Abstract
Respiratory syncytial virus (RSV) is a highly contagious human pathogen that poses a significant threat to children under the age of two, and there is a current need for new small molecule treatments. The Antarctic sponge Suberites sp. is a known source of sesterterpenes, and following an NMR-guided fractionation procedure, it was found to produce several previously unreported metabolites. Neosuberitenone (1), with a new carbon scaffold herein termed the ‘neosuberitane’ backbone, six suberitenone derivatives (2–7), an ansellane-type terpenoid (8), and a highly degraded sesterterpene (9), as well as previously reported suberitenones A (10) and B (11), were characterized. The structures of all of the isolated metabolites including absolute configurations are proposed on the basis of NMR, HRESIMS, optical rotation, and XRD data. The biological activities of the metabolites were evaluated in a range of infectious disease assays. Suberitenones A, B, and F (3) were found to be active against RSV, though, along with other Suberites sp. metabolites, they were inactive in bacterial and fungal screens. None of the metabolites were cytotoxic for J774 macrophages or A549 adenocarcinoma cells. The selectivity of suberitenones A, B, and F for RSV among other infectious agents is noteworthy.
1. Introduction
With the recent emergence of SARS-CoV-2, widespread attention has been drawn to the severity of respiratory illness caused by viral infections. Aside from this and well-known influenza, respiratory syncytial virus (RSV) is another major global pathogen, responsible for bronchiolitis and pneumonia in infants and toddlers under the age of two, as well as severe, sometimes deadly, pneumonia, chronic obstructive pulmonary disease, and asthma in elderly adults [1,2]. Currently there are only two FDA-approved drugs for the treatment of RSV, the guanosine analogue ribavirin and the monoclonal antibody palivizumab [3]; however, both have significant drawbacks. The use of ribavirin is limited to RSV infections in immunocompromised patients owing to its nonspecific activity and toxicity, in conjunction with its relatively high cost [4], while palivizumab is only recommended for prophylactic use in high-risk infants and children [5]. Therefore, there is a current need for new effective and affordable treatments for the widespread RSV pathogen.
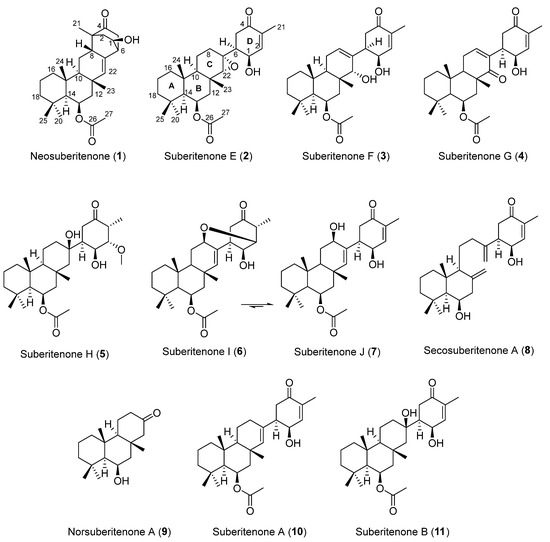
Figure 1.
Terpenoids found in Suberites sp. from Palmer Station, Antarctica.
Figure 1.
Terpenoids found in Suberites sp. from Palmer Station, Antarctica.
Marine sponges collected in the cold waters of Antarctica continue to be a fruitful source of novel bioactive metabolites [6,7,8]. The suberitenones are a class of oxidized sesterterpenes of the ‘suberitane’ carbon skeleton and have been reported from multiple Antarctic Suberites sp. samples as well as Phorbus areolatus collected in the same waters [9,10,11,12]. More recently, anvilone A and B, two metabolites that also share the same carbon skeleton backbone, were reported from a Phorbus sp. sample collected in the temperate waters of Anvil Island, British Columbia [13]. These metabolites have displayed a range of bioactivities, with oxaspirosuberitenone demonstrating mild antibacterial activity against MRSA, isosuberitenone B and 19-episuberitenone B showing weak cytotoxicity against a panel of tumor cell lines, and anvilone A turned on HIV gene expression, showcasing the potential of this class of metabolites for biomedical applications.
As part of our ongoing investigation into the chemistry of Antarctic marine organisms, a Suberites sp. sample collected in 2018 was subjected to a 1H NMR-guided purification procedure using reversed-phase HPLC. The previously reported compounds suberitenones A (10) and B (11) (Figure 1) were both isolated in large quantities from the least polar fractions, while the 1H NMR spectra of earlier eluting fractions showed a number of interesting resonances warranting further analysis. This led to the isolation of nine previously unreported sesterterpene compounds, 1 to 9, of which 1 was made up of a previously unreported carbon skeleton and 6 existed as an interconverting equilibrium between two structures, 6 and 7. Structure elucidation of the isolated compounds and an assessment of their RSV antiviral activity are reported herein.
2. Results and Discussion
Neosuberitenone A (1) was isolated as white crystals, with a molecular formula C27H40O4 (eight double bond equivalents) established by analysis of the (−)-HRESIMS formate adduct ion at m/z 473.2915. The 1D 13C NMR spectrum (125 MHz, CDCl3) gave signals for all 27 carbons (Table 1) and in conjunction with the multiplicity-edited HSQC data, it suggested that 1 contained a ketone, a carboxylate, four sp3 and one sp2 quaternary carbons, seven methine carbons of which one is olefinic and two are oxygen-bearing, seven methylenes, and six methyl groups. These data account for three degrees of unsaturation, therefore 1 must also contain five rings. The 1H NMR data (Table 2) showed signals of two oxygenated methines at δH 4.18 (dd, J = 3.5, 8.7 Hz) and δH 5.51 (br q, J = 3.9 Hz) and an olefin at δH 5.60 (d, J = 3.1 Hz), and it was also observed that the five aliphatic and acetate methyl groups were all singlets, while the remainder of the 1D 1H NMR spectrum was complicated by overlapping aliphatic signals.

Table 1.
13C NMR spectroscopic data of compounds 1–6 and 8.

Table 2.
1H NMR spectroscopic data of compounds 1–6 and 8.
