Diversity and Distribution of Carotenogenic Algae in Europe: A Review
Abstract
1. Introduction
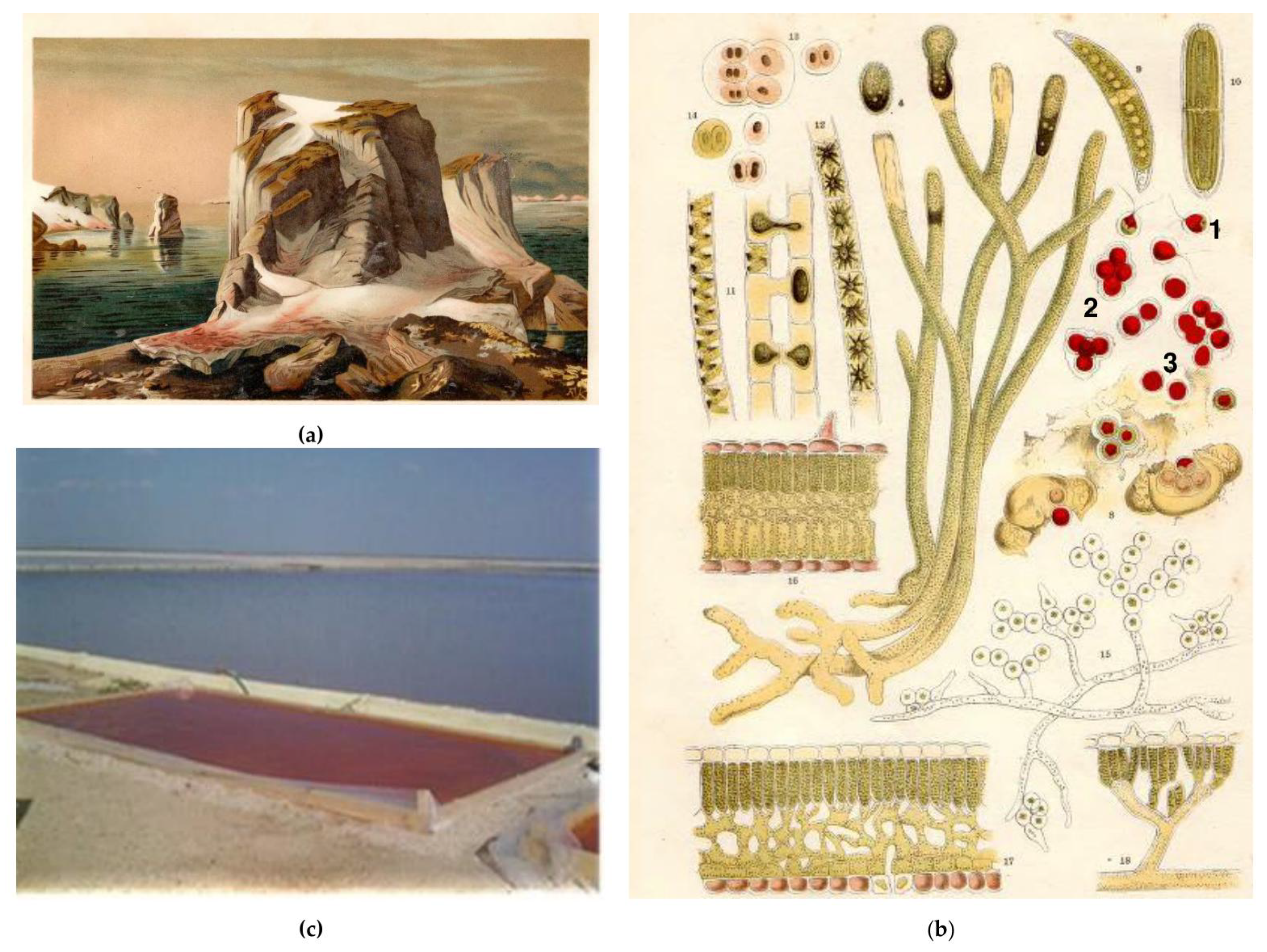
2. A Historical Note
3. Main Concepts and Definitions of the Biology of Carotenogenic Microalgae
3.1. The Difference between Primary and Secondary Carotenoids
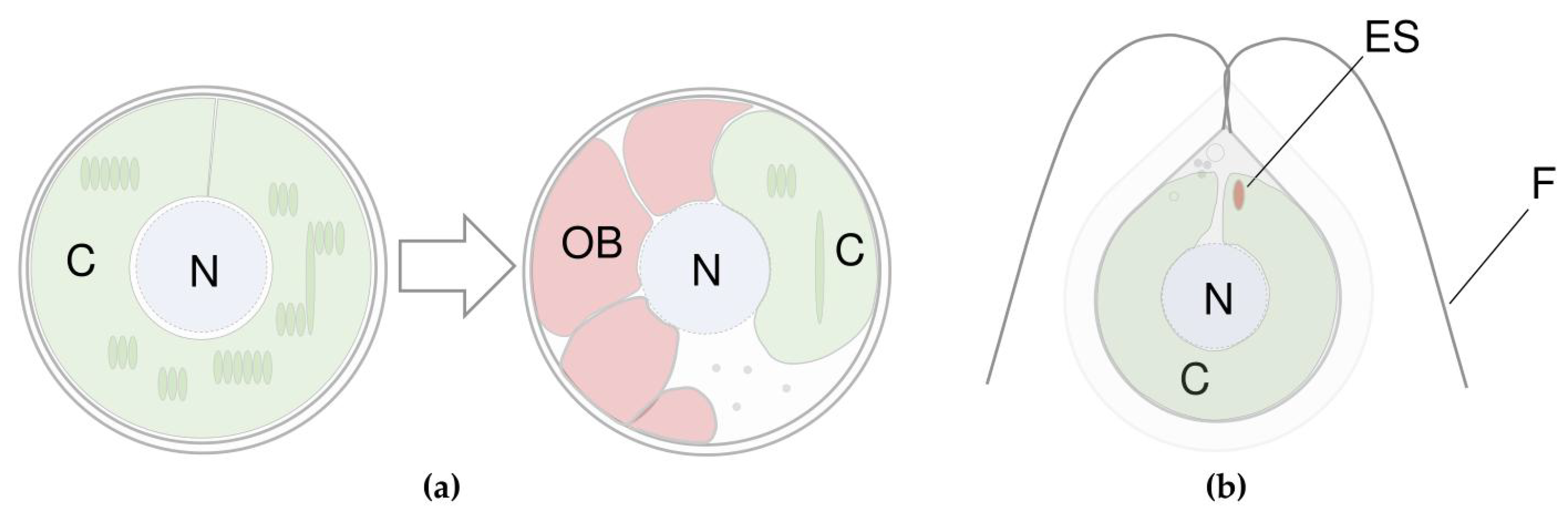
3.2. Where Do Microalgae Store Secondary Carotenoids?
3.3. Which Carotenoids Do Microalgae Accumulate?
3.4. Why Do Microalgae Accumulate Secondary Carotenoids?
3.5. Where Do Carotenogenic Microalgae Live?
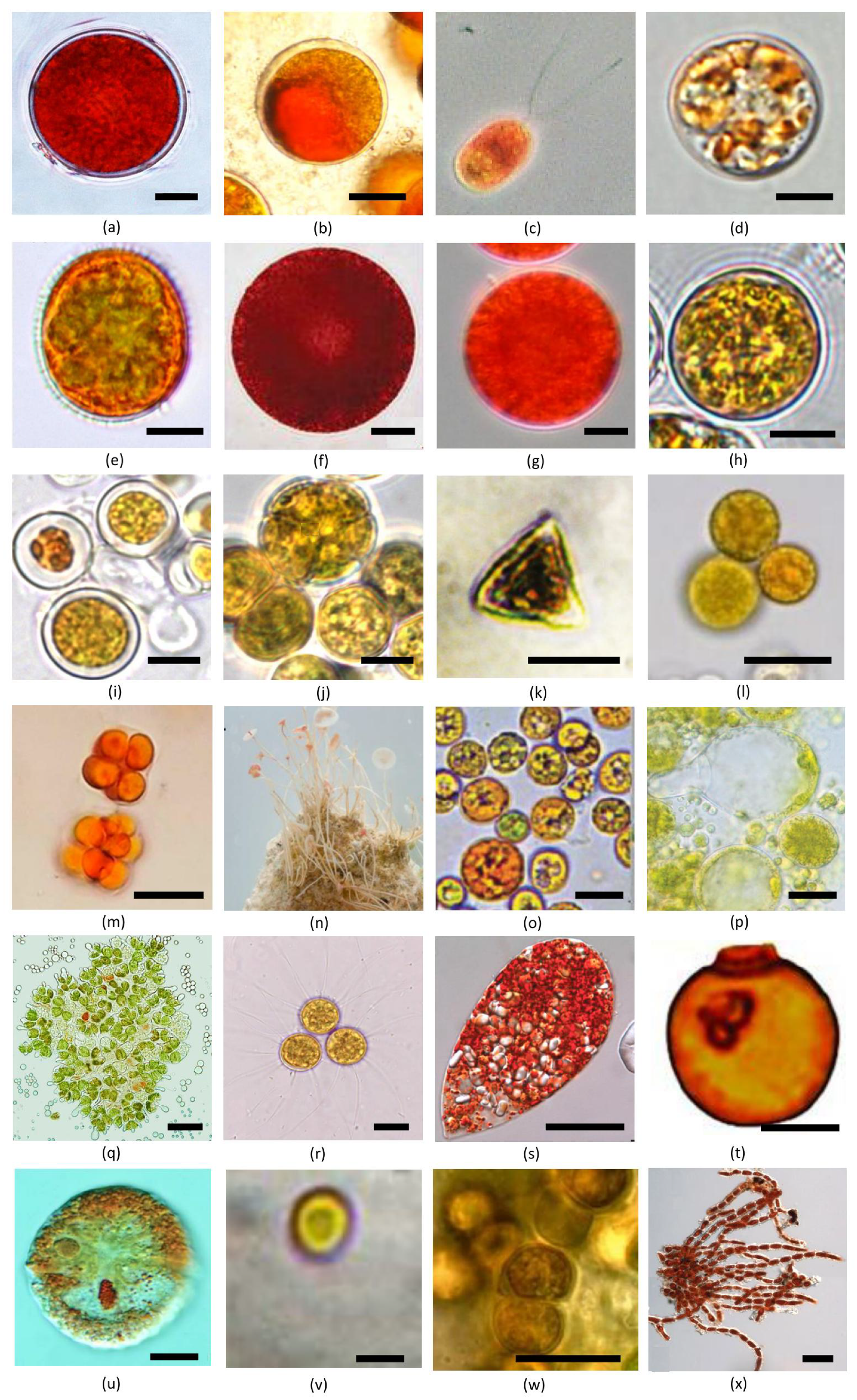
4. Diversity and Distribution of Unicellular Carotenogenic Algae
4.1. Haematococcus Flotow (Chlorophyceae, Chlamydomonadales)
4.2. Ettlia Komárek (Chlorophyceae, Chlamydomonadales)
| Species | Phylum | Order | Predominant Secondary Carotenoid |
|---|---|---|---|
| Acetabularia acetabulum | Chlorophyta | Dasycladales | Astaxanthin |
| Botryococcus braunii race A | Chlorophyta | Trebouxiales | Astaxanthin |
| Botryococcus braunii race B | Chlorophyta | Trebouxiales | Echinenone, botryoxanthines, braunixanthines |
| Botryococcus braunii race L | Chlorophyta | Trebouxiales | Echinenone, and β-carotene |
| Bracteacoccus aggregatus | Chlorophyta | Sphaeropleales | Astaxanthin, adonirubin, and β-carotene |
| Bracteacoccus bullatus | Chlorophyta | Sphaeropleales | Echinenone and astaxanthin |
| Bracteacoccus giganteus | Chlorophyta | Sphaeropleales | Astaxanthaxanthin and canthaxanthin |
| Bracteacoccus minor | Chlorophyta | Sphaeropleales | Astaxanthin and canthaxanthin |
| Chloromonas arctica | Chlorophyta | Chlamydomonadales | NO DATA |
| Chloromonas brevispina | Chlorophyta | Chlamydomonadales | NO DATA |
| Chloromonas rosae | Chlorophyta | Chlamydomonadales | NO DATA |
| Chloromonas rostafinskii | Chlorophyta | Chlamydomonadales | NO DATA |
| Chlainomonas rubra | Chlorophyta | Chlamydomonadales | Astaxanthin |
| Chloromonas nivalis | Chlorophyta | Chlamydomonadales | Astaxanthin |
| Chloromonas hindakii | Chlorophyta | Chlamydomonadales | Astaxanthin |
| Chloromonas krienitzii | Chlorophyta | Chlamydomonadales | Astaxanthin |
| Chlorosarcinopsis bastropiensis | Chlorophyta | Chlamydomonadales | Canthaxanthin |
| Chlorosarcinopsis dissociate | Chlorophyta | Chlamydomonadales | Canthaxanthin |
| Chromochloris zofingiensis | Chlorophyta | Sphaeropleales | Astaxanthin and canthaxanthin |
| Coelastrella rubescens | Chlorophyta | Sphaeropleales | Mixture of xanthophylls 1 and α/β-carotene |
| Coelastrella aeroterrestrica | Chlorophyta | Sphaeropleales | Mixture of xanthophylls 1 |
| Coelastrella terrestris | Chlorophyta | Sphaeropleales | Mixture of xanthophylls 1 |
| Coelastrella oocystiformis | Chlorophyta | Sphaeropleales | Astaxanthin, canthaxanthin, β-carotene |
| Deasonia granata | Chlorophyta | Chlamydomonadales | Mixture of xanthophylls 1,2 |
| Diacronema vlkianum | Haptophyta | Pavlovales | Astaxanthin |
| Dunaliella salina | Chlorophyta | Chlamydomonadales | β-carotene |
| Ettlia carotinosa | Chlorophyta | Chlamydomonadales | Astaxanthin + admixture of adonirubin |
| Euglena rubida | Euglenophyta | Euglenales | Astaxanthin |
| Euglena sanguinea | Euglenophyta | Euglenales | Astaxanthin and adonixanthin |
| Golenkinia brevispicula | Chlorophyta | Sphaeropleales | β-carotene + admixture of astaxanthin |
| Haematococcus lacustris | Chlorophyta | Chlamydomonadales | Astaxanthin |
| Haematococcus rubicundus | Chlorophyta | Chlamydomonadales | Astaxanthin |
| Haematococcus rubens | Chlorophyta | Chlamydomonadales | Astaxanthin |
| Halochlorella rubescens | Chlorophyta | Sphaeropleales | Canthaxanthin, astaxanthin, (β-carotene) |
| Protosiphon botryoides | Chlorophyta | Chlamydomonadales | Astaxanthin |
| Pseudospongiococcum protococcoides | Chlorophyta | Chlamydomonadales | Mixture of xanthophylls 1 |
| Sanguina aurantia | Chlorophyta | Chlamydomonadales | Astaxanthin |
| Sanguina nivaloides | Chlorophyta | Chlamydomonadales | Astaxanthin |
| Tovellia sanguinea | Dinophyta | Gonyaulacales | Astaxanthin |
| Tovellia rubescens | Dinophyta | Gonyaulacales | Astaxanthin |
| Trachelomonas volvocina | Euglenophyta | Euglenales | Astaxanthin |
| Tetraëdron minimum | Chlorophyta | Sphaeropleales | Astaxanthin and adonixanthin |
| Trentepohlia | Chlorophyta | Trentepohliales | β-carotene |
| Rhexinema sarcinoideum | Chlorophyta | Ulvophyceae | Astaxanthin+ admixture of canthaxanthin |
| Species | Country and Region (If Available) |
|---|---|
| Acetabularia acetabulum | Mediterranean and Adriatic Seas |
| Botryococcus braunii | Cambridge (United Kingdom), Maddingley Brick Pits (United Kingdom) Cheshire (United Kingdom), Cumbria (United Kingdom), Brittany (France), Côte-d’Or, Morvan region (France), vicinity of Lingoult (France), Chaumecon Lake and Crescent Lake, Arcachon region (France), Large Lake of Sanguinet (France), barrier lake of Pareloup (France), Grasmere Lake, vicinity of Amieiro (Portugal); Ukraine, Ireland, The Netherlands, Norway, Poland, Romania, Russia 1, Spain, Sweden. |
| Bracteacoccus aggregatus | Yershovskoye Lake, Karelia (Russia 1); Czechia, Germany, Ukraine |
| Bracteacoccus bullatus | Sierra Nevada (Spain), Staro-Berdyansky forestland, Zaporozhye region (Ukraine), Dnipropetrovsk Oblast (Ukraine); Russia 1 |
| Bracteacoccus giganteus | High Ardennes (Belgium); Germany, Russia 1, Ukraine |
| Bracteacoccus minor | Boreč Hill ventaroles (Czechia), Mykhailivska Tsilyna Nature Preserve, Katerynivka (Ukraine); Poland, Romania, Russia 1 |
| Chloromonas arctica | Svalbard Archipelago (Norway) 2 |
