New Naphthalene Derivatives from the Mangrove Endophytic Fungus Daldinia eschscholzii MCZ-18
Abstract
1. Introduction
2. Results and Discussion
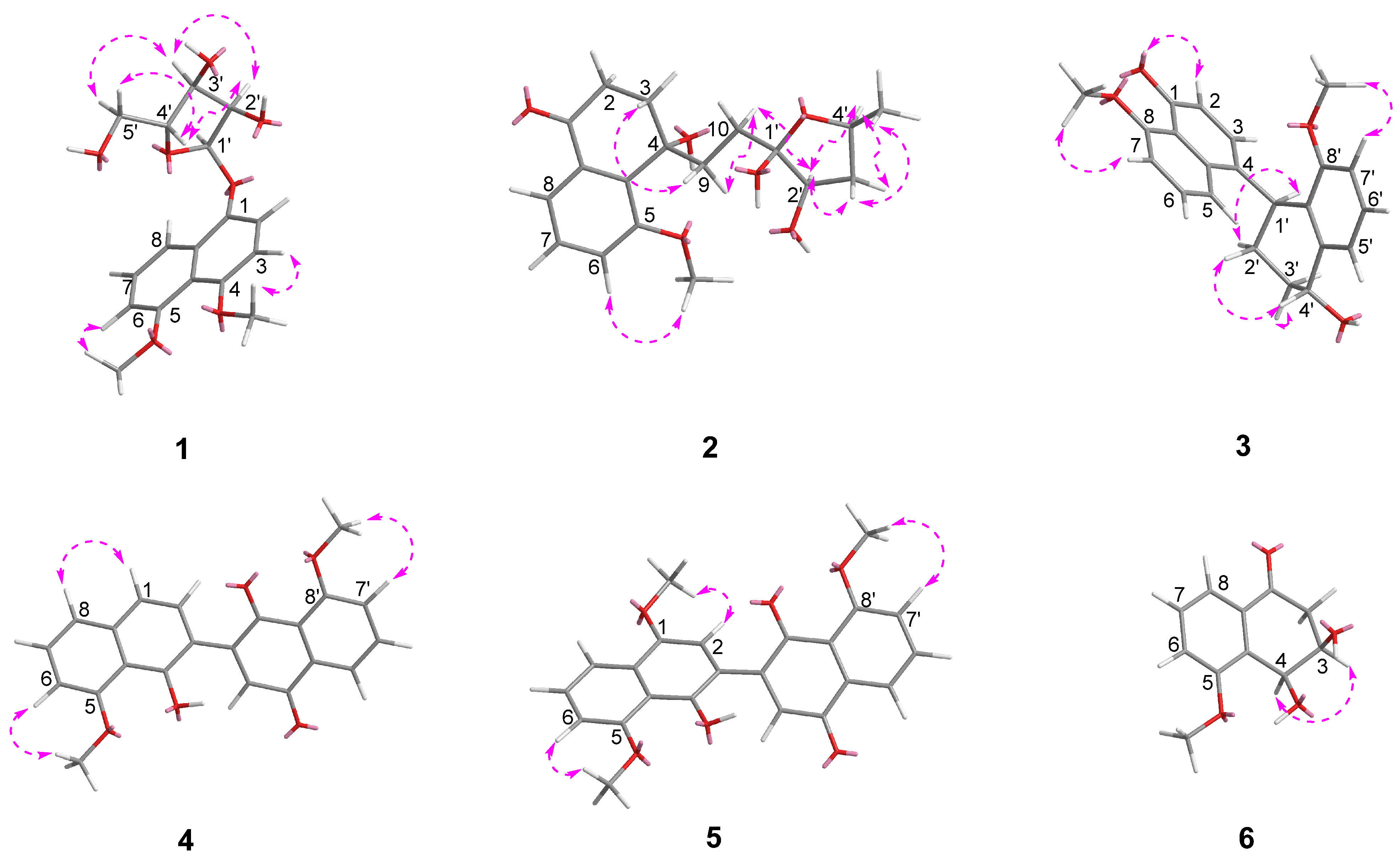
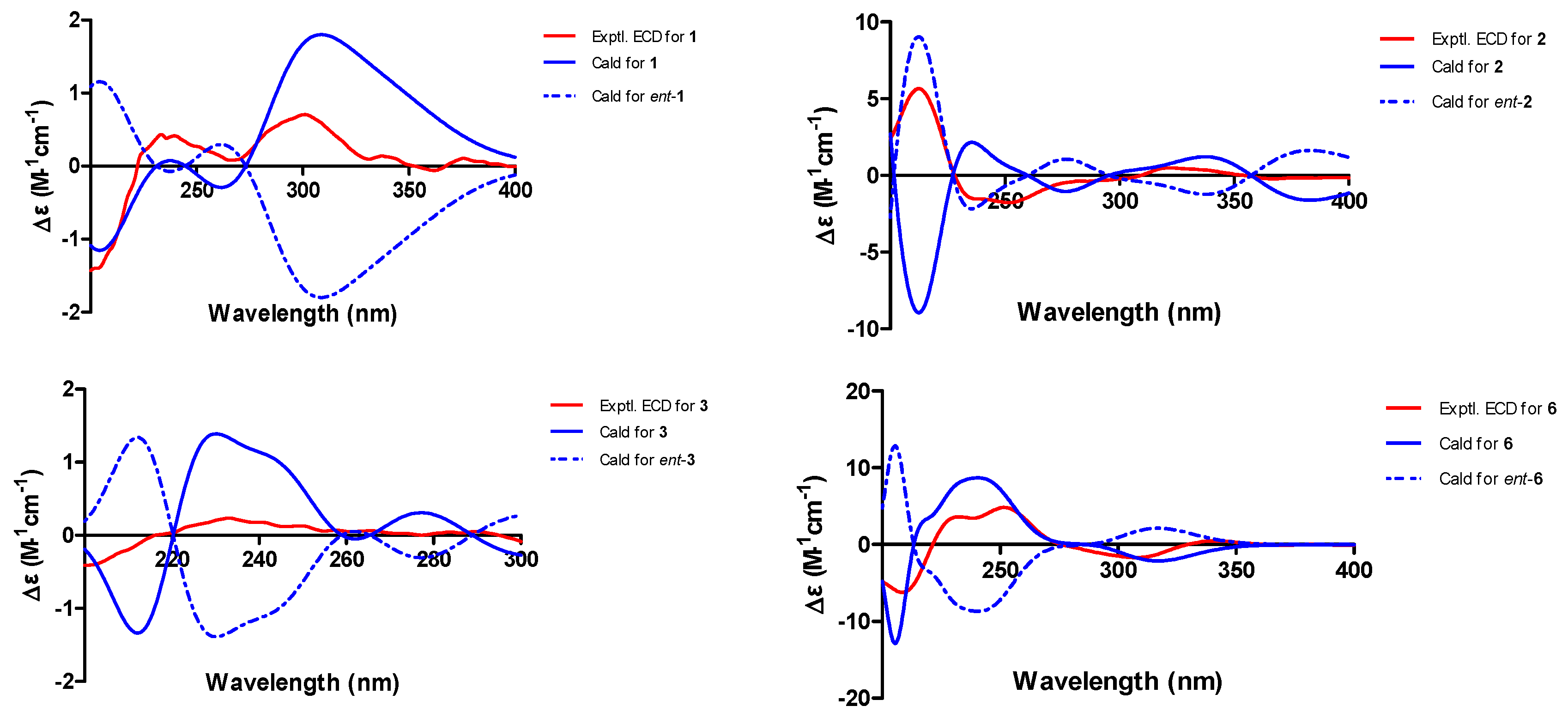
2.1. Structure Elucidation
2.2. Biological Activity
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Fungal Material
3.3. Fermentation, Extraction and Isolation
3.4. ECD Calculations
3.5. Antimicrobial Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, W.; Li, G.; Xu, J. Bio-Active Products from Mangrove Ecosystems. Mar. Drugs 2023, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.J.; Xu, B.; Guo, Y.W. Unusual Secondary Metabolites from the Mangrove Ecosystems: Structures, Bioactivities, Chemical, and Bio-Syntheses. Mar. Drugs 2022, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Biomolecules Produced by Mangrove-Associated Microbes. Curr. Med. Chem. 2011, 18, 5224–5266. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Bioactive Natural Products Derived from Mangrove-Associated Microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Karnchanatat, A.; Petsom, A.; Sangvanich, P.; Piapukiew, J.; Whalley, A.J.S.; Reynolds, C.D.; Gadd, G.M.; Sihanonth, P. A Novel Thermostable Endoglucanase from the Wood-Decaying Fungus Daldinia eschscholzii (Ehrenb.:Fr.) Rehm. Enzym. Microb. Technol. 2008, 42, 404–413. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Ge, H.M.; Zhao, W.; Dong, H.; Xu, Q.; Li, S.H.; Li, J.; Zhang, J.; Song, Y.C.; Tan, R.X. Unprecedented Immunosuppressive Polyketides from Daldinia eschscholzii, a Mantis-Associated Fungus. Angew. Chem. Int. Ed. 2008, 47, 5823–5826. [Google Scholar] [CrossRef] [PubMed]
- Tarman, K.; Palm, G.J.; Porzel, A.; Merzweiler, K.; Arnold, N.; Wessjohann, L.A.; Unterseher, M.; Lindequist, U. Helicascolide C, a New Lactone from an Indonesian Marine Algicolous Strain of Daldinia eschscholzii (Xylariaceae, Ascomycota). Phytochem. Lett. 2012, 5, 83–86. [Google Scholar] [CrossRef]
