Abstract
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia due to impaired insulin secretion, insulin resistance, or both. Oxidative stress and chronic low-grade inflammation play crucial roles in the pathophysiology of diabetes mellitus. There has been a growing interest in applying natural products to improve metabolic derangements without the side effects of anti-diabetic drugs. Microalgae biomass or extract and their bioactive compounds have been applied as nutraceuticals or additives in food products and health supplements. Several studies have demonstrated the therapeutic effects of microalgae and their bioactive compounds in improving insulin sensitivity attributed to their antioxidant, anti-inflammatory, and pancreatic β-cell protective properties. However, a review summarizing the progression in this topic is lacking despite the increasing number of studies reporting their anti-diabetic potential. In this review, we gathered the findings from in vitro, in vivo, and human studies to discuss the effects of microalgae and their bioactive compounds on diabetes mellitus and the mechanisms involved. Additionally, we discuss the limitations and future perspectives of developing microalgae-based compounds as a health supplement for diabetes mellitus. In conclusion, microalgae-based supplementation has the potential to improve diabetes mellitus and be applied in more clinical studies in the future.
1. Introduction
Diabetes mellitus is a metabolic disorder characterized by high blood sugar levels or hyperglycemia that is caused by problems with insulin secretion, insulin action, or both and it is related to carbohydrate, lipid, and protein metabolism derangements [1,2,3]. Diabetes mellitus can be categorized into type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), and gestational diabetes [4,5]. T1DM is an autoimmune disease in which the insulin-producing pancreatic β-cells are damaged resulting in the lack of insulin production [5]. T2DM, which is the most common type of diabetes mellitus, is associated with insulin resistance and impaired insulin production. Genetic risk factors and poor lifestyle, such as physical inactivity and unhealthy diet, impose the risk of developing T2DM [4,6]. Chronic hyperglycemia induces oxidative stress that promotes the destruction of insulin-producing pancreatic β-cells, worsening hyperglycemia. Moreover, chronic low-grade inflammation contributes to the impairment in insulin signaling, thus leading to insulin resistance. Ultimately, the interplay between these factors results in a vicious cycle of hyperglycemia and progressive destruction of pancreatic β-cells.
A study from 2019 reported that around 463 million people within the age range of 20–79 had diabetes mellitus and the prevalence was predicted to increase to 578 million and 700 million in 2030 and 2045, respectively [7]. This would negatively affect the individual’s quality of life and impose an economic burden worldwide since diabetes mellitus is one of the leading causes of premature mortality [8]. Currently, oral medication and insulin therapy are some of the strategies to manage diabetes mellitus but side effects, such as low blood glucose and gastrointestinal problems, have been reported [7,9,10]. Additionally, adopting a healthier lifestyle to improve one’s condition can be difficult; therefore, adherence to lifestyle modifications may decline over time [11,12]. Novel sources of bioactive substances that could alleviate diabetes mellitus with fewer side effects have gained traction over the years [9,13], but further exploration is needed.
Microalgae, which include eukaryotic microalgae and prokaryotic cyanobacteria, are microscopic, unicellular, photosynthetic algae that may thrive in freshwater or saltwater [14,15]. The increased need for functional foods to improve health has led to the incorporation of microalgae into the diet [16]. In that context, microalgae biomass and their bioactive compounds have been added as ingredients in cookies, bread, mayonnaise, ice cream, soft cheeses, smoothies, olive oil, and meat products. Moreover, microalgae biomass and bioactive compound extracts are commercially available as health supplements. For example, Chlorella vulgaris and Arthrospira plantensis biomass, as well as astaxanthin from Haematococcus pluvialis and DHA extracted from Schizochytrium sp. [17]. Microalgae biomass and their bioactive compounds, such as polyunsaturated fatty acids (PUFAs), pigments, amino acids, peptides, scytonemins, pterins and phenolic compounds, have been shown to demonstrate a wide range of therapeutic effects [18]. Importantly, some of the microalgae and bioactive compounds exhibit antioxidant, anti-inflammatory, and immune-modulatory properties, and improve insulin sensitivity that could potentially alleviate diabetes mellitus [18,19,20,21]. Table 1 summarizes the bioactive compounds present in various sources that have been particularly identified in certain microalgae species and their activity on diabetes mellitus. Nevertheless, the FDA has not approved any microalgae-based products for treating or managing diabetes mellitus [22]. Therefore, clinical studies and more research are needed to confirm the anti-diabetic potential of microalgae or their bioactive compounds.

Table 1.
Microalgae-derived bioactive compounds and their effects on diabetes mellitus.
Despite the challenge, research on the anti-diabetic activity of microalgae and their bioactive compounds is increasing and their potential as health supplements for diabetes mellitus is gaining traction; however, a review summarizing the progression in this topic is lacking. Therefore, we summarized the information from in vitro, in vivo, and clinical studies investigating the anti-diabetic activity of microalgae and the bioactive compounds derived from them while explaining the mechanisms involved in producing this effect. This paper also includes the limitations and future perspectives of developing microalgae-based health supplements in managing diabetes mellitus.
2. Effect of Microalgae on Diabetes Mellitus
2.1. In Vitro Study
Investigating the anti-diabetic effects of microalgae or their bioactive compounds in an in vitro setting is mainly focused on determining their ability to inhibit digestive enzymes that are associated with postprandial hyperglycemia or modulate enzymes or transcription factors involved in glucose metabolism (Table 2).

Table 2.
The effects of microalgae and its bioactive compounds in the in vitro studies.
2.1.1. Microalgae Extracts
Arthrospira platensis, Nannochloropsis oculata, Porphyridium purpureum, and Chlorella vulgaris pose potential anti-diabetic properties by inhibiting diabetic-related enzymes and alleviating oxidative stress [79]. In that context, a study reported that these microalgae extracts exhibited α-amylase inhibition [79]. One of the treatment strategies for reducing postprandial hyperglycemia is to inhibit enzymes like α-amylase and β-glucosidase to delay the absorption of glucose and control postprandial blood glucose levels [135]. In addition, it is worth noticing that every species demonstrated antioxidant potential comprising one or two free radical scavenging mechanisms; although the findings from an experiment comparing the antioxidant activities of these microalgae extracts concluded that the results vary depending on the type of assay performed. This reveals that these microalgae do not necessarily have the same underlying mechanisms in scavenging free radicals but they could alleviate oxidative stress.
The author suggested that the polyphenolic and carotenoid contents of the microalgae extracts contributed to these potential anti-diabetic properties mainly due to their antioxidant, anti-inflammatory properties, and anti-apoptotic properties [21]. Phytochemical compositional analysis revealed that all these microalgae extracts had substantial amounts of total phenolic, carotenoid, chlorophyll, and triterpenoid content. In detail, P. purpureum contained the highest amount of phenolic content but was limited to flavan-3-ols, whereas A. platensis had the most diverse phenolic content, such as flavan-3-ols, phenolic acids, and flavonols. Furthermore, P. purpureum and N. oculata had a substantial amount of carotenoids. For instance, P. purpureum contained carotenoids, such as zeaxanthin, β-carotene, and β-cryptoxanthin. As for the total chlorophyll content, A. platensis had the highest chlorophyll content followed by N. oculata, C. vulgaris, and P. purpureum. As for the triterpenoid content, A. platensis had the highest total content which was markedly higher compared to the other species.
Additionally, another in vitro study to measure the inflammation biomarker myeloperoxidase (MPO) release from human neutrophils revealed that A. platensis decreased the MPO release from human neutrophils in a dose-dependent manner [136]. MPO is involved in inflammation and oxidative stress, and its level is correlated to diet-induced insulin resistance and obesity [137]. Therefore, the results from this study further confirm that A. platensis exerts anti-inflammatory properties in alleviating hyperglycemia from an in vitro standpoint. A study comparing glucosidase inhibition among microalgae species isolated from the Amazon revealed that Synechococcus sp. had one of the highest glucosidase inhibitions, thus emerging as a potential anti-diabetic supplement [134]. In that context, the highest concentration of Synechococcus sp. methanolic extract exhibited 90.4% and 96.9% inhibitory activity against α- and β-glucosidase, respectively. The author suggested that the glucosidase inhibition by the microalgae extracts could be due to the pigments in them, such as purified C-phycoerythrin (C-PE) and phycocyanin [138]. Moreover, their extracellular and intracellular polysaccharides could play a part in achieving anti-diabetic effects [139].
2.1.2. Protein Hydrolysate
Villaro et al. investigated the antioxidant and anti-diabetic properties of enzymatic hydrolysates derived from the phycobiliproteins of A. platensis [126]. Proteins isolated from A. platensis could be hydrolyzed into potential bioactive peptides exhibiting antioxidant, dipeptidyl peptidase-IV (DPP-IV), and α-amylase and α-glucosidase inhibitory properties. Inhibition of DPP-IV improves blood glucose fluctuations and enhances glycemic control in diabetes. Blood glucose fluctuations are linked to pancreatic β-cell impairment; therefore, DPP-IV inhibition is an effective strategy to alleviate diabetes [140,141,142]. DPP-IV inhibition controls postprandial insulin secretion by increasing the levels and extending the effects of incretin hormones, namely, glucagon-like peptide 1 (GLP-1) and gastrointestinal insulinotropic peptide (GIP) that regulate insulin secretion in response to nutrients [141,143]. Additionally, it promotes the proliferation of β-cell by inhibiting apoptosis [140]. Results showed that pepsin and ficin hydrolysates had the highest antioxidant capacities compared to other hydrolysates. As for DPP-IV inhibition, alcalase hydrolysates showed the highest capacity compared to other hydrolysates. However, all the tested hydrolysates happened to have limited α-amylase and α-glucosidase inhibitory capacities. The authors suggested that perhaps the concentration used in this experiment was quite low to produce any significant α-amylase and α-glucosidase inhibition.
2.1.3. Oxo-Fatty Acids
Oxo-fatty acids (oFAs), a type of long-chain fatty acid, have been shown to demonstrate peroxisome proliferator-activated receptors (PPARs) agonist activities to regulate energy metabolism [144]. PPARs, such as PPARγ and PPARα, are a family of ligand-dependent transcription factors which function to modulate the expression of genes involved in lipid and glucose metabolism [145]. In that context, PPARγ and PPAR-β/δ activation improves insulin sensitivity and modulates lipid metabolism, respectively [146]. Therefore, targeting PPARs is a common and effective approach to managing diabetes mellitus [132]. Sæther et al. investigated the PPAR agonist activity of oFAs, namely, [(7E)-9-OHE] and [(10E)-9-OHE] derived from C. karianus in hepatocytes and adipocytes [132]. These oFAs exhibited substantial PPAR agonist activity in a dose-dependent manner. Next, the endogenous PPAR target genes activation was studied by treating Huh-7 cells and SGBS adipocytes with oFAs. Generally, it was found that the treatment activates fatty acid catabolism by upregulating CPT1A, ACSL3, PLIN1, and ANGPTL4 gene expressions. CPT1A, ACSL3, and PLIN1 genes function to regulate lipid metabolism [147,148], whereas ANGPTL4 gene regulates energy metabolism, and its upregulation has been reported to improve hyperglycemia in diabetic mice [149,150]. Overall, this result suggests that oFAs modulate energy metabolism in the hepatocytes and adipocytes.
Moreover, the adipogenic potentials of oFAs were studied via adipocyte transcriptomic analyses. Although the focus was to compare the dyslipidemic activities of oFAs, the authors reported that oFAs enhanced insulin-sensitizing adiponectin and leptin gene expressions while suppressing pro-inflammatory cytokines gene expressions due to PPARγ activation. Moreover, IRS1 and SLC2A4 genes were upregulated resulting in improved adipose insulin sensitivity [151,152]. Therefore, the activation of anti-diabetic gene programs is achieved by decreasing pro-inflammatory cytokines and raising insulin-sensitive adipokines. As a result, [(7E)-9-OHE] and [(10E)-9-OHE] derived from C. karianus are referred to as semi-potent dual PPARα/γ agonists with the potential to alleviate hyperglycemia.
2.2. In Vivo Studies
Several studies have evaluated the effects of microalgae or their bioactive compounds on a well-established diabetic rodent model characterized by high blood glucose levels. Table 3 summarizes the findings and the mechanisms involved in alleviating diabetes mellitus.

Table 3.
The effects of microalgae-based supplementations on diabetic animal models.
2.2.1. Arthrospira platensis
Studies have indicated that A. platensis or biomass supplementation alleviates hyperglycemia in diabetic rats mainly due to its antioxidant and anti-inflammatory activities [161,162]. Nasirian et al. supplemented streptozotocin (STZ)-induced diabetic male Wistar rats with 10, 20, or 30 mg/kg body weight (BW) per day of A. platensis extract for 3 weeks [157]. A total of 20 and 30 mg/kg of extracts decreased glucose, triglyceride (TG), cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) levels that were elevated in diabetic rats. Improvement in hyperglycemia and hyperlipidemia was attributed to the trace minerals (TMs) present in A. platensis extract. Chemical compositional analysis confirms that the extract contains zinc (Zn), copper (Cu), iron (Fe), selenium (Se), chromium (Cr), and magnesium (Mg). According to studies, TMs provide insulin-modulatory effects [163]. In addition, TMs potentiate antioxidant activity in diabetes [164]. These doses also increased superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) enzyme concentrations, thus explaining the lowered serum malondialdehyde (MDA) concentrations. The author explains that Zn and Se regulate antioxidant enzyme activities by being a cofactor for SOD and participating in antioxidant reactions, respectively [165,166]. In addition, Cr and Mg play a role in regulating glucose metabolism due to their involvement in insulin signaling [167,168,169]. Moreover, Mg deficiency has been linked to insulin resistance [167,168]. Furthermore, the highest dose decreased plasma concentrations of TNF-α and IL-6. These results imply that the anti-inflammatory effect is related to the antioxidant activities of A. platensis extract. Therefore, TMs improve glucose metabolism and reduce oxidative stress and inflammation.
