Marine-Derived Leads as Anticancer Candidates by Disrupting Hypoxic Signaling through Hypoxia-Inducible Factors Inhibition
Abstract
1. Introduction
1.1. Marine Natural Products in Cancer Therapy
1.2. Hypoxic Signaling in Cancer Development
2. Marine Natural Products Acting as Inhibitors of HIF-1 and HIF-2
2.1. Peptides
2.2. Alkaloids
2.3. Polyketides
2.4. Phenolics
2.5. Terpenes
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayer, A.M.S.; Pierce, M.L.; Howe, K.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine Pharmacology in 2018: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Pharmacol. Res. 2022, 183, 106391. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef]
- Kijjoa, A.; Sawangwong, P. Drugs and Cosmetics from the Sea. Mar. Drugs 2004, 2, 73–82. [Google Scholar] [CrossRef]
- Pecoraro, C.; Terrana, F.; Panzeca, G.; Parrino, B.; Cascioferro, S.; Diana, P.; Giovannetti, E.; Carbone, D. Nortopsentins as Leads from Marine Organisms for Anticancer and Anti-Inflammatory Agent Development. Molecules 2023, 28, 6450. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug Development from Marine Natural Products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.G.M.; Madureira-Carvalho, Á.; Dias-da-Silva, D.; Valentão, P.; Andrade, P.B. Biosynthetic Versatility of Marine-Derived Fungi on the Delivery of Novel Antibacterial Agents against Priority Pathogens. Biomed. Pharmacother. 2021, 140, 111756. [Google Scholar] [CrossRef] [PubMed]
- Robert, J. Comparative Study of Tumorigenesis and Tumor Immunity in Invertebrates and Nonmammalian Vertebrates. Dev. Comp. Immunol. 2010, 34, 915–925. [Google Scholar] [CrossRef]
- Montaser, R.; Luesch, H. Marine Natural Products: A New Wave of Drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef]
- Munro, M.H.G.; Blunt, J.W.; Dumdei, E.J.; Hickford, S.J.H.; Lill, R.E.; Li, S.; Battershill, C.N.; Duckworth, A.R. The Discovery and Development of Marine Compounds with Pharmaceutical Potential. J. Biotechnol. 1999, 70, 15–25. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Cech, N.B.; Oberlies, N.H. From Plant to Cancer Drug: Lessons Learned from the Discovery of Taxol. Nat. Prod. Rep. 2023, 40, 1153–1157. [Google Scholar] [CrossRef]
- Newman, D.J. Natural Products and Drug Discovery. Natl. Sci. Rev. 2022, 9, nwac206. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, W.; Burke, D.C. Contributions to the Study of Marine Products. XXXIX. The Nucleosides of Sponges. III.1 Spongothymidine and Spongouridine. J. Org. Chem. 1955, 20, 1501–1507. [Google Scholar] [CrossRef]
- Lichtman, M.A. A Historical Perspective on the Development of the Cytarabine (7days) and Daunorubicin (3 days) Treatment Regimen for Acute Myelogenous Leukemia: 2013 the 40th Anniversary of 7+3. Blood Cells Mol. Dis. 2013, 50, 119–130. [Google Scholar] [CrossRef]
- Mayer, A.M.S. The Global Marine Pharmaceuticals Pipeline. Available online: https://www.marinepharmacology.org/ (accessed on 24 July 2023).
- Pereira, R.B.; Evdokimov, N.M.; Lefranc, F.; Valentaõ, P.; Kornienko, A.; Pereira, D.M.; Andrade, P.B.; Gomes, N.G.M. Marine-Derived Anticancer Agents: Clinical Benefits, Innovative Mechanisms, and New Targets. Mar. Drugs 2019, 17, 329. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.; Lefranc, F.; Kijjoa, A.; Kiss, R. Can Some Marine-Derived Fungal Metabolites Become Actual Anticancer Agents? Mar. Drugs 2015, 13, 3950–3991. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.; Spanò, V.; Montalbano, A.; Cueto, M.; Díaz Marrero, A.R.; Deniz, I.; Erdoğan, A.; Lukić Bilela, L.; Moulin, C.; Taffin-de-Givenchy, E.; et al. Marine Anticancer Agents: An Overview with a Particular Focus on Their Chemical Classes. Mar. Drugs 2020, 18, 619. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.C.; Wilke, D.V.; Branco, P.C.; Bauermeister, A.; Rezende-Teixeira, P.; Gaudêncio, S.P.; Costa-Lotufo, L. V Enriching Cancer Pharmacology with Drugs of Marine Origin. Br. J. Pharmacol. 2020, 177, 3–27. [Google Scholar] [CrossRef]
- Lefranc, F.; Koutsaviti, A.; Ioannou, E.; Kornienko, A.; Roussis, V.; Kiss, R.; Newman, D. Algae Metabolites: From: In Vitro Growth Inhibitory Effects to Promising Anticancer Activity. Nat. Prod. Rep. 2019, 36, 810–841. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine Natural Products. Nat. Prod. Rep. 1997, 14, 259–302. [Google Scholar] [CrossRef]
- John Faulkner, D. Highlights of Marine Natural Products Chemistry (1972–1999). Nat. Prod. Rep. 2000, 17, 1–6. [Google Scholar] [CrossRef]
- Blunden, G. Biologically Active Compounds from Marine Organisms. Phyther. Res. 2001, 15, 89–94. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Chin, Y.-W.; Swanson, S.M. Discovery of Natural Product Anticancer Agents from Biodiverse Organisms. Curr. Opin. Drug Discov. Devel. 2009, 12, 189–196. [Google Scholar]
- Junttila, M.R.; de Sauvage, F.J. Influence of Tumour Micro-Environment Heterogeneity on Therapeutic Response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Karakashev, S.V.; Reginato, M.J. Progress toward Overcoming Hypoxia-Induced Resistance to Solid Tumor Therapy. Cancer Manag. Res. 2015, 7, 253–264. [Google Scholar] [CrossRef]
- Matuszewska, K.; Pereira, M.; Petrik, D.; Lawler, J.; Petrik, J. Normalizing Tumor Vasculature to Reduce Hypoxia, Enhance Perfusion, and Optimize Therapy Uptake. Cancers 2021, 13, 4444. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Bouleftour, W.; Rowinski, E.; Louati, S.; Sotton, S.; Wozny, A.-S.; Moreno-Acosta, P.; Mery, B.; Rodriguez-Lafrasse, C.; Magne, N. A Review of the Role of Hypoxia in Radioresistance in Cancer Therapy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e934116. [Google Scholar] [CrossRef]
- Codony, V.L.; Tavassoli, M. Hypoxia-Induced Therapy Resistance: Available Hypoxia-Targeting Strategies and Current Advances in Head and Neck Cancer. Transl. Oncol. 2021, 14, 101017. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Tímár, J.; Sebestyén, A.; Kopper, L.; Dankó, T. Hypoxia Signaling in Cancer: From Basics to Clinical Practice. Pathol. Oncol. Res. 2021, 27, 1609802. [Google Scholar] [CrossRef]
- Koh, M.Y.; Powis, G. Passing the Baton: The HIF Switch. Trends Biochem. Sci. 2012, 37, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-L.; Wu, C.; Xiong, Z.-F.; Fang, X. Progress on Hypoxia-Inducible Factor-3: Its Structure, Gene Regulation and Biological Function (Review). Mol. Med. Rep. 2015, 12, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Schönenberger, M.; Kovacs, W. Hypoxia Signaling Pathways: Modulators of Oxygen-Related Organelles. Front. Cell Dev. Biol. 2015, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhang, J.; Huang, G.; Yan, J.; Xu, C.; Dou, Z.; Sun, C.; Zhang, H. The Crosstalk between HIFs and Mitochondrial Dysfunctions in Cancer Development. Cell Death Dis. 2021, 12, 215. [Google Scholar] [CrossRef]
- Germain, K.; Kim, P.K. Pexophagy: A Model for Selective Autophagy. Int. J. Mol. Sci. 2020, 21, 578. [Google Scholar] [CrossRef]
- Kim, J.-A. Peroxisome Metabolism in Cancer. Cells 2020, 9, 1692. [Google Scholar] [CrossRef]
- Tiburcio, P.D.; Choi, H.; Huang, L.E. Complex Role of HIF in Cancer: The Known, the Unknown, and the Unexpected. Hypoxia 2014, 2, 59–70. [Google Scholar] [CrossRef]
- Wicks, E.E.; Semenza, G.L. Hypoxia-Inducible Factors: Cancer Progression and Clinical Translation. J. Clin. Investig. 2022, 132, e159839. [Google Scholar] [CrossRef]
- Sharma, A.; Sinha, S.; Shrivastava, N. Therapeutic Targeting Hypoxia-Inducible Factor (HIF-1) in Cancer: Cutting Gordian Knot of Cancer Cell Metabolism. Front. Genet. 2022, 13, 849040. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for Cancer Therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef]
- Nagle, D.G.; Zhou, Y.-D. Natural Product-Based Inhibitors of Hypoxia-Inducible Factor-1 (HIF-1). Curr. Drug Targets 2006, 7, 355–369. [Google Scholar] [CrossRef]
- Nagle, D.G.; Zhou, Y.-D. Natural Product-Derived Small Molecule Activators of Hypoxia-Inducible Factor-1 (HIF-1). Curr. Pharm. Des. 2006, 12, 2673–2688. [Google Scholar] [CrossRef]
- Ikeda, H.; Kakeya, H. Targeting Hypoxia-Inducible Factor 1 (HIF-1) Signaling with Natural Products toward Cancer Chemotherapy. J. Antibiot. 2021, 74, 687–695. [Google Scholar] [CrossRef]
- Zhong, J.-C.; Li, X.-B.; Lyu, W.-Y.; Ye, W.-C.; Zhang, D.-M. Natural Products as Potent Inhibitors of Hypoxia-Inducible Factor-1α in Cancer Therapy. Chin. J. Nat. Med. 2020, 18, 696–703. [Google Scholar] [CrossRef]
- Manolescu, B.; Oprea, E.; Busu, C.; Cercasov, C. Natural Compounds and the Hypoxia-Inducible Factor (HIF) Signalling Pathway. Biochimie 2009, 91, 1347–1358. [Google Scholar] [CrossRef]
- Ma, Z.; Xiang, X.; Li, S.; Xie, P.; Gong, Q.; Goh, B.-C.; Wang, L. Targeting Hypoxia-Inducible Factor-1, for Cancer Treatment: Recent Advances in Developing Small-Molecule Inhibitors from Natural Compounds. Semin. Cancer Biol. 2022, 80, 379–390. [Google Scholar] [CrossRef]
- Jones, D.T.; Harris, A.L. Small-Molecule Inhibitors of the HIF Pathway and Synthetic Lethal Interactions. Expert Opin. Ther. Targets 2012, 16, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Samanta, P.; Sarkar, R.; Biswas, S.; Saha, P.; Hajra, S.; Bhowmik, A. Targeting HIF-1α by Natural and Synthetic Compounds: A Promising Approach for Anti-Cancer Therapeutics Development. Molecules 2022, 27, 5192. [Google Scholar] [CrossRef]
- Bhattarai, D.; Xu, X.; Lee, K. Hypoxia-Inducible Factor-1 (HIF-1) Inhibitors from the Last Decade (2007 to 2016): A “Structure–Activity Relationship” Perspective. Med. Res. Rev. 2018, 38, 1404–1442. [Google Scholar] [CrossRef] [PubMed]
- Ban, H.S.; Uto, Y.; Won, M.; Nakamura, H. Hypoxia-Inducible Factor (HIF) Inhibitors: A Patent Survey (2011–2015). Expert Opin. Ther. Pat. 2016, 26, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Zhou, Y.-D. Marine Natural Products as Inhibitors of Hypoxic Signaling in Tumors. Phytochem. Rev. 2009, 8, 415–429. [Google Scholar] [CrossRef]
