Abstract
Sarcopenia, a progressive disease characterized by a decline in muscle strength, quality, and mass, affects aging population worldwide, leading to increased morbidity and mortality. Besides resistance exercise, various nutritional strategies, including omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation, have been sought to prevent this condition. This narrative review summarizes the current evidence on the effect and mechanism of n-3 PUFA on musculoskeletal health. Despite conflicting evidence, n-3 PUFA is suggested to benefit muscle mass and volume, with more evident effects with higher supplementation dose (>2 g/day). n-3 PUFA supplementation likely improves handgrip and quadriceps strength in the elderly. Improved muscle functions, measured by walking speed and time-up-to-go test, are also observed, especially with longer duration of supplementation (>6 months), although the changes are small and unlikely to be clinically meaningful. Lastly, n-3 PUFA supplementation may positively affect muscle protein synthesis response to anabolic stimuli, alleviating age-related anabolic resistance. Proposed mechanisms by which n-3 PUFA supplementation improves muscle health include 1. anti-inflammatory properties, 2. augmented expression of mechanistic target of rapamycin complex 1 (mTORC1) pathway, 3. decreased intracellular protein breakdown, 4. improved mitochondrial biogenesis and function, 5. enhanced amino acid transport, and 6. modulation of neuromuscular junction activity. In conclusion, n-3 PUFAs likely improve musculoskeletal health related to sarcopenia, with suggestive effect on muscle mass, strength, physical performance, and muscle protein synthesis. However, the interpretation of the findings is limited by the small number of participants, heterogeneity of supplementation regimens, and different measuring protocols.
1. Introduction
Sarcopenia is an age-related decline in muscle strength, quality, and mass [1]. It is estimated to affect more than 50 million people globally [2]. Not only does it affect musculoskeletal function, leading to falls and fractures [3,4], but it also progresses toward the deterioration in physical function [1], cardiovascular diseases [5], chronic respiratory diseases [6], cognitive impairment [7], dependence [8] and ultimately, increased mortality [9]. The condition undoubtedly increases hospitalization and healthcare costs tremendously with an estimated annual excess cost of £2.5 billion in the United Kingdom [10]. Likewise, the community-dwelling elderly with sarcopenia have more than 2-fold higher direct healthcare costs compared to those without sarcopenia [11]. According to the European Working Group on Sarcopenia in Older People, sarcopenia is diagnosed by the presence of low muscle strength (handgrip strength < 27 kg in men, and <16 kg in women, or chair stand > 15 secs for 5 rises), and reduced muscle quantity (appendicular skeletal muscle mass < 20 kg in men and <15 kg in women, or appendicular skeletal muscle mass/height2 < 7.0 kg/m2 in men or <5.5 kg/m2 in women. The resulting reduction in physical performance indicates its severity, with severe sarcopenia being defined by a reduction in gait speed to 0.8 m/s, short physical performance battery (SPPB) ≤ 8, time-up-and-go test (TUG) > 20 s, and 400-m walk test of ≥6 min [1].
In healthy adults, muscle mass preservation relies on a dynamic balance of muscle protein synthesis (MPS) and muscle protein breakdown (MPB), which oscillates in a dynamic equilibrium of a “fasted-loss/fed-gain” cycle [12]. Resistance exercise and adequateintake of high-quality protein containing essential amino acids, especially leucine, transiently optimize MPS, while fasting can escalate MPB [13]. MPB is also increased in aging due to physiologic and hormonal changes, as well as chronic low-grade inflammation and decreased physical activity that accompany aging [14]. MPB is also increased in various chronic muscle wasting diseases, such as cancer, cachexia, chronic kidney disease, heart failure, and chronic respiratory disease, etc. [15,16,17]. Moreover, aged muscles are less sensitive to exercise and nutrient availability which are strong anabolic stimuli. The resulting anabolic resistance makes the preservation of muscle mass a challenge for the elderly [14,18]. Without disease-specific interventions, resistance exercise remains the primary remedy against sarcopenia [19,20]. Nevertheless, considering the elderly’s limited capacity to engage in physical activities, achieving the required amount of training can be problematic [21]. Hence, developing treatment strategies to increase or maintain the quantity and strength of muscles and overcome anabolic resistance may aid in the perseverance of independence and quality of life. Omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation has gained increasing interest due to its anti-inflammatory properties [22] and biologically plausible mechanism to promote MPS by augmenting the anabolic response to hyperinsulinemia-hyperaminoacidemia and increasing protein kinases in related signaling pathways [22,23,24]. This is supported by promising results from animals [25,26] as well as clinical studies [27,28].
Comprehensively gathering the data from recent meta-analyses with updated findings from novel RCTs, this narrative review summarizes key findings on the effect of n-3 PUFA supplementation on each component of sarcopenia diagnostic criteria. The effect of n-3 PUFA on MPS will also be discussed. Plausible mechanisms by which n-3 PUFA benefits musculoskeletal health are also delineated. This narrative review will provide up-to-date and clinically relevant evidence which will aid clinical decision-making and highlight the gap in current knowledge in this field.
2. n-3 PUFA
n-3 fatty acids comprise a group of various fatty acids containing a double bond between the third and the fourth carbon atoms from the methyl end [29]. n-3 fatty acids can be categorized into monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA), depending on the number of double bonds in their chemical structures [30]. These fatty acids can be also classified by the number of carbon atoms, with short chain containing 6 or fewer carbon atoms, medium chain 12 carbon atoms, long chain fatty acid 14–18 carbon atoms, and very long chain containing 20 or more carbon atoms. Long-chain n-3 PUFA is the majority of n-3 PUFA available in food [31].
While the human body can synthesize n-3 PUFA from alpha-linolenic acid using desaturase and elongase enzymes, this endogenous production of n-3 PUFA only accounts for about 10% of daily PUFA requirement, which is not adequate for the body [32]. Considered an essential fatty acid, n-3 PUFA are mainly derived from external food sources and supplementation [33]. Two types of commonly occurring long-chain n-3 PUFAs in food are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [34], both of which can be found in varying quantities and proportions in marine products. Microalgae oil, a rich and primary source of both EPA and DHA production, is a suitable source of n-3 PUFA for vegetarians [35,36]. Some marine invertebrates, such as oysters, squids and octopus can also synthesize these fatty acids [37,38]. Other marine fishes, such as herring, wild sardine, and mackerel, that feed on these n-3 PUFA producing algae and molluscs are also good sources of dietary n-3 PUFA [31,39]. n-3 PUFA can also be found in the roes of these oily fishes, such as salmon roe [39]. In spite of its availability in various sources, the dietary intake of n-3 PUFA was estimated to be 0.17 g per day, which only equates to about 10–15% of the recommended daily intake in adults over 50 years old [40]. Hence, products containing fish oil, cod liver oil, tuna oil, krill oil, and algal oil, are often used as n-3 PUFA supplementations [31].
n-3 PUFAS have been shown to benefit human health in various ways including metabolic issues [30], cardiovascular health [34], and muscle health [41]. Many of these benefits are largely attributable to n-3 PUFA’s anti-inflammatory properties [42]. Metabolic issues are widely known to be associated with inflammation [30]. n-3 PUFA has been suggested to ameliorate insulin resistance through improved mitochondrial function and modulation of phospholipid membranes [43]. It is also positively correlated with serum adiponectin level, exhibiting protective effects in obese individuals in decelerating the progression of cardiovascular disease and metabolic syndromes [44]. With its cardioprotective effects through blood lipids modulation, vasodilatory effect, blood pressure and heart rate modulation, lowered platelet aggregation, and a reduction of pro-inflammatory biomarkers, n-3 PUFA has been suggested as an intervention for primary and secondary preventions of cardiovascular disease [34,44]. There has been growing interest in the beneficial effects of n-3 PUFA in the prevention and treatment of sarcopenia in the elderly [45], which will be further discussed in detail.
3. Effect of n-3 PUFA Supplementation on Muscle Mass and Volume
Several meta-analyses suggested the positive effects of n-3 PUFA supplementation on the preservation of muscle mass. Although the results remain largely inconclusive and recommendations on the dosage are currently far from clear, dose-dependent effects have been suggested.
A recent meta-analysis found a small increase in skeletal muscle mass of 0.33 kg after n-3 PUFA intervention compared with control in the elderly aged 60 years or above [46]. The estimation was pooled from five small studies (n = 103 for n-3 PUFA groups, and n = 99 for control groups) with low heterogeneity. Specifically, the improvement was only found in participants receiving over 2 g per day of n-3 PUFA with a pooled effect of 0.67 kg, suggesting the dose-effect relationship. However, the findings should be interpreted with caution as one of the included studies used alpha-linolenic acid supplementation as opposed to n-3 PUFA [47]. The meta-analysis also included n-3 PUFA targeted diet intervention with an instructed ratio of n-6 to n-3 PUFA ratio [48,49]. Hence, the real effect of n-3 PUFA as supplementation was obscured. Furthermore, the subgroup analysis in studies with over 2 g per day of n-3 PUFA only involved two studies with limited sample sizes [50,51], one of which was in patients with lung cancer receiving chemotherapy with profoundly different physiology from the general geriatric population. Still, the positive effects of n-3 PUFA were corroborated in another meta-analysis which compiled eight RCTs with 406 older participants [52]. The subjects, nonetheless, mainly had significant comorbidities, for example, cancer [53,54], chronic obstructive pulmonary disease [55,56], and morbid obesity undergoing surgery [57]. These patients were already subjected to chronic inflammation [58], which probably accounted for the exaggerated effect of n-3 PUFA. Moreover, the studies differed significantly in the form of n-3 PUFA supplementation, ranging from fish oils to oral multi-nutritional supplements. The duration varied vastly from 12 days to four months. One study was performed exclusively during hospitalization [55], and another did not specify the dose of n-3 PUFA [54]. However, a sensitivity analysis was not performed to exclude the effects of these methodology discrepancies.
In contrast, Cornish et al. performed a meta-analysis analyzing the effect of n-3 PUFA supplementation on lean body mass reported in 10 RCTs from 433 healthy older adults [59]. There was no difference between n-3 PUFA supplementation compared to control regardless of resistance exercise training. The meta-analysis, however, used lean body mass as opposed to skeletal muscle mass, which is considered a more sensitive and accurate indicator of body functional muscle mass [52]. Due to the variability in the study protocols, a subgroup analysis should also be performed to further explore the effects of different doses and durations of supplementation.