The anti-inflammatory property of A. platensis has been shown to be involved in the protection of pancreatic β-cells. Hyperglycemia causes reactive oxygen species (ROS)-mediated glucose toxicity in pancreatic β-cells that impairs their insulin-producing function, leading to insulin insufficiency [170]. Alloxan-induced diabetic male Wistar or Swiss rats were supplemented with 25, 50, or 100 mg/kg/day (d) of A. platensis powder for 5 or 10 days [136]. It was reported that the 100 mg/kg dose restored the pancreatic islet area and β-cell characteristic and decreased pro-inflammatory TNF-α levels. A separate experiment revealed that 5, 10, and 25 mg/kg doses reduced mice licking time in a formalin test substantially, thus being denoted as anti-inflammatory. Moreover, 50 and 100 mg/kg doses decreased edema, improved tissue architecture, and reduced neutrophil infiltration in the paw tissues of mice. Importantly, serum glucose levels decreased especially on the 10th day. Furthermore, these doses decreased serum TG and TC in a dose-dependent manner. Therefore, these results agree on the anti-inflammatory effects of A. platensis and its role in alleviating hyperglycemia by regulating pro-inflammatory cytokine levels.
In addition, A. platensis extract improves glucose tolerance in diabetic rats by positively affecting the gut microbiota composition. Moreover, different extract types seem to exert different anti-diabetic effects. In that context, male rats that were induced diabetes by a high-fat high-sucrose diet were supplemented with 150 mg/kg/day of ethanol or water extracts of A. platensis for 8 weeks [43]. The results show that the fasting blood glucose (FBG) level was significantly lower in diabetic rats supplemented with water extract of A. platensis compared to non-supplemented diabetic rats. Moreover, an oral glucose tolerance test (OGTT) revealed that both extracts improved glucose tolerance. Interestingly, gut microbiota composition, which was also improved mainly by increasing the abundance of Oscillibacter, was indicated to play a crucial role in attenuating the adverse conditions seen in diabetic rats. These results suggest that A. platensis supplementation, especially its water extract, is efficacious in alleviating hyperglycemia, the hallmark of diabetes mellitus. The author explains that this could be attributed to PUFAs in their extracts that may modulate energy metabolism and improve pancreatic β-cell functions [171]. In addition, it may improve the gut microbiota composition to prevent low-grade chronic inflammation and insulin resistance [172]. In this study, the slight yet significantly higher efficacy of water extract over ethanol extract indicates that some of the bioactive compounds could better dissolve in water compared to ethanol.
Moreover, A. platensis supplementation could modulate energy metabolism by regulating the enzymes involved in glucose metabolism. Furthermore, this supplementation exerts anti-apoptotic properties to protect pancreatic β-cells. Supplementing 500 mg/kg of A. platensis powder twice a week for 2 months to STZ-induced diabetic male Albino rats decreased serum glucose and glycated hemoglobin (HbA1c) concentrations and increased serum insulin concentration [159]. This study revealed that supplementation decreased pyruvate carboxylase (PC) mRNA expression. PC is a crucial gluconeogenic enzyme and its increase due to insulin deficiency may be linked to hyperglycemia [173,174]. The author suggests that the glucose-lowering effect of A. platensis may be due to its ability to inhibit PC expression. Furthermore, supplementation restored the architecture of pancreatic islets and increased glutathione concentration. Moreover, the activities of glutathione-S-transferase (GST), SOD, and catalase were increased. The upregulation of SOD, catalase, and GST mRNA expressions in these rats further corroborates those findings. These enzymes counteract the effects of ROS by neutralizing them to protect pancreatic β-cells [175]. Therefore, this explains the decrease in MDA and TBARS concentrations which are lipid peroxidation biomarkers. The antioxidant activity is credited to the phytochemicals such as phenolic compounds, namely, caffeic acid, coumaric acid, gallic acid, catechin, apigenin, flavonoids, flavonols, and phycocyanin that have antioxidant potential [159]. Moreover, A. platensis supplementation decreased pro-apoptotic Bax, CASP-3, and pro-inflammatory TNF-α mRNA expressions and increased anti-apoptotic Bcl-2 mRNA expression. Additionally, p-p38, p-ERK, and p-JNK MAPKs protein expressions decreased, showing that the activation of p38- and ERK-mediated MAPK signaling was inhibited. A crucial element of the pro-apoptotic signaling pathway imposed on diabetes is the MAPK signaling cascade [176]. MAPK activation may be mediated by ROS and its repression is credited to the antioxidant properties of A. platensis.
2.2.2. Nannochloropsis oculata
N. oculata supplementation was reported to have corrected hyperglycemia and hyperlipidemia in diabetic rats. A total of 10 or 20 mg/kg/day of N. oculata supplementation for 3 weeks on STZ-induced male Wistar diabetic rats resulted in increased BW, serum insulin, and high-density lipoprotein cholesterol (HDL-C), and decreased serum glucose, TG, TC, and LDL-C concentrations [156]. However, the underlying mechanism was unexplored in this study. The authors suggest that the antihyperglycemic properties of N. oculata could be due to its protective effects against oxidative damage on pancreatic β-cell islets or enhanced glucose uptake by peripheral tissues which is comparable to insulin therapy on STZ-induced diabetic rats [21,177]. The pigments in N. oculata could have exerted the previously mentioned antioxidant effects to counteract the oxidative damage caused by STZ on pancreatic islets [178]. Moreover, Se in N. oculata was suggested to play a role in regulating the antioxidant defense system [163,179]. The improvement in lipid profile could mean that lipid metabolism was improved as well as glucose metabolism, mainly by regulating insulin secretion and action. Additionally, the EPA and DHA in N. oculata could have played a role in improving the lipid profile [180].
Another study with a similar study design and doses of N. oculata powder has proven its antihyperglycemic effect by regulating antioxidant enzymes and pro-inflammatory cytokine levels [153]. First, the supplementation increased BW in a dose-dependent manner. The underlying mechanism of this effect is largely unknown, but the authors speculate that it could be due to an improvement in insulin sensitivity and glycemic control [181,182,183]. This was corroborated when serum glucose and insulin in supplemented diabetic rats were also improved in a dose-dependent manner. In addition, serum concentrations of GSH-Px, SOD, and FRAP were increased and serum concentration of MDA was decreased in supplemented rats which suggests the anti-antioxidant activity of N. oculata. Furthermore, serum concentrations of pro-inflammatory markers, such as IL-6, NF-κB, IL-1β and TNF-α, were significantly decreased. Thus, the anti-inflammatory activity of N. oculata could also be credited for the improvement in hyperglycemia in diabetic rats. The authors suggest that carotenoids present in N. oculata could have exerted these bioactivities [178].
2.2.3. Nannochloropsis gaditana
Similar to N. oculata, 10% of N. gadiatana powder supplementation per day for 2 months on STZ-induced diabetic male Wistar rats also improved BW and serum glucose [2]. Furthermore, this study reported an improvement in HbA1c. Additionally, N. gaditana attenuated glycosuria and polyphagia which marked its potential in reversing diabetic complications [184,185]. These effects were credited to the modulatory effect on pancreatic β-cell insulin secretion, thus improving glucose metabolism. In addition, the serum TC and TG levels were decreased in diabetic rats upon N. gaditana supplementation, which could be due to improvement in overall energy metabolism exerted by modulating insulin secretion. Furthermore, it was reported that CAT, glutathione, and SOD enzymes were increased in the liver mitochondria and pancreatic tissues. Moreover, CAT, glutathione, SOD, and GST in the liver tissues were increased. Therefore, the liver mitochondrial MDA and carbonyl proteins concentrations were also decreased. Carbonyl proteins are markers of protein oxidation similar to how MDA is for lipid peroxidation, and these reflect the oxidative status in the rats [186]. It was mentioned that the overwhelming levels of ROS in the mitochondria lead to oxidative stress, and this could underlie the mechanism of diabetes induction by STZ [187,188,189]. Previous studies have reported that N. gaditana scavenges free radicals and prevents lipid peroxidation [190]. Furthermore, the serum concentrations of IL-6 and TNF-α were reduced. This effect was attributed to the bioactivity of carotenoids in N. gaditana [191,192].
2.2.4. Chlorella pyrenoidosa
According to a study, C. pyrenoidosa exerts its anti-diabetic effects by modulating energy metabolism and improving gut microbiota composition [43]. In that context, water and ethanol extracts of C. pyrenoidosa were supplemented to the diet-induced diabetic male rats. Both extract types decreased FBG and improved glucose tolerance denoted by the decreased area under the curve (AUC) in the OGTT. In terms of its effects on the composition of gut microbiota, C. pyrenoidosa restores the microflora balance of the intestine. The supplementation decreased the Firmicutes/Bacteroidetes ratio which is said to be a sign of improvement in the microbiota of the intestine and chronic low-grade inflammation [193,194]. Moreover, some bacterial species that were imbalanced in the gut microbiota of non-supplemented diabetic groups were reversed in the supplemented groups. For example, supplementation decreased Blautia and Turicibacter compositions. Turicibacter is said to cause diabetes and inflammation [195]. Moreover, beneficial bacteria, such as Oscillibacter, Parasutterella, and Ruminococcus species, were maintained by the supplementation. Ruminococcus and Oscillibacter seemed to negatively correlate with AUC FBG. However, these findings are not consistent with other studies and there is a lack of extensive characterization of the gut microbiota in diabetic rat models [196,197]. At every parameter, C. pyrenoidosa performed better than A. platensis suggesting that the former is more efficacious at exerting anti-diabetic effect. The author speculates that the improvement in the abundance of Ruminococcus could underlie the increased hypoglycemia effect of C. pyrenoidosa compared to A. platensis. These effects could be attributed to polyunsaturated fatty acids (PUFAs) found in C. pyrenoidosa [198,199].
2.2.5. Porphyridium cruentum
A study has demonstrated the therapeutic effect of P. cruentum powder on diabetes due to its protective and proliferative effects on pancreatic β-cell islets. STZ-induced male Sprague–Dawley rats that were supplemented with 600, 1200, and 1800 mg/kg/day for 14 days had increased β-cell granulation and the number of pancreatic β-cells. Additionally, 1200 and 1800 mg/kg doses increased pancreatic islets of Langerhans area, but these parameters were markedly reduced by diabetes. The pancreatic β-cells produce and synthesize insulin that most importantly regulates blood glucose levels, and its impairment leads to a lack of insulin synthesis and subsequent metabolic control, leading to hyperglycemia [200]. An increase in the number of pancreatic β-cells may result in enhanced insulin secretion and cell regeneration, which lower blood sugar levels. Therefore, therapeutics targeting the enhancement of pancreatic β-cell regeneration could alleviate diabetes [201,202]. Previous studies have reported the presence of bioactive compounds, such as phycobiliproteins, carotenoids, extracellular polysaccharides, B-phycoerythrin (PE), and omega-3 fatty acids, that could exert antioxidant and anti-inflammatory properties which may attenuate hyperglycemia [67,82,203,204]. Nevertheless, P. cruentum supplementation did not alleviate blood glucose levels of the diabetic rats suggesting that the doses used in this study may not be optimum to impart any hypoglycemic effect.
2.2.6. Dunaliella salina
Ruperez et al. investigated the effects of D. salina extract on the metabolism of STZ-induced male Sprague–Dawley rats [160]. The diabetic rats received 150 mg/kg of D. salina extract at 72, 64, 48, 40, and 24 h before sacrifice. Metabolic fingerprinting, a comprehensive analysis of metabolic profiles, was performed to assess any changes caused by the extract. The results of the study revealed significant alterations in the metabolic profiles of the diabetic rats after receiving D. salina extract. This is due to the fact that the urine fingerprinting PLS-DA scores plot shows a clear separation between supplemented and non-supplemented groups. The extract demonstrated a positive effect on most of the parameters measured, indicating potential therapeutic properties for diabetes management. In that context, there was a slight increase in BW and a decrease in liver size. The author speculates that the extract could have provided better metabolic control resulting in increased liver glycogen utilization which is a sign of improvement in Mauriac syndrome in type 1 diabetes [205]. In addition, supplementation decreased TBARS, increased antioxidant enzymes such as reduced glutathione (GSH), and oxidized glutathione (GSSG), and decreased GSSG/GSH ratio in the liver but not in the blood. The glutathione antioxidant system is dysregulated or imbalanced due to increased ROS production induced by hyperglycemia that leads to insulin resistance and pancreatic β-cell death [206,207,208]. These results show that D. salina has antioxidant properties by reducing markers of oxidative degradation and increasing antioxidant capacity exclusively in the liver. Moreover, a-tocopherol in plasma was increased suggesting that D. salina could protect against damage from free radicals [209]. Furthermore, D. salina has been reported to contain β-carotene and bioactive peptides that exhibit antioxidant activities. Nevertheless, the supplementation did not improve blood glucose levels in this study, but the TG levels were improved. This suggests that the extract exerts an antihyperlipidemic function that could alleviate complications associated with diabetes mellitus. Overall, the findings suggest that D. salina extract has the potential to positively influence the metabolism of diabetic rats.
2.2.7. Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA)
Administration of omega-3 fatty acids has been reported to improve diabetes by regulating lipid metabolism, insulin synthesis, and attenuating oxidative stress and insulin resistance by modulating the expressions of inflammatory and energy metabolism genes [210,211,212,213]. Microalgae are well-known as a good source of omega-3 fatty acids, namely, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [214]. Genetically diabetic male db/db mice supplemented with 1 mg/g of EPA and DHA extracted from Chlorophyceae and Eustigamatophyceae families enhanced their total antioxidant capacity in adipose tissue and plasma [155]. However, the supplementation could not manage to attenuate the increase in lipid peroxidation observed in diabetic mice. Nevertheless, the author explains that previous studies have reported successful alleviation in MDA and promotion of the synthesis of antioxidants in the liver and platelets upon supplementation of microalgae fatty acids [215]. Meanwhile, diabetic rats fed a rodent chow enriched with 2.0% microalgae EPA and DHA had decreased food intake. This effect could be due to the regulatory effect of the fatty acid supplementation on adiponectin synthesis. Adiponectin is an adipokine released by adipose tissue that has been demonstrated to improve insulin sensitivity, control inflammation, and regulate appetite [216,217].