- Govindarajan, G.; Yao, Z.; Zhou, Z.; Zheng, X.; Ma, J.; Kumar, P.S.; Ju, J.; Sun, C. Genome Sequencing of Streptomyces griseus SCSIO PteL053, the Producer of 2,2′-Bipyridine and Actinomycin Analogs, and Associated Biosynthetic Gene Cluster Analysis. J. Mar. Sci. Eng. 2023, 11, 396. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Y.; Xie, Y.; Zhang, C.; Ma, J.; Sun, C.; Ju, J. Identification of the Actinomycin D Biosynthetic Pathway from Marine-Derived Streptomyces costaricanus SCSIO ZS0073. Mar. Drugs 2019, 17, 240. [Google Scholar] [CrossRef] [PubMed]
- Bensaude, O. Inhibiting Eukaryotic Transcription: Which Compound to Choose? How to Evaluate Its Activity? Transcription 2011, 2, 103–108. [Google Scholar] [CrossRef]
- Berra, E.; Richard, D.E.; Gothié, E.; Pouysségur, J. HIF-1-Dependent Transcriptional Activity Is Required for Oxygen-Mediated HIF-1α Degradation. FEBS Lett. 2001, 491, 85–90. [Google Scholar] [CrossRef]
- Wang, G.L.; Semenza, G.L. Characterization of Hypoxia-Inducible Factor 1 and Regulation of DNA Binding Activity by Hypoxia. J. Biol. Chem. 1993, 268, 21513–21518. [Google Scholar] [CrossRef]
- Pagé, E.L.; Robitaille, G.A.; Pouysségur, J.; Richard, D.E. Induction of Hypoxia-Inducible Factor-1α by Transcriptional and Translational Mechanisms J. Biol. Chem. 2002, 277, 48403–48409. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.H.; Patil, K.B.; Parameswaran, P.S.; Naik, C.G.; Jagtap, T.G. Active Pharmaceutical Ingredient (Api) from an Estuarine Fungus, Microdochium nivale (Fr.). J. Environ. Biol. 2011, 32, 653–658. [Google Scholar] [PubMed]
- D’Angelo, G.; Duplan, E.; Vigne, P.; Frelin, C. Cyclosporin A Prevents the Hypoxic Adaptation by Activating Hypoxia-Inducible Factor-1α Pro-564 Hydroxylation. J. Biol. Chem. 2003, 278, 15406–15411. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, K.L.J.; Gloer, J.B.; Cook, J.C.J.; Mizsak, S.A.; Scahill, T.A. Structures of the Didemnins, Antiviral and Cytotoxic Depsipeptides from a Caribbean Tunicate. J. Am. Chem. Soc. 1981, 103, 1857–1859. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Valentaõ, P.; Andrade, P.B.; Pereira, R.B. Plitidepsin to Treat Multiple Myeloma. Drugs Today 2020, 56, 337–347. [Google Scholar] [CrossRef]
- Straight, A.M.; Oakley, K.; Moores, R.; Bauer, A.J.; Patel, A.; Tuttle, R.M.; Jimeno, J.; Francis, G.L. Aplidin Reduces Growth of Anaplastic Thyroid Cancer Xenografts and the Expression of Several Angiogenic Genes. Cancer Chemother. Pharmacol. 2006, 57, 7–14. [Google Scholar] [CrossRef]
- Symersky, J.; Osowski, D.; Walters, D.E.; Mueller, D.M. Oligomycin Frames a Common Drug-Binding Site in the ATP Synthase. Proc. Natl. Acad. Sci. USA 2012, 109, 13961–13965. [Google Scholar] [CrossRef]
- Dame, Z.T.; Islam, M.T.; Helmke, E.; von Tiedemann, A.; Laatsch, H. Oligomycins and Pamamycin Homologs Impair Motility and Induce Lysis of Zoospores of the Grapevine Downy Mildew Pathogen, Plasmopara Viticola. FEMS Microbiol. Lett. 2016, 363, fnw167. [Google Scholar] [CrossRef]
- Nagle, D.G.; Zhou, Y.-D. 2.20—Natural Products as Probes of Selected Targets in Tumor Cell Biology and Hypoxic Signaling. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 651–683. ISBN 978-0-08-045382-8. [Google Scholar]
- Gong, Y.; Agani, F.H. Oligomycin Inhibits HIF-1α Expression in Hypoxic Tumor Cells. Am. J. Physiol. Physiol. 2005, 288, C1023–C1029. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.K.; Chakraborty, M.; Rahman, M.; Gupta, D.R.; Mahmud, N.U.; Rahat, A.A.M.; Sarker, A.; Hannan, M.A.; Rahman, M.M.; Akanda, A.M.; et al. Marine Natural Product Antimycin A Suppresses Wheat Blast Disease Caused by Magnaporthe Oryzae Triticum. J. Fungi 2022, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-S.; Cobessi, D.; Tung, E.Y.; Berry, E.A. Binding of the Respiratory Chain Inhibitor Antimycin to the Mitochondrial Bc1 Complex: A New Crystal Structure Reveals an Altered Intramolecular Hydrogen-Bonding Pattern. J. Mol. Biol. 2005, 351, 573–597. [Google Scholar] [CrossRef]
- Chua, Y.L.; Dufour, E.; Dassa, E.P.; Rustin, P.; Jacobs, H.T.; Taylor, C.T.; Hagen, T. Stabilization of Hypoxia-Inducible Factor-1alpha Protein in Hypoxia Occurs Independently of Mitochondrial Reactive Oxygen Species Production. J. Biol. Chem. 2010, 285, 31277–31284. [Google Scholar] [CrossRef]
- Maeda, M.; Hasebe, Y.; Egawa, K.; Shibanuma, M.; Nose, K. Inhibition of Angiogenesis and HIF-1alpha Activity by Antimycin A1. Biol. Pharm. Bull. 2006, 29, 1344–1348. [Google Scholar] [CrossRef]
- Vlaminck, B.; Toffoli, S.; Ghislain, B.; Demazy, C.; Raes, M.; Michiels, C. Dual effect of echinomycin on hypoxia-inducible factor-1 activity under normoxic and hypoxic conditions. FEBS J. 2007, 274, 5533–5542. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J. Echinomycin, Triostin, and Related Antibiotics. In Mechanism of Action of Antieukaryotic and Antiviral Compounds; Hahn, F.E., Ed.; Springer: Berlin/Heidelberg, Germany, 1979; pp. 173–194. ISBN 978-3-642-46407-2. [Google Scholar]
- Park, Y.-S.; Shin, W.-S.; Kim, C.-S.; Ahn, C.M.; Qi, X.-F.; Kim, S.-K. Molecular and Cellular Toxicological Profiling of DNA Bis-Intercalator, Quinoxaline Compounds: Echinomycin as the Versatile Lead. Mol. Cell. Toxicol. 2018, 14, 9–18. [Google Scholar] [CrossRef]
- Foster, B.J.; Clagett-Carr, K.; Shoemaker, D.D.; Suffness, M.; Plowman, J.; Trissel, L.A.; Grieshaber, C.K.; Leyland-Jones, B. Echinomycin: The First Bifunctional Intercalating Agent in Clinical Trials. Investig. New Drugs 1985, 3, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Koike, K.; Okuda, K.; Hirayama, T.; Ebihara, M.; Takenaka, M.; Nagasawa, H. Solution-Phase Synthesis and Biological Evaluation of Triostin A and Its Analogues. Org. Biomol. Chem. 2016, 14, 2090–2111. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Park, E.J.; Stephen, A.G.; Calvani, M.; Cardellina, J.H.; Monks, A.; Fisher, R.J.; Shoemaker, R.H.; Melillo, G. Echinomycin, a Small-Molecule Inhibitor of Hypoxia-Inducible Factor-1 DNA-Binding Activity. Cancer Res. 2005, 65, 9047–9055. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Marín, L.; Álvarez-Alonso, R.; Redondo, S.; Carvajal, J.; Villamizar, G.; Villar, C.J.; Lombó, F. Biosynthetic Modularity Rules in the Bisintercalator Family of Antitumor Compounds. Mar. Drugs 2014, 12, 2668–2699. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.G.M.; Pereira, R.B.; Andrade, P.B.; Valentão, P. Double the Chemistry, Double the Fun: Structural Diversity and Biological Activity of Marine-Derived Diketopiperazine Dimers. Mar. Drugs 2019, 17, 551. [Google Scholar] [CrossRef]
- Watts, K.R.; Ratnam, J.; Ang, K.-H.; Tenney, K.; Compton, J.E.; McKerrow, J.; Crews, P. Assessing the Trypanocidal Potential of Natural and Semi-Synthetic Diketopiperazines from Two Deep Water Marine-Derived Fungi. Bioorg. Med. Chem. 2010, 18, 2566–2574. [Google Scholar] [CrossRef]
- Yun, K.; Khong, T.T.; Leutou, A.S.; Kim, G.-D.; Hong, J.; Lee, C.-H.; Son, B.W. Cristazine, a New Cytotoxic Dioxopiperazine Alkaloid from the Mudflat-Sediment-Derived Fungus Chaetomium cristatum. Chem. Pharm. Bull. 2016, 64, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.M.; Hilton, S.T.; Mecinović, J.; Motherwell, W.B.; Figg, W.D.; Schofield, C.J. Epidithiodiketopiperazines Block the Interaction between Hypoxia-Inducible Factor-1α (HIF-1α) and P300 by a Zinc Ejection Mechanism. J. Biol. Chem. 2009, 284, 26831–26838. [Google Scholar] [CrossRef] [PubMed]
- Kung, A.L.; Zabludoff, S.D.; France, D.S.; Freedman, S.J.; Tanner, E.A.; Vieira, A.; Cornell-Kennon, S.; Lee, J.; Wang, B.; Wang, J.; et al. Small Molecule Blockade of Transcriptional Coactivation of the Hypoxia-Inducible Factor Pathway. Cancer Cell 2004, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Hahnel, A.; Wichmann, H.; Rot, S.; Kappler, M.; Bache, M.; Vordermark, D. HIF-1α Inhibition by SiRNA or Chetomin in Human Malignant Glioma Cells: Effects on Hypoxic Radioresistance and Monitoring via CA9 Expression. BMC Cancer 2010, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, A.; Hayashi, T.; Kikuchi, N.; Hayashi, A.; Fuseya, C.; Shiozawa, T.; Konishi, I. Hypoxia Upregulates Ovarian Cancer Invasiveness via the Binding of HIF-1α to a Hypoxia-Induced, Methylation-Free Hypoxia Response Element of S100A4 Gene. Int. J. Cancer 2012, 131, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Herath, K.B.; Jayasuriya, H.; Ondeyka, J.G.; Polishook, J.D.; Bills, G.F.; Dombrowski, A.W.; Cabello, A.; Vicario, P.P.; Zweerink, H.; Guan, Z.; et al. Isolation and Structures of Novel Fungal Metabolites as Chemokine Receptor (CCR2) Antagonists. J. Antibiot. 2005, 58, 686–694. [Google Scholar] [CrossRef]
- Indelicato, M.; Pucci, B.; Schito, L.; Reali, V.; Aventaggiato, M.; Mazzarino, M.C.; Stivala, F.; Fini, M.; Russo, M.A.; Tafani, M. Role of Hypoxia and Autophagy in MDA-MB-231 Invasiveness. J. Cell. Physiol. 2010, 223, 359–368. [Google Scholar] [CrossRef]
- Staab, A.; Loeffler, J.; Said, H.M.; Diehlmann, D.; Katzer, A.; Beyer, M.; Fleischer, M.; Schwab, F.; Baier, K.; Einsele, H.; et al. Effects of HIF-1 Inhibition by Chetomin on Hypoxia-Related Transcription and Radiosensitivity in HT 1080 Human Fibrosarcoma Cells. BMC Cancer 2007, 7, 213. [Google Scholar] [CrossRef]
- Greiner, D.; Bonaldi, T.; Eskeland, R.; Roemer, E.; Imhof, A. Reply to “Chaetocin Is a Nonspecific Inhibitor of Histone Lysine Methyltransferases”. Nat. Chem. Biol. 2013, 9, 137. [Google Scholar] [CrossRef]
- Greiner, D.; Bonaldi, T.; Eskeland, R.; Roemer, E.; Imhof, A. Identification of a Specific Inhibitor of the Histone Methyltransferase SU(VAR)3-9. Nat. Chem. Biol. 2005, 1, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lee, J.B.; Indermaur, E.W.; Keung, A.J. Chaetocin Disrupts the SUV39H1–HP1 Interaction Independent of SUV39H1 Methyltransferase Activity. Biochem. J. 2023, 480, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Reece, K.M.; Richardson, E.D.; Cook, K.M.; Campbell, T.J.; Pisle, S.T.; Holly, A.J.; Venzon, D.J.; Liewehr, D.J.; Chau, C.H.; Price, D.K.; et al. Epidithiodiketopiperazines (ETPs) Exhibit in Vitro Antiangiogenic and in Vivo Antitumor Activity by Disrupting the HIF-1α/P300 Complex in a Preclinical Model of Prostate Cancer. Mol. Cancer 2014, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Luo, X.; Lin, M.; Xiao, Z.; Huang, L.; Wang, J.; Zhu, Y.; Liu, Y.; Tao, H. Marine-Fungi-Derived Gliotoxin Promotes Autophagy to Suppress Mycobacteria tuberculosis Infection in Macrophage. Mar. Drugs 2023, 21, 616. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, J.; Zhang, H.; Tong, L.; Zhang, L. Gliotoxin Induced Ferroptosis by Downregulating SUV39H1 Expression in Esophageal Cancer Cells. Recent Pat. Anticancer. Drug Discov. 2023, 18, 397–407. [Google Scholar] [CrossRef]
