Sargassum natans I Algae: An Alternative for a Greener Approach for the Synthesis of ZnO Nanostructures with Biological and Environmental Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of ZnO Nanostructures Synthesized with Extracts of Sargassum natans I Alga
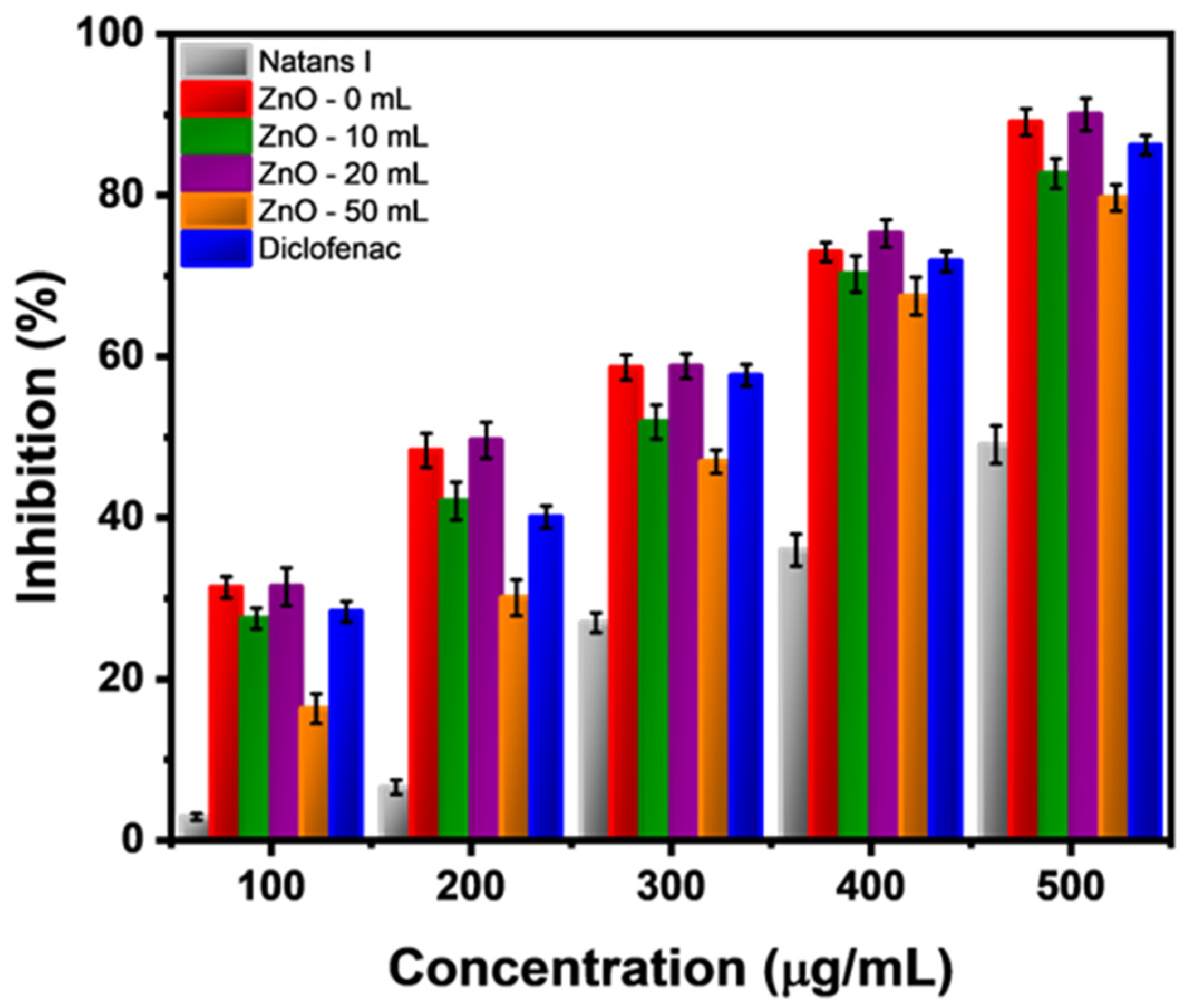
2.2. Anti-Inflammatory Activity of ZnO Nanostructures Synthesized with Extracts of Sargassum natans I Alga
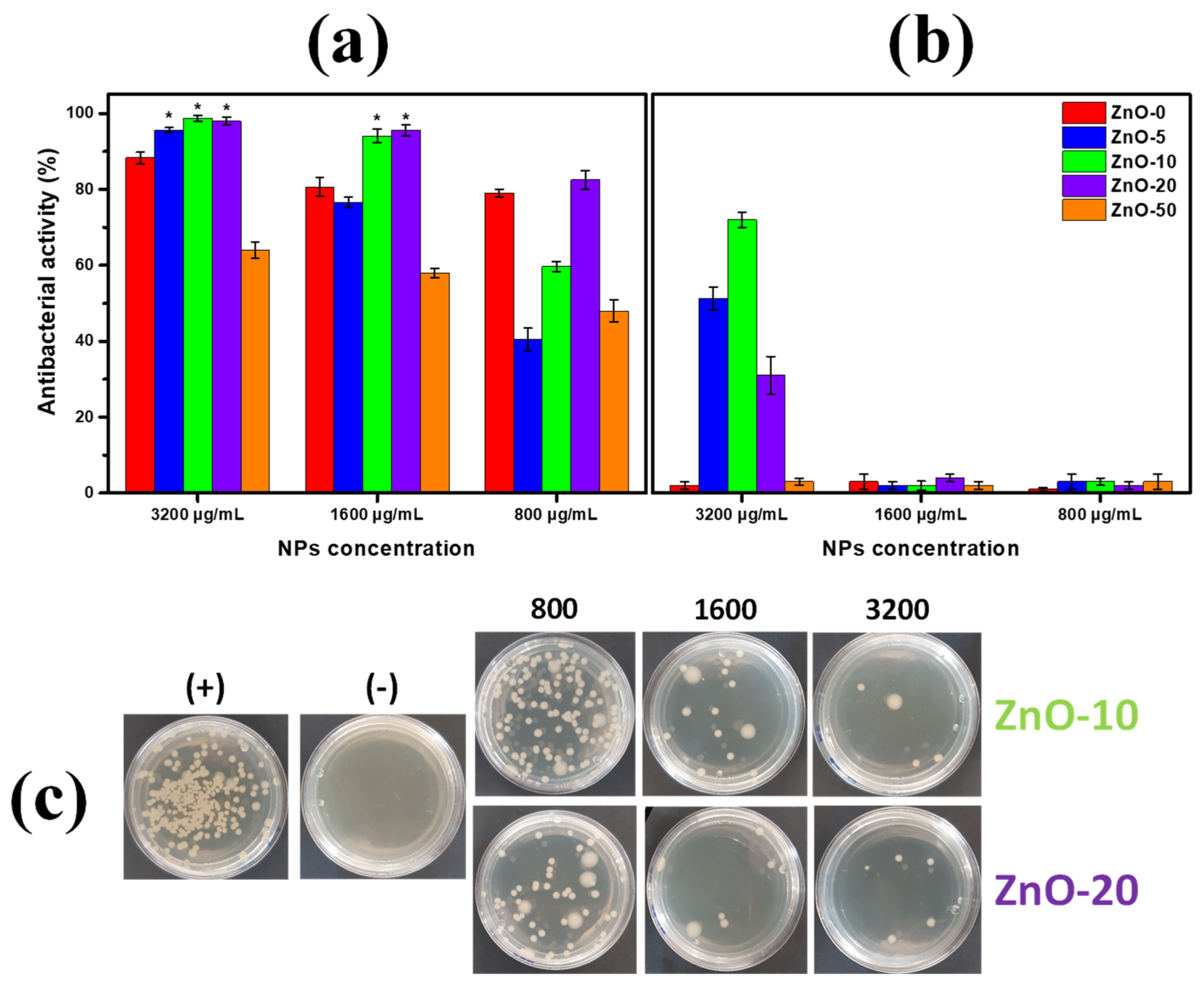
2.3. Antibacterial Activity of ZnO Nanostructures Synthesized with Extracts of Sargassum natans I Alga
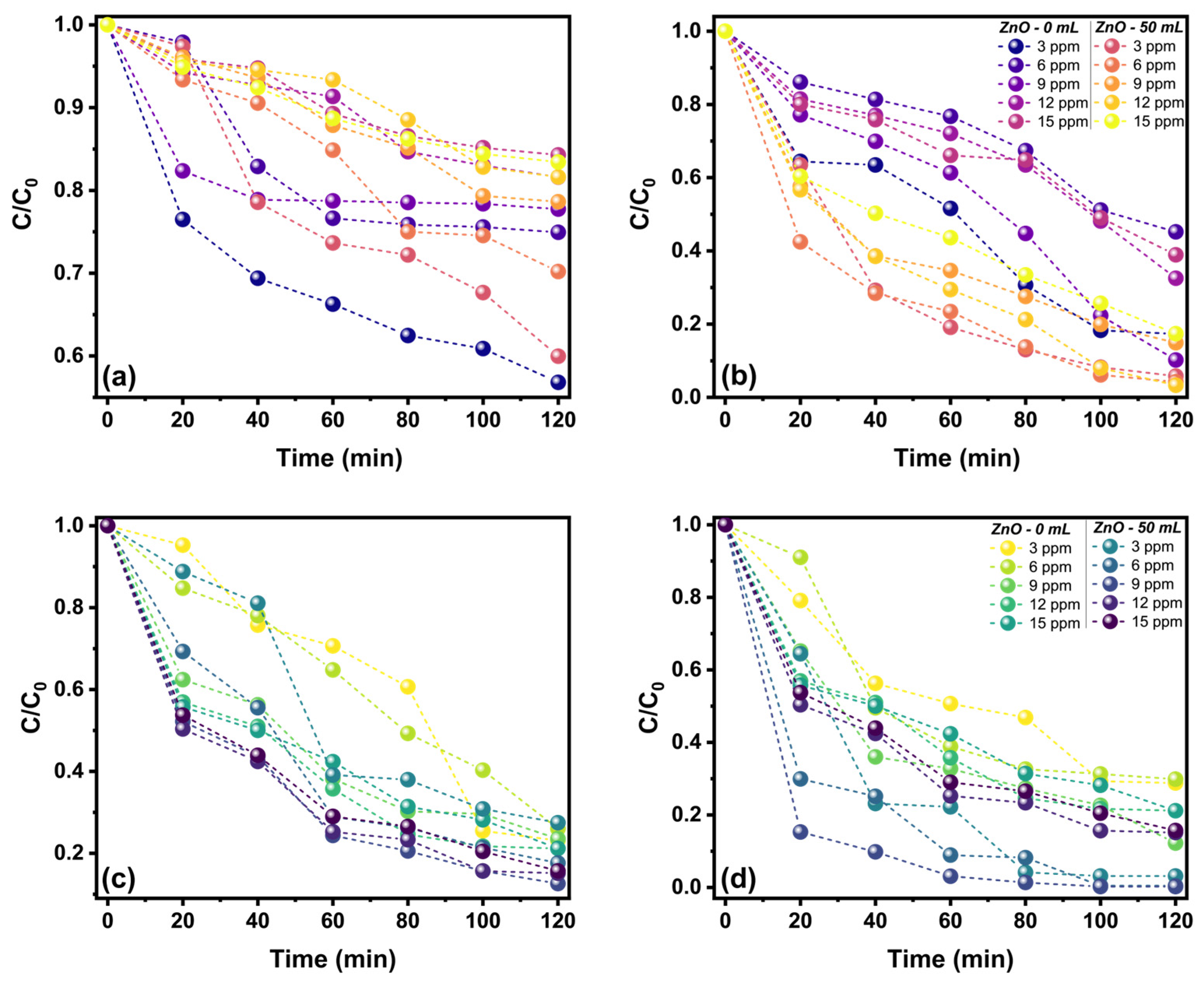
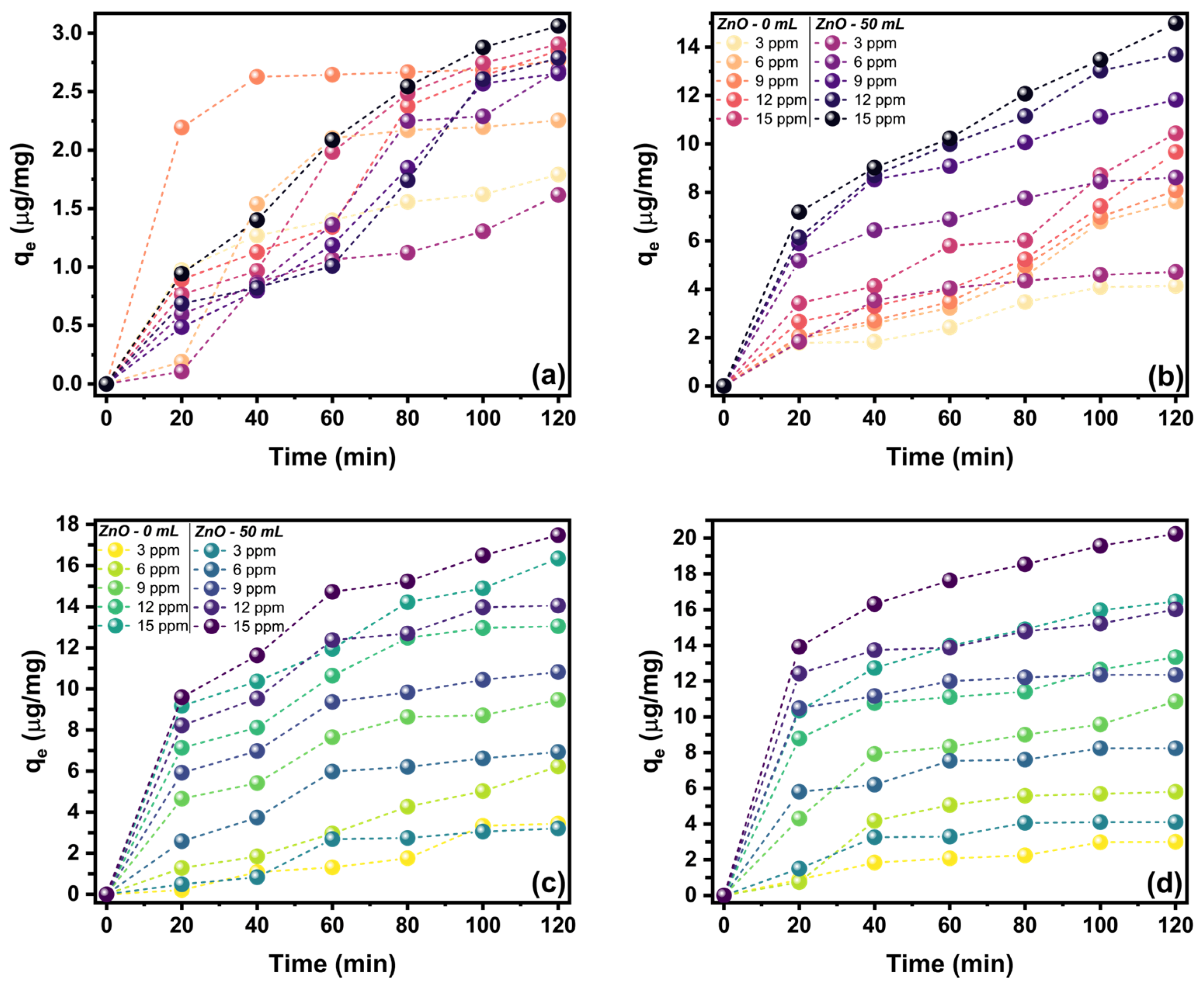
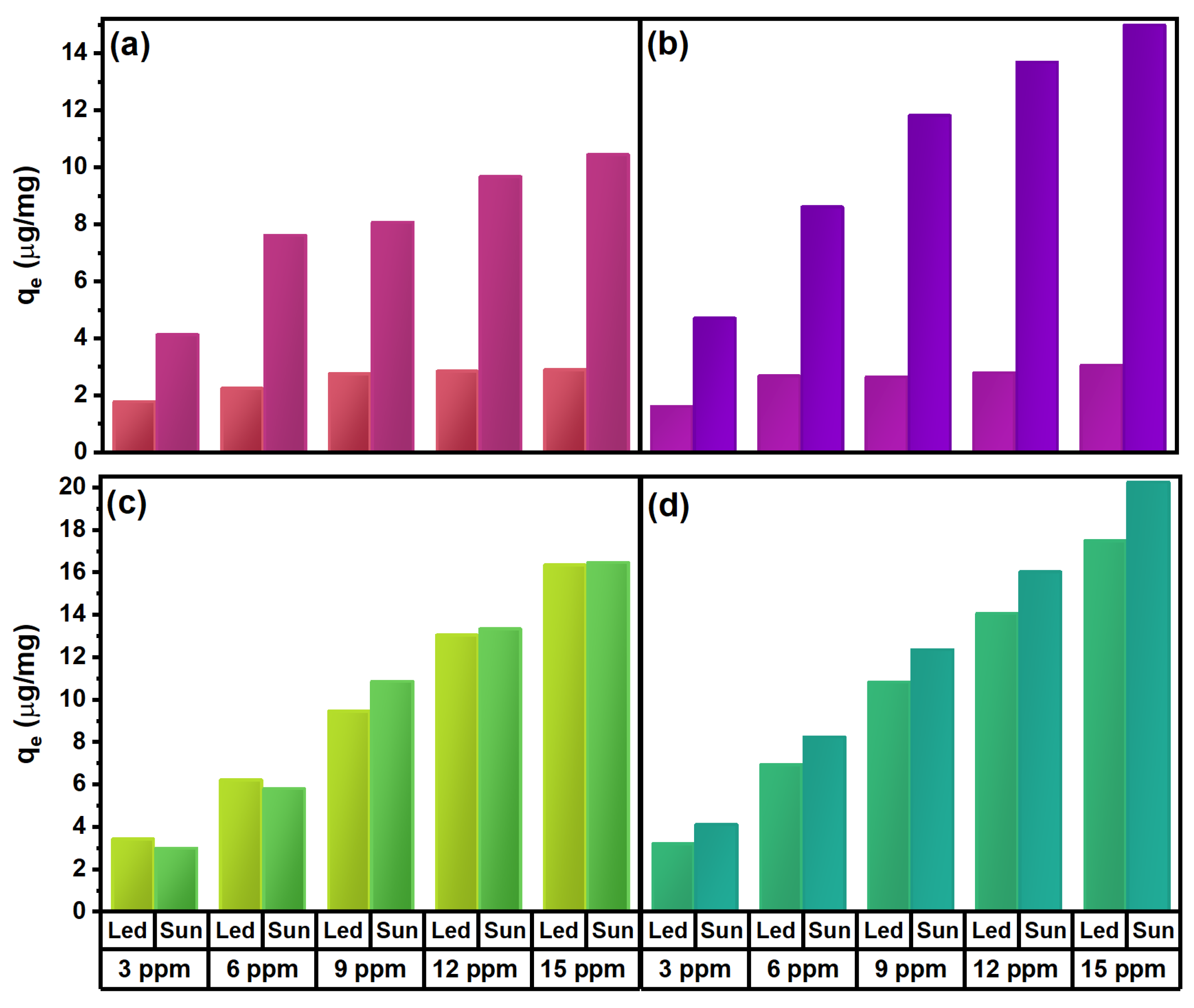
2.4. Photocatalytic Activity of ZnO Nanostructures Synthesized with Extracts of Sargassum natans I Alga
3. Materials and Methods
3.1. Materials
3.2. Preparation of Sargassum natans I Alga Extract
3.3. Synthesis of ZnO Nanostructures Using Sargassum natans I Alga Extract
3.4. Characterization
3.5. Antibacterial Activity
3.6. Study of Anti-Inflammatory Properties
3.7. Evaluation of Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Man Ching Ng, A. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2012, 22, 6526–6535. [Google Scholar] [CrossRef]
- Subhan, M.A.; Neogi, N.; Choudhury, K.P. Industrial Manufacturing Applications of Zinc Oxide Nanomaterials: A Comprehensive Study. Nanomanufacturing 2022, 2, 265–291. [Google Scholar] [CrossRef]
- Zikalala, N.E.; Azizi, S.; Zikalala, S.A.; Kamika, I.; Maaza, M.; Zinatizadeh, A.A.; Mokrani, T.; Kaviyarasu, K. An Evaluation of the Biocatalyst for the Synthesis and Application of Zinc Oxide Nanoparticles for Water Remediation—A Review. Catalysts 2022, 12, 1442. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Napi, M.L.M.; Sultan, S.M.; Ismail, R.; How, K.W.; Ahmad, M.K. Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review. Materials 2019, 12, 2985. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Saravanan, K.; Manogar, P.; Johnson, J.; Vinoth, E.; Mayakannan, M. Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sens. Bio-Sens. Res. 2021, 31, 100399. [Google Scholar] [CrossRef]
- Raj, V.J.; Ghosh, R.; Girigoswami, A.; Girigoswami, K. Application of zinc oxide nanoflowers in environmental and biomedical science. BBA Adv. 2022, 2, 100051. [Google Scholar] [CrossRef]
- Di Mari, G.M.; Mineo, G.; Franzò, G.; Mirabella, S.; Bruno, E.; Strano, V. Low-Cost, High-Yield ZnO Nanostars Synthesis for Pseudocapacitor Applications. Nanomaterials 2022, 12, 2588. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012, 2012, 624520. [Google Scholar] [CrossRef]
