Abstract
Human skin needs additional protection from damaging ultraviolet radiation (UVR: 280–400 nm). Harmful UVR exposure leads to DNA damage and the development of skin cancer. Available sunscreens offer chemical protection from detrimental sun radiation to a certain extent. However, many synthetic sunscreens do not provide sufficient UVR protection due to the lack of photostability of their UV-absorbing active ingredients and/or the lack of ability to prevent the formation of free radicals, inevitably leading to skin damage. In addition, synthetic sunscreens may negatively affect human skin, causing irritation, accelerating skin aging and even resulting in allergic reactions. Beyond the potential negative effect on human health, some synthetic sunscreens have been shown to have a harmful impact on the environment. Consequently, identifying photostable, biodegradable, non-toxic, and renewable natural UV filters is imperative to address human health needs and provide a sustainable environmental solution. In nature, marine, freshwater, and terrestrial organisms are protected from harmful UVR through several important photoprotective mechanisms, including the synthesis of UV-absorbing compounds such as mycosporine-like amino acids (MAAs). Beyond MAAs, several other promising, natural UV-absorbing products could be considered for the future development of natural sunscreens. This review investigates the damaging impact of UVR on human health and the necessity of using sunscreens for UV protection, specifically UV-absorbing natural products that are more environmentally friendly than synthetic UV filters. Critical challenges and limitations related to using MAAs in sunscreen formulations are also evaluated. Furthermore, we explain how the genetic diversity of MAA biosynthetic pathways may be linked to their bioactivities and assess MAAs’ potential for applications in human health.
1. Introduction
The levels of ultraviolet (UV) radiation have continued to increase over the past century [1]. Excessive exposure to UV radiation has been associated with the development of the majority of skin cancers [2,3]. Based on epidemiologic data, the development of melanoma and basal cell carcinoma (BCC) was associated with excessive sun exposure resulting in sunburns [4]. On the other hand, the development of squamous cell carcinoma (SCC) has been associated with a lifetime of prolonged sun exposure, as seen in the example of occupational sun exposure [5]. The negative UVR effect on the skin occurs via mutagen impact on DNA, resulting in the formation of dimeric photoproducts between pyrimidine bases (so-called cyclobutane pyrimidine dimers (CPDs)), leading to DNA base damage [6]. However, different components of UV radiation have varying impacts on the skin, with longer wavelength ultraviolet A (UVA) having a higher penetrance, reaching the dermis skin layer. Alternatively, shorter wavelength ultraviolet B (UVB) mainly impacts the epidermal layer [7]. Importantly, both UV components, UVA and UVB, that reach the Earth’s surface can damage DNA directly and indirectly via reactive oxygen species (ROS), although UVB has a higher mutagenic and carcinogenic impact [3,8].
In nature, organisms have developed different mitigation strategies to protect from damaging UV radiation. Natural products such as mycosporine-like amino acids (MAAs) are major UV-protective agents found in various species living in marine and freshwater environments, including symbiotic and nonsymbiotic species [8,9,10,11,12,13,14]. Marine organisms exposed to severe changes in light irradiance can adapt via photoacclimation [15,16], although they become more susceptible to additional external stressors such as temperature and acidification [15,16]. The mitigation strategies of organisms like corals and sea urchins included the accumulation of more MAAs in tissues exposed to higher UVR [17]. MAAs are UV-absorbing secondary metabolites, which are found to be most commonly represented among aquatic species. These small molecules are involved in photoprotection and reducing UV-induced damage and osmoregulation, and they show promising therapeutic potential [18]. The photoprotective capacities of MAAs are based upon their ability to absorb light in the UV range, including UVA in the range of 315–400 nm and UVB in the range of 280–315 nm, with absorption maxima occurring in the range of 310–362 nm [19,20,21]. There are substantial variations in the composition of MAAs, resulting in variability in species’ UV-absorbing profile [22,23,24], indicating diversity in their UV-absorbing capacity, which is also influenced by seasonal UV fluctuations [25,26,27], environmental stress [20,28,29,30], and nutrient, specifically nitrogen, availability [31,32,33,34].
MAAs are characterized by a high molar extinction coefficient of ε = 28,100–50,000 M−1 cm−1 and the ability to disperse absorbed radiation as heat without the production of free radicals [12,35,36]. Beyond the photoprotective role, MAAs demonstrate antioxidative capacity and can scavenge ROS produced in cells to prevent further DNA damage [37,38,39,40,41,42]. Antioxidants (synthetic or coming from natural resources) are commonly used in modern medicine as bioactive compounds due to their ability to decrease the number of free radicals in cells and tissues [43]. Furthermore, MAAs demonstrate additional biotechnological potentials, including anti-inflammatory, anti-proliferative, and anti-aging properties [44,45,46]. The promising pharmacological properties of MAAs could be further utilized in various biotechnological applications, such as more efficient skin UV protection, which is important for improved skin cancer prevention.
2. Fitzpatrick Phototype and UV Protection Strategies
The skin is the largest organ in the human body, representing approximately 16% of body mass [3]. Higher levels of UV exposure have been associated with increased skin cancer prevalence in humans [47]. Therefore, besides internal UV protection, human skin requires additional external mechanisms to reduce sun-induced DNA damage and potential skin cancer formation. A number of UV-absorbing compounds (i.e., UV-absorbing pigments and other molecules) were found to provide internal UV protection, including additional mechanisms such as increased epidermal thickness, DNA repair mechanisms, and the accumulation of antioxidants [32,48]. For example, the human pigment melanin has a major photoprotective role in reducing sun-induced cancer and function as an antioxidant by scavenging free radicals produced during UVR exposure [48,49,50]. This pigment has two main forms: eumelanin, which is highly UV-protective, and pheomelanin, which has a lower UV-protective capacity [48]. Though the pigment melanin has a strong UV-protective capacity, it does not provide complete protection, but protecting against approximately 50–70% of UVR [48]. People with less eumelanin are more UV-sensitive [3]. Differences in skin pigmentation that impact UV risks are scaled based on the Fitzpatrick scale (Table 1). The Fitzpatrick skin type scale evaluates UV risk and UV exposure tolerance levels, indicating differences in required skin protection. Skin complexion is recognized as one of the most important determinants of UV sensitivity and the risk of developing skin cancer [3]. He and colleagues [51] described the Fitzpatrick skin phototype (skin, eyes, and hair pigmentation) classification system as the most common method to assess sunburns and the subsequent risk of developing skin cancer, primarily basal cell carcinoma (BCC) and malignant melanoma [52]. Consequently, different external sun protection strategies have been recommended depending on the skin phototype (Table 1) [3].

Table 1.
Skin cancer risk based upon Fitzpatrick skin type.
As internal mechanisms for UV protection are often insufficient to prevent UV skin damage, a number of external strategies are used. Different types of strategies are often applied to increase the amount of protection from damaging UVR, including chemical barriers (i.e., sunscreens) and physical barriers (i.e., UV protection clothing, hats, and shade). A large-scale randomized control study with 1600 participants completed in Australia found that the incidence of squamous cell carcinoma and melanoma was significantly reduced in individuals who used sunscreen daily as compared to individuals who used sunscreen on a discretionary basis [53]. Daily use of sunscreen reported a significant decrease (rate ratio 0.62) in actinic keratosis, which is a precursor to the development of squamous cell carcinoma, as compared to controls [54]. Sunscreens were found to be more efficient in reducing skin cancer prevalence compared to UV-protective clothing [55,56], although in some cases, UV-protective clothing was the preferred option [57].
3. Natural UV-Absorbing Compounds
Current chemical protection from UVR is inadequate because synthetic sunscreen products contain active ingredients that may lack photostability [58,59,60]. The photostability of many commonly used chemical UV filters (e.g., oxybenzone, benzophenone-3, which is permitted up to 6% in sunscreen formulations [30]) was tested individually and in combination with other active ingredients [61]. The majority of these compounds showed poor photostability due to photochemical reactions, such as trans-cis isomerization or keto-enol tautomerism, or due to reactions with other UV filters, which produce byproducts [61,62]. Synthetic sunscreens can also negatively impact human health, causing photosensitization and photo irritation, resulting in allergic reactions, free radical formation leading to skin damage, skin irritation, and skin aging acceleration [58,59,60]. Several approaches have been applied during the last decade to improve the photostability of synthetic UV filters, including the use of antioxidants, encapsulation, and the addition of quenching molecules to the sunscreen formulation [63]. For example, avobenzone, a commonly used UVA filter with a very high number of photodegradation products, showed improved photostability in the presence of Vitamins A and C and ubiquinone within the formulation, resulting in an improved SPF value [64]. However, negative environmental impact coming from the application of different synthetic sunscreens remains a problem, as reported in animal and human studies, including the neurotoxic effect of some sunscreen active ingredients [59], endocrine disruption, malformations, coral bleaching, and other detrimental impacts on ecosystems [63,65]. The major issue for these UV filters is their long retention in the environment, slow degradation, and possibly toxicity [58,59]. Consequently, there has been a shift in industry interest toward the use of natural, environmentally friendly UV-absorbing products as UV filters that are biocompatible, biodegradable, and have no toxic properties [63,66].
