Microwave-Assisted Hydrothermal Processing of Rugulopteryx okamurae
Abstract
1. Introduction
2. Results and Discussion
2.1. Raw Material
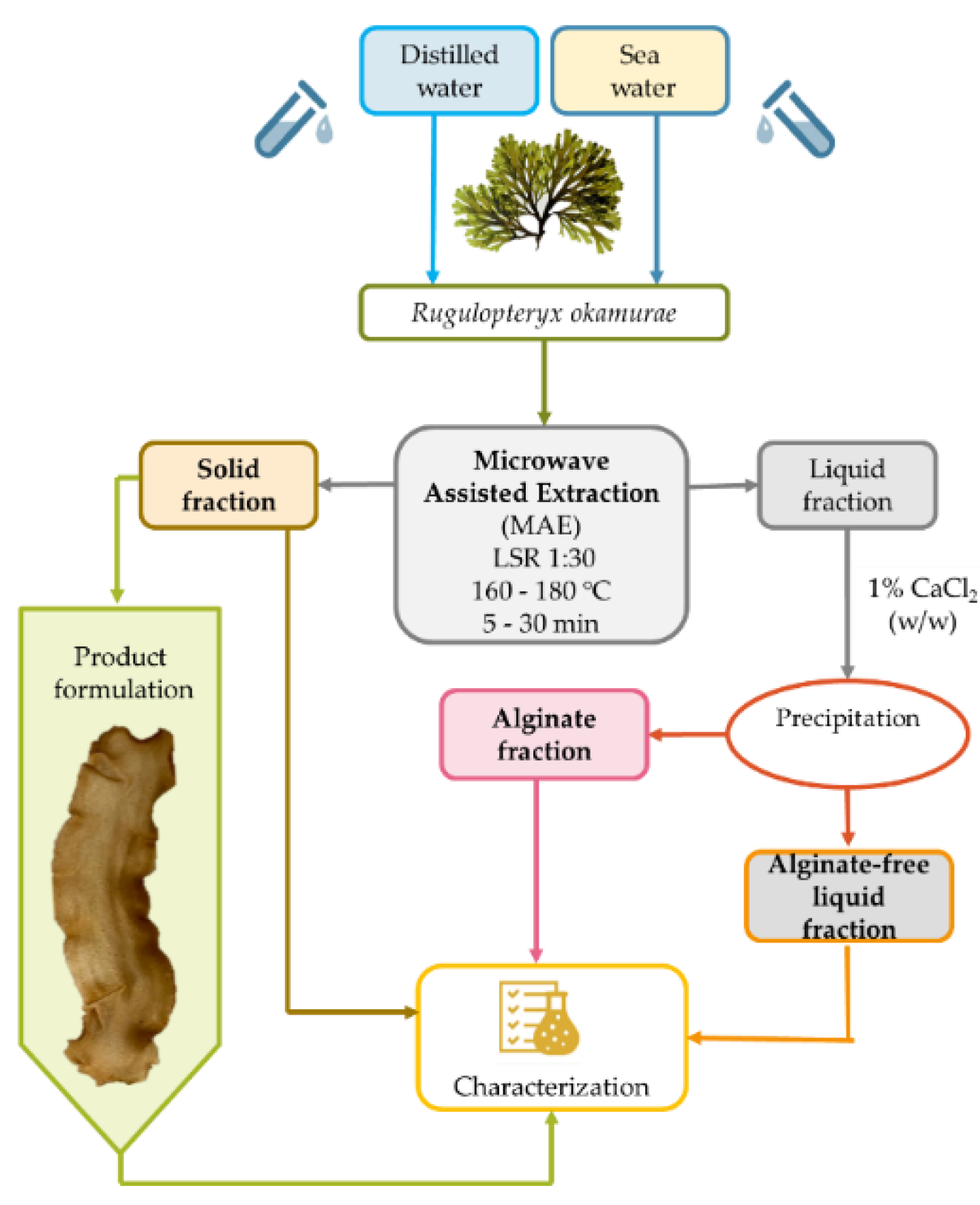
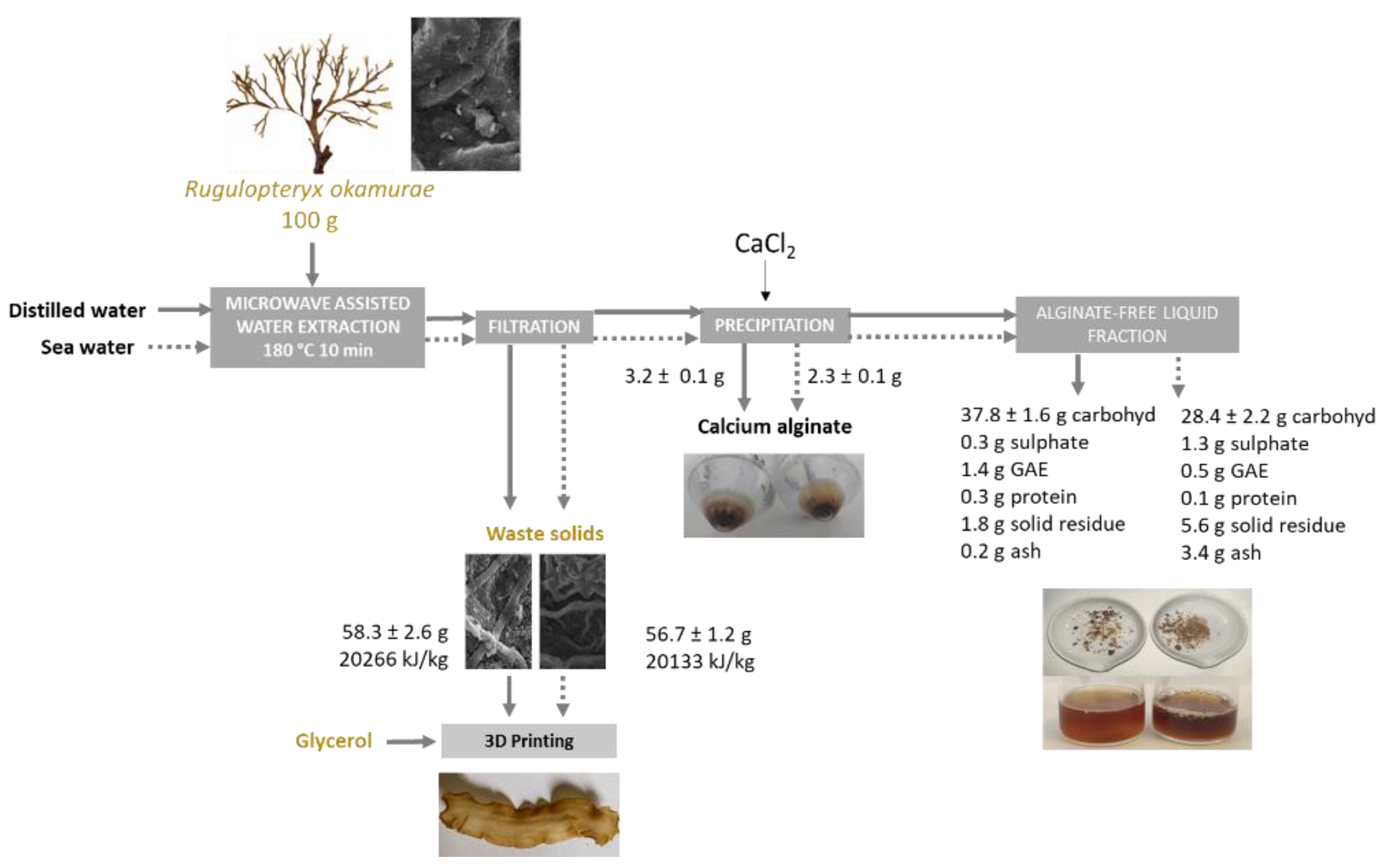
2.2. Microwave-Assisted Extraction
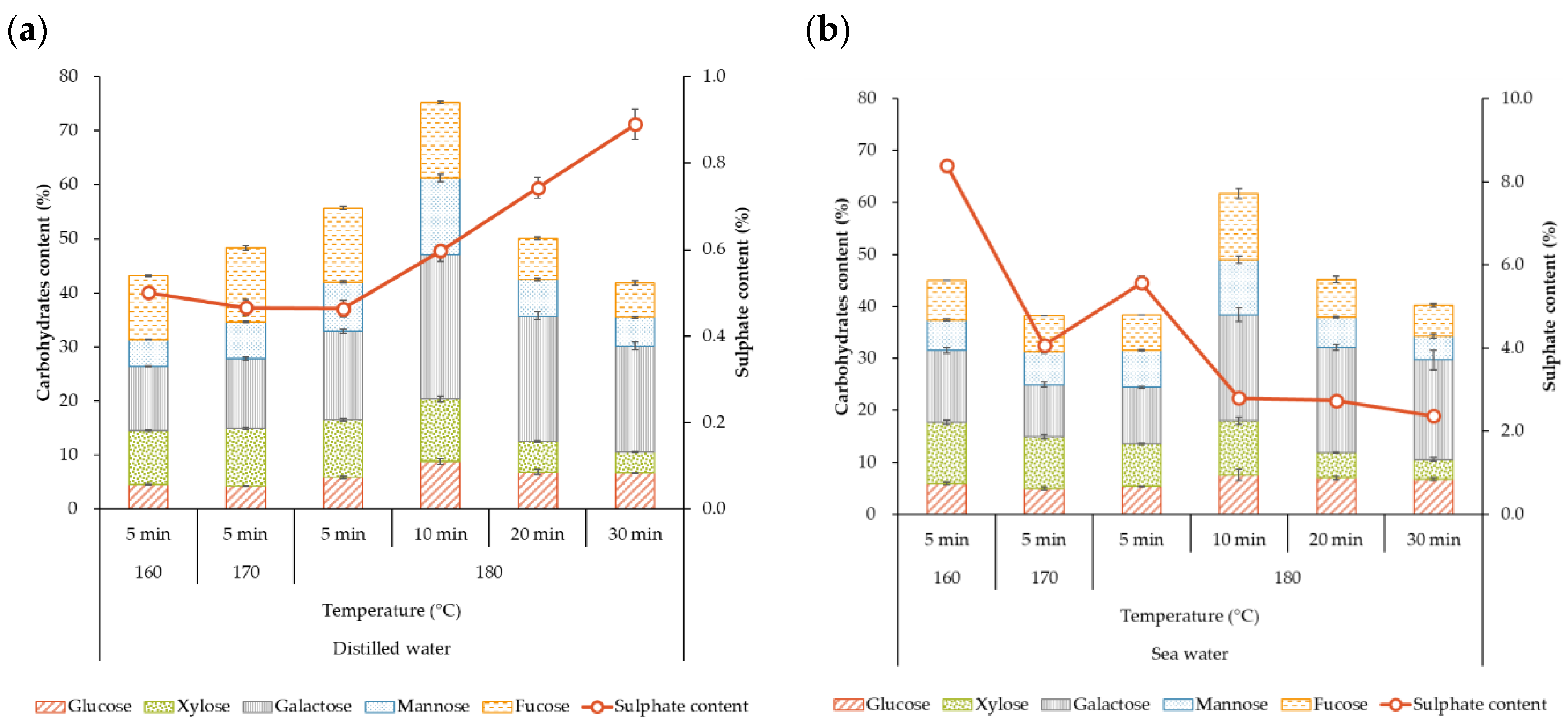
2.2.1. Extraction Yield and Characteristics of the Liquid Fraction
2.2.2. Cytotoxicity
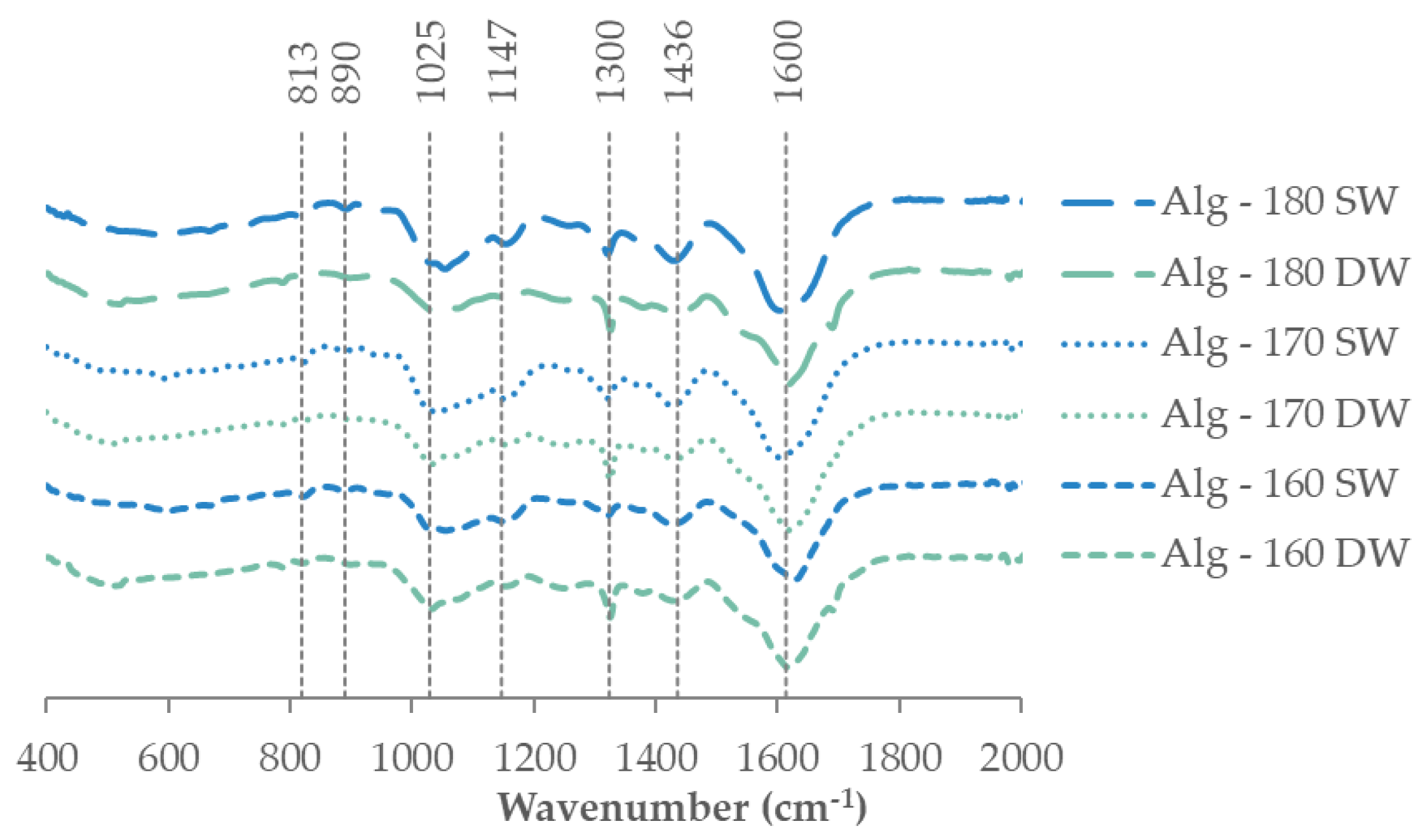
2.2.3. Alginate Recovery and Characterization
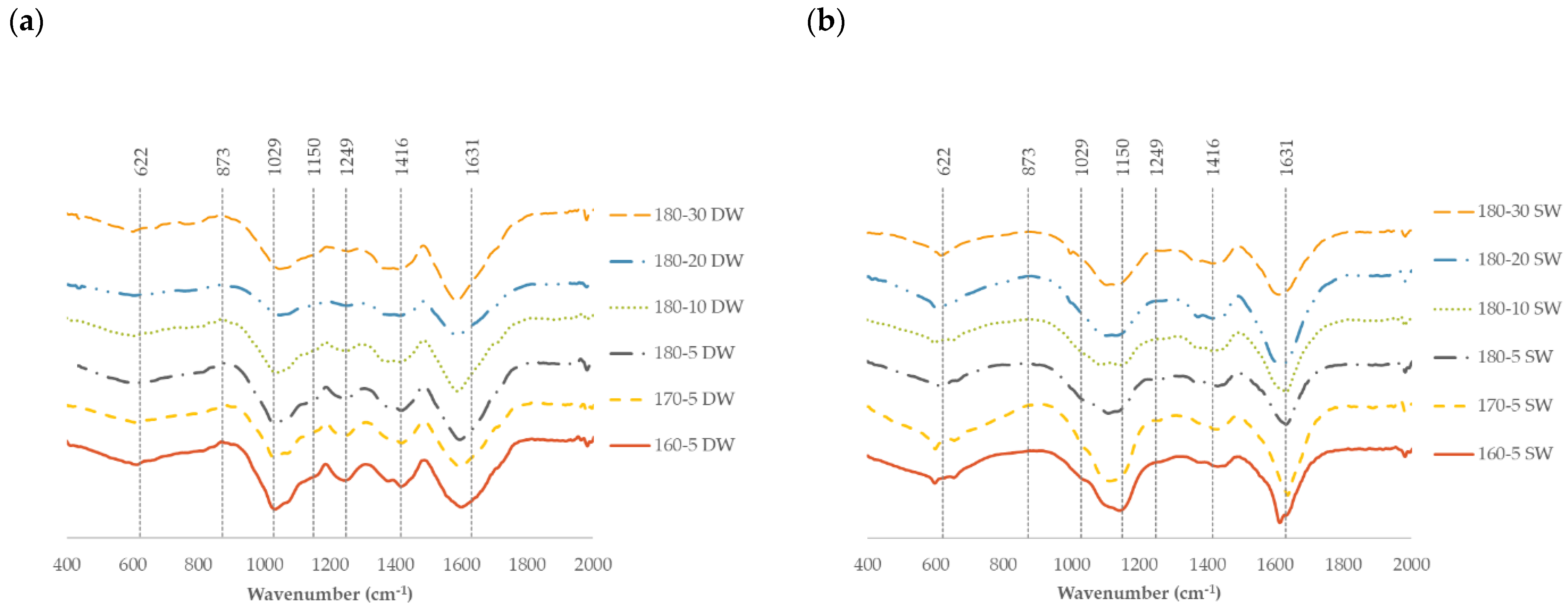
2.3. Characteristics of the Solid Fraction
3. Materials and Methods
3.1. Raw Material
3.2. Microwave Assisted Extraction
3.3. Alginate Precipitation
3.4. Analytical Methods for Raw Material and Solid Residue
3.5. Analytical Methods for Liquid Samples
3.6. Phloroglucinol Content
3.7. Sulfate Content
3.8. Antioxidant Activity
3.9. Soluble Protein
3.10. Oligosaccharide Determination
3.11. Molar Mass Distribution
3.12. Fourier-Transform Infrared Spectroscopy
3.13. Proton Nuclear Magnetic Resonance
3.14. Cell Inhibition Assay
3.15. Rheology
3.16. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, I.K.; Wook, J.L.; Kim, H.S.; De Clerck, O. Taxonomic Reappraisal of Dilophus Okamurae (Dictyotales, Phaeophyta) from the Western Pacific Ocean. Phycologia 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Verlaque, M.; Steen, F.; De Clerck, O. Rugulopteryx (Dictyotales, Phaeophyceae), a Genus Recently Introduced to the Mediterranean. Phycologia 2009, 48, 536–542. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Sempere-Valverde, J.; Ostalé-Valriberas, E.; Martínez, M.; Olaya-Ponzone, L.; Roi González, A.; Espinosa, F.; Sánchez-Moyano, E.; Megina, C.; Parada, J.A. Rugulopteryx Okamurae (E.Y. Dawson) I.K. Hwang, W.J. Lee & H.S. Kim (Dictyotales, Ochrophyta), Alga Exótica “Explosiva” En El Estrecho de Gibraltar. Observaciones Preliminares de Su Distribución e Impacto. [Exotic “Explosive” Alga in the Strait of Gibraltar. Preliminary Observations of Its Distribution and Impact]. Almoraima. Rev. Estud. Campogibraltareños 2018, 49, 99–113. [Google Scholar]
