Enhanced In Vitro Anti-Photoaging Effect of Degraded Seaweed Polysaccharides by UV/H2O2 Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Compositions of SFP and DSFPs
2.2. Molecular Weights and Yields of SFP and DSFPs
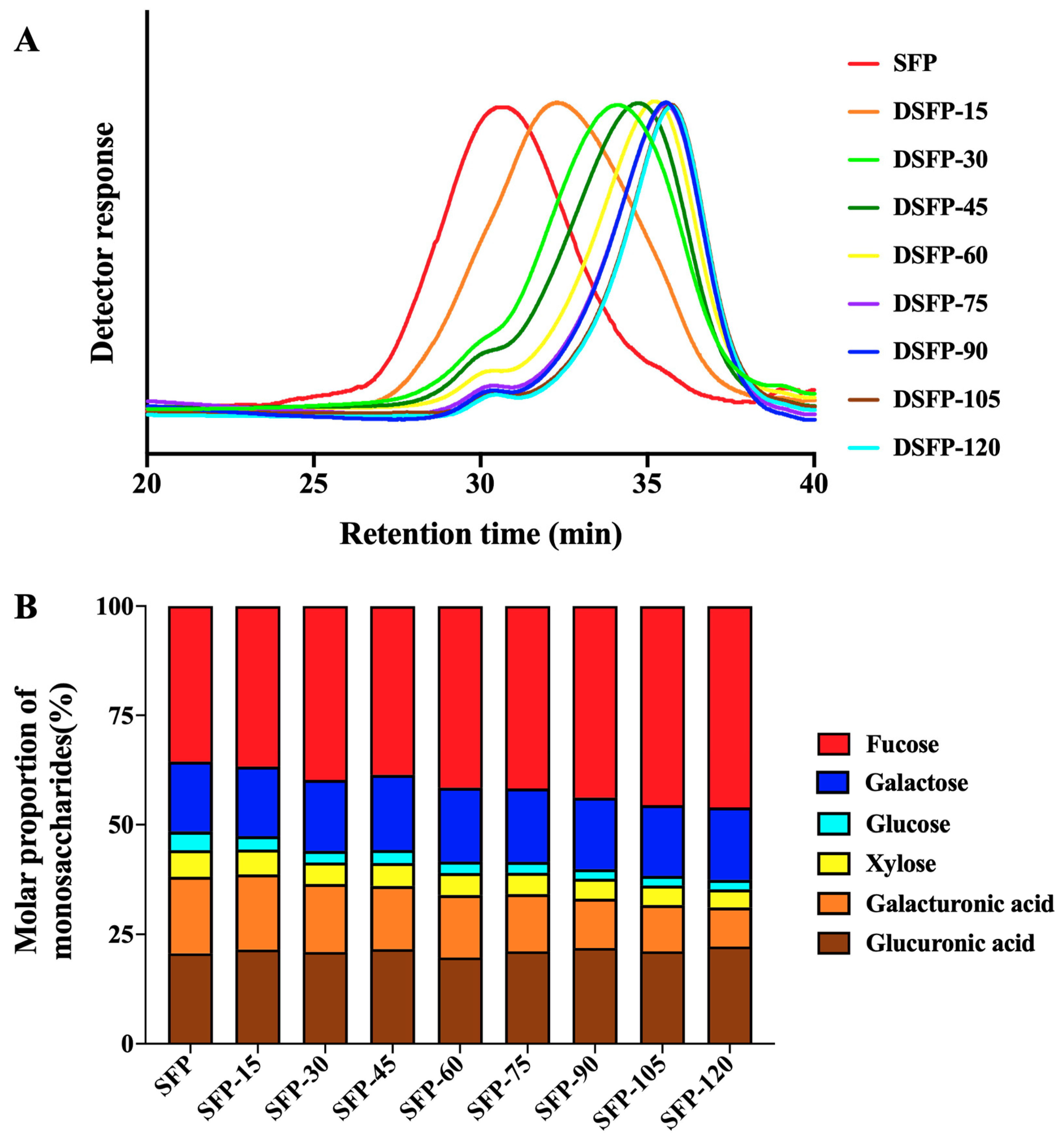
2.3. Monosaccharide Compositions of SFP and DSFPs
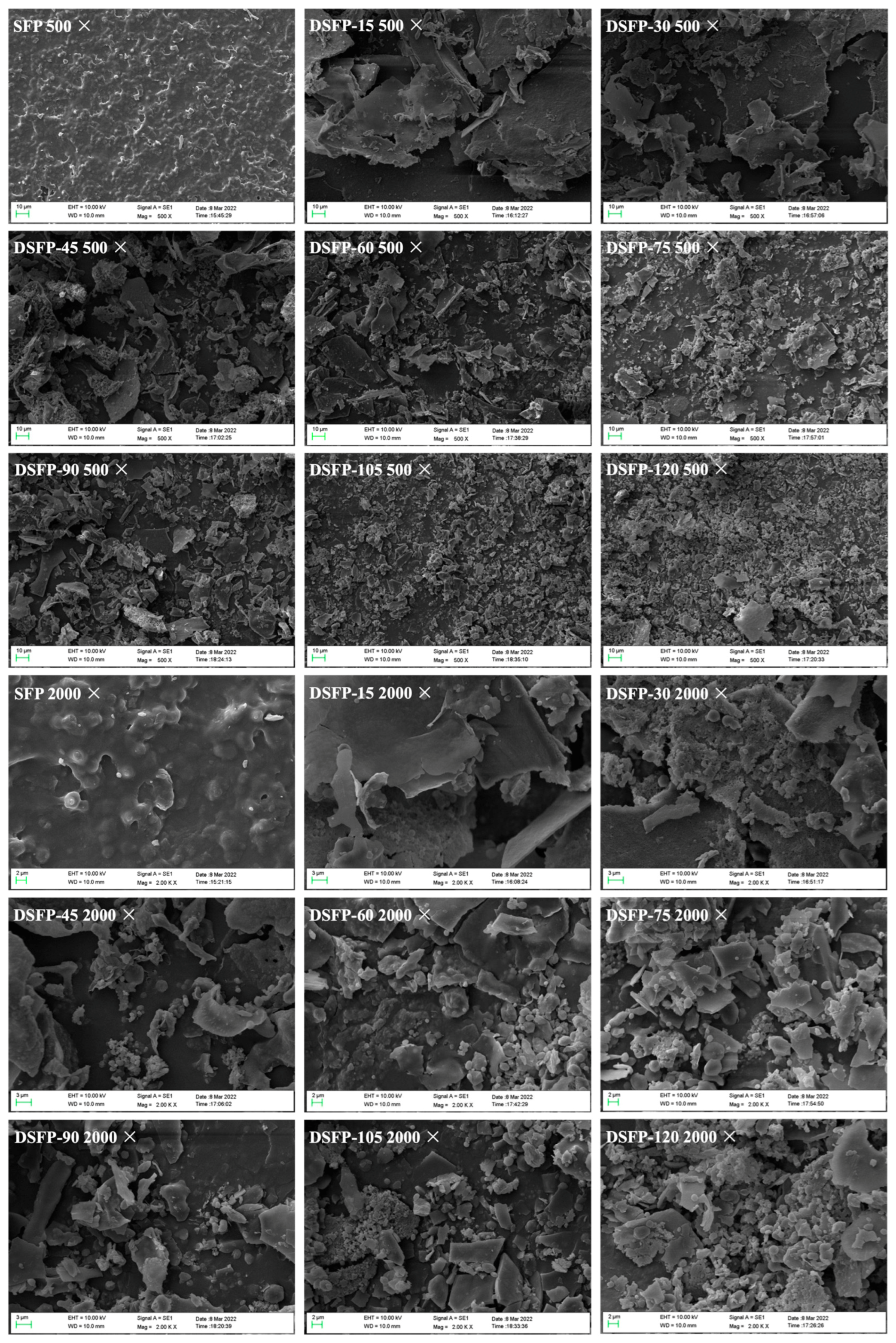
2.4. Scanning Electron Microscope (SEM) Analysis
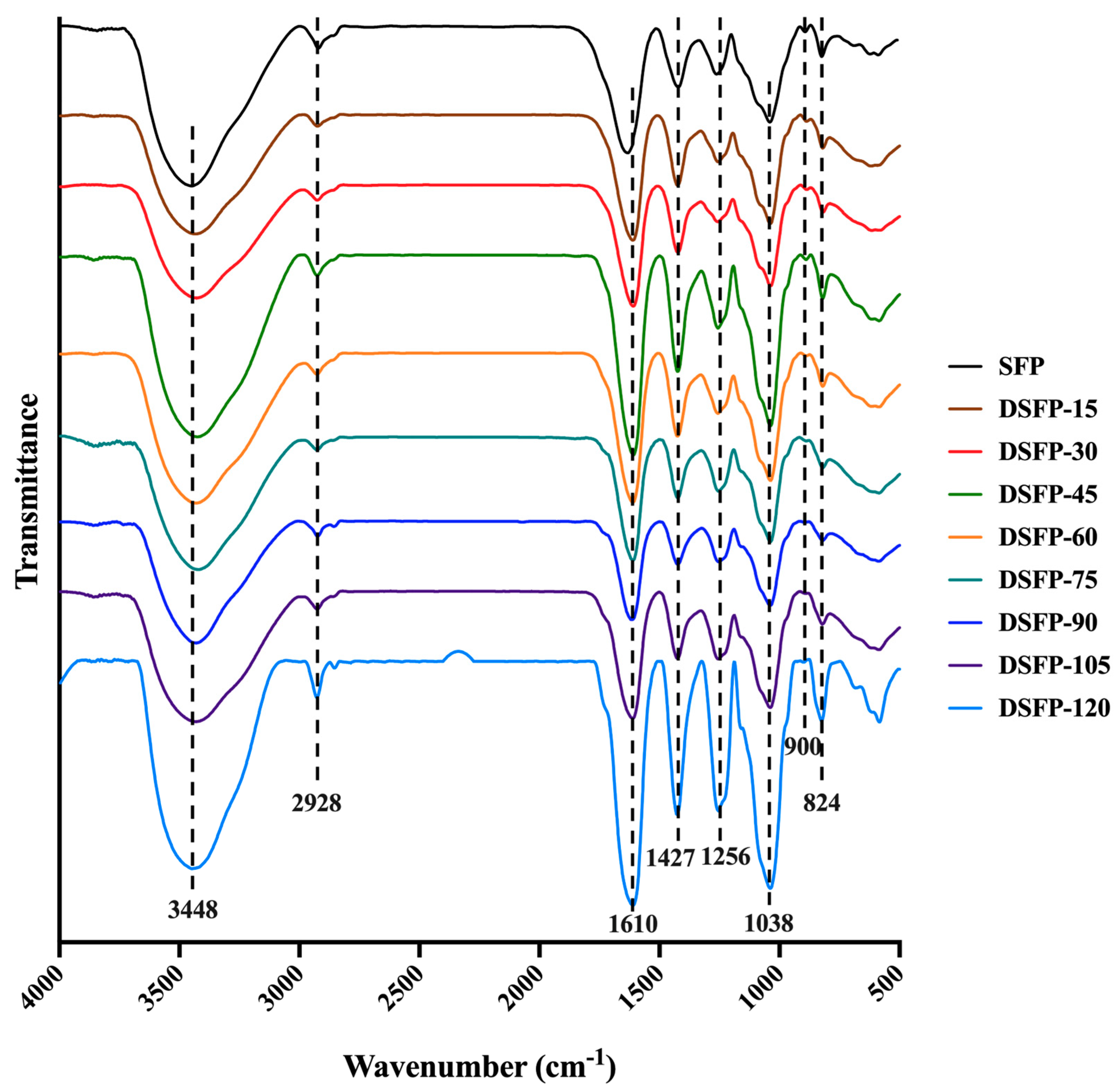
2.5. Fourier Transform Infrared Spectroscopy (FT-IR) Spectra Analysis
2.6. Effects of SFP and DSFPs on the Cell Viability of Photoaged HaCaT Cells
2.7. Effects of SFP and DSFPs on the Level of Hydroxyproline (HYP)
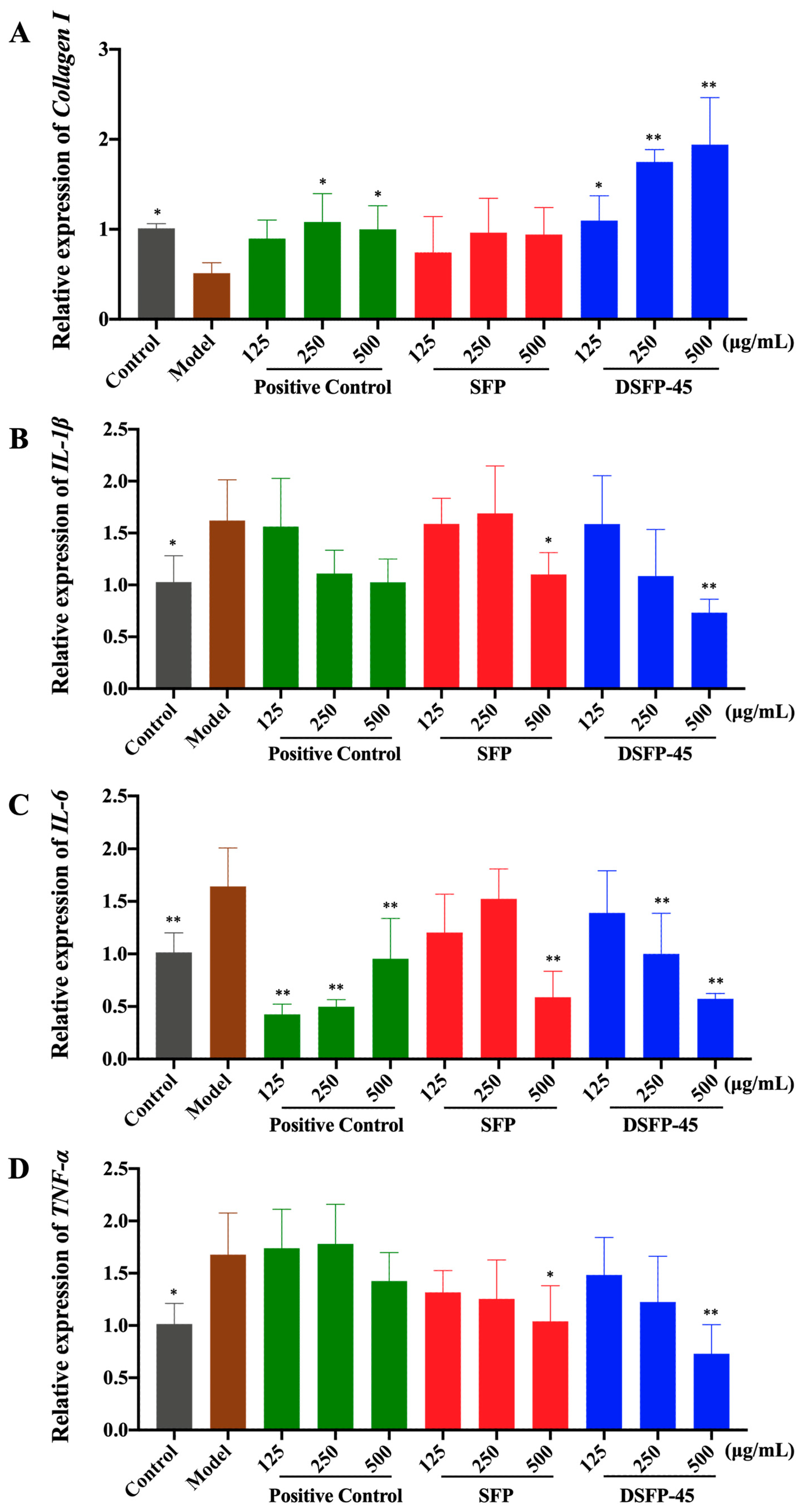
2.8. Effects of SFP and DSFP-45 on the Level and Expression of Collagen I
2.9. Effects of SFP and DSFP-45 on the Levels and Expression of Pro-Inflammatory Cytokines
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation and Degradation of S. fusiforme Polysaccharides
3.3. Determination of Chemical Composition of S. fusiforme Polysaccharides
3.4. Determination of Molecular Weight of S. fusiforme Polysaccharides
3.5. Determination of Monosaccharide Composition of S. fusiforme Polysaccharides
3.6. SEM Analysis of S. fusiforme Polysaccharides
3.7. FT-IR Spectra Analysis of S. fusiforme Polysaccharides
3.8. Anti-Photoaging Activity of S. fusiforme Polysaccharides
3.8.1. Cell Culture and UVB Irradiation
3.8.2. Determination of Cell Viability
3.8.3. Determination of HYP Level
3.8.4. Measurement of Pro-Collagen I α1 Content of HaCaT Cells
3.8.5. Measurement of Pro-Inflammatory Cytokines
3.8.6. Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR) Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Zheng, L.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.L. Preparation methods, biological activities, and potential applications of marine algae oligosaccharides: A review. Food Sci. Hum. Wellness 2023, 12, 359–370. [Google Scholar] [CrossRef]
- Li, X.; Gong, Y.; Yao, W.; Chen, X.; Xian, J.; You, L.; Fardim, P. Structural characterization and protective effects of polysaccharide from Gracilaria lemaneiformis on LPS-induced injury in IEC-6 cells. Food Chem. X 2021, 12, 100157. [Google Scholar] [CrossRef]