Natural products (NPs) have become increasingly popular in the development of sunscreens due to their ability to provide a broad spectrum of UV protection and their advantages over synthetic compounds. These small molecules are derived from natural sources such as medicinal plants, herbs, fungi, and marine organisms, and they possess unique photoprotective properties [67,68,69,70,71]. Some commonly used natural products in sunscreens (Table 2) include flavonoids, polyphenols, terpenoids, melanins, and MAAs, which have been found to have photoprotective and other biological properties [19,67,68,72]. MAAs are highly profuse secondary metabolites found in many marine, freshwater, and terrestrial species [8,9]. Rich sources containing different NPs involved in UV protection are provided, including a number of examples specifically for MAAs (Table 2). More comprehensive details about additional MAAs, their chemical structures, and specific features and resources have been provided in MAA databases and reviews [9,44].
Natural products that are considered UV sunscreens should possess several essential features. One of these key elements is the ability to absorb UV radiation effectively and provide broad-spectrum protection; this means that the compounds should be able to absorb both UVA and UVB radiation. Additionally, the stability of the natural products in the presence of UV light is crucial, as any degradation or decomposition of the compound can lead to a loss of protection [73,74]. The ability to demonstrate a high efficacy even at low concentrations is also desirable as it allows for the practical incorporation of the compounds into sunscreen formulations. Safety is another critical factor that should be considered, as the candidates should not cause adverse effects on the skin, such as cytotoxicity or irritations, and should demonstrate minimal permeation into the systemic circulation [75]. When exploring the photoprotective properties of NPs, various types of models were used, including in vitro human skin keratinocytes (HaCaT cells) when assessing quercetin [76], in vivo mouse models when testing myricetin [77], and cell-free assays when evaluating tannic acid bioactivities [78]. Mycosporine-glycine antioxidant activity was assessed using the DPPH radical scavenging assay to investigate in vivo ROS quenching processes [79], while in vitro human keratinocytes were used for the evaluation of the antioxidant activity of palythine [80].
The solubility of the NP candidate in the solvent system used for the sunscreen formulation is essential to ensure that it can be easily incorporated and evenly distributed throughout the product [81]. MAAs have a high water solubility that allows for their distribution within the cell cytoplasm. There were concerns regarding whether MAA water solubility could present an additional challenge when using these molecules within sunscreen formulations for UVR protection during aquatic activities [18]. However, the main component of all sunscreens is water, and it is critical to have appropriate solubilization of these UV filters [82]. For example, other sunscreen formulations successfully used water-soluble synthetic UV filters such as benzophenone-4 [83], indicating that the hydrophilic nature of MAAs should not prevent their use in sunscreen products.
In summary, natural products offer a promising avenue for the development of safe and effective UV sunscreens. By possessing key features such as UV-absorbing properties, broad-spectrum protection, photostability, high efficacy, safety, and solubility, natural product compounds can be considered viable candidates for sunscreen formulations. Nonetheless, a single compound may not be sufficient for adequate skin protection. Instead, it is recommended to consider a combination of various natural substances [70]. Although numerous products with natural ingredients are readily available in the market, none so far have fulfilled all consumer expectations. Hence, the primary focus of new product development should be on addressing these gaps by aiming to identify and characterize more natural product candidates that can help provide effective sun protection and minimize potential health risks.

Table 2.
Photoprotective natural products with the potential to be used as sunscreen agents due to their UV-absorbing capacities and/or antioxidant properties capable of reducing UV-mediated damage.
Table 2.
Photoprotective natural products with the potential to be used as sunscreen agents due to their UV-absorbing capacities and/or antioxidant properties capable of reducing UV-mediated damage.
| UV-Protective Natural Products | Chemical Structure | Key Features/Bioactive Properties | Source of the Compounds | |
|---|---|---|---|---|
| Flavonoids | Quercetin (C15H10O7) | 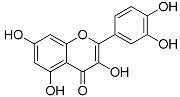 | UV-absorbing, antioxidant-scavenging reactive oxygen species induced by UVA and UVB radiation [76,84] | Fruits and vegetables [85,86,87] |
| Apigenin (C15H10O5) | 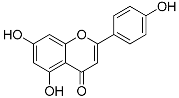 | UV-absorbing, antioxidant against UVA and UVB radiation [88] | Parsley, celery, celeriac, basil, chamomile tea [89,90,91] | |
| Myricetin (C15H10O8) | 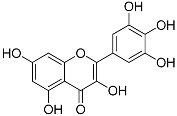 | UV-absorbing, suppressing UVB-induced wrinkle formation [77] | Fruits, vegetables, tea, red wine [92] | |
| Kaempferol (C15H10O6) | 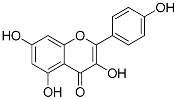 | UV-absorbing, antioxidant [93] | Fruits and vegetables: grapes, tomatoes, broccoli, spinach [94] | |
| Taxifolin (C15H12O7) | 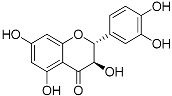 | UVA- and UVB-protective [84,95], antioxidant [96] | Citrus fruits and onion [96] | |
| Polyphenols | (−)-Epigallocatechin gallate (C22H18O11) | 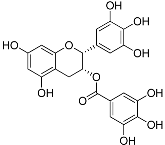 | UV-absorbing [97], antioxidant [98] | Green tea [99] |
| Tannic acid (C76H52O46) | 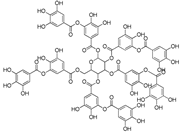 | UV-absorbing, antioxidant [78] | All aerial plant tissues [100] | |
| Resveratrol (C14H12O3) | 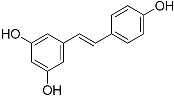 | UV-absorbing [101], antioxidant [102] | Grapes, apples, wine, peanuts, and soy [103,104] | |
| Curcumin (C21H20O6) | 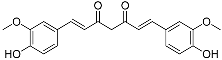 | UV-absorbing [105] anti-inflammatory [106] | Plant Curcuma longa [90] | |
| Terpenoids | α-Tocopherol (C29H50O2) | 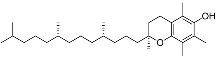 | UV-absorbing (385 nm) [107], antioxidants [108] | Vegetable oils, nuts, and whole grains [109] |
| Astaxanthin (C40H52O4) | 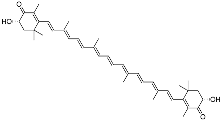 | Antioxidants, prevented UVA-mediated DNA damage [110] | Fungi, bacteria, algae, crustaceans, and some fishes [111,112] | |
| Mycosporines-like amino acids | Mycosporine-glycine (C10H15NO6) | 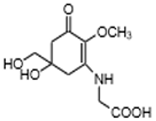 | UV-absorbing, antioxidants [79,113] | Cyanobacteria Chlorogloeopsis sp. PCC6912 [114]; Gloeocapsa sp. [115]; Nostoc commune [116] Macroalgae Acanthophora specifera [22,117], species from genus Bostrychia [118], and Devaleraea [119] Arthropoda, Molluscs, Cnidaria, Echinodermata, Protochordata, Phytoplankton, Nemertinea, Porifera, etc. [9] |
| Shinorine (C13H20N2O8) | 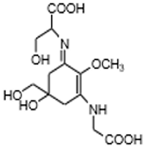 | UV-absorbing, antioxidants [38,120] | Cyanobacteria (Chlorogloeopsis sp. PCC6912 [114]; Gloeocapsa sp. [115]; Nostoc commune [53]) Macroalgae Acanthophora specifera [22], species from the genus Asparagopsis [56,57,58] and Bostrychia [118], and Devaleraea ramentacea [119] Arthropoda, Molluscs, Cnidaria, Echinodermata, Protochordata, Phytoplankton, Nemertinea, Porifera etc. [9] | |
| Porphyra-334 (C14H22N2O8) | 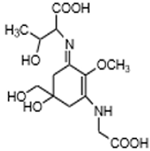 | UV-absorbing, antioxidants [38,120] | Cyanobacteria Nostoc harveyana [52] Macroalgae species from the genus Bostrychia [118] and Porphyra [117] Devaleraea ramentacea [119] Arthropoda, Molluscs, Cnidaria, Echinodermata, Protochordata, Phytoplankton Nemertinea, Porifera etc. [9] | |
| Mycosporine-2-glycine (C12H18N2O7) | 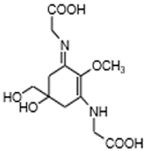 | UV-absorbing, antioxidants [42] | Cyanobacteria Euhalothece sp. LK-1 [121] and Aphanothece halophytica [122] Sea anemone Anthopleura elegantissima [123], dinoflagellate Maristentor dinoferus [124] Molluscs, Cnidaria and others [9] | |
| Palythine (C13H20N2O5;) | 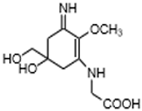 | UV-absorbing, antioxidants [80] | Macroalgae Acanthophora specifera [22,117], Bostrychia species [118] Phytoplankton, Porifera Chordata [9] | |
4. Limitations and Challenges in Using UV-Absorbing MAAs in Sunscreens
Commercially used sunscreens contain synthetic organic and inorganic UVR filters covering a broad range of UVR spectra [18]. Organic UV filters are capable of absorbing UVR, accompanied by inorganic filters such as titanium dioxide (TiO2) and zinc oxide (ZnO), which are also responsible for UVR reflection and scattering [18]. As many synthetic UV filters have a low photostability and a negative effect on the environment, the search for an improved, new generation of UV filters has been ongoing over the last decade [63]. Hundreds of compounds with photoprotective properties were explored, and natural products gained a special interest due to the shift towards environmental safety and raising consumer consciousness [63,125]. MAAs are photoprotective NPs with a supreme potential for use in the new generation of sunscreens due to their abundant presence in marine species, broad UV absorption spectra, and additional roles in actions against osmotic, thermal, and desiccation stress [19,20,30]. MAAs stand out from other photoprotective NPs because of their additional therapeutic properties and ability to accomplish antioxidant, anti-cancer, and anti-inflammatory activities [45]. In addition, the unique ability to perform activation of the Keap1-Nrf2-ARE pathway stimulates cytoprotective gene expression, which is essential for reducing UV-induced damage [38]. Furthermore, MAA mycosporine-2-glycine downregulated gene expression of oxidative stress-induced Cu/Zn-superoxide dismutase 1 and catalase acting on the molecular level and attenuating UVR cellular damage [42].