| Chloromonas brevispina | Tyrol Alps (Austria), Giant Mountains (Czechia), Svalbard Archipelago (Norway) 2 |
| Chlainomonas rubra | Ľadové Lake in the High Tatras and Gossenkölle Lake in the Tyrolean Alps (Austria), Pirin Mountains (Bulgaria) |
| Chloromonas rosae | Tyrol Alps (Austria), Boreč Hill ventaroles (Czechia), Giant Mountains (Czechia) |
| Chloromonas rostafinskii | Stara Planina, Central Balkan Mountains (Bulgaria) |
| Chloromonas nivalis | Alps, Tyrol (Austria), Zheleznitza village, Vitosha Mountains (Bulgaria), Pirin Mountains (Bulgaria), Stara Planina, Central Balkan Mountains (Bulgaria), High Tatra Mountains (Slovakia), Sierra Nevada Mountains (Spain), Pyrenees (Spain, France), Giant Mountains (Czechia), Jeseníky Mountains (Czechia), Mountain Olympus (Greece), Svalbard Archipelago (Norway) 2 |
| Chloromonas hindakii | High Tatra Mountains (Slovakia, Poland), Krkonoše and Jeseníky Mountains (Czechia) |
| Chloromonas krienitzii | Sarntal Alps, South Tyrol (Italy), High Tatra Mountains (Slovakia, Poland), North Pindus (Greece) |
| Chlorosarcinopsis bastropiensis | Ukraine |
| Chlorosarcinopsis dissociata | Snake Islands Tract, Kanevsky Natural Reserve, Cherkasy Oblast (Ukraine) |
| Chromochloris zofingiensis | Ram Oswald near Zofingen (Switzerland), Dalmatia (Former Yugoslavia), Unterengadin (Switzerland), Ortenberg near Marburg/Lahn (Germany), Firenze (Italy), Sklene Teplice (Slovakia); Iceland, Croatia, France, Romania, Bulgaria, Russia 1 |
| Coelastrella rubescens | Pitschberg mountain, South Tirol (Austria), South Tirol (Italy), Rastorguevo Village, Moskovskaya Oblast (Russia 1) |
| Coelastrella aeroterrestrica | Pirin Mountains (Bulgaria), Obergurgl, Tirol (Austria), Kandalaksha bay of the White Sea, Karelia (Russia 1), Odesa Oblast (Ukraine); The Netherlands. |
| Coelastrella terrestris | Sölheimjökull glacier (Iceland), Obergurgl, Tyrol (Austria), Pirin Mountains (Bulgaria), Odesa Oblast (Ukraine); Czechia, Germany, Italy, The Netherlands, Poland, Russia 1, Slovakia, Romania |
| Coelastrella oocystiformis | Windermere (United Kingdom), Boreč Hill ventaroles (Czechia) |
| Deasonia granata | Praha (Czechia), Gomel (Belarus) |
| Diacronema vlkianum | English Channel (France), sea water Ryde, Isle of Wight, England (United Kingdom). Sea coast (Portugal), Kühnhausen near Erfurt (Germany); Ireland, Portugal, Romania, Spain |
| Dunaliella salina | Salt Lake Elton, Volgograd Oblast (Russia 1), Razval, Orenburg Oblast (Russia 1), Arinaga Saltwork, Monzon (Spain), Gran Canaria (Spain). Kuyalnitsky Liman, Odesa oblast (Ukraine), Zmievo Lake, sedimentation pond of Heroyskoe salt works, Kherson Oblast (Ukraine), Genicheskoe Lake, sedimentation pond of Genichesk salt works, Kherson Oblast (Ukraine), IBSS Siwash bay, Crimea (Ukraine), Filatovskaya salt flat, Crimea (Ukraine), Sasyk Lake, Crimea (Ukraine), Saki, Crimea (Ukraine), Lacul Sărat (Romania); Germany, Portugal |
| Ettlia carotinosa | Vicinity of Praha (Czechia); Germany |
| Euglena rubida | Branicki Palace, Białystok (Poland) |
| Euglena sanguinea | Eichenbirkig, Fränkische Schweiz, Bayern (Germany), Spydeberg, Ehrenberg, the county of Østfold (Norway), vicinity of Debden (United Kingdom); Denmark, Belarus, Bulgaria, Czechia, Estonia, Hungary, Italy, Latvia, Moldova, The Netherlands, Poland, Romania, Slovakia, Spain, Ukraine |
| Golenkinia brevispicula | Vicinity of Dortmund (Germany), The Netherlands |
| Haematococcus lacustris | Rozhen village (Bulgaria), Ghent (Belgium), Cefa, Bihor District (Romania), Bruchberg (Germany), Aneboda (Sweden), Basel (Switzerland), Aargau (Switzerland), Zürich (Switzerland), Graubünden (Switzerland), Zbyny, Hirschberg (Czechia), Březová nad Svitavou (Czechia), Třeboň (Czechia), Veverská Bítýška (Czechia), Brno (Czechia), Sevastopol, Crimea (Ukraine), Vlissingen (The Netherlands), Svalbard Archipelago (Norway) 2, coastal zone of the Kandalaksha bay of the White Sea, Karelia (Russia 1), vicinity of Adler and mountainous region of the Central Caucasus (Russia 1), Cambridge (United Kingdom); Ireland, France, Norway, Portugal, Slovakia, Spain |
| Haematococcus rubicundus | Třeboň (Czechia), Wageningen (The Netherlands), Ghent (Belgium), Merelbeke (Belgium), Aaigem (Belgium) Kandalaksha bay of the White Sea, Karelia (Russia 1), Province of Pescara (Italy) |
| Haematococcus rubens | Ghent (Belgium), Tvärminne (Finland) |
| Halochlorella rubescens | Vicinity of Bordeaux (France), Buhta Blagopoluchiya, Bolshoy Slovetskii Island (Russia 1), Lake Solone, Zaporizhzhya Oblast (Ukraine) |
| Protosiphon botryoides | Samara forest, Dnepropetrovsk Region (Ukraine), Františkovy Lázně (Czechia), Lützel-Breitenborn (Germany); United Kingdom, Ireland, Portugal, Spain |
| Pseudospongiococcum protococcoides | Windermere (United Kingdom), Arabat Spit, Crimea (Ukraine) |
| Sanguina aurantia | Svalbard Archipelago (Norway) 2 |
| Sanguina nivaloides | Ötztal Alps (Austria), Sarntal Alps (Italy), High Tatra Mountains (Slovakia), Alps (Slovenia), Urner Alps (Switzerland); Svalbard Archipelago (Norway) 2; Norway |
| Tetraëdron minimum | Þórsmörk (Iceland); United Kingdom, Bulgaria, Czechia, France, Germany, Ireland, The Netherlands, Norway, Portugal, Romania, Russia 1, Slovakia, Spain, Sweden, Ukraine |
| Tovellia sanguinea | Trentino Province (Italy) |
| Tovellia rubescens | Gafanha da Boavista, Ílhavo (Portugal) |
| Trachelomonas volvocina | United Kingdom, Poland; Ireland, Bulgaria, Czechia, Germany, The Netherlands, Romania, Russia, Scandinavia, Slovakia, Spain, Sweden, Ukraine |
