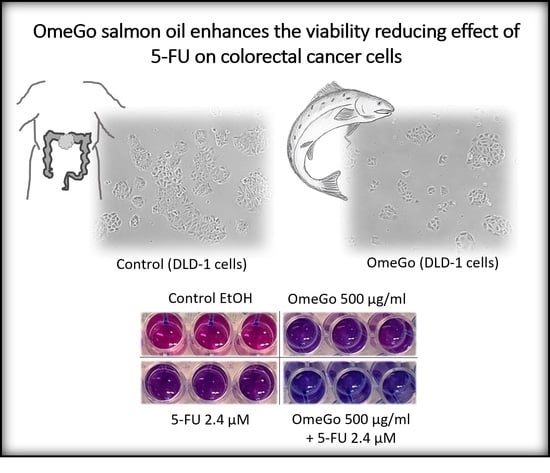
The Salmon Oil OmeGo Reduces Viability of Colorectal Cancer Cells and Potentiates the Anti-Cancer Effect of 5-FU
Abstract
:1. Introduction
2. Results
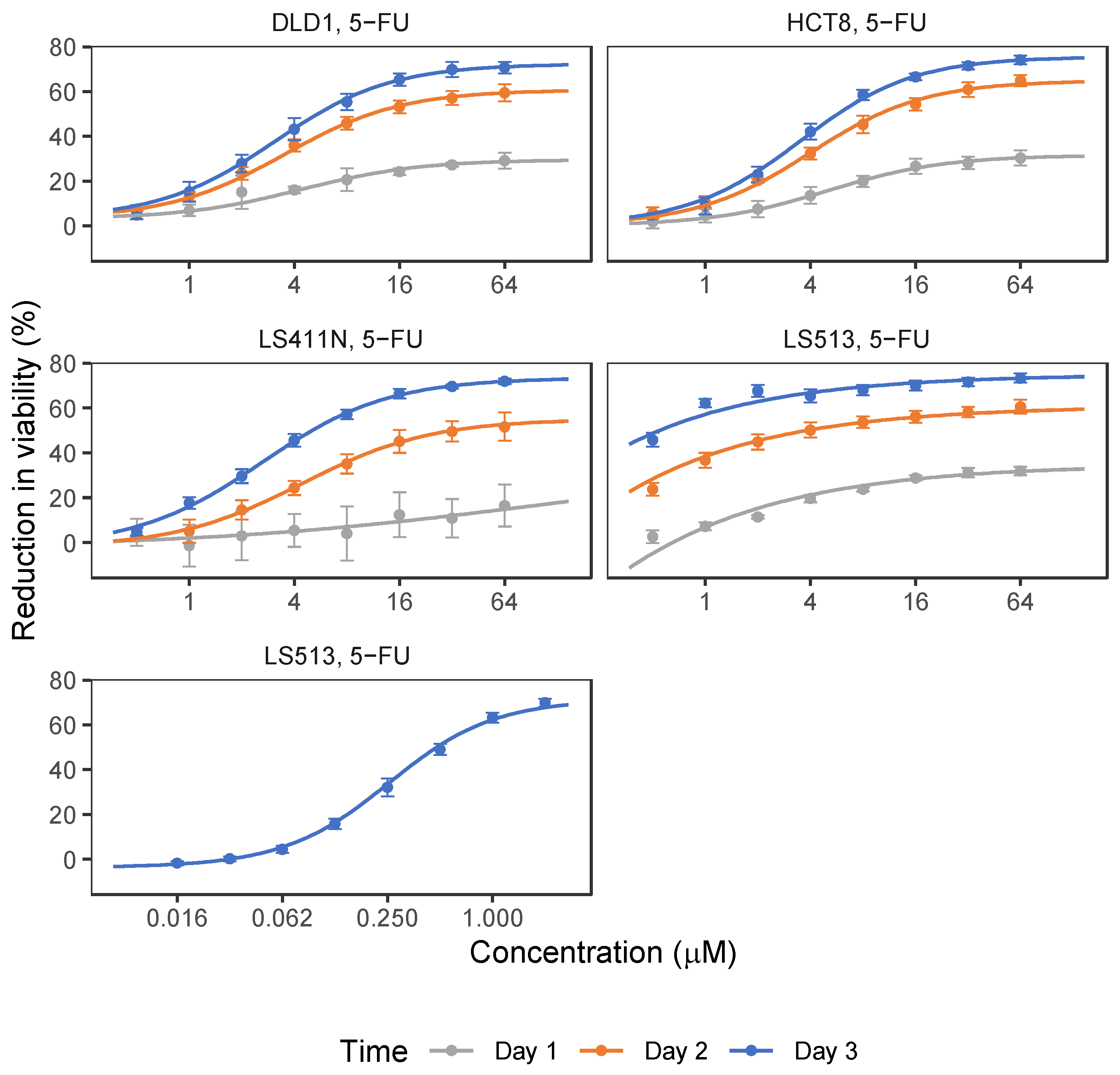
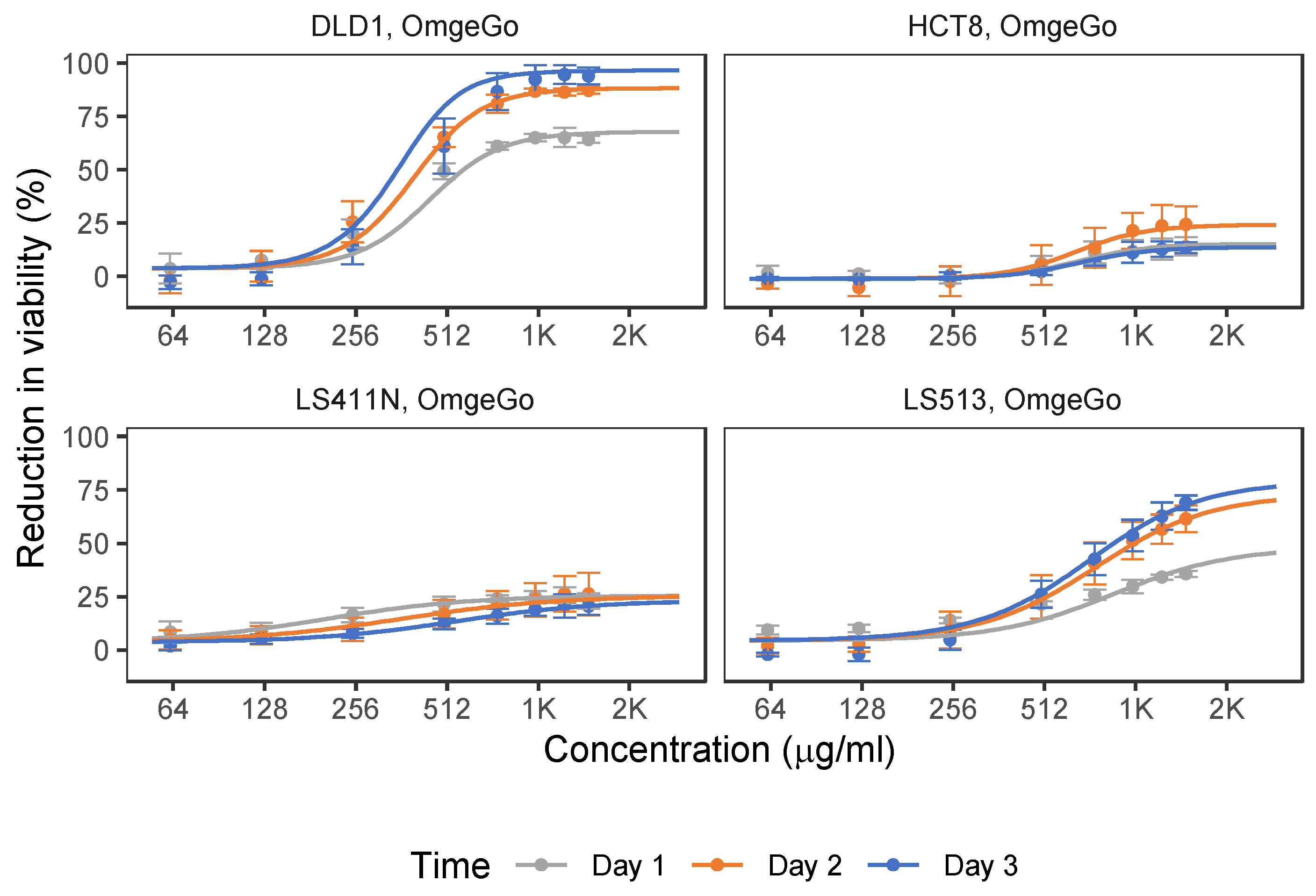
2.1. 5-FU and OmeGo Treatment Reduce Viability of CRC Cell Lines
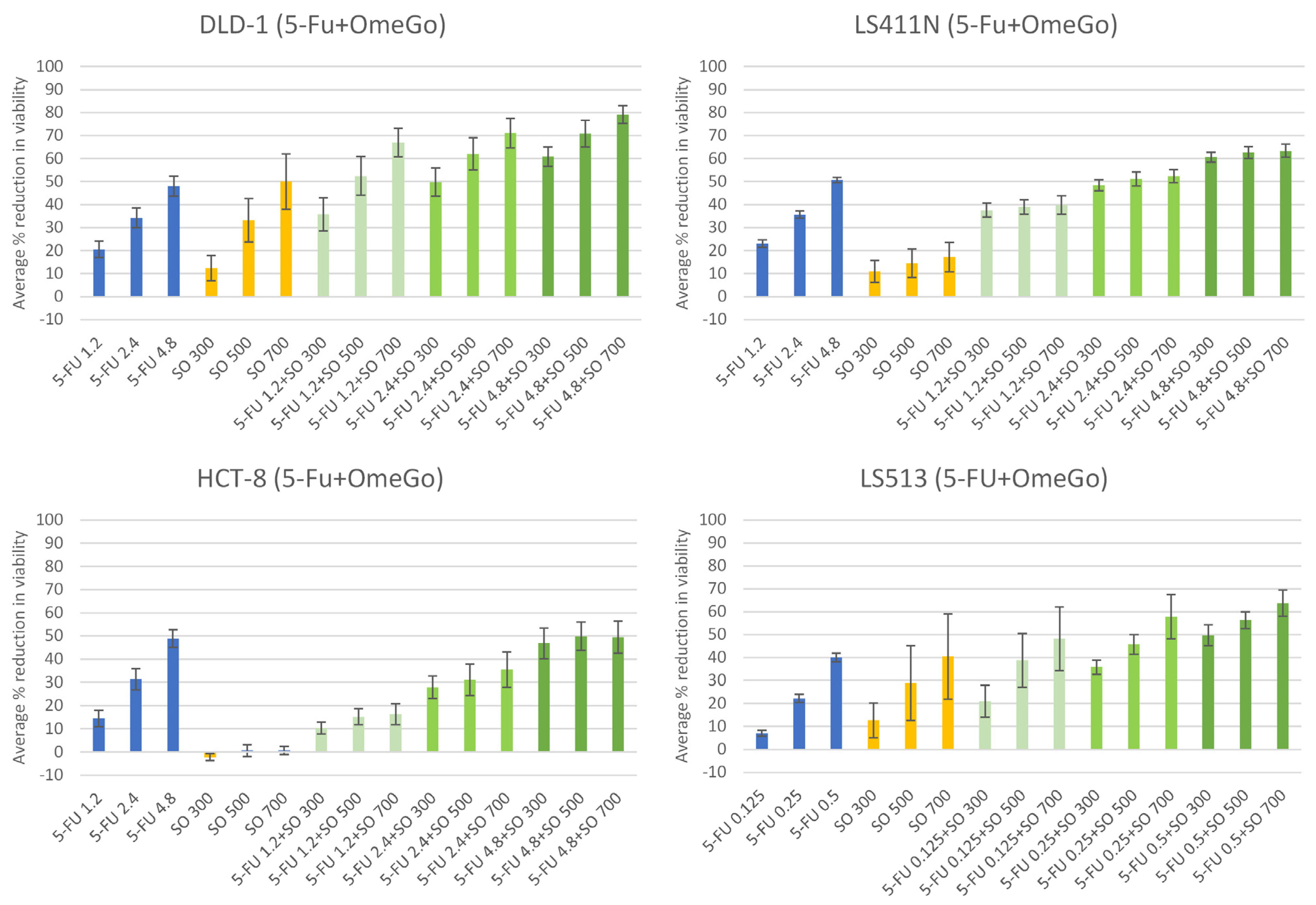
2.2. OmeGo Treatment Potentiates the Anti-Cancer Effect of 5-FU in CRC Cell Lines
2.3. The n-3 PUFAs DHA + EPA Potentiate the Anti-Cancer Effect of 5-FU in CRC Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Culture Conditions, and Chemicals
4.2. Cell Treatment and Resazurin Viability Assay
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larsen, I.K.; Møller, B.; Johannesen, T.B.; Robsahm, T.E.; Grimsrud, T.K.; Larønningen, S.; Seglem, A.H.; Gulbrandsen, J.; Jakobsen, E.; Ursin, G. Cancer in Norway 2019—Cancer Incidence, Mortality, Survival and Prevalence in Norway; Cancer Registry of Norway: Oslo, Norway, 2020. [Google Scholar]
- Danckert, B.; Ferlay, J.; Engholm, G.; Hansen, H.L.; Johannesen, T.B.; Khan, S.; Køtlum, J.E.; Olafsdottir, E.; Schmidt, L.K.H.; Virtanen, A.; et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 8.2. 2019. Available online: http://www.ancr.nu (accessed on 2 October 2023).