- Yao, W.; Qiu, H.; Cheong, K.L.; Zhong, S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int. J. Biol. Macromol. 2022, 221, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Veeraperumal, S.; Qiu, H.; Chen, X.; Cheong, K.L. Anti-cancer effects of Porphyra haitanensis polysaccharides on human colon cancer cells via cell cycle arrest and apoptosis without causing adverse effects in vitro. 3 Biotech 2020, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Hu, C.; Zou, X.; Lin, Y.; Xia, Y.; You, L. Chemistry and immunostimulatory activity of a polysaccharide from Undaria pinnatifida. Food Chem. Toxicol. 2019, 128, 119–128. [Google Scholar] [CrossRef]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.L.; Yu, B.; Chen, J.; Zhong, S. A Comprehensive Review of the Cardioprotective Effect of Marine Algae Polysaccharide on the Gut Microbiota. Foods 2022, 11, 3550. [Google Scholar] [CrossRef]
- Ge, Y.; Ahmed, S.; Yao, W.; You, L.; Zheng, J.; Hileuskaya, K. Regulation effects of indigestible dietary polysaccharides on intestinal microflora: An overview. J. Food Biochem. 2021, 45, e13564. [Google Scholar] [CrossRef]
- Yu, B.; Wang, M.; Teng, B.; Veeraperumal, S.; Cheung, P.C.K.; Zhong, S.; Cheong, K.L. Partially Acid-Hydrolyzed Porphyran Improved Dextran Sulfate Sodium-Induced Acute Colitis by Modulation of Gut Microbiota and Enhancing the Mucosal Barrier. J. Agric. Food Chem. 2023, 71, 7299–7311. [Google Scholar] [CrossRef]
- Malairaj, S.; Veeraperumal, S.; Yao, W.; Subramanian, M.; Tan, K.; Zhong, S.; Cheong, K.L. Porphyran from Porphyra haitanensis Enhances Intestinal Barrier Function and Regulates Gut Microbiota Composition. Mar. Drugs 2023, 21, 265. [Google Scholar] [CrossRef]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 1901–1929. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wassie, T.; Gan, R.; Wu, X. Structural characteristics and immunomodulatory effects of sulfated polysaccharides derived from marine algae. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Xu, S.; Chen, X.; Liu, Y.; Cheong, K.L. Ultrasonic/microwave-assisted extraction, simulated digestion, and fermentation in vitro by human intestinal flora of polysaccharides from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 152, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X. Ultrasonic degradation effects on the physicochemical, rheological and antioxidant properties of polysaccharide from Sargassum pallidum. Carbohydr. Polym. 2020, 239, 116230. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.L.; Li, J.; Zhong, S. Preparation and structure characterization of high-value Laminaria digitata oligosaccharides. Front. Nutr. 2022, 9, 945804. [Google Scholar] [CrossRef]