Lately, Dalle et al. found that neither resistance exercise training alone nor in combination with n-3 PUFA (410 mg DHA + 540 mg EPA) resulted in a gain in leg muscle volume and muscle anabolic sensitivity markers in the elderly with osteoarthritis [60]. However, interleukin-6 (IL-6), an important proinflammatory marker, was decreased, in parallel with a decreasing trend in p65NF-κB, a pro-inflammatory transcription factor, in participants receiving n-3 PUFA. The study, nevertheless, had a small sample size consisting of only 23 individuals. More recently, in a larger RCT involving 63 healthy older adults, six months of 3.9 g daily n-3 PUFA supplementation (300 mg DHA + 675 mg EPA) did not outperform placebo in increasing the leg lean mass and whole-body amino acid metabolic response to exercise [61]. Furthermore, mitochondrial respiration, adenosine triphosphate (ATP) production, and reactive oxygen species (ROS) production remained unchanged. It is worth noting that both studies had relatively short study periods, considering anabolic resistance impeding muscle gain in the elderly. In addition, it is unclear whether the exercise intervention in these studies were sufficient per se to stimulate muscle synthesis in the elderly.
Overall, n-3 PUFA supplementation may have small effects in improving muscle mass via anti-inflammation and increasing protein synthesis which will be further discussed [23,62]. Although dose-dependent relationship was suggested, the effects of different durations of supplementation were inconclusive due to high heterogeneity in research protocol, dose, duration, and form of supplementation as well as the lack of subgroup analysis. Nevertheless, since considerable duration is required to yield a detectable increment in muscle mass, longer duration of treatment should presumably be associated with greater effect. Greater effects were suggested in the population group with chronic wasting conditions and prolonged inflammation, such as cancer and chronic respiratory diseases. This requires further investigations to confirm the effect size in each group of participants, and to identify factors associated with greater benefit.
4. Effect of n-3 PUFA Supplementation on Muscle Strength
To date, meta-analyses on the n-3 PUFA and muscle strength have yielded conflicting results due to protocol heterogeneities such as pooling muscle strength measurement results from different muscles (as opposed to analyzing data from each parameter of muscle strength individually) or pooling outcomes of different forms of omega-3 supplementation. Moreover, these meta-analyses did not measure muscle strength as a primary outcome. Findings from randomized controlled trials are also limited by sample size and the analysis of outcomes from different supplements.
A recently published meta-analysis compared the effect of n-3 PUFA supplementation on lower and upper body strength in healthy elderly [59]. Lower body strength ameliorated after n-3 PUFA supplementation, and so did upper body strength after removing studies with a high risk of bias. While the number of pooled participants was large, this finding should be interpreted with caution as the indicators of muscle strength varied across studies, i.e., isometric torque, isokinetic torque, leg press, and one repetition maximum (1-RM), handgrip strength and chest press, reflecting different aspects of muscle strength and recruitment of different muscle fascicles [63,64,65]. Moreover, each test of muscle strength also differs in its complexity, which results in a varying degree to which training and familiarity to the test can affect the strength improvement [60]. Therefore, these parameters might be too dissimilar to be pooled into one analysis based on rough anatomical regions.
Separately analyzing each parameter of muscle strength, Bird et al. initially reported no significant effect of n-3 PUFA supplementation on handgrip strength from 14 studies [52], but later favored n-3 PUFA after removing two outliers which shows nonsignificant results [66,67]. The studies varied greatly in their duration of intervention, ranging from 26 days [68] to 3 years [69]. A subgroup analysis based on duration would have provided a better understanding. Three studies used mixed nutritional supplements containing n-3 PUFA and thus should be removed in sensitivity analysis [67,70,71]. Another meta-analysis failed to reveal the benefit of n-3 PUFA on handgrip strength in healthy older adults [46]. However, it was limited by the low number of included studies (k = 3) and outcome heterogeneity. One of the studies used mixed oral supplements as opposed to n-3 PUFA alone [72] and hence should be excluded in the sensitivity analysis. Similarly, handgrip strength was found to increase significantly with increasing dietary n-3 PUFA in another meta-analysis [73]. However, the result was pooled from only two studies, one of which combined calcium, gamma-linoleic acid, and EPA as the supplement, as opposed to conventional EPA and DHA [74]. Moreover, muscle strength was not the primary outcome in this meta-analysis.
The effect of n-3 PUFA supplementation on 1-RM maximum chest press pooled from three studies showed a non-significant negative trend [46]. Again, the analysis included a study using mixed oral supplements [72], which should otherwise be excluded from the sensitivity analysis.
Quadriceps maximal voluntary capacity was estimated from 329 participants in 10 studies, mainly involving healthy elderly samples [46]. The analysis revealed a significant increase in quadriceps maximal voluntary capacity in favor of n-3 PUFA. On the contrary, there was no improvement in 1-RM leg strength. Again, the number of included studies was rather small (k = 2), with one of them using alpha-linoleic acid instead of n-3 PUFA [47], and another prescribing healthy diet plan, instead of direct n-3 PUFA supplementation [49]. The analysis, therefore, did not represent the real effect of n-3 PUFA.
More recent trials showed conflicting results. A study in 32 chronic obstructive pulmonary disease patients randomized to receive n-3 PUFA supplementation (1400 mg DHA + 2100 mg EPA, or 1000 mg DHA + 1500 mg EPA) or placebo found no change in handgrip strength after four weeks [75]. However, the trial was limited by a short follow-up period and a small sample size. The participants had preserved muscle mass, which perhaps veiled the effect of n-3 PUFA. Eight weeks of vibration and resistance exercise training combined with whey protein and n-3 PUFA supplementation (1397 mg DHA + 749 mg EPA) significantly raised muscle power during chair rise in healthy older men but not women [76], presumably due to different gender-based responses to training and fewer motor units in females [77]. The improvement in muscle strength coincided with increased serum insulin-like growth factor 1 (IGF-1), which stimulates MPS via mechanistic target of rapamycin complex 1 (mTORC-1) signaling pathway [54,78], and also a significant reduction in pro-inflammatory cytokines, implying an anti-inflammatory effect of n-3 PUFA on skeletal muscle health. Longer-term trials, however, revealed no increase in muscle strength in 107 frail elderly after 24 weeks of supplementation of leucine-enriched protein with n-3 PUFA (1100 mg DHA + 800 mg EPA) [79].
In conclusion, n-3 PUFA supplementation seems to improve muscle strength as evident in the meta-analyses, although findings should be treated with caution due to protocol heterogeneities in the studies included. Moreover, each parameter of muscle strength may respond differently due to differences in the nature of muscle fascicles. While lower body muscle strength may also require a combination of vibration, RET, and protein supplementation, evidence supports the benefit of supplementation on hand grip strength, which is the main parameter used in both initial screening and diagnosis of sarcopenia. Moreover, handgrip strength is a strong predictor of patient outcomes, including hospital length of stay, limitation in activities of daily living, quality of life, and also all-cause mortality in the elderly. It is also related to the strengths of other body parts. Still, it remains unclear which intervention is most beneficial for each individual muscle. A comparison between n-3 and n-3 plus other nutrients will also help to elucidate this. More robust meta-analyses investigating the effect of dose and duration of n-3 PUFA supplementation are required.
5. Effect of n-3 PUFA Supplementation on Physical Performance
Two recently published meta-analyses of RCTs in older individuals revealed that n-3 PUFA supplementation significantly improved TUG by 0.29 s and 0.30 s respectively [46,59], although the latter was limited by the small number of pooled participants of only 73 receiving n-3 PUFA and 63 controls. Moreover, both changes, albeit statistically significant, were arguably too little to be clinically significant.
Walking speed was unaffected by n-3 PUFA supplementation in a meta-analysis of 10 studies in healthy older individuals, regardless of additional exercise intervention, although there was no subgroup analysis based on other parameters such as supplementation regimen [59]. The findings might be undermined as two of the included studies did not directly compare pure n-3 PUFA with passive placebo; one used n-3 PUFA plus vitamin E in the intervention arm [60], and the other used active placebo containing various fatty acids [80]. Another meta-analysis in older adults revealed similar results. Since the included studies had high heterogeneity, a subgroup analysis based on two studies was performed and found an increase in walking speed when supplementation period was over six months. However, one of the two studies compared n-3 PUFA enriched multi-nutritional supplement and placebo, instead of pure n-3 PUFA, which might impact the effect of n-3 PUFA in an unpredictable way [54].
Only one meta-analysis assessed 30-s sit-to-stand performance pooled from six studies with 230 participants [59]. The main analysis showed 1.93 s improvement with n-3 PUFA supplementation. The studies had high heterogeneity, with only one RCT with a high risk of bias showing a large improvement [81], and the effect was no longer significant when the study was removed.
Eight weeks of vibration and resistance exercise training with whey protein and n-3 PUFA supplementation (1397 mg DHA + 749 mg EPA) significantly improved gait speed but not chair rise time [76]. Gait speed difference, however, was very small (0.01 m/s, p = 0.024), and hardly yielded any clinical importance. A longer period of supplementation might yield more applicable results. The long-term effect of n-3 PUFA supplementation in the community-dwelling elderly with low gait speed or limited instrumental activity of daily living was assessed in a large-multicenter RCT which compared multiple physical performance parameters across four parallel groups: n-3 PUFA supplementation (800 mg DHA + 225 mg EPA), n-3 PUFA supplementation with lifestyle intervention, placebo alone, and lifestyle intervention alone (n = 1680) [69]. Six months of low-dose n-3 PUFA supplementation failed to improve repeated chair stand test, walking speed, SPPB, and balance. It might be noteworthy that the study was originally designed to investigate the effect of n-3 PUFA in the prevention of cognitive decline. Similarly, six months of n-3 PUFA (1100 mg DHA + 800 mg EPA) plus leucine-enriched protein did not result in any improvement in SPPB, walking speed, TUG, and chair stand test in the elderly with reduced muscle mass or strength compared to placebo, although the effect of n-3 PUFA alone was not evaluated [79].
In conclusion, n-3 PUFA fatty acid supplementation improved walking speed and TUG test in the meta-analyses. Both are important parameters in the assessment of sarcopenia severity [1]. Gait speed is a quick and easy test to perform in clinics and is also a predictor of poor quality of life and increased mortality in both community-dwelling and diseased elderly [82]. Likewise, poor TUG results are associated with frailty and higher mortality in healthy older adults [83] and those with chronic respiratory diseases [84]. However, the changes caused by n-3 PUFA supplementation were arguably too small to yield clinical significance. Some findings suggested that a longer duration of supplementation might yield more significant results. Pooled results of SPPB, which covers various domains of physical performance, might be more sensitive and helpful in understanding the effect of n-3 PUFA supplementation on physical performance.
6. Effect of n-3 PUFA on MPS
Research on whether n-3 PUFA supplementation enhances MPS and anabolic response yielded controversial results, and meta-analysis on the topic is still lacking. Interpretations of available findings are challenging due to various methodological limitations.