Some of the most abundant MAAs found in nature, including shinorine, porphyra-334, palythine, and mycosporine-glycine (Table 2), have been used in several cosmetic applications as natural sun protection agents. There are 48 patents reported covering the production and/or specific use of MAAs [44]. The sunscreen product Helioguard ®365 contains two MAAs, shonorine and porphyra-334, which were isolated from the red seaweed Porphyra umbilicalis [126]. In Helionori®, palythine, porphyra-334, and shinorine, also isolated from P. umbilicalis, were used in the sunscreen formulation. However, the proportion of MAAs in these formulations was low and also provided more protection in the UVA region (Figure 1) [44]. This critical gap would need to be covered by other UV filters as the skin damage coming from UVB is 1000x higher than that from UVA [127]. As the most abundant MAAs have absorption maxima in UVA, including another abundant MAA with a UVB absorption maximum (such as mycosporine-glycine) into the sunscreen formulations would be highly beneficial. Furthermore, to obtain an improved photoprotective capacity, an increase in the extracted MAA concentration dry weight (DW) content is needed [18,128]. An exponential rise in sun protection factor (SPF) values was observed with the increase in the MAA content (containing palythine, asterina-330, shinorine, porphyra-334, and palythinol) isolated from the red algae Hydropuntia cornea and Gracilariopsis longissima, reaching SPF 7.5 at the highest MAA yield (13.9 mg DW of algae per cm−2) [128]. The total MAA content in H. cornea was 0.8 ± 0.1 mg MAAs g−1 DW, with the main MAA being palythinol (49.2% of the total MAAs), while in G. longissima, there was 1.6 ± 0.1 mg MAAs g−1 DW with dominant MAA Asterina-330 (42.9% of all MAAs). Furthermore, in red macroalgae Gracilaria gracilis, which was exposed to different light conditions, the highest total MAA content was reached under UV lights (133.03 ± 41.54 mg MAAs g−1 DW), demonstrating an increase of 162% compared to the control (cultures exposed to actinic yellow light at 590 nm) [129]. Interestingly, light quality influenced the composition of MAAs, with the highest content of palythine accumulated in the presence of red light (620–670 nm), while the addition of UV (280–400 nm) or blue (400–450 nm) resulted in the highest content of shinorine. Others have also observed the impact of the modulation of UV and visible light on MAA yield and profile [130,131,132,133]. Obviously, obtaining a higher MAA content and specific MAA profiles will improve SPF levels, allowing them to be more competitive with other synthetic UV filters. Manipulating the light conditions further can reveal improved ways to direct the MAA production towards desired MAA compounds in more controlled ways in the future.
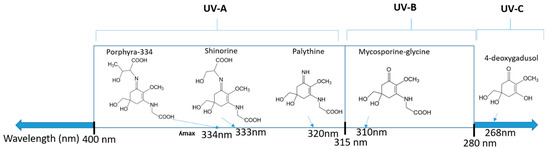
Figure 1.
Chemical structures of mycosporine-like amino acids and their maximum absorbance values placed on the UV wavelength scale.
To enable the widespread use of MAAs, it is also important to successfully apply heterologous expression systems [9]. The limited success here was one of the reasons preventing extensive industry use of MAAs, combined with low extraction yields from natural resources. These existing limitations prompted attempts at chemical synthesis, producing a number of synthetic MAA analogues, which are promising candidates for use in commercial products but have resulted in restricted biological activities compared to the variety of MAAs [10].
5. Investigation of the Efficacy of Natural Products for Use as Sunscreens
Natural products, or novel compounds inspired by natural products, hold enormous potential for developing new sunscreens due to their wide structural diversity. The testing methods for the assessment of the effectiveness of the photoprotection of sunscreens applied topically are indicated by a regulatory norm (ISO24444:2019), which determines the sun protection factor (SPF) [29]. However, the true effectiveness of sunscreens is more variable in practice due to differences in skin phenotype (Table 1), geographical location, meteorological considerations, and, most importantly, the amount and frequency of sunscreen application. A key value for determining SPF is the minimal erythemal dose (MED), defined as the dose of solar radiation that produces sunburn [29]. In fact, the ratio of MED with and without sunscreen is the number reflected by the SPF value. An additional important factor is the UVA protection factor (UVA-PF), obtained on UVA transmittance from in vitro measurements (ISO24443:2020). While formal assessment of the efficacy of new sunscreens is well defined, the development of agents with potential photoprotective properties can occur through a series of pre-clinical models [30].
Several models are available to assess the potential for and efficacy of natural products, such as sunscreens, for human use (Table 3). Each of these different models has advantages and disadvantages. The simplest and most cost-effective model is in vitro testing of the compound for protection against UV-induced cell killing or other biological output in cultured human keratinocytes or immortalized keratinocyte lines (such as HaCaT) [134,135]. While these in vitro assays are easy to perform with equipment usually found in a majority of laboratory settings, they do not reflect the true situation in which keratinocytes are protected by the overlying stratum corneum, which is the outermost layer of the epidermis [136]. Previous work has aimed to address these differences and use a more realistic solar spectrum than that seen at the basal layer of the skin [137]. Other in vitro models use reconstructed human skin or human skin explants. The reconstructed human skin model relies on cultured primary keratinocytes and fibroblasts self-organizing into a structure reminiscent of normal skin [138,139]. Melanocytes, the pigment-producing cells within the skin, may also be included. While this reconstructed skin is closer to human skin, the strata are generally thinner, and the model can be technically challenging. Human skin explants are realistic models [140], but they must be used soon after being excised and require human ethics approval. All in vitro models have the additional advantage of a reduction in the number of animals used for research purposes.