Another study found that supplementation with microalgae EPA and DHA extracted from Chlorophyceae and Eustigmatophyceae families on db/db mice at similar dosages had profound anti-inflammatory properties [158]. In that context, T lymphocyte populations decreased. T lymphocytes have been reported to play a role in the inflammatory processes and adipose tissue insulin resistance underlying the pathogenesis of diabetes mellitus [218,219]. Furthermore, diabetic rats supplemented with microalgae-derived n-3 fatty acids caused a decrease in the levels of pro-inflammatory cytokines IFN-y and TNF-α. Nevertheless, the IL-12 pro-inflammatory cytokines were increased by the fatty acids supplementation. Additionally, supplemented diabetic mice had an increase in anti-inflammatory IL-10 and TGF-β cytokine levels. Moreover, supplemented groups had the least increase in IL-6 cytokine levels compared to other groups. Therefore, microalgae-derived EPA and DHA supplementation exerts a modulatory effect on cytokine levels and the T lymphocyte population, thus improving inflammation in diabetes mellitus.
2.2.8. Polysaccharides
Microalgae-derived polysaccharides have been studied for their antihyperglycemic activity since it slows down gastric emptying and the absorption of glucose in small intestines and protects pancreatic β-cells [82,220,221]. High-glucose high-fat diet, D-gal, and STZ-induced male Kunming diabetic mice supplemented with 150 or 300 mg/kg of C. pyrenoidosa polysaccharides (CPP) for 4 weeks were observed to have improved BW and insulin levels [154]. Additionally, glucose uptake was enhanced in mice supplemented with the higher dosage, performing better than metformin which was included as a positive control in this study. This shows that CPP improves glucose metabolism attributed to their antioxidant activity suggested by the increase in antioxidant enzymes, such as SOD, CAT, and GSH-Px enzymes in tissues and reduction in MDA levels upon supplementation. CPP supplementation also imparts protective effects on tissues that were observed to be injured in AD mice due to STZ and D-gal. In that context, CPP improved pancreatic architecture that could improve β-cell insulin secretion and sensitivity [222]. Additionally, CPP supplementation demonstrated anti-inflammatory effects by decreasing IL-6R mRNA and protein expressions. Moreover, the lower dosage decreased forkhead box O1 (FOXO-1) and increased glucagon-like peptide-1 receptor (GLP-1R). FOXO-1 is a nuclear transcription factor that is involved in mediating insulin levels and current literature suggests that it could be a therapeutic target in alleviating T2DM [223]. Moreover, GLP-1R plays a role in regulating blood glucose levels by enhancing insulin secretion; therefore, its upregulation could alleviate hyperglycemia [224]. Ultimately, the co-modulation of IL-6R/FOXO-1 and GLP-1R/FOXO-1 pathways are suggested to be the underlying mechanisms for the effects imparted by CPP supplementation. A metabolic profiling analysis revealed that phenylpyruvic acid was reduced, correlating with the improvement in glucose uptake.
In another study, the administration of extracellular polysaccharides (Eps) derived from P. cruentum at 150, 300, and 450 mg/kg/day for 14 days on STZ-induced male Sprague–Dawley rats decreased food intake [82]. Furthermore, 300 and 450 mg/kg of EPs managed to reduce blood glucose levels, being as effective as glibenclamide. Apart from slowing down intestinal glucose absorption, EPs may also regulate the secretion of insulin to control blood glucose levels. In addition, supplementation, especially at the highest dosage, improved pancreatic islets’ area and the number of pancreatic β-cells, proving its protective effect against STZ-induced damage on the pancreas. STZ enters the β-cells via glucose transporter 2 (GLUT2) receptors which then induces its toxicity on the pancreas, but EPs seem to compete with STZ for GLUT2 receptors which could explain the protective effect and the attenuation of blood glucose levels [225,226,227]. The author also speculates that this could be due to reduced cytotoxic nitric oxide (NO) and regulating nitric oxide synthase (iNOs) enzyme gene expressions. The highest dosage also improved intestinal villi height similar to fiber supplementation that stimulated the secretion of GLP-2 hormone which is responsible for increasing nutrient absorption and developing and maintaining an optimum gastrointestinal tract condition [228,229]. The results suggest that EPs derived from P. cruentum could be a viable option in managing diabetes.
2.2.9. Astaxanthin
Microalgae pigments, such as chlorophylls, carotenoids (β-carotene and astaxanthin), and phycobilins (phycocyanin, phycoerythrin, and phycoerythrocyanin) are photosynthetic pigments commonly used as dyes in the food, cosmetic, and pharmaceutical industries [230,231]. By protecting pancreatic β-cells, improving insulin resistance (IR), and enhancing insulin secretion, astaxanthin is said to lower blood glucose levels [54,232,233]. Genetically diabetic db/db female mice supplemented with 1.0 mg/mouse/day of astaxanthin derived from marine microalgae for 12 weeks decreased non-fasting blood glucose, improved intraperitoneal glucose tolerance (IPGT), and increased serum insulin levels [55]. Therefore, these results suggest that astaxanthin could preserve pancreatic β-cell function. The author explains that this protective effect is due to the improvement in oxidative stress caused by ROS in the pancreas which is prone to oxidative damage caused by hyperglycemia. Additionally, hyperglycemia leads to the production of advanced glycosylation end products (AGEs), which together with ROS leads to impairment in insulin gene transcription and pancreatic β-cell death [234].
A study carried out by Penislusshiyan et al., investigating the in vitro antioxidant activity of astaxanthin derived from H. pluvialis, explains its mechanism of action [235]. In that context, astaxanthin exhibited significant antioxidant activity attributed to its polyene chain and the C3 methine at its terminal ring moiety [231,236]. Moreover, in silico interaction analysis of astaxanthin with S. cerevisiae α-glucosidase revealed that it interacts with the catalytic site amino acid residue of S. cerevisiae α-glucosidase by hydrogen bonding that indicates their interaction with the active site of this enzyme [235]. The result also reveals that the polyene chain of astaxanthin interacts with a few amino acids at the active site of S. cerevisiae α-glucosidase by hydrophobic interaction. Moreover, astaxanthin interacts with active sites of human α-glucosidases. However, astaxanthin could be viewed as a precursor molecule that could be conjugated with other candidate compounds to increase its efficacy and potency in terms of enhancing its antioxidant activity and the number of interactions to α-glucosidases [235].
2.3. Human Studies
There have not been many well-designed clinical trials examining the effects of various microalgae-based supplementations on diabetic human subjects. In addition, the trials are limited to T2DM patients which could be due to the potential of microalgae or its bioactive compounds in alleviating metabolic disorders. Table 4 summarizes the findings from the human studies.

Table 4.
The effects of microalgae-based supplementation on diabetic patients.
Microalgae Extract
A. platensis supplementation has been shown to improve the glycemic profile of patients with T2DM. Doses of A. platensis as low as 1 or 2 g and/or as high as 8 or 14 g per day improved FBG and glycated protein levels such as HbA1c [237,238,239,240,241]. Moreover, A. platensis supplementation improved lipid profile which is often deranged in diabetics leading to cardiovascular diseases and death [237,238,239,242]. However, the anti-diabetic effects are not dose-dependent which could be due to variations in the severity of diabetes in the patients. In addition, the durations of supplementation were not standardized among the studies and were rather short in certain studies. This limitation could explain the insignificant changes in HbA1c levels in some of these studies since HbA1c is known to take a longer duration for changes to appear. Therefore, the results, although seem promising, are not comparable to one another. Nevertheless, even the highest dosage and the longest test duration did not induce toxicity or side effects on the patients, suggesting its safety to be included as a health supplement.
Furthermore, a study has reported that A. platensis demonstrates a synergistic anti-diabetic effect with metformin while improving lipid profiles in T2DM patients [242]. A total of 2 g of A. platensis administered together with their daily metformin regimen for 3 months managed to decrease the HbA1c, FBS, TC, LDL-C, and TG, and increase the HDL-C levels better than metformin alone. This suggests that A. platensis supplementation could be an additional strategy for managing diabetes without the side effects associated with anti-diabetic drugs.
The antihyperglycemic properties of A. platensis could be attributed to the upregulation of a crucial insulin regulatory mechanism, namely, the adenylate cyclase/cAMP pathway which causes increased cAMP levels followed by upregulation of PKA activity leading to insulin secretion [242]. Moreover, pigments, such as pheophytin, β-carotene, and phycocyanobilin, could inhibit important enzymes associated with diabetes, such α-amylase, α-glucosidase, G6PD, and DPP-IV, thus regulating their activity and attenuating hyperglycemia [7].
3. Limitations
Applying microalgae and their bioactive compounds as health supplements to alleviate diabetes mellitus poses certain limitations. First, only one research has been carried out for most microalgae species or their bioactive compounds. Additionally, the effects of other potential microalgae species or their bioactive compounds on diabetes mellitus are unexplored. Moreover, some of the studies employed only a single dosage of microalgae or their bioactive compounds to assess their anti-diabetic properties. Furthermore, some microalgae dosages were selected based on previous studies on different microalgae species which may not have similar bioactive compound composition or concentration. Therefore, the optimum dosage for most of the test compounds is largely unknown and there is no clear verdict as to how efficacious these dosages are if they were to be considered as health supplements. In addition, the method of inducing diabetes mellitus in animal models varies among studies; therefore, the therapeutic effects of the test compounds cannot be generalized for every type of diabetes. Moreover, the disease animal models may not completely recapitulate the complex pathophysiology of diabetes, especially T2DM. Furthermore, the anti-diabetic mechanisms were only elucidated in some of the studies. Additionally, information on the digestibility or bioavailability of the specific microalgae extracts is lacking, which is crucial since an intact microalgae cell wall could hamper the bioavailability and lessen the potency of the bioactive compounds. Finally, some of the clinical trial study designs face limitations in terms of small sample size, no placebo groups, and short duration of intervention.
Moreover, comprehensive data on the safety of microalgae consumption are not available yet. Extensive toxicological studies are limited to only some species and their bioactive compounds, especially the ones that are commonly consumed, such as A. platensis and astaxanthin, which have been reported as safe for consumption by governmental bodies [243,244]. Generally, A. platensis does not contain toxins, deeming it safe for consumption [45,243,245]. Many in vivo studies include liver enzyme analysis to elucidate the basic toxicity of microalgae or its bioactive compounds. For example, N. oculata, N. gaditana, D. salina and C. vulgaris extracts, fucosterol, and EPA from N. oculata have been reported to be safe in preclinical studies [246,247,248,249,250]. However, there is a lack of evidence on the safety of higher dosages or long-term consumption on human subjects; therefore, their optimal dosage is still inconclusive. Additionally, contamination due to the accumulation of heavy metals from the environment or toxins produced by cyanobacteria in the culture could result in toxicity [45,251]. As a result, adverse effects upon consumption of certain microalgae have been reported [45]. Therefore, the safety of a particular microalgae depends on its source, culture conditions, and quality regulation.
4. Future Perspectives
More studies are needed to elucidate the underlying anti-diabetic mechanisms of microalgae and their bioactive compounds by means of studying the genes and proteins involved. In addition, future studies shall employ omics technology to gain valuable insights into the molecular changes imparted by a particular microalga or its bioactive compounds. In that context, novel biomarkers, therapeutic targets, and upregulated or downregulated pathways responsible for the anti-diabetic effects of the supplementation can be identified. In this review, microalgae supplementations have been shown to alleviate diabetes mellitus mainly due to their antioxidant and anti-inflammatory activities that exert protective effects on pancreatic β-cells and subsequent glycemic control. Moreover, the bioactive compounds inhibit enzymes associated with hyperglycemia. A brief summary of the potential therapeutic effects and the mechanisms of microalgae and their bioactive compounds on hyperglycemia has been illustrated in Figure 1. Therefore, the bioactive compound composition and concentration in the supplements are of particular importance to develop them into health supplements. Hence, future studies shall also focus on optimizing the microalgae culture conditions to maximize the yield of bioactive compounds synthesized and standardize the supplements. Furthermore, the methods of inducing T2DM in animal models, such as STZ and/or HFD, can be further refined by considering the dosage of STZ and/or the nutrient composition of HFD to better represent T2DM. Additionally, in order to deem microalgae or their bioactive compounds as safe and efficacious in managing diabetes mellitus, more human studies are needed. Furthermore, upcoming human studies shall include a larger sample size together with a placebo control group to obtain more reliable results and increase the duration of the experiment to reveal the long-term effects of microalgae supplementation.
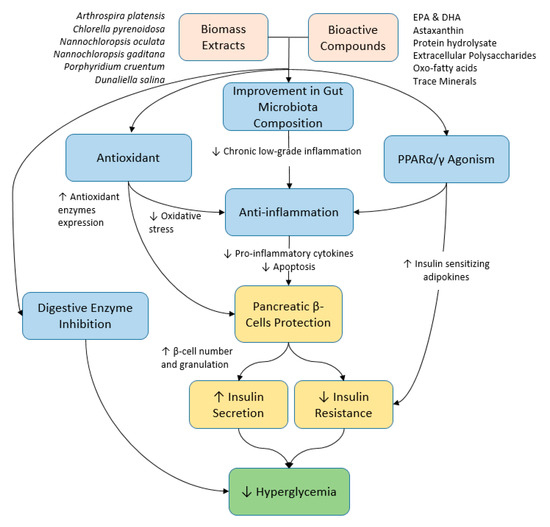
Figure 1.
Summary of the potential therapeutic effects and the mechanisms of microalgae and their bioactive compounds on hyperglycemia.
Author Contributions
Conceptualization, J.K.T. and K.T.S.; writing—original draft preparation, K.T.S.; writing—review and editing, K.T.S., J.K.T., S.M. and J.A.G.; supervision, J.K.T., S.M. and J.A.G.; project administration, J.K.T.; funding acquisition, J.K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education, Malaysia with the Fundamental Research Grant Scheme (FRGS/1/2018/SKK08/UKM/03/1) and the Faculty of Medicine, Universiti Kebangsaan Malaysia, grant number FF-2023-133.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Not applicable.