- Faria, J.; Prestes, A.C.L.; Moreu, I.; Martins, G.M.; Neto, A.I.; Cacabelos, E. Arrival and Proliferation of the Invasive Seaweed Rugulopteryx Okamurae in NE Atlantic Islands. Bot. Mar. 2021, 65, 45–50. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Sempere-Valverde, J.; González, A.R.; Martínez-Chacón, M.; Olaya-Ponzone, L.; Sánchez-Moyano, E.; Ostalé-Valriberas, E.; Megina, C. From Exotic to Invasive in Record Time: The Extreme Impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the Strait of Gibraltar. Sci. Total Environ. 2020, 704, 135408. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Florido, M.; Olaya-Ponzone, L.; Sempere-Valverde, J.; Megina, C. The Invasive Macroalga Rugulopteryx Okamurae: Substrata Plasticity and Spatial Colonization Pressure on Resident Macroalgae. Front. Ecol. Evol. 2021, 9, 631754. [Google Scholar] [CrossRef]
- De la Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Ballesteros, M.; Ruiz-Salvador, Á.R.; Raposo, F.; García-Gómez, J.C.; Borja, R. Turning an Invasive Alien Species into a Valuable Biomass: Anaerobic Digestion of Rugulopteryx okamurae after Thermal and New Developed Low-Cost Mechanical Pretreatments. Sci. Total Environ. 2023, 856, 158914. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamada, H.; Kurata, K. Dictyterpenoids A and B, Two Novel Diterpenoids with Feeding-Deterrent Activity from the Brown Alga Dilophus Okamurae. J. Nat. Prod. 2002, 65, 121–125. [Google Scholar] [CrossRef]
- Casal-Porras, I.; Zubía, E.; Brun, F.G. Dilkamural: A Novel Chemical Weapon Involved in the Invasive Capacity of the Alga Rugulopteryx Okamurae in the Strait of Gibraltar. Estuar. Coast. Shelf Sci. 2021, 257, 107398. [Google Scholar] [CrossRef]
- Ninomiya, M.; Hirohara, H.; Onishi, J.I.; Kusumi, T. Chemical Study and Absolute Configuration of a New Marine Secospatane from the Brown Alga Dilophus okamurae. J. Org. Chem. 1999, 64, 5436–5440. [Google Scholar] [CrossRef]
- Cuevas, B.; Arroba, A.I.; de los Reyes, C.; Gómez-Jaramillo, L.; González-Montelongo, M.C.; Zubía, E. Diterpenoids from the Brown Alga Rugulopteryx okamurae and Their Anti-Inflammatory Activity. Mar. Drugs 2021, 19, 677. [Google Scholar] [CrossRef] [PubMed]