A number of in vivo models have been used to test the photoprotection capacity of sunscreens. Prevention of the ear swelling response of the hairless albino mouse using sunscreen was the work-horse model for a period of time [141]. Other murine models, including the Skh:Hr1 mouse [142], the HGF/SF mouse [143], and various transgenic animals (i.e., BRAF V600E [144], XPA knockout [145]) have also been used. Depending on the model, the readout showing the effect of the compound being tested for photoprotection can be easy and rapid, as in the case of the ear swelling model, or experimentally laborious, as in the case of detecting Tp53 induction or DNA damage in the BRAF or HGF/SF mice, respectively. However, in general, mouse skin lacks the layers of strata that are seen in human skin. Therefore, mouse skin does not represent a perfect model for determining sunscreen efficacy. An additional model that has been used is the porcine skin model. Various different sites on the pig have been tested, including the ear and back [146,147]. Again, the layers of strata vary significantly across the pig, so care must be taken to match the human skin as closely as possible. The porcine models are generally hard to access and expensive in comparison to the murine models. Testing novel sunscreens is most appropriate using the skin of healthy human volunteers [148]. However, accessing volunteers for the testing of new sunscreens requires human ethics approval, clinical support, and specialist equipment that is validated and safe for use in humans, leading to expensive testing. Clearly, the use of pre-clinical models has a place in the development of novel, natural product sunscreens.

Table 3.
Advantages and disadvantages of various models to test novel sunscreen efficacy.
Table 3.
Advantages and disadvantages of various models to test novel sunscreen efficacy.
| Model | Advantages | Disadvantages | |
|---|---|---|---|
| In vitro | Cell culture—primary/immortalized keratinocytes [134,135] |
|
|
| Reconstructed skin [138,139] |
|
| |
| Skin explants [140] |
|
| |
| In vivo | Murine models [141,142,143] |
|
|
| Porcine models [146,147] |
|
| |
| Human volunteers [148] |
|
|
6. Genetics of Marine Organisms Producing MAAs
MAAs are heterologous groups of over 30 small (<400 Da), colorless, hydrophilic molecules with a core structure made of a cyclohexanone or cyclohexenimine ring that is conjugated with an additional radical group [12,149,150,151]. These additional groups added to the MAA core, including further carboxylation and demethylation changes, may alter MAA UV absorption properties [12]. The diversity in the MAAs’ composition and yield, including the UV-absorbing capacity, was detected in various species [12,13,22,122]. MAAs are produced via enzymes encoded by genetically diverse complex enzyme pathways. MAA biosynthesis occurs via two pathways, i.e., the shikimate pathway [17] and/or pentose phosphate pathway, leading to the same MAA precursor 4-deoxygadusol (4-DG), known as a direct precursor of MAAs [152,153]. From 4-DG, MAA biosynthesis leads to the creation of different primary and secondary MAAs (Figure 1) [153,154].
Using genome mining approaches [45,155,156,157], the discovery of MAA biosynthetic pathways occurred through the identification of the gene counterparts in different Gram-positive bacteria [154], cyanobacteria [150,152,156,158,159], and microalgae Symbiodiniaceae [160,161]. All species capable of MAA synthesis were found to have highly similar sequences corresponding to genes from the MAA shikimate or pentose phosphate pathways [160]. The presence of genetic diversity within genes from MAA pathways among marine species indicated the potential for the differential regulation of MAA biosynthetic processes [19,150,156]. Species capable of generating MAAs contained genes from the mys clyster, including dehydrogenase (encoded gene dehydroquinate synthase; DHQS) or a homolog of 2-epi-5-epi-valiolone synthase (EVS; gene mysA) and the oxidoreductase-encoded gene O-methyltransferase (O-MT; gene mysB), needed for the production of 4-DG [152]. Similarly, in the cyanobacterium, Anabaena variabilis ATCC 29413, the existence of certain mys genes resulted in the capacity to generate specific MAAs [152]. For example, the presence of nonribosomalpeptide synthetase (NRPS; encoded by gene mysE) enabled the production of mycosporine-glycine, while the presence of a full 4-gene cluster that included the ATP-grasp homolog gene (mysC) led to the production of shinorine. However, there are levels of variability detected in the order of the genes encoding the enzymes from the MAA biosynthetic cluster [150,154]. In addition, some species were with or without mysE and D-Ala-D-Ala ligase (encoded by gene mysD) from the Nostoc-type mys cluster [162] and were also characterized by multiple copies of specific genes within MAA biosynthetic gene clusters (BGCs) [45,154,156]. The link between the genetic variability of MAA BGC and the functional profile of synthesized MAAs was recently discussed in Brazilian cyanobacteria [150]. However, from 10 analyzed cyanobacterial strains, the only MAAs successfully quantified were shinorine and porphyra 334 and only in two strains, while the levels of these MAAs were influenced by the media used and UV conditions [150]. Simultaneous exposure to photosynthetically active radiation (PAR: 400–700 nm) and UV lights (16 h PAR + UVR: 8 h dark photocycle over 12 days) resulted in the successful induction of MAA production in Antarctic red macroalgae naturally living in shallow waters and the up to 10-fold increase in the MAA yield [163]. Others also reported the variation in the MAA content was impacted by seasonal variation and nutrient conditions [20,25,26,33,122,164,165,166,167,168]. However, a clear link regarding the regulatory processes affecting the MAA biosynthesis, their BGCs up- and down-regulation, and corresponding MAA composition is still missing.
7. Conclusions
Commercially available sunscreens containing synthetic UV filters lack photostability and can result in allergic reactions and inadequate skin protection from damaging UVR. Furthermore, these UV filters pollute our environment and negatively affect living organisms’ delicate balance. Therefore, using natural UV filters should be further explored for the future shift towards sustainable green technologies. MAAs are exceptional candidates among these UV-absorbing compounds, offering skin UVR protection and cosmetic benefits while being ecologically sustainable. A better understanding of regulatory processes and conditions impacting MAA biosynthesis via abiotic factors is critical for the improved and controlled production of MAAs in heterologous expression systems or even when harvesting from the natural environment. Utilizing the advanced pharmacological properties of MAAs and their UV protective capacities may provide additional skin sun protection, creating a new generation of environmentally friendly sunscreens. However, multiple challenges remain unresolved. Substantial knowledge gaps still exist, including the best ways to stimulate and regulate MAA biosynthesis to obtain higher yields and produce targeted MAAs absorbing in both the UVA and UVB ranges. Consequently, further studies are needed to enable controlled MAA production in vivo and in vitro and to improve the amalgamation of MAAs in sunscreen formulations to enhance their future use in UVR protection and skin cancer prevention.