- Du, B.; Zhang, M.; Ye, J.; Wang, D.; Han, J.; Zhang, T. Novel Au Nanoparticle-Modified ZnO Nanorod Arrays for Enhanced Photoluminescence-Based Optical Sensing of Oxygen. Sensors 2023, 23, 2886. [Google Scholar] [CrossRef]
- Khairnar, B.A.; Dabhane, H.A.; Dashpute, R.S.; Girase, M.S.; Nalawade, P.M.; Gaikwad, V.B. Study of biogenic fabrication of zinc oxide nanoparticles and their applications: A review. Inorg. Chem. Commun. 2022, 146, 110155. [Google Scholar] [CrossRef]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185, 1–22. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; de Moraes Ruy Sapata, V.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef] [PubMed]
- Mutukwa, D.; Taziwa, R.T.; Khotseng, L. Antibacterial and Photodegradation of Organic Dyes Using Lamiaceae-Mediated ZnO Nanoparticles: A Review. Nanomaterials 2022, 12, 4469. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, Z.; Nasar, A. Utilization of Azadirachta indica Sawdust as a Potential Adsorbent for the Removal of Crystal Violet Dye. Sustain. Chem. 2023, 4, 110–126. [Google Scholar] [CrossRef]
- Vaiano, V.; De Marco, I. Removal of Azo Dyes from Wastewater through Heterogeneous Photocatalysis and Supercritical Water Oxidation. Separations 2023, 10, 230. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef]
- Deokar, G.K.; Ingale, A.G. Exploring effective catalytic degradation of organic pollutant dyes using environment benign, green engineered gold nanoparticles. Inorg. Chem. Commun. 2023, 151, 110649. [Google Scholar] [CrossRef]
- Bellè, U.; Spini, D.; Del Curto, B.; Pedeferri, M.; Diamanti, M.V. Water-Based Photocatalytic Sol–Gel TiO2 Coatings: Synthesis and Durability. Catalysts 2023, 13, 494. [Google Scholar] [CrossRef]
- Shah, R.K. Efficient photocatalytic degradation of methyl orange dye using facilely synthesized α-Fe2O3 nanoparticles. Arab. J. Chem. 2023, 16, 104444. [Google Scholar] [CrossRef]
- Lingaraja, D.; Praveen Kumar, S.; Aravind, T.; Srinivasan, T.K.; Ramya, S.; Dinesh Ram, G. Green synthesis of SnO2 nanoparticles using Chrysopogon Zizaniodes root extract to degrade the methylene blue dye. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Rasool, A.; Kiran, S.; Gulzar, T.; Abrar, S.; Ghaffar, A.; Shahid, M.; Nosheen, S.; Naz, S. Biogenic synthesis and characterization of ZnO nanoparticles for degradation of synthetic dyes: A sustainable environmental cleaner approach. J. Clean. Prod. 2023, 398, 136616. [Google Scholar] [CrossRef]
- López-Miranda, J.L.; España Sánchez, B.L.; Esparza, R.; Estévez, M. Self-assembly of ZnO nanoflowers synthesized by a green approach with enhanced catalytic, and antibacterial properties. Mater. Chem. Phys. 2022, 289, 126453. [Google Scholar] [CrossRef]