- Luo, Y.; Qiu, L.; Deng, Y.; Yuan, X.-H.; Gao, P. A New Chromone and a New Aliphatic Ester Isolated from Daldinia eschscholtzii. J. Asian Nat. Prod. Res. 2018, 20, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Kongyen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Sakayaroj, J. A New Hydronaphthalenone from the Mangrove-Derived Daldinia eschscholtzii PSU-STD57. Nat. Prod. Res. 2015, 29, 1995–1999. [Google Scholar] [CrossRef]
- Yang, L.-J.; Liao, H.-X.; Bai, M.; Huang, G.-L.; Luo, Y.-P.; Niu, Y.-Y.; Zheng, C.-J.; Wang, C.-Y. One New Cytochalasin Metabolite Isolated from a Mangrove-Derived Fungus Daldinia eschscholtzii HJ001. Nat. Prod. Res. 2018, 32, 208–213. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, J.; Jiang, N.; Lu, Y.H.; Wang, L.; Xu, S.H.; Wang, W.; Zhang, G.F.; Xu, Q.; Ge, H.M.; et al. Immunosuppressive Polyketides from Mantis-Associated Daldinia eschscholzii. J. Am. Chem. Soc. 2011, 133, 5931–5940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Tan, R.; Jiang, N.; Yusupu, K.; Wang, G.; Wang, X.L.; Tan, R.X. Selesconol, a Fungal Polyketide That Induces Stem Cell Differentiation. Org. Lett. 2016, 18, 5488–5491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Liu, W.; Jiang, N.; Wang, X.L.; Wang, G.; Xu, Q.; Tan, R.X. Sequestration of Guest Intermediates by Dalesconol Bioassembly Lines in Daldinia eschscholzii. Org. Lett. 2017, 19, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-X.; Shao, T.-M.; Mei, R.-Q.; Huang, G.-L.; Zhou, X.-M.; Zheng, C.-J.; Wang, C.-Y. Bioactive Secondary Metabolites from the Culture of the Mangrove-Derived Fungus Daldinia eschscholtzii HJ004. Mar. Drugs 2019, 17, 710. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-X.; Zheng, C.-J.; Huang, G.-L.; Mei, R.-Q.; Nong, X.-H.; Shao, T.-M.; Chen, G.-Y.; Wang, C.-Y. Bioactive Polyketide Derivatives from the Mangrove-Derived Fungus Daldinia eschscholtzii HJ004. J. Nat. Prod. 2019, 82, 2211–2219. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, X.; Wu, J.; Wei, C.; Feng, T.; Zhou, D.; Wen, Z.; Xu, J. Immunosuppressive cytochalasins from the mangrove endophytic fungus Phomopsis asparagi DHS-48. Mar. Drugs 2022, 20, 526. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wei, C.; Deng, X.; Chen, D.; Wen, Z.; Xu, J. Epigenetic manipulation induced production of immunosuppressive chromones and cytochalasins from the mangrove endophytic fungus Phomopsis asparagi DHS-48. Mar. Drugs 2022, 20, 616. [Google Scholar] [CrossRef]