Author Contributions
N.R. generated the idea and designed the manuscript. N.R., M.C., G.M.B., D.T.N. and Y.F. contributed to the writing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to thank the four anonymous reviewers, as well as Isidora Skrlin, for their critical review of this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bertram, C.; Hass, R. Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol. Chem. 2008, 389, 211. [Google Scholar] [CrossRef] [PubMed]
- Savoye, I.; Olsen, C.M.; Whiteman, D.C.; Bijon, A.; Wald, L.; Dartois, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Kvaskoff, M. Patterns of Ultraviolet Radiation Exposure and Skin Cancer Risk: The E3N-SunExp Study. J. Epidemiol. 2018, 28, 27. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222. [Google Scholar] [CrossRef]
- Leiter, U.; Eigentler, T.; Garbe, C. Epidemiology of skin cancer. Adv. Exp. Med. Biol. 2014, 810, 120. [Google Scholar]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B 2001, 63, 8. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, H.; Ono, T. The mechanisms of UV mutagenesis. J. Radiat. Res. 2011, 52, 115. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21. [Google Scholar] [CrossRef]
- Rosic, N.N.; Dove, S. Mycosporine-like amino acids from coral dinoflagellates. Appl. Environ. Microbiol. 2011, 77, 8478. [Google Scholar] [CrossRef]
- Sinha, R.P.; Singh, S.P.; Häder, D.P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B Biol. 2007, 89, 29. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. chemical and ecological aspects. Mar. Drugs 2011, 9, 387. [Google Scholar]
- Figueroa, F.L. Mycosporine-Like Amino Acids from Marine Resource. Mar. Drugs 2021, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Exp. Biol. 2008, 46, 7. [Google Scholar]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-Like Amino Acids from Red Macroalgae: UV-Photoprotectors with Potential Cosmeceutical Applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Rosic, N.; Ling, E.Y.S.; Chan, C.-K.K.; Lee, H.C.; Kaniewska, P.; Edwards, D.; Dove, S.; Hoegh-Guldberg, O. Unfolding the secrets of coral-algal symbiosis. ISME J. 2015, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- Kaniewska, P.; Chan, C.-K.K.; Kline, D.; Ling, E.Y.S.; Rosic, N.; Edwards, D.; Hoegh-Guldberg, O.; Dove, S. Transcriptomic Changes in Coral Holobionts Provide Insights into Physiological Challenges of Future Climate and Ocean Change. PLoS ONE 2015, 10, e0139223. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.; Rémond, C.; Mello-Athayde, M.A. Differential impact of heat stress on reef-building corals under different light conditions. Mar. Environ. Res. 2020, 158, 104947. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-Like Amino Acids for Skin Photoprotection. Curr. Med. Chem. 2018, 25, 5512. [Google Scholar] [CrossRef]
- Rosic, N.N. Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 2014, 87, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N.; Braun, C.; Kvaskoff, D. Extraction and Analysis of Mycosporine-Like Amino Acids in Marine Algae. Methods Mol. Biol. 2015, 1308, 119–129. [Google Scholar] [PubMed]
- Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-Protective Compounds in Marine Organisms from the Southern Ocean. Mar. Drugs 2018, 16, 336. [Google Scholar] [CrossRef]
- Parailloux, M.; Godin, S.; Fernandes, S.C.; Lobinski, R. Untargeted Analysis for Mycosporines and Mycosporine-Like Amino Acids by Hydrophilic Interaction Liquid Chromatography (HILIC)—Electrospray Orbitrap MS2/MS3. Antioxidants 2020, 9, 1185. [Google Scholar] [CrossRef] [PubMed]
- Tartarotti, B.; Sommaruga, R. Seasonal and ontogenetic changes of mycosporine-like amino acids in planktonic organisms from an alpine lake. Limnol. Oceanogr. 2006, 51, 1530–1541. [Google Scholar] [CrossRef]
- Al-Utaibi, A.A.; Niaz, G.R.; Al-Lihaibi, S.S. Mycosporine-like amino acids in six scleractinian coral species. Oceanologia 2009, 51, 93–104. [Google Scholar] [CrossRef]
- Raj, S.; Kuniyil, A.M.; Sreenikethanam, A.; Gugulothu, P.; Jeyakumar, R.B.; Bajhaiya, A.K. Microalgae as a Source of Mycosporine-like Amino Acids (MAAs); Advances and Future Prospects. Int. J. Environ. Res. Public Health 2021, 18, 12402. [Google Scholar] [CrossRef]
- Rosic, N. Molecular mechanisms of stress tolerance in cyanobacteria. In Ecophysiology and Biochemistry of Cyanobacteria; Rastogi, R.P., Ed.; Springer Nature Singapore: Singapore, 2021; p. 131. [Google Scholar]
- Rosic, N.; Kaniewska, P.; Chan, C.-K.K.; Ling, E.Y.S.; Edwards, D.; Dove, S.; Hoegh-Guldberg, O. Early transcriptional changes in the reef-building coral Acropora aspera in response to thermal and nutrient stress. BMC Genom. 2014, 15, 1052. [Google Scholar] [CrossRef]
- Singh, A.; Čížková, M.; Bišová, K.; Vítová, M. Exploring Mycosporine-Like Amino Acids (MAAs) as Safe and Natural Protective Agents against UV-Induced Skin Damage. Antioxidants 2021, 10, 683. [Google Scholar] [CrossRef]
- Morey, J.S.; Monroe, E.A.; Kinney, A.L.; Beal, M.; Johnson, J.G.; Hitchcock, G.L.; Van Dolah, F.M. Transcriptomic response of the red tide dinoflagellate, Karenia brevis, to nitrogen and phosphorus depletion and addition. BMC Genom. 2011, 12, 346. [Google Scholar] [CrossRef]
- Korbee, N.; Mata, M.T.; Figueroa, F.L. Photoprotection mechanisms against ultraviolet radiation in Heterocapsa sp. (Dinophyceae) are influenced by nitrogen availability: Mycosporine-like amino acids vs. xanthophyll cycle. Limnol. Oceanogr. 2010, 55, 899. [Google Scholar]
- Singh, S.P.; Klisch, M.; Sinha, R.P.; Häder, D.-P. Effects of abiotic stressors on synthesis of the mycosporine-like amino acid shinorine in the cyanobacterium anabaena variabilis PCC 7937. Photochem. Photobiol. 2008, 84, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Peinado, N.K.; Díaz, R.T.A.; Figueroa, F.L.; Helbling, E.W. Ammonium and uv radiation stimulate the accumulation of mycosporine-like amino acids in porphyra columbina (rhodophyta) from patagonia, argentina1. J. Phycol. 2004, 40, 248. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa, R.P.; Sinha Singh, S.P.; Häder, D.P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537. [Google Scholar] [CrossRef] [PubMed]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. Experimental study of the excited-state properties and photostability of the mycosporine-like amino acid palythine in aqueous solution. Photochem. Photobiol. Sci. 2007, 6, 669–674. [Google Scholar] [CrossRef]
- de la Coba, F.; Aguilera, J.; Figueroa, F.L.; de Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2008, 21, 161–169. [Google Scholar] [CrossRef]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef]
- Kumar, M.S.; Vijaylaxmi, K.K.; Pal, A.K. Antiinflamatuvar and Antioxidant Properties of Spongosorites halichondriodes, a Marine Sponge. Turk. J. Pharm. Sci. 2014, 11, 285. [Google Scholar]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar]
- Rastogi, R.P.; Incharoensakdi, A. UV radiation-induced biosynthesis, stability and antioxidant activity of mycosporine-like amino acids (MAAs) in a unicellular cyanobacterium Gloeocapsa sp. CU2556. J. Photochem. Photobiol. B Biol. 2014, 130, 287–292. [Google Scholar] [CrossRef]
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2-glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Arch. Biochem. Biophys. 2018, 662, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Koltover, V.K. Antioxidant biomedicine: From free radical chemistry to systems biology mechanisms. Russ. Chem. Bull. 2010, 59, 37–42. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N. Recent advances in the discovery of novel marine natural products and mycosporine-like amino acid UV-absorbing compounds. Appl. Microbiol. Biotechnol. 2021, 105, 7053–7067. [Google Scholar] [CrossRef] [PubMed]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Climstein, M.; Doyle, B.; Stapelberg, M.; Rosic, N.; Hertess, I.; Furness, J.; Simas, V.; Walsh, J. Point prevalence of non-melanoma and melanoma skin cancers in Australian surfers and swimmers in Southeast Queensland and Northern New South Wales. PeerJ 2022, 10, e13243. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Eller, M.S.; Geller, A.C.; Yaar, M. The Pathogenesis of Melanoma Induced by Ultraviolet Radiation. N. Engl. J. Med. 1999, 340, 1341–1348. [Google Scholar] [CrossRef]