| Trentepohlia spp. | Ubiquitous in Europe |
| Rhexinema sarcinoideum | Institute of Soil Science and Plant Cultivatio near Puławy (Poland), Chelčice, South Bohemia (Czechia); Ukraine, Russia |
4.3. Dunaliella Teodoresco (Chlorophyceae, Chlamydomonadales)
4.4. Chromochloris Kol and Chodat (Chlorophyceae, Chlamydomonadales)
4.5. Chloromonas Gobi (Chlorophyceae, Chlamydomonadales)
4.6. Chlainomonas Christen (Chlorophyceae, Chlamydomonadales)
4.7. Sanguina Leya, Procházková and Nedbalová (Chlorophyceae, Chlamydomonadales)
4.8. Coelastrella Chodat (Chlorophyceae, Sphaeropleales)
4.9. Bracteacoccus Tereg (Chlorophyceae, Sphaeropleales)
4.10. Halochlorella Dangeard (Chlorophycee, Sphaerapleales)
4.11. Tetraëdron Kützing (Chlorophyceae, Sphaeropleales)
4.12. Deasonia Ettl and Komárek (Chlorophyceae, Chlamydomonadales)
4.13. Chlorosarcinopsis Herndon (Chlorophyceae, Chlamydomonadales)
4.14. Acetabularia Lamouroux (Ulvophyceae, Dasycladales)
4.15. Pseudospongiococcum Gromov and Mamkaeva (Chlorophyceae, Chlamydomonadales)
4.16. Protosiphon Klebs (Chlorophyceae, Chlamydomonadales)
4.17. Botryococcus Kützing (Trebouxiophyceae, Trebouxiales)
4.18. Golenkinia Chodat (Chlorophycee, Sphaerapleales)
4.19. Euglena Ehrenberg (Euglenophyceae, Euglenales)
4.20. Trachelomonas Ehrenberg (Euglenophyceae, Euglenales)
4.21. Tovellia Moestrup, Lindberg and Daugberg (Dinophyceae, Gonyaulacales)
4.22. Diacronema Prauser (Pavlovales)
4.23. Rhexinema Geitler (Ulvophyceae, Helicodictyaceae)
4.24. Trentepohlia Martius (Ulvophyceae, Trentepohliales)
4.25. Other Microalgae
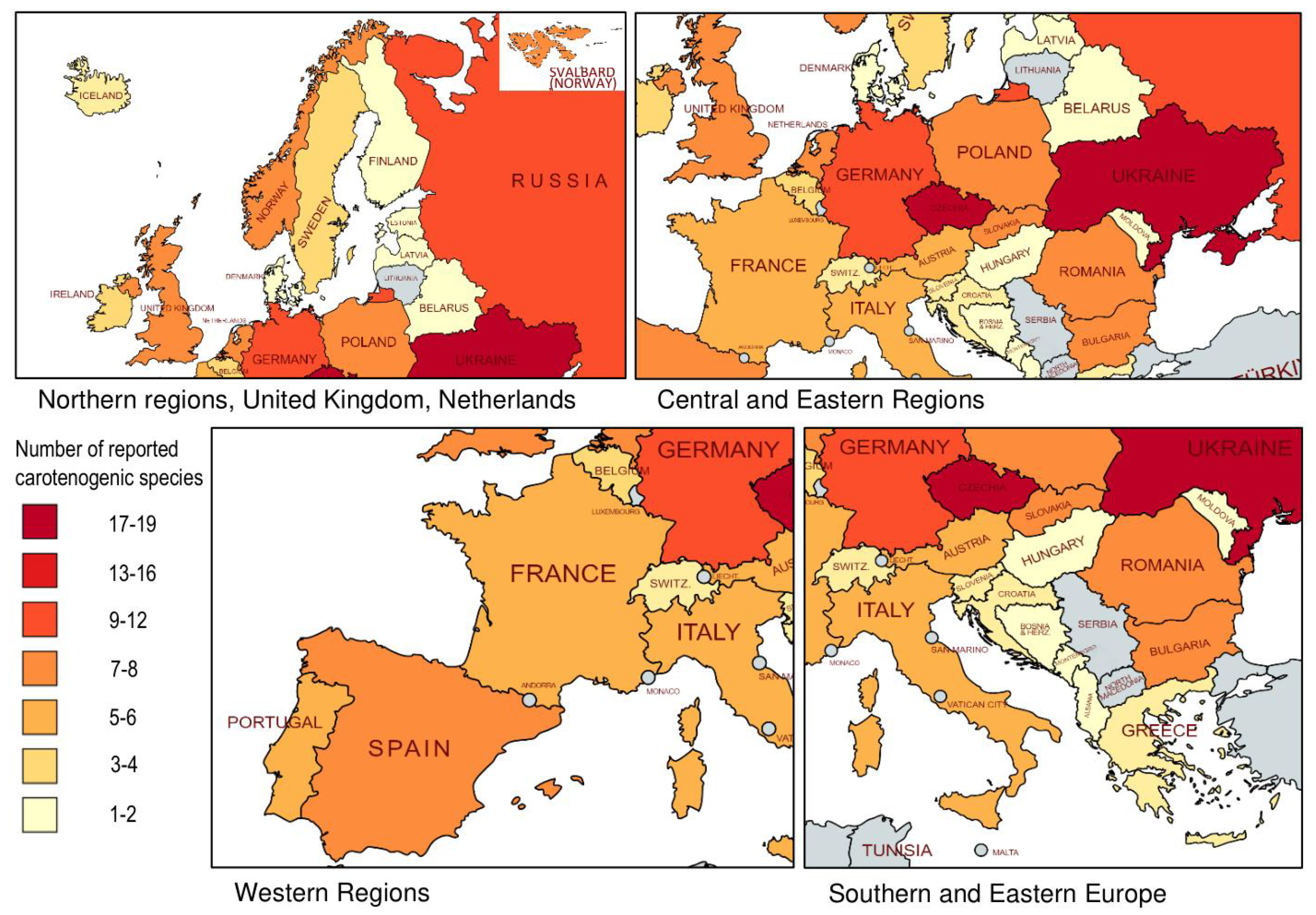
5. Summary of Geographical Distribution
6. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Oren, A. A hundred years of Dunaliella research: 1905–2005. Saline Syst. 2005, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Healy, H.; Driver, R.; Robertson, I.K.; Geraghty, D.P.; Sharman, J.E.; Coombes, J.S. Astaxanthin vs. placebo on arterial stiffness, oxidative stress and inflammation in renal transplant patients (Xanthin): A randomised controlled trial. BMC Nephrol. 2008, 9, 17. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009, 5, 333–342. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Yamashita, E.; Perricone, N.V. High dose astaxanthin lowers blood pressure and increases insulin sensitivity in rats: Are these effects interdependent? Int. J. Med. Sci. 2011, 8, 126–138. [Google Scholar] [CrossRef]
- Gal, A.F.; Andrei, S.; Cernea, C.; Taulescu, M.; Catoi, C. Effects of astaxanthin supplementation on chemically induced tumorigenesis in Wistar rats. Acta Vet. Scand. 2012, 54, 50. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Borowitzka, L.J.; Borowitzka, M.A. β-carotene (provitamin A) production with algae. In Biotechnology of Vitamins, Pigments and Growth Factors; Vandamme, E.J., Ed.; Springer: Dordrecht, The Netherlands, 1989; pp. 15–26. [Google Scholar]
- Polle, J.E.W.; Tran, D.; Ben-Amotz, A. History, distribution, and habitats of algae of the genus Dunaliella Teodoresco (Chlorophyceae). In The Alga Dunaliella, 1st ed.; Ben-Amotz, A., Polle, J.E.W., Rao, D.V.S., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–14. [Google Scholar]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid production from microalgae: Biosynthesis, salinity responses and novel biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef]
- Lu, S.; Li, L. Carotenoid metabolism: Biosynthesis, regulation, and beyond. J. Integr. Plant Biol. 2008, 50, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Khachik, F. Carotenoids in food. In Carotenoids; Britton, G., Pfanden, H., Liaaen-Jensen, S., Eds.; Birkhäuser: Basel, Switzerland, 2009; Volume 5, pp. 45–66. [Google Scholar]
- Johnson, E.A.; Schroeder, W.A. Microbial carotenoids. In Downstream Processing Biosurfactants Carotenoids; Fiechter, A., Ed.; Springer: Berlin, Germany, 1995; pp. 119–178. [Google Scholar]
- Cezare-Gomes, E.A.; del Carmen Mejia-da-Silva, L.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of microalgae carotenoids for industrial application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [PubMed]
- Solovchenko, A.; Chekanov, K. Production of carotenoids using microalgae cultivated in photobioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.-Y., Murthy, H.N., Zhong, J.-J., Eds.; Springer: Dordrecht, Germany, 2014; pp. 63–92. [Google Scholar]
- Isler, O.; Rüegg, R.; Schwieter, U. Carotenoids as food colourants. Pure Appl. Chem. 1967, 14, 245–264. [Google Scholar] [CrossRef]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Lorenz, R.T.A. Technical review of Haematococcus algae. Naturose Tech. Bull. 1999, 60, 1–12. [Google Scholar]
- Akiba, Y.; Sato, K.; Takahashi, K.; Matsushita, K.; Komiyama, H.; Tsunekawa, H.; Nagao, H. Meat color modification in broiler chickens by feeding yeast Phaffia rhodozyma containing high concentrations of astaxanthin. J. Appl. Poult. Res. 2001, 10, 154–161. [Google Scholar] [CrossRef]
- Johnson, E.A.; An, G.H. Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Johnson, E.A.; Villa, T.G.; Lewis, M.J. Phaffia rhodozyma as an astaxanthin source in salmonid diets. Aquaculture 1981, 20, 123–134. [Google Scholar] [CrossRef]
- BCC Research. Available online: https://www.bccresearch.com/ (accessed on 5 December 2022).
- Fortune Buiseness Insights. Available online: https://www.fortunebusinessinsights.com/ (accessed on 5 December 2022).
- MarketsandMarkets. Available online: https://www.marketsandmarkets.com/ (accessed on 5 December 2022).