- Wu, K.; Zhou, L.; Mao, C.; Chu, Y. Solvothermal synthesis of ZnO with controllable morphology. Mater. Lett. 2023, 341, 134161. [Google Scholar] [CrossRef]
- Purcar, V.; Şomoghi, R.; Niţu, S.G.; Nicolae, C.-A.; Alexandrescu, E.; Gîfu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; et al. The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via the Sol–Gel Process. Nanomaterials 2017, 7, 439. [Google Scholar] [CrossRef] [PubMed]
- Blinov, A.V.; Kachanov, M.D.; Gvozdenko, A.A.; Nagdalian, A.A.; Blinova, A.A.; Rekhman, Z.A.; Golik, A.B.; Vakalov, D.S.; Maglakelidze, D.G.; Nagapetova, A.G.; et al. Synthesis and Characterization of Zinc Oxide Nanoparticles Stabilized with Biopolymers for Application in Wound-Healing Mixed Gels. Gels 2023, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Preeti, S.; Vijay, N. Synthesis of nano-ZnO by chemical reduction method and their micro biocide activity against bacterial skin pathogens. Int. J. Life Sci. 2017, 5, 233–240. [Google Scholar]
- Khan, A.U.; Tahir, K.; Albalawi, K.; Khalil, M.Y.; Almarhoon, Z.M.; Zaki, M.E.A.; Latif, S.; Hassan, H.M.A.; Refat, M.S.; Munshi, A.M. Synthesis of ZnO and ZnS nanoparticles and their structural, optical, and photocatalytic properties synthesized via the wet chemical method. Mater. Chem. Phys. 2022, 291, 126667. [Google Scholar] [CrossRef]
- Garino, N.; Limongi, T.; Dumontel, B.; Canta, M.; Racca, L.; Laurenti, M.; Castellino, M.; Casu, A.; Falqui, A.; Cauda, V. A Microwave-Assisted Synthesis of Zinc Oxide Nanocrystals Finely Tuned for Biological Applications. Nanomaterials 2019, 9, 212. [Google Scholar] [CrossRef]
- Khan, M.Z.; Taghavian, H.; Fijalkowski, M.; Militky, J.; Tomkova, B.; Venkataraman, M.; Adach, K. Effect of microwave power on bactericidal and UV protection properties of the ZnO nanorods grown cotton fabrics. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131135. [Google Scholar] [CrossRef]
- Adam, R.E.; Pozina, G.; Willander, M.; Nur, O. Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. Photon-Nanostruct.-Fundam. Appl. 2018, 32, 11–18. [Google Scholar] [CrossRef]
- Belkhaoui, C.; Mzabi, N.; Smaoui, H. Investigations on structural, optical and dielectric properties of Mn doped ZnO nanoparticles synthesized by co-precipitation method. Mater. Res. Bull. 2019, 111, 70–79. [Google Scholar] [CrossRef]
- El-Shazly, A.N.; Rashad, M.M.; Abdel-Aal, E.A.; Ibrahim, I.A.; El-Shahat, M.F.; Shalan, A.E. Nanostructured ZnO photocatalysts prepared via surfactant assisted Co-Precipitation method achieving enhanced photocatalytic activity for the degradation of methylene blue dyes. Environ. Chem. Eng. 2016, 4, 3177–3184. [Google Scholar] [CrossRef]