- Laberge, G.S.; Duvall, E.; Grasmick, Z.; Haedicke, K.; Galan, A.; Leverett, J.; Baswan, S.; Yim, S.; Pawelek, J. Recent Advances in Studies of Skin Color and Skin Cancer. Yale J. Biol. Med. 2020, 93, 69–80. [Google Scholar]
- He, S.Y.; McCulloch, C.E.; Boscardin, W.J.; Chren, M.-M.; Linos, E.; Arron, S.T. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J. Am. Acad. Dermatol. 2014, 71, 731–737. [Google Scholar] [CrossRef]
- Gallagher, R.P.; Hill, G.B.; Bajdik, C.D.; Coldman, A.J.; Fincham, S.; McLean, D.I.; Threlfall, W.J. Sunlight Exposure, Pigmentation Factors, and Risk of Nonmelanocytic Skin Cancer. Arch. Dermatol. 1995, 131, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Williams, G.; Neale, R.; Hart, V.; Leslie, D.; Parsons, P.; Marks, G.C.; Gaffney, P.; Battistutta, D.; Frost, C.; et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: A randomised controlled trial. Lancet 1999, 354, 723. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.C.; Jolley, D.; Marks, R. Reduction of solar keratoses by regular sunscreen use. N. Engl. J. Med. 1993, 329, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.; Beazer, I.R.; Su, S.; Bounsanga, J.; Hon, E.S.; Lipsky, M.S. An Exploration of the Use and Impact of Preventive Measures on Skin Cancer. Healthcare 2022, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Altmeyer, P.; Hoffmann, K. Role of clothes in sun protection. Recent Results Cancer Res. 2002, 160, 15–25. [Google Scholar]
- Berry, E.G.; Bezecny, J.; Acton, M.; Sulmonetti, T.P.; Anderson, D.M.; Beckham, H.W.; Durr, R.A.; Chiba, T.; Beem, J.; Brash, D.E.; et al. Slip versus Slop: A Head-to-Head Comparison of UV-Protective Clothing to Sunscreen. Cancers 2022, 14, 542. [Google Scholar] [CrossRef]
- Wang, J.; Pan, L.; Wu, S.; Lu, L.; Xu, Y.; Zhu, Y.; Guo, M.; Zhuang, S. Recent Advances on Endocrine Disrupting Effects of UV Filters. Int. J. Environ. Res. Public Health 2016, 13, 782. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- de la Coba, F.; Aguilera, J.; Korbee, N.; de Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs). Mar. Drugs 2019, 17, 55. [Google Scholar] [CrossRef]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of sunscreens. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 91–110. [Google Scholar] [CrossRef]
- Couteau, C.; Faure, A.; Fortin, J.; Paparis, E.; Coiffard, L.J. Coiffard, Study of the photostability of 18 sunscreens in creams by measuring the SPF in vitro. J. Pharm. Biomed. Anal. 2007, 44, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Horita, K.; Sousa e Silva, J.P.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; Esteves da Silva, J.C.G.; Sousa Lobo, J.M. Photodegradation of avobenzone: Stabilization effect of antioxidants. J. Photochem. Photobiol. B Biol. 2014, 140, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Bs, C.P.; Smith, J.; Mesinkovska, N.A. The banned sunscreen ingredients and their impact on human health: A systematic review. Int. J. Dermatol. 2020, 59, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Santhra Krishnan, P.; Salian, A.; Dutta, S.; Mandal, S. A roadmap to UV-protective natural resources: Classification, characteristics, and applications. Mater. Chem. Front. 2021, 5, 7696–7723. [Google Scholar]
- Mansuri, R.; Diwan, A.; Kumar, H.; Dangwal, K.; Yadav, D. Potential of Natural Compounds as Sunscreen Agents. Pharmacogn. Rev. 2021, 15, 47–56. [Google Scholar] [CrossRef]
- Wong, H.J.; Mohamad-Fauzi, N.; Rizman-Idid, M.; Convey, P.; Alias, S.A. Protective mechanisms and responses of micro-fungi towards ultraviolet-induced cellular damage. Polar Sci. 2019, 20, 19–34. [Google Scholar] [CrossRef]
- Leach, C.M. Ultraviolet-absorbing substances associated with light-induced sporulation in fungi. Can. J. Bot. 1965, 43, 185–200. [Google Scholar] [CrossRef]
- Korać, R.R.; Khambholja, K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef]
- Wei, M.; Qiu, H.; Zhou, J.; Yang, C.; Chen, Y.; You, L. The Anti-Photoaging Activity of Peptides from Pinctada martensii Meat. Mar. Drugs 2022, 20, 770. [Google Scholar] [CrossRef]
- Ghazi, S. Do the polyphenolic compounds from natural products can protect the skin from ultraviolet rays? Results Chem. 2022, 4, 100428. [Google Scholar] [CrossRef]
- Smaoui, S.; Ben Hlima, H.; Ben Chobba, I.; Kadri, A. Development and stability studies of sunscreen cream formulations containing three photo-protective filters. Arab. J. Chem. 2017, 10, S1216–S1222. [Google Scholar] [CrossRef]
- Yarovaya, L.; Waranuch, N.; Wisuitiprot, W.; Khunkitti, W. Chemical and mechanical accelerated and long-term stability evaluation of sunscreen formulation containing grape seed extract. J. Cosmet. Dermatol. 2022, 21, 6400–6413. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, S.E.; Hu, J.Y.; Wang, S.Q. Sunscreens: A review of health benefits, regulations, and controversies. Dermatol. Clin. 2014, 32, 427–438. [Google Scholar] [CrossRef]
- Zhu, X.; Li, N.; Wang, Y.; Ding, L.; Chen, H.; Yu, Y.; Shi, X. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2017, 37, 209–218. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, K.W.; Kim, H.Y.; Oh, M.H.; Byun, S.; Lim, S.H.; Heo, Y.-S.; Kang, N.J.; Bode, A.M.; Dong, Z.; et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem. Pharmacol. 2010, 79, 1455–1461. [Google Scholar] [CrossRef]
- Daré, R.G.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O. Tannic acid, a promising anti-photoaging agent: Evidences of its antioxidant and anti-wrinkle potentials, and its ability to prevent photodamage and MMP-1 expression in L929 fibroblasts exposed to UVB. Free Radic. Biol. Med. 2020, 160, 342–355. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. Analysis of UV-absorbing photoprotectant mycosporine-like amino acid (MAA) in the cyanobacterium Arthrospira sp. CU2556. Photochem. Photobiol. Sci. 2014, 13, 1016–1024. [Google Scholar] [CrossRef]
- Lawrence, K.; Gacesa, R.; Long, P.; Young, A. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 2017, 178, 1353–1363. [Google Scholar] [CrossRef]
- Lionetti, N.; Rigano, L. The New Sunscreens among Formulation Strategy, Stability Issues, Changing Norms, Safety and Efficacy Evaluations. Cosmetics 2017, 4, 15. [Google Scholar] [CrossRef]
- Del Olmo, M.; Navarro, À.; Garcia, C.; Ehara, T.; Beltran, L. Effects of Structure on the Solubility of UV Filters. Cosmetics 2022, 9, 60. [Google Scholar] [CrossRef]
- Chisvert, A.; Salvador, A. Determination of water-soluble UV-filters in sunscreen sprays by liquid chromatography. J. Chromatogr. A 2002, 977, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Rajnochová Svobodová, A.; Ryšavá, A.; Čížková, K.; Roubalová, L.; Ulrichová, J.; Vrba, J.; Zálešák, B.; Vostálová, J. Effect of the flavonoids quercetin and taxifolin on UVA-induced damage to human primary skin keratinocytes and fibroblasts. Photochem. Photobiol. Sci. 2022, 21, 59. [Google Scholar] [CrossRef]
- Horwitz, R.J. Chapter 30—The Allergic Patient, in Integrative Medicine, 4th ed.; Rakel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; p. 300. [Google Scholar]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Zhao, B.; Wang, L.; Pang, S.; Jia, Z.; Wang, L.; Li, W.; Jin, B. UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Ind. Crop. Prod. 2020, 151, 112483. [Google Scholar] [CrossRef]
- Sánchez-Marzo, N.; Pérez-Sánchez, A.; Ruiz-Torres, V.; Martínez-Tébar, A.; Castillo, J.; Herranz-López, M.; Barrajón-Catalán, E. Antioxidant and Photoprotective Activity of Apigenin and its Potassium Salt Derivative in Human Keratinocytes and Absorption in Caco-2 Cell Monolayers. Int. J. Mol. Sci. 2019, 20, 2148. [Google Scholar] [CrossRef]
- Singh, D.; Kumari, K.; Ahmed, S. CHAPTER 17—Natural herbal products for cancer therapy. In Understanding Cancer; Jain, B., Pandey, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; p. 257. [Google Scholar]
- Venigalla, M.; Gyengesi, E.; Münch, G. Curcumin and Apigenin—novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease. Neural Regen. Res. 2015, 10, 1181. [Google Scholar]
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef]