References
- Zimmet, P.; George Alberti, K.; Magliano, D.J.; Bennett, P.H. Diabetes Mellitus Statistics on Prevalence and Mortality: Facts and Fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef]
- Nacer, W.; Baba Ahmed, F.Z.; Merzouk, H.; Benyagoub, O.; Bouanane, S. Evaluation of the Anti-Inflammatory and Antioxidant Effects of the Microalgae Nannochloropsis Gaditana in Streptozotocin-Induced Diabetic Rats. J. Diabetes Metab. Disord. 2020, 19, 1483–1490. [Google Scholar] [CrossRef]
- Hassan, M.R.; Jamhari, M.N.; Hayati, F.; Ahmad, N.; Zamzuri, M.A.I.A.; Nawi, A.M.; Sharif, K.Y.; Sufri, M.; Ahmad, S.B.; Ismail, N.; et al. Determinants of Glycaemic Control among Type 2 Diabetes Mellitus Patients in Northern State of Kedah, Malaysia: A Cross-Sectional Analysis of 5 Years National Diabetes Registry 2014–2018. Pan Afr. Med. J. 2021, 39, 206. [Google Scholar] [CrossRef]
- Ozougwu, J.C.; Obimba, K.C.; Belonwu, C.D.; Unakalamba, C.B. The Pathogenesis and Pathophysiology of Type 1 and Type 2 Diabetes Mellitus. J. Physiol. Pathophysiol. 2013, 4, 46–57. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Amsah, N.; Isa, M.Z.; Kassim, Z. Poor Glycaemic Control and Its Associated Factors among Type 2 Diabetes Mellitus Patients in Southern Part of Peninsular Malaysia: A Registry-Based Study. Open Access Maced. J. Med. Sci. 2022, 10, 422–427. [Google Scholar] [CrossRef]
- Munawaroh, H.S.H.; Hazmatulhaq, F.; Gumilar, G.G.; Pratiwi, R.N.; Kurniawan, I.; Ningrum, A.; Hidayati, N.A.; Koyande, A.K.; Kumar, P.S.; Show, P.L. Microalgae as a Potential Sustainable Solution to Environment Health. Chemosphere 2022, 295, 133740. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Kaabi, J. Al Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxid. Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Grant, I.D.; Ozanne, S.E.; Reynolds, R.M.; Aiken, C.E. Efficacy and Side Effect Profile of Different Formulations of Metformin: A Systematic Review and Meta-Analysis. Diabetes Ther. 2021, 12, 1901–1914. [Google Scholar] [CrossRef]
- Lambrinou, E.; Hansen, T.B.; Beulens, J.W. Lifestyle Factors, Self-Management and Patient Empowerment in Diabetes Care. Eur. J. Prev. Cardiol. 2019, 26, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Khandelwal, D.; Lal, P.R.; Gupta, Y.; Kalra, S.; Dutta, D. Factors Determining the Success of Therapeutic Lifestyle Interventions in Diabetes—Role of Partner and Family Support. Eur. Endocrinol. 2019, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, C.; Wai, S.T.C.; Zhang, Y.; Portillo, M.P.; Paoli, P.; Wu, Y.; San Cheang, W.; Liu, B.; Carpéné, C.; et al. Regulation of Glucose Metabolism by Bioactive Phytochemicals for the Management of Type 2 Diabetes Mellitus. Crit. Rev. Food Sci. Nutr. 2018, 59, 830–847. [Google Scholar] [CrossRef]
- Laamanen, C.A.; Desjardins, S.M.; Senhorinho, G.N.A.; Scott, J.A. Harvesting Microalgae for Health Beneficial Dietary Supplements. Algal Res. 2021, 54, 102189. [Google Scholar] [CrossRef]
- Ibrahim, T.N.B.T.; Feisal, N.A.S.; Kamaludin, N.H.; Cheah, W.Y.; How, V.; Bhatnagar, A.; Ma, Z.; Show, P.L. Biological Active Metabolites from Microalgae for Healthcare and Pharmaceutical Industries: A Comprehensive Review. Bioresour. Technol. 2023, 372, 128661. [Google Scholar] [CrossRef] [PubMed]
- Gohara-Beirigo, A.K.; Matsudo, M.C.; Cezare-Gomes, E.A.; de Carvalho, J.C.M.; Danesi, E.D.G. Microalgae Trends toward Functional Staple Food Incorporation: Sustainable Alternative for Human Health Improvement. Trends Food Sci. Technol. 2022, 125, 185–199. [Google Scholar] [CrossRef]
- Barros de Medeiros, V.P.; da Costa, W.K.A.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as Source of Functional Ingredients in New-Generation Foods: Challenges, Technological Effects, Biological Activity, and Regulatory Issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef]
- Rajauria, G.; Yuan, Y.V. Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives; Wiley Blackwell: Hoboken, NJ, USA, 2021; ISBN 9781119542582. [Google Scholar]
- Krohn, I.; Menanteau-Ledouble, S.; Hageskal, G.; Astafyeva, Y.; Jouannais, P.; Nielsen, J.L.; Pizzol, M.; Wentzel, A.; Streit, W.R. Health Benefits of Microalgae and Their Microbiomes. Microb. Biotechnol. 2022, 15, 1966–1983. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef]
- Skjånes, K.; Aesoy, R.; Herfindal, L.; Skomedal, H. Bioactive Peptides from Microalgae: Focus on Anti-Cancer and Immunomodulating Activity. Physiol. Plant. 2021, 173, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Rostro-Alanis, M.; Cuéllar-Bermúdez, S.P.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; García-Pérez, J.S.; Chandra, R.; Parra-Saldívar, R. Advancement of Green Process through Microwave-Assisted Extraction of Bioactive Metabolites from Arthrospira Platensis and Bioactivity Evaluation. Bioresour. Technol. 2017, 224, 618–629. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, J.F.; García-Alamilla, P.; Palma-Ramírez, D.; Álvarez-González, C.A.; Paredes-Rojas, J.C.; Márquez-Rocha, F.J. Continuous Microalgal Cultivation for Antioxidants Production. Molecules 2020, 25, 4171. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.; Swick, J.; Ronnenberg, A.G. Vitamin E and Adiponectin: Proposed Mechanism for Vitamin E-Induced Improvement in Insulin Sensitivity. Nutr. Rev. 2011, 69, 155–161. [Google Scholar] [CrossRef]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of Carotenoids and Vitamin E from Their Main Dietary Sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef]
- Millao, S.; Uquiche, E. Antioxidant Activity of Supercritical Extracts from Nannochloropsis Gaditana: Correlation with Its Content of Carotenoids and Tocopherols. J. Supercrit. Fluids 2016, 111, 143–150. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Cuéllar-Bermúdez, S.P.; García-Pérez, J.S.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; Ontiveros-Valencia, A.; Rostro-Alanis, M.; García-García, R.M.; Torres, J.A.; et al. Effect of Supercritical Carbon Dioxide Extraction Parameters on the Biological Activities and Metabolites Present in Extracts from Arthrospira Platensis. Mar. Drugs 2017, 15, 174. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of Microalgal Biomass Profiles as Novel Functional Ingredient for Food Products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and Other Nutrients from Haematococcus Pluvialis—Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Siahbalaei, R.; Kavoosi, G.; Noroozi, M. Manipulation of Chlorella Vulgaris Polyunsaturated ω-3 Fatty Acid Profile by Supplementation with Vegetable Amino Acids and Fatty Acids. Phycol. Res. 2021, 69, 116–123. [Google Scholar] [CrossRef]
- Belury, M.A.; Cole, R.M.; Snoke, D.B.; Banh, T.; Angelotti, A. Linoleic Acid, Glycemic Control and Type 2 Diabetes. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 30–33. [Google Scholar] [CrossRef]
- Hamilton, J.S.; Klett, E.L. Linoleic Acid and the Regulation of Glucose Homeostasis: A Review of the Evidence. Prostaglandins Leukot. Essent. Fat. Acids 2021, 175, 102366. [Google Scholar] [CrossRef] [PubMed]
- Gkioni, M.D.; Andriopoulos, V.; Koutra, E.; Hatziantoniou, S.; Kornaros, M.; Lamari, F.N. Ultrasound-Assisted Extraction of Nannochloropsis Oculata with Ethanol and Betaine: 1,2-Propanediol Eutectic Solvent for Antioxidant Pigment-Rich Extracts Retaining Nutritious the Residual Biomass. Antioxidants 2022, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; García-Beltrán, A.; Kapravelou, G.; Mesas, C.; Cabeza, L.; Perazzoli, G.; Guarnizo, P.; Rodríguez-López, A.; Vallejo, R.A.; Galisteo, M.; et al. In Vivo Nutritional Assessment of the Microalga Nannochloropsis Gaditana and Evaluation of the Antioxidant and Antiproliferative Capacity of Its Functional Extracts. Mar. Drugs 2022, 20, 318. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Marino, T.; Karatza, D.; Musmarra, D. Extraction of Bioactive Compounds Using Supercritical Carbon Dioxide. Molecules 2019, 24, 782. [Google Scholar] [CrossRef]
- Hu, H.; Wang, H.F.; Ma, L.L.; Shen, X.F.; Zeng, R.J. Effects of Nitrogen and Phosphorous Stress on the Formation of High Value LC-PUFAs in Porphyridium Cruentum. Appl. Microbiol. Biotechnol. 2018, 102, 5763–5773. [Google Scholar] [CrossRef]
- Guil-Gucrrcro, J.L.; Belarbi, E.H.; Rebolloso-Fuentes, M.M. Eicosapentaenoic and Arachidonic Acids Purification from the Red Microalga Porphyridium Cruentum. Bioseparation 2000, 9, 299–306. [Google Scholar] [CrossRef]
- Pantami, H.A.; Bustamam, M.S.A.; Lee, S.Y.; Ismail, I.S.; Faudzi, S.M.M.; Nakakuni, M.; Shaari, K. Comprehensive GCMS and LC-MS/MS Metabolite Profiling of Chlorella Vulgaris. Mar. Drugs 2020, 18, 367. [Google Scholar] [CrossRef]
- Gundala, N.K.V.; Naidu, V.G.M.; Das, U.N. Amelioration of Streptozotocin-Induced Type 2 Diabetes Mellitus in Wistar Rats by Arachidonic Acid. Biochem. Biophys. Res. Commun. 2018, 496, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Suresh, Y.; Das, U.N. Protective Action of Arachidonic Acid against Alloxan-Induced Cytotoxicity and Diabetes Mellitus. Prostaglandins Leukot. Essent. Fat. Acids 2001, 64, 37–52. [Google Scholar] [CrossRef]
- Sarbolouki, S.; Javanbakht, M.H.; Derakhshanian, H.; Hosseinzadeh, P.; Zareei, M.; Hashemi, S.B.; Dorosty, A.R.; Eshraghian, M.R.; Djalali, M. Eicosapentaenoic Acid Improves Insulin Sensitivity and Blood Sugar in Overweight Type 2 Diabetes Mellitus Patients: A Double-Blind Randomised Clinical Trial. Singap. Med. J. 2013, 54, 387–390. [Google Scholar] [CrossRef]
- Wan, X.; Li, T.; Zhong, R.; Chen, H.; Xia, X.; Gao, L.; Gao, X.; Liu, B.; Zhang, H.; Zhao, C. Anti-Diabetic Activity of PUFAs-Rich Extracts of Chlorella Pyrenoidosa and Spirulina Platensis in Rats. Food Chem. Toxicol. 2019, 128, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Jia, R.; Yao, Q.; Xu, Y.; Luo, Z.; Luo, X.; Wang, N. Docosahexaenoic Acid Attenuates Adipose Tissue Angiogenesis and Insulin Resistance in High Fat Diet-Fed Middle-Aged Mice via a Sirt1-Dependent Mechanism. Mol. Nutr. Food Res. 2016, 60, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Ashraf ElFar, O.; Billa, N.; Ren Lim, H.; Wayne Chew, K.; Yan Cheah, W.; Siti Halimatul Munawaroh, H.; Balakrishnan, D.; Loke, P. Advances in Delivery Methods of Arthrospira Platensis (Spirulina) for Enhanced Therapeutic Outcomes. Bioengineered 2022, 13, 14681–14718. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Lin, L.; Yang, X.; Pan, Q.; Cheng, X. Antidiabetic Potential of Phycocyanin: Effects on KKAy Mice. Pharm. Biol. 2013, 51, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Li, F.; Li, Q.; Yang, Q.; Zhang, W. Phycocyanin Protects against High Glucose High Fat Diet Induced Diabetes in Mice and Participates in AKT and AMPK Signaling. Foods 2022, 11, 3183. [Google Scholar] [CrossRef]
- Ou, Y.; Ren, Z.; Wang, J.; Yang, X. Phycocyanin Ameliorates Alloxan-Induced Diabetes Mellitus in Mice: Involved in Insulin Signaling Pathway and GK Expression. Chem. Biol. Interact. 2016, 247, 49–54. [Google Scholar] [CrossRef]
- Li, C.; Wu, H.; Xiang, W.; Wu, H.; Wang, N.; Wu, J.; Li, T. Comparison of Production and Fluorescence Characteristics of Phycoerythrin from Three Strains of Porphyridium. Foods 2022, 11, 2069. [Google Scholar] [CrossRef]
- Soni, B.; Visavadiya, N.P.; Madamwar, D. Attenuation of Diabetic Complications by C-Phycoerythrin in Rats: Antioxidant Activity of C-Phycoerythrin Including Copper-Induced Lipoprotein and Serum Oxidation. Br. J. Nutr. 2009, 102, 102–109. [Google Scholar] [CrossRef]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Molecules Impact of Different Storage Methods on Bioactive Compounds in Arthrospira Platensis Biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef] [PubMed]