- de Paula, J.C.; Vallim, M.A.; Teixeira, V.L. What Are and Where Are the Bioactive Terpenoids Metabolites from Dictyotaceae (Phaeophyceae). Rev. Bras. Farmacogn. 2011, 21, 216–228. [Google Scholar] [CrossRef]
- Harada, H.; Kamei, Y. Selective Cytotoxicity of Marine Algae Extracts to Several Human Leukemic Cell Lines. Cytotechnology 1997, 25, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Qian, Z.-J.; Jin, Y.-J.; Kim, G.-O.; Yun, P.-Y.; Cho, T.O. Investigation of α-Glucosidase Inhibitory Activity of Ethanolic Extracts from 19 Species of Marine Macroalgae in Korea. Nat. Prod. Sci. 2012, 18, 130–136. [Google Scholar]
- de la Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Llanos, J.; Mancilla-Leytón, J.M.; Borja, R. Enhancing Methane Production from the Invasive Macroalga Rugulopteryx okamurae through Anaerobic Co-Digestion with Olive Mill Solid Waste: Process Performance and Kinetic Analysis. J. Appl. Phycol. 2021, 33, 4113–4124. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and Golden Seaweed Tides on the Rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef]
- Fernández-Medina, P.; Álvarez-Gallego, C.J.; Caro, I. Yield Evaluation of Enzyme Hydrolysis and Dark Fermentation of the Brown Seaweed Rugulopteryx Okamurae Hydrothermally Pretreated by Microwave Irradiation. J. Environ. Chem. Eng. 2022, 10, 108817. [Google Scholar] [CrossRef]
- Santana, I.; Félix, M.; Guerrero, A.; Bengoechea, C. Processing and Characterization of Bioplastics from the Invasive Seaweed Rugulopteryx okamurae. Polymers 2022, 14, 355. [Google Scholar] [CrossRef]
- Patón, D.; García-Gómez, J.C.; Loring, J.; Torres, A. Composting the Invasive Toxic Seaweed Rugulopteryx okamurae Using Five Invertebrate Species, and a Mini-Review on Composting Macroalgae. Waste Biomass Valorization 2023, 14, 167–184. [Google Scholar] [CrossRef]
- Caxiano, I.N.; Mello, P.A.; Alijó, P.H.; Teixeira, L.V.; Cano, R.F.; Maia, J.G.; Bastos, J.B.; Pavão, M.S. Continuous Design and Economic Analysis of a Sargassum Muticum Biorefinery Process. Bioresour. Technol. 2022, 343, 126152. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Illera, M.; Sánchez, M.; Lodeiro, P.; Torres, M.D.; López-Mosquera, M.E.; Soto, M.; de Vicente, M.S.; Domínguez, H. Integrated Valorization of Sargassum Muticum in Biorefineries. Chem. Eng. J. 2021, 404, 125635. [Google Scholar] [CrossRef]