- Park, K.-S.; Chong, Y.; Kim, M.K. Myricetin: Biological activity related to human health. Appl. Biol. Chem. 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Zhou, S.-S.; Shen, C.-Y.; Jiang, J.-G. Isolation and identification of four antioxidants from Rhodiola crenulata and evaluation of their UV photoprotection capacity in vitro. J. Funct. Foods 2020, 66, 103825. [Google Scholar] [CrossRef]
- Marotta, P.; Grossini, E.; Farruggio, S.; Panella, M. Chapter 25—Antiaging effects of natural agents in the skin: Focus on mitochondria. In Mitochondrial Physiology and Vegetal Molecules; de Oliveira, M.R., Ed.; Academic Press: Cambridge, MA, USA, 2021; p. 557. [Google Scholar]
- Oi, N.; Chen, H.; Kim, M.O.; Lubet, R.A.; Bode, A.M.; Dong, Z. Taxifolin suppresses UV-induced skin carcinogenesis by targeting EGFR and PI3K. Cancer Prev. Res. 2012, 5, 1103–1114. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity-structure relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- OyetakinWhite, P.; Tribout, H.; Baron, E. Protective Mechanisms of Green Tea Polyphenols in Skin. Oxid. Med. Cell. Longev. 2012, 2012, 560682. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.H.; Alex, V.V.; Anto, R.J. 14—Significance of nutraceuticals in cancer therapy. In Evolutionary Diversity as a Source for Anticancer Molecules, Srivastava, A.K., Kannaujiya, V.K., Singh, R.K., Singh, D., Eds.; Academic Press: Cambridge, MA, USA, 2021; p. 309. [Google Scholar]
- Robles, H. Tannic Acid. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; p. 474. [Google Scholar]
- Chan, C.-M.; Huang, C.-H.; Li, H.-J.; Hsiao, C.-Y.; Su, C.-C.; Lee, P.-L.; Hung, C.-F. Protective Effects of Resveratrol against UVA-Induced Damage in ARPE19 Cells. Int. J. Mol. Sci. 2015, 16, 5789–5802. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Liu, G.; Li, J.; Zhang, J.; Cao, Y.; Miao, J. Antioxidant Activity and Mechanism of Resveratrol and Polydatin Isolated from Mulberry (Morus alba L.). Molecules 2021, 26, 7574. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef]
- Kolb, C.A.; Kopecký, J.; Riederer, M.; Pfündel, E.E. UV screening by phenolics in berries of grapevine (Vitis vinifera). Funct. Plant Biol. 2003, 30, 1177–1186. [Google Scholar] [CrossRef]
- Deng, H.; Wan, M.; Li, H.; Chen, Q.; Li, R.; Liang, B.; Zhu, H. Curcumin protection against ultraviolet-induced photo-damage in Hacat cells by regulating nuclear factor erythroid 2-related factor 2. Bioengineered 2021, 12, 9993–10006. [Google Scholar] [CrossRef]
- Adusumilli, N.C.; Mordorski, B.; Nosanchuk, J.; Friedman, J.M.; Friedman, A.J. Curcumin nanoparticles as a photoprotective adjuvant. Exp. Dermatol. 2021, 30, 705–709. [Google Scholar] [CrossRef]
- Saleh, M.M.; Lawrence, K.P.; Jones, S.A.; Young, A.R. The photoprotective properties of α-tocopherol phosphate against long-wave UVA1 (385 nm) radiation in keratinocytes in vitro. Sci. Rep. 2021, 11, 22400. [Google Scholar] [CrossRef] [PubMed]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Astley, S.B. ANTIOXIDANTS|Role of Antioxidant Nutrients in Defense Systems. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; p. 282. [Google Scholar]
- Catanzaro, E.; Bishayee, A.; Fimognari, C. On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer. Mar. Drugs 2020, 18, 544. [Google Scholar] [CrossRef] [PubMed]
- Aneesh, P.; Ajeeshkumar, K.; Lekshmi, R.; Anandan, R.; Ravishankar, C.; Mathew, S. Bioactivities of astaxanthin from natural sources, augmenting its biomedical potential: A review. Trends Food Sci. Technol. 2022, 125, 81–90. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 105–114. [Google Scholar] [CrossRef]
- Portwich, A.; Garcia-Pichel, F. A novel prokaryotic UVB photoreceptor in the cyanobacterium Chlorogloeopsis PCC 6912. Photochem. Photobiol. 2000, 71, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Sommaruga, R.; Garcia-Pichel, F. UV-absorbing mycosporine-like compounds in planktonic and benthic organisms from a high-mountain lake. Fundam. Appl. Limnol. 1999, 144, 255–269. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Bilger, W.; Scherer, S. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J. Bacteriol. 1997, 179, 1940–1945. [Google Scholar] [CrossRef]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 1998, 46, 271–279. [Google Scholar]
- Karsten, U.; Sawall, T.; West, J.; Wiencke, C. Ultraviolet sunscreen compounds in epiphytic red algae from mangroves. Hydrobiologia 2000, 432, 159–171. [Google Scholar] [CrossRef]
- Obermüller, B.; Karsten, U.; Abele, D. Response of oxidative stress parameters and sunscreening compounds in Arctic amphipods during experimental exposure to maximal natural UVB radiation. J. Exp. Mar. Biol. Ecol. 2005, 323, 100–117. [Google Scholar] [CrossRef]
- Ngoennet, S.; Nishikawa, Y.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. A Method for the Isolation and Characterization of Mycosporine-Like Amino Acids from Cyanobacteria. Methods Protoc. 2018, 1, 46. [Google Scholar] [CrossRef] [PubMed]
- Kedar, L.; Kashman, Y.; Oren, A. Mycosporine-2-glycine is the major mycosporine-like amino acid in a unicellular cyanobacterium (Euhalothece sp.) isolated from a gypsum crust in a hypersaline saltern pond. FEMS Microbiol. Lett. 2002, 208, 233–237. [Google Scholar]
- Waditee-Sirisattha, R.; Kageyama, H.; Sopun, W.; Tanaka, Y.; Takabe, T. Identification and upregulation of biosynthetic genes required for accumulation of mycosporine-2-glycine under salt stress conditions in the halotolerant cyanobacterium Aphanothece halophytica. Appl. Environ. Microbiol. 2014, 80, 1763–1769. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C.; Pearse, J.S.; Pearse, V.B. Mycosporine-like Amino Acid Content in Four Species of Sea Anemones in the Genus Anthopleura Reflects Phylogenetic but Not Environmental or Symbiotic Relationships. Biol. Bull. 2002, 203, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Sommaruga, R.; Whitehead, K.; Shick, J.M.; Lobban, C.S. Mycosporine-like Amino Acids in the Zooxanthella-Ciliate Symbiosis Maristentor dinoferus. Protist 2006, 157, 185–191. [Google Scholar] [CrossRef]
- Kim, S.; Seock, Y.-K. Impacts of health and environmental consciousness on young female consumers’ attitude towards and purchase of natural beauty products. Int. J. Consum. Stud. 2009, 33, 627–638. [Google Scholar] [CrossRef]
- Schmidt, E.W. An enzymatic route to sunscreens. ChemBioChem 2011, 12, 363–365. [Google Scholar] [CrossRef]
- Meinhardt, M.; Krebs, R.; Anders, A.; Heinrich, U.; Tronnier, H. Wavelength-dependent penetration depths of ultraviolet radiation in human skin. J. Biomed. Opt. 2008, 13, 044030. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Casas-Arrojo, V.; Abdala-Díaz, R.T.; Figueroa, F.L. UV Photoprotection, Cytotoxicity and Immunology Capacity of Red Algae Extracts. Molecules 2019, 24, 341. [Google Scholar] [CrossRef] [PubMed]
- Ben Ghedifa, A.; Vega, J.; Korbee, N.; Mensi, F.; Figueroa, F.L.; Sadok, S. Effects of light quality on the photosynthetic activity and biochemical composition of Gracilaria gracilis (Rhodophyta). J. Appl. Phycol. 2021, 33, 3413–3425. [Google Scholar] [CrossRef]
- Schneider, G.; Figueroa, F.L.; Vega, J.; Avilés, A.; Horta, P.A.; Korbee, N.; Bonomi-Barufi, J. Effects of UV–visible radiation on growth, photosynthesis, pigment accumulation and UV-absorbing compounds in the red macroalga Gracilaria cornea (Gracilariales, Rhodophyta). Algal Res. 2022, 64, 102702. [Google Scholar] [CrossRef]
- Kräbs, G.; Bischof, K.; Hanelt, D.; Karsten, U.; Wiencke, C. Wavelength-dependent induction of UV-absorbing mycosporine-like amino acids in the red alga Chondrus crispus under natural solar radiation. J. Exp. Mar. Biol. Ecol. 2002, 268, 69–82. [Google Scholar] [CrossRef]
- Bonomi-Barufi, J.; Figueroa, F.L.; Korbee, N.; Momoli, M.M.; Martins, A.P.; Colepicolo, P.; Van Sluys, M.-A.; Oliveira, M.C. How macroalgae can deal with radiation variability and photoacclimation capacity: The example of Gracilaria tenuistipitata (Rhodophyta) in laboratory. Algal Res. 2020, 50, 102007. [Google Scholar] [CrossRef]
- Franklin, L.A.; Kräbs, G.; Kuhlenkamp, R. Blue light and uv-a radiation control the synthesis of mycosporine-like amino acids in Chondrus crispus (florideophyceae). J. Phycol. 2001, 37, 257–270. [Google Scholar] [CrossRef]