- Concil of the European Union. Available online: https://www.consilium.europa.eu/ (accessed on 5 December 2022).
- Goswami, R.K.; Agrawal, K.; Verma, P. An overview of microalgal carotenoids: Advances in the production and its impact on sustainable development. In Bioenergy Research: Evaluating Strategies for Commercialization and Sustainability; Srivastava, N., Srivastava, M., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2021; pp. 105–128. [Google Scholar]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Ntan, C.T.; Chau, H.T.B.; Anwar, C.; ud din Wani, H.M.; Pal, A.; Saini, R.; et al. Algae as an emerging source of bioactive pigments. Biores. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication. National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 12 December 2022).
- Krishnaveni, K.N.; Sudarshan, S.; Alamelu, V.; Vijayarahavan, V.; Vimaladevi, S. Watermelon snow-an alarm of climate change. Biotica Res. Today 2021, 3, 979–981. [Google Scholar]
- Tatlock, J.S. Some mediaeval cases of blood-rain. Class. Philol. 1914, 9, 442–447. [Google Scholar]
- von Marilaun, A.K. Pflanzenleben; Verlag des Bibliographischen Instituts: Leipzig, Germany, 1888. [Google Scholar]
- Hazen, T.E. The life history of Sphaerella lacustris (Haematococcus pluvialis). Mem. Torrey Bot. Club. 1899, 6, 211–246. [Google Scholar]
- Zia-Ul-Haq, M. Historical and introductory aspects of carotenoids. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer: Cham, Switherland, 2021; pp. 1–42. [Google Scholar]
- Droop, M.R. Carotenogenesis in Haematococcus pluvialis. Nature 1955, 175, 42. [Google Scholar] [CrossRef]
- Borovkov, A.B.; Gudvilovich, I.N.; Avsiyan, A.L. Scale-up of Dunaliella salina cultivation: From strain selection to open ponds. J. Appl. Phycol. 2020, 32, 1545–1558. [Google Scholar] [CrossRef]
- Massyuk, N.P. Mass culture of the carotene bearing alga Dunaliella salina. Ukr. Bot. Zh. 1968, 23, 12–19. [Google Scholar]
- Massyuk, N.P.; Abdula, E.G. First experiment of growing carotene-containing algae under semi-industrial conditions. Ukr. Bot. Zh. 1969, 26, 21–27. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Bassi, R.; Pineau, B.; Dainese, P.; Marquardt, J. Carotenoid-binding proteins of photosystem II. Eur. J. Biochem. 1993, 212, 297–303. [Google Scholar] [CrossRef]
- Cunningham, F.X., Jr.; Gantt, E. Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Biol. 1998, 49, 557–583. [Google Scholar] [CrossRef]
- Solovchenko, A.; Neverov, K. Carotenogenic response in photosynthetic organisms: A colorful story. Photosynth. Res. 2017, 133, 31–47. [Google Scholar] [CrossRef]
- Giuliano, G.; Bartley, G.E.; Scolnik, P.A. Regulation of carotenoid biosynthesis during tomato development. Plant Cell 1993, 5, 379–387. [Google Scholar]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.E. Physiology and adaptive significance of secondary carotenogenesis in green microalgae. Russ. J. Plant Physiol. 2013, 60, 1–13. [Google Scholar] [CrossRef]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Jin, E.S.; Lee, C.G.; Polle, J.E. Secondary carotenoid accumulation in Haematococcus (Chlorophyceae): Biosynthesis, regulation, and biotechnology. J. Microbiol. Biotechnol. 2006, 16, 821–831. [Google Scholar]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef]
- Zhekisheva, M.; Boussiba, S.; Khozin-Goldberg, I.; Zarka, A.; Cohen, Z. Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J. Phycol. 2002, 38, 325–331. [Google Scholar] [CrossRef]
- Řezanka, T.; Nedbalová, L.; Sigler, K.; Cepák, V. Identification of astaxanthin diglucoside diesters from snow alga Chlamydomonas nivalis by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. Phytochemistry 2008, 69, 479–490. [Google Scholar] [CrossRef]
- Gu, W.; Xie, X.; Gao, S.; Zhou, W.; Pan, G.; Wang, G. Comparison of different cells of Haematococcus pluvialis reveals an extensive acclimation mechanism during its aging process: From a perspective of photosynthesis. PLoS ONE 2013, 8, e67028. [Google Scholar] [CrossRef]
- Peled, E.; Leu, S.; Zarka, A.; Weiss, M.; Pick, U.; Khozin-Goldberg, I.; Boussiba, S. Isolation of a novel oil globule protein from the green alga Haematococcus pluvialis (Chlorophyceae). Lipids 2011, 46, 851–861. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. Dunaliella: Physiology, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Litvin, F.F.; Sineshchekov, O.A.; Sineshchekov, V.A. Photoreceptor electric potential in the phototaxis of the alga Haematococcus pluvialis. Nature 1978, 271, 476–478. [Google Scholar] [CrossRef]
- Kreimer, G. The green algal eyespot apparatus: A primordial visual system and more? Curr. Genet. 2009, 55, 19–43. [Google Scholar] [CrossRef]
- Okada, S.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Botryoxanthin A, a member of a new class of carotenoids from the green microalga Botryococcus braunii Berkeley. Tetrahedron Lett. 1996, 37, 1065–1068. [Google Scholar] [CrossRef]
- Okada, S.; Tonegawa, I.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Braunixanthins 1 and 2, new carotenoids from the green microalga Botryococcus braunii. Tetrahedron 1997, 53, 11307–11316. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.J.; Chang, J.S. Recent advances in lutein production from microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Biores. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodriguez, H.; Moreno, J.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef]
- Barghini, P.; Giovannini, V.; Fenice, M.; Gorrasi, S.; Pasqualetti, M. High lutein production by a halo-tolerant strain of Dunaliella sp. (Chlorophyceae) isolated from solar salterns in central Italy. J. Environ. Prot. Ecol. 2018, 19, 704–712. [Google Scholar]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Microalgal lutein biosynthesis: Recent trends and challenges to enhance the lutein content in microalgal cell factories. Front. Mar. Sci. 2022, 9, 1015419. [Google Scholar] [CrossRef]
- Saha, S.K.; Ermis, H.; Murray, P. Marine microalgae for potential lutein production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Grandi, S. Lutein and lutein ester content in different types of Tagetes patula and T. erecta. Ind. Crops Prod. 1998, 8, 45–51. [Google Scholar] [CrossRef]
- Gansukh, E.; Mya, K.K.; Jung, M.; Keum, Y.S.; Kim, D.H.; Saini, R.K. Lutein derived from marigold (Tagetes erecta) petals triggers ROS generation and activates Bax and caspase-3 mediated apoptosis of human cervical carcinoma (HeLa) cells. Food Chem. Toxicol. 2019, 127, 11–18. [Google Scholar] [CrossRef]
- Fan, L.; Vonshak, A.; Zarka, A.; Boussiba, S. Does astaxanthin protect Haematococcus against light damage? Z. Nat. C 1998, 53, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, K.; Schastnaya, E.; Neverov, K.; Leu, S.; Boussiba, S.; Zarka, A.; Solovchenko, A. Non-photochemical quenching in the cells of the carotenogenic chlorophyte Haematococcus lacustris under favorable conditions and under stress. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, A.; Chekanov, K.; Zaytsev, P.; Bakhareva, D.; Gorelova, O.; Kochkin, D.; Lobakova, E. Sunscreen effect exerted by secondary carotenoids and mycosporine-like amino acids in the aeroterrestrial chlorophyte Coelastrella rubescens under high light and UV-A irradiation. Plants 2021, 10, 2601. [Google Scholar] [CrossRef]
- Zgonik, V.; Mulec, J.; Eleršek, T.; Ogrinc, N.; Jamnik, P.; Ulrih, N.P. Extremophilic microorganisms in Central Europe. Microorganisms 2021, 9, 2326. [Google Scholar] [CrossRef]
- Stewart, A.; Rioux, D.; Boyer, F.; Gielly, L.; Pompanon, F.; Saillard, A.; Thuiller, W.; Valay, J.-G.; Maréchal, E.; Coissac, E. Altitudinal zonation of green algae biodiversity in the French Alps. Front. Plant Sci. 2021, 12, 679428. [Google Scholar] [CrossRef]
- Hoham, R.W.; Remias, D. Snow and glacial algae: A review. J. Phycol. 2020, 56, 264–282. [Google Scholar] [CrossRef]
- Karsten, U.; Holzinger, A. Green algae in alpine biological soil crust communities: Acclimation strategies against ultraviolet radiation and dehydration. Biodivers. Conserv. 2014, 23, 1845–1858. [Google Scholar] [CrossRef]
- Czygan, C. Blood-rain and blood-snow: Nitrogen-deficient cells of Haematococcus pluvialis and Chlamydomonas nivalis. Arch. Microbiol. 1970, 21, 69–76. [Google Scholar]
- Viala, G. Recherches sur le Chlamydomonas nivalis Wille dans les Pyrénées. Bull. Société Bot. Fr. 1967, 114, 75–79. [Google Scholar] [CrossRef]
- Viala, G. Lastaxanthine chez le Chlamydomonas nivalis Wille. C. R. Hebd. Seances Académie Sci. Ser. D 1966, 263, 1383. [Google Scholar]
- Karsten, U.; Rindi, F. Ecophysiological performance of an urban strain of the aeroterrestrial green alga Klebsormidium sp. (Klebsormidiales, Klebsormidiophyceae). Eur. J. Phycol. 2010, 45, 426–435. [Google Scholar] [CrossRef]
- Karsten, U.; Lembcke, S.; Schumann, R. The effects of ultraviolet radiation on photosynthetic performance, growth and sunscreen compounds in aeroterrestrial biofilm algae isolated from building facades. Planta 2007, 225, 991–1000. [Google Scholar] [CrossRef]
- Kublanovskaya, A.; Baulina, O.; Chekanov, K.; Lobakova, E. The microalga Haematococcus lacustris (Chlorophyceae) forms natural biofilms in supralittoral White Sea coastal rock ponds. Planta 2020, 252, 37. [Google Scholar] [CrossRef]
- Cockell, C.S.; Knowland, J. Ultraviolet radiation screening compounds. Biol. Rev. 1999, 74, 311–345. [Google Scholar] [CrossRef]
- Solovchenko, A.; Baulina, O.; Ptushenko, O.; Gorelova, O. Ultrastructural patterns of photoacclimation and photodamage to photosynthetic algae cell under environmental stress. Physiol. Plant. 2019, 166, 251–263. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef]