- Osloeconomics. Kreft i Norge—Kostnader for Pasientene, Helsetjenesten og Samfunnet; Oslo Economics. 2016. Available online: https://oslocancercluster.no/wp-content/uploads/2016/10/20161004-Kreftkostnader_i_Norge-WEB.pdf (accessed on 2 October 2023).
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- American Society of Clinical Oncology (ASCO). Colorectal Cancer: Types of Treatment. Available online: https://www.cancer.net/cancer-types/colorectal-cancer/types-treatment (accessed on 22 September 2021).
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Eltweri, A.M.; Thomas, A.L.; Metcalfe, M.; Calder, P.C.; Dennison, A.R.; Bowrey, D.J. Potential applications of fish oils rich in omega-3 polyunsaturated fatty acids in the management of gastrointestinal cancer. Clin. Nutr. 2017, 36, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Helsedirektoratet. Fisk Til Middag to Til Tre Ganger i Uken [Nettdokument]. Available online: https://www.helsedirektoratet.no/faglige-rad/kostradene-og-naeringsstoffer/kostrad-for-befolkningen/fisk-til-middag-to-til-tre-ganger-i-uken (accessed on 18 September 2021).
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012, 10, 2815. [Google Scholar] [CrossRef]
- Frøyland, L.; Bentsen, H.; Graff, I.E.; Myhrstad, M.; Paulsen, J.E.; Retterstøl, K.; Ulven, S.M. Evaluation of Negative and Positive Health Effects of n-3 Fatty Acids as Constituents of Food Supplements and Fortified Foods; Norwegian Scientific Committee for Food Safety: Oslo, Norway, 2011; 88p. [Google Scholar]
- Murphy, R.A.; Devarshi, P.P.; Ekimura, S.; Marshall, K.; Hazels Mitmesser, S. Long-chain omega-3 fatty acid serum concentrations across life stages in the USA: An analysis of NHANES 2011–2012. BMJ Open 2021, 11, e043301. [Google Scholar] [CrossRef] [PubMed]
- MacLean, C.H.; Newberry, S.J.; Mojica, W.A.; Khanna, P.; Issa, A.M.; Suttorp, M.J.; Lim, Y.W.; Traina, S.B.; Hilton, L.; Garland, R.; et al. Effects of omega-3 fatty acids on cancer risk: A systematic review. JAMA 2006, 295, 403–415. [Google Scholar] [CrossRef]
- Volpato, M.; Hull, M.A. Omega-3 polyunsaturated fatty acids as adjuvant therapy of colorectal cancer. Cancer Metastasis Rev. 2018, 37, 545–555. [Google Scholar] [CrossRef]
- Song, M.; Zhang, X.; Meyerhardt, J.A.; Giovannucci, E.L.; Ogino, S.; Fuchs, C.S.; Chan, A.T. Marine omega-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut 2017, 66, 1790–1796. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Ye, X.; Zhang, S.; Song, M.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; et al. Marine omega-3 Polyunsaturated Fatty Acid and Fish Intake after Colon Cancer Diagnosis and Survival: CALGB 89803 (Alliance). Cancer Epidemiol. Biomark. Prev. 2018, 27, 438–445. [Google Scholar] [CrossRef]
- Anti, M.; Armelao, F.; Marra, G.; Percesepe, A.; Bartoli, G.M.; Palozza, P.; Parrella, P.; Canetta, C.; Gentiloni, N.; De Vitis, I.; et al. Effects of different doses of fish oil on rectal cell proliferation in patients with sporadic colonic adenomas. Gastroenterology 1994, 107, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- West, N.J.; Clark, S.K.; Phillips, R.K.; Hutchinson, J.M.; Leicester, R.J.; Belluzzi, A.; Hull, M.A. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut 2010, 59, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Courtney, E.D.; Matthews, S.; Finlayson, C.; Di Pierro, D.; Belluzzi, A.; Roda, E.; Kang, J.Y.; Leicester, R.J. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int. J. Color. Dis. 2007, 22, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Cockbain, A.J.; Volpato, M.; Race, A.D.; Munarini, A.; Fazio, C.; Belluzzi, A.; Loadman, P.M.; Toogood, G.J.; Hull, M.A. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut 2014, 63, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chang, Y.N. Omega-3 polyunsaturated fatty acids in the prevention of postoperative complications in colorectal cancer: A meta-analysis. Onco Targets Ther. 2016, 9, 7435–7443. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Bilotto, S.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; Nabavi, S.M. Omega-3 polyunsaturated fatty acids and cancer: Lessons learned from clinical trials. Cancer Metastasis Rev. 2015, 34, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Wang, W.; Zhang, G.; Hammock, B.D. Omega-3 Polyunsaturated Fatty Acids on Colonic Inflammation and Colon Cancer: Roles of Lipid-Metabolizing Enzymes Involved. Nutrients 2020, 12, 3301. [Google Scholar] [CrossRef]