- Mercado, J.M.; Gómez-Jakobsen, F.; Korbee, N.; Aviles, A.; Bonomi-Barufi, J.; Muñoz, M.; Reul, A.; Figueroa, F.L. Analyzing Environmental Factors That Favor the Growth of the Invasive Brown Macroalga Rugulopteryx okamurae (Ochrophyta): The Probable Role of the Nutrient Excess. Mar. Pollut. Bull. 2022, 174, 113315. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, C.; Tamura, S.; Suzuki, M.; Etomi, K.; Nii, N.; Hayashi, J.; Kanemaru, K. Continuous Microwave-Assisted Step-by-Step Extraction of Bioactive Water-Soluble Materials and Fucoidan from Brown Seaweed Undaria pinnatifida Waste. Biomass Convers. Biorefin. 2022, 1–10. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martinez, J.; Meireles, M.A.A. Fundamentals of Microwave Extraction. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: Boston, MA, USA, 2013; pp. 15–52. ISBN 978-1-4614-4830-3. [Google Scholar]
- Biller, P.; Friedman, C.; Ross, A.B. Hydrothermal Microwave Processing of Microalgae as a Pre-Treatment and Extraction Technique for Bio-Fuels and Bio-Products. Bioresour. Technol. 2013, 136, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Cernadas, H.; Flórez-Fernández, N.; González-Muñoz, M.J.; Domínguez, H.; Torres, M.D. Retrieving of High-Value Biomolecules from Edible Himanthalia Elongata Brown Seaweed Using Hydrothermal Processing. Food Bioprod. Process. 2019, 7, 275–286. [Google Scholar] [CrossRef]
- İşçimen, E.M.; Hayta, M. Microwave-Assisted Aqueous Two-Phase System Based Extraction of Phenolics from Pulses: Antioxidant Properties, Characterization and Encapsulation. Ind. Crops Prod. 2021, 173, 114144. [Google Scholar] [CrossRef]
- Mba, O.I.; Kwofie, E.M.; Ngadi, M. Kinetic Modelling of Polyphenol Degradation during Common Beans Soaking and Cooking. Heliyon 2019, 5, e01613. [Google Scholar] [CrossRef]