- Mohamed, A.F.; Abuamara, T.M.M.; Amer, M.E.; EI-Moselhy, L.E.; Gomah, T.A.; Matar, E.R.; Shebl, R.I.; Desouky, S.E.; Abu-Elghait, M. Genetic and Histopathological Alterations in Caco-2 and HuH-7 Cells Treated with Secondary Metabolites of Marine Fungi. J. Gastrointest. Cancer 2022, 53, 480–495. [Google Scholar] [CrossRef]
- Berman, F.W.; Gerwick, W.H.; Murray, T.F. Antillatoxin and Kalkitoxin, Ichthyotoxins from the Tropical Cyanobacterium Lyngbya Majuscula, Induce Distinct Temporal Patterns of NMDA Receptor-Mediated Neurotoxicity. Toxicon 1999, 37, 1645–1648. [Google Scholar] [CrossRef]
- Morgan, J.B.; Liu, Y.; Coothankandaswamy, V.; Mahdi, F.; Jekabsons, M.B.; Gerwick, W.H.; Valeriote, F.A.; Zhou, Y.D.; Nagle, D.G. Kalkitoxin Inhibits Angiogenesis, Disrupts Cellular Hypoxic Signaling, and Blocks Mitochondrial Electron Transport in Tumor Cells. Mar. Drugs 2015, 13, 1552–1568. [Google Scholar] [CrossRef]
- Senter, P.D.; Sievers, E.L. The Discovery and Development of Brentuximab Vedotin for Use in Relapsed Hodgkin Lymphoma and Systemic Anaplastic Large Cell Lymphoma. Nat. Biotechnol. 2012, 30, 631–637. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Dufresne, C.; Cerny, R.L.; Herald, C.L.; Schmidt, J.M. Isolation and Structure of the Cytostatic Linear Depsipeptide Dolastatin 15. J. Org. Chem. 1989, 54, 6005–6006. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Dasari, R.; Chandra, S.; Kiss, R.; Kornienko, A. Marine Invertebrate Metabolites with Anticancer Activities: Solutions to the “Supply Problem”. Mar. Drugs 2016, 14, 98. [Google Scholar] [CrossRef]
- Swain, S.S.; Padhy, R.N.; Singh, P.K. Anticancer Compounds from Cyanobacterium Lyngbya Species: A Review. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 108, 223–265. [Google Scholar] [CrossRef]
- Kerbrat, P.; Dieras, V.; Pavlidis, N.; Ravaud, A.; Wanders, J.; Fumoleau, P. Phase II Study of LU 103793 (Dolastatin Analogue) in Patients with Metastatic Breast Cancer. Eur. J. Cancer 2003, 39, 317–320. [Google Scholar] [CrossRef]
- Marks, R.S.; Graham, D.L.; Sloan, J.A.; Hillman, S.; Fishkoff, S.; Krook, J.E.; Okuno, S.H.; Mailliard, J.A.; Fitch, T.R.; Addo, F. A Phase II Study of the Dolastatin 15 Analogue LU 103793 in the Treatment of Advanced Non-Small-Cell Lung Cancer. Am. J. Clin. Oncol. Cancer Clin. Trials 2003, 26, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Mross, K. Clinical and Pharmacologic Phase I Study of Cemadotin-HCl (LU103793), a Novel Antimitotic Peptide, given as 24-Hour Infusion in Patients with Advanced Cancer. Ann. Oncol. 1998, 9, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Villalona-Calero, M.A.; Baker, S.D.; Hammond, L.; Aylesworth, C.; Eckhardt, S.G.; Kraynak, M.; Fram, R.; Fischkoff, S.; Velagapudi, R.; Toppmeyer, D.; et al. Phase I and Pharmacokinetic Study of the Water-Soluble Dolastatin 15 Analog LU103793 in Patients with Advanced Solid Malignancies. J. Clin. Oncol. 1998, 16, 2770–2779. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Appleman, L.J.; Kirvan-Visovatti, M.; Ryan, D.P.; Regan, E.; Vukelja, S.; Bonate, P.L.; Ruvuna, F.; Fram, R.J.; Jekunen, A.; et al. Phase I and Pharmacokinetic Study of the Dolastatin-15 Analogue Tasidotin (ILX651) Administered Intravenously on Days 1, 3, and 5 Every 3 Weeks in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2005, 11, 7825–7833. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ebbinghaus, S.; Rubin, E.; Hersh, E.; Cranmer, L.D.; Bonate, P.L.; Fram, R.J.; Jekunen, A.; Weitman, S.; Hammond, L.A. A Phase I Study of the Dolastatin-15 Analogue Tasidotin (ILX651) Administered Intravenously Daily for 5 Consecutive Days Every 3 Weeks in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2005, 11, 7807–7816. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Friedman, S.J.; Pettit, G.R.; Hamel, E. Dolastatin 15, a Potent Antimitotic Depsipeptide Derived from Dolabella Auricularia. Interaction with Tubulin and Effects on Cellular Microtubules. Biochem. Pharmacol. 1992, 43, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Lopus, M. Mechanism of Mitotic Arrest Induced by Dolastatin 15 Involves Loss of Tension across Kinetochore Pairs. Mol. Cell. Biochem. 2013, 382, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, R.; Gunasekera, S.P.; Ma, J.J.; Dang, L.H.; Carney, T.J.; Paul, V.J.; Luesch, H. Dolastatin 15 from a Marine Cyanobacterium Suppresses HIF-1α Mediated Cancer Cell Viability and Vascularization. ChemBioChem 2020, 21, 2356–2366. [Google Scholar] [CrossRef]
- Goey, A.K.L.; Chau, C.H.; Sissung, T.M.; Cook, K.M.; Venzon, D.J.; Castro, A.; Ransom, T.R.; Henrich, C.J.; McKee, T.C.; McMahon, J.B.; et al. Screening and Biological Effects of Marine Pyrroloiminoquinone Alkaloids: Potential Inhibitors of the HIF-1α/P300 Interaction. J. Nat. Prod. 2016, 79, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-F.; Fan, H.; Xiong, J.; Wu, S.-B. Discorhabdins and Pyrroloiminoquinone-Related Alkaloids. Chem. Rev. 2011, 111, 5465–5491. [Google Scholar] [CrossRef]
- Harris, E.M.; Strope, J.D.; Beedie, S.L.; Huang, P.A.; Goey, A.K.L.; Cook, K.M.; Schofield, C.J.; Chau, C.H.; Cadelis, M.M.; Copp, B.R.; et al. Preclinical Evaluation of Discorhabdins in Antiangiogenic and Antitumor Models. Mar. Drugs 2018, 16, 241. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.T.S.; Patel, P.R.; Ransom, T.R.; Henrich, C.J.; Mckee, T.C.; Goey, A.K.L.; Cook, K.M.; Figg, W.D.; Mcmahon, J.B.; Schnermann, M.J.; et al. Structural Elucidation and Synthesis of Eudistidine A: An Unusual Polycyclic Marine Alkaloid That Blocks Interaction of the Protein Binding Domains of P300 and HIF-1α. J. Am. Chem. Soc. 2015, 137, 5569–5575. [Google Scholar] [CrossRef]
- Roll, D.M.; Ireland, C.M.; Lu, H.S.M.; Clardy, J. Fascaplysin, an Unusual Antimicrobial Pigment from the Marine Sponge Fascaplysinopsis Sp. J. Org. Chem. 1988, 53, 3276–3278. [Google Scholar] [CrossRef]
- Hörmann, A.; Chaudhuri, B.; Fretz, H. DNA Binding Properties of the Marine Sponge Pigment fascaplysin. Bioorg. Med. Chem. 2001, 9, 917–921. [Google Scholar] [CrossRef]
- Lin, J.; Yan, X.-J.; Chen, H.-M. Fascaplysin, a Selective CDK4 Inhibitor, Exhibit Anti-Angiogenic Activity in Vitro and in Vivo. Cancer Chemother. Pharmacol. 2007, 59, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Muller, L.; Furet, P.; Schoepfer, J.; Stephan, C.; Zumstein-Mecker, S.; Fretz, H.; Chaudhuri, B. Inhibition of Cyclin-Dependent Kinase 4 (Cdk4) by Fascaplysin, a Marine Natural Product. Biochem. Biophys. Res. Commun. 2000, 275, 877–884. [Google Scholar] [CrossRef]
- Bharate, S.B.; Manda, S.; Mupparapu, N.; Battini, N.; Vishwakarma, R.A. Chemistry and Biology of Fascaplysin, a Potent Marine-Derived CDK-4 Inhibitor. Mini Rev. Med. Chem. 2012, 12, 650–664. [Google Scholar] [CrossRef]
- Shafiq, M.I.; Steinbrecher, T.; Schmid, R. Fascaplysin as a Specific Inhibitor for CDK4: Insights from Molecular Modelling. PLoS ONE 2012, 7, e42612. [Google Scholar] [CrossRef]
- Oh, T.-I.; Lee, Y.-M.; Nam, T.-J.; Ko, Y.-S.; Mah, S.; Kim, J.; Kim, Y.; Reddy, R.H.; Kim, Y.J.; Hong, S.; et al. Fascaplysin Exerts Anti-Cancer Effects through the Downregulation of Survivin and HIF-1α and Inhibition of VEGFR2 and TRKA. Int. J. Mol. Sci. 2017, 18, 2074. [Google Scholar] [CrossRef]
- Aguilar-Santos, G. Caulerpin, a New Red Pigment from Green Algae of the Genus Caulerpa. J. Chem. Soc. C 1970, 6, 842–843. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Morgan, J.B.; Coothankandaswamy, V.; Liu, R.; Jekabsons, M.B.; Mahdi, F.; Nagle, D.G. The Caulerpa Pigment Caulerpin Inhibits HIF-1 Activation and Mitochondrial Respiration. J. Nat. Prod. 2009, 1, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Liu, Y.; Morgan, J.B.; Jekabsons, M.B.; Zhou, Y.; Nagle, D.G. Lipophilic 2,5-Disubstituted Pyrroles from the Marine Sponge Mycale Sp. Inhibit Mitochondrial Respiration and HIF-1 Activation. J. Nat. Prod. 2009, 72, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.J.; Faulkner, D.J.; He, C.H.; Van Duyne, G.D.; Clardy, J. Metabolites of the Marine Prosobranch Mollusk Lamellaria Sp. J. Am. Chem. Soc. 1985, 107, 5492–5495. [Google Scholar] [CrossRef]
- Lindquist, N.; Fenical, W.; Van Duyne, G.D.; Clardy, J. New Alkaloids of the Lamellarin Class from the Marine Ascidian Didemnum chartaceum (Sluiter, 1909). J. Org. Chem. 1988, 53, 4570–4574. [Google Scholar] [CrossRef]
- Urban, S.; Capon, R.J. Lamellarin-S: A New Aromatic Metabolite From an Australian Tunicate, Didemnum Sp. Aust. J. Chem. 1996, 49, 711–713. [Google Scholar] [CrossRef]
- Reddy, M.V.R.; Faulkner, D.J.; Venkateswarlu, Y.; Rao, M.R. New Lamellarin Alkaloids from an Unidentified Ascidian from the Arabian Sea. Tetrahedron 1997, 53, 3457–3466. [Google Scholar] [CrossRef]
- Davis, R.A.; Carroll, A.R.; Pierens, G.K.; Quinn, R.J. New Lamellarin Alkaloids from the Australian Ascidian, Didemnum chartaceum. J. Nat. Prod. 1999, 62, 419–424. [Google Scholar] [CrossRef]
- Ham, J.-Y.; Kang, H.-J. A Novel Cytotoxic Alkaloid of Lamellarin Class from a Marine Ascidian Didemnum Sp. Bull. Korean Chem. Soc. 2002, 23, 163–166. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Reddy, V.L.N.; Venkataramana, G.; Ravinder, K.; Srinivasulu, M.; Raju, T.V.; Ravikumar, K.; Chandrasekar, D.; Ramakrishna, S.; Venkateswarlu, Y. New Lamellarin Alkaloids from the Indian Ascidian Didemnum obscurum and Their Antioxidant Properties. J. Nat. Prod. 2004, 67, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Malla Reddy, S.; Srinivasulu, M.; Satyanarayana, N.; Kondapi, A.K.; Venkateswarlu, Y. New Potent Cytotoxic Lamellarin Alkaloids from Indian Ascidian Didemnum obscurum. Tetrahedron 2005, 61, 9242–9247. [Google Scholar] [CrossRef]
- Plisson, F.; Huang, X.-C.; Zhang, H.; Khalil, Z.; Capon, R.J. Lamellarins as Inhibitors of P-Glycoprotein-Mediated Multidrug Resistance in a Human Colon Cancer Cell Line. Chem. Asian J. 2012, 7, 1616–1623. [Google Scholar] [CrossRef]
