± 4.8 | 43.7 ± 3.8 | 51.1 ± 8.4 | 51.6 ± 6.6 |
| 18:4n-3 | 13.9 ± 1.6 | 13.4 ± 1.4 | 15.9 ± 2.7 | 16.1 ± 2.6 |
| 20:5n-3 | 13.4 ± 1.5 | 12.2 ± 1.3 | 16.0 ± 2.8 | 15.6 ± 2.2 |
| n-3 | 115.6 ± 11.9 | 108.3 ± 11.5 | 128.8 ± 26.7 | 128.5 ± 15.8 |
| n-6 | 27.9 ± 3.1 | 25.1 ± 3.2 | 29.2 ± 4.4 | 29.9 ± 2.2 |
| n-6/n-3 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| SFA | 89.1 ± 15.3 | 59.5 ± 7.3 | 55.7 ± 8.1 | 57.8 ± 3.0 |
| MUFA | 42.3 ± 4.5 | 40.5 ± 5.1 | 42.7 ± 6.2 | 44.9 ± 2.7 |
| PUFA | 185.8 ± 19.5 | 173.9 ± 19.8 | 200.7 ± 37.3 | 203.3 ± 20.7 |
| AI | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| TI | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| h/H | 2.7 ± 0.2 | 3.1 ± 0.0 | 3.7 ± 0.1 | 3.7 ± 0.1 |
| Lipid Species Ratio | Control | Light | Frozen | Heated |
|---|---|---|---|---|
| PC 34:2/PC 34:2;O | 70.6 ± 2.6 | 26.8 ± 1.3 | 48.4 ± 16.7 | 72.2 ± 17.4 |
| PC 34:3/PC 34:3;O | 84.2 ± 3.6 | 41.0 ± 2.9 | 84.9 ± 5.2 | 78.7 ± 6.7 |
| PC 34:5/PC 34:5;O | 30.7 ± 6.1 | 23.8 ± 0.5 | 43.9 ± 1.7 | 45.5 ± 2.8 |
| PC 36:4/PC 36:4;O | 61.3 ± 2.0 | 40.9 ± 2.4 | 63.5 ± 7.0 | 64.0 ± 3.4 |
| PC 36:5/PC 36:5;O | 56.9 ± 10.3 | 38.3 ± 8.2 | 64.9 ± 2.7 | 41.4 ± 0.8 |
| PC 36:6/PC 36:6;O | 36.0 ± 4.9 | 29.2 ± 6.8 | 50.6 ± 2.2 | 56.0 ± 2.0 |
| PE 32:1/PE 32:1;O | 809.3 ± 5.2 | 23.0 ± 2.9 | 822.6 ± 65.6 | 685.4 ± 187.3 |
| PE 34:2/PE 34:2;O | 89.8 ± 2.5 | 36.5 ± 2.5 | 77.6 ± 4.0 | 116 ± 46.8 |
| PE 34:3/PE 34:3;O | 58.3 ± 5.6 | 33.4 ± 2.7 | 48.1 ± 2.7 | 105.0 ± 9.7 |
| PE 36:3/PE 36:3;O | 11.1 ± 0.3 | 6.5 ± 0.6 | 11.2 ± 0.7 | 5.1 ± 0.2 |
| Control | Light | Frozen | Heated | |
|---|---|---|---|---|
| ABTS | ||||
| IC40 | 32.0 ± 11.0 | 104.9 ± 6.2 | 91.1 ± 15.3 | 49.4 ± 7.0 |
| TE | 402.8 ± 173.4 | 111.1 ± 6.3 | 131.7 ± 24.1 | 238.1 ± 31.3 |
| DPPH | ||||
| IC20 | 97.8 ± 27.2 | 187.9 ± 15.0 | 103.6 ± 24.6 | 132.1 ± 2.9 |
| TE | 107.1 ± 35.5 | 52.6 ± 4.2 | 98.3 ± 21.0 | 74.5 ± 1.6 |
| Control | Light | Frozen | Heated | |
|---|---|---|---|---|
| ABTS | ||||
| IC40 | 41.4 ± 1.0 | 41.1 ± 0.6 | 29.0 ± 5.4 | 88.5 ± 2.8 |
| TE | 281.0 ± 6.8 | 283.0 ± 3.9 | 411.1 ± 85.2 | 131.4 ± 4.3 |
| DPPH | ||||
| IC20 | 70.6 ± 23.1 | 109.4 ± 10.7 | 90.5 ± 7.0 | 209.4 ± 10.9 |
| TE | 152.5 ± 58.3 | 90.9 ± 8.4 | 109.6 ± 8.1 | 47.3 ± 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, D.; Rey, F.; Gomes, A.; Duarte, L.; Pereira, J.; Pinho, M.; Melo, T.; Domingues, R. Tracing the Impact of Domestic Storage Conditions on Antioxidant Activity and Lipid Profiles in the Edible Microalgae Chlorella vulgaris and Tetraselmis chui. Mar. Drugs 2024, 22, 254. https://doi.org/10.3390/md22060254
Lopes D, Rey F, Gomes A, Duarte L, Pereira J, Pinho M, Melo T, Domingues R. Tracing the Impact of Domestic Storage Conditions on Antioxidant Activity and Lipid Profiles in the Edible Microalgae Chlorella vulgaris and Tetraselmis chui. Marine Drugs. 2024; 22(6):254. https://doi.org/10.3390/md22060254
Chicago/Turabian StyleLopes, Diana, Felisa Rey, Alexandrina Gomes, Luís Duarte, João Pereira, Marisa Pinho, Tânia Melo, and Rosário Domingues. 2024. "Tracing the Impact of Domestic Storage Conditions on Antioxidant Activity and Lipid Profiles in the Edible Microalgae Chlorella vulgaris and Tetraselmis chui" Marine Drugs 22, no. 6: 254. https://doi.org/10.3390/md22060254
APA StyleLopes, D., Rey, F., Gomes, A., Duarte, L., Pereira, J., Pinho, M., Melo, T., & Domingues, R. (2024). Tracing the Impact of Domestic Storage Conditions on Antioxidant Activity and Lipid Profiles in the Edible Microalgae Chlorella vulgaris and Tetraselmis chui. Marine Drugs, 22(6), 254. https://doi.org/10.3390/md22060254