- Procházková, L.; Remias, D.; Bilger, W.; Křížková, H.; Řezanka, T.; Nedbalová, L. Cysts of the snow alga Chloromonas krienitzii (Chlorophyceae) show increased tolerance to ultraviolet radiation and elevated visible light. Front. Plant Sci. 2020, 11, 617250. [Google Scholar] [CrossRef]
- Procházková, L.; Remias, D.; Holzinger, A.; Řezanka, T.; Nedbalová, L. Ecophysiological and morphological comparison of two populations of Chlainomonas sp. (Chlorophyta) causing red snow on ice-covered lakes in the High Tatras and Austrian Alps. Eur. J. Phycol. 2018, 53, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Procházková, L.; Leya, T.; Křížková, H.; Nedbalová, L. Sanguina nivaloides and Sanguina aurantia gen. et spp. nov. (Chlorophyta): The taxonomy, phylogeny, biogeography and ecology of two newly recognised algae causing red and orange snow. FEMS Microbiol. Ecol. 2019, 95, fiz064. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, K.; Fedorenko, T.; Kublanovskaya, A.; Litvinov, D.; Lobakova, E. Diversity of carotenogenic microalgae in the White Sea polar region. FEMS Microbiol. Ecol. 2020, 96, fiz183. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, A.A.; Bakhareva, D.A.; Zaytsev, P.A.; Lobakova, E.S. Characterization of a new halotolerant Arctic strain of the microalga Halochlorella rubescens NAMSU SBB-20. Russ. J. Plant Physiol. 2023, 70. in press. [Google Scholar]
- Ramos, G.J.; de Mattos Bicudo, C.E.; Góes-Neto, A.; Moura, C.W. Hydrodictyaceae (Chlorophyceae, Chlorophyta) do Pantanal dos Marimbus, Chapada Diamantina, Bahia, Brasil. Iheringia Série Botânica 2016, 71, 13–21. [Google Scholar]
- Chekanov, K.; Shibzukhova, K.; Lobakova, E.; Solovchenko, A. Differential responses to UV-A stress recorded in carotenogenic microalgae Haematococcus rubicundus, Bracteacoccus aggregatus, and Deasonia sp. Plants 2022, 11, 1431. [Google Scholar] [CrossRef]
- Cherdchukeattisak, P.; Fraser, P.D.; Purton, S.; Brocklehurst, T.W. Detection and enhancement of ketocarotenoid accumulation in the newly isolated sarcinoid green microalga Chlorosarcinopsis PY02. Biology 2018, 7, 17. [Google Scholar] [CrossRef]
- Costa, J.; Giménez-Casalduero, F.; Melo, R.; Jesus, B. Colour morphotypes of Elysia timida (Sacoglossa, Gastropoda) are determined by light acclimation in food algae. Aquat. Biol. 2012, 17, 81–89. [Google Scholar] [CrossRef]
- Skrebovska, S.V.; Chelebieva, E.S. Pseudospongiococcum protococcoides position detection in the system Chlorophyta. In Proceedings of the International Conference of Young Scientists “Aktualni Problemy Botaniky ta Ekolohii”, Uzhgorod, Ukraine, 19–23 September 2012; Kordyum, E.L., Bezsmertna, O., Voytsekhovich, A., Dymytrova, L., Zav’alova, L., Klymenko, O., Kruglyak, Y., Mosyakin, A., Olshanskyi, I., et al., Eds.; Phytocenter: Kyiv, Ukraine, 2013; pp. 61–62. [Google Scholar]
- Lewis, L.A.; Trainor, F.R. Survival of Protosiphon botryoides (Chlorophyceae, Chlorophyta) from a Connecticut soil dried for 43 years. Phycologia 2012, 51, 662–665. [Google Scholar] [CrossRef]
- Morales-de la Cruz, X.; Mandujano-Chávez, A.; Browne, D.R.; Devarenne, T.P.; Sánchez-Segura, L.; López, M.G.; Lozoya-Gloria, E. In silico and cellular differences related to the cell division process between the A and B Races of the colonial microalga Botryococcus braunii. Biomolecules 2021, 11, 1463. [Google Scholar] [CrossRef]
- Rearte, T.A.; Figueroa, F.L.; Gómez-Serrano, C.; Vélez, C.G.; Marsili, S.; Iorio, A.D.F.; González-Lópezd, C.V.; Cerón-Garcíad, M.C.; Abdala-Díazc, R.T.; Acién-Fernández, F.G. Optimization of the production of lipids and carotenoids in the microalga Golenkinia aff. brevispicula. Algal Res. 2020, 51, 102004. [Google Scholar] [CrossRef]
- van Vuuren, J.S.; Levanets, A. Mass developments of Euglena sanguinea Ehrenberg in South Africa. Afr. J. Aquat. Sci. 2021, 46, 110–122. [Google Scholar] [CrossRef]
- Kouassi, B.A.T.; Ouattara, A.; Da, K.P. Euglenozoa occurring in Adzopé reservoir, Côte d’Ivoire. Turk. J. Bot. 2013, 37, 1176–1187. [Google Scholar] [CrossRef]
- Pandeirada, M.S.; Craveiro, S.C.; Daugbjerg, N.; Moestrup, Ø.; Domingues, P.; Calado, A.J. Studies on woloszynskioid dinoflagellates X: Ultrastructure, phylogeny and colour variation in Tovellia rubescens n. sp. (Dinophyceae). J. Euk. Microbiol. 2019, 66, 937–953. [Google Scholar] [CrossRef]
- Armada, I.; Hachero-Cruzado, I.; Mazuelos, N.; Ríos, J.L.; Manchado, M.; Cañavate, J.P. Differences in betaine lipids and fatty acids between Pseudoisochrysis paradoxa VLP and Diacronema vlkianum VLP isolates (Haptophyta). Phytochemistry 2013, 95, 224–233. [Google Scholar] [CrossRef]
- Sieminiak, D. Filamentous green alga Pleurastum sarcinoideum Groover et Bold—First record in Poland. Oceanol. Hydrobiol. Stud. 2007, 36, 249–254. [Google Scholar]
- Liu, G.; Zhang, Q.; Zhu, H.; Hu, Z. Massive Trentepohlia-bloom in a glacier valley of Mt. Gongga, China, and a new variety of Trentepohlia (Chlorophyta). PLoS ONE 2012, 7, e37725. [Google Scholar] [CrossRef]
- Remias, D. Cell structure and physiology of alpine snow and ice algae. In Plants in Alpine Regions; Lütz, C., Ed.; Springer: Vienna, Austria, 2012; p. 1750185. [Google Scholar]
- Raymond, B.B.; Engstrom, C.B.; Quarmby, L.M. The underlying green biciliate morphology of the orange snow alga Sanguina aurantia. Curr. Biol. 2022, 32, R68–R69. [Google Scholar] [CrossRef]
- Matsuzaki, R.; Hara, Y.; Nozaki, H. A taxonomic study of snow Chloromonas species (Volvocales, Chlorophyceae) based on light and electron microscopy and molecular analysis of cultured material. Phycologia 2014, 53, 293–304. [Google Scholar] [CrossRef]
- Stein, J.R.; Amundsen, C.C. Studies on snow algae and fungi from the front range of Colorado. Can. J. Bot. 1967, 45, 2033–2045. [Google Scholar] [CrossRef]
- Remias, D.; Lütz-Meindl, U.; Lütz, C. Photosynthesis, pigments and ultrastructure of the alpine snow alga Chlamydomonas nivalis. Eur. J. Phycol. 2005, 40, 259–268. [Google Scholar] [CrossRef]
- Komárek, J.; Nedbalová, L. Green cryosestic algae. In Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Springer Science & Business: Dordrecht, The Netherlands, 2007; pp. 321–342. [Google Scholar]
- Nakada, T.; Ota, S. What is the correct name for the type of Haematococcus Flot. (Volvocales, Chlorophyceae)? Taxon 2016, 65, 343–348. [Google Scholar] [CrossRef]
- Allewaert, C.C.; Vanormelingen, P.; Pröschold, T.; Gomez, P.I.; González, M.A.; Bilcke, G.; D’Hondt, S.; Vyverman, W. Species diversity in European Haematococcus pluvialis (Chlorophyceae, Volvocales). Phycologia 2015, 54, 583–598. [Google Scholar] [CrossRef]
- Allewaert, C.C.; Vanormelingen, P.; Daveloose, I.; Verstraete, T.; Vyverman, W. Intraspecific trait variation affecting astaxanthin productivity in two Haematococcus (Chlorophyceae) species. Algal Res. 2017, 21, 191–202. [Google Scholar] [CrossRef]
- Dragoş, N.; Bercea, V.; Bica, A.; Drugă, B.; Nicoară, A.; Coman, C. Astaxanthin production from a new strain of Haematococcus pluvialis grown in batch culture. Ann. Romanian Soc. Cell Biol. 2010, 15, 353–361. [Google Scholar]
- Chekanov, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Sidorov, R.; Solovchenko, A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the White Sea coastal rocks (Russia). Mar. Drugs 2014, 12, 4504–4520. [Google Scholar] [CrossRef]
- Gacheva, G.; Dimitrova, P.; Pilarski, P. New strain Haematococcus cf. pluvialis Rozhen-12-growth, biochemical characteristics and future perspectives. Genet. Plant Physiol. 2015, 5, 29–38. [Google Scholar]
- Chelebieva, E.S.; Dantsyuk, N.V.; Chekanov, K.A.; Chubchikova, I.N.; Drobetskaya, I.V.; Minyuk, G.S.; Lobakova, E.S.; Solovchenko, A.E. Identification and morphological-physiological characterization of astaxanthin producer strains of Haematococcus pluvialis from the Black Sea Region. Appl. Biochem. Microbiol. 2018, 54, 639–648. [Google Scholar] [CrossRef]
- Lang, I.; Hodac, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 124. [Google Scholar] [CrossRef]
- Kostikov, I.Y.; Demchenko, E.N.; Berezovskaya, M.A. Microalgae culture collection at the Taras Shevchenko National University, Kyiv. Catalogue of strains. Chornomorsk. Bot. Z. 2009, 5, 37–79. [Google Scholar] [CrossRef]
- Roach, T.; Böck, N.; Rittmeier, N.; Arc, E.; Kranner, I.; Holzinger, A. Acquisition of desiccation tolerance in Haematococcus pluvialis requires photosynthesis and coincides with lipid and astaxanthin accumulation. Algal Res. 2022, 64, 102699. [Google Scholar] [CrossRef]
- Alanagreh, L.A.; Pegg, C.; Harikumar, A.; Buchheim, M. Assessing intragenomic variation of the internal transcribed spacer two: Adapting the Illumina metagenomics protocol. PLoS ONE 2017, 12, e0181491. [Google Scholar] [CrossRef] [PubMed]
- Klochkova, T.A.; Kwak, M.S.; Han, J.W.; Motomura, T.; Nagasato, C.; Kim, G.H. Cold-tolerant strain of Haematococcus pluvialis (Haematococcaceae, Chlorophyta) from Blomstrandhalvøya (Svalbard). Algae 2013, 28, 185–192. [Google Scholar] [CrossRef]
- Pegg, C.; Wolf, M.; Alanagreh, L.A.; Portman, R.; Buchheim, M.A. Morphological diversity masks phylogenetic similarity of Ettlia and Haematococcus (Chlorophyceae). Phycologia 2015, 54, 385–397. [Google Scholar] [CrossRef]
- Chelebieva, E.S. Screening of unicellular green microalgae as a potential source of natural ketocarotenoids. 3. Introduction into laboratory cultures and by primary estimation of biotechnological potential of Ettlia carotinosa. Mar. Ecol. J. 2011, 2, 96–102. [Google Scholar]