- Urban, S.; Butler, M.S.; Capon, R.J. Lamellarins O and P: New Aromatic Metabolites from the Australian Marine Sponge Dendrilla cactos. Aust. J. Chem. 1994, 47, 1919–1924. [Google Scholar] [CrossRef]
- Huang, X.-C.; Xiao, X.; Zhang, Y.-K.; Talele, T.T.; Salim, A.A.; Chen, Z.-S.; Capon, R.J. Lamellarin O, a Pyrrole Alkaloid from an Australian Marine Sponge, Ianthella Sp., Reverses BCRP Mediated Drug Resistance in Cancer Cells. Mar. Drugs 2014, 12, 3818–3837. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Zhou, Y.-D.; Nagle, D.G. Molecular-Targeted Antitumor Agents. 15. Neolamellarins from the Marine Sponge Dendrilla nigra Inhibit Hypoxia-Inducible Factor-1 Activation and Secreted Vascular Endothelial Growth Factor Production in Breast Tumor Cells. J. Nat. Prod. 2007, 70, 1741–1745. [Google Scholar] [CrossRef]
- Li, G.; Shao, Y.; Pan, Y.; Li, Y.; Wang, Y.; Wang, L.; Wang, X.; Shao, K.; Wang, S.; Liu, N.; et al. Total Synthesis and Biological Evaluation of 7-Hydroxyneolamellarin A as Hypoxia-Inducible Factor-1α Inhibitor for Cancer Therapy. Bioorg. Med. Chem. Lett. 2021, 50, 128338. [Google Scholar] [CrossRef]
- Li, G.; Dong, H.; Ma, Y.; Shao, K.; Li, Y.; Wu, X.; Wang, S.; Shao, Y.; Zhao, W. Structure-Activity Relationships Study of Neolamellarin A and Its Analogues as Hypoxia Inducible Factor-1 (HIF-1) Inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 2327–2331. [Google Scholar] [CrossRef]
- Shin, J.; Rho, J.-R.; Seo, Y.; Lee, H.-S.; Cho, K.W.; Kwon, H.J.; Sim, C.J. Wondonins A and B, New Bis(Dihydroxystyryl)Imidazoles from a Two-Sponge Association. Tetrahedron Lett. 2001, 42, 1965–1968. [Google Scholar] [CrossRef]
- Jun, H.-O.; Kim, Y.; Kwon, Y.-W.; Hong, S.-S.; Kim, K.-W.; Shin, J.; Kim, T.-Y. Wondonin, a Novel Compound, Inhibits Hypoxia-Induced Angiogenesis through Hypoxia-Inducible Factor 1 Alpha. FEBS Lett. 2007, 581, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Oh, J.; Li, F.; Kwon, Y.; Cho, H.; Shin, J.; Lee, S.K.; Kim, S. New Scaffold for Angiogenesis Inhibitors Discovered by Targeted Chemical Transformations of Wondonin Natural Products. ACS Med. Chem. Lett. 2017, 8, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Quiñoà, E.; Crews, P. Phenolic Constituents of Psammaplysilla. Tetrahedron Lett. 1987, 28, 3229–3232. [Google Scholar] [CrossRef]
- Arabshahi, L.; Schmitz, F.J. Brominated Tyrosine Metabolites from an Unidentified Sponge. J. Org. Chem. 1987, 52, 3584–3586. [Google Scholar] [CrossRef]
- Rodriguez, A.D.; Akee, R.K.; Scheuer, P.J. Two Bromotyrosine-Cysteine Derived Metabolites from a Sponge. Tetrahedron Lett. 1987, 28, 4989–4992. [Google Scholar] [CrossRef]
- Piña, I.C.; Gautschi, J.T.; Wang, G.-Y.-S.; Sanders, M.L.; Schmitz, F.J.; France, D.; Cornell-Kennon, S.; Sambucetti, L.C.; Remiszewski, S.W.; Perez, L.B.; et al. Psammaplins from the Sponge Pseudoceratina purpurea: Inhibition of Both Histone Deacetylase and DNA Methyltransferase. J. Org. Chem. 2003, 68, 3866–3873. [Google Scholar] [CrossRef]
- McCulloch, M.W.B.; Coombs, G.S.; Banerjee, N.; Bugni, T.S.; Cannon, K.M.; Harper, M.K.; Veltri, C.A.; Virshup, D.M.; Ireland, C.M. Psammaplin A as a General Activator of Cell-Based Signaling Assays via HDAC Inhibition and Studies on Some Bromotyrosine Derivatives. Bioorg. Med. Chem. 2009, 17, 2189–2198. [Google Scholar] [CrossRef]
- Shin, J.; Lee, H.-S.; Seo, Y.; Rho, J.-R.; Cho, K.W.; Paul, V.J. New Bromotyrosine Metabolites from the Sponge Aplysinella rhax. Tetrahedron 2000, 56, 9071–9077. [Google Scholar] [CrossRef]
- Jung, J.H.; Sim, C.J.; Lee, C.-O. Cytotoxic Compounds from a Two-Sponge Association. J. Nat. Prod. 1995, 58, 1722–1726. [Google Scholar] [CrossRef]
- Li, C.J.; Schmitz, F.J.; Kelly-Borges, M. A New Lysine Derivative and New 3-Bromopyrrole Carboxylic Acid Derivative from Two Marine Sponges. J. Nat. Prod. 1998, 61, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Liu, Y.; Hong, J.; Lee, C.-O.; Cho, H.; Kim, D.-K.; Im, K.S.; Jung, J.H. New Bromotyrosine Derivatives from an Association of Two Sponges, Jaspis wondoensis and Poecillastra wondoensis. J. Nat. Prod. 2003, 66, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, H.S.; Kang, Y.J.; Yoon, S.; Lee, J.; Choi, W.S.; Jung, J.H.; Kim, H.S. Psammaplin A Induces Sirtuin 1-Dependent Autophagic Cell Death in Doxorubicin-Resistant MCF-7/Adr Human Breast Cancer Cells and Xenografts. Biochim. Biophys. Acta 2015, 1850, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.D.; Li, J.; Du, L.; Mahdi, F.; Le, T.P.; Chen, W.L.; Swanson, S.M.; Watabe, K.; Nagle, D.G. Biochemical and Anti-Triple Negative Metastatic Breast Tumor Cell Properties of Psammaplins. Mar. Drugs 2018, 16, 442. [Google Scholar] [CrossRef] [PubMed]
- Leach, B.E.; Ford, J.H.; Whiffen, A.J. Actidione, an Antibiotic from Streptomyces griseus. J. Am. Chem. Soc. 1947, 69, 474. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, L.; Wang, S.; Liu, H.; Gao, J.; Zhao, S. Cycloheximide Acid A, a New Cycloheximide Derivative from Marine Derived Streptomyces Sp. from East China Sea. Rec. Nat. Prod. 2013, 7, 292–295. [Google Scholar]
- Flora, D.O.; Adeyemi, A.I.; George, W.P. Himalomycin A and Cycloheximide-Producing Marine Actinomycete from Lagos Lagoon Soil Sediment. J. Coast. Life Med. 2015, 3, 361–365. [Google Scholar] [CrossRef]
- Semenza, G.L.; Wang, G.L. A Nuclear Factor Induced by Hypoxia via de Novo Protein Synthesis Binds to the Human Erythropoietin Gene Enhancer at a Site Required for Transcriptional Activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef]
- Semenza, G.L.; Roth, P.H.; Fang, H.M.; Wang, G.L. Transcriptional Regulation of Genes Encoding Glycolytic Enzymes by Hypoxia-Inducible Factor 1. J. Biol. Chem. 1994, 269, 23757–23763. [Google Scholar] [CrossRef]
- Kakeya, H.; Takahashi, I.; Okada, G.; Isono, K.; Osada, H. Epolactaene, a Novel Neuritogenic Compound in Human Neuroblastoma Cells, Produced by a Marine Fungus. J. Antibiot. 1995, 48, 733–735. [Google Scholar] [CrossRef]
- Nagumo, Y.; Kakeya, H.; Shoji, M.; Hayashi, Y.; Dohmae, N.; Osada, H. Epolactaene Binds Human Hsp60 Cys442 Resulting in the Inhibition of Chaperone Activity. Biochem. J. 2005, 387, 835–840. [Google Scholar] [CrossRef]
- Ban, H.S.; Shimizu, K.; Minegishi, H.; Nakamura, H. Identification of Heat Shock Protein 60 as the Regulator of the Hypoxia-Inducible Factor Subunit HIF-1α. Pure Appl. Chem. 2012, 84, 2325–2337. [Google Scholar] [CrossRef]
- Spector, I.; Shochet, N.R.; Blasberger, D.; Kashman, Y. Latrunculins--Novel Marine Macrolides That Disrupt Microfilament Organization and Affect Cell Growth: I. Comparison with Cytochalasin D. Cell Motil. Cytoskelet. 1989, 13, 127–144. [Google Scholar] [CrossRef]
- Nèeman, I.; Fishelson, L.; Kashman, T. Isolation of a New Toxin from the Sponge Latrunculia magnifica in the Gulf of Aqaba (Red Sea). Mar. Biol. 1975, 30, 293–296. [Google Scholar] [CrossRef]
- Kashman, Y.; Groweiss, A.; Shmueli, U. Latrunculin, a New 2-Thiazolidinone Macrolide from the Marine Sponge Latrunculia magnifica. Tetrahedron Lett. 1980, 21, 3629–3632. [Google Scholar] [CrossRef]
- Kakou, Y.; Crews, P.; Bakus, G.J. Dendrolasin and Latrunculin A from the Fijian Sponge Spongia mycofijiensis and an Associated Nudibranch Chromodoris Lochi. J. Nat. Prod. 1987, 50, 482–484. [Google Scholar] [CrossRef]
- Okuda, R.K.; Scheuer, P.J. Latrunculin-A, Ichthyotoxic Constituent of the Nudibranch Chromodoris elisabethina. Experientia 1985, 41, 1355–1356. [Google Scholar] [CrossRef]
- Khanfar, M.A.; Youssef, D.T.A.; El Sayed, K.A. 3D-QSAR Studies of Latrunculin-Based Actin Polymerization Inhibitors Using CoMFA and CoMSIA Approaches. Eur. J. Med. Chem. 2010, 45, 3662–3668. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Khanfar, M.A.; Shallal, H.M.; Muralidharan, A.; Awate, B.; Youssef, D.T.A.; Liu, Y.; Zhou, Y.D.; Nagle, D.G.; Shah, G. Latrunculin A and Its C-17-O-Carbamates Inhibit Prostate Tumor Cell Invasion and HIF-1 Activation in Breast Tumor Cells. J. Nat. Prod. 2008, 71, 396–402. [Google Scholar] [CrossRef]
- Shin, I.J.; Park, B.K.; Ahn, Y.T.; Kim, Y.; An, W.G. Actin Disruption Inhibits Hypoxia Inducible Factor-1α Expression via Inactivity of Mdm2-Mediated P70S6K. Mol. Med. Rep. 2010, 3, 815–819. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.-H.; Shin, Y.; Lee, S.-H.; Oh, K.-B.; Lee, S.K.; Shin, J.; Oh, D.-C. Salternamides A-D from a Halophilic Streptomyces Sp. Actinobacterium. J. Nat. Prod. 2015, 78, 836–843. [Google Scholar] [CrossRef]
- Kim, S.-H.; Shin, Y.; Lee, S.K.; Shin, J.; Oh, D.-C. Salternamide E from a Saltern-Derived Marine Actinomycete Streptomyces Sp. Nat. Prod. Sci. 2015, 21, 273–277. [Google Scholar] [CrossRef]
- Bach, D.H.; Kim, S.H.; Hong, J.Y.; Park, H.J.; Oh, D.C.; Lee, S.K. Salternamide a Suppresses Hypoxia-Induced Accumulation of HIF-1α and Induces Apoptosis in Human Colorectal Cancer Cells. Mar. Drugs 2015, 13, 6962–6976. [Google Scholar] [CrossRef] [PubMed]
- Crews, P.; Kakou, Y.; Quinoa, E. Mycothiazole, a Polyketide Heterocycle from a Marine Sponge. J. Am. Chem. Soc. 1988, 110, 4365–4368. [Google Scholar] [CrossRef]
- Cutignano, A.; Bruno, I.; Bifulco, G.; Casapullo, A.; Debitus, C.; Gomez-Paloma, L.; Riccio, R. Dactylolide, a New Cytotoxic Macrolide from the Vanuatu Sponge Dactylospongia Sp. Eur. J. Org. Chem. 2001, 2001, 775–778. [Google Scholar] [CrossRef]
- Meyer, K.J.; Singh, A.J.; Cameron, A.; Tan, A.S.; Leahy, D.C.; O’Sullivan, D.; Joshi, P.; La Flamme, A.C.; Northcote, P.T.; Berridge, M.V.; et al. Mitochondrial Genome-Knockout Cells Demonstrate a Dual Mechanism of Action for the Electron Transport Complex I Inhibitor Mycothiazole. Mar. Drugs 2012, 10, 900–917. [Google Scholar] [CrossRef]
- Morgan, J.B.; Mahdi, F.; Liu, Y.; Coothankandaswamy, V.; Jekabsons, M.B.; Johnson, T.A.; Sashidhara, K.V.; Crews, P.; Nagle, D.G.; Zhou, Y.-D. The Marine Sponge Metabolite Mycothiazole: A Novel Prototype Mitochondrial Complex I Inhibitor. Bioorg. Med. Chem. 2010, 18, 5988–5994. [Google Scholar] [CrossRef]