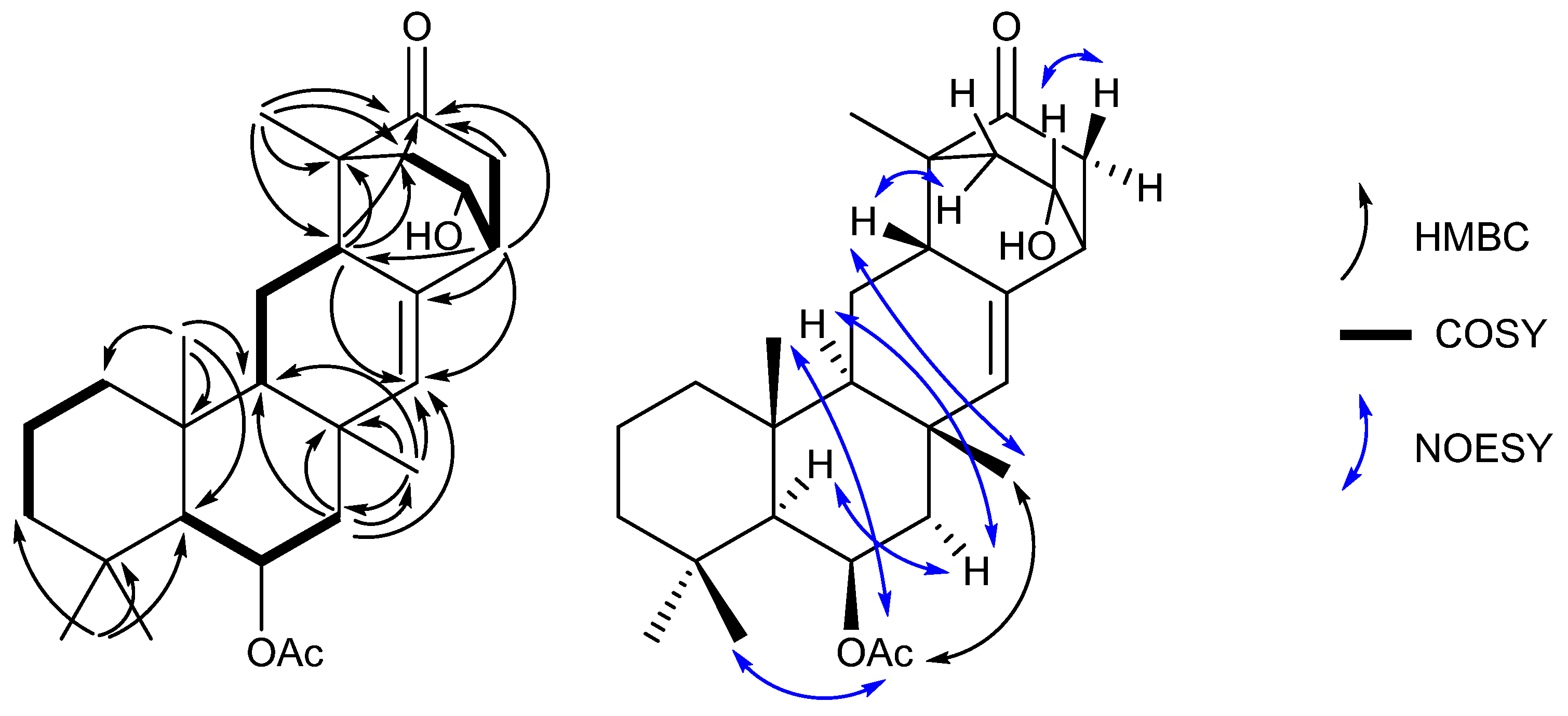
These data, along with correlations in the COSY and HMBC spectra (Table S1), suggested the structure of neosuberitenone A (1) to be closely related to those of the suberitane class of sesterterpenoids (Figure 2). The first spin system was deduced from COSY correlations from H2-16 (δH 0.82 and 1.55) to H2-17 (δH 1.41 and 1.66) and then H2-18 (δH 1.14 and 1.35), which comprise the backbone of ring A. The ring assignment was completed using HMBC correlations from the geminal dimethyl H3-20 and H3-25 (δH 0.92 and 1.01, respectively) to C-14, C-18, and C-19 (δC 57.1, 44.2 and 34.0, respectively), in conjunction with methyl H3-24 (δH 1.28) to C-10, C-15, C-16 (δC 55.1, 38.2 and 41.7, respectively), and C-14. Next, the cycle of ring B was assigned using COSY correlations between H-14 (δH 1.04) and oxygenated H-13 (δH 5.51) and then onto methylene H2-12 (δH 2.09 and 1.44), and HMBC correlations from H2-12 to C-11, C-22, C-23 (δC 35.7, 140.0 and 19.6, respectively), and C-10. Methyl group H3-23 (δH 1.18) showed HMBC correlations to C-10, C-11, C-12, and C-22 so it was therefore placed on quaternary C-12. Ring C, however, showed differences from the suberitane class, lacking the C-8 methylene which was instead replaced by a methine (δH 2.68) that was assigned using COSY correlations to H2-9 (δH 1.75 and 1.26) and H-10 (δH 1.02) and HMBC correlation to olefin C-22.
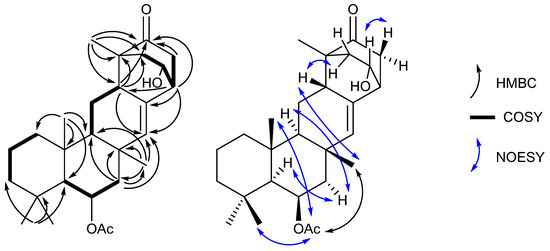
Figure 2.
Key 2D NMR correlations establishing the structure of neosuberitenone A (1).
The remainder of neosuberitenone A (1) consisted of one major spin system between H2-2 (δH 2.13 and 1.64), oxygenated H-1 (δH 4.18), H-6 (δH 2.69), and H2-5 (δH 2.25 and 2.10) deduced from COSY correlations. The HMBC spectrum showed correlations from H-8 to C-2, C-3, and C-4 (δC 42.9, 48.7, and 214.3, respectively) which connected the two units, while the methyl H3-21 (δH 1.01) was placed at C-3 as it showed HMBC correlations to C-2, C-3, C-4, and C-8. This spin system was reasoned to be cyclized between C-4 and C-5 (δC 41.9) as both H2-5 and H-6 showed HMBC correlations to ketone C-4. HMBC correlations from H-6 were able to finalize the ring structure of 1, with correlations to C-7, C-8 (δC 134.3 and 40.8, respectively), and C-22 providing evidence for a bond between C-6 and C-7. The planar structure of 1 was completed by placing the acetyl group on the oxygen of C-13, based on correlations in the NOESY spectrum between H3-27 (2.06) and H3-23, H3-24, and H3-25 which established that the acetyl group was not on the C-1 oxygen.
The stereochemistry of neosuberitenone A (1) was deduced by correlations in the NOESY spectrum. The similar elements of 1 and suberitenone A (10) were determined to have the same stereochemistry, with methyl groups H3-23, H3-24, and H3-25 all deduced to be cofacial with acetyl group H3-27 by NOESY correlations, while correlations between H-12′ to both H-10 and H-14 also placed these protons on the opposite face of the molecule. A correlation between H3-23 to H-8 set the orientation of this center relative to the methyl groups deduced previously, and the correlation from H-8 and H-2′ also suggested these two protons to be syn. As C-3 and C-6 are joined by a two-carbon bridge, this also set the stereochemistry at C-6 as the bridging carbons must be on the same side of the molecule. Finally, correlations between H-1 and H-5 suggested the H-1 proton to be oriented toward C-5 and thus 1 was deemed to have the 1R*,3R*,6R*,8S*,10S*,11S*,13R*,14S*,15R* configuration.