- Ennaji, H.; Bourhia, M.; Taouam, I.; Falaq, A.; Bellahcen, T.O.; Salamatullah, A.M.; Alzahrani, A.; Khalil Alyahya, H.; Ullah, R.; Ibenmoussa, S.; et al. Physicochemical Evaluation of Edible Cyanobacterium Arthrospira Platensis Collected from the South Atlantic Coast of Morocco: A Promising Source of Dietary Supplements. Evid.-Based Complement. Altern. Med. 2021, 2021, 3337231. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Conte, G.M.; Salviati, E.; Pepe, G.; Bertamino, A.; Ostacolo, C.; Sansone, F.; Del Prete, F.; Aquino, R.P.; Campiglia, P. Fast Profiling of Natural Pigments in Different Spirulina (Arthrospira platensis) Dietary Supplements by DI-FT-ICR and Evaluation of Their Antioxidant Potential by Pre-Column DPPH-UHPLC Assay. Molecules 2018, 23, 1152. [Google Scholar] [CrossRef] [PubMed]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Naito, Y.; Hasegawa, G.; Nakamura, N.; Takahashi, J.; Yoshikawa, T. Astaxanthin Protects Beta-Cells against Glucose Toxicity in Diabetic Db/Db Mice. Redox Rep. 2002, 7, 290–293. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S.; Pappa, G.D.; Louli, V.; Magoulas, K.; Voutsas, E. Experimental Design and Optimization of Recovering Bioactive Compounds from Chlorella Vulgaris through Conventional Extraction. Molecules 2022, 27, 29. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Subcritical Water Extraction and Characterization of Bioactive Compounds from Haematococcus Pluvialis Microalga. J. Pharm. Biomed. Anal. 2010, 51, 456–463. [Google Scholar] [CrossRef]
- Todorović, B.; Grujić, V.J.; Krajnc, A.U.; Kranvogl, R.; Ambrožič-Dolinšek, J. Identification and Content of Astaxanthin and Its Esters from Microalgae Haematococcus Pluvialis by HPLC-DAD and LC-QTOF-MS after Extraction with Various Solvents. Plants 2021, 10, 2413. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Total Lipids Content, Lipid Class and Fatty Acid Composition of Ten Species of Microalgae. J Oleo Sci 2020, 69, 1181–1189. [Google Scholar] [CrossRef]
- Lin, H.T.V.; Tsou, Y.C.; Chen, Y.T.; Lu, W.J.; Hwang, P.A. Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes. Mar. Drugs 2017, 15, 113. [Google Scholar] [CrossRef]
- Kou, L.; Du, M.; Zhang, C.; Dai, Z.; Li, X.; Zhang, B. The Hypoglycemic, Hypolipidemic, and Anti-Diabetic Nephritic Activities of Zeaxanthin in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats. Appl. Biochem. Biotechnol. 2017, 182, 944–955. [Google Scholar] [CrossRef]
- Juin, C.; Bonnet, A.; Nicolau, E.; Bérard, J.B.; Devillers, R.; Thiéry, V.; Cadoret, J.P.; Picot, L. UPLC-MSE Profiling of Phytoplankton Metabolites: Application to the Identification of Pigments and Structural Analysis of Metabolites in Porphyridium Purpureum. Mar. Drugs 2015, 13, 2541. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Chien, J.T.; Chen, B.H. Improved High Performance Liquid Chromatographic Method for Determination of Carotenoids in the Microalga Chlorella Pyrenoidosa. J. Chromatogr. A 2006, 1102, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Vaz, B.d.S.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a New Source of Bioactive Compounds in Food Supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Fang, M.; Zheng, J.H.; Ren, D.F.; Lu, J. Optimised Extraction of β-Carotene from Spirulina Platensis and Hypoglycaemic Effect in Streptozotocin-Induced Diabetic Mice. J. Sci. Food Agric. 2016, 96, 1783–1789. [Google Scholar] [CrossRef]
- Peña-Medina, R.L.; Fimbres-Olivarría, D.; Enríquez-Ocaña, L.F.; Martínez-Córdova, L.R.; Del-Toro-Sánchez, C.L.; López-Elías, J.A.; González-Vega, R.I. Erythroprotective Potential of Phycobiliproteins Extracted from Porphyridium Cruentum. Metabolites 2023, 13, 366. [Google Scholar] [CrossRef]
- El-Adl, M.F.; Deyab, M.A.; Ghazal, M.A.; Elsadany, A.Y. Impact of the Microalga Dunaliella Salina (Dunal) Teodoresco Culture and Its β-Carotene Extract on the Development of Salt-Stressed Squash (Cucurbita pepo L. Cv. Mabrouka). Physiol. Mol. Biol. Plants 2022, 28, 749. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Shen, S.; Ji, X.; Chen, F.; Liao, X.; Zhang, H.; Zhang, Y. Inhibitory Effects of Chlorophylls and Its Derivative on Starch Digestion in Vitro. Food Chem. 2023, 413, 135377. [Google Scholar] [CrossRef]
- Semaan, D.G.; Igoli, J.O.; Young, L.; Gray, A.I.; Rowan, E.G.; Marrero, E. In Vitro Anti-Diabetic Effect of Flavonoids and Pheophytins from Allophylus Cominia Sw. on the Glucose Uptake Assays by HepG2, L6, 3T3-L1 and Fat Accumulation in 3T3-L1 Adipocytes. J. Ethnopharmacol. 2018, 216, 8–17. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.J.; Han, J.S. Pheophorbide A from Gelidium Amansii Improves Postprandial Hyperglycemia in Diabetic Mice through α-Glucosidase Inhibition. Phytother. Res. 2019, 33, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Higuchi, O.; Sasaki, M.; Ota, M.; Aida, T.; Takekoshi, H.; Inomata, H.; Miyazawa, T. Removal of Chlorophyll and Pheophorbide from Chlorella Pyrenoidosa by Supercritical Fluid Extraction: Potential of Protein Resource. Biosci. Biotechnol. Biochem. 2021, 85, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, O.; Topsakal, S.; Haligur, M.; Aydogan, A.; Dincoglu, D. Effects of Caffeine and Lycopene in Experimentally Induced Diabetes Mellitus. Pancreas 2016, 45, 579–583. [Google Scholar] [CrossRef]
- Sharavana, G.; Joseph, G.S.; Baskaran, V. Lutein Attenuates Oxidative Stress Markers and Ameliorates Glucose Homeostasis through Polyol Pathway in Heart and Kidney of STZ-Induced Hyperglycemic Rat Model. Eur. J. Nutr. 2017, 56, 2475–2485. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; Martín-Pozuelo, G.; González-Barrio, R.; Navarro-González, I.; Pallarés, F.J.; Santaella, M.; García-Alonso, J.; Sevilla, Á.; Periago-Castón, M.J. Ameliorative Effect of Spinach on Non-Alcoholic Fatty Liver Disease Induced in Rats by a High-Fat Diet. Int. J. Mol. Sci. 2019, 20, 1662. [Google Scholar] [CrossRef]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; El Majdoub, Y.O.; Kounnoun, A.; Miceli, N.; Taviano, M.F.; Mondello, L.; et al. The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef]
- Vieira, M.V.; Turkiewicz, I.P.; Tkacz, K.; Fuentes-Grünewald, C.; Pastrana, L.M.; Fuciños, P.; Wojdyło, A.; Nowicka, P. Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species. Molecules 2021, 26, 7593. [Google Scholar] [CrossRef]
- Zhou, N.; Long, H.; Wang, C.; Zhu, Z.; Yu, L.; Yang, W.; Ren, X.; Liu, X. Characterization of Selenium-Containing Polysaccharide from Spirulina Platensis and Its Protective Role against Cd-Induced Toxicity. Int. J. Biol. Macromol. 2020, 164, 2465–2476. [Google Scholar] [CrossRef]
- Li, T.T.; Huang, Z.R.; Jia, R.B.; Lv, X.C.; Zhao, C.; Liu, B. Spirulina Platensis Polysaccharides Attenuate Lipid and Carbohydrate Metabolism Disorder in High-Sucrose and High-Fat Diet-Fed Rats in Association with Intestinal Microbiota. Food Res. Int. 2021, 147, 110530. [Google Scholar] [CrossRef]
- Setyaningsih, I.; Prasetyo, H.; Agungpriyono, D.R.; Tarman, K. Antihyperglycemic Activity of Porphyridium Cruentum Biomass and Extra-Cellular Polysaccharide in Streptozotocin-Induced Diabetic Rats. Int. J. Biol. Macromol. 2020, 156, 1381–1386. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Sun, L.; Gao, Y. Characterization and Anti-Diabetic Evaluation of Sulfated Polysaccharide from Spirulina Platensis. J. Funct. Foods 2022, 95, 105155. [Google Scholar] [CrossRef]
- Casas-Arrojo, V.; Decara, J.; de los Ángeles Arrojo-Agudo, M.; Pérez-Manríquez, C.; Abdala-Díaz, R.T. Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium Cruentum. (S.F.Gray) Nägeli. Biomolecules 2021, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Wu, L.; Tong, A.; Zhao, L.; Liu, B.; Zhao, C. Physicochemical Characterization of Polysaccharides from Chlorella Pyrenoidosa and Its Anti-Ageing Effects in Drosophila Melanogaster. Carbohydr. Polym. 2018, 185, 120–126. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Collins, C.; Saha, S.K.; Major, I.; Murray, P. Sustainable Production and Pharmaceutical Applications of β-Glucan from Microbial Sources. Microbiol. Res. 2023, 274, 127424. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Raymond, K. Beta-Glucans in the Treatment of Diabetes and Associated Cardiovascular Risks. Vasc. Health Risk Manag. 2008, 4, 1265. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.J.; D’Souza, P.P.; Fazal, F.; Kumar, A.; Bhat, H.P.; Baliga, M.S. Anti-Diabetic Effects of the Indian Indigenous Fruit Emblica Officinalis Gaertn: Active Constituents and Modes of Action. Food Funct. 2014, 5, 635–644. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmed, Z.A.; Mahwi, T.O.; Aziz, T.A. Effect of Quercetin on Postprandial Glucose Excursion after Mono- and Disaccharides Challenge in Normal and Diabetic Rats. J. Diabetes Mellit. 2012, 2012, 82–87. [Google Scholar] [CrossRef]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The Molecular Basis of the Antidiabetic Action of Quercetin in Cultured Skeletal Muscle Cells and Hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [CrossRef]
- Dhanya, R.; Kartha, C.C. Quercetin Improves Oxidative Stress-Induced Pancreatic Beta Cell Alterations via MTOR-Signaling. Mol. Cell. Biochem. 2021, 476, 3879–3887. [Google Scholar] [CrossRef]
- Bellahcen, T.O.; Aamiri, A.; Touam, I.; Hmimid, F.; El Amrani, A.; Cherif, A.; Cherki, M. Evaluation of Moroccan Microalgae: Spirulina platensis as a Potential Source of Natural Antioxidants. J. Complement. Integr. Med. 2020, 17, 20190036. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Q.M.; Hu, H.J.; Yang, L.; Yang, Y.B.; Chou, G.X.; Wang, Z.T. Bioactive Diterpenoids and Flavonoids from the Aerial Parts of Scoparia Dulcis. J. Nat. Prod. 2014, 77, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Cazarolli, L.H.; Folador, P.; Moresco, H.H.; Brighente, I.M.C.; Pizzolatti, M.G.; Silva, F.R.M.B. Mechanism of Action of the Stimulatory Effect of Apigenin-6-C-(2′-O-Alpha-l-Rhamnopyranosyl)-Beta-L-Fucopyranoside on 14C-Glucose Uptake. Chem. Biol. Interact. 2009, 179, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Cho, Y.Y.; Choi, M.S. Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients 2016, 8, 305. [Google Scholar] [CrossRef]
- Wang, N.; Yi, W.J.; Tan, L.; Zhang, J.H.; Xu, J.; Chen, Y.; Qin, M.; Yu, S.; Guan, J.; Zhang, R. Apigenin Attenuates Streptozotocin-Induced Pancreatic β Cell Damage by Its Protective Effects on Cellular Antioxidant Defense. Vitr. Cell. Dev. Biol.-Anim. 2017, 53, 554–563. [Google Scholar] [CrossRef]
- Zhang, B.W.; Li, X.; Sun, W.L.; Xing, Y.; Xiu, Z.L.; Zhuang, C.L.; Dong, Y.S. Dietary Flavonoids and Acarbose Synergistically Inhibit α-Glucosidase and Lower Postprandial Blood Glucose. J. Agric. Food Chem. 2017, 65, 8319–8330. [Google Scholar] [CrossRef]
- Shanak, S.; Bassalat, N.; Albzoor, R.; Kadan, S.; Zaid, H. In Vitro and In Silico Evaluation for the Inhibitory Action of O. basilicum Methanol Extract on α-Glucosidase and α-Amylase. Evid.-Based Complement. Altern. Med. 2021, 2021, 5515775. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jembrek, M.J. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef] [PubMed]
- Vanella, L.; Tibullo, D.; Godos, J.; Pluchinotta, F.R.; Di Giacomo, C.; Sorrenti, V.; Acquaviva, R.; Russo, A.; Li Volti, G.; Barbagallo, I. Caffeic Acid Phenethyl Ester Regulates PPAR’s Levels in Stem Cells-Derived Adipocytes. PPAR Res. 2016, 2016, 7359521. [Google Scholar] [CrossRef]
- Un, J.J.; Lee, M.K.; Yong, B.P.; Jeon, S.M.; Choi, M.S. Antihyperglycemic and Antioxidant Properties of Caffeic Acid in Db/Db Mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef]