- Biesaga, M. Influence of Extraction Methods on Stability of Flavonoids. J. Chromatogr. A 2011, 1218, 2505–2512. [Google Scholar] [CrossRef]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on Phenolic Compounds Stability during Microwave-Assisted Extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, S.; Yu, H.; Guo, Z.; Wang, P.; Li, C.; Li, Z.; Li, P. Salt-assisted acid hydrolysis of chitosan to oligomers under microwave irradiation. Carbohydr. Res. 2005, 340, 2150–2153. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Ustyuzhanina, N.E.; Shashkov, A.S.; Thanh, T.T.T.; Bui, M.L.; Van Tran, T.T.; Bui, V.N.; Nifantiev, N.E.; Usov, A.I. A Sulfated Galactofucan from the Brown Alga Hormophysa cuneiformis (Fucales, Sargassaceae). Carbohydr. Res. 2018, 469, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Jiang, L.; Xu, Y.; Zhou, Y.; Shen, Y.; Xie, W.; Long, Z.; Zhou, J. Synthesis and Anticoagulant Activity of Sodium Alginate Sulfates. Carbohydr. Polym. 2011, 83, 1797–1803. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Monitoring of the Ultrasound Assisted Depolymerisation Kinetics of Fucoidans from Sargassum muticum Depending on the Rheology of the Corresponding Gels. J. Food Eng. 2021, 294, 110404. [Google Scholar] [CrossRef]
- Queffelec, J.; Flórez-Fernández, N.; Dominguez, H.; Torres, M.D. Microwave Hydrothermal Processing of Undaria Pinnatifida for Bioactive Peptides. Bioresour. Technol. 2021, 342, 125882. [Google Scholar] [CrossRef]
- Felenda, J.E.; Turek, C.; Stintzing, F.C. Antiproliferative Potential from Aqueous Viscum album L. Preparations and Their Main Constituents in Comparison with Ricin and Purothionin on Human Cancer Cells. J. Ethnopharmacol. 2019, 236, 100–107. [Google Scholar] [CrossRef]
- Pérez-Larrán, P.; Balboa, E.M.; Torres, M.D.; Domínguez, H. Antioxidant and Antitumoral Properties of Aqueous Fractions from Frozen Sargassum muticum. Waste Biomass Valorization 2020, 11, 1261–1269. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A Green Approach for Alginate Extraction from Sargassum muticum Brown Seaweed Using Ultrasound-Assisted Technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M.; Hifney, A.F.; Abdel-Gawad, K.M. Optimization of Alginate Alkaline Extraction Technology from Sargassum latifolium and Its Potential Antioxidant and Emulsifying Properties. Carbohydr. Polym. 