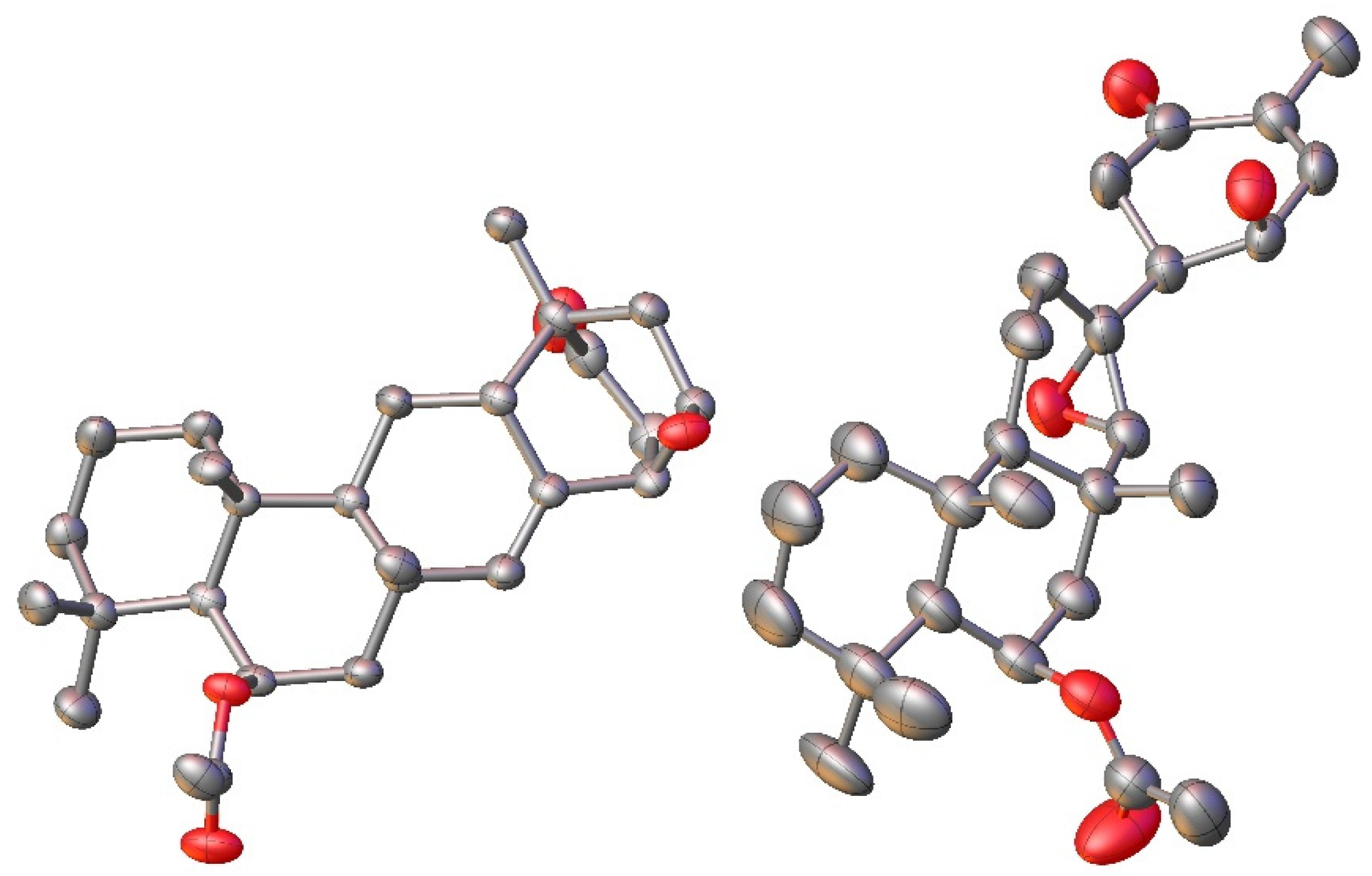
This spectroscopic data analysis was confirmed by single-crystal X-ray diffraction (XRD) studies, with neosuberitenone A (1) forming suitable crystals from 9:1 ACN:H2O. The absolute configuration of 1 was determined to be 1R,3R,6R,8S,10S,11S,13R,14S,15R (Figure 3), which is similar to that deduced by Shin and coworkers on suberitenone A (10) and B (11) using modified Mosher’s method and CD analysis [9]. This terpenoid carbon backbone of 1 is an unprecedented class to date and is termed the ‘neosuberitane’ backbone.
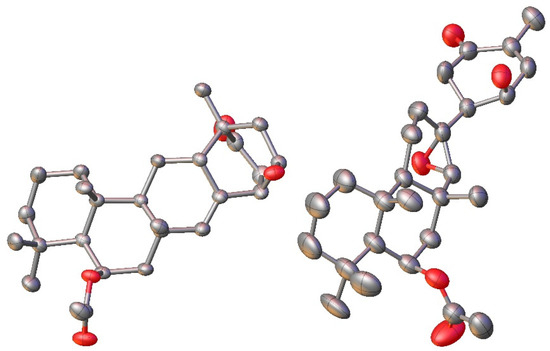
Figure 3.
X-ray crystal structure for 1 (left) and 2 (right). Hydrogens omitted for clarity.
Suberitenone E (2) was also isolated as white crystals, with a molecular formula C27H40O5 established by analysis of the formate adduct ion at m/z 489.2865 in the (−)-HRESIMS data. Analysis of the 1D and 2D NMR data (Table S2) suggested 2 to be structurally related to suberitenone A (10), with major differences present in ring C, notably the absence of the olefin and introduction of a new singlet for H-22 (δH 2.63; δC 70.1). A comparison of the molecular formulas shows 2 to have one extra oxygen atom, all of which points to the presence of an epoxide across the C-7/C-22 bond. Correlations in the NOESY spectrum along with coupling constant analysis were used to deduce that all of the stereocenters shared with suberitenone A were of the same orientation, with a correlation between epoxide H-22 and H3-23 (δH 1.27) suggesting a syn relationship and therefore establishing the configuration of both C-7 and C-22. Single-crystal XRD confirmed this interpretation and established the absolute configuration as 1R,6S,7R,10R,11R,13R,14S,15R,22R, as in Figure 3.
Suberitenone F (3) was isolated as a white film, with a molecular formula C27H40O5 established by analysis of the formate adduct ion at m/z 489.2841 in the (−)-HRESIMS data and is thus isomeric with suberitenone E (2). The major noticeable differences in the NMR data are related to the resonances of ring C, markedly the presence of an olefinic 1H resonance (δH 5.67) that showed COSY correlations to H2-9 (δH 2.12). The 2D NMR correlations (Table S3) inferred this signal to be from H-8 and thus suggest a C-7/C-8 double bond, as found in that of suberitenone C (12, Figure 4). Similar to 2, the NMR data gave evidence for an oxymethine at C-22 (δH 3.15; δC 75.3) and therefore placed a hydroxyl group at this center, completing the planar structure of 3. Correlations in the NOESY spectrum were used to deduce the chiral centers, where the key correlation between H-22 and H3-23 (δH 0.99) showed these two to be related syn to each other, leading to the 22R assignment. All other observed correlations confirmed the remaining configurations to be the same as those deduced by XRD on 2.