- Bahari, N.; Hashim, N.; Abdan, K.; Md Akim, A.; Maringgal, B.; Al-Shdifat, L. Role of Honey as a Bifunctional Reducing and Capping/Stabilizing Agent: Application for Silver and Zinc Oxide Nanoparticles. Nanomaterials 2023, 13, 1244. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.S.; Bajpai, V.; Khan, M.I.; Elboughdiri, N.; Shanableh, A.; Luque, R. An eco-friendly approach on green synthesis, bio-engineering applications, and future outlook of ZnO nanomaterial: A critical review. Environ. Res. 2023, 221, 114807. [Google Scholar] [CrossRef]
- Zeghoud, S.; Hemmami, H.; Ben Seghir, B.; Ben Amor, I.; Kouadri, I.; Rebiai, A.; Messaoudi, M.; Ahmed, S.; Pohl, P.; Simal-Gandara, J. A review on biogenic green synthesis of ZnO nanoparticles by plant biomass and their applications. Mater. Today Commun. 2022, 33, 104747. [Google Scholar] [CrossRef]
- Mahdi Ismail, S.M.; Ahmed, S.M.; Abdulrahman, A.F.; AlMessiere, M.A. Characterization of green synthesized of ZnO nanoparticles by using pinus brutia leaves extracts. J. Mol. Struct. 2023, 1280, 135063. [Google Scholar] [CrossRef]
- Singh, K.; Nancy Singh, G.; Singh, J. Sustainable synthesis of biogenic ZnO NPs for mitigation of emerging pollutants and pathogens. Environ. Res. 2023, 219, 114952. [Google Scholar] [CrossRef]
- Ragavendran, C.; Kamaraj, C.; Jothimani, K.; Priyadharsan, A.; Anand Kumar, D.; Natarajan, D.; Malafaia, G. Eco-friendly approach for ZnO nanoparticles synthesis and evaluation of its possible antimicrobial, larvicidal and photocatalytic applications. Sustain. Mater. Technol. 2023, 36, e00597. [Google Scholar] [CrossRef]
- Alprol, A.E.; Mansour, A.T.; El-Beltagi, H.S.; Ashour, M. Algal Extracts for Green Synthesis of Zinc Oxide Nanoparticles: Promising Approach for Algae Bioremediation. Materials 2023, 16, 2819. [Google Scholar] [CrossRef] [PubMed]
- López Miranda, J.L.; Celis, L.B.; Estévez, M.; Chávez, V.; van Tussenbroek, B.I.; Uribe-Martínez, A.; Cuevas, E.; Rosillo Pantoja, I.; Masia, L.; Cauich-Kantun, C. Commercial Potential of Pelagic Sargassum spp. in Mexico. Front. Mar. Sci. 2021, 8, 1692. [Google Scholar] [CrossRef]
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; Van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, L.V.; Leal-Bautista, R. Massive influx of pelagic Sargassum spp. on the coasts of the Mexican Caribbean 2014–2020: Challenges and opportunities. Water 2020, 12, 2908. [Google Scholar] [CrossRef]
- Robledo, D.; Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Vásquez-Elizondo, R.M.; Qui-Minet, Z.N.; Salazar-Garibay, A. Challenges and opportunities in relation to Sargassum events along the Caribbean Sea. Front. Mar. Sci. 2021, 8, 699664. [Google Scholar] [CrossRef]