- Walsh, T.J.; Standiford, H.C.; Reboli, A.C.; John, J.F.; Mulligan, M.E.; Ribner, B.S.; Montgomerie, J.Z.; Goetz, M.B.; Mayhall, C.G.; Rimland, D. Randomized Double-Blinded Trial of Rifampin with Either Novobiocin or Trimethoprim-Sulfamethoxazole against Methicillin-Resistant Staphylococcus Aureus Colonization: Prevention of Antimicrobial Resistance and Effect of Host Factors on Outcome. Antimicrob. Agents Chemother. 1993, 37, 1334–1342. [Google Scholar] [CrossRef]
- Eder, J.P.; Wheeler, C.A.; Teicher, B.A.; Schnipper, L.E. A Phase I Clinical Trial of Novobiocin, a Modulator of Alkylating Agent Cytotoxicity. Cancer Res. 1991, 51, 510–513. [Google Scholar]
- Zhou, J.; Gelot, C.; Pantelidou, C.; Li, A.; Yücel, H.; Davis, R.E.; Färkkilä, A.; Kochupurakkal, B.; Syed, A.; Shapiro, G.I.; et al. A First-in-Class Polymerase Theta Inhibitor Selectively Targets Homologous-Recombination-Deficient Tumors. Nat. Cancer 2021, 2, 598–610. [Google Scholar] [CrossRef]
- Hoeksema, H.; Johnson, J.L.; Hinman, J.W. Structural Studies on Streptonivicin,1 a New Antibiotic. J. Am. Chem. Soc. 1955, 77, 6710–6711. [Google Scholar] [CrossRef]
- Smith, C.G.; Dietz, A.; Sokolski, W.T.; Savage, G.M. Streptonivicin, a New Antibiotic. I. Discovery and Biologic Studies. Antibiot. Chemother. 1956, 6, 135–142. [Google Scholar]
- Hoeksema, H.; Bergy, M.E.; Jackson, W.G.; Shell, J.W.; Hinman, J.W.; Fonken, A.E.; Boyack, G.A.; Caron, E.L.; Ford, J.H.; Devries, W.H.; et al. Streptonivicin, a New Antibiotic. II. Isolation and Characterization. Antibiot. Chemother. 1956, 6, 143–148. [Google Scholar]
- Dalisay, D.S.; Williams, D.E.; Wang, X.L.; Centko, R.; Chen, J.; Andersen, R.J. Marine Sediment-Derived Streptomyces Bacteria from British Columbia, Canada Are a Promising Microbiota Resource for the Discovery of Antimicrobial Natural Products. PLoS ONE 2013, 8, e77078. [Google Scholar] [CrossRef]
- Syed, A.; Filandr, F.; Patterson-Fortin, J.; Bacolla, A.; Ravindranathan, R.; Zhou, J.; McDonald, D.T.; Albuhluli, M.E.; Verway-Cohen, A.; Newman, J.A.; et al. Novobiocin Blocks Nucleic Acid Binding to Polθ and Inhibits Stimulation of Its ATPase Activity. Nucleic Acids Res. 2023, 51, 9920–9937. [Google Scholar] [CrossRef]
- Conde, R.; Belak, Z.R.; Nair, M.; O’Carroll, R.F.; Ovsenek, N. Modulation of Hsf1 Activity by Novobiocin and Geldanamycin. Biochem. Cell Biol. 2009, 87, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Katschinski, D.M.; Le, L.; Heinrich, D.; Wagner, K.F.; Hofer, T.; Schindler, S.G.; Wenger, R.H. Heat Induction of the Unphosphorylated Form of Hypoxia-Inducible Factor-1α Is Dependent on Heat Shock Protein-90 Activity. J. Biol. Chem. 2002, 277, 9262–9267. [Google Scholar] [CrossRef] [PubMed]
- Lupescu, A.; Bissinger, R.; Herrmann, T.; Oswald, G.; Jilani, K.; Lang, F. Induction of Suicidal Erythrocyte Death by Novobiocin. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 33, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, R.; Zhao, R.; Chen, G.; Cai, Y.; Jin, J. A Novel Function of Novobiocin: Disrupting the Interaction of HIF 1α and P300/CBP through Direct Binding to the HIF1α C-Terminal Activation Domain. PLoS ONE 2013, 8, e62014. [Google Scholar] [CrossRef] [PubMed]
- Egorov, E.A.; Alekhina, V.A.; Volobueva, T.M.; Fedoreev, S.A.; Mishchenko, N.P.; Kol’tsova, E.A. Histochrome, a new antioxidant, in the treatment of ocular diseases. Vestn. Oftalmol. 1999, 115, 34–35. [Google Scholar]
- Mishchenko, N.P.; Fedoreev, S.A.; Bagirova, V.L. Histochrome: A New Original Domestic Drug. Pharm. Chem. J. 2003, 37, 48–52. [Google Scholar] [CrossRef]
- Hwang, J.-W.; Park, J.-H.; Park, B.-W.; Kim, H.; Kim, J.-J.; Sim, W.-S.; Mishchenko, N.P.; Fedoreyev, S.A.; Vasileva, E.A.; Ban, K.; et al. Histochrome Attenuates Myocardial Ischemia-Reperfusion Injury by Inhibiting Ferroptosis-Induced Cardiomyocyte Death. Antioxidants 2021, 10, 1624. [Google Scholar] [CrossRef]
- Artyukov, A.A.; Popov, A.M.; Tsybulsky, A.V.; Krivoshapko, O.N.; Polyakova, N.V. Pharmacological Activity of Echinochrome a Alone and in the Biologically Active Additive Timarin. Biochem. Suppl. Ser. B Biomed. Chem. 2013, 7, 237–242. [Google Scholar] [CrossRef]
- Munn, C.A. Mac On the Chromatology of the Blood of Some Invertebrates. J. Cell Sci. 1885, s2-25, 469–490. [Google Scholar] [CrossRef]
- Service, M.; Wardlaw, A.C. Echinochrome-A as a Bactericidal Substance in the Coelomic Fluid of Echinus esculentus (L.). Comp. Biochem. Physiol. Part B Comp. Biochem. 1984, 79, 161–165. [Google Scholar] [CrossRef]
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Ni, J. Emodin: A Review of Its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef]
- Stompor-Gorący, M. The Health Benefits of Emodin, a Natural Anthraquinone Derived from Rhubarb-A Summary Update. Int. J. Mol. Sci. 2021, 22, 9522. [Google Scholar] [CrossRef]
- de Mattos-Shipley, K.M.J.; Simpson, T.J. The “emodin Family” of Fungal Natural Products-Amalgamating a Century of Research with Recent Genomics-Based Advances. Nat. Prod. Rep. 2023, 40, 174–201. [Google Scholar] [CrossRef]
- Greco, G.; Turrini, E.; Catanzaro, E.; Fimognari, C. Marine Anthraquinones: Pharmacological and Toxicological Issues. Mar. Drugs 2021, 19, 272. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Taktaz, F.; Ayatollahi, S.A.; Kijjoa, A. Anthraquinones and Their Analogues from Marine-Derived Fungi: Chemistry and Biological Activities. Mar. Drugs 2022, 20, 474. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.M.; Dethoup, T.; Singburaudom, N.; Gales, L.; Silva, A.M.S.; Kijjoa, A. Eurocristatine, a New Diketopiperazine Dimer from the Marine Sponge-Associated Fungus Eurotium cristatum. Phytochem. Lett. 2012, 5, 717–720. [Google Scholar] [CrossRef]
- Tuli, H.S.; Aggarwal, V.; Tuorkey, M.; Aggarwal, D.; Parashar, N.C.; Varol, M.; Savla, R.; Kaur, G.; Mittal, S.; Sak, K. Emodin: A Metabolite That Exhibits Anti-Neoplastic Activities by Modulating Multiple Oncogenic Targets. Toxicol. Vitr. 2021, 73, 105142. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Fang, Y.; Jia, W.; Wang, Y.; Sun, J.; Tao, D. Emodin Relieves Hypoxia-Triggered Injury via Elevation of MicroRNA-25 in PC-12 Cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2678–2687. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.K.; Song, Y.H.; Jeong, S.J.; Lee, H.J.; Jung, J.H.; Kim, B.; Song, H.S.; Huh, J.E.; Kim, S.H. Emodin Inhibits Proinflammatory Responses and Inactivates Histone Deacetylase 1 in Hypoxic Rheumatoid Synoviocytes. Biol. Pharm. Bull. 2011, 34, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shan, C.; Wu, Z.; Yu, H.; Yang, A.; Tan, B. Emodin Alleviated Pulmonary Inflammation in Rats with LPS-Induced Acute Lung Injury through Inhibiting the MTOR/HIF-1α/VEGF Signaling Pathway. Inflamm. Res. 2020, 69, 365–373. [Google Scholar] [CrossRef]
- Qi, L.; Fu, Q.; Du, C.; Wu, D.; Zhang, G.; Yuan, B.; Yan, L. Amelioration of Hypoxia and LPS-Induced Intestinal Epithelial Barrier Dysfunction by Emodin through the Suppression of the NF-ΚB and HIF-1α Signaling Pathways. Int. J. Mol. Med. 2014, 34, 1629–1639. [Google Scholar] [CrossRef]
- Lv, B.; Zheng, K.; Sun, Y.; Wu, L.; Qiao, L.; Wu, Z.; Zhao, Y.; Zheng, Z. Network Pharmacology Experiments Show That Emodin Can Exert a Protective Effect on MCAO Rats by Regulating Hif-1α/VEGF-A Signaling. ACS Omega 2022, 7, 22577–22593. [Google Scholar] [CrossRef]
- Hu, L.; Cui, R.; Liu, H.; Wang, F. Emodin and Rhein Decrease Levels of Hypoxia-Inducible Factor-1a in Human Pancreatic Cancer Cells and Attenuate Cancer Cachexia in Athymic Mice Carrying These Cells. Oncotarget 2017, 8, 88008–88020. [Google Scholar] [CrossRef][Green Version]
- Shi, G.H.; Zhou, L. Emodin Suppresses Angiogenesis and Metastasis in Anaplastic Thyroid Cancer by Affecting TRAF6-Mediated Pathways in Vivo and in Vitro. Mol. Med. Rep. 2018, 18, 5191–5197. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Heo, K.; Kim, J.S.; Im, J.W.; Lee, S.M.; Cho, M.; Kang, D.H.; Heo, J.; Lee, J.W.; Choi, C.W.; et al. Emodin Attenuates Radioresistance Induced by Hypoxia in HepG2 Cells via the Enhancement of PARP1 Cleavage and Inhibition of JMJD2B. Oncol. Rep. 2015, 33, 1691–1698. [Google Scholar] [CrossRef]
- Deboer, C.; Meulman, P.A.; Wnuk, R.J.; Peterson, D.H. Geldanamycin, a New Antibiotic. J. Antibiot. 1970, 23, 442–447. [Google Scholar] [CrossRef]
- Yi, K.-X.; Xie, Q.-Y.; Ma, Q.-Y.; Yang, L.; Dai, H.-F.; Zhao, Y.-X.; Hao, Y.-E. Diverse Ansamycin Derivatives from the Marine-Derived Streptomyces Sp. ZYX-F-97 and Their Antibacterial Activities. Fitoterapia 2024, 173, 105814. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, M.; Qiu, Y.; Liu, X.; Wang, C.; Chen, J.; Zhang, H.; Wei, B.; Yu, Y.; Ying, Y.; et al. α-Glucosidase Inhibitors from Two Mangrove-Derived Actinomycetes. Molecules 2023, 28, 3822. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Lian, X.-Y.; Zhang, Z. Cytotoxic Metabolites from the Marine-Associated Streptomyces Sp. ZZ1944. Phytochemistry 2022, 201, 113292. [Google Scholar] [CrossRef] [PubMed]
- Nong, X.-H.; Tu, Z.-C.; Qi, S.-H. Ansamycin Derivatives from the Marine-Derived Streptomyces Sp. SCSGAA 0027 and Their Cytotoxic and Antiviral Activities. Bioorg. Med. Chem. Lett. 2020, 30, 127168. [Google Scholar] [CrossRef]
- Whitesell, L.; Mimnaugh, E.G.; De Costa, B.; Myers, C.E.; Neckers, L.M. Inhibition of Heat Shock Protein HSP90-Pp60v-Src Heteroprotein Complex Formation by Benzoquinone Ansamycins: Essential Role for Stress Proteins in Oncogenic Transformation. Proc. Natl. Acad. Sci. USA 1994, 91, 8324–8328. [Google Scholar] [CrossRef] [PubMed]
- Roe, S.M.; Prodromou, C.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. Structural Basis for Inhibition of the Hsp90 Molecular Chaperone by the Antitumor Antibiotics Radicicol and Geldanamycin. J. Med. Chem. 1999, 42, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, C.; Menéndez, J.C. Chapter 14—Miscellaneous Small- Molecule and Biological Approaches to Targeted Cancer Therapy. In Medicinal Chemistry of Anticancer Drugs, 3rd ed.; Avendaño, C., Menéndez, J.C., Eds.; Elsevier: Boston, MA, USA, 2023; pp. 743–822. ISBN 978-0-12-818549-0. [Google Scholar]