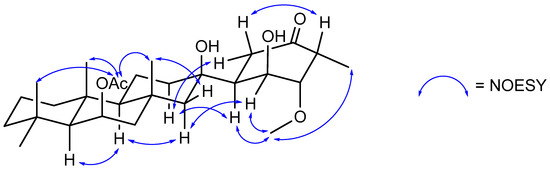
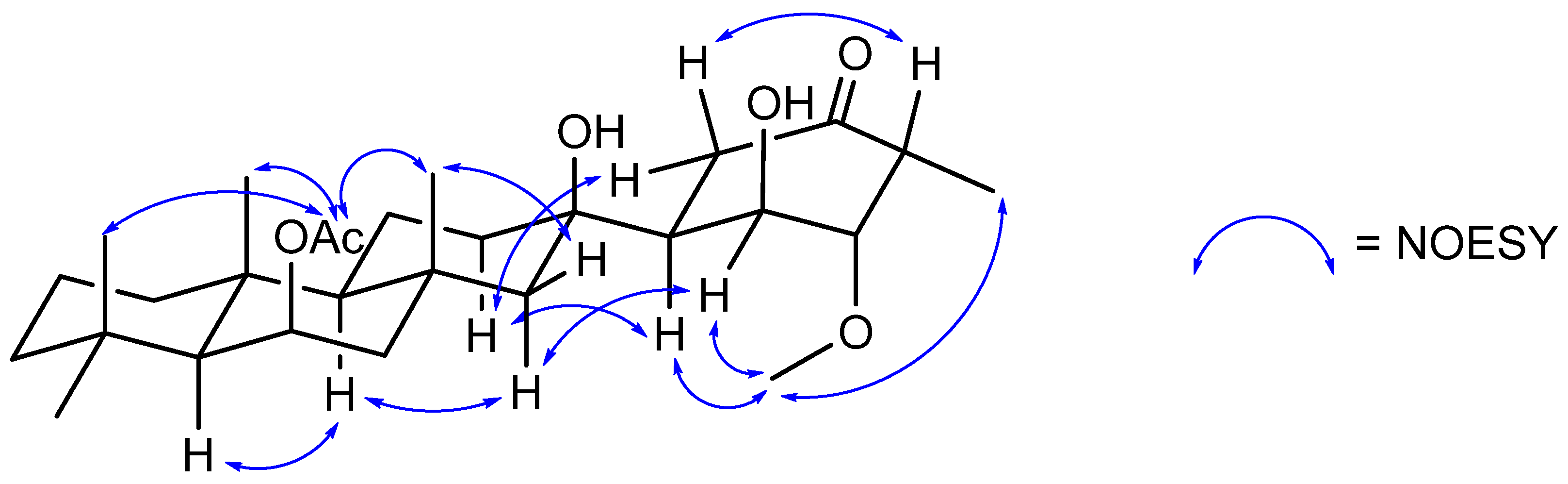
Figure 4.
Key NOESY correlations establishing the relative stereochemistry of suberitenone H (5).
Suberitenone G (4) was isolated as a white film, with a molecular formula C27H38O5 established by analysis of the deprotonated molecule at m/z 441.2645 in the (−)-HRESIMS data. The NMR data (in MeOD as decomposition was observed in CDCl3, Table S4) for 4 suggested the molecule to be related to suberitenone A (10); however, the C ring olefin resonances were further deshielded moving from δH 5.18; δC 138.9 to δH 6.78; δC 147.2. Combined with the observation of a second α,β-unsaturated ketone (δC 205.5) that showed HMBC correlations from this new olefin methine, it was suggested that 4 contained a enone in ring C as well as ring D. A HMBC correlation from H3-23 (δH 1.27) to the new ketone placed it as C-22, while COSY correlations between H-10 (δH 1.71) and H2-9 (δH 2.52) to olefin H-8 (δH 6.78) confirmed the ring C enone orientation. The configuration of the stereocenters of 4 was deduced from NOESY correlations to be the same as that of 2, as was previously deduced by XRD.
Suberitenone H (5) was isolated as a white film, with a molecular formula C28H46O6 established by analysis of the formate adduct ion at m/z 523.3284 in the (−)-HRESIMS data. Analysis of the 1H and 13C NMR data suggested 5 to be similar to suberitenone B (11), hydrated across the C-7/C-22 bond with tertiary alcohol at C-7. However, 5 exhibited several differences in the resonances associated with ring D including the loss of the olefinic 13C/1H signals, splitting of the methyl signal into a doublet, and the gain of an aliphatic methine, an oxymethine, and a methoxy signal. The doublet methyl H3-21 (δH 1.02) correlated in the COSY spectrum to H-3 (δH 3.03) which correlated with H-2 (δH 3.52). The signal of H-2 and methoxy H3-28 showed mutual HMBC to the other carbons for C-28 and C-2 (δC 59.1 and 88.6, respectively) and this accounts for the loss of the C-2/C-3 double bond. The remainder of ring D remains the same as suberitenone B, assigned on the basis of COSY and HMBC correlations (Table S5).
The stereochemistry of suberitenone H (5) was deduced by a combination of J coupling constant analysis and correlations in the NOESY spectrum. The similar elements of 5 and suberitenone B (11) were determined to have the same configurations as above, with methyl groups H3-23 (δH 1.37), H3-24 (δH 1.23), and H3-25 (δH 1.04) all deduced to be cofacial with acetyl group H3-27 (δH 2.03) by NOESY correlations, while a correlation between H-22 (δH 1.81) and H3-23 also placed this proton syn. In contrast, H-22′ (δH 1.15) showed a NOESY correlation to H-10 (δH 0.99) which shared a correlation with H-14 (δH 1.14), placing these two on the opposite side of the molecule. With the large coupling constant (J = 13.7) between H-5 (δH 2.65) and H-6 (δH 1.81), these protons share a pseudo-axial–axial relationship, and as has been seen for previously isolated suberitenones [12], this suggested C-6 (δC 48.3) to have an S configuration. The relatively smaller coupling constant between H-6 and H-1 (J = 3.3) suggested C-1 (δH 66.6) to also have the S configuration. The cofacial relationship of these two protons is further affirmed by both showing NOESY correlations with methoxy H3-28, which also correlated to H3-21, suggesting that this group was on the same face. In addition, a NOESY correlation between pseudo-axial H-3 and H-5 provided the final evidence to assign 2S and 3R to the ring. The last chiral center, C-7, is a tertiary alcohol and was assigned an R configuration, the same as that for suberitenone B. The chemical shift is consistent with the previously isolated molecules, while the NOESY correlations between H-1 and H-22′ and H-8′ with both H-5′ and H-6 mirror those used diagnostically for isosuberitenone B [12].
Suberitenone I (6) was isolated as a white film with a molecular formula C27H40O5 established by analysis of the formate adduct ion at m/z 523.3284 in the (−)-HRESIMS data, therefore being isomeric with 2. Like suberitenone H (5), the 1H and 13C NMR data showed no evidence of a ring D double bond, and instead, the signal for H3-21 (δH 1.19) was a doublet, and the chemical shift of C-2 and H-2 (δC 79.9, δH 4.03) suggested the center was oxygenated (Table S6). Based on COSY correlations throughout the spin system with H-10 (δH 1.13) and H2-9 (δH 1.90 and 1.67), H-8 was assigned (δH 3.79, δC 70.8) and both the 13C and 1H chemical shifts suggest the center was also oxygen-bearing. The planar structure of 6 was finally deduced by a key HMBC correlation from H-2 to C-8, thus providing evidence for an oxa-bicyclo[3.3.1]nonane moiety and providing the correct number of degrees of unsaturation.
While NMR data were collected on purified suberitenone I (6), the signals decreased in intensity while those of a new species grew in intensity. This more-polar compound was separable by reversed-phase HPLC (Figure S44), and from analysis of the signals, it was clear the new species was a suberitenone-type molecule, with the same enone D ring as suberitenone A (10), and COSY/HSQC correlations revealed C-8 (δH 4.30) to be hydroxylated (Table S7). Thus, suberitenone J (7) is the oxaspiro-ring-opened isomer, existing in equilibrium with 6. When the two were separated by reversed-phase HPLC, both reverted to an equilibrium mixture of ~3:1 6:7 over 24 h.
To determine the configuration of the chiral centers, key NOESY correlations and the 1H NMR coupling constants for both compounds were taken into account. In the NOESY spectra of both compounds, a key correlation between H-8 and H-10 placed these two protons syn, establishing the orientation of C-8 relative to the suberitane scaffold. As with oxaspirosuberitenone, the extra ring distorts the D ring away from a pseudo-chair conformation in suberitenone I (6) and thus, coupling constants cannot be understood to rapidly assign configurations. This is not the case for suberitenone J (7) where the pseudo-axial–axial coupling constant of H-5 and H-6 (J = 13.7 Hz) established that C-6 shares the same configuration as the previously isolated congeners and the same can be applied between H-6 and H-1 (J = 4.3 Hz) to establish C-1. Finally, correlations to H3-21 from H-2 were used to assign the orientation of the methyl group on C-3, which is in accordance with the other molecules isolated from this organism.