- Zhang, X.; Aweya, J.J.; Huang, Z.; Kang, Z.; Bai, Z.; Li, K.; He, X.; Liu, Y.; Chen, X.; Cheong, K.L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 2020, 234, 115894. [Google Scholar] [CrossRef] [PubMed]
- Fleita, D.; El-Sayed, M.; Rifaat, D. Evaluation of the antioxidant activity of enzymatically-hydrolyzed sulfated polysaccharides extracted from red algae; Pterocladia capillacea. LWT Food Sci. Technol. 2015, 63, 1236–1244. [Google Scholar] [CrossRef]
- Imran, M.; Poduval, P.B.; Ghadi, S.C. Bacterial degradation of algal polysaccharides in marine ecosystem. In Marine Pollution and Microbial Remediation; Springer: Singapore, 2017; pp. 189–203. [Google Scholar]
- Chen, X.; Sun-Waterhouse, D.; Yao, W.; Li, X.; Zhao, M.; You, L. Free radical-mediated degradation of polysaccharides: Mechanism of free radical formation and degradation, influence factors and product properties. Food Chem. 2021, 365, 130524. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, Y.; Chang, S.; Qiu, H.; You, L. Degradation kinetic models and mechanism of isomaltooligosaccharides by hydroxyl radicals in UV/H2O2 system. Carbohydr. Polym. 2023, 300, 120240. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Li, Y.; Li, X.; You, L.; Kulikouskaya, V.; Hileuskaya, K. Degradation of polysaccharides from Sargassum fusiforme using UV/H2O2 and its effects on structural characteristics. Carbohydr. Polym. 2020, 230, 115647. [Google Scholar] [CrossRef]
- Gong, Y.; Ma, Y.; Cheung, P.C.K.; You, L.; Liao, L.; Pedisić, S.; Kulikouskaya, V. Structural characteristics and anti-inflammatory activity of UV/H2O2-treated algal sulfated polysaccharide from Gracilaria lemaneiformis. Food Chem. Toxicol. 2021, 152, 112157. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Sun-Waterhouse, D.; Zhu, B.; You, L.; Hileuskaya, K. Polysaccharides from Sargassum fusiforme after UV/H2O2 degradation effectively ameliorate dextran sulfate sodium-induced colitis. Food Funct. 2021, 12, 11747–11759. [Google Scholar] [CrossRef]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.S.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Beckenbach, L.; Baron, J.M.; Merk, H.F.; Loffler, H.; Amann, P.M. Retinoid treatment of skin diseases. Eur. J. Dermatol. 2015, 25, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Katsambas, A.D. The role of topical retinoids in the treatment of photoaging. Drugs 2005, 65, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Huang, Y.; Oshima, K.; Yearsley, M.; Zhang, J.; Arnold, M.; Yu, J.; Wang, L. The immunomodulatory potential of natural compounds in tumor-bearing mice and humans. Crit. Rev. Food Sci. Nutr. 2019, 59, 992–1007. [Google Scholar] [CrossRef]