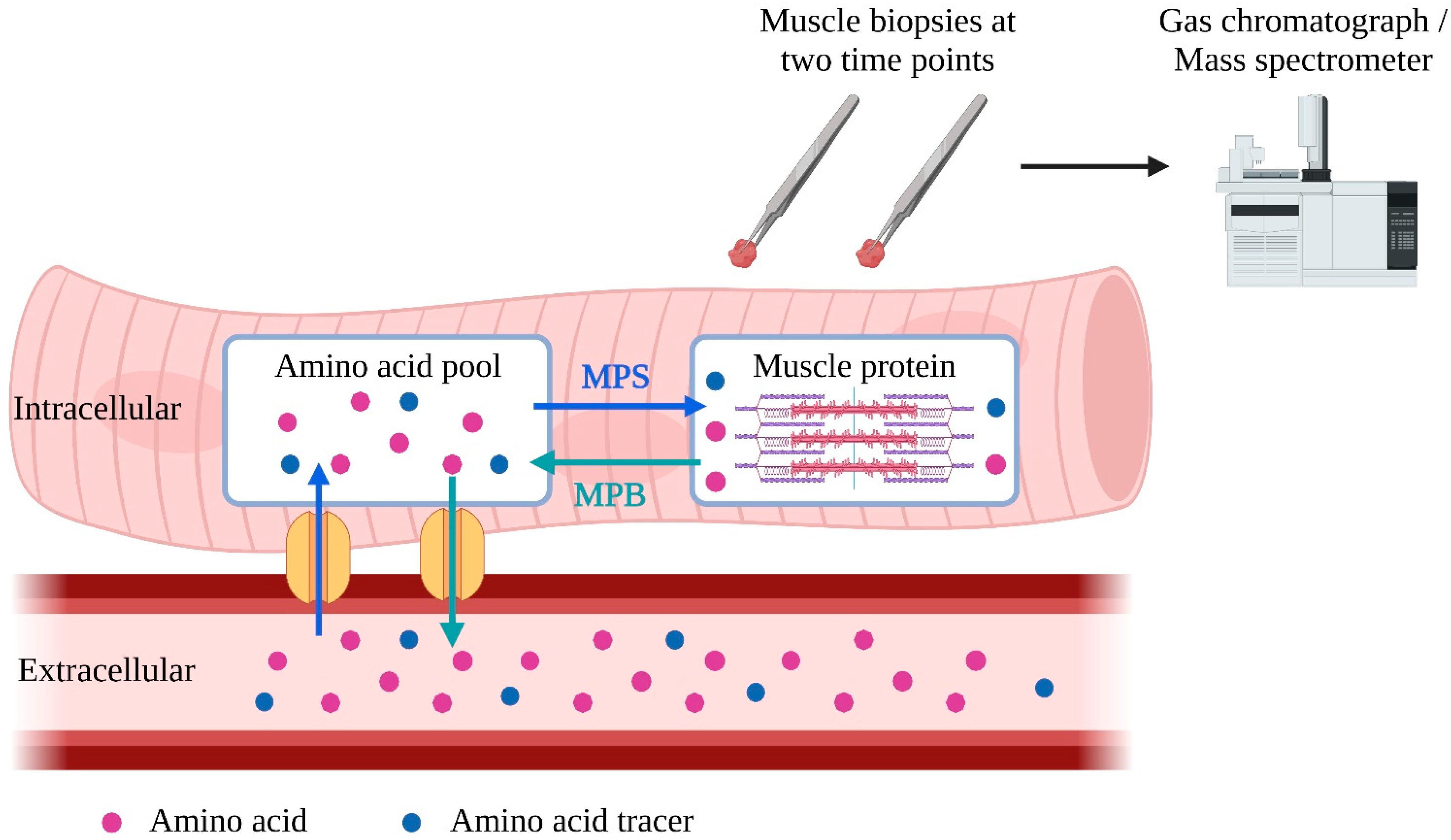
While in vivo MPB measurement remains more complicated and invasive, MPS is commonly measured in research as fractional synthetic rate (FSR) [85]. After being injected into the bloodstream, isotope-labeled amino acid tracers enter muscle cells, where they are incorporated into muscle proteins during MPS [86,87]. Two subsequent muscle biopsies are taken to determine the rate of incorporation of amino acid tracers into muscle protein to calculate FSR [88] (Figure 1). Alternatively, it is possible to use deuterium-labeled alanine produced from ingested deuterium-labeled as tracers, enabling a more extended period for FSR measurement [89].
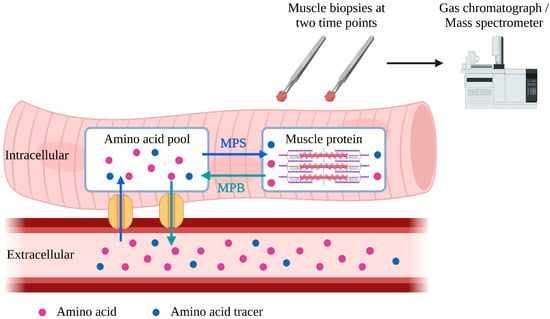
Figure 1.
Schematic representation of measurement of fractional synthetic rate (FSR). To measure muscle protein synthesis (MPS), tracer amino acids can be introduced into the bloodstream through injection or labeling from ingested deuterated water. These tracer amino acids are taken up by the intracellular amino acid pool and used for MPS. To calculate FSR, the amount of tracer amino acid incorporated into muscle protein per unit of time is analyzed in two muscle samples. MPS = muscle protein synthesis; MPB = muscle protein breakdown. The blue arrows represent the incorporation of amino acids into the intracellular muscle pool and intracellular protein. The green arrows represent the release of amino acids of muscle protein and intracellular amino acid pool into the blood stream.
Among the earliest clinical trials on MPS and n-3 PUFA were studies by Smith et al. which demonstrated that although n-3 PUFA supplementation (1500 mg DHA + 1860 mg EPA) did not alter the baseline muscle protein FSR compared to placebo, it augmented MPS response to hyperaminoacidemia-hyperinsulinemia anabolic stimulation as well as increased the expression of mTORC-1 signaling pathway. This effect was more evident in healthy young men compared to older individuals (50% vs. 30%) [23,28]. The increase in MPS was consistent with the increase in mTORC-1 signaling pathway expression. Positive effects of n-3 PUFA supplementation (2030 mg DHA + 2970 mg EPA) were also demonstrated in other population groups. n-3 PUFA supplementation increased the myofibrillar protein FSR in healthy young women before, during, and after leg immobilization compared to placebo, resulting in a better recovery of muscle disuse atrophy after two weeks of immobilization [90]. High dose n-3 PUFA (1400 mg DHA + 2100 mg EPA) in patients with chronic obstructive pulmonary disease improved anabolic response with approximately 15% increase in net protein synthesis [75]. Short-term effects of both high and low doses n-3 PUFA supplementation (1000 mg DHA + 1500 mg EPA) in reducing protein breakdown were also demonstrated. Finally, after 4 months of supplementation with n-3 PUFA (1200 mg DHA + 2700 mg EPA), mitochondrial and sarcoplasmic FSR were increased before exercise, while mitochondrial and myofibrillar FSR increased post-exercise [91]. The increase in MPS was parallel to the upregulation of genes that promote protein synthesis and protein folding and assembly, as well as the downregulation of myostatin, which is responsible for MPB, and other atrophy-related genes. The change in protein metabolism and genetic expression after exercise might be attributable to the reduction of anabolic resistance mediated by transcriptional changes and the alteration of anabolic signaling proteins [91,92].
On the contrary, other studies did not reiterate these results. A randomized controlled trial showed no significant increase in the FSR in healthy young men who had n-3 PUFA supplementation (900 mg DHA + 3500 mg EPA) compared to the control group having coconut oil, although the results should be interpreted with caution as the baseline FSR was not measured or recorded [93]. The lack of significant positive effects was also demonstrated in the independently living elderly after 6 months of n-3 PUFA supplementation (1200 mg DHA + 2700 mg EPA) [61]. In another study, 18 weeks of n-3 PUFA supplementation (600 mg DHA + 2100 mg EPA) contributed to a significant increase in muscle strength and quality in elderly females in the absence of an increase in MPS [27]. Another RCT failed to manifest the benefit of n-3 PUFA (1100 mg DHA + 800 mg EPA) and leucine-enriched protein supplementation, compared to leucine-enriched protein alone or placebo, in increasing MPS [79]. However, MPS was measured in only 40% of the participants. In a cohort who had hemodialysis with chronic inflammation, 2.9 g of n-3 PUFA supplementation (2:1 ratio of EPA:DHA), when compared to placebo, mitigated forearm muscle protein breakdown without significant effect on whole body protein metabolism [94]. Since the study was conducted in hemodialysis patients with many physiologic disturbances, the result might not truly represent the general geriatric population.
Overall, n-3 PUFA supplementation may positively affect MPS response to anabolic stimuli, offering a potential remedy for anabolic resistance. However, existing trials were mainly limited by the small numbers of participants and the heterogeneity of n-3 PUFA regimen, including the types of amino acid tracers, the duration between two muscle biopsies, and the physiologic condition under which muscle biopsies were taken. The lack of complete evaluation of MPS, including the lack of baseline measurement in one study also poses challenges in data interpretation. More rigorous trials with consistent outcome measures are needed to elucidate the relationship between n-3 PUFA and MPS.