Secosuberitenone A (8) was isolated as a white film, with a molecular formula of C25H38O3 established by analysis of the formate adduct ion at m/z 431.2814 in the (−)-HRESIMS data. The 1D and 2D NMR data suggested major differences present in ring C and also lacked the signals of the acetyl group which suggested that the ring B hydroxyl did not bear an acetyl group as in the previously isolated metabolites (Table S8). The most significant difference with other suberitenones was the presence of two terminal vinylidene moieties, thereby accounting for all seven degrees of unsaturation and therefore suggesting that ring C was not cyclized. The 1H signals for H2-23 (δH 5.03 and 4.77) showed HMBC correlations to C-10, C-11, and C-12 (δC 57.5, 144.4, and 47.8, respectively); therefore, this replaced the methyl group normally present there, whereas vinylidene H2-22 (δH 5.16 and 4.92) showed correlations to C-1, C-6, C-7, and C-8 (δC 63.6, 45.5, 148.8, and 34.2, respectively) which revealed it to be adjacent to ring D. Both sets of correlations lead to the conclusion that compound 8 lacks the cyclization bond from C-10 to C-22 typically formed in the terpene backbone cyclization to form the suberitane scaffold (Figure 5), instead forming the ansellane backbone first observed in ansellone A [14]. The configuration of chiral centers in 8 were deduced from NOESY correlations to be the same as that of 2 as was previously deduced by XRD.
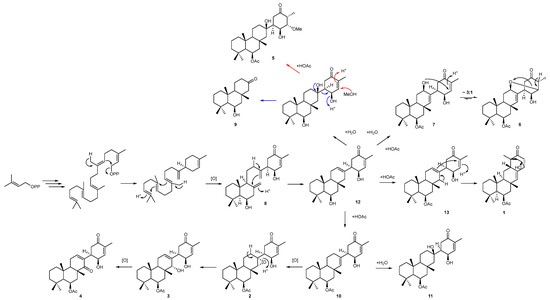
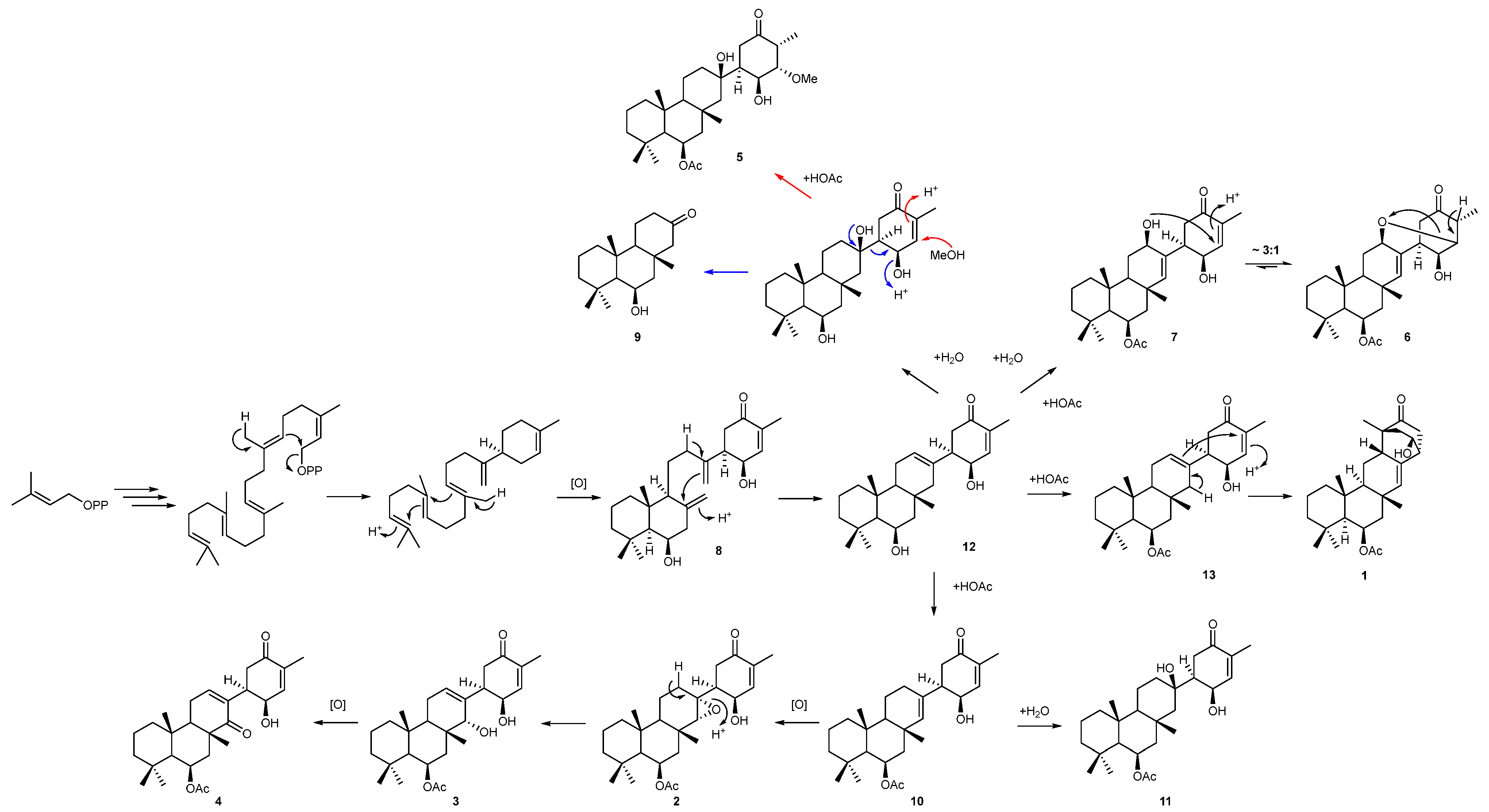
Figure 5.
Proposed biogenesis of suberitane and neosuberitane backbone based on the compounds isolated in this study. Blue arrows indicate the degradation path to 9 while red arrows indicate the methanol addition pathway leading to 5.
Norsuberitenone A (9) was isolated as a white crystalline solid with the molecular formula C18H30O2 deduced from analysis of the protonated molecular at m/z 279.2320 (+)-HRESIMS data. Norsuberitenone A is significantly smaller than previously isolated suberitenones, and the NMR spectra lacked signals associated with the D ring or the acetyl group. Using HMBC correlations (Table S9) from H3-15, H3-17, and H3-18, rings A and B were deduced to be the same as the suberitenone A (with a hydroxyl in place of the acetyl group at C-8); however, ring C was determined to have a ketone at C-2 (δC 215.1) based on HMBC correlations from H2-1 (δH 2.21 and 1.90), H2-3 (δH 2.37 and 2.33), and H-4 (δH 2.05). This position is the tertiary alcohol carbon that bridges to ring D in suberitenone B (11); therefore, it is likely that 9 forms as a degradation product from this (blue arrows, Figure 5). The stereochemistry of the chiral centers was assigned the same configuration as the other congeners based on correlations in the NOESY spectrum.