- Rani, I.; Kumar, S.; Sharma, B.; Prasad, R.; Kaur, S.; Sharma, P.; Agnihotri, N. Elucidation of underlying molecular mechanism of 5-Fluorouracil chemoresistance and its restoration using fish oil in experimental colon carcinoma. Mol. Cell Biochem. 2021, 476, 1517–1527. [Google Scholar] [CrossRef]
- Calviello, G.; Di Nicuolo, F.; Serini, S.; Piccioni, E.; Boninsegna, A.; Maggiano, N.; Ranelletti, F.O.; Palozza, P. Docosahexaenoic acid enhances the susceptibility of human colorectal cancer cells to 5-fluorouracil. Cancer Chemother. Pharmacol. 2005, 55, 12–20. [Google Scholar] [CrossRef]
- Yang, T.; Fang, S.; Zhang, H.X.; Xu, L.X.; Zhang, Z.Q.; Yuan, K.T.; Xue, C.L.; Yu, H.L.; Zhang, S.; Li, Y.F.; et al. N-3 PUFAs have antiproliferative and apoptotic effects on human colorectal cancer stem-like cells. J. Nutr. Biochem. 2013, 24, 744–753. [Google Scholar] [CrossRef]
- Vasudevan, A.; Yu, Y.; Banerjee, S.; Woods, J.; Farhana, L.; Rajendra, S.G.; Patel, A.; Dyson, G.; Levi, E.; Maddipati, K.R.; et al. Omega-3 fatty acid is a potential preventive agent for recurrent colon cancer. Cancer Prev. Res. 2014, 7, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.H.; Zhang, L.M.; Mu, Q.T.; Lou, Y.R.; Gong, Z.H.; Shi, Y.J.; Ouyang, G.F.; Zhang, Y. The effect of combination treatment with docosahexaenoic acid and 5-fluorouracil on the mRNA expression of apoptosis-related genes, including the novel gene, in gastric cancer cells. In Vitro Cell. Dev. Biol. Anim. 2009, 45, 69–74. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, F.; Witte, T.R.; Hardman, W.E.; Claudio, P.P. Omega-3 Eicosapentaenoic Acid Decreases CD133 Colon Cancer Stem-Like Cell Marker Expression While Increasing Sensitivity to Chemotherapy. PLoS ONE 2013, 8, e69760. [Google Scholar] [CrossRef] [PubMed]
- Ocal, O.; Naziroglu, M. Eicosapentaenoic acid enhanced apoptotic and oxidant effects of cisplatin via activation of TRPM2 channel in brain tumor cells. Chem. Biol. Interact. 2022, 359, 109914. [Google Scholar] [CrossRef] [PubMed]
- Dupertuis, Y.M.; Boulens, N.; Angibaud, E.; Briod, A.S.; Viglione, A.; Allémann, E.; Delie, F.; Pichard, C. Antitumor Effect of 5-Fluorouracil-Loaded Liposomes Containing n-3 Polyunsaturated Fatty Acids in Two Different Colorectal Cancer Cell Lines. AAPS PharmSciTech 2021, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E.; Moyer, M.P.; Cameron, I.L. Dietary fish oil sensitizes A549 lung xenografts to doxorubicin chemotherapy. Cancer Lett. 2000, 151, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Granci, V.; Cai, F.; Lecumberri, E.; Clerc, A.; Dupertuis, Y.M.; Pichard, C. Colon cancer cell chemosensitisation by fish oil emulsion involves apoptotic mitochondria pathway. Br. J. Nutr. 2013, 109, 1188–1195. [Google Scholar] [CrossRef]
- Jordan, A.; Stein, J. Effect of an omega-3 fatty acid containing lipid emulsion alone and in combination with 5-fluorouracil (5-FU) on growth of the colon cancer cell line Caco-2. Eur. J. Nutr. 2003, 42, 324–331. [Google Scholar] [CrossRef]
- Rani, I.; Sharma, B.; Kumar, S.; Kaur, S.; Agnihotri, N. Apoptosis mediated chemosensitization of tumor cells to 5-fluorouracil on supplementation of fish oil in experimental colon carcinoma. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef]
- Samdal, H.; Sandmoe, M.A.; Olsen, L.C.; Jarallah, E.A.H.; Hoiem, T.S.; Schonberg, S.A.; Pettersen, C.H.H. Basal level of autophagy and MAP1LC3B-II as potential biomarkers for DHA-induced cytotoxicity in colorectal cancer cells. FEBS J. 2018, 285, 2446–2467. [Google Scholar] [CrossRef]
- Hofseth BioCare. OmeGo® Delivers All the Nutritional Benefits of Consuming Fish. Available online: https://hofsethbiocare.com/products/salmon-oil (accessed on 19 August 2022).