7. Mechanism of n-3 PUFA on Skeletal Muscle Health
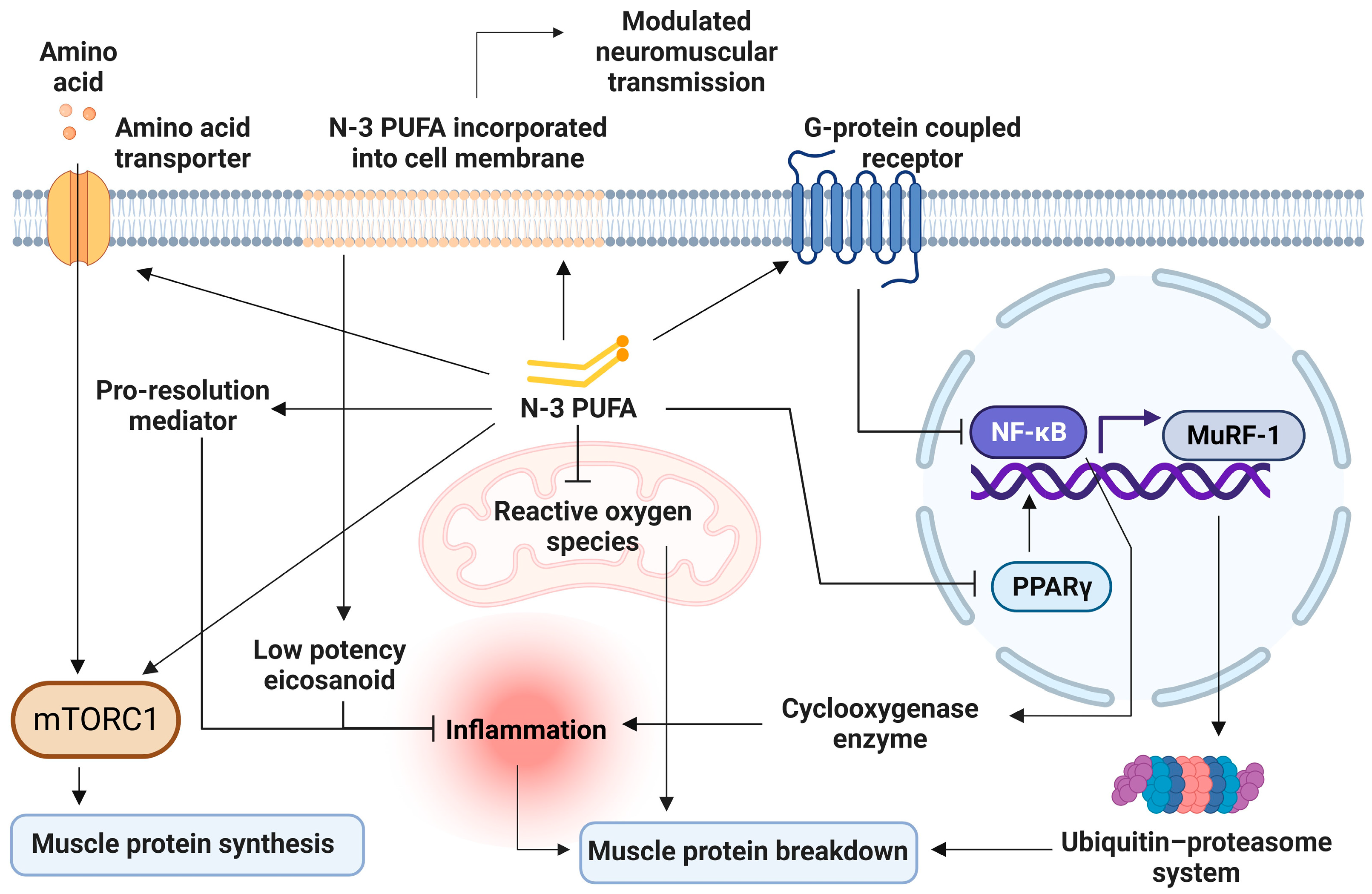
To date, meta-analysis on the mechanism of n-3 PUFA on skeletal muscle health remains scarce. RCTs in animals and human have suggested different fragments of entwining mechanisms on how n-3 PUFA can benefit skeletal muscle health and function, which are 1. Reducing inflammation, 2. Increasing MPS through mTORc1 pathway, 3. Decreasing MPB through ubiquitin-proteasome system (UPS) and autophagy lysosome system (ALS), 4. Improving mitochondrial function, 5. Increasing cellular amino acid transport, and 6. Optimizing membrane fluidity (Figure 2).
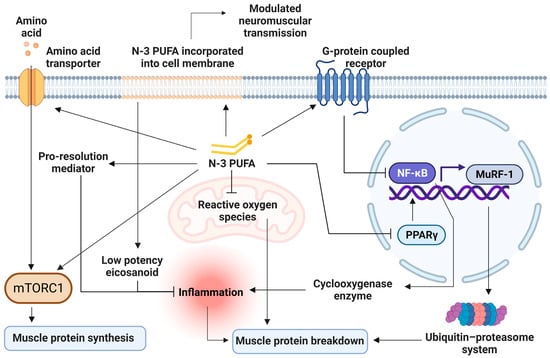
Figure 2.
Cellular mechanisms of n-3 PUFA on skeletal muscles. n-3 PUFA displaces n-6 PUFA on membranes, resulting in less inflammatory mediators production, and produces pro-resolution mediators. It also reduces cyclooxygenase production through the inhibition of nuclear factor kappa B (NF-κB). Decreased inflammation leads to decreased muscle protein breakdown. NF-κB inhibition also suppresses the ubiquitin-proteasome system through the downregulation of the muscle ring finger-1 (MuRF-1) gene. n-3 PUFA activates the mTORC-1 signaling pathway, stimulating muscle protein synthesis by phosphorylation of involving protein kinases. n-3 PUFA increases amino acid transporter expressions, allowing more substrates for protein synthesis. n-3 PUFA improves mitochondrial function and reduces free radicle production, which leads to muscle breakdown. mTORC-1 = Mechanistic target of rapamycin complex 1; MuRF-1 = muscle ring finger-1; NF-κB = Nuclear factor kappa B; n-3 PUFA = omega-3 polyunsaturated fatty acid; PPAR-γ = peroxisome proliferator-activated receptor gamma.
The most important mechanism is probably anti-inflammation [95]. Recently, an umbrella meta-analysis found reduced serum C-reactive protein (CRP), tumor necrosis factor α (TNFα), and IL-6 concentrations in healthy and diseased elderly participants receiving n-3 PUFA supplementation [96,97,98,99], with higher heterogeneity among middle-aged adults [27,66,80]. The anti-inflammatory effect of n-3 PUFA arose from EPA and DHA’s ability to displace arachidonic acid, a principal substrate for potent inflammatory mediators [100]. Twelve weeks of n-3 PUFA supplementation increases EPA and DHA components by approximately three folds in muscle cell membranes, while decreasing n-6 to n-3 ratios by 3 folds [101]. A higher n-6 to n-3 ratio in plasma was found to be associated with increased inflammatory markers such as CRP, IL-6, and TNFα [102]. EPA and DHA are metabolized into eicosanoids with lower inflammatory potency [22]. In addition, n-3 PUFA lessened cyclooxygenase-2 (COX-2) production via the inhibition of nuclear factor kappa B (NF-κB) [103] through the activation of peroxisome proliferator-activated receptor gamma (PPAR-γ) [104,105] and the stimulation of G-protein coupled receptor GPR120 [22,106]. The consumption of COX inhibitor was previously found to benefit skeletal muscle function [107], lower the risk of sarcopenia [108], and increase muscle fiber size in older adults [109]. However, due to its potential side effects, COX inhibitor was not prescribed for long-term use in the elderly [110]. The inhibition of NF-κB also reduces downstream stimulation of muscle ring finger-1 (MuRF-1) gene, which encodes for muscle-specific E3 ubiquitin ligase, the key enzyme of UPS, the main intracellular protein degradation machinery [65,111]. Furthermore, EPA and DHA are also substrates for pro-resolution mediators such as resolvins, protectins and maresins [112], which limit leukocyte trafficking, increase the clearance of inflammatory debris, and reduce inflammatory cytokine production [113]. While both EPA and DHA share anti-inflammatory effects, DHA has been found to suppress a wider array of inflammatory genes and cytokine responses compared to EPA in monocytes under chronic inflammation stimulation. However, their effects are similar in unstimulated conditions [114,115].
Another mechanism by which n-3 PUFA enhances skeletal muscle health is through protein kinase activation in the mTORC-1 pathway, the key pathway in muscle synthesis [116,117,118]. n-3 PUFA supplementation elevated focal adhesion kinase (FAK) expression [119], which in turn, activated the mTORC1 signaling pathway, initiating MPS [120]. Likewise, there was a 30–50% increase in MPS in response to anabolic stimuli along with a parallel increase of approximately 50% of mTORSer2448 concentrations and its downstream product in muscle cells after eight weeks of n-3 PUFA supplementation [23,24]. Microarray analyses from best responders to six months of n-3 PUFA supplementation showed decreased inhibition of the mTORC-1 signaling pathway compared to participants receiving a placebo [92].
n-3 PUFA decreases protein breakdown by UPS and ALS [121]. UPS is the major pathway in cellular protein breakdown, including myofibrillar proteins. The rate-limiting step of UPS in the muscles is the ubiquitination of two E3 ubiquitin ligases, muscle atrophy F-box (MAFbx) and MuRF1 [122]. EPA can reduce MuRF1 expression by preventing NF-κB nuclear binding, and finally suppressing muscle protein degradation [123,124]. DHA restricted UPS activity through the obstruction of proteasome catalytic sites by excessive loads of DHA-mediated oxidized proteins [125]. Six months of n-3 PUFA supplementation in older adults suppressed the expression of ubiquitin-mediated proteolysis from microarray analysis [92]. ALS is responsible for the degradation of protein complexes and malfunctioned organelles [121]. DHA was found to decrease the rate of autophagosome formation, and therefore, slow down ALS [126]. However, ALS operates at a basal rate during normal physiology, and its role in sarcopenia pathogenesis remains uncertain.
Many authors believe that when compared with DHA, EPA is responsible for improved muscle protein turnover [45,76]. Incubation with 50 micromolar EPA, but not DHA, has been shown to increase C2C12 myotubes protein synthesis and decrease protein breakdown in mice [127]. However, at higher doses (300–700 micromolar), DHA can more effectively decrease muscle protein breakdown [128]. It is noteworthy that in both studies, the dose is much higher than the doses typically used in clinical trials [45].
n-3 PUFA improves mitochondrial biogenesis and function by increasing glycolytic capacity, basal oxidative metabolism, oxygen consumption, total mitochondrial metabolism, and mitochondrial content in muscle cells [129,130,131]. An in vivo study showed that n-3 PUFA supplementation increases mitochondrial and peroxisomal density, in addition to peroxisome enzymes involved in fatty acid oxidation [131]. Older adults receiving n-3 PUFA showed significantly higher expressions of UCP3 and UQCRC1 which are the key components of the electron transport chain [92]. n-3 PUFA supplementation increases mitochondrial adenosine diphosphate sensitivity [132], leading to declined ROS production by 20–25% [133,134]. Reduced ROS emission with n-3 supplementation is in accordance with a higher rate of mitochondrial and sarcoplasmic protein synthesis [91]. It also decreases susceptibility to oxidative damage [132], which precipitates MPB [135]. Furthermore, n-3 PUFA supplementation maintains adenosine diphosphate-stimulated mitochondrial respiration and mitochondrial protein synthesis during leg immobilization, which would otherwise decrease during prolonged immobilization [90]. The finding implies the role of n-3 PUFA in preventing muscle disuse atrophy.
n-3 PUFA might improve cellular amino acid transport. Expression of L-amino acid transporter (LAT) and sodium-coupled neural amino acid transporter-2 was increased in pigs fed with diets rich in n-3 PUFA, enabling more substrate for MPS [136]. There was also a trend toward an increase in LAT-1 expression in young women receiving high dose n-3 PUFA during two weeks of leg immobilization with a slower decline of muscle mass compared to placebo (p = 0.06) [90].