A proposed biogenesis of newly isolated metabolites 1 to 11 is presented in Figure 5. Both 1 and 4 can be envisaged to form from common intermediate 12 (desacetoxy suberitenone C = suberitenol C) [10] by either an intramolecular cyclization or oxidation reaction, respectively. Compound 2 forms from suberitenone A (10) by a further epoxidation reaction of the C7/C22 double bond, while 5 is likely to be a methanol addition product of suberitenone B (11). While we cannot rule out 5 as an artifact of isolation, it would be unusual that only suberitenone B, and none of the other six α,β-unsaturated ketones, added methanol. Furthermore, stirring pure isolated suberitenone B in methanol for one week did not produce any detectable amount of 5. Metabolite 9 bearing only 18 carbon atoms is likely to be a ‘heptanorsesquiterpene’ formed by the elimination of the suberitenone B D ring. The purification procedure used avoided the use of ethyl acetate, which proved the acetyl group at C-13 to be biogenerated.
The newly isolated compounds alongside suberitenones A (10) and B (11) were assessed for their ability to inhibit RSV gene expression and their effect on the cell viability (Table 3). Neosuberitenone (1) was the only compound that did not show any viral inhibitory effects at 25 µg/mL (6, 8, and 9 were not tested due to a lack of sample supply), suggesting that the ring D must be appended by a single bond to result in activity. Suberitenones A and B demonstrated the strongest antiviral potential of the tested compounds, with IC50 values of 7.9 and 3.5 µM, respectively, while suberitenone F (3) was also active (IC50 = 9.8 µM). The other isolated compounds had IC50 values ranging from 10.9–20.5 µM (Table S12), therefore suggesting that oxidation about ring C and removing the enone double bond of ring D are not likely to be alterations that would result in an increase in activity for future structure–activity relationship studies. The CC50 values were ~4–5 fold higher than the IC50 values for the newly isolated compounds (Table 3). Suberitenone B (11) showed the highest activity and greatest selectivity index.

Table 3.
RSV antiviral (IC50) and cytotoxicity (CC50) activities of 2–5, 10, and 11 (means ± SEM of triplicate samples). Calculated from data in Figure S12 using Prism. Selectivity index = CC50/IC50.
Suberites sp. compounds were also submitted to a broad range of infectious disease screening. No antibacterial activity was detected against the ESKAPE pathogens, nor antifungal activity against several Candida strains. The cytotoxicity was assayed against the murine macrophage J774 cell line, where no significant activity (EC50 below 10 µM) was observed for any compound. Taken with low cytotoxicity, the selectivity of suberitenones A and B (10, 11) for RSV among other infectious diseases is noteworthy.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were measured using an AutoPol IV polarimeter at 589 nm. UV/Vis spectra were extracted from HPLC chromatograms. NMR spectra were acquired using either a Varian Inova 500 spectrophotometer or a Varian 600 MHz broadband spectrophotometer. The residual solvent peak was used as an internal chemical shift reference (CD3OD: δC 49.0; δH 3.31, CDCl3: δC 77.0; δH 7.26). High-resolution mass spectrometry−liquid chromatography data were obtained on an Agilent 6540 QTOF LCMS with electrospray ionization detection. Reversed-phase HPLC was performed on a Shimadzu LC20-AT system equipped with a photodiode array detector (M20A) using a preparative Phenominex C18 column (5 μm, 100 Å, 250 × 21.2 mm; 9 mL/min) or on a semipreparative Phenominex C18 column (10 μm, 100 Å, 250 × 10 mm; 4 mL/min). All of the solvents used for column chromatography were of HPLC grade, and H2O was distilled. Solvent mixtures are reported as % v/v unless otherwise stated.
3.2. Biological Material, Extraction and Isolation
Sponge specimens were collected in the austral fall, 2018, within a 3.5 km radius of Palmer Station, Antarctica (64° 46.50’ S; 64° 03.30’ W). The frozen sponge was freeze-dried (1.35 kg wet weight and 200 g dry weight) and then crushed by hand before being extracted in MeOH (1.6 L) twice overnight. The extracts were combined, were then passed through an HP20 column (250 mL), pre-equilibrated in H2O, and combined following elution. The eluent was then diluted with an equal volume of H2O and passed back through the column twice, followed by a 750 mL H2O wash. The column was then eluted with 750 mL portions of (1) 75% Me2CO/H2O and (2) Me2CO (fractions A1 and A2, respectively). Fraction A1 (5 g) was then reconstituted in MeOH (25 mL), filtered, and fractionated generating fractions (B1–B25) by repeated preparative C18 HPLC (9 mL/min) using the following method: 70% can/H2O (0.1% CH2O2) for 6 min, a linear gradient to 100% ACN over 8 min, and 100% ACN isocratic for 11 mins. Fraction B25 contained suberitenone A (~500 mg), while fraction B23 contained suberitenone B (~250 mg). Fraction B20 was purified using semipreparative C18 HPLC using a linear gradient from 50% MeOH/H2O (0.1% CH2O2) to 100% MeOH over 25 min to afford 1 (5.6 mg) and 2 (1.6 mg), while fractions B15, B16, B17, B18, B19, and B21 were also purified by the same method to afford 6 (0.8 mg), 4 (3.4 mg), 8 (0.8 mg) 3 (1.9 mg), 9 (0.8 mg), and 5 (6.5 mg), respectively.
Neosuberitenone A (1): white crystals; [α]22D 10.5 (c 0.2, MeOH); UV (MeOH/H2O) λmax 210, 235 (sh) nm; 1H and 13C NMR spectra (CDCl3), see Table 1 and Table 2; (−)-HRESIMS m/z 473.2915 [M + HCOO]− (calcd for C28H41O6, 473.2909; Δ 1.32 ppm).
Suberitenone E (2): white crystals; [α]22D −42 (c 0.1, MeOH); UV (MeOH/H2O) λmax 231 nm; 1H and 13C NMR spectra (CD3OD), see Table 1 and Table 2; (−)-HRESIMS m/z 489.2865 [M + HCOO]− (calcd for C28H41O7, 489.2858; Δ 1.58 ppm).
Suberitenone F (3): white film; [α]22D −45 (c 0.1, MeOH); UV (MeOH/H2O) λmax 233 nm; 1H and 13C NMR spectra (CD3OD), see Table 1 and Table 2; (−)-HRESIMS m/z 489.2841 [M + HCOO]− (calcd for C28H41O7, 489.2858; Δ 3.34 ppm).
Suberitenone G (4): white film; [α]22D −62 (c 0.2, MeOH); UV (MeOH/H2O) λmax 233 nm; 1H and 13C NMR spectra (CD3OD), see Table 1 and Table 2; (−)-HRESIMS m/z 441.2645 [M − H]− (calcd for C27H37O5, 441.2646; Δ 0.42 ppm).
Suberitenone H (5): white film; [α]22D −9.0 (c 0.2, MeOH); UV (MeOH/H2O) λmax 233, 280 (sh) nm; 1H and 13C NMR spectra (CD3OD), see Table 1 and Table 2; (−)-HRESIMS m/z 523.3284 [M + HCOO]− (calcd for C29H47O8, 523.3276; Δ 1.36 ppm).
Suberitenone I (6): white film; [α]22D 7.7 (c 0.1, MeOH, 3:1 mixture with 7); UV (MeOH/H2O) λmax 210 nm; 1H and 13C NMR spectra (CD3OD), see Table 1 and Table 2; (−)-HRESIMS m/z 489.2855 [M + HCOO]− (calcd for C28H41O7, 489.2858; Δ 0.54 ppm).