- Mabjeesh, N.J.; Post, D.E.; Willard, M.T.; Kaur, B.; Van Meir, E.G.; Simons, J.W.; Zhong, H. Geldanamycin Induces Degradation of Hypoxia-Inducible Factor 1alpha Protein via the Proteosome Pathway in Prostate Cancer Cells. Cancer Res. 2002, 62, 2478–2482. [Google Scholar] [PubMed]
- Suzuki, Y.; Kondo, Y.; Hara, S.; Kimata, R.; Nishimura, T. Effect of the Hsp90 Inhibitor Geldanamycin on Androgen Response of Prostate Cancer under Hypoxic Conditions. Int. J. Urol. 2010, 17, 281–285. [Google Scholar] [CrossRef]
- Alqawi, O.; Moghaddas, M.; Singh, G. Effects of Geldanamycin on HIF-1alpha Mediated Angiogenesis and Invasion in Prostate Cancer Cells. Prostate Cancer Prostatic Dis. 2006, 9, 126–135. [Google Scholar] [CrossRef]
- van der Bilt, J.D.W.; Soeters, M.E.; Duyverman, A.M.M.J.; Nijkamp, M.W.; Witteveen, P.O.; van Diest, P.J.; Kranenburg, O.; Borel Rinkes, I.H.M. Perinecrotic Hypoxia Contributes to Ischemia/Reperfusion-Accelerated Outgrowth of Colorectal Micrometastases. Am. J. Pathol. 2007, 170, 1379–1388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koga, F.; Tsutsumi, S.; Neckers, L.M. Low Dose Geldanamycin Inhibits Hepatocyte Growth Factor and Hypoxia-Stimulated Invasion of Cancer Cells. Cell Cycle 2007, 6, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.V.; Baek, J.H.; Zhang, H.; Diez, R.; Cole, R.N.; Semenza, G.L. RACK1 Competes with HSP90 for Binding to HIF-1alpha and Is Required for O(2)-Independent and HSP90 Inhibitor-Induced Degradation of HIF-1alpha. Mol. Cell 2007, 25, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Mejia, E.J.; Loveridge, S.T.; Stepan, G.; Tsai, A.; Jones, G.S.; Barnes, T.; White, K.N.; Drašković, M.; Tenney, K.; Tsiang, M.; et al. Study of Marine Natural Products Including Resorcyclic Acid Lactones from Humicola fuscoatra That Reactivate Latent HIV-1 Expression in an in Vitro Model of Central Memory CD4+ T Cells. J. Nat. Prod. 2014, 77, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Grkovic, T.; Whitson, E.L.; Rabe, D.C.; Gardella, R.S.; Bottaro, D.P.; Linehan, W.M.; McMahon, J.B.; Gustafson, K.R.; McKee, T.C. Identification and Evaluation of Soft Coral Diterpenes as Inhibitors of HIF-2α Induced Gene Expression. Bioorg. Med. Chem. Lett. 2011, 21, 2113–2115. [Google Scholar] [CrossRef]
- McKee, T.C.; Rabe, D.; Bokesch, H.R.; Grkovic, T.; Whitson, E.L.; Diyabalanage, T.; Van Wyk, A.W.W.; Marcum, S.R.; Gardella, R.S.; Gustafson, K.R.; et al. Inhibition of Hypoxia Inducible Factor-2 Transcription: Isolation of Active Modulators from Marine Sponges. J. Nat. Prod. 2012, 75, 1632–1636. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Bloor, S.J. Xesto- and Halenaquinone Derivatives from a Sponge, Adocia Sp., from Truk lagoon. J. Org. Chem. 1988, 53, 3922–3925. [Google Scholar] [CrossRef]
- Concepción, G.P.; Foderaro, T.A.; Eldredge, G.S.; Lobkovsky, E.; Clardy, J.; Barrows, L.R.; Ireland, C.M. Topoisomerase II-Mediated DNA Cleavage by Adocia- and Xestoquinones from the Philippine Sponge Xestospongia Sp. J. Med. Chem. 1995, 38, 4503–4507. [Google Scholar] [CrossRef]
- Du, L.; Mahdi, F.; Datta, S.; Jekabsons, M.B.; Zhou, Y.-D.; Nagle, D.G. Structures and Mechanisms of Antitumor Agents: Xestoquinones Uncouple Cellular Respiration and Disrupt HIF Signaling in Human Breast Tumor Cells. J. Nat. Prod. 2012, 75, 1553–1559. [Google Scholar] [CrossRef]
- Cao, S.; Foster, C.; Brisson, M.; Lazo, J.S.; Kingston, D.G.I. Halenaquinone and Xestoquinone Derivatives, Inhibitors of Cdc25B Phosphatase from a Xestospongia Sp. Bioorg. Med. Chem. 2005, 13, 999–1003. [Google Scholar] [CrossRef]
- Isaac, B.G.; Ayer, S.W.; Elliott, R.C.; Stonard, R.J. Herboxidiene: A Potent Phytotoxic Polyketide from Streptomyces Sp. A7847. J. Org. Chem. 1992, 57, 7220–7226. [Google Scholar] [CrossRef]
- Damayanti, E.; Nisa, K.; Handayani, S.; Dewi, R.T.; Mustofa, M.; Dinoto, A.; Dinoto, A.; Widada, J. Cytotoxicity and Molecular Mechanism of Marine-Derived Streptomyces Sp. Gmy01 on Human Lung Cancer Cell Line A549. J. Appl. Pharm. Sci. 2021, 11, 46–55. [Google Scholar] [CrossRef]
- Hasegawa, M.; Miura, T.; Kuzuya, K.; Inoue, A.; Won Ki, S.; Horinouchi, S.; Yoshida, T.; Kunoh, T.; Koseki, K.; Mino, K.; et al. Identification of SAP155 as the Target of GEX1A (Herboxidiene), an Antitumor Natural Product. ACS Chem. Biol. 2011, 6, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Kaida, D.; Motoyoshi, H.; Tashiro, E.; Nojima, T.; Hagiwara, M.; Ishigami, K.; Watanabe, H.; Kitahara, T.; Yoshida, T.; Nakajima, H.; et al. Spliceostatin A Targets SF3b and Inhibits Both Splicing and Nuclear Retention of Pre-MRNA. Nat. Chem. Biol. 2007, 3, 576–583. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, Y.; Shin, J.Y.; Sohng, J.K.; Kwon, H.J. Antiangiogenic Activity of Herboxidiene via Downregulation of Vascular Endothelial Growth Factor Receptor-2 and Hypoxia-Inducible Factor-1α. Arch. Pharm. Res. 2015, 38, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liu, Y.; Jia, H.; Zhou, Y.D.; Nagle, D.G. Benzochromenones from the Marine Crinoid Comantheria Rotula Inhibit Hypoxia-Inducible Factor-1 (HIF-1) in Cell-Based Reporter Assays and Differentially Suppress the Growth of Certain Tumor Cell Lines. J. Nat. Prod. 2007, 70, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, S.B.; Reis, M.; Sousa, M.L.; Ribeiro, T.; Almeida, J.R.; Pereira, S.; Antunes, J.; Rosa, F.; Vasconcelos, V.; Achour, L.; et al. The Marine Seagrass Halophila stipulacea as a Source of Bioactive Metabolites against Obesity and Biofouling. Mar. Drugs 2020, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, K.H.; Jung, H.J.; Kwon, H.J. Matairesinol Inhibits Angiogenesis via Suppression of Mitochondrial Reactive Oxygen Species. Biochem. Biophys. Res. Commun. 2012, 421, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin Extracts from Fucales Characterized by HPLC-DAD-ESI-MSn: Approaches to Hyaluronidase Inhibitory Capacity and Antioxidant Properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.-K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological Activities and Potential Health Benefits. BioFactors 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Lopes, G.; Andrade, P.B.; Valentão, P. Phlorotannins: Towards New Pharmacological Interventions for Diabetes Mellitus Type 2. Molecules 2017, 22, 56. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Xiao, Z.; Tang, Y.; Hong, P.; Sun, S.; Zhou, C.; Qian, Z.J. Inhibition Effects of 7-Phloro-Eckol from Ecklonia cava on Metastasis and Angiogenesis Induced by Hypoxia through Regulation of AKT/MTOR and ERK Signaling Pathways. Arab. J. Chem. 2021, 14, 103187. [Google Scholar] [CrossRef]
- Hodges, T.W.; Hossain, C.F.; Kim, Y.P.; Zhou, Y.D.; Nagle, D.G. Molecular-Targeted Antitumor Agents: The Saururus cernuus Dineolignans Manassantin B and 4-O-Demethylmanassantin B Are Potent Inhibitors of Hypoxia-Activated HIF-1. J. Nat. Prod. 2004, 67, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Hossain, C.F.; Kim, Y.P.; Baerson, S.R.; Zhang, L.; Bruick, R.K.; Mohammed, K.A.; Agarwal, A.K.; Nagle, D.G.; Zhou, Y.D. Saururus cernuus Lignans—Potent Small Molecule Inhibitors of Hypoxia-Inducible Factor-1. Biochem. Biophys. Res. Commun. 2005, 333, 1026–1033. [Google Scholar] [CrossRef]
- Dai, J.; Liu, Y.; Zhou, Y.D.; Nagle, D.G. Cytotoxic Metabolites from an Indonesian Sponge Lendenfeldia Sp. J. Nat. Prod. 2007, 70, 1824–1826. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Yang, J.; Yang, X.; Zhang, J.; Zhao, Z. Type A Trichothecene Metabolic Profile Differentiation, Mechanisms, Biosynthetic Pathways, and Evolution in Fusarium Species—A Mini Review. Toxins 2023, 15, 446. [Google Scholar] [CrossRef]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From Simple to Complex Mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Shin, H.W.; Chun, Y.S.; Leutou, A.S.; Son, B.W.; Park, J.W. Diacetoxyscirpenol as a New Anticancer Agent to Target Hypoxiainducible Factor 1. Oncotarget 2016, 7, 62107–62122. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, W.; Kuca, K. From Hypoxia and Hypoxia-Inducible Factors (HIF) to Oxidative Stress: A New Understanding of the Toxic Mechanism of Mycotoxins. Food Chem. Toxicol. 2020, 135, 110968. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Kawachi, T.; Sato, H.; Setiawan, A.; Kobayashi, M. Marine Spongian Sesquiterpene Phenols, Dictyoceratin-C and Smenospondiol, Display Hypoxia-Selective Growth Inhibition against Cancer Cells. Bioorg. Med. Chem. Lett. 2014, 24, 3155–3157. [Google Scholar] [CrossRef]
- Nakamura, H.; Deng, S.; Kobayashi, J.; Ohizumi, Y.; Hirata, Y. Dictyoceratin-A and -B, Novel Antimicrobial Terpenoids from the Okinawan Marine Sponge Hipposponqia Sp. Tetrahedron 1986, 42, 4197–4201. [Google Scholar] [CrossRef]
- Kushlan, D.M.; Faulkner, D.J.; Parkanyi, L.; Clardy, J. Metabolites of the Palauan Sponge Dactylospongia Sp. Tetrahedron 1989, 45, 3307–3312. [Google Scholar] [CrossRef]
- Shen, Y.C.; Hsieh, P.W. New Sesquiterpene Hydroquinones from a Taiwanese Marine Sponge Polyfibrospongia australis. J. Nat. Prod. 1997, 60, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Gao, Z.; Thomas, S.J.; Hecht, S.M.; Lazo, J.S.; Kingston, D.G.I. Marine Sesquiterpenoids That Inhibit the Lyase Activity of DNA Polymerase Beta. J. Nat. Prod. 2004, 67, 1716–1718. [Google Scholar] [CrossRef] [PubMed]
- Sumii, Y.; Kotoku, N.; Fukuda, A.; Kawachi, T.; Arai, M.; Kobayashi, M. Structure-Activity Relationship and in Vivo Anti-Tumor Evaluations Ofdictyoceratin-A and-C,Hypoxia-Selective Growth Inhibitors from Marine Sponge. Mar. Drugs 2015, 13, 7419–7432. [Google Scholar] [CrossRef] [PubMed]
- Sumii, Y.; Kotoku, N.; Fukuda, A.; Kawachi, T.; Sumii, Y.; Arai, M.; Kobayashi, M. Enantioselective Synthesis of Dictyoceratin-A (Smenospondiol) and -C, Hypoxia-Selective Growth Inhibitors from Marine Sponge. Bioorg. Med. Chem. 2015, 23, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, T.; Tanaka, S.; Fukuda, A.; Sumii, Y.; Setiawan, A.; Kotoku, N.; Kobayashi, M.; Arai, M. Target Identification of the Marine Natural Products Dictyoceratin-A and -C as Selective Growth Inhibitors in Cancer Cells Adapted to Hypoxic Environments. Mar. Drugs 2019, 17, 163. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.N.; Perzanowski, H.P.; Ross, R.A.; Erdman, T.R.; Scheuer, P.J.; Finer, J.; Clardy, J. Recent Research in Marine Natural Products: The Puupehenones. Pure Appl. Chem. 1979, 51, 1893–1900. [Google Scholar] [CrossRef]