- Lopez-Miranda, J.L.; Molina, G.A.; González-Reyna, M.A.; España-Sánchez, B.L.; Esparza, R.; Silva, R.; Estévez, M. Antibacterial and Anti-Inflammatory Properties of ZnO Nanoparticles Synthesized by a Green Method Using Sargassum Extracts. Int. J. Mol. Sci. 2023, 24, 1474. [Google Scholar] [CrossRef]
- López-Miranda, J.L.; Molina, G.A.; Esparza, R.; González-Reyna, M.A.; Silva, R.; Estévez, M. Green Synthesis of Homogeneous Gold Nanoparticles Using Sargassum spp. Extracts and Their Enhanced Catalytic Activity for Organic Dyes. Toxics 2021, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, J.L.; Esparza, R.; González-Reyna, M.A.; España-Sánchez, B.L.; Hernandez-Martinez, A.R.; Silva, R.; Estévez, M. Sargassum influx on the Mexican Coast: A source for synthesizing silver nanoparticles with catalytic and antibacterial properties. Appl. Sci. 2021, 11, 4638. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Salazar-Garibay, A.; Serviere-Zaragoza, E.; Méndez-Rodríguez, L.C.; Robledo, D. Species composition and chemical characterization of Sargassum influx at six different locations along the Mexican Caribbean coast. Sci. Total Environ. 2021, 795, 148852. [Google Scholar] [CrossRef]
- Ahammed, K.R.; Ashaduzzaman, M.; Paul, S.C.; Nath, M.R.; Bhowmik, S.; Saha, O.; Rahaman, M.M.; Bhowmik, S.; Aka, T.D. Microwave assisted synthesis of zinc oxide (ZnO) nanoparticles in a noble approach: Utilization for antibacterial and photocatalytic activity. SN Appl. Sci. 2020, 2, 955. [Google Scholar] [CrossRef]
- Chen, H.; Gao, M.; Huang, H. Biosynthesis of zinc oxide nanoparticles and their catalytic and disinfection evaluation. Mater. Res. Express 2019, 6, 085081. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Markiewicz, E.; Jesionowski, T. Structural Characterisation of ZnO Particles Obtained by the Emulsion Precipitation Method. J. Nanomater. 2012, 2012, 656353. [Google Scholar] [CrossRef]
- Mondal, P. Oxygen vacancy induced anomalous Raman mode in intrinsic ZnO film. Vib. Spectrosc. 2019, 103, 102939. [Google Scholar] [CrossRef]
- Das, J.; Pradhan, S.K.; Sahu, D.R.; Mishra, D.K.; Sarangi, S.N.; Nayak, B.B.; Verma, S.; Roul, B.K. Micro-Raman and XPS studies of pure ZnO ceramics. Phys. B Condens. Matter. 2010, 405, 2492–2497. [Google Scholar] [CrossRef]
- Windisch, C.F., Jr.; Exarhos, G.J.; Yao, C.; Wang, L.-Q. Raman study of the influence of hydrogen on defects in ZnO. J. Appl. Phys. 2007, 101, 123711. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.; Park, B.J.; Han, J.W.; Lee, H.-S.; Park, J.H.; Lee, W. Precise control of surface oxygen vacancies in ZnO nanoparticles for extremely high acetone sensing response. J. Adv. Ceram. 2022, 11, 769–783. [Google Scholar] [CrossRef]