Suberitenone J (7): white film; [α]22D 7.7 (c 0.1, MeOH, 3:1 mixture with 7); UV (MeOH/H2O) λmax 232 nm; 1H and 13C NMR (CD3OD) could not be fully assigned due to rapid conversion to 6 upon purification. The observed signals that differed from 6 are reported in Table S7; (−)-HRESIMS m/z 489.2855 [M + HCOO]− (calcd for C28H41O7, 489.2858; Δ 0.54 ppm).
Secosuberitenone A (8): white film; [α]22D −11.7 (c 0.1, MeOH); UV (MeOH/H2O) λmax 230 nm; 1H and 13C NMR spectra (CDCl3), see Table 1 and Table 2; (−)-HRESIMS m/z 431.2814 [M + HCOO]– (calcd for C26H39O5, 431.2803; Δ −2.51 ppm).
Norsuberitenone A (9): white crystalline solid; [α]22D 6.3 (c 0.1, MeOH); UV (MeOH/H2O) λmax 210, 237, 285 (sh) nm; 1H and 13C NMR spectra (CD3OD), see Table S9; (+)-HRESIMS m/z 279.2320 [M + H]+ (calcd for C18H31O2, 279.2319; Δ 0.38 ppm).
3.3. X-ray Crystallography
Crystallographic data for the structures reported in this article were deposited at the Cambridge Crystallographic Data Center under the deposition numbers CCDC 2164632 (1) and 2164633 (2). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/ (accessed on 5 April 2022). XRD data were measured on Bruker D8 Venture PHOTON II CMOS diffractometer equipped with a Cu Kα INCOATEC ImuS micro-focus source (λ = 1.54178 Å). Indexing was performed using APEX4 (difference vectors method) [15]. Data integration and reduction were performed using SaintPlus [16]. Absorption correction was performed by the multi-scan method implemented in SADABS [17]. Space group was determined using XPREP implemented in APEX3 [15]. The structure was solved using SHELXT [18] and refined using SHELXL-2018/3 (full-matrix least-squares on F2) [19] through the OLEX2 interface program [20]. The ellipsoid plot was completed with Platon [21].
Neosuberitenone A (1): A hydrogen atom of the -OH group was found from a difference Fourier map and was freely refined. All remaining hydrogen atoms were refined using a riding model. Crystal data: C27H40O4, M = 428.59 g/mol, monoclinic, space group C2, a = 24.7343(6) Å, b = 6.27570(10) Å, c = 18.2166(4) Å, V = 2308.80(9) Å3, Z = 4, T = 100.00 K, μ(Cu Kα) = 0.636 mm−1, ρcalc = 1.233 g/cm3, 21421 reflections measured (5.942° ≤ 2θ ≤ 159.866°), 4852 independent reflections (Rint = 0.0409, Rsigma = 0.0334), which were used in all of the calculations. The final R1 was 0.0330 (I >= 2σ(I)) and the wR2 was 0.0881 (all data). Flack parameter: 0.06(6). A full table of these parameters for the crystal structure can be found in the supplementary information.
Suberitenone E (2): A hydrogen atom of the -OH group was found from a difference Fourier map and was refined with distance restraint. All remaining hydrogen atoms were refined using a riding model. Crystal data: C27H40O5, M = 444.59 g/mol, monoclinic, space group P21, a = 6.7821(2) Å, b = 8.9780(3) Å, c = 20.5457(6) Å, V = 1245.06(7) Å3, Z = 2, T = 298.00 K, μ(Cu Kα) = 0.638 mm−1, ρcalc = 1.186 g/cm3, 28530 reflections measured (8.648° ≤ 2θ ≤ 158.82°), 5185 independent reflections (Rint = 0.0572, Rsigma = 0.0426), which were used in all of the calculations. The final R1 was 0.0465 (I >= 2σ(I)) and the wR2 was 0.1325 (all data). Flack parameter: 0.04(11). A full table of these parameters for the crystal structure can be found in the Supplementary Materials.
3.4. RSV Antiviral Assay
A549 (CCL-185, ATCC) cells were seeded at 1.6 × 104 cells in 100 μL of 5% FBS/1X penicillin–streptomycin/F12 medium per well in 96-well µClear® black plates with a clear bottom (Greiner 655090). The cells were infected with 400 PFU/well of a recombinant RSV encoding Renilla luciferase as an additional transcription unit (rA2-Rluc [22]) in a total volume of 50 µL/well and allowed to adsorb for 1 h. Purified compounds were serially diluted two-fold in triplicate and 50 µL/well of the diluted extracts were added to the infected cells. After further incubation at 37 °C and 5% CO2 for 24 h, the supernatants were removed and cells were lysed using Renilla lysis buffer (Promega). Luciferase activity was measured with the Renilla Luciferase reagent (Promega) and BioTek Synergy Mx microplate luminometer using Gen5 version 2.00.18 software. The RSV antiviral effect for the test samples was determined by normalizing to the DMSO-treated control samples and multiplying by 100 to obtain the percent of control. Statistical analysis was performed using GraphPad Prism software version 9.4.1.
3.5. Cytotoxicity Assay
To test for cell viability, duplicate 96-well plates of uninfected A549 cells were set up in parallel with the RSV antiviral assay. Extracts were diluted and used to treat cells as described above for the RSV antiviral assay. The negative control for cytotoxicity was 2% DMSO while the positive was 2 µM (final concentration) of PDKI inhibitor. After the 24 h incubation period, the cells were processed for an MTT Proliferation Assay (Provost and Wallert Research) according to the manufacturer’s protocol. Absorbance at 570 nm was measured by a microplate luminometer and standardized to control as above. Two percent DMSO and 2 µM PDK1 inhibitor (CalBioChem) were used as negative and positive controls for cell death, respectively. Statistical analysis was performed using GraphPad Prism software version 9.4.1.
4. Conclusions
In summary, a 1H NMR-guided fractionation procedure of the polar extract constituents of the Antarctic sponge Suberites sp. resulted in the isolation of nine new sesterterpenoids; neosuberitenone A (1), suberitenones E–J (2–7), secosuberitenone A (8), and norsuberitenone A (9) along with large quantities of suberitenones A (10) and B (11). The previously unreported compounds showed various oxidations and ring formations never before reported from this class of compounds, including the new neosuberitane terpenoid carbon skeleton solved by a combination of NMR interpretation and XRD data. Although the compounds did not show activity in antibacterial or antifungal assays, they showed the ability to inhibit RSV viral transcription with relatively low levels of cytotoxicity. The new compounds demonstrated weaker activity than suberitenones A and B and provide important information for future structure–activity relationship studies and structure optimization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/1660-3397/21/2/107/s1. Figures S1–S7. NMR spectra and MS data for neosuberitenone A (1); Figures S8–S14. NMR spectra and MS data for suberitenone E (2); Figures S15–S21. NMR spectra and MS data for suberitenone F (3); Figures S22–S28. NMR spectra and MS data for suberitenone G (4); Figures S29–S35. NMR spectra and MS data for suberitenone H (5); Figures S36–S44. NMR spectra and MS data for suberitenone I (6) and suberitenone J (7); Figures S45–S51. NMR spectra and MS data for secosuberitenone A (8); Figures S52–S58. NMR spectra and MS data for norsuberitenone A (9); Figure S59. Ellipsoid plot of neosuberitenone A (1); Figure S60. Ellipsoid plot of suberitenone E (2); Figure S61. Antiviral activity and cytotoxicity of selected compounds. A549 Cells were infected with rA2-Rluc for 1 h (a) or left uninfected (b) then treated in triplicate with serially diluted purified compounds. 24 h post-infection, antiviral activity was determined by Renilla luciferase assay (a) and cytotoxicity was measured by MTT assay (b). Values are normalized to DMSO-treated cells. Shown are means ± SEM; Table S1. NMR data for neosuberitenone A (1); Table S2. NMR data for suberitenone E (2); Table S3. NMR data for suberitenone F (3); Table S4. NMR data for suberitenone G (4); Table S5. NMR data for suberitenone H (5); Table S6. NMR data for suberitenone I (6); Table S7. NMR data for suberitenone J (7); Table S8. NMR data for secosuberitenone A (8); Table S9. NMR data for norsuberitenone A (9); Table S10. Crystal data for neosuberitenone A (1); Table S11. Crystal data for suberitenone E (2); Table S12. Antiviral activity against RSV.