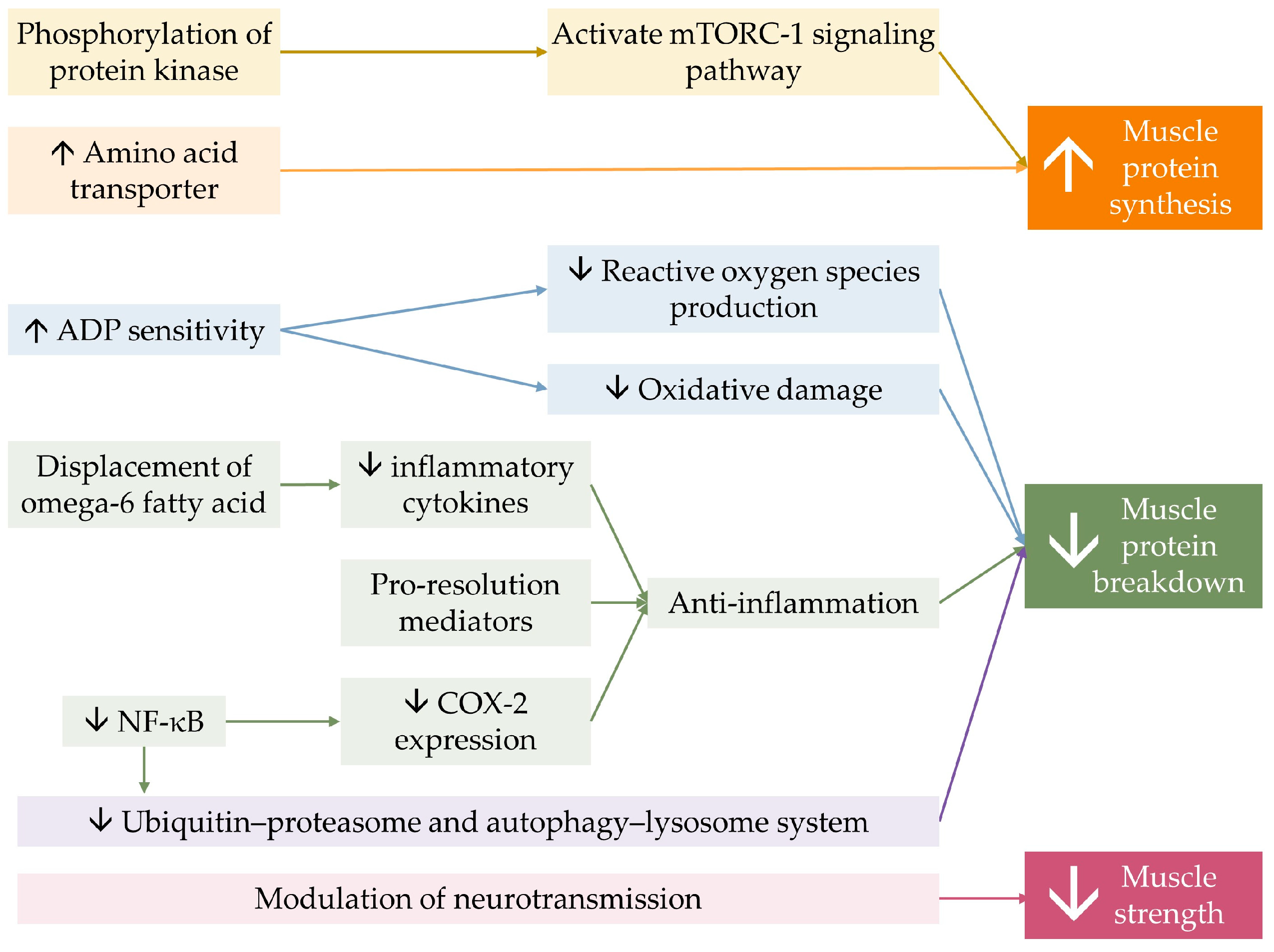
Lastly, optimizing membrane fluidity by n-3 PUFA, owing to its ability to incorporate into phospholipid bilayers of the cell membrane, could possibly influence the membrane’s property and flexibility, resulting in the modulation of neurotransmitter transmission [101,137,138]. This mechanism is believed to be attributable to DHA, which is present in higher concentrations than EPA in neuromuscular tissue [139]. n-3 PUFA supplementation combined with strength training was found to increase skeletal muscle activation levels and decrease electromechanical response time in older women, leading to greater improvement in muscle strength and functional capacity, thereby enhancing the effect of resistance training [81]. The benefit of n-3 PUFA on muscle contractility in response to neural stimuli was suspected to explain muscle strength improvement independent of muscle mass and volume [59,60,81]. Figure 3 demonstrates a conceptual diagram summarizing the effect of n-3 PUFA on musculoskeletal health.
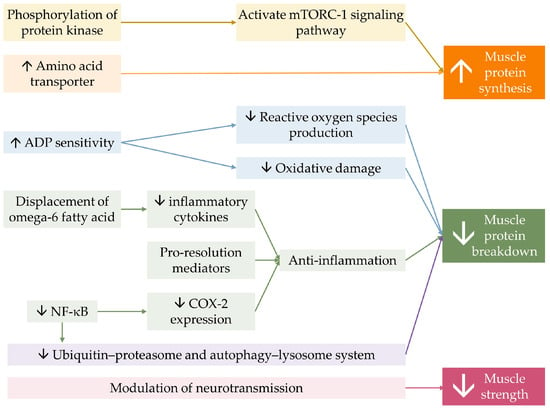
Figure 3.
Conceptual summary of the mechanism of n-3 PUFA on musculoskeletal health. n-3 PUFA activates mTORC-1 signaling pathway, by phosphorylation of involving protein kinases. It also increases the expression amino of acid transporter, allowing more substrates for protein synthesis. n-3 PUFA improves mitochondrial function by increasing mitochondrial Adenosine diphosphate (ADP) sensitivity. n-3 PUFA displaces omega-6 fatty acid on membranes, resulting in less production of inflammatory mediators, and produces pro-resolution mediators, constituting less muscle protein breakdown. Omega-3 reduces cyclooxygenase-2 (COX-2) expression through the inhibition of nuclear factor kappa B. n-3 PUFA directly decreases intracellular protein breakdown. Together, they result in a greater muscle quantity. Lastly, n-3 PUFA modulates neurotransmission, resulting in greater muscle strength. The ↑ symbol represents an increase, while the ↓ symbol represents a decrease.
8. Gap of Knowledge and Future Research Direction
Various clinical trials have attempted to explore the effect of n-3 PUFA supplementation on different aspects of musculoskeletal health. However, controversies and a large gap of knowledge still exist due to the heterogeneity of results which probably stem from different protocols of supplementation, and additional physical and nutritional intervention given alongside n-3 PUFA. There is a paucity of the data on the comparative effect of EPA versus DHA on musculoskeletal health and function since most clinical trials used combined EPA and DHA in varying proportions as their interventions without any trials directly comparing the effect of pure EPA versus pure DHA. More importantly, the difference in an individual’s response might lead to greatly varying results, especially when trials are pooled together.
Since n-3 PUFA is relatively safe and widely accessible, n-3 PUFA supplements might be a promising and cost-effective strategy for muscle preservation in the elderly. Future research focusing on how the effect of n-3 PUFA supplementation can be maximized will provide a more tangible clinical application, i.e., the most effective form and dose of n-PUFA, an optimal duration of supplementation, appropriate additional physical training, factors affecting the response to n-3 PUFA supplementation (i.e., age, gender, baseline EPA, DHA level), and conditions that will most benefit from the supplementation (healthy vs. with chronic muscle wasting diseases). The parameters used for the measurement of the effect of n-3 PUFA on muscles and physical function should be sensitive and reflect important health outcomes. Larger trials exploring the effect of n-3 PUFA on MPS should implement more homogeneous protocols of MPS measurement (i.e., the choice of amino acid tracer, the duration of measurement, and the presence of anabolic stimulation during measurement). Future rigorous meta-analyses focusing on each parameter of sarcopenia are needed. Sensitivity analyses and subgroup analyses based on the dose, duration, and types of n-3 PUFA supplementation, as well as demographic data such as gender and health status, should be performed to more clearly delineate the effect of n-3 PUFA supplementation. Meta-regression should also be done to identify the factors associated with greater benefit.
9. Conclusions
Sarcopenia can lead to various negative health outcomes. The diagnosis of sarcopenia relies on the reduction in muscle mass and muscle strength, with a decrease in physical function indicating severe sarcopenia. Compiling the most up-to-date evidence from meta-analyses and RCTs, this narrative review found the benefits of n-3 PUFA in various aspects of sarcopenia. n-3 PUFA supplementation provides small benefits in improving muscle mass with a possible dose-effect relationship. In general, the effect of n-3 PUFA supplementation on muscle strength was shown to be positive. Nonetheless, each parameter of muscle strength may respond differently. It also demonstrated pooled benefits on physical function, in particular walking speed and TUG test. However, the changes were too small to bear clinical significance. Longer duration of supplementation tends to be more favorable. n-3 PUFA may positively affect MPS response to anabolic stimuli. Mechanisms by which n-3 PUFA supplementation improves muscle health are 1. Anti-inflammation, 2. Increased expression of mTORC1 pathway, 3. Decreased UPS and ALS, 4. Enhanced mitochondrial biogenesis and function, 5. Improved amino acid transport, and 6. Improved neuromuscular junction activity. However, relevant trials differ profoundly in their research protocols, supplementation regimens, and outcome measurements, resulting in highly heterogeneous results. Robust meta-analyses with thorough subgroup analysis are required.