- Amalan, V.; Vijayakumar, N.; Indumathi, D.; Ramakrishnan, A. Antidiabetic and Antihyperlipidemic Activity of P-Coumaric Acid in Diabetic Rats, Role of Pancreatic GLUT 2: In Vivo Approach. Biomed. Pharmacother. 2016, 84, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.; El-Twab, S.M.A.; Yousef, A.I.; Reheim, E.S.A.; Ashour, M.B. Modulation of Hyperglycemia and Dyslipidemia in Experimental Type 2 Diabetes by Gallic Acid and P-Coumaric Acid: The Role of Adipocytokines and PPARγ. Biomed. Pharmacother. 2018, 105, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition Mechanism of Ferulic Acid against α-Amylase and α-Glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic Acid Exerts Its Antidiabetic Effect by Modulating Insulin-Signalling Molecules in the Liver of High-Fat Diet and Fructose-Induced Type-2 Diabetic Adult Male Rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Zhang, N.; Ji, Z.; Ma, Z.; Fu, Q.; Qu, R.; Ma, S. Ferulic Acid Attenuates Diabetes-Induced Cognitive Impairment in Rats via Regulation of PTP1B and Insulin Signaling Pathway. Physiol. Behav. 2017, 182, 93–100. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, J.; Li, H. Ferulic Acid Confers Protection on Islet β Cells and Placental Tissues of Rats with Gestational Diabetes Mellitus. Cell. Mol. Biol. 2020, 66, 37–41. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Q.; Zhao, T. Preventive Effects of Kaempferol on High-Fat Diet-Induced Obesity Complications in C57BL/6 Mice. Biomed. Res. Int. 2020, 2020, 4532482. [Google Scholar] [CrossRef]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative Effect of Kaempferol, a Flavonoid, on Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D. Flavonol Kaempferol Improves Chronic Hyperglycemia-Impaired Pancreatic Beta-Cell Viability and Insulin Secretory Function. Eur. J. Pharmacol. 2011, 670, 325–332. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, W.; Maechler, P.; Liu, D. Small Molecule Kaempferol Modulates PDX-1 Protein Expression and Subsequently Promotes Pancreatic β-Cell Survival and Function via CREB. J. Nutr. Biochem. 2013, 24, 638–646. [Google Scholar] [CrossRef]
- Wu, J.B.; Kuo, Y.H.; Lin, C.H.; Ho, H.Y.; Shih, C.C. Tormentic Acid, a Major Component of Suspension Cells of Eriobotrya Japonica, Suppresses High-Fat Diet-Induced Diabetes and Hyperlipidemia by Glucose Transporter 4 and AMP-Activated Protein Kinase Phosphorylation. J. Agric. Food Chem. 2014, 62, 10717–10726. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, H.; Duan, W.; Mu, D.; Zhang, L. Maslinic Acid Reduces Blood Glucose in KK-Ay Mice. Biol. Pharm. Bull. 2007, 30, 2075–2078. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Chen, Y.P.; Mao, L.F.; Shang, J.; Sun, H.B.; Zhang, L.Y. Maslinic Acid Modulates Glycogen Metabolism by Enhancing the Insulin Signaling Pathway and Inhibiting Glycogen Phosphorylase. Chin. J. Nat. Med. 2014, 12, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. Inhibitory Effect of Corosolic Acid on α-Glucosidase: Kinetics, Interaction Mechanism, and Molecular Simulation. J. Sci. Food Agric. 2019, 99, 5881–5889. [Google Scholar] [CrossRef]
- Miura, T.; Ueda, N.; Yamada, K.; Fukushima, M.; Ishida, T.; Kaneko, T.; Matsuyama, F.; Seino, Y. Antidiabetic Effects of Corosolic Acid in KK-Ay Diabetic Mice. Biol. Pharm. Bull. 2006, 29, 585–587. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Kumar, V.; Prakash, O. Enzymes Inhibition and Antidiabetic Effect of Isolated Constituents from Dillenia Indica. BioMed Res. Int. 2013, 2013, 382063. [Google Scholar] [CrossRef]
- Gomes Castro, A.J.; Silva Frederico, M.J.; Cazarolli, L.H.; Bretanha, L.C.; Tavares, L.D.C.; Buss, Z.D.S.; Dutra, M.F.; Pacheco De Souza, A.Z.; Pizzolatti, M.G.; Silva, F.R.M.B. Betulinic Acid and 1,25(OH)2 Vitamin D₃ Share Intracellular Signal Transduction in Glucose Homeostasis in Soleus Muscle. Int. J. Biochem. Cell Biol. 2014, 48, 18–27. [Google Scholar] [CrossRef]
- de Melo, C.L.; Queiroz, M.G.R.; Fonseca, S.G.C.; Bizerra, A.M.C.; Lemos, T.L.G.; Melo, T.S.; Santos, F.A.; Rao, V.S. Oleanolic Acid, a Natural Triterpenoid Improves Blood Glucose Tolerance in Normal Mice and Ameliorates Visceral Obesity in Mice Fed a High-Fat Diet. Chem. Biol. Interact. 2010, 185, 59–65. [Google Scholar] [CrossRef]
- Salah El Dine, R.; Ma, Q.; Kandil, Z.A.; El-Halawany, A.M. Triterpenes as Uncompetitive Inhibitors of α-Glucosidase from Flowers of Punica Granatum L. Nat. Prod. Res. 2014, 28, 2191–2194. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic Acid Improves Hepatic Insulin Resistance via Antioxidant, Hypolipidemic and Anti-Inflammatory Effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef]
- Teodoro, T.; Zhang, L.; Alexander, T.; Yue, J.; Vranic, M.; Volchuk, A. Oleanolic Acid Enhances Insulin Secretion in Pancreatic Beta-Cells. FEBS Lett. 2008, 582, 1375–1380. [Google Scholar] [CrossRef]
- Nataraju, A.; Saini, D.; Ramachandran, S.; Benshoff, N.; Liu, W.; Chapman, W.; Mohanakumar, T. Oleanolic Acid, a Plant Triterpenoid, Significantly Improves Survival and Function of Islet Allograft. Transplantation 2009, 88, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Etsassala, N.G.E.R.; Badmus, J.A.; Waryo, T.T.; Marnewick, J.L.; Cupido, C.N.; Hussein, A.A.; Iwuoha, E.I. Alpha-Glucosidase and Alpha-Amylase Inhibitory Activities of Novel Abietane Diterpenes from Salvia Africana-Lutea. Antioxidants 2019, 8, 421. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.M.; Yee, S.T.; Choi, J.; Choi, M.S.; Do, G.M.; Jeon, S.M.; Yeo, J.; Kim, M.J.; Seo, K.I.; Lee, M.K. Ursolic Acid Enhances the Cellular Immune System and Pancreatic Beta-Cell Function in Streptozotocin-Induced Diabetic Mice Fed a High-Fat Diet. Int. Immunopharmacol. 2009, 9, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Villaró, S.; Jiménez-Márquez, S.; Musari, E.; Bermejo, R.; Lafarga, T. Production of Enzymatic Hydrolysates with in Vitro Antioxidant, Antihypertensive, and Antidiabetic Properties from Proteins Derived from Arthrospira Platensis. Food Res. Int. 2023, 163, 112270. [Google Scholar] [CrossRef]
- Cunha, S.A.; Coscueta, E.R.; Nova, P.; Silva, J.L.; Pintado, M.M. Bioactive Hydrolysates from Chlorella Vulgaris: Optimal Process and Bioactive Properties. Molecules 2022, 27, 2505. [Google Scholar] [CrossRef]
- Li, Y.; Aiello, G.; Fassi, E.M.A.; Boschin, G.; Bartolomei, M.; Bollati, C.; Roda, G.; Arnoldi, A.; Grazioso, G.; Lammi, C. Investigation of Chlorella Pyrenoidosa Protein as a Source of Novel Angiotensin I-Converting Enzyme (ACE) and Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Peptides. Nutrients 2021, 13, 1624. [Google Scholar] [CrossRef]
- He, W.; Xie, J.; Xia, Z.; Chen, X.; Xiao, J.; Cao, Y.; Liu, X. A Novel Peptide Derived from Haematococcus Pluvialis Residue Exhibits Anti-Aging Activity in Caenorhabditis Elegans via the Insulin/IGF-1 Signaling Pathway. Food Funct. 2023, 14, 5576–5588. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Ai, W.; Zhang, F.; Yang, K.; Wang, L.; Zhu, X.; Gao, P.; Shu, G.; Jiang, Q.; et al. Phytol Increases Adipocyte Number and Glucose Tolerance through Activation of PI3K/Akt Signaling Pathway in Mice Fed High-Fat and High-Fructose Diet. Biochem. Biophys. Res. Commun. 2017, 489, 432–438. [Google Scholar] [CrossRef]
- Moldes-Anaya, A.; Sæther, T.; Uhlig, S.; Nebb, H.I.; Larsen, T.; Eilertsen, H.C.; Paulsen, S.M. Two Isomeric C16 Oxo-Fatty Acids from the Diatom Chaetoceros Karianus Show Dual Agonist Activity towards Human Peroxisome Proliferator-Activated Receptors (PPARs) α/γ. Mar. Drugs 2017, 15, 148. [Google Scholar] [CrossRef]
- Sæther, T.; Paulsen, S.M.; Tungen, J.E.; Vik, A.; Aursnes, M.; Holen, T.; Hansen, T.V.; Nebb, H.I. Synthesis and Biological Evaluations of Marine Oxohexadecenoic Acids: PPARα/γ Dual Agonism and Anti-Diabetic Target Gene Effects. Eur. J. Med. Chem. 2018, 155, 736–753. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kim, Y.I.; Furuzono, T.; Takahashi, N.; Yamakuni, K.; Yang, H.E.; Li, Y.; Ohue, R.; Nomura, W.; Sugawara, T.; et al. 10-Oxo-12(Z)-Octadecenoic Acid, a Linoleic Acid Metabolite Produced by Gut Lactic Acid Bacteria, Potently Activates PPARγ and Stimulates Adipogenesis. Biochem. Biophys. Res. Commun. 2015, 459, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Gradíssimo, D.G.; da Silva, V.C.O.; Xavier, L.P.; Do Nascimento, S.V.; Valadares, R.B.d.S.; Faustino, S.M.M.; Schneider, M.P.C.; Santos, A.V. Glucosidase Inhibitors Screening in Microalgae and Cyanobacteria Isolated from the Amazon and Proteomic Analysis of Inhibitor Producing Synechococcus sp. GFB01. Microorganisms 2021, 9, 1593. [Google Scholar] [CrossRef] [PubMed]
- Kawee-Ai, A.; Kim, A.T.; Kim, S.M. Inhibitory Activities of Microalgal Fucoxanthin against α-Amylase, α-Glucosidase, and Glucose Oxidase in 3T3-L1 Cells Linked to Type 2 Diabetes. J. Oceanol. Limnol. 2019, 37, 928–937. [Google Scholar] [CrossRef]
- Joventino, I.P.; Alves, H.G.R.; Neves, L.C.; Pinheiro-Joventino, F.; Leal, L.K.A.M.; Neves, S.A.; Ferreira, F.V.; Brito, G.A.C.; Viana, G.B. The Microalga Spirulina Platensis Presents Anti-Inflammatory Action as Well as Hypoglycemic and Hypolipidemic Properties in Diabetic Rats. J. Complement. Integr. Med. 2012, 9, 17. [Google Scholar] [CrossRef]
- Qaddoumi, M.G.; Alanbaei, M.; Hammad, M.M.; Al Khairi, I.; Cherian, P.; Channanath, A.; Thanaraj, T.A.; Al-Mulla, F.; Abu-Farha, M.; Abubaker, J. Investigating the Role of Myeloperoxidase and Angiopoietin-like Protein 6 in Obesity and Diabetes. Sci. Rep. 2020, 10, 6170. [Google Scholar] [CrossRef]
- Munawaroh, H.S.H.; Gumilar, G.G.; Nurjanah, F.; Yuliani, G.; Aisyah, S.; Kurnia, D.; Wulandari, A.P.; Kurniawan, I.; Ningrum, A.; Koyande, A.K.; et al. In-Vitro Molecular Docking Analysis of Microalgae Extracted Phycocyanin as an Anti-Diabetic Candidate. Biochem. Eng. J. 2020, 161, 107666. [Google Scholar] [CrossRef]
- Yi, Z.; Su, Y.; Brynjolfsson, S.; Olafsdóttir, K.; Fu, W. Bioactive Polysaccharides and Their Derivatives from Microalgae: Biosynthesis, Applications, and Challenges. Stud. Nat. Prod. Chem. 2021, 71, 67–85. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Kim, Y.; Kim, E.Y. Effect of DPP-IV Inhibitors on Glycemic Variability in Patients with T2DM: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 13296. [Google Scholar] [CrossRef]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in Diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef]
- Jadhav, P.B.; Jadhav, S.B.; Zehravi, M.; Mubarak, M.S.; Islam, F.; Jeandet, P.; Khan, S.L.; Hossain, N.; Rashid, S.; Ming, L.C.; et al. Virtual Screening, Synthesis, and Biological Evaluation of Some Carbohydrazide Derivatives as Potential DPP-IV Inhibitors. Molecules 2023, 28, 149. [Google Scholar] [CrossRef]
- Chong, S.C.; Sukor, N.; Robert, S.A.; Ng, K.F.; Kamaruddin, N.A. Endogenous GLP-1 Levels Play an Important Role in Determining the Efficacy of DPP-IV Inhibitors in Both Prediabetes and Type 2 Diabetes. Front. Endocrinol. 2022, 13, 1012412. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of Energy Metabolism by Long-Chain Fatty Acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, E.; Amodeo, P.; Vitale, R.M. Marine Natural and Nature-Inspired Compounds Targeting Peroxisome Proliferator Activated Receptors (PPARs). Mar. Drugs. 2023, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Gupta, P.; Saini, A.; Kaushal, C.; Sharma, S. The Peroxisome Proliferator-Activated Receptor: A Family of Nuclear Receptors Role in Various Diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236. [Google Scholar] [CrossRef]
- Gao, H.; Geng, T.; Huang, T.; Zhao, Q. Fish Oil Supplementation and Insulin Sensitivity: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2017, 16, 131. [Google Scholar] [CrossRef]
- Padanad, M.S.; Konstantinidou, G.; Venkateswaran, N.; Melegari, M.; Rindhe, S.; Mitsche, M.; Yang, C.; Batten, K.; Huffman, K.E.; Liu, J.; et al. Fatty Acid Oxidation Mediated by Acyl-CoA Synthetase Long Chain 3 Is Required for Mutant KRAS Lung Tumorigenesis. Cell Rep. 2016, 16, 1614–1628. [Google Scholar] [CrossRef]
- Kersten, S. Role and Mechanism of the Action of Angiopoietin-like Protein ANGPTL4 in Plasma Lipid Metabolism. J. Lipid Res. 2021, 62, 100150. [Google Scholar] [CrossRef]
- Xu, A.; Lam, M.C.; Chan, K.W.; Wang, Y.; Zhang, J.; Boo, R.L.C.; Xu, J.Y.; Chen, B.; Chow, W.S.; Tso, A.W.K.; et al. Angiopoietin-like Protein 4 Decreases Blood Glucose and Improves Glucose Tolerance but Induces Hyperlipidemia and Hepatic Steatosis in Mice. Proc. Natl. Acad. Sci. USA 2005, 102, 6086–6091. [Google Scholar] [CrossRef]
- Lagathu, C.; Yvan-Charvet, L.; Bastard, J.P.; Maachi, M.; Quignard-Boulangé, A.; Capeau, J.; Caron, M. Long-Term Treatment with Interleukin-1β Induces Insulin Resistance in Murine and Human Adipocytes. Diabetologia 2006, 49, 2162–2173. [Google Scholar] [CrossRef]