- Singla, R.K.; Wang, X.; Gundamaraju, R.; Joon, S.; Tsagkaris, C.; Behzad, S.; Khan, J.; Gautam, R.; Goyal, R.; Rakmai, J.; et al. Natural products derived from medicinal plants and microbes might act as a game-changer in breast cancer: A comprehensive review of preclinical and clinical studies. Crit. Rev. Food Sci. Nutr. 2022, 1–45. [Google Scholar] [CrossRef]
- Kwon, N.; Vinayagam, R.; Do, G.S.; Lee, K.E.; Kang, S.G. Protective Effects of Fermented Houttuynia cordata Against UVA and H2O2-Induced Oxidative Stress in Human Skin Keratinocytes. Appl. Biochem. Biotechnol. 2023, 195, 3027–3046. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Q.; Lei, L.; Li, F.; Zhao, J.; Yin, R.; Ming, J. The anthocyanin extracts from purple-fleshed sweet potato exhibited anti-photoaging effects on ultraviolent B-irradiated BALB/c-nu mouse skin. J. Funct. Foods 2020, 64, 103640. [Google Scholar] [CrossRef]
- Wei, M.; Qiu, H.; Zhou, J.; Yang, C.; Chen, Y.; You, L. The Anti-Photoaging Activity of Peptides from Pinctada martensii Meat. Mar. Drugs 2022, 20, 770. [Google Scholar] [CrossRef]
- Roh, E.; Kim, J.E.; Kwon, J.Y.; Park, J.S.; Bode, A.M.; Dong, Z.; Lee, K.W. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit. Rev. Food Sci. Nutr. 2017, 57, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Gao, Q.; Ma, C.-w.; Ge, Y.; You, L.; Liu, R.H.; Fu, X.; Liu, D. Effect of polysaccharides from Tremella fuciformis on UV-induced photoaging. J. Funct. Foods 2016, 20, 400–410. [Google Scholar] [CrossRef]
- Yao, W.; Chen, X.; Li, X.; Chang, S.; Zhao, M.; You, L. Current trends in the anti-photoaging activities and mechanisms of dietary non-starch polysaccharides from natural resources. Crit. Rev. Food Sci. Nutr. 2021, 62, 9021–9035. [Google Scholar] [CrossRef]
- Ye, Y.; Ji, D.; You, L.; Zhou, L.; Zhao, Z.; Brennan, C. Structural properties and protective effect of Sargassum fusiforme polysaccharides against ultraviolet B radiation in hairless Kun Ming mice. J. Funct. Foods 2018, 43, 8–16. [Google Scholar] [CrossRef]
- Ji, D.; You, L.; Ren, Y.; Wen, L.; Zheng, G.; Li, C. Protective effect of polysaccharides from Sargassum fusiforme against UVB-induced oxidative stress in HaCaT human keratinocytes. J. Funct. Foods 2017, 36, 332–340. [Google Scholar] [CrossRef]