- Bortolotti, M.; Lutterotti, L.; Lonardelli, I. ReX: A computer program for structural analysis using powder diffraction data. J. Appl. Crystallogr. 2009, 42, 538–539. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.; Abdulkareem, A.; Tijani, J.; Shuaib, D.; Mohammed, A.; Sumaila, A. Comparative study of crystallite size using Williamson-Hall and Debye-Scherrer plots for ZnO nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045013. [Google Scholar] [CrossRef]
- Li, T.; Dang, N.; Zhang, W.; Liang, W.; Yang, F. Determining the degree of [001] preferred growth of Ni(OH)2 nanoplates. Nanomaterials 2018, 8, 991. [Google Scholar] [CrossRef]
- Luo, S.; Chen, R.; Xiang, L.; Wang, J. Hydrothermal synthesis of (001) facet highly exposed ZnO plates: A new insight into the effect of citrate. Crystals 2019, 9, 552. [Google Scholar] [CrossRef]
- Quek, J.-A.; Sin, J.-C.; Lam, S.-M.; Mohamed, A.R.; Zeng, H. Bioinspired green synthesis of ZnO structures with enhanced visible light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2020, 31, 1144–1158. [Google Scholar] [CrossRef]
- Raula, M.; Rashid, M.H.; Paira, T.K.; Dinda, E.; Mandal, T.K. Ascorbate-assisted growth of hierarchical ZnO nanostructures: Sphere, spindle, and flower and their catalytic properties. Langmuir 2010, 26, 8769–8782. [Google Scholar] [CrossRef]
- Phukan, S.; Mahanta, A.; Rashid, M.H. Size-tunable ZnO nanotapes as an efficient catalyst for oxidative chemoselective CB bond cleavage of arylboronic acids. Appl. Catal. A Gen. 2018, 562, 58–66. [Google Scholar] [CrossRef]
- Ekennia, A.C.; Uduagwu, D.N.; Nwaji, N.N.; Oje, O.O.; Emma-Uba, C.O.; Mgbii, S.I.; Olowo, O.J.; Nwanji, O.L. Green synthesis of biogenic zinc oxide nanoflower as dual agent for photodegradation of an organic dye and tyrosinase inhibitor. J. Inorg. Organomet. Polym. Mater. 2021, 31, 886–897. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures via a sol–gel synthesis of solvent driven shape-controlled crystal growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.K. Band gap engineering, quantum confinement, defect mediated broadband visible photoluminescence and associated quantum States of size tuned zinc oxide nanostructures. Optik 2020, 221, 165337. [Google Scholar] [CrossRef]
- Moustaoui, H.; Saber, J.; Djeddi, I.; Liu, Q.; Diallo, A.T.; Spadavecchia, J.; Lamy de la Chapelle, M.; Djaker, N. Shape and Size Effect on Photothermal Heat Elevation of Gold Nanoparticles: Absorption Coefficient Experimental Measurement of Spherical and Urchin-Shaped Gold Nanoparticles. J. Phys. Chem. C 2019, 123, 17548–17554. [Google Scholar] [CrossRef]