Author Contributions
Conceptualization, M.N.T., C.D.A., J.B.M. and B.J.B.; formal analysis, J.B., S.S.H.O., M.N.T., K.C.T. and B.J.B.; Funding acquisition, C.D.A., J.B.M. and B.J.B.; investigation, J.B., S.S.H.O., M.N.T. and K.C.T.; methodology, J.B., S.S.H.O., K.C.T. and B.J.B.; project administration, B.J.B.; resources, M.N.T., C.D.A., J.B.M. and B.J.B.; supervision, M.N.T., C.D.A. and B.J.B.; writing—original draft, J.B.; writing—review and editing, S.S.H.O., M.N.T., K.C.T., C.D.A., J.B.M. and B.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
The research in Antarctica was funded by the National Science Foundation awards PLR-1341333 (CDA and JBM) and PLR-1341339 (BJB); a portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) Facility, supported by the National Science Foundation Cooperative Agreement DMR-1644779 and the State of Florida. ESKAPE pathogen screening was supported by NIH grant AI154922 and Candida screening by NIH grant AT010939.
Data Availability Statement
Data generated in the process of this research are available in the Supplementary Materials.
Acknowledgments
The authors would like to thank Jim R. Rocca at the University of Florida for his help in acquiring NMR data, where a portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) Facility. We would also like to acknowledge the assistance of the USF core facilities for XRD, Lukasz Wojtas, and mass spectrometry, Laurent Calcul. Support for the field work in Antarctica from National Science Foundation’s Antarctic Organisms and Ecosystems Program is gratefully acknowledged. The ASC support staff at Palmer Station, Antarctica, are acknowledged for the outstanding logistical services they provide to facilitate our field work. We would also like to thank Sarah Kennedy and Les Shaw for running the ESKAPE assay and Sarah Dietrick for running the Candida assay. JBM wishes to acknowledge support from an endowed Professorship in Polar and Marine Biology from the University of Alabama at Birmingham.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to resolve a typo. This change does not affect the scientific content of the article.
References
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Lopardo, G.; Scarpellini, B.; Stein, R.T.; Ribeiro, D. Systematic review on respiratory syncytial virus epidemiology in adults and the elderly in Latin America. Int. J. Infect. Dis. 2020, 90, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, M.A.; Leyva-Grado, V.H. Overview of current therapeutics and novel candidates against influenza, respiratory syncytial virus, and middle east respiratory syndrome coronavirus infections. Front. Microbiol. 2019, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Pan, Y.; Jiang, S.; Lu, L. Respiratory Syncytial Virus entry inhibitors targeting the F protein. Viruses 2013, 5, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Domachowske, J.B.; Anderson, E.J.; Goldstein, M. The future of Respiratory Syncytial Virus disease prevention and treatment. Infect. Dis. Ther. 2021, 10, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.S.; Mutka, T.; Vesley, B.; Amsler, M.O.; McClintock, J.B.; Amsler, C.D.; Perman, J.A.; Singh, M.P.; Maiese, W.M.; Zaworotko, M.J.; et al. Norselic acids A-E, highly oxidized anti-infective steroids that deter mesograzer predation, from the Antarctic sponge Crella sp. J. Nat. Prod. 2009, 72, 1842–1846. [Google Scholar] [CrossRef] [PubMed]
- Knestrick, M.A.; Wilson, N.G.; Roth, A.; Adams, J.H.; Baker, B.J. Friomaramide, a highly modified linear hexapeptide from an Antarctic sponge, inhibits Plasmodium falciparum liver-stage development. J. Nat. Prod. 2019, 82, 2354–2358. [Google Scholar] [CrossRef] [PubMed]
- Shilling, A.J.; Witowski, C.G.; Maschek, J.A.; Azhari, A.; Vesely, B.; Kyle, D.E.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Spongian diterpenoids derived from the Antarctic sponge Dendrilla antarctica are potent inhibitors of the Leishmania parasite. J. Nat. Prod. 2020, 83, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Seo, Y.; Rho, J.R.; Baek, E.; Kwon, B.-M.; Jeong, T.S.; Bok, S.H. Suberitenones A and B: Sesterterpenoids of an unprecedented skeletal class from the Antarctic sponge Suberites sp. J. Org. Chem. 1995, 60, 7582–7588. [Google Scholar] [CrossRef]
- Lee, H.-S.; Ahn, J.-W.; Lee, Y.-H.; Rho, J.-R.; Shin, J. New Sesterterpenes from the Antarctic Sponge Suberites sp. J. Nat. Prod. 2004, 67, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Marrero, A.R.; Brito, I.; Cueto, M.; San-Martin, A.; Darias, J. Suberitane network, a taxonomical marker for Antarctic sponges of the genus Suberites? Novel sesterterpenes from Suberites caminatus. Tetrahedron Lett. 2004, 45, 4707–4710. [Google Scholar] [CrossRef]
- Solanki, H.; Angulo-Preckler, C.; Calabro, K.; Kaur, N.; Lasserre, P.; Cautain, B.; de la Cruz, M.; Reyes, F.; Avila, C.; Thomas, O.P. Suberitane sesterterpenoids from the Antarctic sponge Phorbas areolatus (Thiele, 1905). Tetrahedron Lett. 2018, 59, 3353–3356. [Google Scholar] [CrossRef]
- Wang, M.; Tietjen, I.; Chen, M.; Williams, D.E.; Daoust, J.; Brockman, M.A.; Andersen, R.J. Sesterterpenoids isolated from the sponge Phorbas sp. activate latent HIV-1 provirus expression. J. Org. Chem. 2016, 81, 11324–11334. [Google Scholar] [CrossRef] [PubMed]
- Daoust, J.; Fontana, A.; Merchant, C.E.; de Voogd, N.J.; Patrick, B.O.; Kieffer, T.J.; Andersen, R.J. Ansellone A, a Sesterterpenoid Isolated from the Nudibranch Cadlina luteromarginata and the Sponge Phorbas sp., Activates the cAMP Signaling Pathway. Org. Lett. 2010, 12, 3208–3211. [Google Scholar] [CrossRef] [PubMed]
- APEX4, 2015.9; Bruker AXS Inc.: Madison, WI, USA, 2022.
- SAINT, 8.35A; Bruker AXS Inc.: Madison, WI, USA, 2016.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Fuentes, S.; Crim, R.L.; Beeler, J.; Teng, M.N.; Golding, H.; Khurana, S. Development of a simple, rapid, sensitive, high-throughput luciferase reporter based microneutralization test for measurement of virus neutralizing antibodies following Respiratory Syncytial Virus vaccination and infection. Vaccine 2013, 31, 3987–3994. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).