- Orosa, M.; Torres, E.; Fidalgo, P.; Abalde, J. Production and analysis of secondary carotenoids in green algae. J. Appl. Phycol. 2000, 12, 553–556. [Google Scholar] [CrossRef]
- Chelebieva, E.S.; Minyuk, G.S.; Drobetskaya, I.V.; Chubchikova, I.N. Physiological and biochemical characteristics of Ettlia carotinosa Komárek 1989 (Chlorophyceae) under experimental stress condition. Mar. Ecol. J. 2013, 12, 78. [Google Scholar]
- Xu, Y.; Ibrahim, I.M.; Wosu, C.I.; Ben-Amotz, A.; Harvey, P.J. Potential of new isolates of Dunaliella salina for natural β-carotene production. Biology 2018, 7, 14. [Google Scholar] [CrossRef]
- Nemtseva, N.V.; Selivanova, E.A.; Ignatenko, M.E.; Sharapova, N.V. Characterization of a novel Dunaliella salina (Chlorophyta) strain and the assessment of its cultivation parameters. Russ. J. Plant Physiol. 2013, 60, 529–535. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Selivanova, E.A.; Chekanov, K.A.; Sidorov, R.A.; Nemtseva, N.V.; Lobakova, E.S. Induction of secondary carotenogenesis in new halophile microalgae from the genus Dunaliella (Chlorophyceae). Biochemistry 2015, 80, 1508–1513. [Google Scholar] [CrossRef]
- Ionescu, V.; Năstăsescu, M.; Spiridon, L.; Bulgăreanu, V.A. The biota of Romanian saline lakes on rock salt bodies: A review. Int. J. Salt Lake Res. 1998, 7, 45–80. [Google Scholar] [CrossRef]
- Pasiuga, O.S.; Antonenko, S.P.; Komaristaya, V.P.; Rudas, A.N. Variability of cultural and morphological traits of Dunaliella salina Teod. from different habitats. J. V. N. Karazin Kharkiv Nat. Univ. Ser. Biol. 2013, 18, 54–63. [Google Scholar]
- Minicheva, G.G.; Kalashnik, K.S. Formation of phytoperiphyton on the hydrotechnical structures of the connecting channel Black Sea—Kuyalnitsky Liman. Mar. Ecol. J. 2020, 1, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Solonenko, A.M. Algae of different biotopes of the Arabat Spit, Azov Sea (Ukraine). Int. J. Algae 2016, 18, 247–256. [Google Scholar] [CrossRef]
- Fučíková, K.; Lewis, L.A. Intersection of Chlorella, Muriella and Bracteacoccus: Resurrecting the genus Chromochloris Kol et Chodat (Chlorophyceae, Chlorophyta). Fottea 2012, 12, 83–93. [Google Scholar] [CrossRef]
- Kopecký, J.; Schoefs, B.; Loest, K.; Štys, D.; Pulz, O. Microalgae as a source for secondary carotenoid production: A screening study. Algol. Stud. Archiv. Hydrobiol. 2000, 98, 153–168. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Y.; Bai, F.; Liu, J. The oleaginous astaxanthin-producing alga Chromochloris zofingiensis: Potential from production to an emerging model for studying lipid metabolism and carotenogenesis. Biotechnol. Biofuels 2021, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.E.; Ross, M.E.; Jubeau, S.; Montalescot, V.; Stanley, M.S. Progress towards a targeted biorefinery of Chromochloris zofingiensis: A review. Biomass Convers. Biorefin. 2022, 1–26. [Google Scholar] [CrossRef]
- Minyuk, G.; Sidorov, R.; Solovchenko, A. Effect of nitrogen source on the growth, lipid, and valuable carotenoid production in the green microalga Chromochloris zofingiensis. J. Appl. Phycol. 2020, 32, 923–935. [Google Scholar] [CrossRef]
- Reichelt, N.; Leger, D.; Daubert, M.; Ruffino, P.; Pröschold, T.; Darienko, T. Epigenomic stability assessment during cryopreservation and physiology among various strains of Chromochloris zofingiensis (Chlorophyceae) and their genetic variability revealed by AFLP and MS-AFLP. J. Appl. Phycol. 2021, 33, 2327–2340. [Google Scholar] [CrossRef]
- Pelah, D.; Sintov, A.; Cohen, E. The effect of salt stress on the production of canthaxanthin and astaxanthin by Chlorella zofingiensis grown under limited light intensity. World J Microbiol. Biotechnol. 2004, 20, 483–486. [Google Scholar] [CrossRef]
- Ip, P.F.; Chen, F. Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem. 2005, 40, 733–738. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Y.; Ding, W.; Mao, X.; Li, Y.; Gerken, H.; Liu, J. Astaxanthin is ketolated from zeaxanthin independent of fatty acid synthesis in Chromochloris zofingiensis. Plant Physiol. 2020, 183, 883–897. [Google Scholar] [CrossRef]
- Schoefs, B.; Rmiki, N.E.; Rachadi, J.; Lemoine, Y. Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids. FEBS Lett. 2001, 500, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Hindák, F. Taxonomic position of the chlorococcal alga Chlorella zofingiensis Dönz 1934 (Chlorophyceae). Algol. Stud. 1982, 40, 13–23. [Google Scholar]
- Remias, D.; Karsten, U.; Lütz, C.; Leya, T. Physiological and morphological processes in the Alpine snow alga Chloromonas nivalis (Chlorophyceae) during cyst formation. Protoplasma 2010, 243, 73–86. [Google Scholar] [CrossRef]
- Procházková, L.; Remias, D.; Řezanka, T.; Nedbalová, L. Ecophysiology of Chloromonas hindakii sp. nov. (Chlorophyceae), causing orange snow blooms at different light conditions. Microorganisms 2019, 7, 434. [Google Scholar] [CrossRef]
- Procházková, L.; Nedbalová, L. Snow algal blooms: Melting mountain and polar snow as a challenging habitat. Czech Polar Rep. 2020, 10, 130–131. [Google Scholar]
- Remias, D.; Procházková, L.; Holzinger, A.; Nedbalová, L. Ecology, cytology and phylogeny of the snow alga Scotiella cryophila K-1 (Chlamydomonadales, Chlorophyta) from the Austrian Alps. Phycologia 2018, 57, 581–592. [Google Scholar] [CrossRef]
- Remias, D.; Pichrtová, M.; Pangratz, M.; Lütz, C.; Holzinger, A. Ecophysiology, secondary pigments and ultrastructure of Chlainomonas sp. (Chlorophyta) from the European Alps compared with Chlamydomonas nivalis forming red snow. FEMS Microbiol. Ecol. 2016, 92, fiw030. [Google Scholar] [CrossRef]
- Hoham, R.W.; Mullet, J.E. The life history and ecology of the snow alga Chloromonas cryophila sp. nov. (Chlorophyta, Volvocales). Phycologia 1977, 16, 53–68. [Google Scholar] [CrossRef]
- Nedbalová, L.; Kociánová, M.; Lukavský, J. Ecology of snow algae in the Giant Mts. Opera Corcontica 2008, 45, 59–68. [Google Scholar]
- Segawa, T.; Matsuzaki, R.; Takeuchi, N.; Akiyoshi, A.; Navarro, F.; Sugiyama, S.; Yonezawa, T.; Mori, H. Bipolar dispersal of red-snow algae. Nature Commun. 2018, 9, 3094. [Google Scholar] [CrossRef] [PubMed]
- Procházková, L.; Remias, D.; Řezanka, T.; Nedbalová, L. Chloromonas nivalis subsp. tatrae, subsp. nov. (Chlamydomonadales, Chlorophyta): Re–examination of a snow alga from the High Tatra Mountains (Slovakia). Fottea 2018, 18, 1. [Google Scholar] [CrossRef]
- Lukavský, J.; Furnadzhieva, S.; Nedbalová, L. First record of cryoseston in the Vitosha Mountains (Bulgaria). Nova Hedwig. 2009, 88, 97–110. [Google Scholar] [CrossRef]
- Kol, E. Roter Schnee von Scotiella in der Hohen Tátra. Ann. Hist. Nat. Mus. Nat. Hung. Pars Bot 1965, 57, 145–148. [Google Scholar]
- Cepák, V.; Lukavský, J. Cryoseston in the Sierra Nevada Mountains (Spain). Nova Hedwig. 2012, 94, 163–173. [Google Scholar] [CrossRef]
- Remias, D.; Albert, A.; Lütz, C. Effects of realistically simulated, elevated UV irradiation on photosynthesis and pigment composition of the alpine snow alga Chlamydomonas nivalis and the arctic soil alga Tetracystis sp. (Chlorophyceae). Photosynthetica 2010, 48, 269–277. [Google Scholar] [CrossRef]
- Holzinger, A.; Allen, M.C.; Deheyn, D.D. Hyperspectral imaging of snow algae and green algae from aeroterrestrial habitats. J. Photochem. Photobiol. B Biol. 2016, 162, 412–420. [Google Scholar] [CrossRef]
- Lukavský, J.; Cepák, V. Cryoseston in Stara Planina (Balkan) Mountains, Bulgaria. Acta Bot. Croat. 2010, 69, 163–171. [Google Scholar]
- Cepák, V.; Lukavský, J. Cryoseston of the Pirin Mountains, Bulgaria. Acta Bot. Croat. 2013, 72, 257–268. [Google Scholar] [CrossRef]
- Cepák, V.; Kvíderová, J.; Lukavský, J. The first description of snow algae on Mount Olympus (Greece). Nova Hedwig. 2016, 103, 457–473. [Google Scholar] [CrossRef]
- Procházková, L.; Matsuzaki, R.; Řezanka, T.; Nedbalová, L.; Remias, D. The snow alga Chloromonas kaweckae sp. nov. (Volvocales, Chlorophyta) causes green surface blooms in the High Tatras (Slovakia) and tolerates high irradiance. J. Phycol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Barcytė, D.; Hodač, L.; Nedbalová, L.; Elster, J. Chloromonas arctica sp. nov., a psychrotolerant alga from snow in the High Arctic (Chlamydomonadales, Chlorophyta). Int. J. Syst. Evol. Microboiol. 2018, 68, 851–859. [Google Scholar] [CrossRef]
- Škaloud, P. Species composition and diversity of aero-terrestrial algae and cyanobacteria of the Boreč Hill ventaroles. Fottea 2009, 9, 65–80. [Google Scholar] [CrossRef]
- Hoham, R.W. New findings in the life history of the snow alga, Chlainomonas rubra (Stein et Brooke) comb. nov. (Chlorophyta, Volvocales). Syesis 1974, 7, 239–247. [Google Scholar]
- Procházková, L.; Remias, D.; Holzinger, A.; Řezanka, T.; Nedbalová, L. Ecophysiological and ultrastructural characterisation of the circumpolar orange snow alga Sanguina aurantia compared to the cosmopolitan red snow alga Sanguina nivaloides (Chlorophyta). Polar Biol. 2021, 44, 105–117. [Google Scholar] [CrossRef]
- Minyuk, G.; Chelebieva, E.; Chubchikova, I.; Dantsyuk, N.; Drobetskaya, I.; Sakhon, E.; Chekanov, K.; Solovchenko, A. Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae 2017, 32, 245–259. [Google Scholar] [CrossRef]
- Kaufnerová, V.; Eliáš, M. The demise of the genus Scotiellopsis Vinatzer (Chlorophyta). Nova Hedwig. 2013, 97, 415–428. [Google Scholar] [CrossRef]
- Doppler, P.; Kriechbaum, R.; Käfer, M.; Kopp, J.; Remias, D.; Spadiut, O. Coelastrella terrestris for adonixanthin production: Physiological characterization and evaluation of secondary carotenoid productivity. Mar. Drugs 2022, 20, 175. [Google Scholar] [CrossRef]