2017, 157, 1903–1912. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR Spectra of Alginic Acid Block Fractions in Three Species of Brown Seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Holdt, S.L.; De Francisci, D.; Alvarado-Morales, M.; Mishra, H.N.; Angelidaki, I. Extraction of Alginate from Sargassum muticum: Process Optimization and Study of Its Functional Activities. J. Appl. Phycol. 2016, 28, 3625–3634. [Google Scholar] [CrossRef]
- Cano-Vicent, A.; Hashimoto, R.; Takayama, K.; Serrano-Aroca, Á. Biocompatible Films of Calcium Alginate Inactivate Enveloped Viruses Such as SARS-CoV-2. Polymers 2022, 14, 1483. [Google Scholar] [CrossRef]
- Larsen, B.; Salem, D.M.S.A.; Sallam, M.A.E.; Mishrikey, M.M.; Beltagy, A.I. Characterization of the Alginates from Algae Harvested at the Egyptian Red Sea Coast. Carbohydr. Res. 2003, 338, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and Characterization of an Alginate from the Brown Seaweed Sargassum turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137. [Google Scholar] [CrossRef]
- Garrett, B.; Matharu, A.S.; Hurst, G.A. Using Greener Gels To Explore Rheology. J. Chem. Educ. 2017, 94, 500–504. [Google Scholar] [CrossRef]
- Trigueros, E.; Sanz, M.T.; Alonso-Riaño, P.; Beltrán, S.; Ramos, C.; Melgosa, R. Recovery of the Protein Fraction with High Antioxidant Activity from Red Seaweed Industrial Solid Residue after Agar Extraction by Subcritical Water Treatment. J. Appl. Phycol. 2021, 33, 1181–1194. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; De-Paula, J.C.; Pereira, L.O.D.S.; Marquez, U.M.L. Amino Acid Composition, Protein Content and Calculation of Nitrogen-to-Protein Conversion Factors for 19 Tropical Seaweeds. Phycol. Res. 2002, 50, 233–241. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of Solubre, Cell-Wall-Bound and Exuded Phlorotannins in the Brown Alga Fucus vesiculosus, with Implications on Their Ecological Functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef]