- Velsankar, K.; Venkatesan, A.; Muthumari, P.; Suganya, S.; Mohandoss, S.; Sudhahar, S. Green inspired synthesis of ZnO nanoparticles and its characterizations with biofilm, antioxidant, anti-inflammatory, and anti-diabetic activities. J. Mol. Struct. 2022, 1255, 132420. [Google Scholar] [CrossRef]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.-M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-J.; Horng, J.-L.; Yu, C.-H.; Fang, C.-Y.; Yeh, Y.-H.; Lin, L.-Y. Toxic effects of silver and copper nanoparticles on lateral-line hair cells of zebrafish embryos. Aquat. Toxicol. 2019, 215, 105273. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef]
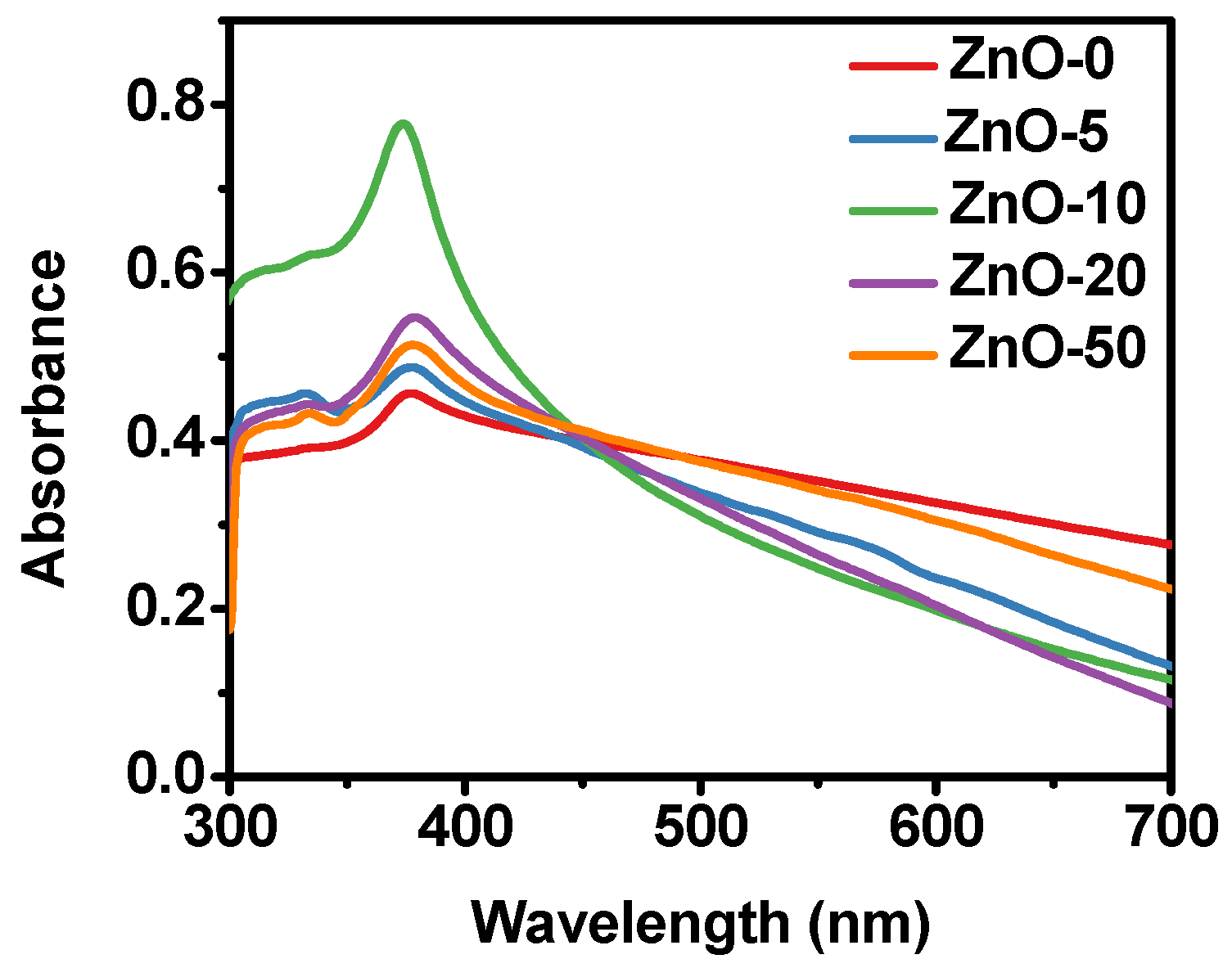

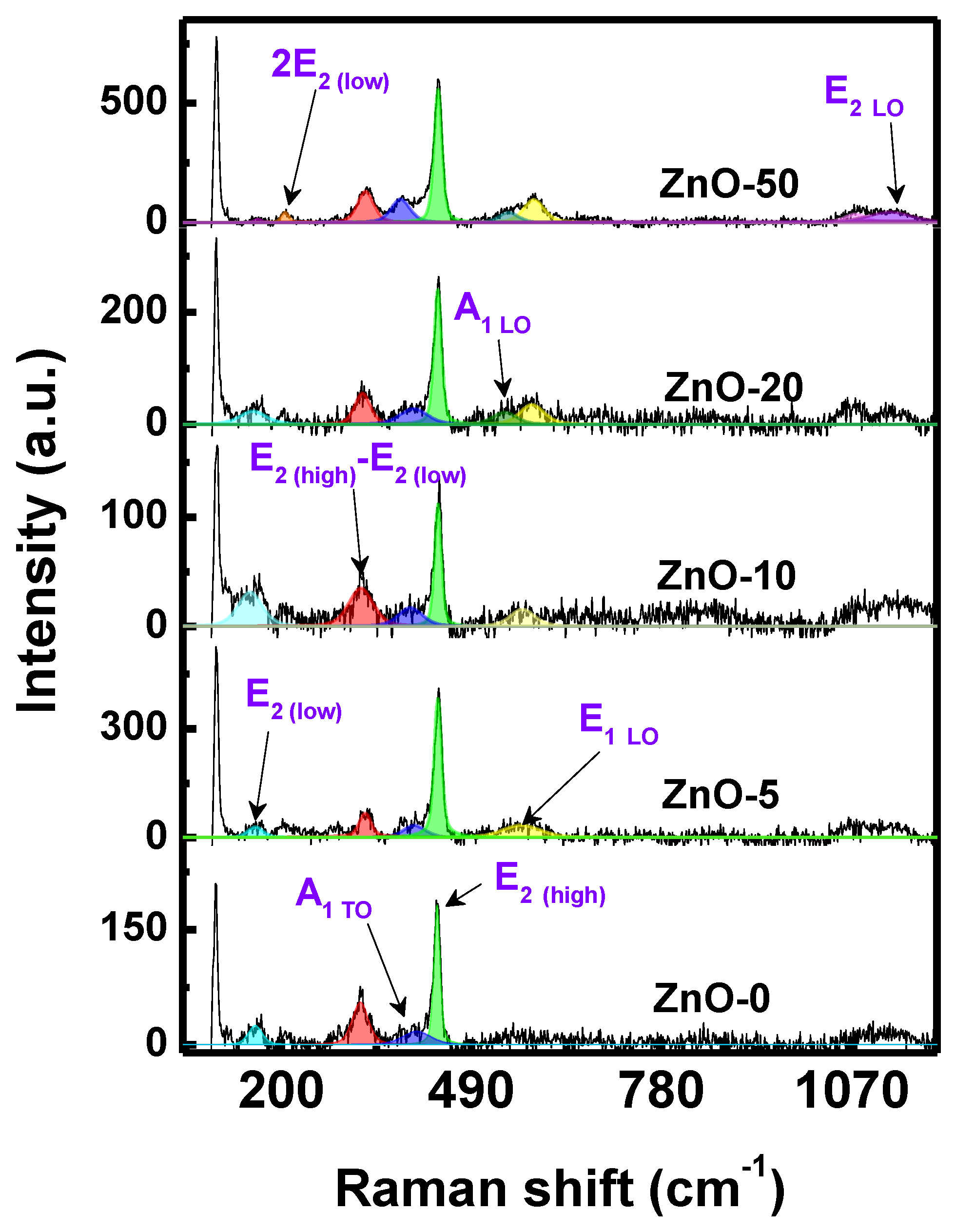
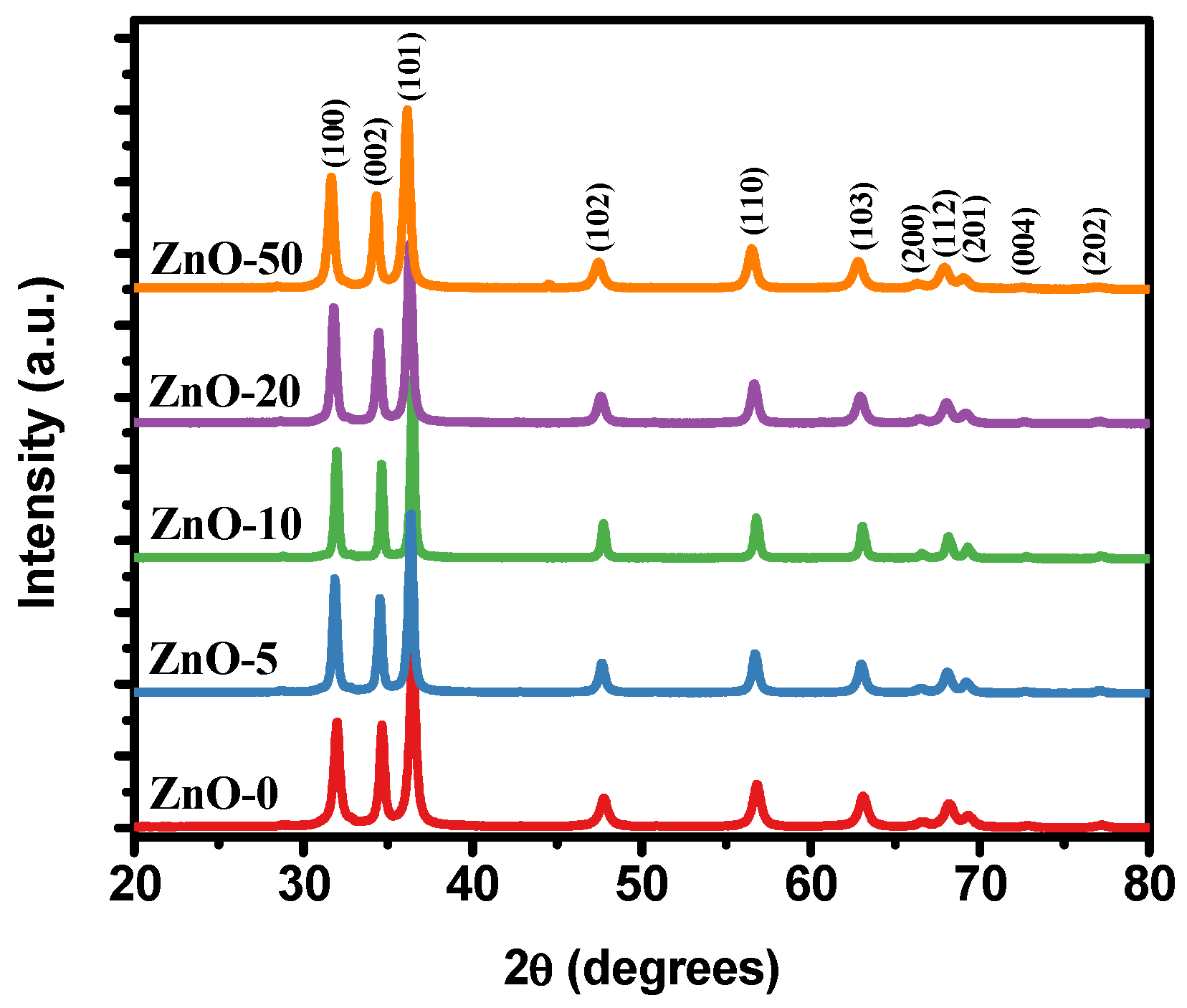
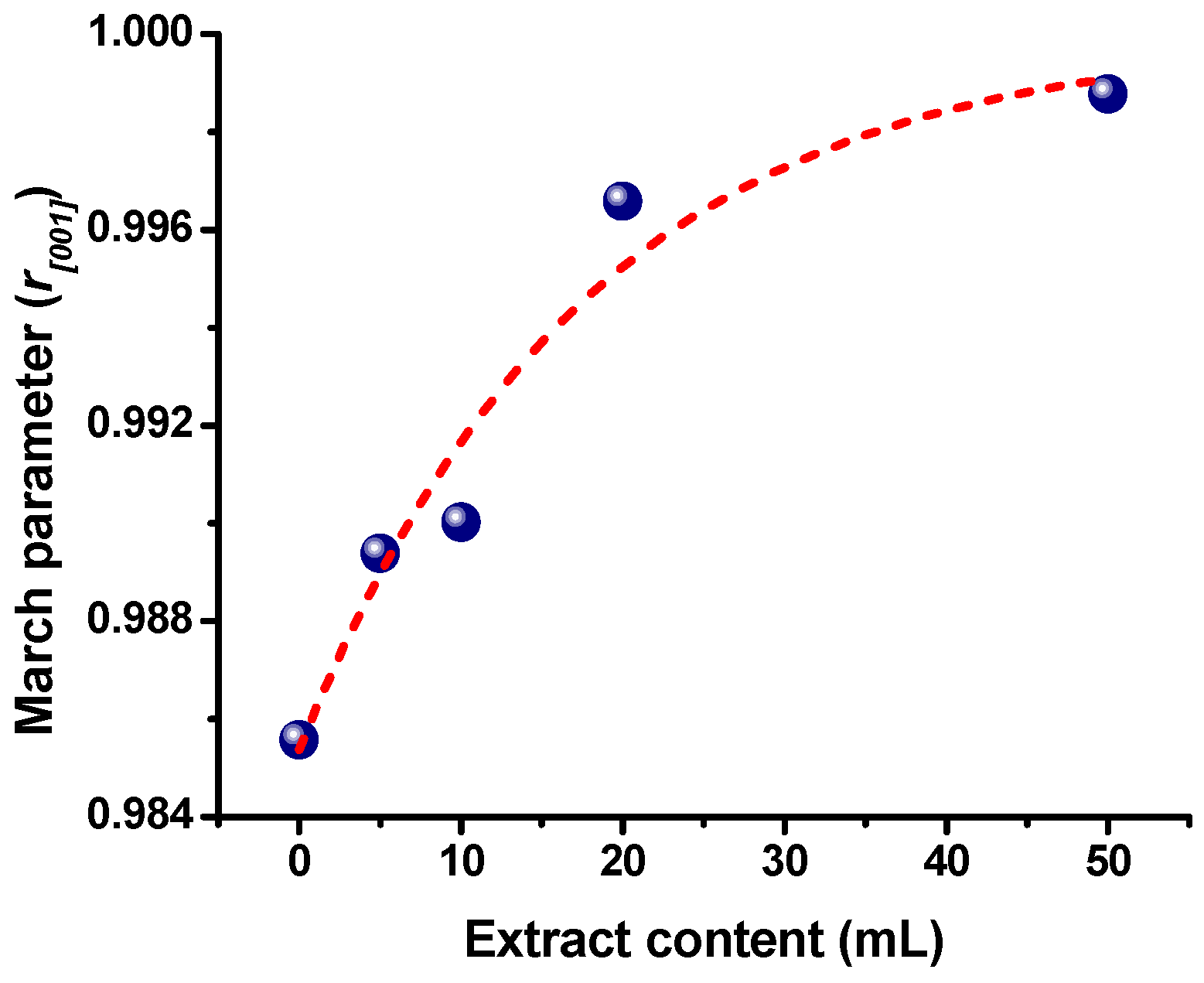

| Sample | Lattice Parameters (nm) | Crystallite Size (nm) | |
|---|---|---|---|
| a | c | ||
| ZnO-0 | 0.32423 | 0.51924 | 36.31 |
| ZnO-5 | 0.32507 | 0.52069 | 28.46 |
| ZnO-10 | 0.32520 | 0.52091 | 24.53 |
| ZnO-20 | 0.32498 | 0.52061 | 21.52 |
| ZnO-50 | 0.326228 | 0.52252 | 20.94 |
| Concentration (μg/mL) | Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| Natans I | ZnO-0 | ZnO-10 | ZnO-20 | ZnO-50 | Diclofenac | |
| 100 | 2.92 | 31.39 | 27.52 | 31.47 | 16.32 | 28.35 |
| 200 | 6.59 | 48.35 | 42.09 | 49.62 | 30.08 | 40.10 |
| 300 | 26.98 | 58.64 | 51.87 | 58.79 | 46.97 | 57.65 |
| 400 | 36.00 | 72.95 | 70.24 | 75.26 | 67.50 | 71.80 |
| 500 | 49.09 | 89.07 | 82.70 | 90.01 | 79.68 | 86.21 |
| Concentration (μg/mL) | ||||||
| IC50 | 511.13 | 228.10 | 264.78 | 222.78 | 311.59 | 253.76 |
| IC90 | 839.81 | 514.02 | 553.59 | 503.09 | 555.35 | 525.13 |
| Methyl Violet | Malachite Green | |||||
|---|---|---|---|---|---|---|
| Kinetic Model | Sample | Constant and Correlation Coefficient | Led | Solar | Led | Solar |
| PFO | ZnO-0 | k1 | 0.0286 | 0.0127 | 0.0228 | 0.0249 |
| qcal1 | 4.0004 | 10.0972 | 14.5111 | 9.7051 | ||
| R2 | 0.9064 | 0.8568 | 0.9617 | 0.8848 | ||
| ZnO-50 | k1 | 0.0269 | 0.0210 | 0.0269 | 0.0306 | |
| qcal1 | 3.8371 | 14.0896 | 15.9001 | 15.5991 | ||
| R2 | 0.9268 | 0.9633 | 0.9744 | 0.9552 | ||
| PSO | ZnO-0 | k2 | 0.0003 | 0.0004 | 0.0015 | 0.0029 |
| h | 0.0353 | 0.1490 | 0.6148 | 1.0080 | ||
| qcal2 | 11.1111 | 19.1571 | 20.0401 | 18.7970 | ||
| R2 | 0.1229 | 0.4994 | 0.9652 | 0.9968 | ||
| ZnO-50 | k2 | 0.0013 | 0.0012 | 0.0017 | 0.0031 | |
| h | 0.0512 | 0.4399 | 0.7487 | 1.5615 | ||
| qcal2 | 6.2972 | 19.5313 | 21.2314 | 22.3214 | ||
| R2 | 0.9267 | 0.9489 | 0.9896 | 0.9971 | ||
| IPD | ZnO-0 | kid | 0.2877 | 0.8857 | 1.4368 | 1.4667 |