- Hofseth BioCare. Cardio Salmon Oil—Nutritional Content. Available online: https://www.amazon.com/Hofseth-BioCare-Salmon-Capsules-Vitamin/dp/B0BQRVD4G8#immersive-view_1702498410332 (accessed on 3 February 2023).
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Framroze, B.; Heggdal, H. An in vitro study to explore the modulation of eosinophil effector function in human allergic peripheral blood eosinophils using enzymatically extracted salmonid oil. Funct. Foods Health Dis. 2020, 10, 357–367. [Google Scholar] [CrossRef]
- Bargut, T. Fish oil has beneficial effects on allergen-induced airway inflammation and hyperreactivity in mice. PLoS ONE 2013, 8, e75059. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Framroze, B. The Effect of Dietary Oil Capsules on Reducing Serum Concentrations of Oxidized Low Density Lipoprotein- β2-Glycoprotein-I Complex. J. Nutr. Food Sci. 2013, 3, 225. [Google Scholar] [CrossRef]
- Abe, Y.; Sakuyama, N.; Sato, T.; Kishine, K.; Nagayasu, K.; Nakatani, A.; Kitajima, M.; Watanabe, T.; Nishimura, K.; Ochiai, T.; et al. Evaluation of the 5-fluorouracil plasma level in patients with colorectal cancer undergoing continuous infusion chemotherapy. Mol. Clin. Oncol. 2019, 11, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Jiang, Y.; Yang, Y.; Zheng, Y.; Zheng, J.; Jiang, H.; Zhang, S.; Lin, L.; Zheng, J.; Zhang, S.; et al. Determining the optimal 5-FU therapeutic dosage in the treatment of colorectal cancer patients. Oncotarget 2016, 7, 81880–81887. [Google Scholar] [CrossRef] [PubMed]
- Violette, S.; Poulain, L.; Dussaulx, E.; Pepin, D.; Faussat, A.M.; Chambaz, J.; Lacorte, J.M.; Staedel, C.; Lesuffleur, T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int. J. Cancer 2002, 98, 498–504. [Google Scholar] [CrossRef] [PubMed]
- de la Cueva, A.; Ramirez de Molina, A.; Alvarez-Ayerza, N.; Ramos, M.A.; Cebrian, A.; Del Pulgar, T.G.; Lacal, J.C. Combined 5-FU and ChoKalpha inhibitors as a new alternative therapy of colorectal cancer: Evidence in human tumor-derived cell lines and mouse xenografts. PLoS ONE 2013, 8, e64961. [Google Scholar] [CrossRef]
- Sam, M.R.; Esmaeillou, M.; Shokrgozar, M.A. Fish-Oil-Derived DHA-mediated Enhancement of Apoptosis in Acute Lymphoblastic Leukemia Cells is Associated with Accumulation of p53, Downregulation of Survivin, and Caspase-3 Activation. Nutr. Cancer 2017, 69, 64–73. [Google Scholar] [CrossRef]
- Xue, M.; Wang, Q.; Zhao, J.; Dong, L.; Ge, Y.; Hou, L.; Liu, Y.; Zheng, Z. Docosahexaenoic acid inhibited the Wnt/beta-catenin pathway and suppressed breast cancer cells in vitro and in vivo. J. Nutr. Biochem. 2014, 25, 104–110. [Google Scholar] [CrossRef]
- Jing, K.; Song, K.S.; Shin, S.; Kim, N.; Jeong, S.; Oh, H.R.; Park, J.H.; Seo, K.S.; Heo, J.Y.; Han, J.; et al. Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild-type p53. Autophagy 2011, 7, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Long, Y.; Cheng, W.; Huang, Y.; Li, J.; Li, Y.; Li, X.; Guo, X.; Li, Y.; Li, G.; et al. Visfatin inhibits colon cancer cell apoptosis and decreases chemosensitivity to 5-FU by promoting the SDF-1/CXCR4/Akt axis. Int. J. Oncol. 2022, 60, 75. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhu, H.; Zhou, S.; Wu, T.; Wu, H.; Yang, H.; Mao, H.; SekharKathera, C.; Janardhan, A.; Edick, A.M.; et al. Wnt pathway is involved in 5-FU drug resistance of colorectal cancer cells. Exp. Mol. Med. 2018, 50, 101. [Google Scholar] [CrossRef] [PubMed]