- Acosta-Rangel, A.; Sánchez-Polo, M.; Polo, A.M.S.; Rivera-Utrilla, J.; Berber-Mendoza, M.S. Sulfonamides degradation assisted by UV, UV/H2O2 and UV/K2S2O8: Efficiency, mechanism and byproducts cytotoxicity. J. Environ. Manag. 2018, 225, 224–231. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Zhu, B.; Sun-Waterhouse, D.; You, L. Insights into the mechanisms underlying the degradation of xylooligosaccharides in UV/H2O2 system. Carbohydr. Polym. 2023, 317, 121091. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zheng, J.; Ye, X. Ultrasonic-assisted citrus pectin modification in the bicarbonate-activated hydrogen peroxide system: Chemical and microstructural analysis. Ultrason. Sonochemistry 2019, 58, 104576. [Google Scholar] [CrossRef]
- Shen, C.; Wen, Y.; Kang, X.; Liu, W. H2O2-induced surface modification: A facile, effective and environmentally friendly pretreatment of chitosan for dyes removal. Chem. Eng. J. 2011, 166, 474–482. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, M.; Liu, F.; Luo, Y.; Chen, L.; Meng, M.; Wang, X.; Zhang, Y. Preparation and inhibition on α-d-glucosidase of low molecular weight polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2016, 93, 27–33. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Zhu, R.; Yu, J.; Lu, W.; Pan, C.; Yao, W.; Gao, X. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohydr. Polym. 2016, 147, 114–124. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, L.; Xu, Y.; Wang, L.; Teng, X.; Li, X.; Dai, J. Characterization and biological activities of a novel polysaccharide isolated from raspberry (Rubus idaeus L.) fruits. Carbohydr. Polym. 2015, 132, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Huo, D.; Cao, C.; Li, Y.; Liang, Y.; Li, B.; Li, L. Preliminary characterization, antioxidant and α-glucosidase inhibitory activities of polysaccharides from Mallotus furetianus. Carbohydr. Polym. 2019, 215, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, S.; Wang, Q.; Lu, X.; Lin, L.; Tian, Y.; Xiao, J.; Zheng, B. Characterization and hypoglycemic activity of a β-pyran polysaccharides from bamboo shoot (Leleba oldhami Nakal) shells. Carbohydr. Polym. 2016, 144, 438–446. [Google Scholar] [CrossRef]
- Liu, P.; Xue, J.; Tong, S.; Dong, W.; Wu, P. Structure Characterization and Hypoglycaemic Activities of Two Polysaccharides from Inonotus obliquus. Molecules 2018, 23, 1948. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.E.; Park, J.E.; Cho, Y.H.; Lim, D.S.; Kim, A.J.; Moh, S.H.; Lee, J.H.; Lee, J.S. Alpha-neoendorphin can reduce UVB-induced skin photoaging by activating cellular autophagy. Arch. Biochem. Biophys. 2020, 689, 108437. [Google Scholar] [CrossRef]