- Jager, J.; Grémeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.F. Interleukin-1β-Induced Insulin Resistance in Adipocytes through Down-Regulation of Insulin Receptor Substrate-1 Expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Fereidouni, A.; Khaleghian, A.; Mousavi-Niri, N.; Moradikor, N. The Effects of Supplementation of Nannochloropsis Oculata Microalgae on Biochemical, Inflammatory and Antioxidant Responses in Diabetic Rats. Biomol. Concepts 2022, 13, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Gao, X.; Chen, R.; Lu, S.; Wan, X.; Farag, M.A.; Zhao, C. Metabolomics and Biochemical Insights on the Regulation of Aging-Related Diabetes by a Low-Molecular-Weight Polysaccharide from Green Microalga Chlorella Pyrenoidosa. Food Chem. X 2022, 14, 100316. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Pliego, L.E.; Martínez-Carrillo, B.E.; Reséndiz-Albor, A.A.; Valdés-Ramos, R. Effect on Adipose Tissue of Diabetic Mice Supplemented with N-3 Fatty Acids Extracted from Microalgae. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Nasirian, F.; Sarir, H.; Moradi-Kor, N. Antihyperglycemic and Antihyperlipidemic Activities of Nannochloropsis Oculata Microalgae in Streptozotocin-Induced Diabetic Rats. Biomol. Concepts 2019, 10, 37–43. [Google Scholar] [CrossRef]
- Nasirian, F.; Dadkhah, M.; Moradi-Kor, N.; Obeidavi, Z. Effects of Spirulina Platensis Microalgae on Antioxidant and Anti-Inflammatory Factors in Diabetic Rats. Diabetes Metab. Syndr. Obes. 2018, 11, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Pliego, L.E.; Martínez-Carrillo, B.E.; Reséndiz-Albor, A.A.; Arciniega-Martínez, I.M.; Escoto-Herrera, J.A.; Rosales-Gómez, C.A.; Valdés-Ramos, R. Effect of Supplementation with N-3 Fatty Acids Extracted from Microalgae on Inflammation Biomarkers from Two Different Strains of Mice. J. Lipids 2018, 2018, 4765358. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.M.; Lebda, M.A.; Nasr, S.M.; Shoukry, M. Spirulina Platensis Prevents Hyperglycemia in Rats by Modulating Gluconeogenesis and Apoptosis via Modification of Oxidative Stress and MAPK-Pathways. Biomed. Pharmacother. 2017, 92, 1085–1094. [Google Scholar] [CrossRef]
- Ruperez, F.J.; Garcia-Martinez, D.; Baena, B.; Maeso, N.; Vallejo, M.; Angulo, S.; Garcia, A.; Ibañez, E.; Señorans, F.J.; Cifuentes, A.; et al. Dunaliella Salina Extract Effect on Diabetic Rats: Metabolic Fingerprinting and Target Metabolite Analysis. J. Pharm. Biomed. Anal. 2009, 49, 786–792. [Google Scholar] [CrossRef]
- Bitam, A.; Aissaoui, O. Spirulina Platensis, Oxidative Stress, and Diabetes; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128157763. [Google Scholar]
- Bohórquez-Medina, S.L.; Bohórquez-Medina, A.L.; Benites Zapata, V.A.; Ignacio-Cconchoy, F.L.; Toro-Huamanchumo, C.J.; Bendezu-Quispe, G.; Pacheco-Mendoza, J.; Hernandez, A.V. Impact of Spirulina Supplementation on Obesity-Related Metabolic Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. NFS J. 2021, 25, 21–30. [Google Scholar] [CrossRef]
- Godavari, A.; Manickamoorthi, N. Role of Micronutrients and Trace Elements in Diabetes Mellitus: A Review. In Structure and Health Effects of Natural Products on Diabetes Mellitus; Springer: Singapore, 2021; pp. 297–307. [Google Scholar] [CrossRef]
- Akhuemokhan, I.K.; Eregie, A.; Fasanmade, O.A. Diabetes Prevention and Management: The Role of Trace Minerals. Afr. J. Diabetes Med. 2013, 21, 37–41. [Google Scholar]
- Battin, E.E.; Brumaghim, J.L. Antioxidant Activity of Sulfur and Selenium: A Review of Reactive Oxygen Species Scavenging, Glutathione Peroxidase, and Metal-Binding Antioxidant Mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef]
- Chen, M.D.; Liou, S.J.; Lin, P.I.Y.; Yang, V.C.; Alexander, P.S.; Lin, W.H. Effects of Zinc Supplementation on the Plasma Glucose Level and Insulin Activity in Genetically Obese (Ob/Ob) Mice. Biol. Trace Elem. Res. 1998, 61, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Nazem, M.R.; Asadi, M.; Adelipour, M.; Jabbari, N.; Allameh, A. Zinc Supplementation Ameliorates Type 2 Diabetes Markers through the Enhancement of Total Antioxidant Capacity in Overweight Patients. Postgrad. Med. J. 2022, 99, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Viktorínová, A.; Tošerová, E.; Križko, M.; Ďuračková, Z. Altered Metabolism of Copper, Zinc, and Magnesium Is Associated with Increased Levels of Glycated Hemoglobin in Patients with Diabetes Mellitus. Metabolism 2009, 58, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Zhang, X.H.; Russell, J.C.; Hulver, M.; Cefalu, W.T. Chromium Picolinate Enhances Skeletal Muscle Cellular Insulin Signaling In Vivo in Obese, Insulin-Resistant JCR:LA-Cp Rats. J. Nutr. 2006, 136, 415–420. [Google Scholar] [CrossRef]
- Robertson, R.P.; Harmon, J.S. Diabetes, Glucose Toxicity, and Oxidative Stress: A Case of Double Jeopardy for the Pancreatic Islet β Cell. Free Radic. Biol. Med. 2006, 41, 177–184. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Y.; Yang, C.; Liu, B.; Huang, Y. Hypotensive, Hypoglycaemic and Hypolipidaemic Effects of Bioactive Compounds from Microalgae and Marine Micro-Organisms. Int. J. Food Sci. Technol. 2015, 50, 1705–1717. [Google Scholar] [CrossRef]
- Wen, L.; Ley, R.E.; Volchkov, P.Y.; Stranges, P.B.; Avanesyan, L.; Stonebraker, A.C.; Hu, C.; Wong, F.S.; Szot, G.L.; Bluestone, J.A.; et al. Innate Immunity and Intestinal Microbiota in the Development of Type 1 Diabetes. Nature 2008, 455, 1109–1113. [Google Scholar] [CrossRef]
- Kiesel, V.A.; Sheeley, M.P.; Coleman, M.F.; Cotul, E.K.; Donkin, S.S.; Hursting, S.D.; Wendt, M.K.; Teegarden, D. Pyruvate Carboxylase and Cancer Progression. Cancer Metab. 2021, 9, 20. [Google Scholar] [CrossRef]
- Hughey, C.C.; Crawford, P.A. Pyruvate Carboxylase Wields a Double-Edged Metabolic Sword. Cell Metab. 2019, 29, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Jörns, A.; Tiedge, M.; Lenzen, S.; Munday, R. Effect of Superoxide Dismutase, Catalase, Chelating Agents, and Free Radical Scavengers on the Toxicity of Alloxan to Isolated Pancreatic Islets in Vitro. Free Radic. Biol. Med. 1999, 26, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Hamid, Z.A.; Budin, S.B. The Potential Role of Flavonoids in Ameliorating Diabetic Cardiomyopathy via Alleviation of Cardiac Oxidative Stress, Inflammation and Apoptosis. Int. J. Mol. Sci. 2021, 22, 5094. [Google Scholar] [CrossRef] [PubMed]
- Abo-Shady, A.M.; Gheda, S.F.; Ismail, G.A.; Cotas, J.; Pereira, L.; Abdel-Karim, O.H. Antioxidant and Antidiabetic Activity of Algae. Life 2023, 13, 460. [Google Scholar] [CrossRef]
- Andriopoulos, V.; Lamari, F.N.; Hatziantoniou, S.; Kornaros, M. Production of Antioxidants and High Value Biomass from Nannochloropsis Oculata: Effects of PH, Temperature and Light Period in Batch Photobioreactors. Mar. Drugs 2022, 20, 552. [Google Scholar] [CrossRef]
- Casanova, P.; Monleon, D. Role of Selenium in Type 2 Diabetes, Insulin Resistance and Insulin Secretion. World J. Diabetes 2023, 14, 147. [Google Scholar] [CrossRef]
- Kosmalski, M.; Pękala-Wojciechowska, A.; Sut, A.; Pietras, T.; Luzak, B. Dietary Intake of Polyphenols or Polyunsaturated Fatty Acids and Its Relationship with Metabolic and Inflammatory State in Patients with Type 2 Diabetes Mellitus. Nutrients 2022, 14, 1083. [Google Scholar] [CrossRef]
- Wu, G.; Bai, Z.; Wan, Y.; Shi, H.; Huang, X.; Nie, S. Antidiabetic Effects of Polysaccharide from Azuki Bean (Vigna angularis) in Type 2 Diabetic Rats via Insulin/PI3K/AKT Signaling Pathway. Food Hydrocoll. 2020, 101, 105456. [Google Scholar] [CrossRef]
- Sundaram, R.; Nandhakumar, E.; Haseena Banu, H. Hesperidin, a Citrus Flavonoid Ameliorates Hyperglycemia by Regulating Key Enzymes of Carbohydrate Metabolism in Streptozotocin-Induced Diabetic Rats. Toxicol. Mech. Methods 2019, 29, 644–653. [Google Scholar] [CrossRef]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Zhang, K.; Pan, S.; Nie, S. Hypoglycemic and Hypolipidemic Effects of Glucomannan Extracted from Konjac on Type 2 Diabetic Rats. J. Agric. Food Chem. 2019, 67, 5278–5288. [Google Scholar] [CrossRef]
- Kreilmeier-Berger, T.; Zeugswetter, F.K.; Blohm, K.O.; Schwendenwein, I.; Baszler, E.; Ploderer, B.; Burgener, I.A.; Künzel, F. Successful Insulin Glargine Treatment in Two Pet Guinea Pigs with Suspected Type 1 Diabetes Mellitus. Animals 2021, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Teng, J.; Dong, R.; Ban, Q.; Yang, L.; Du, K.; Wang, Y.; Pu, H.; Yang, C.S.; Ren, Z. Alleviating Effects and Mechanisms of Action of Large-Leaf Yellow Tea Drinking on Diabetes and Diabetic Nephropathy in Mice. Food Sci. Hum. Wellness 2023, 12, 1660–1673. [Google Scholar] [CrossRef]
- Yaas, A.A.; Al-Shakour, A.A.; Mansour, A.A. Assessment of Serum Level of Protein Carbonyl as a Marker of Protein Oxidation in Patients with Type 2 Diabetes Mellitus. AL-Kindy Coll. Med. J. 2022, 18, 190–195. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; de Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; John, A. Streptozotocin-Induced Cytotoxicity, Oxidative Stress and Mitochondrial Dysfunction in Human Hepatoma HepG2 Cells. Int. J. Mol. Sci. 2012, 13, 5751–5767. [Google Scholar] [CrossRef] [PubMed]
- Tayanloo-Beik, A.; Kiasalari, Z.; Roghani, M. Paeonol Ameliorates Cognitive Deficits in Streptozotocin Murine Model of Sporadic Alzheimer’s Disease via Attenuation of Oxidative Stress, Inflammation, and Mitochondrial Dysfunction. J. Mol. Neurosci. 2022, 72, 336–348. [Google Scholar] [CrossRef]
- Bendaoud, A.; Baba Ahmed, F.Z.; Merzouk, H.; Bouanane, S.; Bendimerad, S. Effects of Dietary Microalgae Nannochloropsis Gaditana on Serum and Redox Status in Obese Rats Subjected to a High Fat Diet. Phytothérapie 2019, 17, 177–187. [Google Scholar] [CrossRef]
- Cichoński, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef]
- Gallego, R.; Valdés, A.; Suárez-Montenegro, Z.J.; Sánchez-Martínez, J.D.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Anti-Inflammatory and Neuroprotective Evaluation of Diverse Microalgae Extracts Enriched in Carotenoids. Algal Res. 2022, 67, 102830. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Govoni, S.; Coppola, A.; Gazzaruso, C. The Role of Gut Microbiota in Obesity, Diabetes Mellitus, and Effect of Metformin: New Insights into Old Diseases. Curr. Opin. Pharmacol. 2019, 49, 1–5. [Google Scholar] [CrossRef]
- Lambeth, S.M.; Carson, T.; Lowe, J.; Ramaraj, T.; Leff, J.W.; Luo, L.; Bell, C.J.; Shah, V.O. Composition, Diversity and Abundance of Gut Microbiome in Prediabetes and Type 2 Diabetes. J. Diabetes Obes. 2015, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jones-Hall, Y.L.; Kozik, A.; Nakatsu, C. Correction: Ablation of Tumor Necrosis Factor Is Associated with Decreased Inflammation and Alterations of the Microbiota in a Mouse Model of Inflammatory Bowel Disease. PLoS ONE 2015, 10, e0125309. [Google Scholar] [CrossRef]
- Ibrahim, K.S.; Bourwis, N.; Dolan, S.; Lang, S.; Spencer, J.; Craft, J.A. Characterisation of Gut Microbiota of Obesity and Type 2 Diabetes in a Rodent. Biosci. Microbiota Food Health 2021, 40, 65–74. [Google Scholar] [CrossRef]
- Song, Y.; Wu, M.S.; Tao, G.; Lu, M.W.; Lin, J.; Huang, J.Q. Feruloylated Oligosaccharides and Ferulic Acid Alter Gut Microbiome to Alleviate Diabetic Syndrome. Food Res. Int. 2020, 137, 109410. [Google Scholar] [CrossRef]
- Wan, X.; Li, T.; Liu, D.; Chen, Y.; Liu, Y.; Liu, B.; Zhang, H.; Zhao, C. Effect of Marine Microalga Chlorella Pyrenoidosa Ethanol Extract on Lipid Metabolism and Gut Microbiota Composition in High-Fat Diet-Fed Rats. Mar. Drugs 2018, 16, 498. [Google Scholar] [CrossRef]
- Widyaningrum, D.; Prianto, A.D. Chlorella as a Source of Functional Food Ingredients: Short Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 794, 012148. [Google Scholar] [CrossRef]
- Perego, C.; Da Dalt, L.; Pirillo, A.; Galli, A.; Catapano, A.L.; Norata, G.D. Cholesterol Metabolism, Pancreatic β-Cell Function and Diabetes. Biochim. Biophys. Acta-Mol. 2019, 1865, 2149–2156. [Google Scholar] [CrossRef]