- Bracht, K.; Nicholls, A.M.; Liu, Y.; Bodmer, W.F. 5-Fluorouracil response in a large panel of colorectal cancer cell lines is associated with mismatch repair deficiency. Br. J. Cancer 2010, 103, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Rani, I.; Vaiphei, K.; Agnihotri, N. Supplementation of fish oil augments efficacy and attenuates toxicity of 5-fluorouracil in 1,2-dimethylhydrazine dihydrochloride/dextran sulfate sodium-induced colon carcinogenesis. Cancer Chemother. Pharmacol. 2014, 74, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Piccioni, E.; Merendino, N.; Calviello, G. Dietary polyunsaturated fatty acids as inducers of apoptosis: Implications for cancer. Apoptosis 2009, 14, 135–152. [Google Scholar] [CrossRef]
- Serini, S.; Fasano, E.; Piccioni, E.; Cittadini, A.R.; Calviello, G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem. Res. Toxicol. 2011, 24, 2093–2105. [Google Scholar] [CrossRef]
- Schonberg, S.A.; Lundemo, A.G.; Fladvad, T.; Holmgren, K.; Bremseth, H.; Nilsen, A.; Gederaas, O.; Tvedt, K.E.; Egeberg, K.W.; Krokan, H.E. Closely related colon cancer cell lines display different sensitivity to polyunsaturated fatty acids, accumulate different lipid classes and downregulate sterol regulatory element-binding protein 1. FEBS J. 2006, 273, 2749–2765. [Google Scholar] [CrossRef]
- Karlenius, T.C.; Shah, F.; Yu, W.C.; Hawkes, H.J.; Tinggi, U.; Clarke, F.M.; Tonissen, K.F. The selenium content of cell culture serum influences redox-regulated gene expression. Biotechniques 2011, 50, 295–301. [Google Scholar] [CrossRef]
- Vega-Avila, E.; Pugsley, M.K. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc. West. Pharmacol. Soc. 2011, 54, 10–14. [Google Scholar]
- Fu, Y.; Yang, G.; Zhu, F.; Peng, C.; Li, W.; Li, H.; Kim, H.G.; Bode, A.M.; Dong, Z.; Dong, Z. Antioxidants decrease the apoptotic effect of 5-Fu in colon cancer by regulating Src-dependent caspase-7 phosphorylation. Cell Death Dis. 2014, 5, e983. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, K.; Monsen, V.T.; Hakvag Pettersen, C.H.; Overland, H.B.; Pettersen, G.; Samdal, H.; Tesfahun, A.N.; Lundemo, A.G.; Bjorkoy, G.; Schonberg, S.A. DHA-induced stress response in human colon cancer cells—Focus on oxidative stress and autophagy. Free Radic. Biol. Med. 2016, 90, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Gasmi, A.; Lenchyk, L.; Shanaida, M.; Zafar, S.; Mujawdiya, P.K.; Lysiuk, R.; Antonyak, H.; Noor, S.; Akram, M.; et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules 2022, 27, 7167. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, Y.M.; Hong, S. Astaxanthin suppresses the metastasis of colon cancer by inhibiting the MYC-mediated downregulation of microRNA-29a-3p and microRNA-200a. Sci. Rep. 2019, 9, 9457. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur. J. Biochem. 1981, 115, 207–216. [Google Scholar] [CrossRef]