- Wu, J.; Chen, D.; Li, Q.; Feng, T.; Xu, J. Metabolomics-Guided Discovery of New Dimeric Xanthones from Co-Cultures of Mangrove Endophytic Fungi Phomopsis asparagi DHS-48 and Phomopsis sp. DHS-11. Mar. Drugs 2024, 22, 102. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lin, Y.; Zhou, S.; Vrijmoed, L.L.P. Metabolites of Mangrove Endophytic Fungus 3920 from the South China Sea. Acta Sci. Nat. Univ. Sunyatseni 2005, 6, 137–138. [Google Scholar]
- Lu, M.M.; Chen, Y.C.; Liu, H.X.; Li, S.N.; Li, H.H.; Tao, M.H.; Hao, Z.B.; Zhang, W.M. Secondary Metabolites of Endophytic Daldinia eschscholtzii A630 from Pogostemon cablin and Their Cytotoxic Activities. Nat. Prod. Res. Dev. 2018, 30, 1176–1180. [Google Scholar] [CrossRef]
- Takeya, T.; Kondo, H.; Otsuka, T.; Tomita, K.; Okamoto, I.; Tamura, O. A Novel Construction of Dibenzofuran-1,4-Diones by Oxidative Cyclization of Quinone-Arenols. Org. Lett. 2007, 9, 2807–2810. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Yokoyama, E.; Takahashi, T.; Uzawa, J.; Morooka, N.; Tsunoda, H.; Tatsuno, T. Studies on the Metabolites of Penicillium Diversum Var. Aureum. I. Chem. Pharm. Bull. 1986, 34, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, A.K.; Sorensen, J.L. Polyketides Produced by Daldinia loculata Cultured from Northern Manitoba. Tetrahedron Lett. 2011, 52, 1697–1699. [Google Scholar] [CrossRef]
- Deng, Q.; Li, G.; Sun, M.; Yang, X.; Xu, J. A New Antimicrobial Sesquiterpene Isolated from Endophytic Fungus Cytospora sp. from the Chinese Mangrove Plant Ceriops tagal. Nat. Prod. Res. 2020, 34, 1404–1408. [Google Scholar] [CrossRef]