Author Contributions
Conceptualization, A.T. and M.I.; Methodology, A.T.; Writing—Original Draft Preparation, A.T.; Writing—Review & Editing, N.P.; Visualization, N.P.; Supervision, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This is a review paper. Data were utilized from open-access resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef]
- Harvey, N.C.; Orwoll, E.; Kwok, T.; Karlsson, M.K.; Rosengren, B.E.; Ribom, E.; Cauley, J.A.; Cawthon, P.M.; Ensrud, K.; Liu, E.; et al. Sarcopenia Definitions as Predictors of Fracture Risk Independent of FRAX(®), Falls, and BMD in the Osteoporotic Fractures in Men (MrOS) Study: A Meta-Analysis. J. Bone Miner. Res. 2021, 36, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 13, 3519. [Google Scholar] [CrossRef] [PubMed]
- Nagano, A.; Wakabayashi, H.; Maeda, K.; Kokura, Y.; Miyazaki, S.; Mori, T.; Fujiwara, D. Respiratory Sarcopenia and Sarcopenic Respiratory Disability: Concepts, Diagnosis, and Treatment. J. Nutr. Health Aging 2021, 25, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Beeri, M.S.; Leugrans, S.E.; Delbono, O.; Bennett, D.A.; Buchman, A.S. Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J. Am. Geriatr. Soc. 2021, 69, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Koyanagi, A.; Cereda, E.; Maggi, S.; Barbagallo, M.; Dominguez, L.J.; Smith, L. Sarcopenia reduces quality of life in the long-term: Longitudinal analyses from the English longitudinal study of ageing. Eur. Geriatr. Med. 2022, 13, 633–639. [Google Scholar] [CrossRef]
- Zhang, X.M.; Chen, D.; Xie, X.H.; Zhang, J.E.; Zeng, Y.; Cheng, A.S. Sarcopenia as a predictor of mortality among the critically ill in an intensive care unit: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 339. [Google Scholar] [CrossRef]
- Pinedo-Villanueva, R.; Westbury, L.D.; Syddall, H.E.; Sanchez-Santos, M.T.; Dennison, E.M.; Robinson, S.M.; Cooper, C. Health Care Costs Associated With Muscle Weakness: A UK Population-Based Estimate. Calcif. Tissue Int. 2019, 104, 137–144. [Google Scholar] [CrossRef]
- Steffl, M.; Sima, J.; Shiells, K.; Holmerova, I. The increase in health care costs associated with muscle weakness in older people without long-term illnesses in the Czech Republic: Results from the Survey of Health, Ageing and Retirement in Europe (SHARE). Clin. Interv. Aging 2017, 12, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
- Trommelen, J.; Betz, M.W.; van Loon, L.J.C. The Muscle Protein Synthetic Response to Meal Ingestion Following Resistance-Type Exercise. Sports Med. 2019, 49, 185–197. [Google Scholar] [CrossRef]
- Atherton, P.J.; Smith, K. Muscle protein synthesis in response to nutrition and exercise. J. Physiol. 2012, 590, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Curcio, F.; Testa, G.; Liguori, I.; Papillo, M.; Flocco, V.; Panicara, V.; Galizia, G.; Della-Morte, D.; Gargiulo, G.; Cacciatore, F.; et al. Sarcopenia and Heart Failure. Nutrients 2020, 12, 211. [Google Scholar] [CrossRef]
- Sancho-Muñoz, A.; Guitart, M.; Rodríguez, D.A.; Gea, J.; Martínez-Llorens, J.; Barreiro, E. Deficient muscle regeneration potential in sarcopenic COPD patients: Role of satellite cells. J. Cell. Physiol. 2021, 236, 3083–3098. [Google Scholar] [CrossRef]
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 2021, 34, 1347–1372. [Google Scholar] [CrossRef]
- Burd, N.A.; Gorissen, S.H.; van Loon, L.J. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport. Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Papadimitriou, K.; Voulgaridou, G.; Georgaki, E.; Tsotidou, E.; Zantidou, O.; Papandreou, D. Exercise and Nutrition Impact on Osteoporosis and Sarcopenia-The Incidence of Osteosarcopenia: A Narrative Review. Nutrients 2021, 13, 4499. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Rehabilitation Nutrition for Injury Recovery of Athletes: The Role of Macronutrient Intake. Nutrients 2020, 12, 2449. [Google Scholar] [CrossRef]
- Collado-Mateo, D.; Lavín-Pérez, A.M.; Peñacoba, C.; Del Coso, J.; Leyton-Román, M.; Luque-Casado, A.; Gasque, P.; Fernández-Del-Olmo, M.; Amado-Alonso, D. Key Factors Associated with Adherence to Physical Exercise in Patients with Chronic Diseases and Older Adults: An Umbrella Review. Int. J. Environ. Res. Public. Health 2021, 18, 2023. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Chen, Y.-C.; Tzeng, H.-P.; Chiang, M.-T. Fish oil enriched ω-3 fatty acids ameliorates protein synthesis/degradation imbalance, inflammation, and wasting in muscles of diet-induced obese rats. J. Funct. Foods 2021, 87, 104755. [Google Scholar] [CrossRef]
- You, J.S.; Park, M.N.; Song, W.; Lee, Y.S. Dietary fish oil alleviates soleus atrophy during immobilization in association with Akt signaling to p70s6k and E3 ubiquitin ligases in rats. Appl. Physiol. Nutr. Metab. 2010, 35, 310–318. [Google Scholar] [CrossRef]
- Da Boit, M.; Sibson, R.; Sivasubramaniam, S.; Meakin, J.R.; Greig, C.A.; Aspden, R.M.; Thies, F.; Jeromson, S.; Hamilton, D.L.; Speakman, J.R.; et al. Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 151–158. [Google Scholar] [CrossRef]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Sinha, S.; Haque, M.; Lugova, H.; Kumar, S. The Effect of Omega-3 Fatty Acids on Insulin Resistance. Life 2023, 13, 1322. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Nara, T.Y. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 145–150. [Google Scholar] [CrossRef]
- Bjerve, K.S.; Brubakk, A.M.; Fougner, K.J.; Johnsen, H.; Midthjell, K.; Vik, T. Omega-3 fatty acids: Essential fatty acids with important biological effects, and serum phospholipid fatty acids as markers of dietary omega 3-fatty acid intake. Am. J. Clin. Nutr. 1993, 57, 801S–805S, discussion 805S–806S. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.L.; Powers, S.; Napier, J.A.; Sayanova, O. Heterotrophic Production of Omega-3 Long-Chain Polyunsaturated Fatty Acids by Trophically Converted Marine Diatom Phaeodactylum tricornutum. Mar. Drugs 2016, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Stiefvatter, L.; Frick, K.; Lehnert, K.; Vetter, W.; Montoya-Arroyo, A.; Frank, J.; Schmid-Staiger, U.; Bischoff, S.C. Potentially Beneficial Effects on Healthy Aging by Supplementation of the EPA-Rich Microalgae Phaeodactylum tricornutum or Its Supernatant—A Randomized Controlled Pilot Trial in Elderly Individuals. Mar. Drugs 2022, 20, 716. [Google Scholar]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef]
- Rato, A.; Pereira, L.F.; Joaquim, S.; Gomes, R.; Afonso, C.; Cardoso, C.; Machado, J.; Gonçalves, J.F.M.; Vaz-Pires, P.; Magnoni, L.J.; et al. Fatty Acid Profile of Pacific Oyster, Crassostrea gigas, Fed Different Ratios of Dietary Seaweed and Microalgae during Broodstock Conditioning. Lipids 2019, 54, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.M.; Hallaråker, H.; Sæbø, P.C.; Innis, S.M.; Kelley, K.M.; Sanoshy, K.D.; Berger, A.; Maki, K.C. Bioavailability of long chain omega-3 polyunsaturated fatty acids from phospholipid-rich herring roe oil in men and women with mildly elevated triacylglycerols. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.K.; Bowen, K.J.; Mozaffarian, D.; Kris-Etherton, P.M.; Skulas-Ray, A.C. Total Long-Chain n-3 Fatty Acid Intake and Food Sources in the United States Compared to Recommended Intakes: NHANES 2003-2008. Lipids 2017, 52, 917–927. [Google Scholar] [CrossRef]
- Gray, S.R.; Mittendorfer, B. Fish oil-derived n-3 polyunsaturated fatty acids for the prevention and treatment of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 104–109. [Google Scholar] [CrossRef]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef]
- Gao, K.; Chen, L.; Yang, M.; Han, L.; Yiguang, S.; Zhao, H.; Chen, X.; Hu, W.; Liang, H.; Luo, J.; et al. Marine n-3 PUFA protects hearts from I/R injury via restoration of mitochondrial function. Scand. Cardiovasc. J. 2015, 49, 264–269. [Google Scholar] [CrossRef]
- Gray, B.; Steyn, F.; Davies, P.S.; Vitetta, L. Omega-3 fatty acids: A review of the effects on adiponectin and leptin and potential implications for obesity management. Eur. J. Clin. Nutr. 2013, 67, 1234–1242. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Turnover in Health, Disuse, and Disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Chiu, W.C.; Hsu, Y.P.; Lo, Y.L.; Wang, Y.H. Effects of Omega-3 Fatty Acids on Muscle Mass, Muscle Strength and Muscle Performance among the Elderly: A Meta-Analysis. Nutrients 2020, 12, 3739. [Google Scholar] [CrossRef] [PubMed]
- Cornish, S.M.; Chilibeck, P.D. Alpha-linolenic acid supplementation and resistance training in older adults. Appl. Physiol. Nutr. Metab. 2009, 34, 49–59. [Google Scholar] [CrossRef]
- Edholm, P.; Strandberg, E.; Kadi, F. Lower limb explosive strength capacity in elderly women: Effects of resistance training and healthy diet. J. Appl. Physiol. 2017, 123, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, E.; Edholm, P.; Ponsot, E.; Wåhlin-Larsson, B.; Hellmén, E.; Nilsson, A.; Engfeldt, P.; Cederholm, T.; Risérus, U.; Kadi, F. Influence of combined resistance training and healthy diet on muscle mass in healthy elderly women: A randomized controlled trial. J. Appl. Physiol. 2015, 119, 918–925. [Google Scholar] [CrossRef]
- Logan, S.L.; Spriet, L.L. Omega-3 Fatty Acid Supplementation for 12 Weeks Increases Resting and Exercise Metabolic Rate in Healthy Community-Dwelling Older Females. PLoS ONE 2015, 10, e0144828. [Google Scholar] [CrossRef]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 2011, 117, 1775–1782. [Google Scholar] [CrossRef]
- Bird, J.K.; Troesch, B.; Warnke, I.; Calder, P.C. The effect of long chain omega-3 polyunsaturated fatty acids on muscle mass and function in sarcopenia: A scoping systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 46, 73–86. [Google Scholar] [CrossRef]