| Cell Line | Days | 5-FU (µM) | OmeGo (µg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC20 | ±SE | IC30 | ±SE | IC50 | ±SE | IC20 | ±SE | IC30 | ±SE | IC50 | ±SE | ||
| DLD-1 | 3 | 1.68 | 0.01 | 2.56 | 0.01 | 4.99 | 0.01 | 353.10 | 1.40 | 410.22 | 1.34 | 519.28 | 1.03 |
| HCT-8 | 3 | 2.02 | 0.001 | 3.06 | 0.01 | 5.90 | 0.01 | 464.66 | 1.66 | 537.80 | 1.59 | 676.73 | 3.11 |
| LS411N * | 3 | 1.38 | 0.01 | 2.20 | 0.01 | 4.56 | 0.02 | 278.06 | 2.39 | 370.96 | 2.76 | 583.60 | 5.19 |
| LS513 | 3 | 0.15 | 0.00 | 0.216 | 0.00 | 0.40 | 0.00 | 534.85 | 3.75 | 680.84 | 5.97 | 994.97 | 12.34 |
| Cell Line | Days | ε Intercept | 5-FU | OmeGo | 5-FU × OmeGo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect % | ±SD | p-Value | Effect % | ±SD | p-Value | Effect % | ±SD | p-Value | |||
| DLD-1 | 3 | 8.55 | 8.94 | 0.77 | 1.17 × 10−26 | 6.17 | 0.52 | 9.94 × 10−28 | −0.36 | 0.17 | 3.85 × 10−2 |
| HCT-8 | 3 | 2.82 | 9.82 | 0.79 | 3.80 × 10−29 | −0.02 | 0.53 | 9.67 × 10−1 | 0.07 | 0.18 | 6.88 × 10−1 |
| LS411N | 3 | 15.30 | 7.70 | 0.77 | 6.14 × 10−21 | 1.23 | 0.52 | 1.90 × 10−2 | 0.30 | 0.17 | 8.09 × 10−2 |
| LS513 | 3 | −2.17 | 88.46 | 8.14 | 9.55 × 10−24 | 6.29 | 0.57 | 3.64 × 10−24 | −6.10 | 1.84 | 1.00 × 10−3 |
| Cell Line | Days | ε Intercept | 5-FU | DHA + EPA | 5-FU × DHA + EPA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect % | ±SD | p-Value | Effect % | ±SD | p-Value | Effect % | ±SD | p-Value | |||
| DLD-1 | 3 | −10.86 | 12.45 | 2.07 | 4.32 × 10−9 | 13.79 | 1.36 | 2.15 × 10−21 | −1.68 | 0.46 | 3.00 × 10−4 |
| HCT-8 | 3 | −20.26 | 13.76 | 2.19 | 1.00 × 10−9 | 9.03 | 1.46 | 1.65 × 10−9 | −1.53 | 0.49 | 1.80 × 10−3 |
| LS411N | 3 | −0.99 | 10.74 | 2.07 | 3.49 × 10−7 | 10.60 | 1.36 | 7.47 × 10−14 | −1.40 | 0.46 | 2.20 × 10−3 |
| LS513 | 3 | −33.60 | 150.76 | 21.93 | 2.62 × 10−11 | 14.05 | 1.50 | 7.72 × 10−19 | −17.31 | 4.81 | 4.00 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pettersen, C.H.H.; Samdal, H.; Sætrom, P.; Wibe, A.; Hermansen, E.; Schønberg, S.A. The Salmon Oil OmeGo Reduces Viability of Colorectal Cancer Cells and Potentiates the Anti-Cancer Effect of 5-FU. Mar. Drugs 2023, 21, 636. https://doi.org/10.3390/md21120636
Pettersen CHH, Samdal H, Sætrom P, Wibe A, Hermansen E, Schønberg SA. The Salmon Oil OmeGo Reduces Viability of Colorectal Cancer Cells and Potentiates the Anti-Cancer Effect of 5-FU. Marine Drugs. 2023; 21(12):636. https://doi.org/10.3390/md21120636
Chicago/Turabian StylePettersen, Caroline H. H., Helle Samdal, Pål Sætrom, Arne Wibe, Erland Hermansen, and Svanhild A. Schønberg. 2023. "The Salmon Oil OmeGo Reduces Viability of Colorectal Cancer Cells and Potentiates the Anti-Cancer Effect of 5-FU" Marine Drugs 21, no. 12: 636. https://doi.org/10.3390/md21120636
APA StylePettersen, C. H. H., Samdal, H., Sætrom, P., Wibe, A., Hermansen, E., & Schønberg, S. A. (2023). The Salmon Oil OmeGo Reduces Viability of Colorectal Cancer Cells and Potentiates the Anti-Cancer Effect of 5-FU. Marine Drugs, 21(12), 636. https://doi.org/10.3390/md21120636