| Ci | −0.2981 | −0.6289 | 1.0477 | 1.9028 | ||
| Ri | 1.1026 | 1.0602 | 0.9359 | 0.8844 | ||
| R2 | 0.9169 | 0.9152 | 0.9688 | 0.9216 | ||
| ZnO-50 | kid | 0.2943 | 1.3199 | 1.5789 | 1.7721 | |
| Ci | −0.1915 | 0.4331 | 1.2347 | 2.9096 | ||
| Ri | 1.0625 | 0.9711 | 0.9294 | 0.8563 | ||
| R2 | 0.9728 | 0.9903 | 0.9650 | 0.9118 | ||
| Methyl Violet | Malachite Green | |||||
|---|---|---|---|---|---|---|
| Isotherm | Sample | Parameter | Led | Solar | Led | Solar |
| Langmuir | ZnO-0 | qm | 3.5224 | 16 | 625 | −97.0874 |
| kL | 0.3395 | 0.1253 | 0.0018 | −0.0100 | ||
| R2 | 0.9927 | 0.9567 | −0.2732 | 0.1412 | ||
| ZnO -50 | qm | 3.6805 | 33.1126 | −181.8182 | 714.2857 | |
| kL | 0.3047 | 0.0578 | −0.0060 | 0.0019 | ||
| R2 | 0.9737 | 0.9616 | 0.2660 | 0.3246 | ||
| Freundlich | ZnO-0 | kF | 1.2829 | 2.4177 | 1.1418 | 0.8976 |
| 1/n | 0.3193 | 0.5582 | 0.9739 | 1.0870 | ||
| R2 | 0.9451 | 0.924 | 0.9957 | 0.9869 | ||
| ZnO-50 | kF | 1.1860 | 2.2121 | 1.0254 | 1.3941 | |
| 1/n | 0.3633 | 0.7321 | 1.0564 | 0.9877 | ||
| R2 | 0.7944 | 0.9788 | 0.9981 | 0.9997 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Miranda, J.L.; Mares-Briones, F.; Molina, G.A.; González-Reyna, M.A.; Velázquez-Hernández, I.; España-Sánchez, B.L.; Silva, R.; Esparza, R.; Estévez, M. Sargassum natans I Algae: An Alternative for a Greener Approach for the Synthesis of ZnO Nanostructures with Biological and Environmental Applications. Mar. Drugs 2023, 21, 297. https://doi.org/10.3390/md21050297
López-Miranda JL, Mares-Briones F, Molina GA, González-Reyna MA, Velázquez-Hernández I, España-Sánchez BL, Silva R, Esparza R, Estévez M. Sargassum natans I Algae: An Alternative for a Greener Approach for the Synthesis of ZnO Nanostructures with Biological and Environmental Applications. Marine Drugs. 2023; 21(5):297. https://doi.org/10.3390/md21050297
Chicago/Turabian StyleLópez-Miranda, Jose Luis, Fabian Mares-Briones, Gustavo A. Molina, M. A. González-Reyna, Isaac Velázquez-Hernández, Beatriz Liliana España-Sánchez, Rodolfo Silva, Rodrigo Esparza, and Miriam Estévez. 2023. "Sargassum natans I Algae: An Alternative for a Greener Approach for the Synthesis of ZnO Nanostructures with Biological and Environmental Applications" Marine Drugs 21, no. 5: 297. https://doi.org/10.3390/md21050297
APA StyleLópez-Miranda, J. L., Mares-Briones, F., Molina, G. A., González-Reyna, M. A., Velázquez-Hernández, I., España-Sánchez, B. L., Silva, R., Esparza, R., & Estévez, M. (2023). Sargassum natans I Algae: An Alternative for a Greener Approach for the Synthesis of ZnO Nanostructures with Biological and Environmental Applications. Marine Drugs, 21(5), 297. https://doi.org/10.3390/md21050297