- Kamisuki, S.; Ishimaru, C.; Onoda, K.; Kuriyama, I.; Ida, N.; Sugawara, F.; Yoshida, H.; Mizushina, Y. Nodulisporol and Nodulisporone, Novel Specific Inhibitors of Human DNA Polymerase λ from a Fungus, Nodulisporium sp. Bioorganic Med. Chem. 2007, 15, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-L.; Zheng, J.-J.; Cao, F.; Wang, C.; Wang, C.-Y. Chemical Constituents of the Gorgonian-Derived Fungus Chaetomium globosum. Chem. Nat. Compd. 2017, 53, 199–202. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Hashimoto, T.; Takaoka, S.; Kan, Y.; Asakawa, Y. A 10-Phenyl-[11]-Cytochalasan from a Species of Daldinia. Phytochemistry 1996, 42, 173–176. [Google Scholar] [CrossRef]
- Chang, C.; Chang, H.; Cheng, M.; Liu, T.; Hsieh, S.; Yuan, G.; Chen, I. Inhibitory Effects of Constituents of an Endophytic Fungus Hypoxylon investiens on Nitric Oxide and Interleukin-6 Production in RAW264.7 Macrophages. Chem. Biodivers. 2014, 11, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Couché, E.; Fkyerat, A.; Tabacchi, R. Stereoselective Synthesis of cis- and trans-3,4-Dihydro-3,4,8-trihydroxynaphthalen-1(2H)-one. Helv. Chim. Acta 2009, 92, 903–917. [Google Scholar] [CrossRef]
- Boonyaketgoson, S.; Trisuwan, K.; Bussaban, B.; Rukachaisirikul, V.; Phongpaichit, S. Isochromanone Derivatives from the Endophytic Fungus Fusarium sp. PDB51F5. Tetrahedron Lett. 2015, 56, 5076–5078. [Google Scholar] [CrossRef]
- Krohn, K.; Bahramsari, R.; Flörke, U.; Ludewig, K.; Kliche-Spory, C.; Michel, A.; Aust, H.-J.; Draeger, S.; Schulz, B.; Antus, S. Dihydroisocoumarins from Fungi: Isolation, Structure Elucidation, Circular Dichroism and Biological Activity. Phytochemistry 1997, 45, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Guo, Z.; Li, Q.; Zhang, D.; She, Z.; Vrijmoed, L.L.P. A New Griseofulvin Derivative from the Mangrove Endophytic Fungus Sporothrix Sp. Chem. Nat. Compd. 2010, 46, 363–365. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Shaala, L.A.; Genta-Jouve, G. Asperopiperazines A and B: Antimicrobial and Cytotoxic Dipeptides from a Tunicate-Derived Fungus Aspergillus sp. DY001. Mar. Drugs 2022, 20, 451. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, G.; Huang, H.; Yu, P. Chromone Derivatives from Halenia elliptica and Their Anti-HBV Activities. Phytochemistry 2012, 75, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liao, H.; Liang, D. Chemical constitutes from Clerodendrum japonicum. China J. Chin. Mater. Medica 2018, 43, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- Amagata, T.; Tanaka, M.; Yamada, T.; Doi, M.; Minoura, K.; Ohishi, H.; Yamori, T.; Numata, A. Variation in Cytostatic Constituents of a Sponge-Derived Gymnascella dankaliensis by Manipulating the Carbon Source. J. Nat. Prod. 2007, 70, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, G.; Deng, Q.; Zheng, D.; Yang, X.; Xu, J. Cytotoxic Constituents from the Mangrove Endophytic Pestalotiopsis sp. Induce G0/G1 Cell Cycle Arrest and Apoptosis in Human Cancer Cells. Nat. Prod. Res. 2018, 32, 2968–2972. [Google Scholar] [CrossRef]
- Wei, C.W.; Sun, C.X.; Feng, Z.; Zhang, X.X.; Xu, J. Four New Chromones from the Endophytic Fungus Phomopsis asparagi DHS-48 Isolated from the Chinese Mangrove Plant Rhizophora mangle. Mar. Drugs 2021, 19, 348. [Google Scholar] [CrossRef]


| Position | 1 a,d | 2 a,c | 3 b,c | 4 b,c | 5 b,c | 6 a,c |
|---|---|---|---|---|---|---|
| 1 | 7.33, d (7.6) | |||||
| 2 | 6.84, d (6.8) | 2.46, m | 6.59, d (8.0) | 7.35, d (7.6) | 6.72, s | Ha 3.18, dd (16.9, 2.8) |
| Hb 2.64, dd (16.9, 3.2) | ||||||
| 3 | 7.18, d (6.8) | Ha 2.76, m | 6.37, d (8.0) | 4.37, dd (3.2, 2.8) | ||
| Hb 2.63, m | ||||||
| 4 | 5.13, d (2.8) | |||||
| 5 | 7.91, d (8.7) | |||||
| 6 | 6.96, d (6.1) | 7.32, dd (8.1, 1.0) | 7.45, t (8.4, 7.7) | 6.82, d (7.6) | 6.87, d (7.7) | 7.29, d (8.1) |
| 7 | 7.39, t (6.5, 6.4) | 7.42, t (8.1, 7.8) | 6.86, d (7.7) | 7.37, t (7.8) | 7.39, t (8.3, 8.0) | 7.42, t (8.1, 7.8) |
| 8 | 8.00, d (6.4) | 7.60, dd (7.8, 1.0) | 7.43, d (7.6) | 7.87, d (7.9) | 7.56, d (7.8) | |
| 9 | Ha 2.54, m | |||||
| Hb 2.33, m | ||||||
| 10 | Ha 2.56, m | |||||
| Hb 2.23, m | ||||||
| 1′ | 5.63, d (3.6) | |||||
| 2′ | 4.24, m (overlap) | 4.15, t (7.5) | Ha 2.38, m; Hb 1.86, m | |||
| 3′ | 4.15, dd (6.6, 3.1) | Ha 2.00,m | Ha 1.83, m; Hb 1.78, m | 7.06, s | 7.05, s | |
| Hb 1.83, m | ||||||
| 4′ | 4.23, m (overlap) | 4.29, m | 4.87, t (2.7) | |||