- Colgrave, M.L.; Allingham, P.G.; Jones, A. Hydroxyproline quantification for the estimation of collagen in tissue using multiple reaction monitoring mass spectrometry. J. Chromatogr. A 2008, 1212, 150–153. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, Y.; Zhu, Y.; Chen, Z.; Mu, W.; Zhao, C. Synthesis of bioactive oligosaccharides and their potential health benefits. Crit. Rev. Food Sci. Nutr. 2023, 1–13. [Google Scholar] [CrossRef]
- Xie, X.; Cheong, K.L. Recent advances in marine algae oligosaccharides: Structure, analysis, and potential prebiotic activities. Crit. Rev. Food Sci. Nutr. 2022, 62, 7703–7717. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef]
- Terraz, C.; Toman, D.; Delauche, M.; Ronco, P.; Rossert, J. δEF1 Binds to a Far Upstream Sequence of the Mouse Pro-α1(I) Collagen Gene and Represses Its Expression in Osteoblasts. J. Biol. Chem. 2001, 276, 37011–37019. [Google Scholar] [CrossRef]
- Unsöld, C.; Pappano, W.N.; Imamura, Y.; Steiglitz, B.M.; Greenspan, D.S. Biosynthetic Processing of the Pro-α1(V)2Pro-α2(V) Collagen Heterotrimer by Bone Morphogenetic Protein-1 and Furin-like Proprotein Convertases. J. Biol. Chem. 2002, 277, 5596–5602. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S.K.; Bae, S.; Kim, H.; Park, Y.; Chu, N.K.; Kim, S.G.; Kim, H.R.; Hwang, Y.; Kang, J.S.; et al. The anti-inflammatory effect of alloferon on UVB-induced skin inflammation through the down-regulation of pro-inflammatory cytokines. Immunol. Lett. 2013, 149, 110–118. [Google Scholar] [CrossRef]
- Yao, W.; Kong, Q.; You, L.; Zhong, S.; Hileuskaya, K. Polysaccharides from brown seaweed: Physicochemical properties, absorption in the intestine, and beneficial effects on intestinal barrier. Food Frontiers 2023. [Google Scholar] [CrossRef]
- Li, L.; Hwang, E.; Ngo, H.T.T.; Seo, S.A.; Lin, P.; Gao, W.; Liu, Y.; Yi, T.H. Ribes nigrum L. Prevents UVB-mediated Photoaging in Human Dermal Fibroblasts: Potential Antioxidant and Antiinflammatory Activity. Photochem. Photobiol. 2018, 94, 1032–1039. [Google Scholar] [CrossRef]
- Xiao, T.; Chen, Y.; Song, C.; Xu, S.; Lin, S.; Li, M.; Chen, X.; Gu, H. Possible treatment for UVB-induced skin injury: Anti-inflammatory and cytoprotective role of metformin in UVB-irradiated keratinocytes. J. Dermatol. Sci. 2021, 102, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Patriota, R.C.R.; Rodrigues, C.J.; Cuce, L.C. Intense pulsed light in photoaging: A clinical, histopathological and immunohistochemical evaluation. An. Bras. Dermatol. 2011, 86, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jayawardena, T.U.; Hyun, J.; Wang, K.; Fu, X.; Xu, J.; Gao, X.; Park, Y.; Jeon, Y.J. Antioxidant and anti-photoaging effects of a fucoidan isolated from Turbinaria ornata. Int. J. Biol. Macromol. 2023, 225, 1021–1027. [Google Scholar] [CrossRef]