- Amade, P.; Chevelot, L.; Perzanowski, H.P.; Scheuer, P.J. A Dimer of Puupehenone. Helv. Chim. Acta 1983, 66, 1672–1675. [Google Scholar] [CrossRef]
- Nasu, S.S.; Yeung, B.K.S.; Hamann, M.T.; Scheuer, P.J.; Kelly-Borges, M.; Goins, K. Puupehenone-Related Metabolites from Two Hawaiian Sponges, Hyrtios Spp. J. Org. Chem. 1995, 60, 7290–7292. [Google Scholar] [CrossRef]
- Piña, I.C.; Sanders, M.L.; Crews, P. Puupehenone Congeners from an Indo-Pacific Hyrtios Sponge. J. Nat. Prod. 2003, 66, 2–6. [Google Scholar] [CrossRef]
- Robinson, S.J.; Hoobler, E.K.; Riener, M.; Loveridge, S.T.; Tenney, K.; Valeriote, F.A.; Holman, T.R.; Crews, P. Using Enzyme Assays to Evaluate the Structure and Bioactivity of Sponge-Derived Meroterpenes. J. Nat. Prod. 2009, 72, 1857–1863. [Google Scholar] [CrossRef]
- Kohmoto, S.; McConnell, O.J.; Wright, A.; Koehn, F.; Thompson, W.; Lui, M.; Snader, K.M. Puupehenone, a Cytotoxic Metabolite from a Deep Water Marine Sponge, Stronglyophora hartmani. J. Nat. Prod. 1987, 50, 336. [Google Scholar] [CrossRef]
- Coval, S.J.; Conover, M.A.; Mierzwa, R.; King, A.; Puar, M.S.; Phife, D.W.; Pai, J.-K.; Burrier, R.E.; Ahn, H.-S.; Boykow, G.C.; et al. Wiedendiol-A and -B, Cholesteryl Ester Transfer Protein Inhibitors from the Marine Sponge Xestospongia wiedenmayeri. Bioorg. Med. Chem. Lett. 1995, 5, 605–610. [Google Scholar] [CrossRef]
- Ueda, K.; Ueta, T.; Siwu, E.R.O.; Kita, M.; Uemura, D. Haterumadienone: A New Puupehenone Congener from an Okinawan Marine Sponge, Dysidea Sp. Chem. Lett. 2005, 34, 1530–1531. [Google Scholar] [CrossRef]
- Utkina, N.K.; Denisenko, V.A.; Krasokhin, V.B. Diplopuupehenone, a New Unsymmetrical Puupehenone-Related Dimer from the Marine Sponge Dysidea Sp. Tetrahedron Lett. 2011, 52, 3765–3768. [Google Scholar] [CrossRef]
- Hagiwara, K.; Garcia Hernandez, J.E.; Harper, M.K.; Carroll, A.; Motti, C.A.; Awaya, J.; Nguyen, H.-Y.; Wright, A.D. Puupehenol, a Potent Antioxidant Antimicrobial Meroterpenoid from a Hawaiian Deep-Water Dactylospongia Sp. Sponge. J. Nat. Prod. 2015, 78, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Amagata, T.; Whitman, S.; Johnson, T.A.; Stessman, C.C.; Loo, C.P.; Lobkovsky, E.; Clardy, J.; Crews, P.; Holman, T.R. Exploring Sponge-Derived Terpenoids for Their Potency and Selectivity against 12-Human, 15-Human, and 15-Soybean Lipoxygenases. J. Nat. Prod. 2003, 66, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.E.; González-Iriarte, M.; Barrero, A.F.; Salvador-Tormo, N.; Muñoz-Chápuli, R.; Medina, M.A.; Quesada, A.R. Study of Puupehenone and Related Compounds as Inhibitors of Angiogenesis. Int. J. Cancer 2004, 110, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Douat-Casassus, C.; Marchand-Geneste, N.; Diez, E.; Aznar, C.; Picard, P.; Geoffre, S.; Huet, A.; Bourguet-Kondracki, M.-L.; Gervois, N.; Jotereau, F.; et al. Covalent Modification of a Melanoma-Derived Antigenic Peptide with a Natural Quinone Methide. Preliminary Chemical, Molecular Modelling and Immunological Evaluation Studies. Mol. BioSyst. 2006, 2, 240–249. [Google Scholar] [CrossRef]
- Mohammed, K.A.; Hossain, C.F.; Zhang, L.; Bruick, R.K.; Zhou, Y.D.; Nagle, D.G. Laurenditerpenol, a New Diterpene from the Tropical Marine Alga Laurencia intricata That Potently Inhibits HIF-1 Mediated Hypoxic Signaling in Breast Tumor Cells. J. Nat. Prod. 2004, 67, 2002–2007. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Hulot, G.; Tursch, B.; Declercq, J.P.; Germain, G.; van Meerssche, M. Chemical Studies of Marine Invertebrates. XXXVII(1). Three Novel Meroditerpenoids from the Sponge Strongylophora Durissima(2). Bull. Sociétés Chim. Belg. 1978, 87, 917–926. [Google Scholar] [CrossRef]
- McHardy, L.M.; Warabi, K.; Andersen, R.J.; Roskelley, C.D.; Roberge, M. Strongylophorine-26, a Rho-Dependent Inhibitor of Tumor Cell Invasion That Reduces Actin Stress Fibers and Induces Nonpolarized Lamellipodial Extensions. Mol. Cancer Ther. 2005, 4, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Noda, A.; Sakai, E.; Kato, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Yokosawa, H.; Tsukamoto, S. Strongylophorines, Meroditerpenoids from the Marine Sponge Petrosia corticata, Function as Proteasome Inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 2650–2653. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, K.A.; Jadulco, R.C.; Bugni, T.S.; Harper, M.K.; Sturdy, M.; Ireland, C.M. Strongylophorines: Natural Product Inhibitors of Hypoxia-Inducible Factor-1 Transcriptional Pathway. J. Med. Chem. 2008, 51, 1402–1405. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liu, Y.; Zhou, Y.-D.; Nagle, D.G. Hypoxia-Selective Antitumor Agents: Norsesterterpene Peroxides from the Marine Sponge Diacarnus levii Preferentially Suppress the Growth of Tumor Cells under Hypoxic Conditions. J. Nat. Prod. 2007, 70, 130–133. [Google Scholar] [CrossRef]
- Erdogan-Orhan, I.; Sener, B.; de Rosa, S.; Perez-Baz, J.; Lozach, O.; Leost, M.; Rakhilin, S.; Meijer, L. Polyprenyl-Hydroquinones and -Furans from Three Marine Sponges Inhibit the Cell Cycle Regulating Phosphatase CDC25A. Nat. Prod. Res. 2004, 18, 1–9. [Google Scholar] [CrossRef]
- Tasdemir, D.; Bugni, T.S.; Mangalindan, G.C.; Concepción, G.P.; Harper, M.K.; Ireland, C.M. Cytotoxic Bromoindole Derivatives and Terpenes from the Philippine Marine Sponge Smenospongia Sp. Z. Naturforsch. C 2002, 57, 914–922. [Google Scholar] [CrossRef]
- Cimino, G.; De Stefano, S.; Minale, L. Polyprenyl Derivatives from the Sponge Ircinia spinosula: 2-Polyprenylbenzoquinones, 2-Polyprenylbenzoquinols, Prenylated Furans and a C-31 Difuranoterpene. Tetrahedron 1972, 28, 1315–1324. [Google Scholar] [CrossRef]
- McPhail, K.; Davies-Coleman, M.T.; Coetzee, P. A New Furanosesterterpene from the South African Nudibranch Hypselodoris capensis and a Dictyoceratida Sponge. J. Nat. Prod. 1998, 61, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Erdoǧan, I.; Şener, B. Two Metabolites from Tbe Marine Sponge Spongia officinalis L. Acta Pharm. Turc. 2001, 43, 17–19. [Google Scholar]
- Prawat, H.; Mahidol, C.; Kaweetripob, W.; Wittayalai, S.; Ruchirawat, S. Iodo–Sesquiterpene Hydroquinone and Brominated Indole Alkaloids from the Thai Sponge Smenospongia Sp. Tetrahedron 2012, 68, 6881–6886. [Google Scholar] [CrossRef]
- Arai, M.; Kawachi, T.; Setiawan, A.; Kobayashi, M. Hypoxia-Selective Growth Inhibition of Cancer Cells by Furospinosulin-1, a Furanosesterterpene Isolated from an Indonesian Marine Sponge. ChemMedChem 2010, 5, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Kawachi, T.; Kotoku, N.; Nakata, C.; Kamada, H.; Tsunoda, S.; Tsutsumi, Y.; Endo, H.; Inoue, M.; Sato, H.; et al. Furospinosulin-1, Marine Spongean Furanosesterterpene, Suppresses the Growth of Hypoxia-Adapted Cancer Cells by Binding to Transcriptional Regulators P54(Nrb) and LEDGF/P75. ChemBioChem 2016, 17, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kashman, Y.; Zviely, M. Furospongolide, a New C21 Furanoterpene from a Marine Organism. Experientia 1980, 36, 1279. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, R.; Mao, S.-C.; Morgan, J.B.; Jekabsons, M.B.; Zhou, Y.-D.; Nagle, D.G. Molecular-Targeted Antitumor Agents. 19. Furospongolide from a Marine Lendenfeldia Sp. Sponge Inhibits Hypoxia-Inducible Factor-1 Activation in Breast Tumor Cells. J. Nat. Prod. 2008, 71, 1854–1860. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Villani, G.; Varcamonti, M.; Sayem, S.M.A.; van Soest, R.; Gavagnin, M. Bioactive Terpenes from Spongia Officinalis. J. Nat. Prod. 2011, 74, 1241–1247. [Google Scholar] [CrossRef]
- Li, J.; Du, L.; Kelly, M.; Zhou, Y.-D.; Nagle, D.G. Structures and Potential Antitumor Activity of Sesterterpenes from the Marine Sponge Hyrtios communis. J. Nat. Prod. 2013, 76, 1492–1497. [Google Scholar] [CrossRef]
- Albizati, K.F.; Holman, T.; Faulkner, D.J.; Glaser, K.B.; Jacobs, R.S. Luffariellolide, an Anti-Inflammatory Sesterterpene from the Marine Sponge Luffariella Sp. Experientia 1987, 43, 949–950. [Google Scholar] [CrossRef]
- Tasdemir, D.; Concepción, G.P.; Mangalindan, G.C.; Harper, M.K.; Hajdu, E.; Ireland, C.M. New Terpenoids from a Cacospongia Sp. from the Philippines. Tetrahedron 2000, 56, 9025–9030. [Google Scholar] [CrossRef]
- Elkhayat, E.; Edrada, R.; Ebel, R.; Wray, V.; van Soest, R.; Wiryowidagdo, S.; Mohamed, M.H.; Müller, W.E.G.; Proksch, P. New Luffariellolide Derivatives from the Indonesian Sponge Acanthodendrilla Sp. J. Nat. Prod. 2004, 67, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Foster, C.; Lazo, J.S.; Kingston, D.G.I. Sesterterpenoids and an Alkaloid from a Thorectandra Sp. as Inhibitors of the Phosphatase Cdc25B. Bioorg. Med. Chem. 2005, 13, 5094–5098. [Google Scholar] [CrossRef]
- Blanchard, J.L.; Epstein, D.M.; Boisclair, M.D.; Rudolph, J.; Pal, K. Dysidiolide and Related γ-Hydroxy Butenolide Compounds as Inhibitors of the Protein Tyrosine Phosphatase, CDC25. Bioorg. Med. Chem. Lett. 1999, 9, 2537–2538. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z.; Lin, S.; Zheng, W.; Wang, R.; Jin, S.; Chen, J.; Jin, L.; Li, Y. Revealing a Natural Marine Product as a Novel Agonist for Retinoic Acid Receptors with a Unique Binding Mode and Inhibitory Effects on Cancer Cells. Biochem. J. 2012, 446, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Minamida, Y.; Matsuura, H.; Ishii, T.; Miyagi, M.; Shinjo, Y.; Sato, K.; Kamada, T.; Mihara, Y.; Togashi, I.; Sugimoto, K.; et al. New Acetogenin Katsuurallene from Laurencia Saitoi Collected from Katsuura, Japan. Nat. Prod. Bioprospect. 2022, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Díaz-Marrero, A.R.; Cen-Pacheco, F.; Sifaoui, I.; Reyes-Batlle, M.; Souto, M.L.; Daranas, A.H.; Piñero, J.E.; Fernández, J.J. Evaluation of Oxasqualenoids from the Red Alga Laurencia viridis against Acanthamoeba. Mar. Drugs 2019, 17, 420. [Google Scholar] [CrossRef]