- Orosa, M.; Valero, J.F.; Herrero, C.; Abalde, J. Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other green microalgae under N-starvation and high light conditions. Biotechnol. Lett. 2001, 23, 1079–1085. [Google Scholar] [CrossRef]
- Vinatzer, G. Neue Bodenalgen aus den Dolomiten. Plant Syst. Evol. 1975, 123, 213–235. [Google Scholar] [CrossRef]
- Gärtner, G. The culture collection of algae at the Botanical Institute of the University at Innsbruck (Austria). Ber. Nat. Med. Ver. Innsbr. 1985, 72, 33–52. [Google Scholar]
- Stirk, W.A.; Ördög, V.; Novák, O.; Rolčík, J.; Strnad, M.; Bálint, P.; van Staden, J. Auxin and cytokinin relationships in 24 microalgal strains. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef]
- Tschaikner, A.; Ingolić, E.; Stoyneva, M.P.; Gärtner, G. Autosporulation in the soil alga Coelastrella terrestris (Chlorophyta, Scenedesmaceae, Scenedesmoideae). Phytol. Balc. 2007, 13, 29–34. [Google Scholar]
- Mikhailyuk, T.I.; Vinogradova, O.M.; Glaser, K.; Rybalka, N.; Demchenko, E.M.; Karsten, U. Algae of biological soil crusts from sand dunes of the Danube Delta biosphere reserve (Odesa Region, Ukraine). Algologia 2021, 31, 25–62. [Google Scholar] [CrossRef]
- Uzunov, B.A.; Stoyneva, M.P.; Gärtner, G.; Kofler, W. First record of Coelastrella species (Chlorophyta: Scenedesmaceae) in Bulgaria. Ber. Naturwiss.-Med. Ver. Innsb. 2008, 95, 27–34. [Google Scholar]
- Tschaikner, A.G.; Kofler, W. Coelastrella aeroterrestrica sp. nov.(Chlorophyta, Scenedesmoideae) a new, obviously often overlooked aeroterrestrial species. Algol. Stud. 2008, 128, 11–20. [Google Scholar] [CrossRef]
- Corato, A.; Le, T.T.; Baurain, D.; Jacques, P.; Remacle, C.; Franck, F. A fast-growing oleaginous strain of Coelastrella capable of astaxanthin and canthaxanthin accumulation in phototrophy and heterotrophy. Life 2022, 12, 334. [Google Scholar] [CrossRef]
- Dimitrova, P.; Marinova, G.; Alexandrov, S.; Iliev, I.; Pilarski, P. Biochemical characteristics of a newly isolated strain Coelastrella sp. BGV cultivated at different temperatures and light intensities. Annu. Univ. Sofía St. Kliment Ohridski Fac. Biol. 2017, 102, 139–146. [Google Scholar]
- Goecke, F.; Noda, J.; Paliocha, M.; Gislerød, H.R. Revision of Coelastrella (Scenedesmaceae, Chlorophyta) and first register of this green coccoid microalga for continental Norway. World J. Microbiol. Biotechnol. 2020, 36, 149. [Google Scholar] [CrossRef]
- Minyuk, G.S.; Chelebieva, E.S.; Chubchikova, I.N. Secondary carotenogenesis of the green microalga Bracteacoccus minor (Chodat) Petrova (Chlorophyta) in a two-stage culture. Int. J. Algae 2014, 16, 354–368. [Google Scholar] [CrossRef]
- Chubchikova, I.N.; Drobetskaya, I.V.; Minyuk, G.S.; Dantsyuk, N.V.; Chelebiyeva, E.S. Screening of green microalgae as a potential source of natural ketocarotenoids 2. Features of growth and secondary carotenogenesis in the representatives of the genus Bacteacoccus (Clorophyceae). Marine Ecol. J. 2011, 10, 91–97. [Google Scholar]
- Chekanov, K.; Litvinov, D.; Fedorenko, T.; Chivkunova, O.; Lobakova, E. Combined production of astaxanthin and β-carotene in a new strain of the microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) cultivated in photobioreactor. Biology 2021, 10, 643. [Google Scholar] [CrossRef]
- Lukavský, J.; Kopecký, J.; Kubáč, D.; Kvíderová, J.; Procházková, L.; Řezanka, T. The alga Bracteacoccus bullatus (Chlorophyceae) isolated from snow, as a source of oil comprising essential unsaturated fatty acids and carotenoids. J. Appl. Phycol. 2023, in press. [Google Scholar]
- Czerwik-Marcinkowska, J.; Mrozińska, T. Algae and cyanobacteria in caves of the Polish Jura. Pol. Bot. J. 2011, 56, 203–243. [Google Scholar]
- Garbacki, N.; Ector, L.; Kostikov, I.; Hoffmann, L. Contribution à l’étude de la flore des grottes de Belgique. Belg. J. Bot. 1999, 131, 43–76. [Google Scholar]
- Kostikov, I.; Romanenko, P.; Demchenko, E.; Darienko, T.M.; Mikhayljuk, T.I.; Rybchinskiy, O.V.; Solonenko, A.M. Phytosotsiologichniy; Phytocenter: Kyiv, Ukraine, 2001. (In Ukrainian) [Google Scholar]
- Levanets, A.A. Soil algae of «Mykhailivska Tsilyna» (branch of the Ukrainian Steppe Nature Reserve). Int. J. Algae 1999, 1, 61–70. [Google Scholar] [CrossRef]
- Maltsev, Y.I.; Maltseva, I.A.; Maltseva, S.Y.; Kulikovskiy, M.S. Biotechnological potential of a new strain of Bracteacoccus bullatus (Sphaeropleales, Chlorophyta) as a promising producer of omega-6 polyunsaturated fatty acids. Russ. J. Plant Physiol. 2020, 67, 185–193. [Google Scholar] [CrossRef]
- Mamaeva, A.; Namsaraev, Z.; Maltsev, Y.; Gusev, E.; Kulikovskiy, M.; Petrushkina, M.; Filimonova, A.; Sorokin, B.; Zotko, N.; Vinokurov, V.; et al. Simultaneous increase in cellular content and volumetric concentration of lipids in Bracteacoccus bullatus cultivated at reduced nitrogen and phosphorus concentrations. J. Appl. Phycol. 2018, 30, 2237–2246. [Google Scholar] [CrossRef]
- Jo, S.W.; Hong, J.W.; Do, J.M.; Na, H.; Kim, J.J.; Park, S.I.; Kim, Y.-S.; Kim, I.-S.; Yoon, H.S. Nitrogen deficiency-dependent abiotic stress enhances carotenoid production in indigenous green microalga Scenedesmus rubescens KNUA042, for use as a potential resource of high value products. Sustainability 2020, 12, 5445. [Google Scholar] [CrossRef]
- Chelebieva, E.; Minyuk, G.; Chubchikova, I. Features of secondary carotenogenesis in a green microalgae Scenedesmus rubescens (Dangeard) Kessler et al. under two-stage batch culture. Sci. Notes V.I. Vernadsky Crime. Fed. Univ. Ser. Biol. Chem. 2013, 26, 175–187. [Google Scholar]
- Dangeard, P. Sur quelques algues vertes marines nouvelles observées en culture. Botaniste 1966, 49, 5–45. [Google Scholar]
- Solonenko, A.; Iarovyi, S.; Iarova, T. Salt marsh seaweeds of Lake Solone coast (Zaporizhzhya Region). Visnyk Lviv Univ. Ser. Biol. 2010, 52, 13–20. [Google Scholar]
- Doppler, P.; Kornpointner, C.; Halbwirth, H.; Remias, D.; Spadiut, O. Tetraedron minimum, first reported member of hydrodictyaceae to accumulate secondary carotenoids. Life 2021, 11, 107. [Google Scholar] [CrossRef]
- Temraleeva, A.D.; Moskalenko, S.V.; Bachura, Y.M. Morphology, ecology, and 18S rDNA phylogeny of the green microalgal order Protosiphonales (Chlorophyceae, Chlorophyta). Microbiology 2017, 86, 159–169. [Google Scholar] [CrossRef]
- Shibzukhova, K.A.; Gavrilova, O.V.; Chivkunova, O.B.; Sidorov, R.A.; Solovchenko, A.E.; Lobakova, E.S. Estimation of biotechnological potential and clarification of taxonomic status of Parietochloris genus microalgae (Trebouxiophyceae) from the CALU collection. Mosc. Univ. Biol. Sci. Bull. 2017, 72, 137–141. [Google Scholar] [CrossRef]
- Temraleeva, A.D.; Moskalenko, S.V. Application of morphological and molecular systematics for identification of green microalgae of the genus Chlorococcum and some closely related taxa. Microbiology 2019, 88, 27–38. [Google Scholar] [CrossRef]
- Cherdchukeattisak, P. Classification and evaluation of the soil green microalgae Chlorosarcinopsis spp. for kor ketocarotenoid production. Master’s Thesis, Silpakorn University, Bangkok, Thailand, 2015. [Google Scholar]
- Groover, R.D.; Bold, H.C. Phycological Studies—VIII. In The Taxonomy and Comparative Physiology of the Chlorosarcinales and Certain Other Edaphic Algae; The University of Texas Publications: Austin, TX, USA, 1969. [Google Scholar]
- Darienko, T.M. Rare and new for Ukraine algae from the soils of reserves of the Mountain Crimea. Int. J. Algae 2000, 2, 86–101. [Google Scholar] [CrossRef]
- Kleinig, H.; Egger, K. Ketocarotinoidester in Acetabularia mediterranea Lam. Phytochemistry 1967, 6, 611–619. [Google Scholar] [CrossRef]
- Czeczuga, B. Characteristic carotenoids in some phytobenthos species in the coastal area of the Adriatic Sea. Acta Soc. Bot. Pol. 1986, 55, 601–609. [Google Scholar] [CrossRef]
- Havurinne, V.; Tyystjärvi, E. Photosynthetic sea slugs induce protective changes to the light reactions of the chloroplasts they steal from algae. Elife 2020, 9, e57389. [Google Scholar] [CrossRef]
- Chubchikova, I.N.; Minyuk, G.S.; Drobetskaya, I.V.; Dantsyuk, N.V. Chlorococcal microalgae as source of natural secondary carotenoids. Ekol. Morya 2009, 77, 77–83. [Google Scholar]
- Gromov, B.V.; Mamkaeva, K.A. Morphology and ultrastructure of some chlorococcal algae from the collection of algal strains in Leningrad University. I. Pseudospongiococcum protococcoides gen. nov. sp. nov. Algol. Stud. 1974, 10, 1–9. [Google Scholar]
- Kleinig, H. Carotenoids of siphonous green algae: A chemotaxonomical study. J. Phycol. 1969, 5, 281–284. [Google Scholar] [CrossRef]
- Maltseva, I.A.; Maltsev, Y.I.; Solonenko, A.N. Soil algae of the oak groves of the steppe zone of Ukraine. Int. J. Algae 2017, 19, 215–226. [Google Scholar] [CrossRef]
- Grung, M.; Metzger, P.; Liaaen-jensen, S. Primary and secondary carotenoids in two races of the green alga Botryococcus braunii. Biochem. Syst. Ecol. 1989, 17, 263–269. [Google Scholar] [CrossRef]
- Rao, A.R.; Dayananda, C.; Sarada, R.; Shamala, T.R.; Ravishankar, G.A. Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Biores. Technol. 2007, 98, 560–564. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathnarayana Gokare, R.; Ravi, S.; Bikkina, P.N.; Su, Y.; Lei, B. Botryococcus as an alternative source of carotenoids and its possible applications—An overview. Crit. Rev. Biotechnol. 2018, 38, 541–558. [Google Scholar] [CrossRef]
- Matsuura, H.; Watanabe, M.M.; Kaya, K. Echinenone production of a dark red-coloured strain of Botryococcus braunii. J. Appl. Phycol. 2012, 24, 973–977. [Google Scholar] [CrossRef]