| 5′ | Ha 3.71, dd (9.7, 3.8) Hb 3.65, dd (9.7, 3.2) | 1.21, d (6.3) | 7.11, d (7.6) | |||
| 6′ | 7.32, t (7.9) | 7.78, dd (7.6, 2.9) | 7.77, dd (7.6, 1.0) | |||
| 7′ | 6.79, d (8.0) | 7.68, t (8.2, 7.8) | 7.68, t (8.3, 7.7) | |||
| 8′ | 7.31, d (8.2) | 7.32, d (8.1) | ||||
| 1-OCH3 | 3.96, s | |||||
| 4-OCH3 | 3.87, s | |||||
| 5-OCH3 | 3.92, s | 3.89, s | 4.05, s | 4.03, s | 3.93, s | |
| 8-OCH3 | 4.08, s | |||||
| 8′-OCH3 | 3.49, s | 4.01, s | 4.01, s | |||
| 4-OH | 9.82, s | 9.42, s |
| Position | 1 a,d | 2 a,c | 3 b,c | 4 b,c | 5 b,c | 6 a,c |
|---|---|---|---|---|---|---|
| 1 | 148.6, C | 199.8, C | 152.8, C | 118.7, CH | 147.9, C | 198.9, C |
| 2 | 108.2, CH | 38.6, CH2 | 109.4, CH | 128.8, CH | 107.2, CH | 41.8, CH2 |
| 3 | 113.1, CH | 36.6, CH2 | 126.6, CH | 116.2, C | 114.9, C | 71.4, CH |
| 4 | 153.6, C | 84.8, C | 131.5, C | 152.8, C | 146.4, C | 65.1, CH |
| 4a | 131.7, C | 134.6, C | 134.1, C | 114.8, C | 115.4, C | 131.2, C |
| 5 | 158.0, C | 159.3, C | 117.9, CH | 156.7, C | 156.5, C | 160.1, C |
| 6 | 108.4, CH | 118.6, CH | 125.5, CH | 104.6, CH | 105.6, CH | 117.3, CH |
| 7 | 127.2, CH | 130.3, CH | 103.8, CH | 126.8, CH | 126.4, CH | 130.4, CH |
| 8 | 116.5, CH | 120.1, CH | 156.9, C | 121.9, CH | 116.0, CH | 118.7, CH |
| 8a | 119.7, C | 134.7, C | 115.6, C | 137.4, C | 129.0, C | 133.8, C |
| 9 | 36.1, CH2 | |||||
| 10 | 35.1, CH2 | |||||
| 1′ | 103.9, CH | 115.8, C | 33.7, CH | 183.3, C | 183.3, C | |
| 2′ | 73.7, CH | 75.0, CH | 23.5, CH2 | 150.4, C | 151.0, C | |
| 3′ | 71.2, CH | 40.4, CH2 | 26.7, CH2 | 134.5, CH | 134.5, CH | |
| 4′ | 87.6, CH | 73.3, CH | 67.3, CH | 185.4, C | 185.4, C | |
| 4a′ | 140.4, C | 134.5, C | 134.4, C | |||
| 5′ | 63.3, CH2 | 22.4, CH3 | 121.6, CH | 118.7, CH | 118.7, CH | |
| 6′ | 127.2, CH | 134.6, CH | 134.5, CH | |||
| 7′ | 110.2, CH | 117.8, CH | 117.8, CH | |||
| 8′ | 157.2, C | 159.7, C | 159.7, C | |||
| 8a′ | 128.2, C | 121.0, C | 121.2, C | |||
| 1-OCH3 | 56.0, CH3 | |||||
| 4-OCH3 | 57.7, CH3 | |||||
| 5-OCH3 | 56.9, CH3 | 56.1, CH3 | 56.2, CH3 | 56.2, CH3 | 56.5, CH3 | |