- Ouyang, Q.; Li, Y.; Mei, S.; Zhang, Q.; Li, X.; Luo, H.; Zhu, Y.; Wu, K. Protective effects of GLHP from Gracilaria lemaneiformis against UVB-induced photodamage in human immortalized keratinocytes cells and BALB/c mice. Exp. Gerontol. 2021, 155, 111550. [Google Scholar] [CrossRef]
- Kim, Y.I.; Oh, W.S.; Song, P.H.; Yun, S.; Kwon, Y.S.; Lee, Y.J.; Ku, S.K.; Song, C.H.; Oh, T.H. Anti-Photoaging Effects of Low Molecular-Weight Fucoidan on Ultraviolet B-Irradiated Mice. Mar. Drugs 2018, 16, 286. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Liu, M.; Chen, X.; You, L.; Ma, Y.; Hileuskaya, K. Effects of UV/H2O2 degradation and step gradient ethanol precipitation on Sargassum fusiforme polysaccharides: Physicochemical characterization and protective effects against intestinal epithelial injury. Food Res. Int. 2022, 155, 111093. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; You, L.; Ma, Y.; Zhao, Z.; Kulikouskaya, V. Influence of UV/H2O2 treatment on polysaccharides from Sargassum fusiforme: Physicochemical properties and RAW 264.7 cells responses. Food Chem. Toxicol. 2021, 153, 112246. [Google Scholar] [CrossRef]
- Dodgson, K.; Price, R. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]


| Total Carbohydrate (%) | Protein (%) | Reducing Sugar (%) | Uronic Acids (%) | Sulfate (%) | |
|---|---|---|---|---|---|
| SFP | 50.42 ± 1.09 a | 2.72 ± 0.05 b | 1.29 ± 0.29 a | 35.52 ± 0.47 f | 6.14 ± 0.97 a |
| DSFP-15 | 52.12 ± 2.64 ab | 2.56 ± 0.83 ab | 1.41 ± 0.18 a | 32.24 ± 0.19 d | 5.96 ± 0.47 a |
| DSFP-30 | 62.04 ± 2.62 c | 1.54 ± 0.22 ab | 2.71 ± 0.75 b | 34.29 ± 0.35 def | 6.26 ± 0.67 ab |
| DSFP-45 | 59.44 ± 2.95 bc | 1.87 ± 0.47 ab | 3.09 ± 0.66 bc | 33.70 ± 0.96 def | 5.76 ± 0.15 a |
| DSFP-60 | 60.40 ± 2.83 bc | 1.30 ± 0.50 ab | 3.91 ± 0.50 c | 34.80 ± 1.80 ef | 6.41 ± 0.26 ab |
| DSFP-75 | 60.82 ± 2.78 bc | 1.36 ± 0.25 ab | 5.16 ± 1.07 d | 32.92 ± 1.43 de | 7.12 ± 0.27 bc |
| DSFP-90 | 72.14 ± 3.96 d | 1.80 ± 0.44 ab | 5.66 ± 0.48 d | 29.88 ± 1.16 c | 8.15 ± 0.30 de |
| DSFP-105 | 71.47 ± 5.99 d | 1.31 ± 0.13 a | 5.86 ± 0.68 d | 22.51 ± 1.16 b | 7.46 ± 0.40 cd |
| DSFP-120 | 66.51 ± 4.55 cd | 1.25 ± 0.27 ab | 6.16 ± 0.25 d | 19.46 ± 1.42 a | 8.81 ± 0.50 e |