- Metzger, P.; Largeau, C. Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 2005, 66, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Tonegawa, I.; Okada, S.; Murakami, M.; Yamaguchi, K. Pigment composition of the green microalga Botryococcus braunii Kawaguchi-1. Fish. Sci. 1998, 64, 305–308. [Google Scholar] [CrossRef]
- Senousy, H.H.; Beakes, G.W.; Hack, E. Phylogenetic placement of Botryococcus braunii (Trebouxiophyceae) and Botryococcus sudeticus isolate UTEX 2629 (Chlorophyceae). J. Phycol. 2004, 40, 412–423. [Google Scholar] [CrossRef]
- Knights, B.A.; Brown, A.C.; Conway, E.; Middleditch, B.S. Hydrocarbons from the green form of the freshwater alga Botryococcus braunii. Phytochemistry 1970, 9, 1317–1324. [Google Scholar] [CrossRef]
- Maxwell, J.R.; Douglas, A.G.; Eglinton, G.; McCormick, A. The botryococcenes—Hydrocarbons of novel structure from the alga Botryococcus braunii, Kützing. Phytochemistry 1968, 7, 2157–2171. [Google Scholar] [CrossRef]
- Metzger, P.; Villarreal-Rosales, E.; Casadevall, E.; Coute, A. Hydrocarbons, aldehydes and triacylglycerols in some strains of the arace of the green alga Botryococcus braunii. Phytochemistry 1989, 28, 2349–2353. [Google Scholar] [CrossRef]
- Metzger, P.; Templier, J.; Largeau, C.; Casadevall, E. An n-alkatriene and some n-alkadienes from the A race of the green alga Botryococcus braunii. Phytochemistry 1986, 25, 1869–1872. [Google Scholar] [CrossRef]
- Metzger, P.; Berkaloff, C.; Casadevall, E.; Coute, A. Alkadiene-and botryococcene-producing races of wild strains of Botryococcus braunii. Phytochemistry 1985, 24, 2305–2312. [Google Scholar] [CrossRef]
- Metzger, P.; Pouet, Y.; Summons, R. Chemotaxonomic evidence for the similarity between Botryococcus braunai L race and Botryococcus neglectus. Phytochemistry 1997, 44, 1071–1075. [Google Scholar] [CrossRef]
- Metzger, P.; Casadevall, E.; Coute, A. Botryococcene distribution in strains of the green alga Botryococcus braunii. Phytochemistry 1988, 27, 1383–1388. [Google Scholar] [CrossRef]
- Tsarenko, P.M.; Borysova, O.V.; Korkhovyi, V.I.; Blume, Y.B. High-efficiency Ukrainian strains of microalgae for biodiesel fuel production (Overview). Open Agric. J. 2020, 14, 209–218. [Google Scholar] [CrossRef]
- Stasiuk, L.D. Confocal laser scanning fluorescence microscopy of Botryococcus alginite from boghead oil shale, Boltysk, Ukraine: Selective preservation of various micro-algal components. Org. Geochem. 1999, 30, 1021–1026. [Google Scholar] [CrossRef]
- Chekanov, K.; Lukyanov, A.; Boussiba, S.; Aflalo, C.; Solovchenko, A. Modulation of photosynthetic activity and photoprotection in Haematococcus pluvialis cells during their conversion into haematocysts and back. Photosynth. Res. 2016, 128, 313–323. [Google Scholar] [CrossRef]
- Rearte, T.A.; Vélez, C.G.; Beligni, M.V.; Figueroa, F.L.; Gómez, P.I.; Flaig, D.; de Iorio, A.F. Biological characterization of a strain of Golenkinia (Chlorophyceae) with high oil and carotenoid content induced by increased salinity. Algal Res. 2018, 33, 218–230. [Google Scholar] [CrossRef]
- Stockenreiter, M.; Haupt, F.; Graber, A.K.; Seppälä, J.; Spilling, K.; Tamminen, T.; Stibor, H. Functional group richness: Implications of biodiversity for light use and lipid yield in microalgae. J. Phycol. 2013, 49, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the classification, nomenclature, and diversity of eukaryotes. J. Euk. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef] [PubMed]
- Kottuparambil, S.; Thankamony, R.L.; Agusti, S. Euglena as a potential natural source of value-added metabolites. A review. Algal Res. 2019, 37, 154–159. [Google Scholar] [CrossRef]
- Yao, R.; Fu, W.; Du, M.; Chen, Z.X.; Lei, A.P.; Wang, J.X. Carotenoids biosynthesis, accumulation, and applications of a model microalga Euglena gracilis. Mar. Drugs 2022, 20, 496. [Google Scholar] [CrossRef]
- Grung, M.; Liaaen-Jensen, S. Algal carotenoids 52; secondary carotenoids of algae 3; carotenoids in a natural bloom of Euglena sanguinea. Biochem. Syst. Ecol. 1993, 21, 757–763. [Google Scholar] [CrossRef]
- Gerber, S.; Häder, D.P. Effects of enhanced UV-B irradiation on the red coloured freshwater flagellate Euglena sanguinea. FEMS Microbiol. Ecol. 1944, 13, 177–184. [Google Scholar] [CrossRef]
- Czeczuga, B. Carotenoids in Euglena rubida Mainx. Comp. Biochem. Physiol. B. Comp. Biochem. 1974, 48, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Zimba, P.V.; Rowan, M.; Triemer, R. Identification of euglenoid algae that produce ichthyotoxin(s). J. Fish Dis. 2004, 27, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Zimba, P.V.; Huang, I.S.; Gutierrez, D.; Shin, W.; Bennett, M.S.; Triemer, R.E. Euglenophycin is produced in at least six species of euglenoid algae and six of seven strains of Euglena sanguinea. Harmful Algae 2017, 63, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Green, J. The occurence of astaxanthin in the euglinoid Trachelomonas volvocina. Comp. Biochem. Physiol. 1963, 9, 313–316. [Google Scholar] [CrossRef]
- Wołowski, K.; Grabowska, M. Trachelomonas species as the main component of the euglenophyte community in the Siemianówka Reservoir (Narew River, Poland). Int. J. Limnol. 2007, 43, 207–218. [Google Scholar] [CrossRef]
- Poniewozik, M.; Juráň, J. Extremely high diversity of euglenophytes in a small pond in eastern Poland. Plant Ecol. Evol. 2018, 151, 18–34. [Google Scholar] [CrossRef]
- Frassanito, R.; Flaim, G.; Mancini, I.; Guella, G. High production of unexpected carotenoids in Dinophyceae. Astaxanthin esters from the freshwater dinoflagellate Tovellia sanguinea. Biochem. Syst. Ecol. 2006, 34, 843–853. [Google Scholar] [CrossRef]
- Spitale, D.; Tardio, M.; Cantonati, M. Competition between a planktonic diatom and a dinoflagellate during enclosure experiments in a mountain lake. Phycologia 2005, 44, 320–327. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Hansen, G.; Daugbjerg, N.; Flaim, G.; D’andrea, M. Studies on woloszynskioid dinoflagellates II: On Tovellia sanguinea sp. nov., the dinoflagellate responsible for the reddening of Lake Tovel, N. Italy. Eur. J. Phycol. 2006, 41, 47–65. [Google Scholar] [CrossRef]
- Hansen, G.; Flaim, G. Dinoflagellates of the Trentino Province, Italy. J. Limnol. 2007, 66, 107–141. [Google Scholar] [CrossRef]
- Donato, M.; Vilela, M.H.; Bandarra, N.M. Fatty acids, sterols, α-tocopherol and total carotenoids composition of Diacronema vlkianum. J. Food Lipids 2003, 10, 267–276. [Google Scholar] [CrossRef]
- Durmaz, Y.A.Ş.A.R.; Donato, M.; Monteiro, M.; Gouveia, L.; Nunes, M.L.; Gama Pereira, T.; Gökpınar, Ş.; Bandarra, N.M. Effect of temperature on α-tocopherol, fatty acid profile, and pigments of Diacronema vlkianum (Haptophyceae). Aquac. Int. 2009, 17, 391–399. [Google Scholar] [CrossRef]
- Bendif, E.M.; Probert, I.; Herve, A.; Billard, C.; Goux, D.; Lelong, C.; Cadoret, J.-P.; Veron, B. Integrative taxonomy of the Pavlovophyceae (Haptophyta): A reassessment. Protist 2011, 162, 738–761. [Google Scholar] [CrossRef] [PubMed]
- Darienko, T.; Pröschold, T. Toward a monograph of non-marine Ulvophyceae using an integrative approach (Molecular phylogeny and systematics of terrestrial Ulvophyceae II). Phytotaxa 2017, 324, 1–41. [Google Scholar] [CrossRef]
- Lukešová, A. Three filamentous green algae isolated from soil. Arch. Protistenknd. 1991, 139, 69–83. [Google Scholar] [CrossRef]
- Rindi, F.; Guiry, M.D. Diversity, life history, and ecology of Trentepohlia and Printzina (Trentepohliales, Chlorophyta) in urban habitats in western Ireland. J. Phycol. 2002, 38, 39–54. [Google Scholar] [CrossRef]
- Rindi, F.; Guiry, M.D.; Critchley, A.T.; Ar Gall, E. The distribution of some species of Trentepohliaceae (Trentepohliales, Chlorophyta) in France. Cryptogam.-Algol. 2003, 24, 133–144. [Google Scholar]
- Chen, L.; Zhang, L.; Liu, T. Concurrent production of carotenoids and lipid by a filamentous microalga Trentepohlia arborum. Biores. Technol. 2016, 214, 567–573. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Zhang, W.; Liu, T. Comparative analysis of growth and carotenoid accumulation of Trentepohlia arborum in aerial, subaerial, and aquatic cultivation. J. Appl. Phycol. 2015, 27, 1079–1087. [Google Scholar] [CrossRef]
- Aburai, N.; Ohkubo, S.; Miyashita, H.; Abe, K. Composition of carotenoids and identification of aerial microalgae isolated from the surface of rocks in mountainous districts of Japan. Algal Res. 2013, 2, 237–243. [Google Scholar] [CrossRef]
- Chen, B.; Wan, C.; Mehmood, M.A.; Chang, J.S.; Bai, F.; Zhao, X. Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products—A review. Bioresour. Technol. 2017, 244, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Mikhailyuk, T.I. Terrestrial algae from the granite outcrops of river valleys of the Ukraine. Int. J. Algae 2013, 15, 311–330. [Google Scholar] [CrossRef]
- Burczyk, J.; Czygan, F.C. Ocurrence of carotenoids and sporopollenin in the cell wall of Chlorella fusca and of its mutants. Z. Pflanzenphysiol. 1983, 111, 169–174. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chekanov, K. Diversity and Distribution of Carotenogenic Algae in Europe: A Review. Mar. Drugs 2023, 21, 108. https://doi.org/10.3390/md21020108
Chekanov K. Diversity and Distribution of Carotenogenic Algae in Europe: A Review. Marine Drugs. 2023; 21(2):108. https://doi.org/10.3390/md21020108
Chicago/Turabian StyleChekanov, Konstantin. 2023. "Diversity and Distribution of Carotenogenic Algae in Europe: A Review" Marine Drugs 21, no. 2: 108. https://doi.org/10.3390/md21020108
APA StyleChekanov, K. (2023). Diversity and Distribution of Carotenogenic Algae in Europe: A Review. Marine Drugs, 21(2), 108. https://doi.org/10.3390/md21020108