| 8-OCH3 | 56.2, CH3 | |||||
| 8′-OCH3 | 55.6, CH3 | 56.5, CH3 | 56.5, CH3 |
| MIC(μg/mL) | |||||
|---|---|---|---|---|---|
| Compound | P. aeruginosa | E. faecalis | MRSA | E.coli | C. albicans |
| 1 | 6.25 | 25 | 12.5 | 6.25 | 25 |
| 2 | 50 | >50 | 50 | >50 | >50 |
| 3 | 25 | 25 | 25 | 12.5 | >50 |
| 4 | 25 | 50 | 50 | >50 | >50 |
| 5 | 12.5 | 50 | 25 | >50 | 50 |
| 6 | >50 | >50 | 50 | 50 | >50 |
| 7 | 12.5 | 50 | 25 | 25 | 50 |
| 8 | 25 | >50 | 25 | 50 | 12.5 |
| 9 | >50 | 25 | >50 | >50 | 25 |
| 10 | 50 | >50 | 50 | 50 | >50 |
| 11 | 50 | 25 | 50 | 50 | >50 |
| 12 | 25 | 25 | 25 | >50 | 25 |
| 13 | 25 | 12.5 | 12.5 | 25 | 25 |
| 14 | 25 | 25 | 25 | 25 | 25 |
| 15 | 50 | 25 | 50 | 25 | 25 |
| 16 | 12.5 | 25 | 12.5 | 6.25 | 50 |
| 17 | 12.5 | 12.5 | 6.25 | 12.5 | 25 |
| 18 | >50 | >50 | 12.5 | 25 | >50 |
| 19 | 12.5 | 25 | 25 | 12.5 | >50 |
| 20 | >50 | >50 | 50 | 50 | >50 |
| 21 | 50 | >50 | 50 | 50 | >50 |
| 22 | >50 | >50 | 25 | 50 | >50 |
| 23 | >50 | >50 | 25 | 25 | >50 |
| 24 | 50 | >50 | >50 | >50 | >50 |
| Ciprofloxacin | 0.78 | 0.625 | 0.3125 | 0.625 | |
| Amphotericin B | 0.78 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Feng, T.; Wen, Z.; Li, Q.; Chen, B.; Liu, P.; Xu, J. New Naphthalene Derivatives from the Mangrove Endophytic Fungus Daldinia eschscholzii MCZ-18. Mar. Drugs 2024, 22, 242. https://doi.org/10.3390/md22060242
Xu Z, Feng T, Wen Z, Li Q, Chen B, Liu P, Xu J. New Naphthalene Derivatives from the Mangrove Endophytic Fungus Daldinia eschscholzii MCZ-18. Marine Drugs. 2024; 22(6):242. https://doi.org/10.3390/md22060242
Chicago/Turabian StyleXu, Zhiyong, Ting Feng, Zhenchang Wen, Qing Li, Biting Chen, Pinghuai Liu, and Jing Xu. 2024. "New Naphthalene Derivatives from the Mangrove Endophytic Fungus Daldinia eschscholzii MCZ-18" Marine Drugs 22, no. 6: 242. https://doi.org/10.3390/md22060242
APA StyleXu, Z., Feng, T., Wen, Z., Li, Q., Chen, B., Liu, P., & Xu, J. (2024). New Naphthalene Derivatives from the Mangrove Endophytic Fungus Daldinia eschscholzii MCZ-18. Marine Drugs, 22(6), 242. https://doi.org/10.3390/md22060242