- Dodgson, K.S. Determination of Inorganic Sulphate in Studies on the Enzymic and Non-Enzymic Hydrolysis of Carbohydrate and Other Sulphate Esters. Biochem. J. 1961, 78, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Von Gadow, A.; Joubert, E.; Hansmam, C.F. Comparison of the Antioxidant Activity of Rooibos Tea (Aspalathus Linearis) with Green, Oolong and Black Tea. Food Chem. 1997, 60, 7377. [Google Scholar]
- Silva, C.; Torres, M.D.; Chenlo, F.; Moreira, R. Rheology of aqueous mixtures of tragacanth and guar gums: Effects of temperature and polymer ratio. Food Hydrocoll. 2017, 69, 293–300. [Google Scholar]






| Fraction | Content | Metal | Content |
|---|---|---|---|
| Moisture (%) | 8.06 ± 0.16 | Ca (mg/g) | 15.90 ± 1.33 |
| Ash (%) | 11.56 ± 0.68 | Mg (mg/g) | 3.20 ± 0.12 |
| Protein (%) | 16.43 ± 0.70 | Fe (mg/g) | 0.55 ± 0.06 |
| Lipid (%) | 6.17 ± 0.15 | Na (mg/g) | 0.53 ± 0.02 |
| Acid insoluble residue, AIR (%) | 30.81 ± 1.00 | K (mg/g) | 0.40 ± 0.02 |
| Extractives (%) | Cu (µg/g) | 23.98 ± 0.98 | |
| Ethanol (96%) | 11.24 ± 0.14 | As (µg/g) | 5.82 ± 0.40 |
| Hexane | 1.73 ± 0.66 | Pb (µg/g) | 0.97 ± 0.02 |
| MeOH + Acetone + H2O (3:1:1) | 12.73 ± 0.27 | Cd (µg/g) | 0.15 ± 0.05 |
| Carbohydrates (%) | B (mg/g) | 0.041 ± 0.001 | |
| Glucose | 11.69 ± 0.12 | Cu (mg/g) | 0.034 ± 0.001 |
| Galactose | 2.76 ± 0.07 | Hg (µg/g) | 0.016 ± 0.001 |
| Fucose | 6.38 ± 0.02 | ||
| Mannose | 1.91 ± 0.05 | ||
| Xylose | 0.68 ± 0.02 | ||
| Acetyl groups | 2.36 ± 0.01 | ||
| Uronic acids (glucuronic acid eq.) | 1.65 ± 0.01 | ||
| Sulfate (%) | 2.79 ± 0.07 |
| Samples 1 | Emax (% Inhibition) | IC50 (mg/mL) |
|---|---|---|
| 160 DW | 65 ± 2 | 0.048 ± 0.01 |
| 160 SW | 60 ± 1 | 0.689 ± 0.13 |
| 170 SW | 53 ± 1 | 0.317 ± 0.02 |
| 180 SW | 60 ± 3 | 0.715 ± 0.14 |
| Cisplatin | 94 ± 1 | 0.80 ± 0.03 µM |
| Alginate | M/G | FM | FG | FMM | FGG | FMG |
|---|---|---|---|---|---|---|
| Alg-160 DW | 0.56 c | 0.36 c | 0.64 b | 0.25 c | 0.53 b | 0.11 a |
| Alg-170 DW | 0.69 b | 0.41 b | 0.59 c | 0.29 b | 0.47 c | 0.12 a |
| Alg-180 DW | 0.82 a | 0.45 a | 0.55 d | 0.69 a | 0.42 d | 0.13 a |
| Alg-160 SW | 0.47 d | 0.32 d | 0.68 a | 0.24 c | 0.60 a | 0.08 b |