| Molecular Weight (kDa) | Yield (%) | |
|---|---|---|
| SFP | 271 | - |
| DSFP-15 | 128 | 65.7 |
| DSFP-30 | 59 | 56.0 |
| DSFP-45 | 43 | 56.7 |
| DSFP-60 | 36 | 48.3 |
| DSFP-75 | 31 | 33.3 |
| DSFP-90 | 28 | 33.3 |
| DSFP-105 | 28 | 38.3 |
| DSFP-120 | 26 | 30.0 |
| Fraction | Molar Proportion of Monosaccharides (%) | |||||
|---|---|---|---|---|---|---|
| Fucose | Galactose | Glucose | Xylose | Galacturonic Acid | Glucuronic Acid | |
| SFP | 35.58 | 15.96 | 4.19 | 6.12 | 17.53 | 20.62 |
| DSFP-15 | 36.70 | 15.84 | 3.05 | 5.69 | 17.20 | 21.51 |
| DSFP-30 | 39.73 | 16.16 | 2.67 | 4.91 | 15.58 | 20.95 |
| DSFP-45 | 38.61 | 17.06 | 2.98 | 5.26 | 14.46 | 21.62 |
| DSFP-60 | 41.50 | 16.86 | 2.60 | 5.02 | 14.29 | 19.73 |
| DSFP-75 | 41.70 | 16.69 | 2.51 | 4.89 | 13.10 | 21.11 |
| DSFP-90 | 43.84 | 16.24 | 2.20 | 4.54 | 11.27 | 21.91 |
| DSFP-105 | 45.47 | 16.14 | 2.23 | 4.44 | 10.57 | 21.14 |
| DSFP-120 | 46.04 | 16.46 | 2.20 | 4.11 | 8.95 | 22.23 |
| Gene | Primer (5′-3′) | |
|---|---|---|
| GAPDH | Forward | TCCACTGGCGTCTTCACCACCAT |
| Reverse | GGAGGCATTGCTGATGATCTTGAGG | |
| collagen I | Forward | CAAGGTGTTGTGCGATGACG |
| Reverse | TGGTTTCTTGGTCGGTGGG | |
| IL-1β | Forward | CTGTACCTGTCCTGCGTGTT |
| Reverse | AGACGGGCATGTTTTCTGCT | |
| IL-6 | Forward | CTGACCCAACCACAAATGC |
| Reverse | TCTGAGGTGCCCATGCTAC | |
| TNF-α | Forward | GCTGCACTTTGGAGTGATCG |
| Reverse | CTTGTCACTCGGGGTTCGAG | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, W.; Yong, J.; Lv, B.; Guo, S.; You, L.; Cheung, P.C.-K.; Kulikouskaya, V.I. Enhanced In Vitro Anti-Photoaging Effect of Degraded Seaweed Polysaccharides by UV/H2O2 Treatment. Mar. Drugs 2023, 21, 430. https://doi.org/10.3390/md21080430
Yao W, Yong J, Lv B, Guo S, You L, Cheung PC-K, Kulikouskaya VI. Enhanced In Vitro Anti-Photoaging Effect of Degraded Seaweed Polysaccharides by UV/H2O2 Treatment. Marine Drugs. 2023; 21(8):430. https://doi.org/10.3390/md21080430
Chicago/Turabian StyleYao, Wanzi, Jiayu Yong, Bingxue Lv, Siyu Guo, Lijun You, Peter Chi-Keung Cheung, and Viktoryia I. Kulikouskaya. 2023. "Enhanced In Vitro Anti-Photoaging Effect of Degraded Seaweed Polysaccharides by UV/H2O2 Treatment" Marine Drugs 21, no. 8: 430. https://doi.org/10.3390/md21080430
APA StyleYao, W., Yong, J., Lv, B., Guo, S., You, L., Cheung, P. C.-K., & Kulikouskaya, V. I. (2023). Enhanced In Vitro Anti-Photoaging Effect of Degraded Seaweed Polysaccharides by UV/H2O2 Treatment. Marine Drugs, 21(8), 430. https://doi.org/10.3390/md21080430