- Koutsaviti, A.; Daskalaki, M.G.; Agusti, S.; Kampranis, S.C.; Tsatsanis, C.; Duarte, C.M.; Roussis, V.; Ioannou, E. Thuwalallenes A–E and Thuwalenynes A–C: New C15 Acetogenins with Anti-Inflammatory Activity from a Saudi Arabian Red Sea Laurencia Sp. Mar. Drugs 2019, 17, 644. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Miao, F.-P.; Li, K.; Ji, N.-Y. Sesquiterpenes and Acetogenins from the Marine Red Alga Laurencia okamurai. Fitoterapia 2012, 83, 518–522. [Google Scholar] [CrossRef]
- Ji, N.-Y.; Li, X.-M.; Xie, H.; Ding, J.; Li, K.; Ding, L.-P.; Wang, B.-G. Highly Oxygenated Triterpenoids from the Marine Red Alga Laurencia mariannensis (Rhodomelaceae). Helv. Chim. Acta 2008, 91, 1940–1946. [Google Scholar] [CrossRef]
- Blunt, J.W.; Hartshorn, M.P.; McLennan, T.J.; Munro, M.H.G.; Robinson, W.T.; Yorke, S.C. Thyrsiferol: A Squalene-Derived Metabolite of Laurencia Thyrsifera. Tetrahedron Lett. 1978, 19, 69–72. [Google Scholar] [CrossRef]
- Mahdi, F.; Falkenberg, M.; Ioannou, E.; Roussis, V.; Zhou, Y.D.; Nagle, D.G. Thyrsiferol Inhibits Mitochondrial Respiration and HIF-1 Activation. Phytochem. Lett. 2011, 4, 75–78. [Google Scholar] [CrossRef]
- Rudi, A.; Goldberg, I.; Stein, Z.; Benayahu, Y.; Schleyer, M.; Kashman, Y. Sodwanones A-C, Three New Triterpenoids from a Marine Sponge. Tetrahedron Lett. 1993, 34, 3943–3944. [Google Scholar] [CrossRef]
- Rudi, A.; Kashman, Y.; Benayahu, Y.; Schleyer, M. Sodwanones A-F, New Triterpenoids from the Marine Sponge Axinella weltneri. J. Nat. Prod. 1994, 57, 1416–1423. [Google Scholar] [CrossRef]
- Rudi, A.; Goldberg, I.; Stein, Z.; Kashman, Y.; Benayahu, Y.; Schleyer, M.; Garcia Gravalos, M.D. Sodwanones G, H, and I, New Cytotoxic Triterpenes from a Marine Sponge. J. Nat. Prod. 1995, 58, 1702–1712. [Google Scholar] [CrossRef]
- Rudi, A.; Aknin, M.; Gaydou, E.M.; Kashman, Y. Sodwanones K, L, and M; New Triterpenes from the Marine Sponge Axinella weltneri. J. Nat. Prod. 1997, 60, 700–703. [Google Scholar] [CrossRef]
- Rudi, A.; Yosief, T.; Schleyer, M.; Kashman, Y. Several New Isoprenoids from Two Marine Sponges of the Family Axinellidae. Tetrahedron 1999, 55, 5555–5566. [Google Scholar] [CrossRef]
- Carletti, I.; Long, C.; Funel, C.; Amade, P. Yardenone A and B: New Cytotoxic Triterpenes from the Indian Ocean Sponge Axinella Cf. Bidderi. J. Nat. Prod. 2003, 66, 25–29. [Google Scholar] [CrossRef]
- Funel, C.; Berrué, F.; Roussakis, C.; Fernandez Rodriguez, R.; Amade, P. New Cytotoxic Steroids from the Indian Ocean Sponge Axinella Cf. Bidderi. J. Nat. Prod. 2004, 67, 491–494. [Google Scholar] [CrossRef]
- Dai, J.; Fishback, J.A.; Zhou, Y.-D.; Nagle, D.G. Sodwanone and Yardenone Triterpenes from a South African Species of the Marine Sponge Axinella Inhibit Hypoxia-Inducible Factor-1 (HIF-1) Activation in Both Breast and Prostate Tumor Cells. J. Nat. Prod. 2006, 69, 1715–1720. [Google Scholar] [CrossRef]
- Rudi, A.; Stein, Z.; Goldberg, I.; Yosief, T.; Kashman, Y.; Schleyer, M. Yardenone and Abudinol Two New Triterpenes from the Marine Sponge Ptilocaulis spiculifer. Tetrahedron Lett. 1998, 39, 1445–1448. [Google Scholar] [CrossRef]
- Tabudravu, J.N.; Jaspars, M. Stelliferin Riboside, a Triterpene Monosaccharide Isolated from the Fijian Sponge Geodia globostellifera. J. Nat. Prod. 2001, 64, 813–815. [Google Scholar] [CrossRef]
- Liu, W.K.; Ho, J.C.K.; Che, C.T. Apoptotic Activity of Isomalabaricane Triterpenes on Human Promyelocytic Leukemia HL60 Cells. Cancer Lett. 2005, 230, 102–110. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Q.; Peng, X.; Zhou, C.; Zhong, Y.; Chen, X.; Qiu, Y.; Jin, M.; Gong, M.; Kong, D. Stellettin B Induces G1 Arrest, Apoptosis and Autophagy in Human Non-Small Cell Lung Cancer A549 Cells via Blocking PI3K/Akt/MTOR Pathway. Sci. Rep. 2016, 6, 27071. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Q.; Zhang, L.; Zhong, Y.; Fan, G.; Zhang, Z.; Wang, R.; Jin, M.; Qiu, Y.; Kong, D. Stellettin B Induces Apoptosis in Human Chronic Myeloid Leukemia Cells via Targeting PI3K and Stat5. Oncotarget 2017, 8, 28906–28921. [Google Scholar] [CrossRef]
- Tasdemir, D.; Mangalindan, G.C.; Concepción, G.P.; Verbitski, S.M.; Rabindran, S.; Miranda, M.; Greenstein, M.; Hooper, J.N.A.; Harper, M.K.; Ireland, C.M. Bioactive Isomalabaricane Triterpenes from the Marine Sponge Rhabdastrella globostellata. J. Nat. Prod. 2002, 65, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lin, B.J.; Chen, C.H.; Nguyen, N.L.; Hsieh, T.H.; Su, J.H.; Chen, M.C. Stellettin B Induces Cell Death in Bladder Cancer via Activating the Autophagy/DAPK2/Apoptosis Signaling Cascade. Mar. Drugs 2023, 21, 73. [Google Scholar] [CrossRef]
- Tsai, T.C.; Wu, W.T.; Lin, J.J.; Su, J.H.; Wu, Y.J. Stellettin B Isolated from Stelletta Sp. Reduces Migration and Invasion of Hepatocellular Carcinoma Cells through Reducing Activation of the MAPKs and FAK/PI3K/AKT/MTOR Signaling Pathways. Int. J. Cell Biol. 2022, 2022, 4416611. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, H.; Tian, X.; Lin, H.; Wang, M.; Yao, M. Three New Cytotoxic Isomalabaricane Triterpenes from the Marine Sponge Stelletta tenuis. Fitoterapia 2015, 106, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-A.; Zhou, Q.; Guo, W.-Z.; Qiu, Y.; Wang, R.; Jin, M.; Zhang, W.; Li, K.; Yamori, T.; Dan, S.; et al. In Vitro Antitumor Activity of Stellettin B, a Triterpene from Marine Sponge Jaspis stellifera, on Human Glioblastoma Cancer SF295 Cells. Mar. Drugs 2014, 12, 4200–4213. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chen, N.F.; Lin, P.Y.; Su, J.H.; Chen, B.H.; Kuo, H.M.; Sung, C.S.; Sung, P.J.; Wen, Z.H.; Chen, W.F. Anti-Invasion and Antiangiogenic Effects of Stellettin B through Inhibition of the Akt/Girdin Signaling Pathway and VEGF in Glioblastoma Cells. Cancers 2019, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.J.; Jean, Y.H.; Shih, P.C.; Cheng, S.Y.; Kuo, H.M.; Lee, Y.T.; Lai, Y.C.; Tseng, C.C.; Chen, W.F.; Wen, Z.H. Stellettin B-Induced Oral Cancer Cell Death via Endoplasmic Reticulum Stress–Mitochondrial Apoptotic and Autophagic Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 8813. [Google Scholar] [CrossRef]
- Bukowski, R.; Vaughn, C.; Bottomley, R.; Chen, T. Phase II Study of Anguidine in Gastrointestinal Malignancies: A Southwest Oncology Group Study. Cancer Treat. Rep. 1982, 66, 381–383. [Google Scholar] [PubMed]
- Yap, H.Y.; Murphy, W.K.; DiStefano, A.; Blumenschein, G.R.; Bodey, G.P. Phase II Study of Anguidine in Advanced Breast Cancer. Cancer Treat. Rep. 1979, 63, 789–791. [Google Scholar] [PubMed]
- Dosik, G.M.; Barlogie, B.; Johnston, D.A.; Murphy, W.K.; Drewinko, B. Lethal and Cytokinetic Effects of Anguidine on a Human Colon Cancer Cell Line. Cancer Res. 1978, 38, 3304–3309. [Google Scholar] [PubMed]
- Chang, A.Y.; Kim, K.; Boucher, H.; Bonomi, P.; Stewart, J.A.; Karp, D.D.; Blum, R.H. A Randomized Phase II Trial of Echinomycin, Trimetrexate, and Cisplatin plus Etoposide in Patients with Metastatic Nonsmall Cell Lung Carcinoma: An Eastern Cooperative Oncology Group Study (E1587). Cancer 1998, 82, 292–300. [Google Scholar] [CrossRef]
- Schilsky, R.L.; Faraggi, D.; Korzun, A.; Vogelzang, N.; Ellerton, J.; Wood, W.; Henderson, I.C. Phase II Study of Echinomycin in Patients with Advanced Breast Cancer: A Report of Cancer and Leukemia Group B Protocol 8641. Investig. New Drugs 1991, 9, 269–272. [Google Scholar] [CrossRef]
- Marshall, M.E.; Wolf, M.K.; Crawford, E.D.; Taylor, S.; Blumenstein, B.; Flanigan, R.; Meyers, F.J.; Hynes, H.E.; Barlogie, B.; Eisenberger, M. Phase II Trial of Echinomycin for the Treatment of Advanced Renal Cell Carcinoma. A Southwest Oncology Group Study. Investig. New Drugs 1993, 11, 207–209. [Google Scholar] [CrossRef]
- Bailey, C.M.; Liu, Y.; Peng, G.; Zhang, H.; He, M.; Sun, D.; Zheng, P.; Liu, Y.; Wang, Y. Liposomal Formulation of HIF-1α Inhibitor Echinomycin Eliminates Established Metastases of Triple-Negative Breast Cancer. Nanomedicine 2020, 29, 102278. [Google Scholar] [CrossRef]
- Comas, L.; Polo, E.; Domingo, M.P.; Hernández, Y.; Arias, M.; Esteban, P.; Martínez-Lostao, L.; Pardo, J.; Martínez de la Fuente, J.; Gálvez, E.M. Intracellular Delivery of Biologically-Active Fungal Metabolite Gliotoxin Using Magnetic Nanoparticles. Materials 2019, 12, 1092. [Google Scholar] [CrossRef]
- Manh Hung, L.V.; Song, Y.W.; Cho, S.K. Effects of the Combination of Gliotoxin and Adriamycin on the Adriamycin-Resistant Non-Small-Cell Lung Cancer A549 Cell Line. Mar. Drugs 2018, 16, 105. [Google Scholar] [CrossRef]
- Turbyville, T.J.; Wijeratne, E.M.K.; Liu, M.X.; Burns, A.M.; Seliga, C.J.; Luevano, L.A.; David, C.L.; Faeth, S.H.; Whitesell, L.; Gunatilaka, A.A.L. Search for Hsp90 Inhibitors with Potential Anticancer Activity: Isolation and SAR Studies of Radicicol and Monocillin I from Two Plant-Associated Fungi of the Sonoran Desert. J. Nat. Prod. 2006, 69, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Dethe, D.H.; Sau, S.K. Total Synthesis of (+)-Strongylophorines 2 and 9. Org. Lett. 2019, 21, 3799–3803. [Google Scholar] [CrossRef]

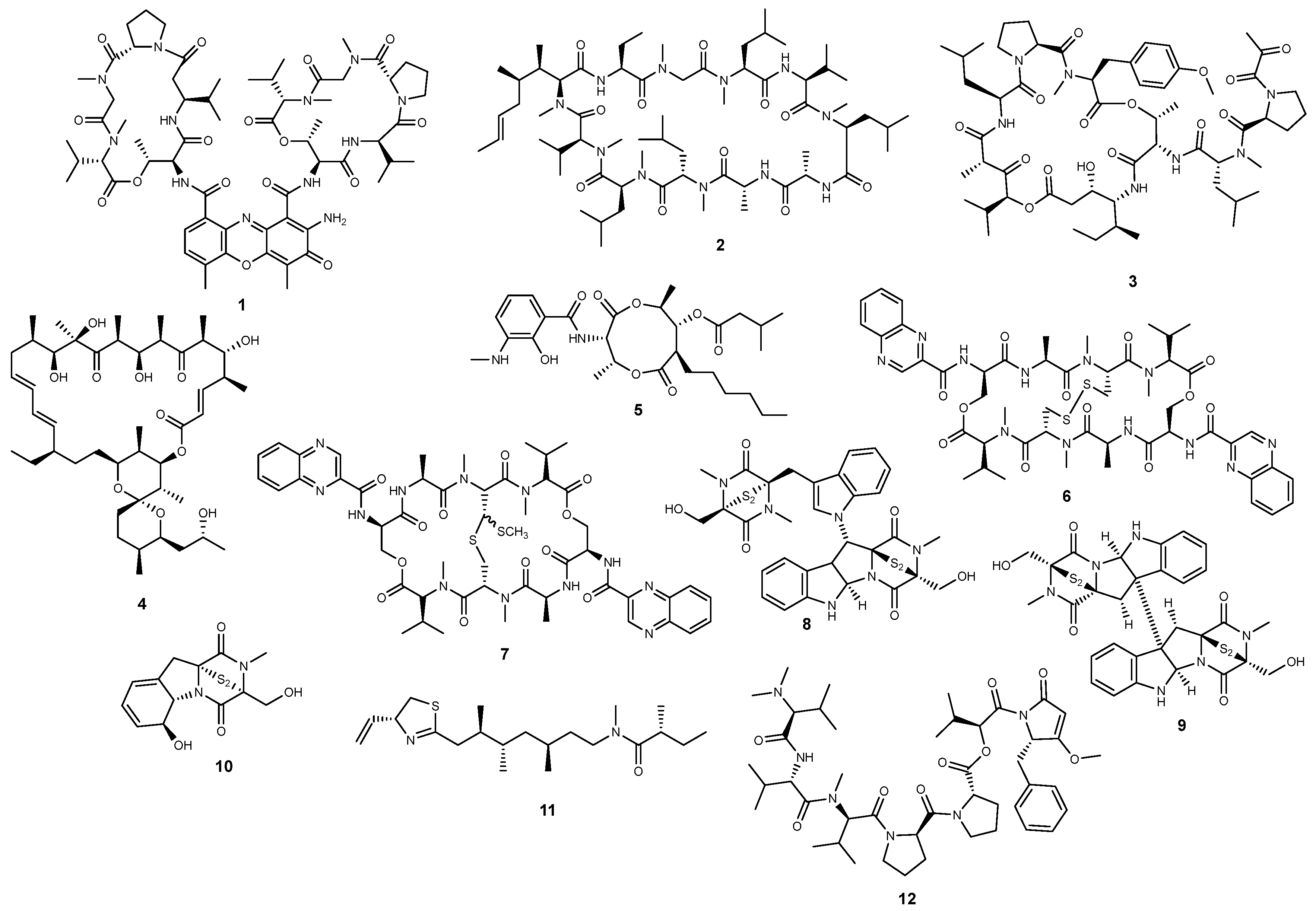
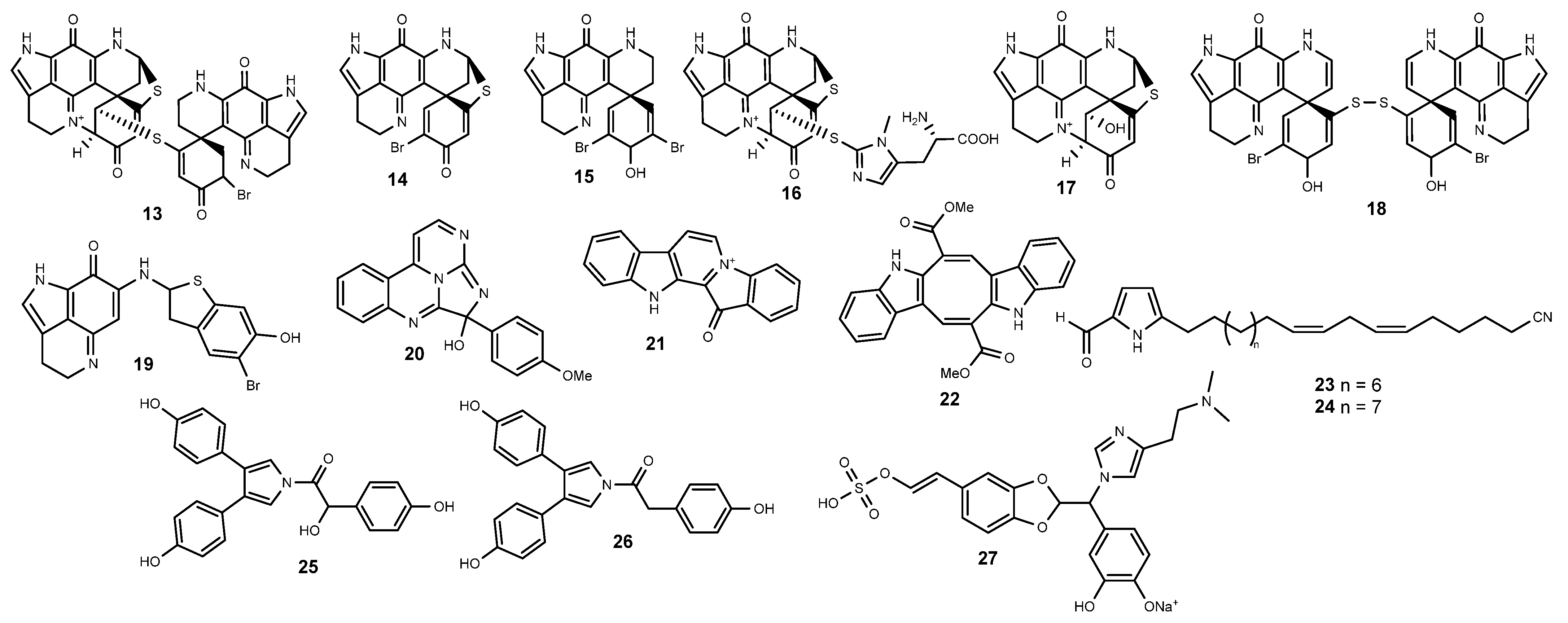
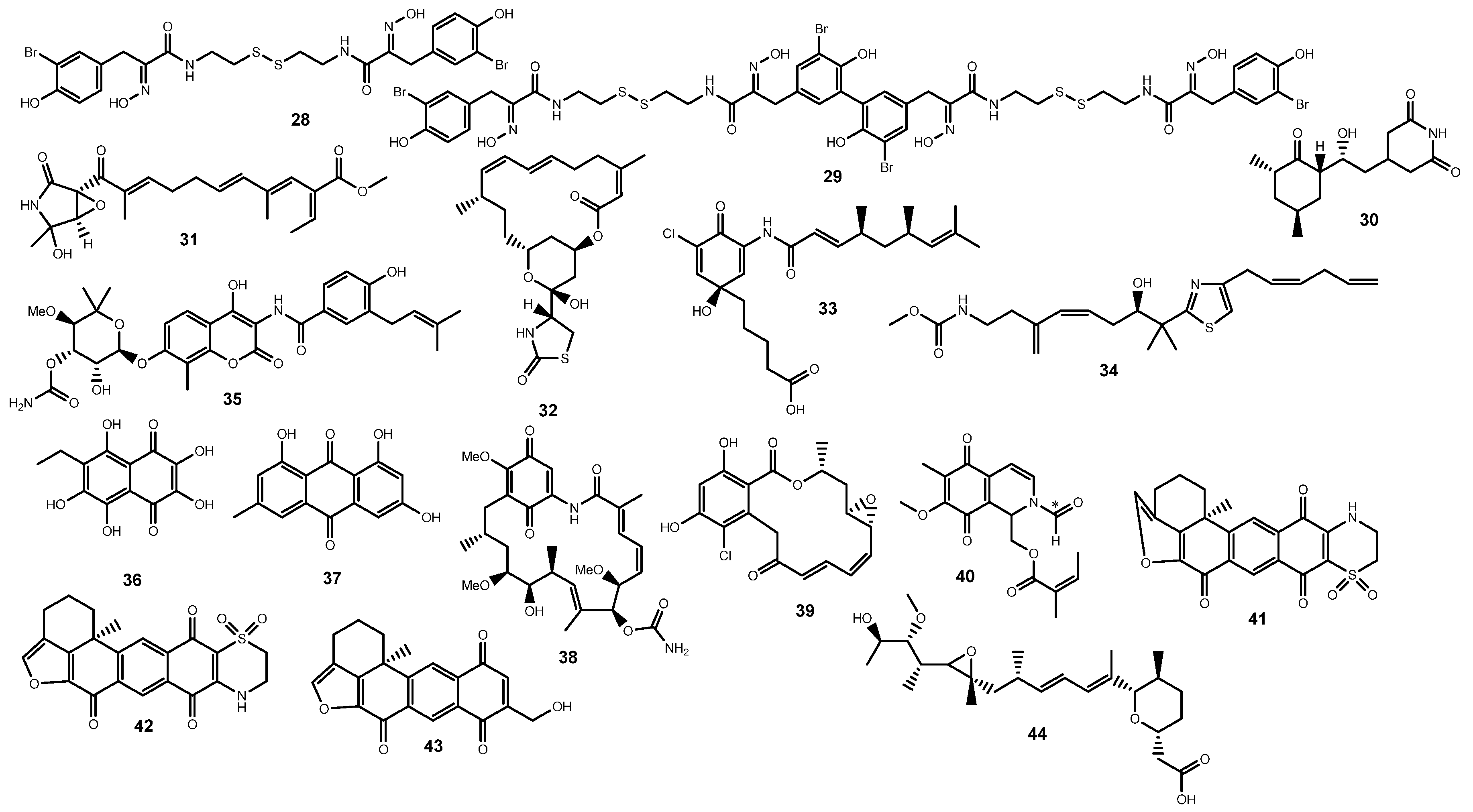

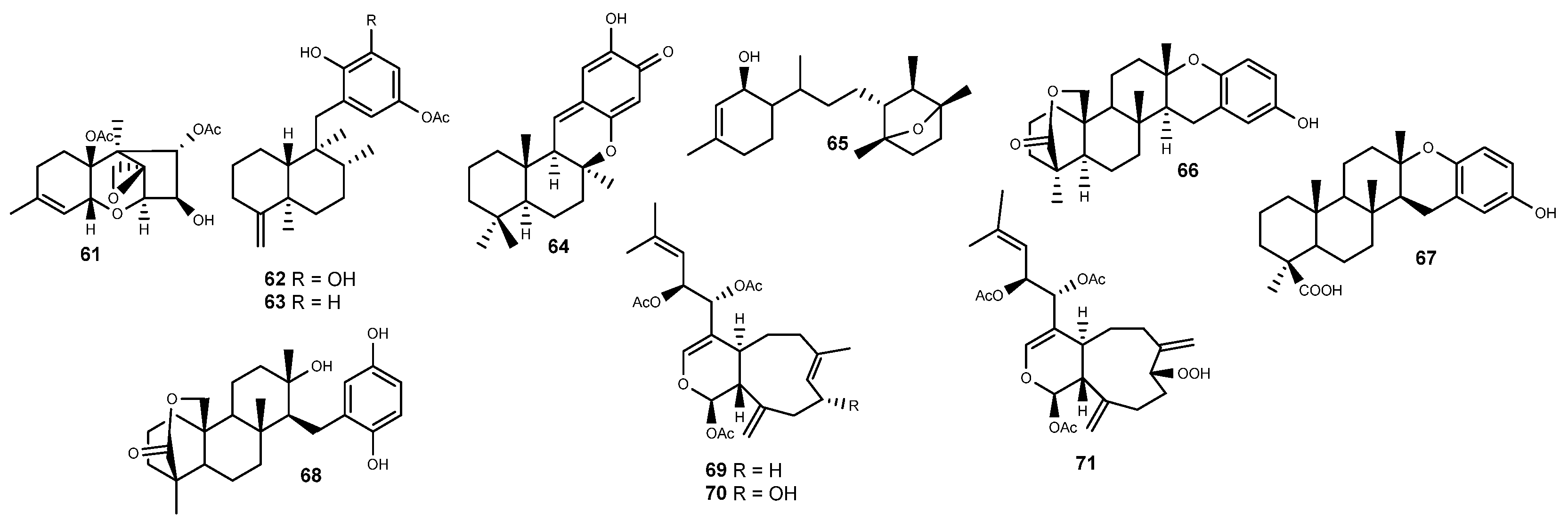
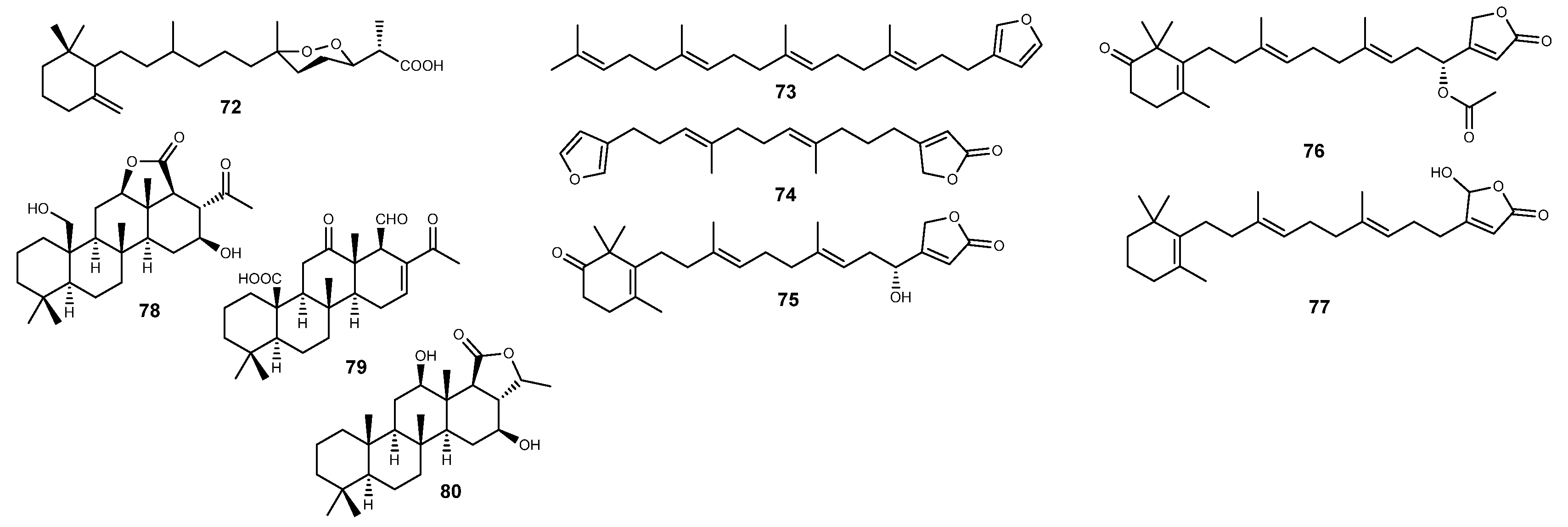
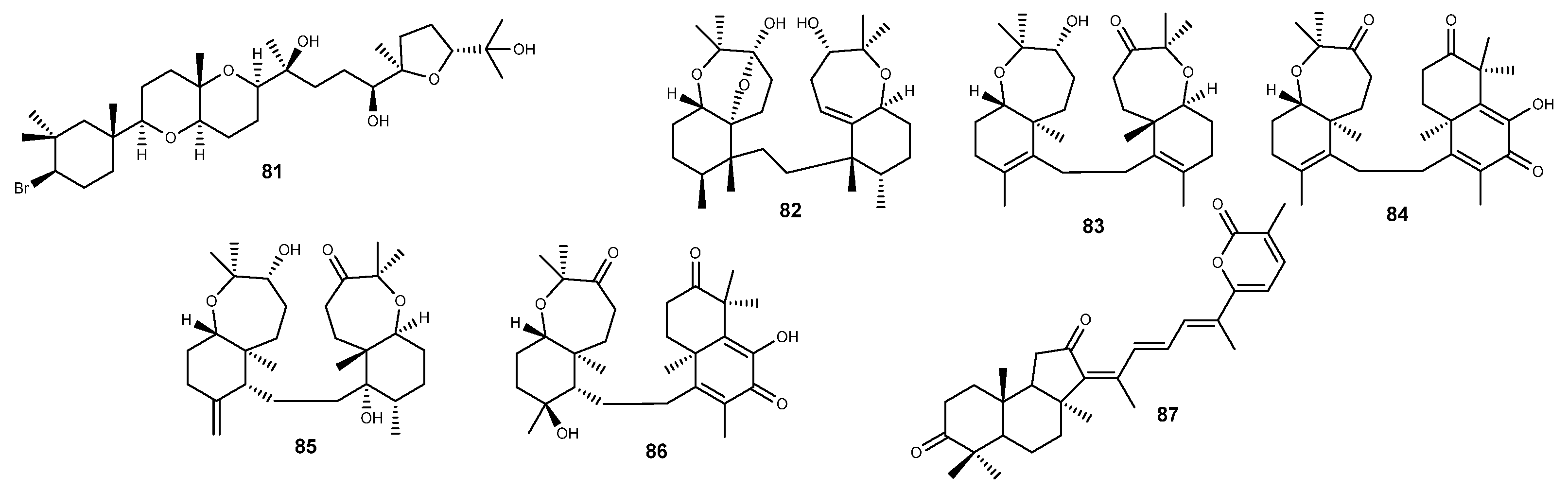
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, M.R.; Andrade, P.B.; Lefranc, F.; Gomes, N.G.M. Marine-Derived Leads as Anticancer Candidates by Disrupting Hypoxic Signaling through Hypoxia-Inducible Factors Inhibition. Mar. Drugs 2024, 22, 143. https://doi.org/10.3390/md22040143
Garcia MR, Andrade PB, Lefranc F, Gomes NGM. Marine-Derived Leads as Anticancer Candidates by Disrupting Hypoxic Signaling through Hypoxia-Inducible Factors Inhibition. Marine Drugs. 2024; 22(4):143. https://doi.org/10.3390/md22040143
Chicago/Turabian StyleGarcia, Maria Rita, Paula B. Andrade, Florence Lefranc, and Nelson G. M. Gomes. 2024. "Marine-Derived Leads as Anticancer Candidates by Disrupting Hypoxic Signaling through Hypoxia-Inducible Factors Inhibition" Marine Drugs 22, no. 4: 143. https://doi.org/10.3390/md22040143
APA StyleGarcia, M. R., Andrade, P. B., Lefranc, F., & Gomes, N. G. M. (2024). Marine-Derived Leads as Anticancer Candidates by Disrupting Hypoxic Signaling through Hypoxia-Inducible Factors Inhibition. Marine Drugs, 22(4), 143. https://doi.org/10.3390/md22040143