| Alg-170 SW | 0.58 c | 0.37 c | 0.63 b | 0.28 b | 0.54 b | 0.09 b |
| Alg-180 SW | 0.69 b | 0.41 b | 0.59 c | 0.30 b | 0.48 c | 0.11 a |
| T (°C) | Nitrogen (%, w/w) | Carbon (%, w/w) | Hydrogen (%, w/w) | HHV (kJ/kg) | |
|---|---|---|---|---|---|
| Distilled water | 160 1 | 3.28 ± 0.16 a | 43.16 ± 0.21 c | 5.89 ± 0.30 a | 17,508 ± 62 f |
| 170 1 | 3.31 ± 0.09 a | 44.07 ± 0.08 b | 6.10 ± 0.18 a | 16,102 ± 188 h | |
| 180 1 | 3.39 ± 0.01 a | 45.44 ± 0.46 a | 6.03 ± 0.08 a | 17,867 ± 48 e | |
| 180 * | 4.10 ± 0.13 | 46.84 ± 0.50 | 6.32 ± 0.18 | 19,123 ± 56 c | |
| 180 ** | 4.10 ± 0.07 | 49.58 ± 0.14 | 6.17 ± 0.15 | 20,266 ± 98 a | |
| 180 *** | 4.11 ± 0.01 | 49.34 ± 0.13 | 6.11 ± 0.06 | 20,141 ± 102 a | |
| Sea water | 160 1 | 2.83 ± 0.04 c | 38.94 ± 0.59 e | 5.16 ± 0.01 b | 16,045 ± 150 h |
| 170 1 | 2.86 ± 0.05 c | 38.82 ± 0.42 e | 5.26 ± 0.05 b | 18,413 ± 177 d | |
| 180 1 | 3.13 ± 0.06 b | 41.42 ± 0.28 d | 5.43 ± 0.03 a, b | 16,900 ± 108 g | |
| 180 * | 4.36 ± 0.08 | 46.80 ± 0.50 | 6.00 ± 0.11 | 19,085 ± 121 c | |
| 180 ** | 4.35 ± 0.17 | 48.57 ± 0.46 | 6.11 ± 0.03 | 19,843 ± 103 b | |
| 180 *** | 4.20 ± 0.02 | 49.19 ± 0.23 | 6.25 ± 0.08 | 20,133 ± 99 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira-Anta, T.; Flórez-Fernández, N.; Torres, M.D.; Mazón, J.; Dominguez, H. Microwave-Assisted Hydrothermal Processing of Rugulopteryx okamurae. Mar. Drugs 2023, 21, 319. https://doi.org/10.3390/md21060319
Ferreira-Anta T, Flórez-Fernández N, Torres MD, Mazón J, Dominguez H. Microwave-Assisted Hydrothermal Processing of Rugulopteryx okamurae. Marine Drugs. 2023; 21(6):319. https://doi.org/10.3390/md21060319
Chicago/Turabian StyleFerreira-Anta, Tania, Noelia Flórez-Fernández, Maria Dolores Torres, José Mazón, and Herminia Dominguez. 2023. "Microwave-Assisted Hydrothermal Processing of Rugulopteryx okamurae" Marine Drugs 21, no. 6: 319. https://doi.org/10.3390/md21060319
APA StyleFerreira-Anta, T., Flórez-Fernández, N., Torres, M. D., Mazón, J., & Dominguez, H. (2023). Microwave-Assisted Hydrothermal Processing of Rugulopteryx okamurae. Marine Drugs, 21(6), 319. https://doi.org/10.3390/md21060319