- Aredes, M.A.; da Camara, A.O.; de Paula, N.S.; Fraga, K.Y.D.; do Carmo, M.; Chaves, G.V. Efficacy of ω-3 supplementation on nutritional status, skeletal muscle, and chemoradiotherapy toxicity in cervical cancer patients: A randomized, triple-blind, clinical trial conducted in a middle-income country. Nutrition 2019, 67–68, 110528. [Google Scholar] [CrossRef]
- Akita, H.; Takahashi, H.; Asukai, K.; Tomokuni, A.; Wada, H.; Marukawa, S.; Yamasaki, T.; Yanagimoto, Y.; Takahashi, Y.; Sugimura, K.; et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: Prospective randomized control study. Clin. Nutr. ESPEN 2019, 33, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, T.; Marui, S.; Miura, E.; Sugiura, M.; Matsuyama, W.; Aoshima, Y.; Kasamatsu, N.; Ogiku, M.; Ikematsu, Y. Effect of eicosapentaenoic acid on prevention of lean body mass depletion in patients with exacerbation of chronic obstructive pulmonary disease: A prospective randomized controlled trial. Clin. Nutr. ESPEN 2018, 28, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Laviano, A.; Lonnqvist, F.; Muscaritoli, M.; Öhlander, M.; Schols, A. Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: A randomized, controlled trial. J. Cachexia Sarcopenia Muscle 2018, 9, 28–40. [Google Scholar] [CrossRef]
- Bakker, N.; van den Helder, R.S.; Geenen, R.W.F.; Hunfeld, M.A.; Cense, H.A.; Demirkiran, A.; Houdijk, A.P.J. Four Weeks of Preoperative Omega-3 Fatty Acids Reduce Liver Volume: A Randomised Controlled Trial. Obes. Surg. 2019, 29, 2037–2044. [Google Scholar] [CrossRef]
- Ritchie, A.I.; Wedzicha, J.A. Definition, Causes, Pathogenesis, and Consequences of Chronic Obstructive Pulmonary Disease Exacerbations. Clin. Chest Med. 2020, 41, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Cornish, S.M.; Cordingley, D.M.; Shaw, K.A.; Forbes, S.C.; Leonhardt, T.; Bristol, A.; Candow, D.G.; Chilibeck, P.D. Effects of Omega-3 Supplementation Alone and Combined with Resistance Exercise on Skeletal Muscle in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2221. [Google Scholar] [CrossRef]
- Dalle, S.; Van Roie, E.; Hiroux, C.; Vanmunster, M.; Coudyzer, W.; Suhr, F.; Bogaerts, S.; Van Thienen, R.; Koppo, K. Omega-3 Supplementation Improves Isometric Strength But Not Muscle Anabolic and Catabolic Signaling in Response to Resistance Exercise in Healthy Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 406–414. [Google Scholar] [CrossRef]
- Kunz, H.E.; Michie, K.L.; Gries, K.J.; Zhang, X.; Ryan, Z.C.; Lanza, I.R. A Randomized Trial of the Effects of Dietary n3-PUFAs on Skeletal Muscle Function and Acute Exercise Response in Healthy Older Adults. Nutrients 2022, 14, 3537. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Lee, S.E.K.; Lira, C.A.B.; Nouailhetas, V.L.A.; Vancini, R.L.; Andrade, M.S. Do isometric, isotonic and/or isokinetic strength trainings produce different strength outcomes? J. Bodyw. Mov. Ther. 2018, 22, 430–437. [Google Scholar] [CrossRef]
- Guilhem, G.; Cornu, C.; Guével, A. Muscle architecture and EMG activity changes during isotonic and isokinetic eccentric exercises. Eur. J. Appl. Physiol. 2011, 111, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Frantz, J.D.; Tawa, N.E., Jr.; Melendez, P.A.; Oh, B.C.; Lidov, H.G.; Hasselgren, P.O.; Frontera, W.R.; Lee, J.; Glass, D.J.; et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Hutchins-Wiese, H.L.; Kleppinger, A.; Annis, K.; Liva, E.; Lammi-Keefe, C.J.; Durham, H.A.; Kenny, A.M. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J. Nutr. Health Aging 2013, 17, 76–80. [Google Scholar] [CrossRef] [PubMed]
- van der Meij, B.S.; Langius, J.A.; Smit, E.F.; Spreeuwenberg, M.D.; von Blomberg, B.M.; Heijboer, A.C.; Paul, M.A.; van Leeuwen, P.A. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J. Nutr. 2010, 140, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Hossain, T.; Phillips, B.E.; Doleman, B.; Lund, J.N.; Williams, J.P. A double-blind randomized controlled trial of the effects of eicosapentaenoic acid supplementation on muscle inflammation and physical function in patients undergoing colorectal cancer resection. Clin. Nutr. 2020, 39, 2055–2061. [Google Scholar] [CrossRef]
- Rolland, Y.; Barreto, P.S.; Maltais, M.; Guyonnet, S.; Cantet, C.; Andrieu, S.; Vellas, B. Effect of Long-Term Omega 3 Polyunsaturated Fatty Acid Supplementation with or without Multidomain Lifestyle Intervention on Muscle Strength in Older Adults: Secondary Analysis of the Multidomain Alzheimer Preventive Trial (MAPT). Nutrients 2019, 11, 1931. [Google Scholar] [CrossRef]
- Nilsson, M.I.; Mikhail, A.; Lan, L.; Di Carlo, A.; Hamilton, B.; Barnard, K.; Hettinga, B.P.; Hatcher, E.; Tarnopolsky, M.G.; Nederveen, J.P.; et al. A Five-Ingredient Nutritional Supplement and Home-Based Resistance Exercise Improve Lean Mass and Strength in Free-Living Elderly. Nutrients 2020, 12, 2391. [Google Scholar] [CrossRef]
- Fietkau, R.; Lewitzki, V.; Kuhnt, T.; Hölscher, T.; Hess, C.F.; Berger, B.; Wiegel, T.; Rödel, C.; Niewald, M.; Hermann, R.M.; et al. A disease-specific enteral nutrition formula improves nutritional status and functional performance in patients with head and neck and esophageal cancer undergoing chemoradiotherapy: Results of a randomized, controlled, multicenter trial. Cancer 2013, 119, 3343–3353. [Google Scholar] [CrossRef]
- Strike, S.C.; Carlisle, A.; Gibson, E.L.; Dyall, S.C. A High Omega-3 Fatty Acid Multinutrient Supplement Benefits Cognition and Mobility in Older Women: A Randomized, Double-blind, Placebo-controlled Pilot Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 236–242. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Hooper, L.; Sivakaran, R.; Hayhoe, R.P.G.; Welch, A. The Relationship Between Omega-3, Omega-6 and Total Polyunsaturated Fat and Musculoskeletal Health and Functional Status in Adults: A Systematic Review and Meta-analysis of RCTs. Calcif. Tissue Int. 2019, 105, 353–372. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.C.; Coetzer, H.; de Winter, R.; Gericke, G.; van Papendorp, D.H. Calcium, gamma-linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging 1998, 10, 385–394. [Google Scholar] [CrossRef]
- Engelen, M.; Jonker, R.; Sulaiman, H.; Fisk, H.L.; Calder, P.C.; Deutz, N.E.P. ω-3 polyunsaturated fatty acid supplementation improves postabsorptive and prandial protein metabolism in patients with chronic obstructive pulmonary disease: A randomized clinical trial. Am. J. Clin. Nutr. 2022, 116, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Haß, U.; Kochlik, B.; Herpich, C.; Rudloff, S.; Norman, K. Effects of an Omega-3 Supplemented, High-Protein Diet in Combination with Vibration and Resistance Exercise on Muscle Power and Inflammation in Old Adults: A Pilot Randomized Controlled Trial. Nutrients 2022, 14, 4274. [Google Scholar] [CrossRef] [PubMed]
- Landen, S.; Hiam, D.; Voisin, S.; Jacques, M.; Lamon, S.; Eynon, N. Physiological and molecular sex differences in human skeletal muscle in response to exercise training. J. Physiol. 2023, 601, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Murphy, C.H.; Flanagan, E.M.; De Vito, G.; Susta, D.; Mitchelson, K.A.J.; Castro, E.d.M.; Senden, J.M.G.; Goessens, J.P.B.; Miklosz, A.; Chabowski, A.; et al. Does supplementation with leucine-enriched protein alone and in combination with fish-oil-derived n-3 PUFA affect muscle mass, strength, physical performance, and muscle protein synthesis in well-nourished older adults? A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2021, 113, 1411–1427. [Google Scholar] [CrossRef]
- Cornish, S.M.; Myrie, S.B.; Bugera, E.M.; Chase, J.E.; Turczyn, D.; Pinder, M. Omega-3 supplementation with resistance training does not improve body composition or lower biomarkers of inflammation more so than resistance training alone in older men. Nutr. Res. 2018, 60, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Rodacki, C.L.; Rodacki, A.L.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef]
- Abellan van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef]
- Ascencio, E.J.; Cieza-Gómez, G.D.; Carrillo-Larco, R.M.; Ortiz, P.J. Timed up and go test predicts mortality in older adults in Peru: A population-based cohort study. BMC Geriatr. 2022, 22, 61. [Google Scholar] [CrossRef]
- Albarrati, A.M.; Gale, N.S.; Munnery, M.M.; Reid, N.; Cockcroft, J.R.; Shale, D.J. The Timed Up and Go test predicts frailty in patients with COPD. NPJ Prim. Care Respir. Med. 2022, 32, 24. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R.; Kim, I.-Y.; Park, S.; Ferrando, A. Tracing metabolic flux to assess optimal dietary protein and amino acid consumption. Exp. Mol. Med. 2022, 54, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Chinkes, D.L.; Wolfe, R.R. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E753–E764. [Google Scholar] [CrossRef]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef]
- Brook, M.S.; Din, U.; Tarum, J.; Selby, A.; Quinlan, J.; Bass, J.J.; Gharahdaghi, N.; Boereboom, C.; Abdulla, H.; Franchi, M.V.; et al. Omega-3 supplementation during unilateral resistance exercise training in older women: A within subject and double-blind placebo-controlled trial. Clin. Nutr. Espen 2021, 46, 394–404. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Small, A.C.; Greig, C.A.; Husi, H.; Ross, J.A.; Stephens, N.A.; Fearon, K.C.; Preston, T. A novel oral tracer procedure for measurement of habitual myofibrillar protein synthesis. Rapid Commun. Mass. Spectrom. 2013, 27, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- McGlory, C.; Gorissen, S.H.M.; Kamal, M.; Bahniwal, R.; Hector, A.J.; Baker, S.K.; Chabowski, A.; Phillips, S.M. Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 2019, 33, 4586–4597. [Google Scholar] [CrossRef] [PubMed]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging 2017, 9, 1096–1129. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Smith, G.I.; Kelly, S.C.; Julliand, S.; Reeds, D.N.; Mittendorfer, B. Effect of dietary n-3 PUFA supplementation on the muscle transcriptome in older adults. Physiol. Rep. 2016, 4, e12785. [Google Scholar] [CrossRef] [PubMed]