- Gulston, M.; Knowland, J. Illumination of human keratinocytes in the presence of the sunscreen ingredient Padimate-O and through an SPF-15 sunscreen reduces direct photodamage to DNA but increases strand breaks. Mutat. Res. 1999, 444, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Duale, N.; Olsen, A.-K.; Christensen, T.; Butt, S.T.; Brunborg, G. Octyl Methoxycinnamate Modulates Gene Expression and Prevents Cyclobutane Pyrimidine Dimer Formation but not Oxidative DNA Damage in UV-Exposed Human Cell Lines. Toxicol. Sci. 2010, 114, 272–284. [Google Scholar] [CrossRef]
- Menon, G.K. New insights into skin structure: Scratching the surface. Adv. Drug Deliv. Rev. 2002, 54, S3–S17. [Google Scholar] [CrossRef]
- Schuch, A.P.; Moraes, M.C.S.; Yagura, T.; Menck, C.F.M. Highly Sensitive Biological Assay for Determining the Photoprotective Efficacy of Sunscreen. Environ. Sci. Technol. 2014, 48, 11584. [Google Scholar] [CrossRef]
- Bernerd, F.; Vioux, C.; Asselineau, D. Evaluation of the protective effect of sunscreens on in vitro reconstructed human skin exposed to UVB or UVA irradiation. Photochem. Photobiol. 2000, 71, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Bernerd, F.; Vioux, C.; Lejeune, F.; Asselineau, D. The sun protection factor (SPF) inadequately defines broad spectrum photoprotection: Demonstration using skin reconstructed in vitro exposed to UVA, UVBor UV-solar simulated radiation. Eur. J. Dermatol. 2003, 13, 242–249. [Google Scholar] [PubMed]
- Mouret, S.; Bogdanowicz, P.; Haure, M.-J.; Castex-Rizzi, N.; Cadet, J.; Favier, A.; Douki, T. Assessment of the Photoprotection Properties of Sunscreens by Chromatographic Measurement of DNA Damage in Skin Explants. Photochem. Photobiol. 2010, 87, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.A.; Forbes, P.D.; Ludwigsen, K. Sunscreen testing using the mouse ear model. Photo-dermatology 1989, 6, 131–136. [Google Scholar]
- Ley, R.D.; Fourtanier, A. Sunscreen protection against ultraviolet radiation-induced pyrimidine dimers in mouse epidermal DNA. Photochem. Photobiol. 1997, 65, 1007–1011. [Google Scholar] [CrossRef]
- Klug, H.L.P.; Tooze, J.A.; Graff-Cherry, C.; Anver, M.R.; Noonan, F.; Fears, T.R.; Tucker, M.A.; De Fabo, E.C.; Merlino, G. Sunscreen prevention of melanoma in man and mouse. Pigment. Cell Melanoma Res. 2010, 23, 835–837. [Google Scholar] [CrossRef]
- Viros, A.; Sanchez-Laorden, B.; Pedersen, M.; Furney, S.J.; Rae, J.; Hogan, K.; Ejiama, S.; Girotti, M.R.; Cook, M.; Dhomen, N.; et al. Ultraviolet radiation accelerates BRAF-driven melanomagenesis by targeting TP53. Nature 2014, 511, 478–482. [Google Scholar] [CrossRef]
- Horiki, S.; Miyauchi-Hashimoto, H.; Tanaka, K.; Nikaido, O.; Horio, T. Protective effects of sunscreening agents on photocarcinogenesis, photoaging, and DNA damage in XPA gene knockout mice. Arch. Dermatol. Res. 2000, 292, 511–518. [Google Scholar] [CrossRef]
- Duracher, L.; Blasco, L.; Abdel Jaoued, A.; Vian, L.; Marti-Mestres, G. Irradiation of skin and contrasting effects on absorption of hydrophilic and lipophilic compounds. Photochem. Photobiol. 2009, 85, 1459–1467. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Xue, C.; Zhou, S.; Lan, F.; Bi, L.; Xu, H.; Yang, X.; Zeng, F.-D. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol. Lett. 2009, 191, 1–8. [Google Scholar] [CrossRef]
- Liardet, S.; Scaletta, C.; Panizzon, R.; Hohlfeld, P.; Laurent-Applegate, L. Protection against pyrimidine dimers, p53, and 8-hydroxy-2’-deoxyguanosine expression in ultraviolet-irradiated human skin by sunscreens: Difference between UVB + UVA and UVB alone sunscreens. J. Investig. Dermatol. 2001, 117, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Dextro, R.B.; Delbaje, E.; Geraldes, V.; Pinto, E.; Long, P.F.; Fiore, M.F. Exploring the Relationship between Biosynthetic Gene Clusters and Constitutive Production of Mycosporine-like Amino Acids in Brazilian Cyanobacteria. Molecules 2023, 28, 1420. [Google Scholar] [CrossRef] [PubMed]
- Milito, A.; Castellano, I.; Damiani, E. From Sea to Skin: Is There a Future for Natural Photoprotectants? Mar. Drugs 2021, 19, 379. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef]
- Portwich, A.; Garcia-Pichel, F. Biosynthetic pathway of mycosporines (mycosporine-like amino acids) in the cyanobacterium Chlorogloeopsis sp. strain PCC 6912. Phycologia 2003, 42, 384–392. [Google Scholar]
- Miyamoto, K.T.; Komatsu, M.; Ikeda, H. Discovery of gene cluster for mycosporine-like amino acid biosynthesis from Actinomycetales microorganisms and production of a novel mycosporine-like amino acid by heterologous expression. Appl. Environ. Microbiol. 2014, 80, 5028–5036. [Google Scholar] [CrossRef]
- Rosic, N. Genome Mining as an Alternative Way for Screening the Marine Organisms for Their Potential to Produce UV-Absorbing Mycosporine-like Amino Acid. Mar. Drugs 2022, 20, 478. [Google Scholar] [CrossRef]
- D’Agostino, P.M.; Woodhouse, J.N.; Liew, H.T.; Sehnal, L.; Pickford, R.; Wong, H.L.; Burns, B.P.; Neilan, B.A. Bioinformatic, phylogenetic and chemical analysis of the UV-absorbing compounds scytonemin and mycosporine-like amino acids from the microbial mat communities of Shark Bay, Australia. Environ. Microbiol. 2019, 21, 702–715. [Google Scholar] [CrossRef]
- Ambrosino, L.; Tangherlini, M.; Colantuono, C.; Esposito, A.; Sangiovanni, M.; Miralto, M.; Sansone, C.; Chiusano, M.L. Bioinformatics for Marine Products: An Overview of Resources, Bottlenecks, and Perspectives. Mar. Drugs 2019, 17, 576. [Google Scholar] [CrossRef]
- Mogany, T.; Kumari, S.; Swalaha, F.M.; Bux, F. In silico analysis of enzymes involved in mycosporine-like amino acids biosynthesis in Euhalothece sp.: Structural and functional characterization. Algal Res. 2022, 66, 102806. [Google Scholar] [CrossRef]
- Llewellyn, C.A.; Greig, C.; Silkina, A.; Kultschar, B.; Hitchings, M.D.; Farnham, G. Mycosporine-like amino acid and aromatic amino acid transcriptome response to UV and far-red light in the cyanobacterium Chlorogloeopsis fritschii PCC 6912. Sci. Rep. 2020, 10, 20638. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N. Phylogenetic analysis of genes involved in mycosporine-like amino acid biosynthesis in symbiotic dinoflagellates. Appl. Microbiol. Biotechnol. 2012, 94, 29–37. [Google Scholar] [CrossRef]
- Shoguchi, E. Gene clusters for biosynthesis of mycosporine-like amino acids in dinoflagellate nuclear genomes: Possible recent horizontal gene transfer between species of Symbiodiniaceae (Dinophyceae). J. Phycol. 2022, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Garcia-Pichel, F. An ATP-Grasp Ligase Involved in the Last Biosynthetic Step of the Iminomycosporine Shinorine in Nostoc punctiforme ATCC 29133. J. Bacteriol. 2011, 193, 5923–5928. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Wiencke, C. Induction of sunscreen compounds in Antarctic macroalgae by different radiation conditions. Mar. Biol. 2002, 141, 619–627. [Google Scholar]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and temperature effects on bioactivity in diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef]
- Waditee-Sirisattha, R.; Kageyama, H.; Fukaya, M.; Rai, V.; Takabe, T. Nitrate and amino acid availability affects glycine betaine and mycosporine-2-glycine in response to changes of salinity in a halotolerant cyanobacterium Aphanothece halophytica. FEMS Microbiol. Lett. 2015, 362, fnv198. [Google Scholar] [CrossRef]
- Nishida, Y.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Monthly Variation and Ultraviolet Stability of Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Phycology 2021, 1, 119–128. [Google Scholar] [CrossRef]
- Weiss, E.L.; Cape, M.R.; Pan, B.J.; Vernet, M.; James, C.C.; Smyth, T.J.; Ha, S.Y.; Iriarte, J.L.; Mitchell, B.G. The distribution of mycosporine-like amino acids in phytoplankton across a Southern Ocean transect. Front. Mar. Sci. 2022, 9, 2133. [Google Scholar] [CrossRef]
- Jofre, J.; Celis-Plá, P.S.M.; Figueroa, F.L.; Navarro, N.P. Seasonal Variation of Mycosporine-Like Amino Acids in Three Subantarctic Red Seaweeds. Mar. Drugs 2020, 18, 75. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).