- Yong, J.; Johnson, J.D.; Arvan, P.; Han, J.; Kaufman, R.J. Therapeutic Opportunities for Pancreatic β-Cell ER Stress in Diabetes Mellitus. Nat. Rev. Endocrinol. 2021, 17, 455–467. [Google Scholar] [CrossRef]
- Semwal, D.K.; Kumar, A.; Aswal, S.; Chauhan, A.; Semwal, R.B. Protective and Therapeutic Effects of Natural Products against Diabetes Mellitus via Regenerating Pancreatic β-Cells and Restoring Their Dysfunction. Phytother. Res. 2021, 35, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, U.H.; Lee, S.B.; Jeong, G.T.; Kim, S.K. Improvement of Unsaturated Fatty Acid Production from Porphyridium Cruentum Using a Two-Phase Culture System in a Photobioreactor with Light-Emitting Diodes (LEDs). J. Microbiol. Biotechnol. 2021, 31, 456–463. [Google Scholar] [CrossRef]
- Liberti, D.; Imbimbo, P.; Giustino, E.; D’Elia, L.; Ferraro, G.; Casillo, A.; Illiano, A.; Pinto, G.; Di Meo, M.C.; Alvarez-Rivera, G.; et al. Inside out Porphyridium Cruentum: Beyond the Conventional Biorefinery Concept. ACS Sustain. Chem. Eng. 2023, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M.; Howard, C.P. Improper Insulin Compliance May Lead to Hepatomegaly and Elevated Hepatic Enzymes in Type 1 Diabetic Patients. Diabetes Care 2004, 27, 619–620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salgıntaş, H.H.; Dönmez, N.; Özsan, M. The Effect of Curcumin on the Antioxidant System in Diabetic Rats. J. Hell. Vet. Med. Soc. 2022, 72, 3279–3284. [Google Scholar] [CrossRef]
- Arab Sadeghabadi, Z.; Abbasalipourkabir, R.; Mohseni, R.; Ziamajidi, N. Investigation of Oxidative Stress Markers and Antioxidant Enzymes Activity in Newly Diagnosed Type 2 Diabetes Patients and Healthy Subjects, Association with IL-6 Level. J. Diabetes Metab. Disord. 2019, 18, 437–443. [Google Scholar] [CrossRef]
- Lutchmansingh, F.K.; Hsu, J.W.; Bennett, F.I.; Badaloo, A.V.; Norma, M.A.; Georgiana, M.G.S.; Rosemarie, A.W.P.; Jahoor, F.; Boyne, M.S. Glutathione Metabolism in Type 2 Diabetes and Its Relationship with Microvascular Complications and Glycemia. PLoS ONE 2018, 13, e0198626. [Google Scholar] [CrossRef]
- Zafar, H.; Mirza, I.A.; Hussain, W.; Fayyaz, M. Comparative Efficacy of Tocotrienol and Tocopherol for Their Anti Diabetic Effects Coupling And Condensation of Azo Compounds and Other Aromatic Compounds View Project Candida Auris Outbreak Report from Pakistan: A Success Story of Infection Control in Tertiary Care Hospital View Project Comparative Efficacy of Tocotrienol and Tocopherol for Their Anti Diabetic Effects. Biomed. J. Sci. Tech. Res. 2021, 38, 30835–30840. [Google Scholar] [CrossRef]
- Wei, W.; Hu, M.; Huang, J.; Yu, S.; Li, X.; Li, Y.; Mao, L. Anti-Obesity Effects of DHA and EPA in High Fat-Induced Insulin Resistant Mice. Food Funct. 2021, 12, 1614–1625. [Google Scholar] [CrossRef]
- Song, X.; Tian, S.; Liu, Y.; Shan, Y. Effects of Omega-3 PUFA Supplementation on Insulin Resistance and Lipid Metabolism in Patients with T2DM: A Systematic Review and Meta-Analysis. Curr. Dev. Nutr. 2020, 4, 77. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine N−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Q.; Liao, X.; Elbelt, U.; Weylandt, K.H. The Effects of Omega-3 Fatty Acids in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Prostaglandins Leukot. Essent. Fat. Acids 2022, 182, 102456. [Google Scholar] [CrossRef]
- Mishra, N.; Gupta, E.; Singh, P. Application of Microalgae Metabolites in Food and Pharmaceutical Industry Screening of Stevia Rebaundiana, Therapeutic Benefits beyond Sweetness, by Using In Vitro Systems View Project Potential Use of Brown Algae Isochrysis Galbana in Bakery Products View Project; Academic Press: Cambridge, MA, USA, 2021; ISBN 9780128202845. [Google Scholar]
- Haimeur, A.; Mimouni, V.; Ulmann, L.; Martineau, A.S.; Messaouri, H.; Pineau-Vincent, F.; Tremblin, G.; Meskini, N. Fish Oil and Microalga Omega-3 as Dietary Supplements: A Comparative Study on Cardiovascular Risk Factors in High-Fat Fed Rats. Lipids 2016, 51, 1037–1049. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, X.; Chen, D.; Li, Z. The Controversial Role of Adiponectin in Appetite Regulation of Animals. Nutrients 2021, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, P.; Calvo, P.; Pujol, A.; Rivera, R.; Berga, F.; Fortuny, R.; Costa-Bauza, A.; Grases, F.; Masmiquel, L. Daily Phytate Intake Increases Adiponectin Levels among Patients with Diabetes Type 2: A Randomized Crossover Trial. Nutr. Diabetes 2023, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.F.; Haines, D.; Dashti, A.A.; El-Shazly, S.; Al-Najjar, F. Correlation between Heat Shock Proteins, Adiponectin, and T Lymphocyte Cytokine Expression in Type 2 Diabetics. Cell Stress Chaperones 2018, 23, 955–965. [Google Scholar] [CrossRef]
- Kintscher, U.; Hartge, M.; Hess, K.; Foryst-Ludwig, A.; Clemenz, M.; Wabitsch, M.; Fischer-Posovszky, P.; Barth, T.F.E.; Dragun, D.; Skurk, T.; et al. T-Lymphocyte Infiltration in Visceral Adipose Tissue. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1304–1310. [Google Scholar] [CrossRef]
- Lunn, J.; Buttriss, J.L. Carbohydrates and Dietary Fibre. Nutr. Bull. 2007, 32, 21–64. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Liu, H.; Su, J.; Lan, C.Q.; Zhong, M.; Hu, X. Production, Isolation and Bioactive Estimation of Extracellular Polysaccharides of Green Microalga Neochloris Oleoabundans. Algal Res. 2020, 48, 101883. [Google Scholar] [CrossRef]
- Mandaliya, D.K.; Seshadri, S. Short Chain Fatty Acids, Pancreatic Dysfunction and Type 2 Diabetes. Pancreatology 2019, 19, 617–622. [Google Scholar] [CrossRef]
- Benchoula, K.; Arya, A.; Parhar, I.S.; Hwa, W.E. FoxO1 Signaling as a Therapeutic Target for Type 2 Diabetes and Obesity. Eur. J. Pharmacol. 2021, 891, 173758. [Google Scholar] [CrossRef]
- Malhotra, K.; Katsanos, A.H.; Lambadiari, V.; Goyal, N.; Palaiodimou, L.; Kosmidou, M.; Krogias, C.; Alexandrov, A.V.; Tsivgoulis, G. GLP-1 Receptor Agonists in Diabetes for Stroke Prevention: A Systematic Review and Meta-Analysis. J. Neurol. 2020, 267, 2117–2122. [Google Scholar] [CrossRef]
- Amin, A.; Lotfy, M.; Mahmoud-Ghoneim, D.; Adeghate, E.; Al-Akhras, M.A.; Al-Saadi, M.; Al-Rahmoun, S.; Hameed, R. Pancreas-Protective Effects of Chlorella in STZ-Induced Diabetic Animal Model: Insights into the Mechanism. J. Diabetes Mellit. 2011, 1, 36–45. [Google Scholar] [CrossRef]
- Seedevi, P.; Ramu Ganesan, A.; Moovendhan, M.; Mohan, K.; Sivasankar, P.; Loganathan, S.; Vairamani, S.; Shanmugam, A. Anti-Diabetic Activity of Crude Polysaccharide and Rhamnose-Enriched Polysaccharide from G. lithophila on Streptozotocin (STZ)-Induced in Wistar Rats. Sci. Rep. 2020, 10, 556. [Google Scholar] [CrossRef]
- Szkudelski, T. The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Baccari, M.C.; Vannucchi, M.G.; Idrizaj, E. Glucagon-Like Peptide-2 in the Control of Gastrointestinal Motility: Physiological Implications. Curr. Protein Pept. Sci. 2022, 23, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Feng, M.; Zhu, X.; Long, J.; Zhou, Z.; Liu, S. WR-GLP2, a Glucagon-like Peptide 2 from Hybrid Crucian Carp That Protects Intestinal Mucosal Barrier and Inhibits Bacterial Infection. Fish Shellfish Immunol. 2022, 122, 29–37. [Google Scholar] [CrossRef]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and Studies on Lipid and Pigments of Microalgae: A Review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Xiao, J.; Wang, M.; Chen, F.; Cheng, K.W. Multi-Mechanistic Antidiabetic Potential of Astaxanthin: An Update on Preclinical and Clinical Evidence. Mol. Nutr. Food Res. 2021, 65, 2100252. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Tambat, V.S.; Chen, C.W.; Chauhan, A.S.; Kumar, P.; Vadrale, A.P.; Huang, C.Y.; Dong, C.D.; Singhania, R.R. Recent Advancements in Astaxanthin Production from Microalgae: A Review. Bioresour. Technol. 2022, 364, 128030. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194. [Google Scholar] [CrossRef]
- Sakayanathan, P.; Loganathan, C.; Iruthayaraj, A.; Periyasamy, P.; Poomani, K.; Periasamy, V.; Thayumanavan, P. Biological Interaction of Newly Synthesized Astaxanthin-s-Allyl Cysteine Biconjugate with Saccharomyces Cerevisiae and Mammalian α-Glucosidase: In Vitro Kinetics and in Silico Docking Analysis. Int. J. Biol. Macromol. 2018, 118, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Beutner, S.; Bloedorn, B.; Frixel, S.; Blanco, I.H.; Hoffmann, T.; Martin, H.D.; Mayer, B.; Noack, P.; Ruck, C.; Schmidt, M.; et al. Quantitative Assessment of Antioxidant Properties of Natural Colorants and Phytochemicals: Carotenoids, Flavonoids, Phenols and Indigoids. The Role of β-Carotene in Antioxidant Functions. J. Sci. Food Agric. 2001, 81, 559–568. [Google Scholar] [CrossRef]
- Kaur, K.; Sachdeva, R.; Grover, K. Effect of Supplementation of Spirulina on Blood Glucose and Lipid Profile of the Non-Insulin Dependent Diabetic Male Subjects. J. Dairy. Foods Home Sci. 2008, 27, 202–208. [Google Scholar]
- Anitha, L.; Chandralekha, K. Effect of Supplementation of Spirulina on Blood Glucose, Glycosylated Hemoglobin and Lipid Profile of Male Non-Insulin Dependent Diabetics. Asian J. Exp. Biol. Sci. 2010, 1, 36–46. [Google Scholar]
- Mani, U.V.; Desai, S.; Iyer, U. Studies on the Long-Term Effect of Spirulina Supplementation on Serum Lipid Profile and Glycated Proteins in NIDDM Patients. J. Nutraceuticals Funct. Med. Foods 2000, 2, 25–32. [Google Scholar] [CrossRef]
- Alam, A.; Quamri, S.; Fatima, S.; Roqaiya, M.; Ahmad, Z. Efficacy of Spirulina (Tahlab) in Patients of Type 2 Diabetes Mellitus (Ziabetus Shakri)—A Randomized Controlled Trial. J. Diabetes Metab. 2016, 7, 1–5. [Google Scholar] [CrossRef]
- Beihaghi, M.; Ghodrati Azadi, H.; Taherzadeh, Z.; Bahrami, H. reza The Effects of Oral Administration of Spirulina Platensis (Cultured Iranian) on Blood Glucose and Glycosylated Hemoglobin Blood in Type Ii Diabetes Mellitus Patients. Iran. J. Diabetes Metab. 2017, 16, 183–190. [Google Scholar]
- Karizi, S.R.; Armanmehr, F.; Azadi, H.G.; Zahroodi, H.S.; Ghalibaf, A.A.M.; Bazzaz, B.S.F.; Abbaspour, M.; Boskabadi, J.; Eslami, S.; Taherzadeh, Z. A Randomized, Double-Blind Placebo-Controlled Add-on Trial to Assess the Efficacy, Safety, and Anti-Atherogenic Effect of Spirulina Platensis in Patients with Inadequately Controlled Type 2 Diabetes Mellitus. Phytother. Res. 2023, 37, 1435–1448. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Niccolai, A.; Tredici, M.R.; Biondi, N.; Rodolfi, L.; Lodovici, M.; D’Ambrosio, M.; Mori, G.; Luceri, C. Safety Evaluations and Lipid-Lowering Activity of an Arthrospira Platensis Enriched Diet: A 1-Month Study in Rats. Food Res. Int. 2017, 102, 380–386. [Google Scholar] [CrossRef]
- Brendler, T.; Williamson, E.M. Astaxanthin: How Much Is Too Much? A Safety Review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira Platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 2023, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- González-Arceo, M.; Gómez-Zorita, S.; Aguirre, L.; Portillo, M.P. Effect of Microalgae and Macroalgae Extracts on Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 2017. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Harwanto, D.; Tirtawijaya, G.; Negara, B.F.S.P.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism. Mar. Drugs 2021, 19, 545. [Google Scholar] [CrossRef]
- Kagan, M.L.; Matulka, R.A. Safety Assessment of the Microalgae Nannochloropsis Oculata. Toxicol. Rep. 2015, 2, 617–623. [Google Scholar] [CrossRef]
- Kagan, M.L.; Sullivan, D.W.; Gad, S.C.; Ballou, C.M. Safety Assessment of EPA-Rich Polar Lipid Oil Produced from the Microalgae Nannochloropsis Oculata. Int. J. Toxicol. 2014, 33, 459–474. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Aly, H.F.; Salama, A.A.A. Toxicity Assessment of the Green Dunaliella Salina Microalgae. Toxicol. Rep. 2019, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.S. Bioremediation of Heavy Metals Using Microalgae: Recent Advances and Mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).