- McGlory, C.; Wardle, S.L.; Macnaughton, L.S.; Witard, O.C.; Scott, F.; Dick, J.; Bell, J.G.; Phillips, S.M.; Galloway, S.D.R.; Hamilton, D.L.; et al. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol. Rep. 2016, 4, e12715. [Google Scholar] [CrossRef]
- Deger, S.M.; Hung, A.M.; Ellis, C.D.; Booker, C.; Bian, A.; Chen, G.; Abumrad, N.N.; Ikizler, T.A. High Dose Omega-3 Fatty Acid Administration and Skeletal Muscle Protein Turnover in Maintenance Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2016, 11, 1227–1235. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Kavyani, Z.; Musazadeh, V.; Fathi, S.; Hossein Faghfouri, A.; Dehghan, P.; Sarmadi, B. Efficacy of the omega-3 fatty acids supplementation on inflammatory biomarkers: An umbrella meta-analysis. Int. Immunopharmacol. 2022, 111, 109104. [Google Scholar] [CrossRef]
- Lin, N.; Shi, J.-j.; Li, Y.-M.; Zhang, X.-Y.; Chen, Y.; Calder, P.C.; Tang, L.-J. What is the impact of n-3 PUFAs on inflammation markers in Type 2 diabetic mellitus populations: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2016, 15, 133. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, B.; Li, X.; Hui, H.; Zhou, Y.; Li, N.; Xie, X. N-3 Polyunsaturated Fatty Acids can Reduce IL-6 and TNF Levels in Patients with Cancer. Br. J. Nutr. 2022, 129, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.; Couture, P.; Leclerc, M.; Charest, A.; Marin, J.; Lépine, M.C.; Talbot, D.; Tchernof, A.; Lamarche, B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED) Study. Am. J. Clin. Nutr. 2016, 104, 280–287. [Google Scholar] [CrossRef]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef]
- Gerling, C.J.; Mukai, K.; Chabowski, A.; Heigenhauser, G.J.F.; Holloway, G.P.; Spriet, L.L.; Jannas-Vela, S. Incorporation of Omega-3 Fatty Acids Into Human Skeletal Muscle Sarcolemmal and Mitochondrial Membranes Following 12 Weeks of Fish Oil Supplementation. Front. Physiol. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, N.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Rousinou, G.; Toutouza, M.; Stefanadis, C. Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin. Chim. Acta 2010, 411, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Habib, A.; Lubrano, L.; Del Turco, S.; Lazzerini, G.; Bourcier, T.; Weksler, B.B.; De Caterina, R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc. Natl. Acad. Sci. USA 2006, 103, 15184–15189. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Tsukamoto, I. n-3 polyunsaturated fatty acids stimulate osteoclastogenesis through PPARγ-mediated enhancement of c-Fos expression, and suppress osteoclastogenesis through PPARγ-dependent inhibition of NFkB activation. J. Nutr. Biochem. 2015, 26, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Krey, G.; Braissant, O.; L’Horset, F.; Kalkhoven, E.; Perroud, M.; Parker, M.G.; Wahli, W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol. Endocrinol. 1997, 11, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Fountain, W.A.; Naruse, M.; Claiborne, A.; Stroh, A.M.; Gries, K.J.; Jones, A.M.; Minchev, K.; Lester, B.E.; Raue, U.; Trappe, S.; et al. Low-dose aspirin and COX inhibition in human skeletal muscle. J. Appl. Physiol. 2020, 129, 1477–1482. [Google Scholar] [CrossRef]
- Landi, F.; Cruz-Jentoft, A.J.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilSIRENTE study. Age Ageing 2013, 42, 203–209. [Google Scholar] [CrossRef]
- Trappe, T.A.; Ratchford, S.M.; Brower, B.E.; Liu, S.Z.; Lavin, K.M.; Carroll, C.C.; Jemiolo, B.; Trappe, S.W. COX Inhibitor Influence on Skeletal Muscle Fiber Size and Metabolic Adaptations to Resistance Exercise in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1289–1294. [Google Scholar] [CrossRef]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Kitajima, Y.; Yoshioka, K.; Suzuki, N. The ubiquitin-proteasome system in regulation of the skeletal muscle homeostasis and atrophy: From basic science to disorders. J. Physiol. Sci. 2020, 70, 40. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef]
- So, J.; Wu, D.; Lichtenstein, A.H.; Tai, A.K.; Matthan, N.R.; Maddipati, K.R.; Lamon-Fava, S. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: A randomized, double-blind, crossover study. Atherosclerosis 2021, 316, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Vors, C.; Allaire, J.; Marin, J.; Lépine, M.C.; Charest, A.; Tchernof, A.; Couture, P.; Lamarche, B. Inflammatory gene expression in whole blood cells after EPA vs. DHA supplementation: Results from the ComparED study. Atherosclerosis 2017, 257, 116–122. [Google Scholar] [CrossRef]
- Gingras, A.A.; White, P.J.; Chouinard, P.Y.; Julien, P.; Davis, T.A.; Dombrowski, L.; Couture, Y.; Dubreuil, P.; Myre, A.; Bergeron, K.; et al. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J. Physiol. 2007, 579, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Rabanal-Ruiz, Y.; Korolchuk, V.I. mTORC1 and Nutrient Homeostasis: The Central Role of the Lysosome. Int. J. Mol. Sci. 2018, 19, 818. [Google Scholar] [CrossRef]
- Kamolrat, T.; Gray, S.R.; Thivierge, M.C. Fish oil positively regulates anabolic signalling alongside an increase in whole-body gluconeogenesis in ageing skeletal muscle. Eur. J. Nutr. 2013, 52, 647–657. [Google Scholar] [CrossRef]
- McGlory, C.; Galloway, S.D.; Hamilton, D.L.; McClintock, C.; Breen, L.; Dick, J.R.; Bell, J.G.; Tipton, K.D. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot. Essent. Fatty Acids 2014, 90, 199–206. [Google Scholar] [CrossRef]
- Rindom, E.; Vissing, K. Mechanosensitive Molecular Networks Involved in Transducing Resistance Exercise-Signals into Muscle Protein Accretion. Front. Physiol. 2016, 7, 547. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeon, J.H.; Lee, M.J. Docosahexaenoic Acid, a Potential Treatment for Sarcopenia, Modulates the Ubiquitin-Proteasome and the Autophagy-Lysosome Systems. Nutrients 2020, 12, 2597. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef]
- Khal, J.; Tisdale, M.J. Downregulation of muscle protein degradation in sepsis by eicosapentaenoic acid (EPA). Biochem. Biophys. Res. Commun. 2008, 375, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, A.S.; Tisdale, M.J. Downregulation of ubiquitin-dependent proteolysis by eicosapentaenoic acid in acute starvation. Biochem. Biophys. Res. Commun. 2001, 285, 598–602. [Google Scholar] [CrossRef]
- Shin, S.K.; Kim, J.H.; Lee, J.H.; Son, Y.H.; Lee, M.W.; Kim, H.J.; Noh, S.A.; Kim, K.P.; Kim, I.G.; Lee, M.J. Docosahexaenoic acid-mediated protein aggregates may reduce proteasome activity and delay myotube degradation during muscle atrophy in vitro. Exp. Mol. Med. 2017, 49, e287. [Google Scholar] [CrossRef]
- Woodworth-Hobbs, M.E.; Hudson, M.B.; Rahnert, J.A.; Zheng, B.; Franch, H.A.; Price, S.R. Docosahexaenoic acid prevents palmitate-induced activation of proteolytic systems in C2C12 myotubes. J. Nutr. Biochem. 2014, 25, 868–874. [Google Scholar] [CrossRef]
- Kamolrat, T.; Gray, S.R. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem. Biophys. Res. Commun. 2013, 432, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Q.W.; Zheng, P.P.; Zhang, J.S.; Huang, F.R. DHA inhibits protein degradation more efficiently than EPA by regulating the PPARγ/NFκB pathway in C2C12 myotubes. Biomed. Res. Int. 2013, 2013, 318981. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Conn, C.A.; Trujillo, K.A. Conjugated linoleic acid or omega 3 fatty acids increase mitochondrial biosynthesis and metabolism in skeletal muscle cells. Lipids Health Dis. 2012, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.R.; Nabavi, S.F.; Nabavi, S.M.; Jardim, F.R. Omega-3 polyunsaturated fatty acids and mitochondria, back to the future. Trends Food Sci. Technol. 2017, 67, 76–92. [Google Scholar] [CrossRef]
- Neschen, S.; Moore, I.; Regittnig, W.; Yu, C.L.; Wang, Y.; Pypaert, M.; Petersen, K.F.; Shulman, G.I. Contrasting effects of fish oil and safflower oil on hepatic peroxisomal and tissue lipid content. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E395–E401. [Google Scholar] [CrossRef]
- Herbst, E.A.; Paglialunga, S.; Gerling, C.; Whitfield, J.; Mukai, K.; Chabowski, A.; Heigenhauser, G.J.; Spriet, L.L.; Holloway, G.P. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J. Physiol. 2014, 592, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Pharaoh, G.; Brown, J.; Ranjit, R.; Ungvari, Z.; Van Remmen, H. Reduced adenosine diphosphate sensitivity in skeletal muscle mitochondria increases reactive oxygen species production in mouse models of aging and oxidative stress but not denervation. JCSM Rapid Commun. 2021, 4, 75–89. [Google Scholar] [CrossRef]
- Miotto, P.M.; McGlory, C.; Bahniwal, R.; Kamal, M.; Phillips, S.M.; Holloway, G.P. Supplementation with dietary ω-3 mitigates immobilization-induced reductions in skeletal muscle mitochondrial respiration in young women. FASEB J. 2019, 33, 8232–8240. [Google Scholar] [CrossRef]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Duan, Y.; Li, Y.; Tang, Y.; Geng, M.; Oladele, O.A.; Kim, S.W.; Yin, Y. Effects of dietary n-6:n-3 PUFA ratio on fatty acid composition, free amino acid profile and gene expression of transporters in finishing pigs. Br. J. Nutr. 2015, 113, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Dietary (n-3) Fatty Acids and Brain Development12. J. Nutrition 2007, 137, 855–859. [Google Scholar] [CrossRef]
- Salem, N., Jr.; Litman, B.; Kim, H.Y.; Gawrisch, K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef]
- Igarashi, M.; Ma, K.; Gao, F.; Kim, H.W.; Greenstein, D.; Rapoport, S.I.; Rao, J.S. Brain lipid concentrations in bipolar disorder. J. Psychiatr. Res. 2010, 44, 177–182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).